Abstract
Molecular tumor testing has transformed the treatment of patients with malignancies and is helping catalyze the development of novel therapeutic strategies. In a recent issue of The New England Journal of Medicine, Arakawa et al. describe two cases of pediatric lung cancer resulting from mother-to-infant transmission, diagnosed remote from delivery.1
Molecular testing of patient tumors has evolved over the last decade, with incorporation of comprehensive characterization of disease as a standard component of treatment, helping usher in an era of personalized cancer therapy. This evolution has permitted oncologists to make treatment recommendations based on genomic aberrations and suspected molecular drivers of disease, using novel targeted drugs and immunotherapy. Importantly, the implications of improved molecular characterization of disease in the cancer arena extends beyond therapy alone and may include genetic evidence of cancer transmission.
As the average maternal age in the United States continues to increase, the incidence of maternal cancer during pregnancy, currently estimated at 1 in 1,000, is anticipated to rise.2 There have been 18 reported cases of maternal-to-fetal transmission in the literature, prior to the publication by Arakawa et al., encompassing hematologic malignancies, skin cancer, and other solid tumors.1 Although the biologic properties of transmission remain unclear, two principal hypotheses exist: (1) transplacental hematogenous dissemination and (2) direct transmission (via contact or aspiration) at the time of delivery (Figure 1A).3 Although the first reported case of maternal transmission of malignancy was described in 1866, to date, marginal gains have been made in defining the underlying biology, which is particularly important given the implications as they relate to delayed presentation in affected children. In a recent issue of The New England Journal of Medicine, Arakawa et al. detail two cases of maternal-to-fetal transmission of cervical carcinoma, presenting as lung cancer, with the intent of highlighting the importance of tumor molecular testing in unraveling these clinical mysteries (Figure 1B).1
Figure 1. Transmission of cervical cancer from mothers to infants.
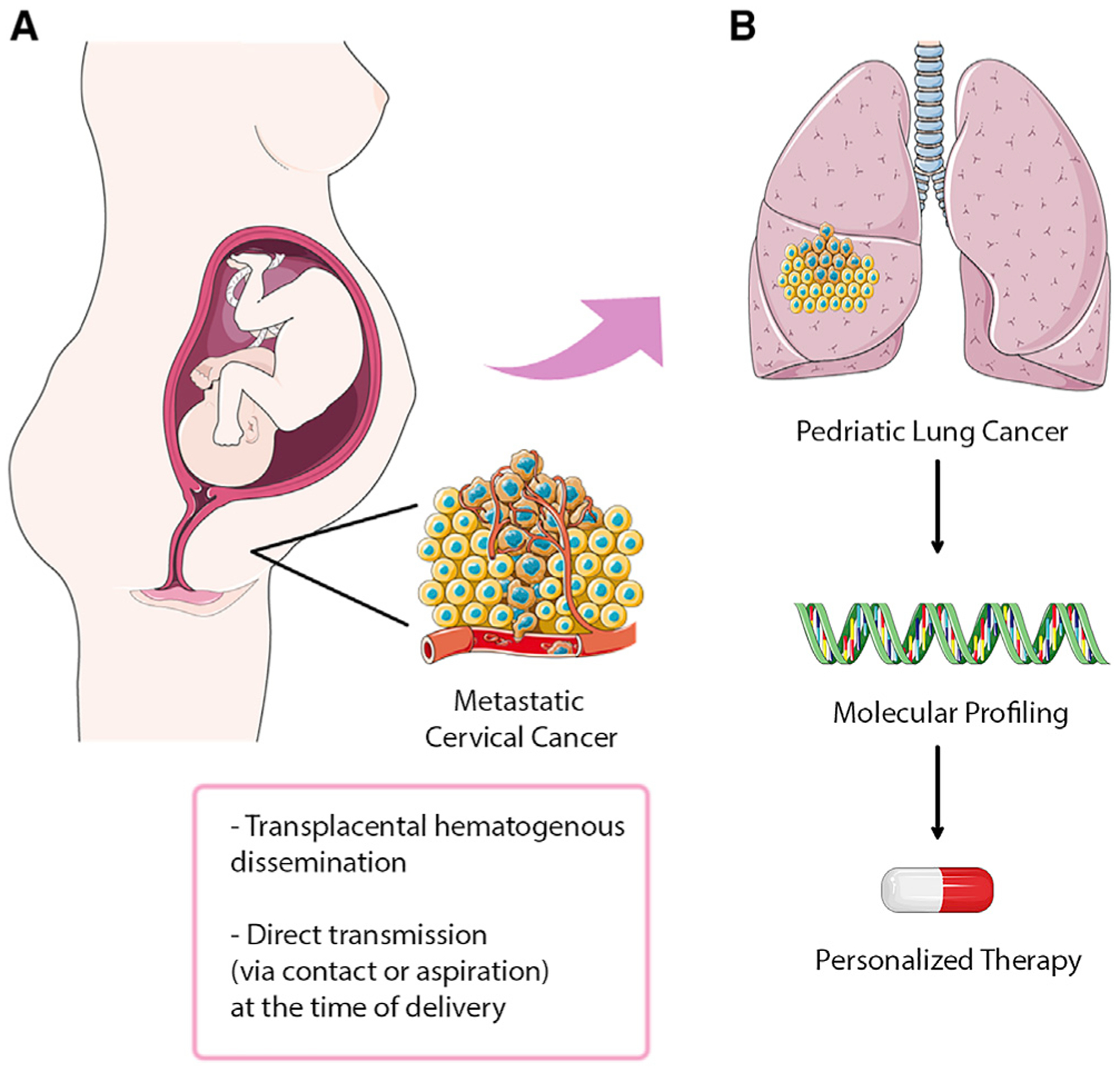
(A) Potential routes for cancer transmission by transplacental hematogenous dissemination or direct transmission at the time of delivery. (B) Next-generation sequencing of the pediatric lung tumors confirmed maternal transmission and led to personalized therapy (image credit: adapted form Servier Medical Art by Servier).
Arakawa and colleagues performed next-generation sequencing of paired samples of tumor and normal tissue to detect mutations in 114 cancer-related genes as part of an established prospective clinical trial.4 As detailed in the manuscript, both children presented remote from delivery (ages 23 months and 6 years) with pulmonary symptoms including cough and chest pain. Interestingly, the mother of patient 1 had an unremarkable appearing cervix, with negative cervical cytology seven months prior to delivery. The mother was diagnosed with cervical carcinoma three months after the infant’s birth, and ultimately succumbed from recurrent, metastatic neuroendocrine cervical cancer. The comparison of gene profiles in matched tumor and normal tissue obtained from both the mother and child confirmed that maternal transmission of tumor to the infant occurred—with identification of identical pathogenic KRAS:G13D and P53:E285K mutations. Furthermore, 47 exonic single-nucleotide polymorphism (SNP) alleles carried by the mother but not inherited in the child’s germline were identified in the tumor, and the tumor in the (male) child lacked the Y chromosome.1
Patient 2 was diagnosed with a 6 cm hilar lung mass (biopsy-proven adenocarcinoma), and his mother was known to have a polypoid cervical mass at the time of vaginal delivery, although it was presumed to be benign based on negative cytology. Following delivery, biopsy of the cervical mass confirmed a malignant adenocarcinoma, and the mother ultimately died from her disease two years after primary surgery. Analogously, comparison of gene profiles in matched tumor and normal tissue obtained from both the mother and child confirmed maternal transmission—with identical KRAS:G12D and STK11:splice site mutations and 38 exonic SNP alleles carried by the mother but not in the child’s germline—and the tumor in the child lacked the Y chromosome.
Interestingly, although both mothers died of disease progression despite multi-modal therapy, the children remain alive, without evidence of disease recurrence at the time of publication. Historically, metastatic or recurrent cervical cancer is an aggressive malignancy, with preferred systemic treatment options encompassing combination platinum, taxane, and the anti-angiogenic agent bevacizumab.5 As outlined in the report by Arakawa et al., the first patient was found to have a neuroendocrine cervical carcinoma, which is notoriously difficult to treat, although recent reports suggest benefit with immune checkpoint inhibition in particular patients with high-grade disease.6,7 Patient 1 was described as having a mixed response following cytotoxic chemotherapy and was ultimately treated with single-agent nivolumab on clinical trial with a complete response. It isunclear if this malignancy exhibited mismatch repair deficiency or was microsatellite unstable-high (MSI-high), both predictors of response to immune checkpoint inhibition. Assessment of a resected pulmonary nodule after 14 cycles of nivolumab illustrated tertiary lymphoid formation without viable tumor, and a high fraction of B cells, as well as CD4+ and CD8+ T cells. Furthermore, response was seen even in the absence of PD-1 and PD-L1 expression by immunohistochemistry, which would not be anticipated.8 Conversely, patient 2 was treated with several lines of combination systemic chemotherapy, followed by a total left pneumonectomy, and remains without evidence of disease 15 months after surgical resection.
The etiology of this “atypical” response to therapy, in the context of what can be considered metastatic cervical cancer, in the two pediatric patients described in the publication is difficult to explain. The rarity of maternal-to-fetal transmission of cancer is likely reflective of the efficacy of the placental barrier (syncytiotrophoblast cells) as well as immune-surveillance.9 As described by Arakawa et al., the tumor in patient 1 did not contain human leukocyte antigen (HLA) class I alleles, which may have rendered the cancer cells immunologically inert, leading to cell growth, propagation and metastases. However, this would have argued against a favorable response to immune checkpoint therapy, though perhaps HLA class II alleles might have been operative once checkpoint inhibitors were administered. Conversely, the presence of HLA class I alleles may have resulted in relative “tumor control” in patient 2, explaining the delayed presentation at six years of age, reflecting indolent tumor growth.
In addition to the above, the authors comment on the implications of vaginal delivery on maternal-to-fetal transmission of malignancy, stating that cesarean section should be recommended for mother with uterine cervical cancer. It is well established that women with visible cervical lesions, reflective of malignancy, should undergo cesarean section. The transmission of HPV-related disease, via genital contact at the time of delivery, is a well-established paradigm, with reports of laryngeal papillomatosis in children dating back to 1956.10 In the two reported cases published by Arakawa et al., the diagnosis of cervical cancer was not made until well after delivery, although in patient 2, the authors reported a visible cervical lesion. These findings highlight the importance of comprehensive pelvic exam at the start of pregnancy, including cytologic evaluation and high-risk HPV testing. Furthermore, in the presence of a visible lesion, biopsy should be performed, rather than cytologic evaluation, which can result in false negative results.
More broadly, the report by Arakawa and colleagues calls into question the potential number of pediatric malignancies that may be related to maternal cancers. As discussed above, patient 2 presented six years following delivery, and without incorporation of molecular testing, the “hereditary” connection would have likely remained undiscovered. The application of molecular profiling may hold great promise in improving our understanding of maternal-to-fetal transmission of malignancy, whether it be a result of direct contact during delivery or hematogenous spread.
ACKNOWLEDGMENTS
This work was supported by the National Cancer Institute (P30 CA023100) and the Joan and Irwin Jacobs Fund (philanthropic fund).
Footnotes
DECLARATION OF INTERESTS
Dr. Eskander receives research funding from Clovis Oncology, Merck, and AstraZenca, as well as consultant and/or speaker fees and/or advisory board from AstraZeneca, GSK/Tesaro, Myriad, Merck, and the GOG Foundation.
Dr. Kurzrock receives research funding from Genentech, Merck Serono, Pfizer, Boehringer Ingelheim, TopAlliance, Takeda, Incyte, Debiopharm, Medimmune, Sequenom, Foundation Medicine, Konica Minolta, Grifols, Omniseq, and Guardant, as well as consultant and/or speaker fees and/or advisory board for X-Biotech, Neomed, Pfizer, Actuate Therapeutics, Roche, Turning Point Therapeutics, TD2/Volastra, Bicara Therapeutics, Inc.; has an equity interest in IDbyDNA and CureMatch Inc; serves on the Board of CureMatch and CureMetrix; and is a co-founder of CureMatch.
References
- 1.Arakawa A, Ichikawa H, Kubo T, Motoi N, Kumamoto T, Nakajima M, Yonemori K, Noguchi E, Sunami K, Shiraishi K, et al. (2021). Vaginal Transmission of Cancer from Mothers with Cervical Cancer to Infants.N. Engl. J. Med 384, 42–50. [DOI] [PubMed] [Google Scholar]
- 2.Teksam M, McKinney A, Short J, Casey SO, and Truwit CL (2004). Intracranial metastasis via transplacental (vertical) transmission of maternal small cell lung cancer to fetus: CT and MRI findings. Acta Radiol. 45, 577–579. [DOI] [PubMed] [Google Scholar]
- 3.Herskovic E, Ryan M, Weinstein J, and Wadhwani NR (2014). Maternal to fetal transmission of cervical carcinoma. Pediatr. Radiol 44, 1035–1038. [DOI] [PubMed] [Google Scholar]
- 4.Sunami K, Ichikawa H, Kubo T, Kato M, Fujiwara Y, Shimomura A, Koyama T, Kakishima H, Kitami M, Matsushita H,et al. (2019). Feasibility and utility of a panel testing for 114 cancer-associated genes in a clinical setting: A hospital-based study. Cancer Sci. 110, 1480–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tewari KS, Sill MW, Penson RT, Huang H, Ramondetta LM, Landrum LM, Oaknin A, Reid TJ, Leitao MM, Michael HE, et al. (2017). Bevacizumab for advanced cervical cancer: final overall survival and adverse event analysis of a randomised, controlled, open-label, phase 3 trial (Gynecologic Oncology Group 240). Lancet 390, 1654–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eskander RN, Elvin J, Gay L, Ross JS, Miller VA, and Kurzrock R (2020). Unique Genomic Landscape of High-Grade Neuroendocrine Cervical Carcinoma: Implications for Rethinking Current Treatment Paradigms. JCO Precis Oncol 4, 972–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel SP, Othus M, Chae YK, Giles FJ, Hansel DE, Singh PP, Fontaine A, Shah MH, Kasi A, Baghdadi TA, et al. (2020). A Phase II Basket Trial of Dual Anti-CTLA-4 and Anti-PD-1 Blockade in Rare Tumors (DART SWOG 1609) in Patients with Nonpancreatic Neuroendocrine Tumors. Clin. Cancer Res 26, 2290–2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung HC, Ros W, Delord JP, Perets R, Italiano A, Shapira-Frommer R, Manzuk L, Piha-Paul SA, Xu L, Zeigenfuss S, et al. (2019). Efficacy and Safety of Pembrolizumab in Previously Treated Advanced Cervical Cancer: Results From the Phase II KEYNOTE-158 Study. J. Clin. Oncol 37, 1470–1478. [DOI] [PubMed] [Google Scholar]
- 9.Isoda T, Ford AM, Tomizawa D, van Delft FW, De Castro DG, Mitsuiki N, Score J, Taki T, Morio T, Takagi M, et al. (2009). Immunologically silent cancer clone transmission from mother to offspring. Proc. Natl. Acad. Sci. USA 106, 17882–17885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Syrjänen S, and Puranen M (2000). Human papillomavirus infections in children: the potential role of maternal transmission.Crit. Rev. Oral Biol. Med 11, 259–274. [DOI] [PubMed] [Google Scholar]


