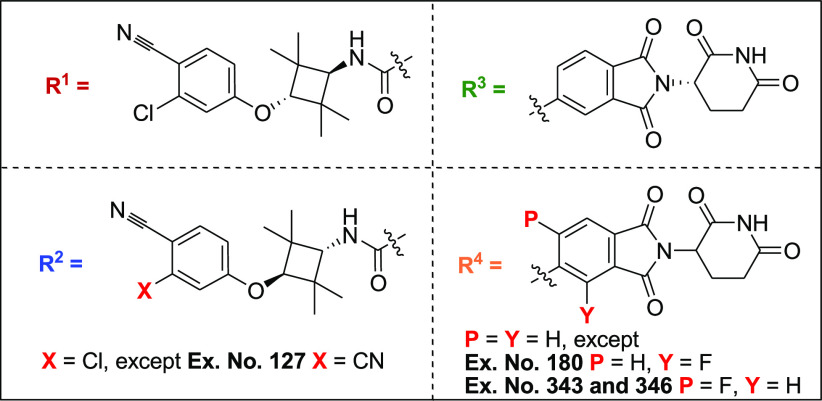
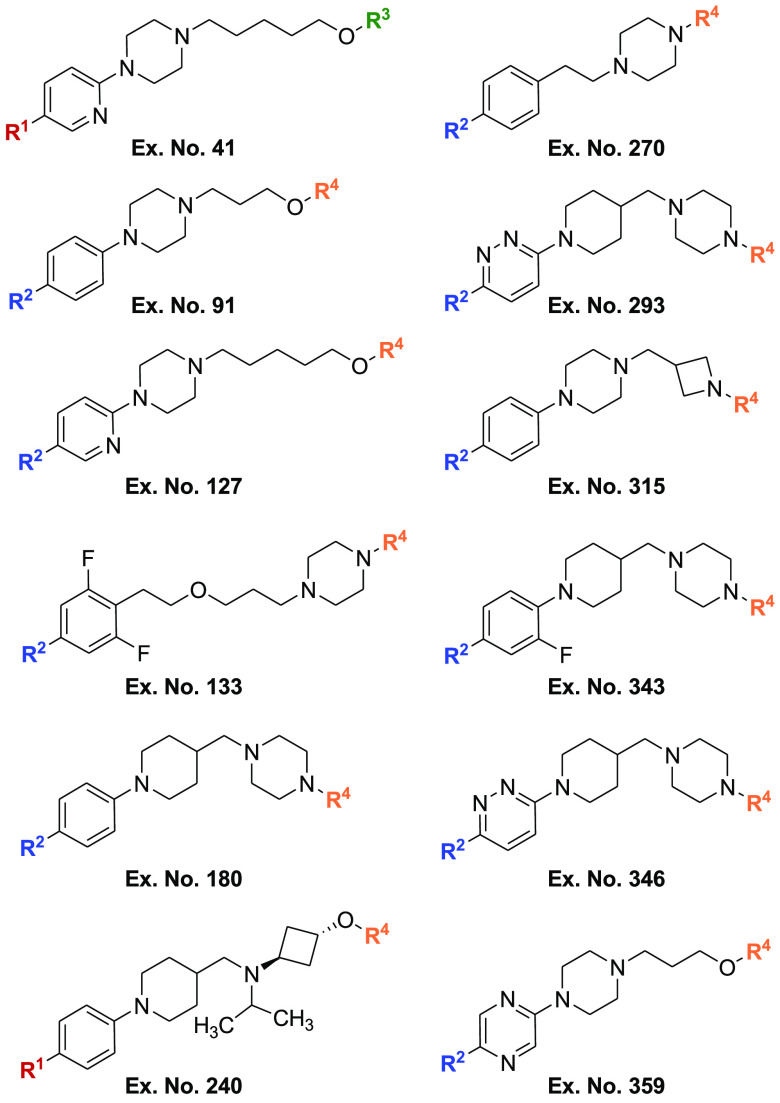
Important Compound Classes
Title
Compounds and Methods for the Targeted Degradation of Androgen Receptor
Patent Publication Number
US 2021/0009528 A1
Publication Date
January 14, 2021
Priority Application
US 16/938,864
Priority Date
July 24, 2020
Inventors
Crew, A. P.; Hornberger, K. R.; Zimmermann, K.; Wang, J.; Berlin, M.; Crews, C. M.; Dong, H.; Snyder, L. B.
Assignee Company
Arvinas Operations, Inc., New Haven, CT, United States
Disease Area
Androgen receptor
Biological Target
Prostate cancer
Summary
Prostate cancer (PCa) accounts for 26% of the most common cancers (lung and colorectal) diagnosed in men and is prevalent in the male urinary system. Although long-term declines in mortality have halted for prostate cancer, in 2021, it is estimated that 248 530 new cases of prostate cancer will be diagnosed in the United States and that 34 130 people will die from the disease (see: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2021/cancer-facts-and-figures-2021.pdf, accessed February 17, 2021). The 2021 projections are based on currently available mortality and incidence data and may not reflect the full impact of SARS-CoV-2 virus on cancer cases and deaths.
PCa is treated with curative intent through radiation therapy or surgery; however, treatments fail in 30% of patients within 10 years, which results in metastatic disease. The resistance to androgen deprivation therapy (ADT) and the non-androgen receptor (AR)-driven tumors that emerge are particularly deadly and are becoming more prevalent; this represents a major challenge in the treatment of the disease. The androgen receptor or nuclear receptor subfamily 3, group C, gene 4 (AR or NR3C4) and its endogenous ligands, androgens, play a pivotal role in the growth and development of the normal prostate gland and also in the progression of PCa. The prolonged exposure to ADT leads many patients to develop castration-resistant prostate cancer (CRPC), which is driven typically by the retained and enhanced AR signaling. Consequently, the AR is considered to be the primary target for PCa treatment, which has led to the development of novel AR-pathway-directed therapies, including abiraterone acetate, apalutamide, enzalutamide, and darolutamide.
However, evidence suggests that ADT may exert a negative effect not only on cognitive function but also on the central nervous system (CNS). In addition, AR inhibitors such as enzalutamide and apalutamide may also affect CNS function. In clinical trials of enzalutamide, abiraterone acetate, and apalutamide, patients with CRPC reported CNS-related adverse events.
CNS-related adverse events may lead to an increased morbidity, diminished treatment adherence, reduced quality of life, and decreased efficacy of cancer treatment due to dose interruptions or reductions.
The AR gene is located on the chromosome Xq and has three domains: an aminoterminal domain (NTD) containing activation functional domains; a DNA binding domain (DBD); and a carboxyl-terminal domain (CTD) which includes the ligand-binding domain (LBD). The AR protein in the absence of ligand is located in the cytoplasm and is associated with heat-shock and other chaperone proteins. The binding of AR with androgens lead to a conformational change and exposure to the nuclear localization signal (NLS).
The translocation of the androgen complex to the nucleus causes it to dimerize, bind to AREs, and facilitate modulation of gene transcription. Additionally, AR could be activated in a ligand-independent manner such as via different growth factors, phosphorylation, or following interaction with coactivators.
Understanding the mechanism of action of these compounds is crucial, and several clinical trials are currently exploring AR-targeted therapy. However, the progress of drug resistance always compromises successful treatment, and urgent solutions are needed for the problems of existing drugs.
The present Patent Highlight showcases bifunctional compounds which find utility as modulators of targeted ubiquitination. Proteolysis-targeting chimera (PROTAC) may have a VHL ligand at one end of the molecule, which binds to the VHL E3 ubiquitin ligase, and on the other end a moiety, which binds a target protein (AR) such that degradation of the target protein/polypeptide is triggered. There are over 600 E3 ubiquitin ligases in humans, which necessitate substrate specificity for ubiquitination. They are more attractive therapeutic targets than general proteasome inhibitors due to their specificity for certain protein substrates. The development of ligands of E3 ligase has proven challenging, in part due to the required disruption of protein–protein interactions. However, recent developments have provided specific ligands which bind to these ligases. Furthermore, protein–protein interactions are notoriously difficult to target using small molecules due to their large contact surfaces and the shallow grooves or flat interfaces that are accessible. It is often essential for most small molecule drugs to bind enzymes or receptors in tight and well-defined pockets. Nutlins, the first small molecule E3 ligase inhibitors, have led to additional compounds that target inhibitors of apoptosis proteins (IAPs) SCFCdc4, and SCFMet30; however, the field remains underdeveloped. The present invention exhibits a broad range of pharmacological activities associated with the degradation/inhibition of targeted polypeptides.
Key Structures
Biological Assay
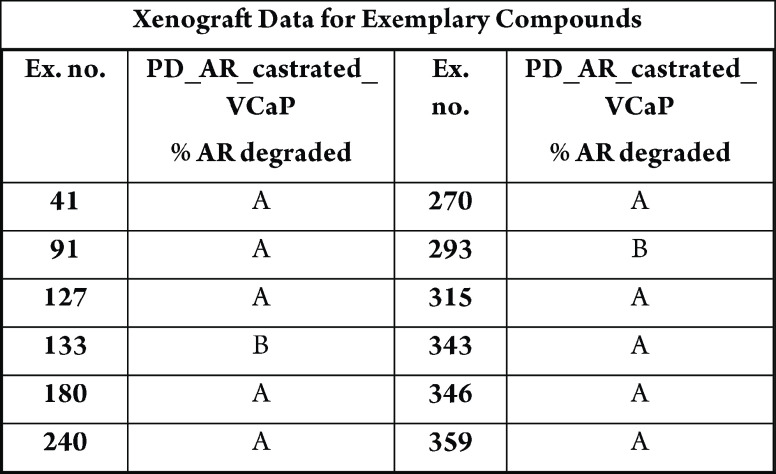
Caspase-Glo assay, CellTiter-Glo luminescent cell viability assay, PSA ELISA, and tumor growth inhibition in VCaP Xenograft Model.
Biological Data
The table below shows degradation of AR-maximum inhibition (%) AR ELISA in LNCaP and/or VCaP cells, where A > 50% and B < 50%.
Recent Review Articles
-
1.
Tian J.; Chi C.; Bian G.; Wang X.; Yu B.; Guo F.. Basic Clin. Pharmacol. Toxicol. 2021, 128, 195..
-
2.
Pisano C.; Tucci M.; Di Stefano R. F.; Turco F.; Scagliotti G. V.; Di Maio M.; Buttigliero C.. Crit. Rev. Oncol. Hematol. 2021, 103185..
-
3.
Formaggio N.; Rubin M. A.; Theurillat J.. Oncogene 2021, 40, 1205..
-
4.
Luan H.; Xu P.; Meng Y.; Li Z.; Bian J.. Bioorg. Med. Chem. 2020, 28, 115554..
-
5.
Hu X.; Chai X.; Wang X.; Pang J.; Fu W.; Duan M.; Li D.; Hou T.. Drug Discov. Today 2020, 25, 1453..
-
6.
Salvi S.; Bonafe M.; Bravaccini S.. Semin. Cancer Biol. 2020, 60, 132..
The author declares no competing financial interest.