Abstract
Introduction:
Bleeding episodes in patients who have haemophilia A (HA), a hereditary bleeding disorder caused by a deficiency in factor VIII (FVIII), are treated or prophylactically prevented with infusions of exogenous FVIII. Neutralizing antibodies, referred to as inhibitors, against infusion products are a major complication experienced by up to 30% of patients who have severe HA. Bypassing agents (BPA), a class of therapeutics given to patients who have inhibitors, bypass the need for FVIII in the coagulation cascade, and long-term inhibitor eradication is accomplished using immune tolerance induction therapy (ITI). Data examining the antibody levels in patients receiving BPA and ITI are limited.
Aim:
Measure anti-FVIII antibody levels in specimens from patients receiving ITI or BPA in order to evaluate the anti-FVIII antibody response in those patients.
Methods:
Specimens were tested using the CDC-modified Nijmegen-Bethesda assay (NBA) and the CDC fluorescence immunoassay (FLI) for anti-FVIII IgG1 and IgG4.
Results:
NBA-negative specimens from patients undergoing ITI or receiving BPAs have a higher frequency of anti-FVIII IgG4 positivity compared with the previously published level for NBA-negative HA patients. Analysis of anti-FVIII antibody levels in serial samples from patients undergoing ITI reveals that antibodies can persist even after the patient's NBA result falls into the negative range.
Conclusions:
Measurement of anti-FVIII antibodies may be a useful means to better contextualize NBA results in specimens from patients receiving BPA or ITI. In addition, assessment of anti-FVIII antibody levels has the potential to improve inhibitor surveillance and clinical decision-making related to the progress of ITI.
Keywords: factor VIII, factor VIII deficiency, haemophilia A, immunoassay, immunology, inherited blood coagulation disorders
1 ∣. INTRODUCTION
Haemophilia A (HA), an X-linked inherited bleeding disorder characterized by a defect in coagulation factor VIII (FVIII), affects roughly 25,000 people in the United States.1 Bleeding episodes in patients who have HA are commonly treated or prophylactically prevented with infusions of exogenous FVIII. A significant complication associated with FVIII infusion therapy is the development of neutralizing alloantibodies (inhibitors) against the infused product. Inhibitors interfere with the function of the infusion product and/or expedite its clearance, thereby nullifying the therapeutic effects of treatment. Patients who develop inhibitors present unique challenges to the healthcare system, including increased morbidity, the need for alternative therapies, more vigilant monitoring and increased cost of treatment, which can exceed one million U.S. dollars annually.2
The Nijmegen-Bethesda assay (NBA)3 to detect FVIII inhibitors utilizes in vitro reactions to measure the degree to which test-plasmas inhibit FVIII activity in plasma from a healthy donor, upon mixing. Techniques to directly detect anti-FVIII antibodies using fluorescence immunoassays (FLI),4-7 enzyme-linked immunosorbent assays (ELISA)8,9 and surface plasmon resonance (SPR)10,11 have been developed more recently. Direct antibody detection methods are more sensitive and less susceptible to false-positive results caused by non-specific inhibitors of coagulation12 compared with the NBA, which reports inhibition of clotting without a means to assess FVIII immunoreactivity. Data using direct antibody detection methods indicate that the presence of anti-FVIII IgG4 and IgG1 are the best indicators that a clinically relevant, functional inhibitor is present.6,8 Inhibitor testing using direct antibody detection can serve as useful means to confirm results obtained using traditional clotting methods, particularly when the results approach the positive threshold. To this end, the Centers for Disease Control and Prevention's (CDC) Division of Blood Disorders (DBD) integrated a FLI into the FVIII inhibitor testing algorithm to confirm low-positive NBA results on samples tested in the Community Counts inhibitor surveillance program, a public health surveillance program run by CDC’s DBD in collaboration with the American Thrombosis and Hemostasis Network and the United States Hemophilia Treatment Center Network.13
Strategies to treat patients who develop FVIII inhibitors include on-demand or prophylactic administration of bypassing agents (BPA) such as recombinant factor VIIa (FVIIa, NovoSeven®) or activated prothrombin complex concentrates (FEIBA®), and long-term eradication of inhibitors is accomplished using immune tolerance induction therapy (ITI) with FVIII-containing products.14 BPAs function to stop or prevent bleeding episodes in patients who have HA and inhibitors by bypassing the requirement for FVIII in the coagulation cascade, while ITI utilizes frequent high-dose FVIII infusions to accomplish the goal of tolerizing the patient's immune system to FVIII. Inhibitor status in patients receiving ITI and/or BPAs is monitored by evaluating functional outputs such as FVIII infusion kinetics and FVIII inhibitor titres, typically without regard for anti-factor VIII antibody levels. Direct measurement of the antibodies responsible for FVIII inhibition may be a useful supplement to traditional assessments of ITI progress because it provides a more objective readout of the status of the immune response and due to the potential for inhibitor results obtained using clot-based testing methods to be compromised by BPAs or high levels of on-board FVIII used in ITI. Conversely, the clinical significance of antibodies that persist beyond inhibitor eradication is unknown and there are currently limited data in the literature describing anti-FVIII antibody profiles in HA patients receiving ITI and/or BPAs or in patients who have eradicated or transient inhibitors. The goal of the current study is to examine results obtained using the NBA and FLI for FVIII inhibitors in specimens from patients treated with ITI or BPAs in order to better contextualize inhibitor results in patients treated with those therapies and to improve FVIII inhibitor surveillance.
2 ∣. MATERIALS AND METHODS
2.1 ∣. Subjects
Specimens included in the study were from patients enrolled in Community Counts surveillance13 who have HA and indicated ITI or a BPA without ITI (NovoSeven® or FEIBA®) as their current treatment for one or more specimens at the time of draw. Emicizumab was not used as a criterion for inclusion as a BPA due to its ability to interfere with the NBA, but some serial specimens came from patients taking emicizumab as indicated. Participants were not required to give informed consent for surveillance specimens.
2.2 ∣. Nijmegen-Bethesda and chromogenic Bethesda assays
The CDC-modified NBA was performed as previously described.15 The chromogenic Bethesda assay was performed by an identical method while using a bovine-derived FVIII assay (Siemens Factor VIII Chromogenic Assay, Siemens, Marburg, Germany). NBA and CBA results ≥0.5 NBU were considered positive.
2.3 ∣. Fluorescence immunoassay
The anti-FVIII FLI was performed as previously described.6 Briefly, plasma was spun at 20,000xg for 4 minutes, diluted 1:30 in phosphate-buffered saline containing 1% dried milk (PBSM) and incubated with FVIII-conjugated SeroMap beads (Luminex Corporation, Austin, TX, USA). Anti-FVIII antibodies were detected by incubating beads with biotin-conjugated anti-human IgG1 or IgG4 followed by streptavidin-conjugated R-phycoerythrin on a Bio-Plex 200 suspension array system (Bio-Rad Laboratories, Hercules, CA, USA), and results were reported as median fluorescence intensity (MFI). Positivity thresholds are recalculated as necessary to accommodate reagent lot changes and were set at 2 standard deviations above the mean MFIs obtained from testing specimens from ≥50 healthy donors.6 Over the course of data collection for the current study, positive thresholds varied from 14.6 to 23.7 and 8.3 to 12.5 MFI for FVIII IgG1 and IgG4, respectively. FLI results are reported as positive or negative relative to the positivity threshold in use at the time of the test and those for serial samples are meant to demonstrate trends rather than strict quantitation because the results were collected over time and results can vary between test runs. The beads used in the current study were conjugated to Kogenate (Bayer Healthcare, Tarrytown, NY, USA) and may not detect antibodies specific for epitopes unique to other FVIII treatment products.
A group of results previously reported for the FLI on specimens from patients enrolled in the Hemophilia Inhibitor Research Study16 were divided by history of inhibitor and positive or negative NBA results to produce a group with both negative NBA and negative history who had not undergone either ITI or BPA treatment for comparison with the study groups. This group had a FLI positivity rate of 6 of 281 (2.1%) for IgG4 and 64 of 281 (22.8%) for IgG1.
2.4 ∣. Statistics
Fisher's exact test was used to evaluate differences in categorical data.
3 ∣. RESULTS AND DISCUSSION
Specimens from HA patients who indicated ITI or BPAs as their current therapy were tested by the NBA and for anti-FVIII IgG1 and IgG4 in order to characterize the correlation between results obtained using a functional inhibitor assay and those obtained using direct antibody detection in the context of ITI or BPA usage without ITI (Table 1 and Figure 1). As expected, most specimens from 31 patients who indicated ITI as their current therapy and NBA were also positive by FLI for anti-FVIII IgG1 (83.9%) or IgG4 (96.8%). Examination of NBA-negative specimens from 45 patients who indicated current ITI revealed that 22.2% and 46.7% of specimens were positive for anti-FVIII IgG1 or IgG4, respectively. Similarly, most of the NBA-positive specimens from 57 non-ITI patients who indicated BPA usage at the time of specimen collection were positive by FLI for anti-FVIII IgG1 (89.5%) or IgG4 (93.0%), and analysis of 30 NBA-negative specimens from patients using BPAs revealed that anti-FVIII IgG1 or IgG4 was present in 26.7% and 50.0% of specimens, respectively (Table 1 and Figure 1). These data were compared with the subset of NBA-negative specimens from patients with negative history of inhibitor in our previous study, in which anti-FVIII IgG4 was present in 6 (2.1%) of 281 NBA-negative specimens (p < 0.0001)6 from patients enrolled in the Hemophilia Inhibitor Research Study,16 demonstrating that NBA-negative specimens from patients who are undergoing ITI or using BPAs are positive for anti-FVIII IgG4 at much higher frequencies than NBA-negative specimens from those who have never had an inhibitor. Although this observation is not unexpected, these data highlight the fact that all negative NBA results are not necessarily equivalent and raise the question: what is the relationship between antibody positivity and NBA results over time in patients who have persistent antibodies despite negative NBA results?
TABLE 1.
Summary of fluorescence immunoassay (FLI) results for anti-FVIII IgG1 and IgG4 on specimens from subjects who indicated current treatment type as Immune Tolerance Induction (ITI) or bypassing agent (BPA) without ITI.
| % Positive for Anti-Factor VIII by FLI |
||||||||
|---|---|---|---|---|---|---|---|---|
| Current Treatment Type ITI |
Current Treatment Type BPA |
|||||||
| n | IgG1 | IgG4 | IgG1 and 4 | n | IgG1 | IgG4 | IgG1 and 4 | |
| All | 76 | 47.4 | 67.1 | 44.7 | 87 | 67.8 | 78.1 | 60.9 |
| NBA-positive | 31 | 83.9 | 96.8 | 80.6 | 57 | 89.5 | 93.0 | 82.5 |
| NBA-negative | 45 | 22.2 | 46.7 | 20.0 | 30 | 26.7 | 50.0 | 20.0 |
FIGURE 1.
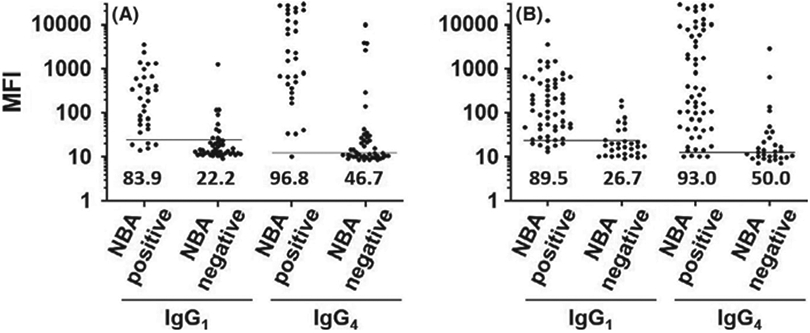
Levels of anti-Factor VIII (FVIII) antibodies in specimens from patients who have haemophilia A and were receiving Immune Tolerance Induction (ITI) or bypassing agents (BPA) at the time of specimen collection. Scatter plots show median fluorescence intensities for anti-FVIII IgG1 or IgG4 in specimens that were positive or negative by the Nijmegen-Bethesda Assay in subjects who indicated that their current treatment type was ITI (A) or bypassing agent (B). Per cent positives are listed above the x-axis. Horizontal lines indicate assay positivity thresholds.
Anti-FVIII antibody levels were assessed in serial specimens from 35 of the ITI subjects shown in Figure 1 and Table 1. The FLI results for serial specimens (Table 2) were concordant with the results of a functional assay for each serial specimen in 13 of the subjects (Patients 1–13), including specimens from 5 subjects (Patients 1–5) whose NBA/CBA status changed during the course of sample collection. Notably, 22 of the 35 subjects (Table 2; Subjects 14–35) had detectable anti-FVIII IgG1 and/or IgG4 in specimens that tested negative by NBA/CBA. In addition, 10 of those 22 subjects (Patients 26–35) transitioned from NBA/CBA negative to positive while maintaining a positive FLI for anti-FVIII IgG4 and/or IgG1, indicating that the positive FLI may be a signal that a patient's inhibitor had not been eradicated or that the patient may be at risk for inhibitor re-emergence. Next, an examination of serial specimens from 44 of the BPA subjects shown in Figure 1 and Table 1 was conducted to assess antibody levels in patients who have a history of an inhibitor, but who were not undergoing ITI (Table 3). FLI results were concordant with NBA results for each serial specimen in 20 subjects (Patients 1–20), including 3 patients whose NBA status changed over the course of the study, while 24 subjects (Patients 21–44) had conflicting NBA and FLI results in at least one serial sample.
TABLE 2.
Fluorescence Immunoassay and Nijmegen-Bethesda assay (NBA) results on specimens from subjects who indicated current treatment type as Immune Tolerance Induction (ITI) on one or more specimens. Positive results are in bold.
| Patient | Severity | Peak Titre | Age (yrs.) | ITI at Draw | BPA at draw | NBU | IgG1 | IgG4 |
|---|---|---|---|---|---|---|---|---|
| 1 | severe | 11.5 | 1.4 | Y | Y | 7.5 | 409 | 73 |
| 2.4 | Y | Y | 0 | 15 | 10.5 | |||
| 3.4 | Y | N | 0 | 15 | 7.5 | |||
| 2 | severe | 32 | 12.9 | Y | Y | 1.6 | 117 | 17.5 |
| 14.1 | Y | Y | 0.2 | 12 | 9.5 | |||
| 15.1 | Y | N | 0.1 | 11.5 | 12 | |||
| 15.7 | N | Y | 9.0* | 55 | 23 | |||
| 16.1 | N | Y | 1.0* | 7 | 12.8 | |||
| 3 | ND | 6.5 | 4.9 | Y | Y | 2.9 | 325.5 | 13.5 |
| 5.7 | Y | Y | 0 | 11 | 10.5 | |||
| 7.0 | Y | N | 0 | 11 | 9 | |||
| 4 | severe | 131.8 | 4.6 | Y | Y | 6.9 | 52.5 | 1913 |
| 5.7 | Y | Y | 1.1 | 41.5 | 90.8 | |||
| 6.7 | Y | Y | 0.4 | 19 | 10.5 | |||
| 7.7 | Y | Y | 0.1 | 9.5 | 9.5 | |||
| 5 | severe | 66 | 17.6 | Y | Y | 0.9 | 149.8 | 29.5 |
| 18.8 | Y | Y | 1 | 13 | 164.5 | |||
| 19.9 | Y | N | 1.5 | 215.5 | 690.5 | |||
| 21.0 | Y | N | 0.2 | 11.5 | 10.5 | |||
| 6 | severe | 86 | 10.2 | Y | Y | 2.6 | 8.5 | 338 |
| 12.2 | Y | Y | 30 | 359 | 8183 | |||
| 13.0 | Y | Y | 23.2 | 53 | 1134 | |||
| 7 | severe | 583 | 48.3 | Y | Y | 1.1 | 202.8 | 179 |
| 50.6 | Y | Y | 8.8 | 19 | 11219.5 | |||
| 8 | severe | 394.2 | 17.2 | Y | Y | 95.7 | 266.5 | 15884.8 |
| 18.2 | Y | Y | 4.8 | 44.5 | 7842 | |||
| 19.1 | Y | Y | 3.4 | 14 | 2487 | |||
| 20.0 | Y | Y | 57.8 | 193.5 | 22838 | |||
| 20.3 | N | Y | 110.3* | 276.5 | 23985 | |||
| 9 | severe | ND | 1.7 | N | N | 251.3 | 291.5 | 21365.5 |
| 1.8 | N | N | 181.1 | 744 | 24812 | |||
| 2.4 | Y | Y | 98.9* | 108.5 | 27476 | |||
| 2.8 | Y | Y | 231.4* | 193 | 26583 | |||
| 10 | severe | 4.9 | 6.4 | Y | Y | 0 | 18.5 | 9.5 |
| 7.5 | Y | Y | 0.1 | 13.5 | 11.5 | |||
| 8.6 | Y | N | 0 | 9 | 8.5 | |||
| 11 | severe | 7.6 | 2.7 | N | Y | 0.2 | 15.5 | 10.5 |
| 3.9 | N | Y | 0 | 16.5 | 12.5 | |||
| 5.1 | Y | N | 0 | 12 | 11 | |||
| 12 | severe | 0.6 | 1.4 | Y | Y | 0 | 11.5 | 9 |
| 2.4 | Y | Y | 0 | 9.5 | 9.5 | |||
| 3.4 | Y | Y | 0 | 11 | 8.5 | |||
| 4.6 | Y | N | 0.1 | 11.5 | 9.5 | |||
| 5.7 | Y | N | 0.2 | 9.5 | 12.5 | |||
| 13 | severe | 8.6 | 4.8 | Y | N | 0.1 | 11 | 8.5 |
| 5.5 | Y | N | 0.2 | 12.5 | 9.5 | |||
| 14 | moderate | 2.6 | 6.7 | Y | N | 0.1 | 12.5 | 18.5 |
| 8.3 | Y | Y | 0 | 15.5 | 23.5 | |||
| 15 | severe | 35.2 | 8.9 | N | N | 0.1 | 31.5 | 20.5 |
| 9.9 | Y | N | 0 | 42.5 | 32.5 | |||
| 10.7 | N | N | 0.2 | 11.8 | 77.5 | |||
| 11.0 | N | N | 0 | 10 | 11.3 | |||
| 11.9 | N | Y | 0.1* | 12 | 313 | |||
| 16 | severe | 2.4 | 2.1 | Y | Y | 0 | 39 | 43 |
| 2.6 | N | N | 0 | 11 | 26.5 | |||
| 3.1 | Y | N | 0.1 | 12 | 9.5 | |||
| 3.5 | Y | N | 0 | 9 | 10 | |||
| 17 | severe | 1.0 | 1.8 | Y | Y | 0.4 | 21.5 | 41 |
| 2.9 | Y | Y | 0.1 | 21 | 20.5 | |||
| 3.8 | Y | N | 0.1 | 10 | 11.5 | |||
| 4.7 | N | N | 0.1 | 7.8 | 198.8 | |||
| 18 | severe | 4 | 1.3 | Y | Y | 0.5 | 100 | 117 |
| 2.5 | Y | Y | 0.1 | 13 | 75.5 | |||
| 3.7 | Y | N | 0.1 | 13 | 291 | |||
| 19 | severe | 27.2 | 3.8 | Y | Y | 8 | 40.5 | 13548 |
| 4.7 | Y | Y | 0 | 26 | 4131 | |||
| 4.9 | N | N | 0 | 21.5 | 5059 | |||
| 6.0 | Y | Y | 0 | 24 | 3600 | |||
| 7.1 | Y | Y | 0 | 23.5 | 3801 | |||
| 20 | severe | 1.4 | 6.9 | N | N | 0.6 | 11 | 254.8 |
| 8.3 | Y | Y | 1.1 | 20 | 375.5 | |||
| 9.1 | Y | Y | 0.5 | 11.8 | 54.5 | |||
| 9.9 | Y | N | 0.1 | 13.5 | 139 | |||
| 21 | severe | 436.8 | 7.5 | Y | Y | 0.3 | 31.5 | 53.5 |
| 10.0 | Y | N | 0 | 14.5 | 15.5 | |||
| 22 | severe | 2 | 72.1 | Y | Y | 0.7 | 75.3 | 97.5 |
| 74.1 | Y | Y | 0.3 | 90 | 28.5 | |||
| 23 | severe | 7.4 | 2.3 | Y | Y | 0.3 | 13 | 198.5 |
| 3.0 | Y | Y | 0.1 | 22 | 64 | |||
| 4.2 | Y | N | 0.1 | 27 | 2645.8 | |||
| 24 | severe | 323 | 2.3 | Y | Y | 0.3 | 17 | 606.5 |
| 3.4 | Y | Y | 0 | 15 | 204 | |||
| 5.0 | N | N | 0.1 | 13.5 | 30.5 | |||
| 6.7 | Y | N | 0 | 12.5 | 10.5 | |||
| 25 | severe | 16.2 | 5.3 | Y | Y | 0.1 | 27 | 23.5 |
| 6.4 | Y | N | 0.2 | 8.5 | 12 | |||
| 26 | severe | ND | 1.4 | Y | N | 0.1 | 1255 | 3915.5 |
| 1.6 | Y | N | 1.3 | 227 | 297 | |||
| 27 | severe | 250 | 4.9 | Y | Y | 0.7 | 82.5 | 10213.5 |
| 6.6 | Y | Y | 7.9 | 65.5 | 12826.5 | |||
| 7.6 | Y | Y | 0.3 | 70 | 6895.5 | |||
| 8.6 | Y | Y | 0.4 | 115 | 9956.5 | |||
| 9.6 | N | N | 0.2 | 10 | 4588.5 | |||
| 10.6 | N | Y | 0.8* | 13.3 | 3696.3 | |||
| 28 | severe | 160 | 1.9 | N | N | 5.3 | 19 | 1176.5 |
| 4.1 | Y | Y | 0.1 | 56 | 35.5 | |||
| 5.0 | Y | N | 0.3 | 12.5 | 31.5 | |||
| 6.1 | N | Y | 11.2* | 11.5 | 6256.5 | |||
| 29 | severe | 18 | 19.6 | Y | N | 0.1 | 314 | 8276 |
| 20.8 | Y | Y | 1.5 | 1317.5 | 7299.5 | |||
| 22.2 | N | Y | 34.5* | 258 | 1341 | |||
| 30 | severe | 8 | 1.4 | Y | Y | 0.2 | 58.5 | 70 |
| 3.7 | N | N | 1.1 | 69 | 666.5 | |||
| 4.7 | Y | Y | 3.6 | 51 | 3328.5 | |||
| 31 | moderate | unknown | 3.8 | N | N | 0 | 8.5 | 11 |
| 5.8 | N | N | 0 | 27.5 | 10 | |||
| 6.5 | Y | Y | 0.6 | 143 | 503.5 | |||
| 7.0 | Y | Y | 0.2 | 33.5 | 34.5 | |||
| 7.6 | N | Y | 0.2 | 13.5 | 19 | |||
| 32 | severe | 12124 | 8.0 | Y | Y | 21.5 | 10.5 | 3453 |
| 9.2 | Y | Y | 15.3 | 19 | 5695 | |||
| 10.7 | Y | Y | 1.4 | 12 | 469 | |||
| 11.8 | Y | N | 0.2 | 19 | 17.5 | |||
| 12.8 | Y | N | 0.7 | 17.5 | 270 | |||
| 33 | severe | 11.5 | 2.1 | Y | Y | 0 | 87 | 43.5 |
| 3.4 | Y | Y | 0.3 | 134.5 | 17 | |||
| 4.7 | Y | N | 2.3 | 425.5 | 41 | |||
| 5.2 | N | N | 1.1 | 32.5 | 33 | |||
| 6.0 | N | Y | 7.8* | 137 | 120.5 | |||
| 34 | moderate | 16 | 10.7 | Y | Y | 1.9 | 23.5 | 20 |
| 11.3 | Y | Y | 0.4 | 20 | 77 | |||
| 12.4 | Y | Y | 1.8 | 38 | 9251 | |||
| 13.5 | Y | N | 0.5 | 15.5 | 451 | |||
| 14.5 | N | N | 9.9 | 16.5 | 8412.5 | |||
| 35 | severe | 832 | 21.8 | Y | Y | 34.4 | 21.5 | 5134.5 |
| 23.4 | Y | Y | 0.1 | 10 | 16.5 | |||
| 24.5 | Y | Y | 0.1 | 12.5 | 15 | |||
| 26.2 | Y | N | 0 | 10.5 | 12.5 | |||
| 26.6 | N | Y | 0.6* | 16.5 | 137 |
Abbreviation: ND, Data not collected
Result is in Chromogenic Bethesda Units and was obtained using Chromogenic Bethesda assay due to the presence of emicizumab in the patient sample.
TABLE 3.
Fluorescence Immunoassay and Nijmegen-Bethesda assay (NBA) results on specimens from subjects who indicated the use of a bypassing agent as current treatment type on one or more specimens. Positive results are in bold.
| Patient | Severity | Peak Titre | Age | Hist ITI on IVF | BP agent | NBU | IgG1 | IgG4 |
|---|---|---|---|---|---|---|---|---|
| 1 | severe | 54.4 | 1.9 | Yes | NovoSeven | 30.2 | 22 | 24181.5 |
| 3.8 | NovoSeven | 0.5 | 54.5 | 1739.5 | ||||
| 5.7 | NovoSeven | 2.3* | 18.0 | 959.5 | ||||
| 2 | severe | 200.0 | 17.1 | Yes | FEIBA | 6.2 | 67.5 | 149.5 |
| 18.7 | FEIBA/NovoSeven | 0.7 | 28.0 | 10.5 | ||||
| 18.8 | FEIBA/NovoSeven | 0.5 | 25.5 | 10.0 | ||||
| 19.8 | NovoSeven | 1.1* | 78.0 | 15.5 | ||||
| 3 | mild | 41.0 | 58.4 | No | FEIBA | 1 | 42.5 | 12.8 |
| 61.5 | FEIBA | 0.3 | 20.0 | 11.5 | ||||
| 62.4 | FEIBA | 0.5 | 21.0 | 12.8 | ||||
| 4 | severe | 1.0 | 60.7 | No | FEIBA | 0.6 | 45.0 | 24.5 |
| 61.6 | FEIBA | 0.8 | ND | ND | ||||
| 63.7 | FEIBA | 0.5 | 27.5 | 43.5 | ||||
| 64.9 | 2.0* | 13.5 | 139.5 | |||||
| 5 | severe | 30.0 | 25.7 | Yes | 1.2 | 10.0 | 15.0 | |
| 26.7 | 0.9 | 16.0 | 40.5 | |||||
| 27.9 | 0.8 | 27.5 | 31.3 | |||||
| 28.9 | NovoSeven | 0.5 | 24.0 | 40.0 | ||||
| 29.9 | NovoSeven | 0.4 | 8.5 | 7.5 | ||||
| 6 | severe | 128.0 | 40.9 | Yes | FEIBA | 0.9 | 30.0 | 10.8 |
| 41.9 | FEIBA | 0.6 | 21.8 | 11.0 | ||||
| 43.0 | 0.8 | 39.5 | 15.3 | |||||
| 44.0 | FEIBA | 0.6 | 56.5 | 13.5 | ||||
| 45.0 | NovoSeven | 10.9* | 117.0 | 9113.5 | ||||
| 7 | severe | 282.2 | 9.8 | yes | FEIBA | 0.7 | 23.0 | 103.0 |
| 10.8 | 0.7* | 47.0 | 186.5 | |||||
| 8 | severe | 2174.0 | 36.0 | No | 1.3 | 25.8 | 219.5 | |
| 37.1 | 0.9 | 13.0 | 65.8 | |||||
| 38.3 | 1 | 28.0 | 131.0 | |||||
| 39.4 | NovoSeven | 0.7 | 20.0 | 401.5 | ||||
| 9 | mild | 3.0 | 45.2 | No | 1.4 | 72.0 | 12.0 | |
| 45.6 | NovoSeven | 0.9 | 159.5 | 14.0 | ||||
| 10 | severe | 317.0 | 17.7 | Yes | FEIBA | 1.5 | 88.5 | 40.0 |
| 18.8 | FEIBA | 0.8 | 22.0 | 25.8 | ||||
| 19.9 | 1.5 | 13.3 | 88.3 | |||||
| 21.4 | FEIBA/NovoSeven | 0.9 | 43.0 | 106.0 | ||||
| 11 | severe | 65.0 | 23.0 | No | 0.9 | 25.8 | 91.3 | |
| 24.1 | 1.5 | 24.5 | 246.3 | |||||
| 25.0 | FEIBA/NovoSeven | 1 | 16.0 | 97.8 | ||||
| 12 | severe | ND | 23.7 | Yes | 0.9 | 44.8 | 28.3 | |
| 24.7 | 1.8 | 41.5 | 44.5 | |||||
| 25.8 | 4.7 | 13.0 | 418.0 | |||||
| 26.7 | FEIBA | 1.2 | 90.5 | 68.8 | ||||
| 27.8 | FEIBA | 7.1 | 20.0 | 672.0 | ||||
| 13 | severe | ND | 1.7 | ND | 2.9 | 15.0 | 749.0 | |
| 2.4 | FEIBA | 1.3 | 259.5 | 137.0 | ||||
| 3.2 | 2.4 | 8.0 | 714.5 | |||||
| 14 | moderate | 9.6 | 49.2 | ND | 2.9 | 46.5 | 8.5 | |
| 50.7 | 1.5 | 324.5 | 11.0 | |||||
| 51.7 | FEIBA | 1.5 | 186.5 | 10.0 | ||||
| 15 | severe | 7.2 | 10.6 | No | 0.7 | 104.8 | 11.0 | |
| 12.1 | 0.2 | 10.5 | 9.5 | |||||
| 12.9 | 0 | 8.0 | 9.0 | |||||
| 13.9 | FEIBA | 0 | 22.5 | 10.5 | ||||
| 14.6 | FEIBA | 0.1 | 8.5 | 6.0 | ||||
| 16 | moderate | 0.7 | 25.3 | No | 0.2 | 9 | 8.5 | |
| 28.5 | NovoSeven | 0.2 | 10.0 | 7.0 | ||||
| 17 | severe | ND | 27.1 | No | 0 | 9.5 | 8.5 | |
| 28.3 | FEIBA | 0 | 10.5 | 7.0 | ||||
| 18 | severe | 1.5 | 10.3 | No | FEIBA | 0 | 23.5 | 9.0 |
| 11.2 | 0 | 18.5 | 10.0 | |||||
| 12.0 | FEIBA | 0 | 11.5 | 8.5 | ||||
| 12.8 | FEIBA | 0 | 10.0 | 10.0 | ||||
| 13.7 | 0* | 9.5 | 7.0 | |||||
| 19 | severe | ND | 39.9 | No | FEIBA | 0.1 | 8.0 | 7.5 |
| 41.0 | FEIBA | 0 | 10.5 | 8.0 | ||||
| 42.0 | FEIBA | 0.1 | 7.5 | 8.0 | ||||
| 20 | mild | ND | 73.1 | No | 0.1 | 10.5 | 11.0 | |
| 74.7 | FEIBA | 0.2 | 10.0 | 10.5 | ||||
| 21 | severe | 560.0 | 18.6 | Yes | FEIBA | 0.3 | 28.5 | 11.5 |
| 19.8 | FEIBA | 0.3 | 59.0 | 13.0 | ||||
| 22 | severe | ND | 28.0 | Unknown | FEIBA | 0.7 | 18.0 | 33.5 |
| 29.1 | FEIBA | 1 | 13.0 | 14.5 | ||||
| 30.0 | 0.2* | 10.0 | 45.5 | |||||
| 23 | mild | 10.2 | 68.1 | No | 1.3 | 16.0 | 321.0 | |
| 69.2 | 1 | 107.8 | 60.5 | |||||
| 70.2 | 0.6 | 43.8 | 33.8 | |||||
| 71.6 | NovoSeven | 0.5 | 77.0 | 44.0 | ||||
| 72.6 | NovoSeven | 0.4 | 59.0 | 32.0 | ||||
| 24 | severe | 15.0 | 13.0 | No | 0.4 | 9.0 | 121.5 | |
| 14.5 | 2.1 | 9.0 | 704.5 | |||||
| 15.9 | FEIBA | 0.5 | 47.0 | 373.5 | ||||
| 16.7 | FEIBA | 0 | 11.0 | 50.5 | ||||
| 25 | moderate | ND | 18.2 | ND | FEIBA | 0.6 | 167.8 | 105.5 |
| 21.3 | FEIBA | 0.3 | 11.0 | 19.5 | ||||
| 26 | mild | 4.2 | 25.2 | No | 0.8 | 41.5 | 8.5 | |
| 26.4 | 0.7 | 70.0 | 12.3 | |||||
| 27.7 | NovoSeven | 0.8 | 20.0 | 10.5 | ||||
| 27 | severe | 5.5 | 38.7 | Yes | 3.3 | 33.0 | 1688.5 | |
| 39.6 | FEIBA | 1.3 | 211.0 | 230.5 | ||||
| 40.6 | FEIBA | 0 | 38.5 | 155.0 | ||||
| 28 | moderate | 10.0 | 15.7 | ND | 0.3 | 7.5 | 69.5 | |
| 16.7 | 1.9 | 29.5 | 85.3 | |||||
| 17.5 | 1.7 | 43.8 | 281.0 | |||||
| 18.7 | FEIBA | 0.3 | 16.5 | 36.5 | ||||
| 19.6 | FEIBA | 0.2 | 9 | 28 | ||||
| 29 | mild | 10.0 | 64.0 | No | 0.2 | 47.5 | 9.5 | |
| 65.0 | 0.2 | 67.0 | 10.0 | |||||
| 66.1 | 0.1 | 44.0 | 10.5 | |||||
| 67.1 | NovoSeven | 0 | 20.0 | 10.0 | ||||
| 68.3 | 0 | 7.5 | 8.5 | |||||
| 30 | severe | 658.0 | 2.8 | Yes | 40.4 | 68.5 | 15848.5 | |
| 3.8 | FEIBA | 0.1 | 17.0 | 15.5 | ||||
| 31 | severe | 256.0 | 34.6 | Yes | NovoSeven | 0.3 | 77.0 | 16.5 |
| 35.4 | NovoSeven | 0.3 | 12.5 | 11.5 | ||||
| 32 | moderate | 64.0 | 27.3 | No | 0.2 | 56.5 | 8.5 | |
| 29.5 | NovoSeven | 0 | 10.5 | 9.3 | ||||
| 30.5 | NovoSeven | 0 | 10.0 | 9.0 | ||||
| 33 | mild | 5.0 | 9.2 | Yes | 0.8 | 307.3 | 10.0 | |
| 10.3 | 0.4 | 255.5 | 10.5 | |||||
| 12.9 | NovoSeven | 0.4 | 42.0 | 12.5 | ||||
| 34 | moderate | ND | 45.7 | No | 0.2 | 31.5 | 17.0 | |
| 48.0 | NovoSeven | 0 | 24.0 | 18.5 | ||||
| 49.8 | NovoSeven | 0 | 7.0 | 12.0 | ||||
| 35 | severe | 102.0 | 19.2 | Yes | 69 | 65.0 | 24462.5 | |
| 20.5 | NovoSeven | 0 | 40.5 | 2872.0 | ||||
| 36 | severe | 10.8 | 3.3 | Yes | 0 | 14.5 | 19.0 | |
| 4.7 | 0.1 | 6.5 | 9.0 | |||||
| 5.7 | 0.3 | 7.5 | 7.0 | |||||
| 6.7 | 0.1 | |||||||
| 7.7 | NovoSeven | 0.1 | 12.0 | 9.0 | ||||
| 37 | mild | ND | 52.4 | Unknown | 0.3 | 364.5 | 11.0 | |
| 53.7 | NovoSeven | 0.1 | 187.5 | 9.5 | ||||
| 38 | moderate | 4.5 | 69.9 | No | 2.1 | 15.5 | 566.5 | |
| 72.4 | FEIBA | 0.2 | 138.5 | 655.0 | ||||
| 73.4 | FEIBA | 0.2 | 23.5 | 15.5 | ||||
| 39 | severe | 14.4 | 1.7 | Yes | 0.4 | 9.5 | 30.5 | |
| 5.1 | NovoSeven | 0.1 | 15.5 | 13.5 | ||||
| 40 | moderate | 9.5 | 20.4 | Unknown | NovoSeven | 0.4 | 14.0 | 14.0 |
| 21.3 | NovoSeven | 0.1 | ND | ND | ||||
| 41 | severe | ND | 60.9 | No | FEIBA | 0.3 | 8.0 | 46.0 |
| 63.6 | FEIBA | 0.1 | 10.0 | 135.0 | ||||
| 42 | severe | 604.0 | 8.1 | Yes | FEIBA | 3.2 | 38.0 | 110.5 |
| 8.8 | FEIBA/NovoSeven | 1.8 | 173.5 | 45.3 | ||||
| 10.0 | 1.3 | 86.0 | 73.5 | |||||
| 10.9 | FEIBA | 0.4 | 63.0 | 27.0 | ||||
| 43 | severe | 234.1 | 19.5 | Yes | FEIBA | 0 | 10.0 | 53.0 |
| 21.8 | FEIBA | 0.1 | 9.0 | 10.0 | ||||
| 44 | severe | 29.2 | 18.7 | No | 3.4 | 15.0 | 3311.5 | |
| 20.1 | 1.1 | 21.5 | 88.0 | |||||
| 22.2 | NovoSeven | 0 | 20.0 | 109.5 | ||||
| 23.7 | NovoSeven | 0.2 | 8.0 | 97.5 |
Abbreviations: IVF, Initial Visit Form; ND, Data not collected.
Result is in Chromogenic Bethesda Units and was obtained using the Chromogenic Bethesda assay due to the presence of emicizumab in the patient sample.
The mechanisms by which ITI facilitates inhibitor eradication are not well defined. Hypotheses to define those mechanisms focus on 3 main areas, memory B-cell inhibition, T-cell function and the development anti-idiotypic antibodies.17 First, memory B cells, long-lived immune cells that can be rapidly reactivated into antibody-producing plasma cells upon re-exposure to antigen, specific for FVIII have been shown to be absent or present at low levels in patients who have undergone successful ITI,18 which is an observation that supports similar findings in an earlier study using a murine model of HA showing that high doses of FVIII can inhibit memory B-cell differentiation into antibody secreting plasma cells.19 The second area of hypotheses aimed at describing the underlying mechanisms responsible for successful ITI focuses on T-cell function. CD4+ T cells, a subgroup of lymphocytes that participate in the immune response by directing the function of other immune cells, are thought to become functionally inactive or undergo activation induced cell death (reviewed in Schep et al.17) when they are subjected to repeated interactions with an antigen in the absence of costimulatory signals.20 Notably, there is evidence indicating that functional inactivation of T cells in this manner can lead to the generation of regulatory T-cell (Treg) precursors.21 Production of Tregs, which help to maintain tolerance to self-antigens, with specificity to FVIII is thought to contribute to the success of ITI through mediation of T-cell and B-cell functions.17,22 The third potential mechanism hypothesized to play a role in the success of ITI involves the development of anti-idiotype antibodies. The unique structure of an antibody's variable region allows for antibody/antigen interactions and defines the antibody idiotype. Given their unique structure, antibody variable regions can themselves elicit an immune response leading to the generation of antibodies that bind to the variable region of other antibodies. In the case of a FVIII inhibitor, there is the potential for the development of anti-idiotype antibodies that bind to the variable region of the inhibitor, thereby preventing it from binding to and imposing inhibition on FVIII. In addition, anti-idiotype antibodies have the potential to initiate B-cell suppression or apoptosis by crosslinking receptors on B cells responsible for inhibitor production.17
The clinical significance of anti-FVIII IgG4 in patients who have an inhibitor history and a negative NBA result is unclear. Potential explanations for the observation that antibodies can persist beyond eradication or spontaneous resolution of a clinically relevant inhibitor include the possibility that the antibodies detected are subclinical, non-neutralizing and/or low-affinity. Additionally, it is possible that false-negative NBA results can explain the NBA-FLI discrepancies observed in the current study, and on-board BPAs and the frequent high doses of FVIII used for ITI may compromise the NBA results. A previous study in our laboratory showed that FVIII:C in plasma spiked with a conventional FVIII infusion product was inactivated by the heat treatment utilized by the CDC NBA at levels up to 125 u/dL,23 but it is unclear whether the frequent high doses of infused FVIII received by ITI patients would be affected in the same manner. Similar studies for BPAs have not been done. We have recently shown, however, that IgG4 positivity was 28.8% among previously positive patients with current negative chromogenic Bethesda results when they were receiving emicizumab and no other FVIII or BPA product (Miller et al., submitted), suggesting that this phenomenon is not an artefact of the NBA.
The current criteria to define success of ITI in HA rely on the results of FVIII pharmacokinetics and functional FVIII inhibitor testing.24 The data from the current study indicate that patients who have received ITI and achieved a negative functional inhibitor result may still harbour anti-FVIII antibodies and likely a reservoir of B cells capable of anti-FVIII antibody production. Given the complex immunological mechanisms underlying the production and eradication FVIII inhibitors and the amount of time and resources invested in the therapy, it may be beneficial to include a direct measurement of anti-FVIII IgG4 as a parameter to periodically assess the progress of ITI for HA. Further studies are required to elucidate the underlying factors responsible for dictating whether antibodies that persist beyond “eradication” have a role in inhibitor re-emergence.
ACKNOWLEDGEMENTS
This work was supported by the CDC Foundation through grants from Pfizer Pharmaceuticals and Baxter Healthcare. The authors would like to thank their partners at Hemophilia Treatment Centers Network and the American Thrombosis and Hemostasis Network and the patients in the Community Counts registry. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Funding information
CDC Foundation
Footnotes
CONFLICTS OF INTEREST
All authors agree with the manuscript's content and its submission to Haemophilia. The authors declare no conflicts of interest.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Soucie JM, Miller CH, Dupervil B, Le B, Buckner TW. Occurrence rates of haemophilia among males in the United States based on surveillance conducted in specialized haemophilia treatment centres. Haemophilia. 2020;26(3):487–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guh S, Grosse SD, McAlister S, Kessler CM, Soucie JM. Healthcare expenditures for males with haemophilia and employer-sponsored insurance in the United States, 2008. Haemophilia. 2012;18(2):268–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verbruggen B, Novakova I, Wessels H, Boezeman J, van den Berg M, Mauser-Bunschoten E. The Nijmegen modification of the Bethesda assay for factor VIII: C inhibitors: improved specificity and reliability. Thromb Haemost. 1995;73(2):247–251. [PubMed] [Google Scholar]
- 4.Lavigne-Lissalde G, Tarrade C, Lapalud P, et al. Simultaneous detection and epitope mapping of anti-factor VIII antibodies. Thromb Haemost. 2008;99(6):1090–1096. [DOI] [PubMed] [Google Scholar]
- 5.Krudysz-Amblo J, Parhami-Seren B, Butenas S, et al. Quantitation of anti-factorVIIIantibodiesinhumanplasma.Blood.2009;113(11):2587–2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boylan B, Rice AS, Dunn AL, et al. Characterization of the anti-factor VIII immunoglobulin profile in patients with hemophilia A by use of a fluorescence-based immunoassay. J Thromb Haemost. 2015;13(1):47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zakarija A, Harris S, Rademaker AW, et al. Alloantibodies to factor VIII in haemophilia. Haemophilia. 2011;17(4):636–640. [DOI] [PubMed] [Google Scholar]
- 8.Whelan SF, Hofbauer CJ, Horling FM, et al. Distinct characteristics of antibody responses against factor VIII in healthy individuals and in different cohorts of hemophilia A patients. Blood. 2013;121(6):1039–1048. [DOI] [PubMed] [Google Scholar]
- 9.Gilles JG, Arnout J, Vermylen J, Saint-Remy JM. Anti-factor VIII antibodies of hemophiliac patients are frequently directed towards nonfunctional determinants and do not exhibit isotypic restriction. Blood. 1993;82(8):2452–2461. [PubMed] [Google Scholar]
- 10.Lewis KB, Hughes RJ, Epstein MS, et al. Phenotypes of allo- and autoimmune antibody responses to FVIII characterized by surface plasmon resonance. PLoS One. 2013;8(5):e61120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nguyen PC, Lewis KB, Ettinger RA, et al. High-resolution mapping of epitopes on the C2 domain of factor VIII by analysis of point mutants using surface plasmon resonance. Blood. 2014;123(17):2732–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rampersad AG, Boylan B, Miller CH, Shapiro A. Distinguishing lupus anticoagulants from factor VIII inhibitors in haemophilic and non-haemophilic patients. Haemophilia. 2018;24(5):807–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manco-Johnson MJ, Byams VR, Recht M, et al. Community counts: Evolution of a national surveillance system for bleeding disorders. Am J Hematol. 2018;93(6):E137–E140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leissinger CA. Prevention of bleeds in hemophilia patients with inhibitors: emerging data and clinical direction. Am J Hematol. 2004;77(2):187–193. [DOI] [PubMed] [Google Scholar]
- 15.Miller CH, Platt SJ, Rice AS, Kelly F, Soucie JM. Validation of Nijmegen-Bethesda assay modifications to allow inhibitor measurement during replacement therapy and facilitate inhibitor surveillance. J Thromb Haemost. 2012;10(6):1055–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soucie JM, Miller CH, Kelly FM, et al. A study of prospective surveillance for inhibitors among persons with haemophilia in the United States. Haemophilia. 2014;20(2):230–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schep SJ, Schutgens REG, Fischer K, Boes ML. Review of immune tolerance induction in hemophilia A. Blood Rev. 2018;32(4):326–338. [DOI] [PubMed] [Google Scholar]
- 18.van Helden PM, Kaijen PH, Fijnvandraat K, van den Berg HM, Voorberg J. Factor VIII-specific memory B cells in patients with hemophilia A. J Thromb Haemost. 2007;5(11):2306–2308. [DOI] [PubMed] [Google Scholar]
- 19.Hausl C, Ahmad RU, Sasgary M, et al. High-dose factor VIII inhibits factor VIII-specific memory B cells in hemophilia A with factor VIII inhibitors. Blood. 2005;106(10):3415–3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen TC, Cobbold SP, Fairchild PJ, Waldmann H. Generation of anergic and regulatory T cells following prolonged exposure to a harmless antigen. J Immunol. 2004;172(10):5900–5907. [DOI] [PubMed] [Google Scholar]
- 21.Kalekar LA, Schmiel SE, Nandiwada SL, et al. CD4(+) T cell anergy prevents autoimmunity and generates regulatory T cell precursors. Nat Immunol. 2016;17(3):304–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133(5):775–787. [DOI] [PubMed] [Google Scholar]
- 23.Payne AB, Ellingsen D, Driggers J, Bean CJ, Miller CH. Evaluation of pre-analytic heat treatment protocol used in the CDC Nijmegen-Bethesda assay for heat inactivation of extended half-life haemophilia treatment products. Haemophilia. 2020;26(1):e28–e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dimichele DM, Hoots WK, Pipe SW, Rivard GE, Santagostino E. International workshop on immune tolerance induction: consensus recommendations. Haemophilia. 2007;13(Suppl 1):1–22. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


