Abstract
Rett Syndrome (RTT) is a genetic disorder that is caused by mutations in the x-linked gene coding for methyl-CpG-biding-protein 2 (MECP2) and that mainly affects females. Male and female transgenic mouse models of RTT have been studied extensively, and we have learned a great deal regarding RTT neuropathology and how MeCP2 deficiency may be influencing brain function and maturation. In this manuscript we review what is known concerning structural and coinciding functional and behavioral deficits in RTT and in mouse models of MeCP2 deficiency. We also introduce our own corroborating data regarding behavioral phenotype and morphological alterations in volume of the cortex and striatum and the density of neurons, aberrations in experience-dependent plasticity within the barrel cortex and the impact of MeCP2 loss on glial structure. We conclude that regional structural changes in genetic models of RTT show great similarity to the alterations in brain structure of patients with RTT. These region-specific modifications often coincide with phenotype onset and contribute to larger issues of circuit connectivity, progression, and severity. Although the alterations seen in mouse models of RTT appear to be primarily due to cell-autonomous effects, there are also non-cell autonomous mechanisms including those caused by MeCP2-deficient glia that negatively impact healthy neuronal function. Collectively, this body of work has provided a solid foundation on which to continue to build our understanding of the role of MeCP2 on neuronal and glial structure and function, its greater impact on neural development, and potential new therapeutic avenues.
Keywords: Rett; MeCP2; Behavior; Developmental disorder; Molecular biology; Neuropathology; Morphometry; Plasticity; Barrel field; Glutamate receptors, review
1. Introduction
Rett syndrome (RTT) is an x-linked neurodevelopmental disorder primarily affecting females that is caused by mutations in the gene encoding for methyl-CpG binding protein 2 (MeCP2). Patients with RTT first experience a developmental regression, followed by the onset of various motor stereotypies, autonomic dysfunction and cognitive dysfunction (Anderson, Wong, Jacoby, Downs, & Leonard, 2014; Berger-Sweeney, 2011; Chahrour & Zoghbi, 2007; Neul et al., 2010). Recent work in genetic rodent models of RTT has yielded information as to how these neuropathological and behavioral changes evolve over development. In Mecp2-heterozygous and especially Mecp2-null mice, the effects of MeCP2 loss on structure and function within all of these areas is similar to what has been observed in patients with RTT. Most prominently, Mecp2-null male mice in both Bird and Jaenisch models show decreases in brain size and in volume of various grey matter regions (hippocampus, cortex, striatum, and cerebellum) and in white matter tracts including the anterior commissure, fornix and fimbria (Belichenko, Belichenko, Li, Mobley, & Francke, 2008). And as others have elegantly reviewed, dendritic complexity and spine density is
In this manuscript, we review regional structural changes and their relationship to functional and behavioral alterations observed in mouse models of MeCP2 deficiency. We also include new data from our group on structural alterations in the MeCP2 deficient mouse model that corroborate those published by others while also providing insights into the temporal progression of region-specific neuropathology. Possible hypotheses that explain these alterations in developmental trajectories along with appropriate human correlates are discussed. In general, structural abnormalities in neurons emerge at a time in development that coincides with an active period of synaptogenesis and maturation in the specific brain regions where they reside (Huttenlocher, 1999; Paolicelli et al., 2011b; Tagawa, Kanold, Majdan, & Shatz, 2005; Tau & Peterson, 2010).
We also review what is known about structural and functional deficits in glia and their impact on neuronal structure and function. Understanding how structural changes come about (either through cell autonomous or non-cell autonomous mechanisms) and how these modifications impact functionality of various regions and circuits and ultimately behavioral phenotype is paramount for designing effective treatments for patients with RTT.
2. Emergence of structural and morphological changes postnatally coincides with MeCP2 expression
2.1. Age dependent structural changes in the MeCP2-deficient brain
MeCP2 expression is observed in fetal development and the timing of expression suggests that it follows the time course of neuronal differentiation and maturation. In mice, MeCP2 expression is first observed at embryonic day (E) 10–12 in the spinal cord and brainstem and in the marginal zone and subplate of the cerebral cortex (Shahbazian, Antalffy, Armstrong, & Zoghbi, 2002). MeCP2 expression is observed in thalamus, striatum, hippocampus and hypothalamus around E14-E16 (Shahbazian et al., 2002). By the early postnatal period MeCP2 is expressed in all cortical layers while in the granule cell layer of the cerebellum MeCP2 expression is delayed until the third postnatal week (Mullaney, Johnston, & Blue, 2004; Shahbazian et al., 2002) once granule cells have migrated from the external granule cell layer and synaptic contacts are being established. The fact that the onset of MeCP2 expression in cerebellar granule cells does not start until these neurons begin to form synapses fits with the notion that MeCP2 influences the formation and maintenance of synaptic connections. In fact, there is ample evidence for synaptic deficits in RTT. Dendritic arborization and spine density are diminished throughout the brain in girls with RTT with greater reductions observed in the prefrontal cortex (Subramaniam, Naidu, & Reiss, 1997). In Mecp2tm1.1Bird-null and Mecp2tm1Jae null mouse models alterations in cortical thickness, in striatal and cortical volume and in neuronal spine density are also reported (Belichenko et al., 2008; Belichenko et al., 2009; Fukuda, Itoh, Ichikawa, Washiyama, & Goto, 2005; Wood & Shepherd, 2010; Stuss, Boyd, Levin, & Delaney, 2012) (Table 1, Fig. 1). Others have reported reductions in spine density in Mecp2tm1.1Jae-heterozygous mice from 3 months onward (Rietveld, Stuss, McPhee, & Delaney, 2015). Such synaptic and dendritic modifications likely lead to the consistent neuropathological and MRI findings of smaller brain volumes in RTT (Murakami, Courchesne, Haas, Press, & Yeung-Courchesne, 1992; Reiss et al., 1993).
Table 1.
Timeline of neuropathology seen in various regions within the MeCP2-deficient brain along with behavioral and human correlates to these neuropathological findings. BFC, barrel field cortex; M1/S1, primary motor and somatosensory cortex; V1, primary visual cortex; HC, hippocampus; STR, striatum; SNpc, substantia nigra pars compacta; AMY, amygdala; Bird, Mecp2tm1.1Bird; Jae, Mecp2-tm1.1Jaenish; 308, Mecp2308.
| Region | Structure | Model | Geno | Age in weeks | Neuropathological results | Behavioral implications/correlates | Human correlates |
|---|---|---|---|---|---|---|---|
| Cortex | M1/S1 | Jae | null (−/y) | 2 | Decreases in pyramidal cell presence (Stuss et al., 2012) | n/a | Unknown |
| M1/S1 | Bird | null (−/y) | 2 | Increased NMDA receptor density compared to WT (Blue et al., 2011) | n/a | Similar in patients (Blue, Naidu, & Johnston, 1999 | |
| BFC | Bird | null (−/y) | 2 | Decreased size/area/volume, dendritic complexity; greater inhibitory tone, increased GABA receptor efficiency (Lee et al., 2017; Moroto et al., 2013) | n/a | n/a | |
| BFC | Bird | het (−/+) | 2 | Size/area/volume decreased (Belichenko et al., 2009) | n/a | n/a | |
| M1/S1 | Bird | null (−/y) | 3 | cortical thinning, neuronal density increases and spine density decreases in both Layer 2/3 and 5 (Fukuda et al., 2005) | Impaired motor coordination, somatosensation | Decreased cortical volume (Carter et al., 2008) | |
| M1/S1 | Bird | null (−/y) | 11 | Decreased NMDA receptor density (compared to WT) (Blue et al., 2011) | n/a | Similar in patients (Blue et al., 1999) | |
| M1/S1 | Jae | null (−/y) | 11 | Spine density decreases in Layer 5 (Stuss et al., 2012) | n/a | Unknown | |
| M1/S1 | Jae | het (−/+) | 12 | Spine density decreases; Decreased soma size, nucleus size, branching complexity and dendritic length in Mecp2 negative cells (Rietveld et al., 2015) | n/a | Unknown | |
| VC | Bird | null (−/y) | 4 to 5 | Accelerated maturation/premature closing of critical window; NMDA receptor maturation is faster, increased GABA in PV+ interneurons (Krishnan et al., 2015) | Impaired visual acuity | Decreased acuity with age (von Tetzchner et al., 1996) | |
| PFC | Jae | null | 5 to 7 | Decreased spine density, hypoconnective, decreased excitatory post-synaptic currents, increase in NR2B subunit expression (delayed NMDA receptor maturation) (Krishnan et al., 2015; Sceniak et al., 2016) | n/a | Unknown | |
| PFC | 308 | 308/y | n/a | Over-pruning of cortical-LA synapses, decreased FOS expression; no AMPA reorganization that is normally seen in development (Howell et al., 2017) | n/a | Unknown | |
| Hippocampus | HC | Bird | null (−/y) | 3 | Decreases in dendritic complexity and spine density, impaired LTP and LTD only after phenotype onset, impaired synaptic scaling (Fukuda et al., 2005; Stuss et al., 2012; Wood & Shepherd, 2010; Moretti et al., 2006; Gogliotti et al., 2016) | Contextual fear conditioning is impaired | Unknown |
| HC | 308 | 308/y | 4 to 6 | No change in dendritic structure (Moretti et al., 2006) | Spatial memory impaired at 20 weeks of age | Unknown | |
| HC | Bird | null (−/y) | 4 to 8 | Increase in NR2B subunit protein and decrease in NR2A subunit; AMPA/NMDA receptor ration does not increase after LTP as it does in WT. Increase in Glu1A (AMPA subunit) in experimentally naïve slice culture; decreases in metabotropic glutamate receptors mGluR5 and mGluR7 (Asaka et al., 2006; Li et al., 2016, Gogliotti et al., 2016, 2017) | Impaired contextual learning | Unknown | |
| Basal Ganglia | STR | Bird | null (−/y) | 4 | Decrease in DA and TH, increase in D2 receptors (Wong et al., 2018) | Decreased locomotion, altered gait, impaired rotarod performance | Increase in D2 receptors in young patients (Chiron et al., 1993; Wenk et al., 1997) |
| SNpc | Bird | null (−/y) | 5 | Decrease in TH, no decrease in DA (Panayotis et al., 2011) | Hyperlocomotion, impaired motor coordination | DAT decreased in ST in patients (Wong et al., 1998) | |
| STR | Bird | null (−/y) | 7 to 10 | Decrease in D2 receptors (Wong et al., 2018) | Parkinsonian-like features | Low but normal D2 levels in older patients (Naidu et al., 2001) | |
| STR | Bird | het (−/+) | 52 | Decrease in DA, changes in membrane properties in Mecp2-cells (Gantz et al., 2011) | Motor dysfunction present well before at 3 months | Unknown |
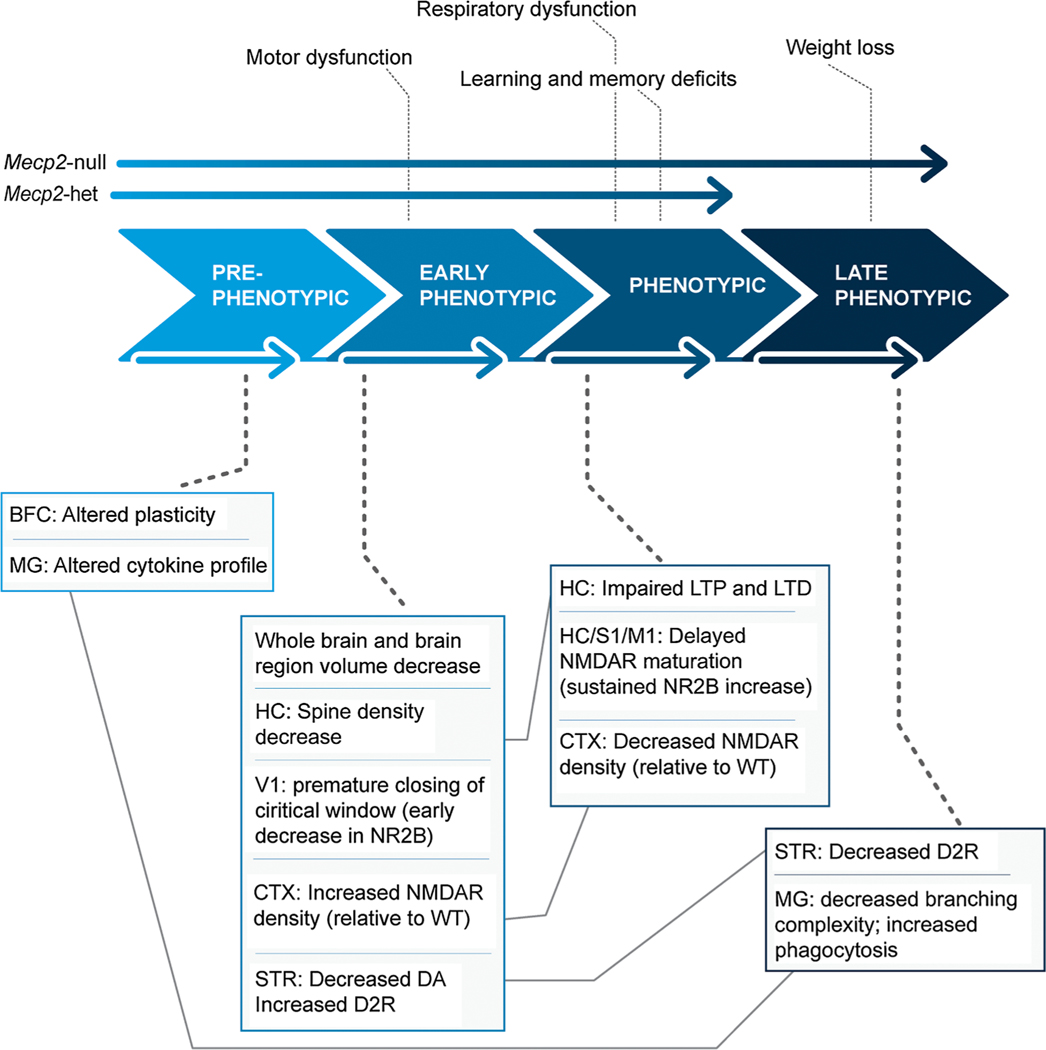
Fig. 1.
Schematic depiction of dynamic structural and functional neuropathology and coinciding behavioral phenotype of MeCP2-deficient mice. In vivo features are listed above the large phase arrows and the ex vivo features (postmortem and in vitro) below them. Only Mecp2-null mice experience significant weight loss. Abbreviations: BFC = barrel field cortex, MG = microglia; HC = hippocampus, V1 = primary visual cortex, CTX = cerebral cortex, STR = striatum, S1 = primary somatosensory cortex, M1 = primary motor cortex NR2B = NMDA2B receptors, DA = dopamine, D2R = Dopamine D2 receptors, LTP = long term potentiation, LTD = long term depression.
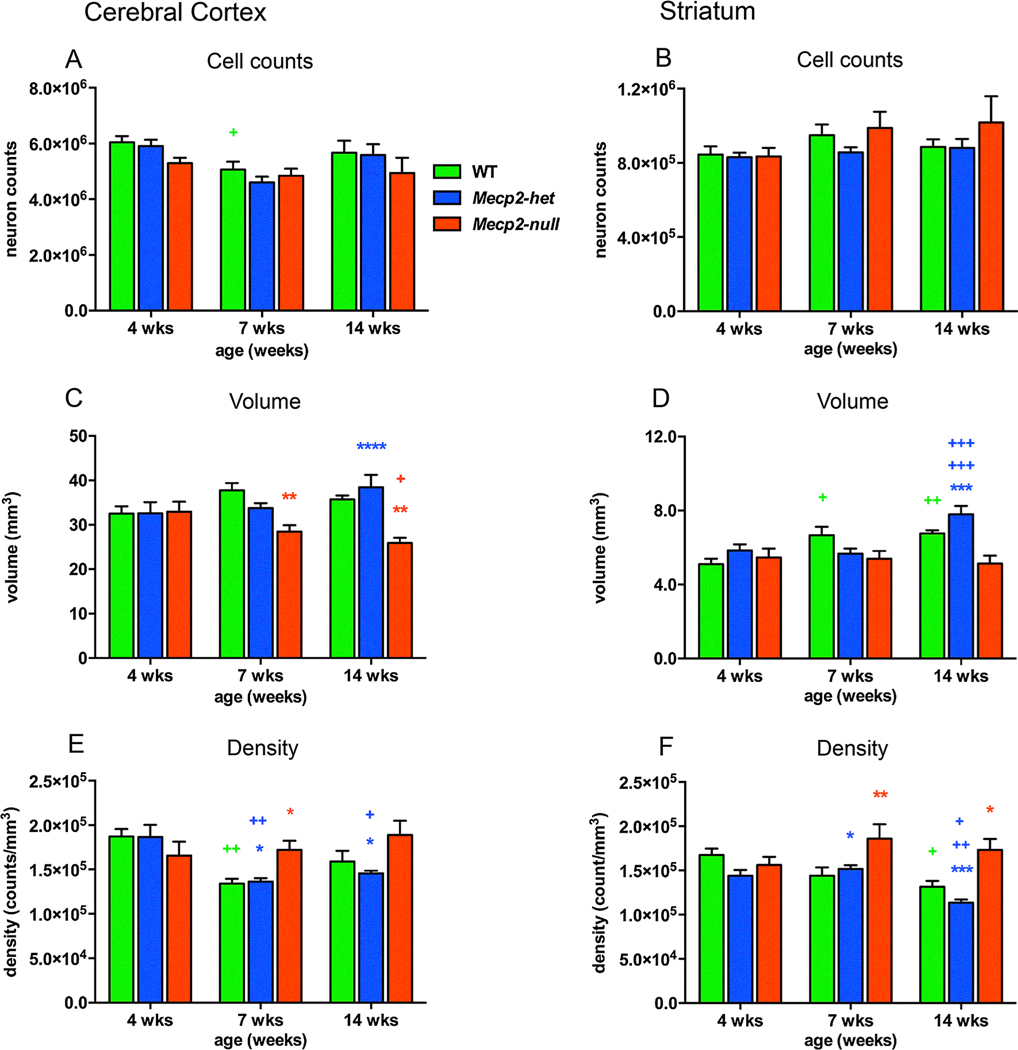
To better describe the relationship between MeCP2 expression and neuronal changes we performed our own quantitative study to determine the impact of MeCP2 insufficiency on morphologic development in cerebral cortex and striatum in the Mecp2tm1.1Bird model. We used unbiased stereological methods to count the number of neurons and glial cells, measure the volume and calculate density in Mecp2-null, Mecp2-heterozygous and WT mice that were 4, 7 and 14 weeks of age (methods included in supplement). As has been reported previously, we did not observed any genotypic differences in neuron number in cortex (Fig. 2A) and striatum, (Fig. 2B). However, developmental trajectories for volume growth did vary by genotype. In WT and Mecp2-heterozygous mice, volume either remained steady (cortex) or increased with age (striatum), while volume tended to decrease with age in Mecp2-null mice so that at 7 and 14 weeks of age, cross-sectional volume of the cortex in Mecp2-null mice was significantly smaller than WT and Mecp2-heterozygous mice (Fig. 2C) and striatal volume was significantly smaller than Mecp2-heterozygous mice at 14 weeks of age (Fig. 2D). As a result of the age dependent changes in volume, the density of neurons was increased in Mecp2-null mice compared to WT and to Mecp2-heterozygous mice at 7 weeks and 14 weeks of age (Supplemental Table 2, Fig. 2E, F). The present results of decreased volume and increased neuronal density in Mecp2-null mice are similar to previous findings in this and other mouse models of MeCP2 deficiency, although there are timeframe and regional differences (Allemang-Grand et al., 2017; Belichenko et al., 2008; Stearns et al., 2007; Ward, Kolodny, Nag, & Berger-Sweeney, 2009). Taken together these findings implicate MeCP2 deficiency in the failure for proper dendritic and axonal outgrowth of neurons that leads to decreases in the amount of neuropil over time.
Fig. 2.
The cerebral cortex and striatum of Mecp2-null mice exhibit reductions in cross sectional volume and concomitant increases in neuronal density compared to WT and Mecp2-heterozygous mice. Age differences are depicted with green+ for WT mice, blue+ for Mecp2-het mice and red+ for Mecp2-null mice. Similarly, genotype differences are red* for WT versus Mecp2-null mice and blue* for Mecp2-heterozygous (Mecp2-het) versus Mecp2-null mice. The numbers of symbols refer to p values (+ or *p < 0.05, ++ or **p < 0.01, +++ or ***p < 0.001, ++++ or ****p < 0.0001). No genotype differences were observed in the number of neurons in the cerebral cortex (A) or striatum (B) across development although the number of cortical neurons dropped by 16% in WT mice from 4 to 7 weeks of age (+). (C) Cortical volume decreased from 4 to 14 weeks of age (+) in Mecp2-null mice and was significantly lower than WT mice at 7 and 14 weeks of age (**for both ages) and Mecp2-het mice at 14 weeks of age (****). (D) Volume of the striatum increased with age in WT mice from 4 to 7 weeks ( + ) and from 4 to 14 weeks (++) and from 4 to 14 and 7–14 in Mecp2-het mice (+++ for both) but not in Mecp2-null mice. As a result, striatal volume was decreased by 34% in Mecp2-null mice compared to Mecp2-het mice at 14 weeks of age (***). (E) The density of neurons in the cerebral cortex decreased with age in WT from 4 to 7 weeks (++) and from 4 to 7 (++) and 4–14 weeks (+) in Mecp2-het mice but not in Mecp2-null mice. Neuronal density in cortex was significantly higher in Mecp2-null mice compared to WT mice at 7 weeks of age (*) and compared to Mecp2-het mice at 7 and 14 weeks of age (**for both ages). (F) The density of neurons in the striatum also decreased with age in WT from 4 to 14 weeks (+) and from 4 to 14 and from 7 to 14 weeks (+, ++ respectively) in Mecp2-het mice but not in Mecp2-null mice. Neuronal density in striatum was significantly higher in Mecp2-null mice compared to WT mice at 7 (**) and 14 weeks of age (*) and compared to Mecp2-het mice at 7 (*) and 14 weeks of age (***). WT: n = 6; Mecp2-het: n = 6–7; Mecp2-null: n = 6–8.
2.2. MeCP2 expression in Mecp2-heterozygous mice across lifespan
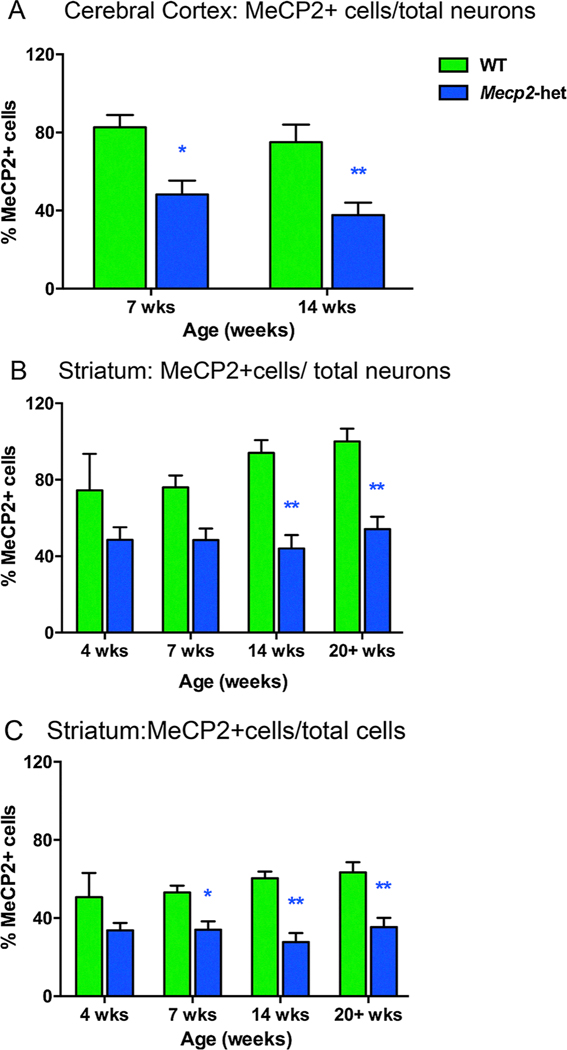
X-chromosome inactivation (XCI) is a factor that affects the severity of symptoms and presumably brain structure in girls with RTT. One study found that in Mecp2tm1.1Jae-het mice, MeCP2 negative cells within the cortex predominately display decreases in soma size, nucleus size, branching complexity, and dendritic length (Rietveld et al., 2015). When X chromosome inactivation (XCI) skewed towards very low MeCP2 expression, MeCP2 negative cells in these Mecp2tm1.1Bird-hetereozygous mice had larger somas and nuclei (Rietveld et al., 2015). Vice versa when XCI skewed toward high MeCP2 expression, MeCP2 negative cell somas and nuclei were smaller, raising the possibility of cell non-autonomous effects such as competition for synaptic connections that influence cell size and morphology (Rietveld et al., 2015). Our group looked at this issue by determining the proportion of neurons expressing MeCP2 in Mecp2-heterozygous mice and determining whether the proportions change over development. We found that in both cortex and striatum of Mecp2-heterozygous mice about 40–50% of the neurons were MeCP2 positive and that ratio remained relatively steady over the different ages (Fig. 3A, 3B, Supplemental Table 3). In contrast, 75–100% of neurons in WT were MeCP2 positive. The percentage of all cells (neurons + glia) expressing MeCP2 + in Mecp2-heterozygous mice ranged from 28 to 35% compared to a 51–63% range for WT mice; the differences were significant at 7, 14 and 20+ weeks of age (Supplemental Table 3, Fig. 3C). The results were different than those from our previous analysis (Metcalf, Mullaney, Johnston, & Blue, 2006). We believe the differences in the studies may be due to the less variability in MeCP2 immunostaining and in neuronal staining with MBA2 compared to NeuN immunostaining. These percentages are consistent with the notion that XCI is approximately 50–50 in the Mecp2-heterozygous mice and in patients with RTT, suggesting that X-inactivation is normally not skewed in Mecp2-heterozygous mice.
Fig. 3.
Approximately 50% of neurons expressed MeCP2 in the cerebral cortex and striatum of Mecp2-heterozygous mice. (A) In Mecp2-het mice, approximately 50% of the total number of neurons were MeCP2+ at 7 weeks of age and 14 weeks of age (*, ** respectively versus WT). (B) Likewise, the percentage of MeCP2+ cells found in the striatum of Mecp2-het mice was approximately 40–50% of the total number of neurons at all ages studied and was significantly different from WT mice at 14 and 20+ weeks (**for both 14 and 20+ weeks). (C) When glial and neuron counts were added the percentage of MeCP2+ cells was 28–35% of the total number of cells in Mecp2-het mice and significantly different from WT mice at 7, 14 and 20+ weeks of age (*, **, **, respectively). WT: n = 6; Mecp2-het: n = 5. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
3. Basal ganglia and the motor phenotype
3.1. Basal ganglia morphological and motor impairments emerge coincidentally.
It is generally reported that morphological changes become apparent around four weeks of age in the basal ganglia of Mecp2-null mice and Mecp2-null rats and this timing coincides with when motor dysfunction emerges (Bhattacherjee et al., 2018; Gantz, Ford, Neve, & Williams, 2011; Guy, Hendrich, Holmes, Martin, & Bird, 2001; Kao, Su, Carlson, & Liao, 2015; Panayotis et al., 2011; Schaevitz, Gómez, Zhen, & Berger-Sweeney, 2013; Stearns et al., 2007; Veeraragavan et al., 2015; Wegener et al., 2014). Striatal volume exhibits a correlative relationship with phenotype (i.e. as volume decreases, phenotype worsens) in addition to other functionally-related regions including primary somatosensory cortex (S1) and primary motor cortex (M1) (Allemang-Grand et al., 2017). These correlative relationships cannot tell us which change comes first but does strengthen the notion of structural-functional linkages in this disorder
In addition to these structural-functional linkages, dopaminergic neurotransmission is affected by MeCP2 deficiency. At P28 there are not only changes in neuronal morphology but also decreases in dopamine levels and in tyrosine hydroxylase (TH) mRNA in the rostral striatum, nucleus accumbens, and ventral midbrain in Mecp2-null mice (Kao et al., 2015). Others report that the number of TH immunoreactive cells is reduced within the substantia nigra pars compacta at P35 and P55 despite DA levels being normal in Mecp2-null mice (Panayotis et al., 2011). Furthermore, several experiments demonstrated that loss of MeCP2 either in all TH+ cells or just in striatal neurons leads to psychomotor deficits in the open field and rotarod tests (Samaco et al., 2009; Su, Kao, Huang, & Liao, 2015) and conversely, selective preservation of striatal MeCP2 prevents these deficits (Su et al., 2015). Taken together with the morphometric findings of decreased volume in the striatum, we think it likely that volumetric declines are due to decreases in dendritic complexity and that dopamine deficiencies are not due to cell loss but rather a consequence of decreased DA production from lowered TH activity.
Unlike their Mecp2-null male counterparts, changes in dopaminergic cell functionality within the striatum of Mecp2tm1.1Bird-hetereozygous mice occur well before motor phenotype onset. At one month of age, MeCP2 negative cells in Mecp2tm1.1Bird-hetereozygous mice show both functional (decreases in capacitance and increase in resistance) and structural (process length and complexity) deficits. However, decreases in dopamine release from axon terminals in the striatum are not observed until 9–12 months of age in Mecp2tm1.1Bird-hetereozygous mice (Gantz et al., 2011) (Table 1, Fig. 1) These data corroborates what has been learned in PD - that a critical threshold of dysfunction or loss must be reached in order for motor impairments to occur.
In late-phenotypic (P49-P70) Mecp2tm1.1Bird-null mice and Mecp2tm1.1Bird-heterozygous mice of the same age D2 receptor density is decreased compared to WT in the striatum (Wong et al., 2018) (Table 1, Fig. 1). These data concur with patient literature demonstrating low-to-normal levels of D2 receptors in older patients (Naidu et al., 2001) and decreased D2 receptor density in 15–30 years old women with RTT (Wong et al., 2018). Yet, increases in D2 density have been reported in a single photon emission computed tomography (SPECT) study where the age range was 4–15 years (Chiron et al., 1993). The D2 receptor findings suggest age-related changes in D2 receptors such that patients may have higher densities than normal in the first decade of life but lower densities as they approach adulthood. Thus it appears that dopamine and basal ganglia-related pathology continues to evolve after phenotype onset and that dopaminergic pathways may be more susceptible to deterioration than other structures that do not show the same late-stage phenotypic changes.
3.2. Confirmation of motor deficits in MeCP2 deficient mice
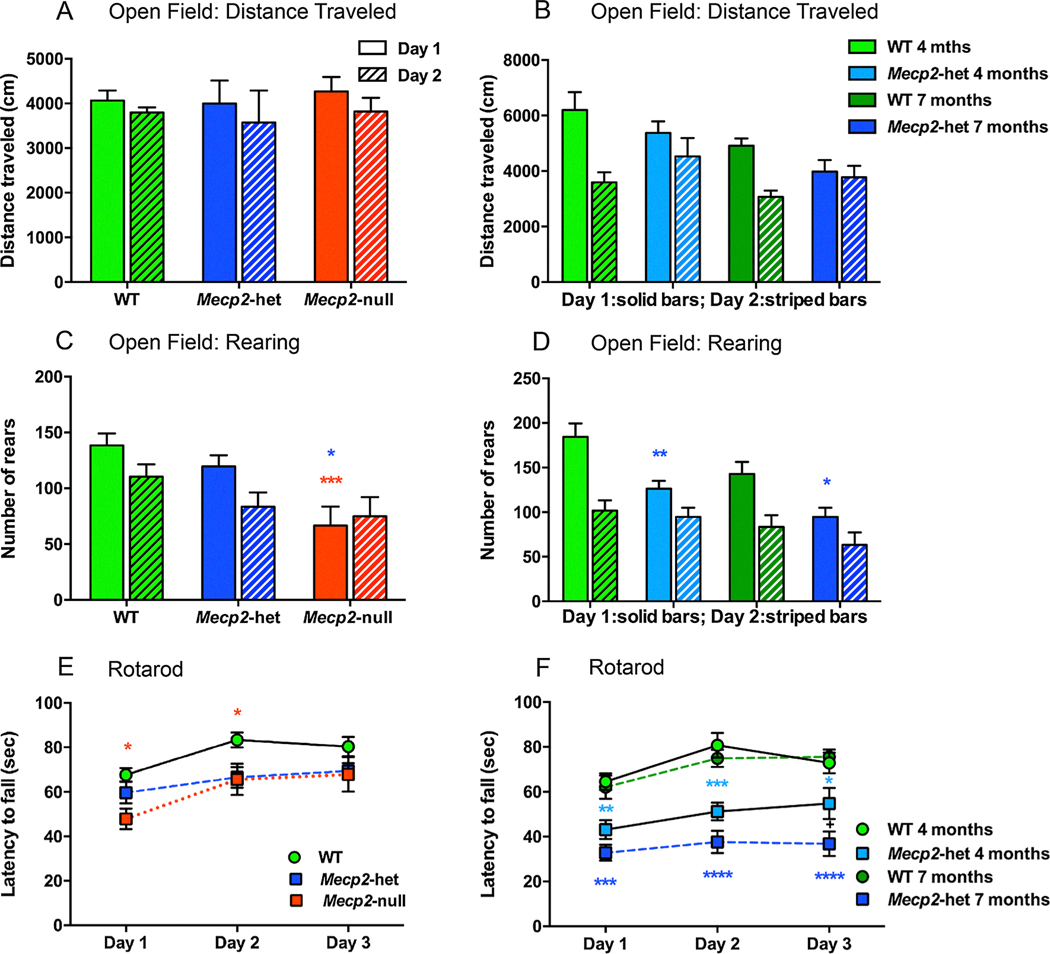
We performed our own analysis of motoric behavior in juvenile Mecp2tm1.1Bird-null and hetereozygous mice and (5–7 weeks old) and in newly symptomatic (4 months old) and fully symptomatic (7 months old) Mecp2tm1.1Bird-hetereozygous mice. Distance traveled and the number or rears over a 30 min period in two daily sessions in an Open Field test served as an index of spontaneous locomotor activity and the number of rears executed over that time period was used to assess exploratory behavior. In contrast to other reports of decreased distance traveled in an open field in Mecp2tm1.1Bird-null and Mecp2tm1.1Bird -heterozygous mice (Alvarez-Saavedra, Sáez, Kang, Zoghbi, & Young, 2007; Guy et al., 2001; Jugloff et al., 2008; Samaco et al., 2013) we did not observe differences in the distance traveled in an open field for Mecp2-heterozygous and Mecp2-null mice at 5–7 weeks of age (Supplemental Table 4, Fig. 4A) or at 4 and 7 months of age (Supplemental Table 4, Fig. 4B). Consistent with other studies (Jugloff et al., 2008; Kondo et al., 2008; Lonetti et al., 2010; Pelka et al., 2006; Pratte, Panayotis, Ghata, Villard, & Roux, 2011; Stearns et al., 2007) we also observed significant reductions in the numbers of rears made by 5–7 weeks old Mecp2-null and 4 and 7 months old Mecp2-heterozygous mice compared to WT mice on the first day tested (Fig. 4C, D). Another difference we noted was that the 5–7 weeks old WT and Mecp2-heterozygous mice habituated to the open field on the second day of testing by rearing less while Mecp2-null mice did not (Fig. 4C). We also saw less habituation in the 4 and 7 months old Mecp2-heterozygous than in corresponding WT female mice (Fig. 4D). These data illustrate that on both activity measures (distance and rearing), mice become less active as they age, and that Mecp2-heterozygous mice fail to habituate.
Fig. 4.
Open field analysis and rotarod tests revealed decreased exploratory behavior and decreased motor learning in Mecp2-heterozygous and Mecp2-null mice aged 5–7 weeks of age and in Mecp2-heterozygous mice that were 4 or 7 months of age. At 5–7 weeks of age, distanced traveled in the open field by Mecp2-null mice (n = 8) and Mecp2-heterozygous mice (n = 6) was not different from WT mice (n = 14) (Fig. 5A), nor were there differences in distanced traveled between 4 and 7 months old Mecp2-heterozygous (n = 8) and WT mice (n = 8) (Fig. 5B). Fig. 5C At 5–7 weeks of age, Mecp2-null mice showed decreases in rearing behavior compared to WT mice (red***) and Mecp2-het mice (blue*). Fig. 5D Both 4 and 7 months old Mecp2-het mice exhibited less rearing than WT mice (**, * respectively). Fig. 5E Compared to 5–7 weeks old WT mice, Mecp2-null mice showed initial deficits in rotarod performance that dissipated with training (* both Day 1 and Day 2). Rotorod performance in Mecp2-heterozygous mice was not different from the WT at 5–7 weeks of age but did show persistent deficits at 4 (light blue**, ***, * compared to 4 months old WT mice) and 7 months of age (Fig. 5F; blue***, ****, **** compared to 7 months old WT mice)). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
We used the rotarod test to assess motor coordination, balance, equilibrium, and motor learning by measuring the ability of mice to improve motor performance with repeated exposure. Similar to other studies (Jugloff et al., 2008; Kondo et al., 2008; Lonetti et al., 2010; Pelka et al., 2006; Pratte et al., 2011; Stearns et al., 2007), we found decreased performance in this test in all cohorts of MeCP2 deficient mice. Motor learning was impaired in 5–7 weeks Mecp2-null mice as they showed no improvement on subsequent days of training (Fig. 4E). These results indicate that both Mecp2-null and Mecp2-heterozygous mice have motor learning and coordination deficits. Because older Mecp2-heterozygous mice become obese we were concerned that weight was a confounding variable for their poor performance in the open field studies. Therefore a subset of mice was weight-matched (i.e., 8 wt versus 4 Mecp2-heterozygous mice) and Open Field and Rotarod analyses performed. The differences persisted on both tests indicating that weight did not confound our initial findings.
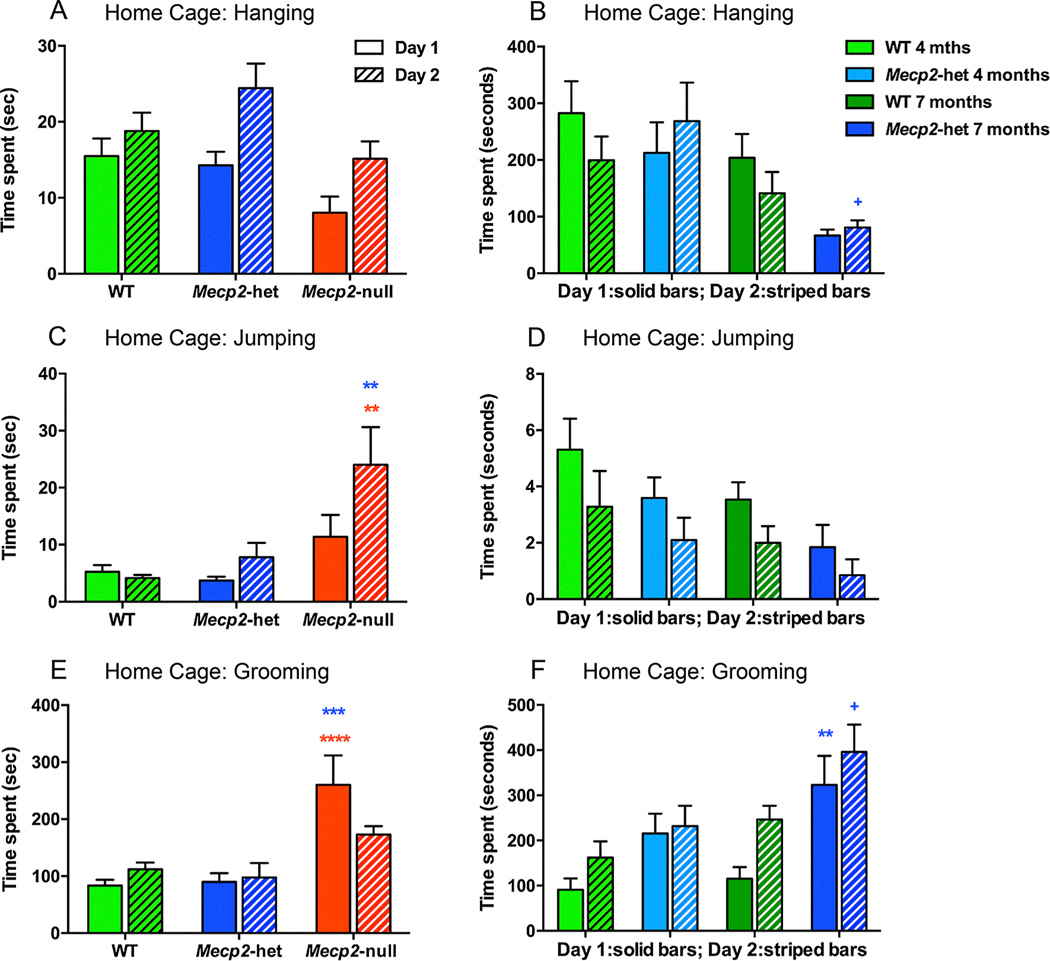
A novel behavioral testing paradigm to this study was measuring general behavioral activity including hanging, jumping and grooming over two consecutive days in a home cage setting. Mecp2-null mice that were 5–7 weeks old spent less time hanging (Fig. 5A) and more time jumping (Fig. 5C) and grooming (Fig. 5E) than age-matched WT and Mecp2-heterozygous mice. There were also significant age effects for hanging and grooming for the 4 month versus 7 month old Mecp2-heterozyous mice as the older mice spent less time hanging and more time grooming than the younger mice (Fig. 5B and 5F). The 7 months old Mecp2-heterozygous mice also spent significantly more time grooming than the WT mice (Fig. 5F). Overall, these data suggest that 5–7 weeks old mice have impaired motor coordination (less hanging) and exhibit an increase in repetitive-like behaviors (grooming and jumping). Age had a clear impact on activity levels in these mice and additional differences between WT and Mecp2-heterozygous mice were evident as the adult mice aged.
Fig. 5.
Home cage behaviors including hanging, jumping and grooming were altered in juvenile Mecp2-heterozygous and Mecp2-null mice aged 5–7 weeks of age and in Mecp2-heterozygous mice that were 4 or 7 months of age. (A) Mecp2-null mice that were 5–7 weeks old (n = 8) spent less time hanging than WT mice (n = 14). (B) Likewise, 7 months old Mecp2-het mice (n = 8) spent less time hanging than 4 months old Mecp2-het mice (n = 8, blue + p < 0.05). (C) Juvenile Mecp2-null mice spent more time jumping than WT (red **) and Mecp2-het mice (blue**). (D) While hanging behavior diminished from 4 to 7 months in age and was less in Mecp2-het mice than WT, there were not significant post hoc p values. (E) Juvenile Mecp2-null mice spent more time grooming than WT (red ****) and Mecp2-het mice (blue***).(F) Similarly, 7 months old Mecp2-het mice spent more time grooming than 4 months old Mecp2-het mice (n = 8, blue + p < 0.05) and than 7 months old WT mice (blue**). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Decreased hanging time has only been reported previously in Mecp2tm1Bird-hypomorph mice (Kerr, Alvarez-Saavedra, Sáez, Saona, & Young, 2008) and excessive grooming in Mecp2tm1.1Bird-heterozygous mice (Guy et al., 2001; Samaco et al., 2008). Increased grooming and jumping are repetitive behaviors that may be a mouse correlate of repetitive, non-purposeful hand wringing behaviors in girls with Rett syndrome. Over-grooming is also a feature of other disease models where repetitive behavior is a feature such as autism spectrum disorder (ASD) and obsessive-compulsive disorder (OCD). In these ASD and OCD models the basal ganglia has been shown to be involved in grooming behavior suggesting a similar cause of pathological grooming in RTT models (Aldridge, Berridge, & Rosen, 2004; Brodkin et al., 2014; Graybiel & Grafton, 2015; Graybiel & Saka, 2002; Graybiel, 2008). In addition, striatal increases in dopamine and decreases in dopamine transporter have generally been linked to excessive self-grooming behavior. Thus, it seems that changes in either direction in dopamine function may cause increased repetitive behavior.
In summary, the results of our behavioral analyses showed significant motor deficits in Mecp2-null and Mecp2-heterozygous mice. Our results and those of other studies of Mecp2tm1.1Bird mice found that motor impairments onset with structural deficits in Mecp2-null mice and lag behind the emergence of structural deficits in Mecp2-heterozygous mice (Belichenko et al., 2008, 2009; Gantz et al., 2011; Kao et al., 2015; Samaco et al., 2009). Interestingly, and exception to this rule is the Mecp2R168x-heterozygous female mice that overall shows a less severe and slower progressing phenotype but with an earlier onset of psychomotor dysfunction than heterozygous female mice in other models (Schaevitz et al., 2013; Wegener et al., 2014). Furthermore, motor deficits may be more complex than we currently believe as recent findings of Mecp2-heterozygous female mice show normal ability to learn a motor skill at week 4 but begin to show psychomotor regression in a learned motor skill at 9 weeks of age (Veeraragavan et al., 2015) well before locomotor or rotarod deficits are routinely observed. Taken together, motor function and complex motor skills track with structural and functional changes of the basal ganglia, however mice may also rely on other structures or circuits for exploratory or skill learning behavior.
3.3. Pharmacological recovery of motor function
As one of the most consistent hallmark phenotypic features of MeCP2-deficient mice, motor function is generally utilized as a primary outcome measure by which to evaluate preclinical pharmacological interventions. L-dopa alone only rescues locomotor behavior in open field in older P60 Mecp2-null mice but not in the younger P30 or P45 Mecp2-null mice (Panayotis et al., 2011). The addition of benserazide, a dopamine decarboxylase inhibitor to a daily oral L-dopa regimen (similar to PD dosing schemes) improves motor function (mobility, gait, overall phenotype score) at 7 weeks of age (P49) but the improvements are transient and no effect is observed at 10 weeks of age (Szczesna et al., 2014).
Other non-dopaminergic mechanisms approaches have been used to target universal neuronal and behavioral pathology in the MeCP2 deficient mice. Administration of the NMDA receptor antagonist ketamine from P15 or P30 leads to modest improvements in accelerating rotarod and stereotypic paw clasping behavior (Patrizi et al., 2016) but the treated mice never approach the performance of WT mice. Low-dose ketamine that increase BDNF levels has shown modest efficacy in preserving or rescuing motor dysfunction (Johnson et al., 2012; Patrizi et al., 2016). Growth factors such as insulin growth factor-1 (IGF-1) and brain-derived neurotrophic factor (BDNF) have also been targeted and have been shown to improve open field locomotion (Johnson et al., 2012; Schmid et al., 2012; Tropea et al., 2009). Specifically, BDNF receptor (TrkB) agonists improve motor function in Mecp2tm1.1Jae-null males but not Mep2tm1.1Bird-null males (Johnson et al., 2012; Schmid et al., 2012). Environmental enrichment that increased BDNF levels improved performance in Mecp2-heterozygous female mice in accelerating rotarod but had no impact on mobility or locomotor deficits (Kondo et al., 2008) suggesting a role for BDNF therapy in motor skills but not innate mobility or gait. There have been a substantial subset of drugs taken to clinical trials in recent years including desipramine (SNRI) and saritozan (5-HT1a agonist) that have been predominately pursued for their impact on respiratory function and have shown little efficacy in improving motor phenotype in mice or correlative symptoms in patients (Kaufmann, Stallworth, Everman, & Skinner, 2016; Pozzo-Miller, Pati, & Percy, 2015).
4. Hippocampal structural changes result in impaired synaptic plasticity mechanisms
4.1. Dendritic morphology and receptor changes
Changes in hippocampal dendritic and synaptic morphology resulting from MeCP2 deficiency have been well documented. At P21 and onward, dendritic complexity as well as dendritic spine density in hippocampal neurons is reduced (Belichenko et al., 2009; Chapleau et al., 2009). Others have shown that glutamatergic synapse density directly corresponds to MeCP2 levels (Chao, Zoghbi, & Rosenmund, 2007). Recently, Nguyen and colleagues demonstrated direct consequence of the lack of MeCP2 expression on hippocampal morphology. Using a Cre/Tamoxifen system to knock out MeCP2 expression starting at either 5 or 10 weeks of age led to reductions in CA1 thickness and dendritic complexity when analyzed 10 weeks after the start of Mecp2 silencing (Nguyen et al., 2012). Thus inducing MeCP2 deficiency in adulthood well after hippocampal structure and circuitry has formed can have a lasting impact.
Glutamate receptors also show dysregulation within the hippocampus and contribute to abnormal functionality. Symptomatic Mecp2-null male mice 6–10 weeks of age exhibit increased expression of the “immature” NR2B subunit of NMDA while expression of the “mature” NR2A subunit is reduced compared to the WT (Asaka, Jugloff, Zhang, Eubanks, & Fitzsimonds, 2006) indicating a delay in NMDA receptor maturation (NR2B to NR2A switch; Table 1, Fig. 1). Experimentally naïve Mecp2tm1.1Bird-null preparations show increased AMPA receptor subunit Glu1A expression and as a consequence function as if potentiated (Li, Xu, & Pozzo-Miller, 2016) (Table 1, Fig. 1). Pyramidal neuron spine synapses from these mice also are larger with enhanced surface levels of Glu1A-containing receptors and do not grow in size or incorporate Glu1A subunits after long term potentiation (LTP). These results showing altered LTP in Mecp2tm1.1Bird-null mice and altered expression levels of proteins involved in AMPA receptor trafficking, suggest that AMPA receptors could be targets for therapy in RTT (Li et al., 2016). Metabotropic glutamate receptors within and outside of the hippocampus have also been implicated in learning and memory dysfunction of these mice. Both RNA and protein expression of mGluR5 and mGluR7 is reduced in the hippocampus in phenotypic/symptomatic Mecp2tm1.1.Bird-null mice (Gogliotti et al., 2016, 2017). Likewise, postmortem analyses of patients with RTT revealed decreased expression of these receptors compared to controls (Gogliotti et al., 2016, 2017) (Table 1, Fig. 1) suggesting a valid, potentially translatable therapeutic target.
4.2. Functional consequences of hippocampal structure abnormalities
As a consequence of these morphological and receptor changes, MeCP2-deficient mice display impairments in mechanisms of plasticity. Specifically, deficits in both short-term plasticity and long-term plasticity mechanisms have been observed. Decreased paired-pulse inhibition ratios and shorter excitatory post-synaptic periods indicate abnormal short-term plasticity in MeCP2-deficient mice (Asaka et al., 2006; Moretti, 2006; Nelson, Kavalali, & Monteggia, 2006). Decreased or absent LTP and long-term depression (LTD) have also been observed. Generally, these deficits in plasticity mechanism do not emerge in Mecp2tm1.1Bird-null or Mecp2stop/y mice until after motor symptom onset (Asaka et al., 2006; Weng, McLeod, Bailey, & Cobb, 2011), and the overall phenotype severity positively correlates with the magnitude of LTP impairment (Weng et al., 2011). Interestingly, although LTP can be induced by high-frequency tetanus, the magnitude is reduced in MeCP2-deficient mice and LTP cannot be induced with theta burst stimulation, which is thought to be more physiologically relevant (Asaka et al., 2006), suggesting that LTP deficits in vivo may be more profound than originally thought. Moreover, awake, behaving Mecp2-heterozygous mice partaking in learning and memory tasks show deficits after down-regulation of MeCP2 by shRNA or genetic deletion of Mecp2 blocks bicuculline-induced synaptic scaling indicating another role for MeCP2 in plasticity at the level of behavior (Qiu et al., 2012).
Abnormal LTP and LTD hippocampal plasticity mechanisms underlie impairments in hippocampal-dependent learning and memory paradigms including novel objection recognition/novel object location, social memory recognition, contextual fear conditioning and Morris water maze reported in both full deletion (Mecp2tm1.1Bird-null and Mecp21lox-null) (Gogliotti et al., 2016, 2017; Hao et al., 2015; Schaevitz, Moriuchi, Nag, Mellot, & Berger-Sweeney, 2010; Stearns et al., 2007) and truncation (Mecp2308/y Mecp2R168x) models (Moretti, 2006; Schaevitz et al., 2013). Similar to other aspects of the RTT phenotype reported, these learning and memory impairments onset earlier in Mecp2-null mice (4–6 weeks of age) and later in Mecp2-heterozygous mice (18–20 weeks). Mecp2308/y mice, which generally show a later onset and less severe phenotype, also show hippocampal learning and memory dysfunction within the same window in which other phenotypic features emerge, approximately 20 weeks of age.
Recovery of these deficits is possible through many mechanisms but several that are relevant to this review include receptor-specific targets and deep brain stimulation. NMDAR antagonist memantine improves short-term but not long-term plasticity, while ketamine, another NMDAR antagonist, enhances spontaneous and evoked potentials in visual cortex along with ameliorating many neurobehavioral dysfunctions (Patrizi et al., 2016). Positive allosteric modulators for both mGluR5 and mGluR7 recover hippocampal-dependent learning and memory and aspects of motor and respiratory function in Mecp2tm1.1.Bird-null mice (Gogliotti et al., 2017; Tao et al., 2016). While negative allosteric modulation of mGluR5 does not improve contextual fear conditioning behavior, it does correct inhibitory avoidance behavior and CA1 soma size suggesting a complex role for metabotropic glutamate receptor dysregulation (Tao et al., 2016). Recently it has been demonstrated that deep brain stimulation of the fimbria-fornix in Mecp2tm1.1Bird-hetereozygous mice can rescue LTP, excitatory inhibitory balance and hippocampal-dependent learning memory tasks including the Morris water maze and contextual fear conditioning (Hao et al., 2015; Lu et al., 2016; Pohodich et al., 2018). All of these mechanisms of recovery demonstrate that aberrant structural and functional changes are directly tied to these cognitive dysfunctions and transient repair or rescue is sufficient.
4.3. MeCP2-deficiency in adulthood results in cognitive dysfunction
Many aspects of hippocampal dysfunction and corresponding learning and memory deficits can be both induced and recovered in adult mice. MeCP2 suppression in late adolescence or adulthood leads to deficits in long-term memory and reductions in dendritic complexity, pyramidal cell layer thickness and expression of synaptic proteins (Nguyen et al., 2012). A knock down/reduction of MeCP2 expression (as opposed to full knockout) in adulthood also causes long-term memory impairments but no changes in dendritic morphology/complexity when examined four weeks post-knockdown (Gulmez Karaca, Brito, Zeuch, & Oliveira, 2018). It may be that a complete loss of MeCP2 expression is needed to see changes in mature neuronal dendritic structure. This hypothesis is supported by studies that have demonstrated in the case of missense or truncated Mecp2 sequences where the phenotype and pathology are less severe, dendritic structure is preserved even in the presence of deficits in contextual fear conditioning, Morris water maze and social learning (Moretti, 2006). In experiments of MeCP2 re-expression or administration of exogenous MeCP2, performance on novel object recognition tasks improved (Garg et al., 2013) but no other cognitive aspects were tested. However, given the current body of work, it is likely that many of the structural and resulting functional deficits within the hippocampus will be recovered.
5. Disrupted plasticity results in perturbed cortical development
5.1. Development and plasticity in barrel field cortex is disrupted by MeCP2 deficiency
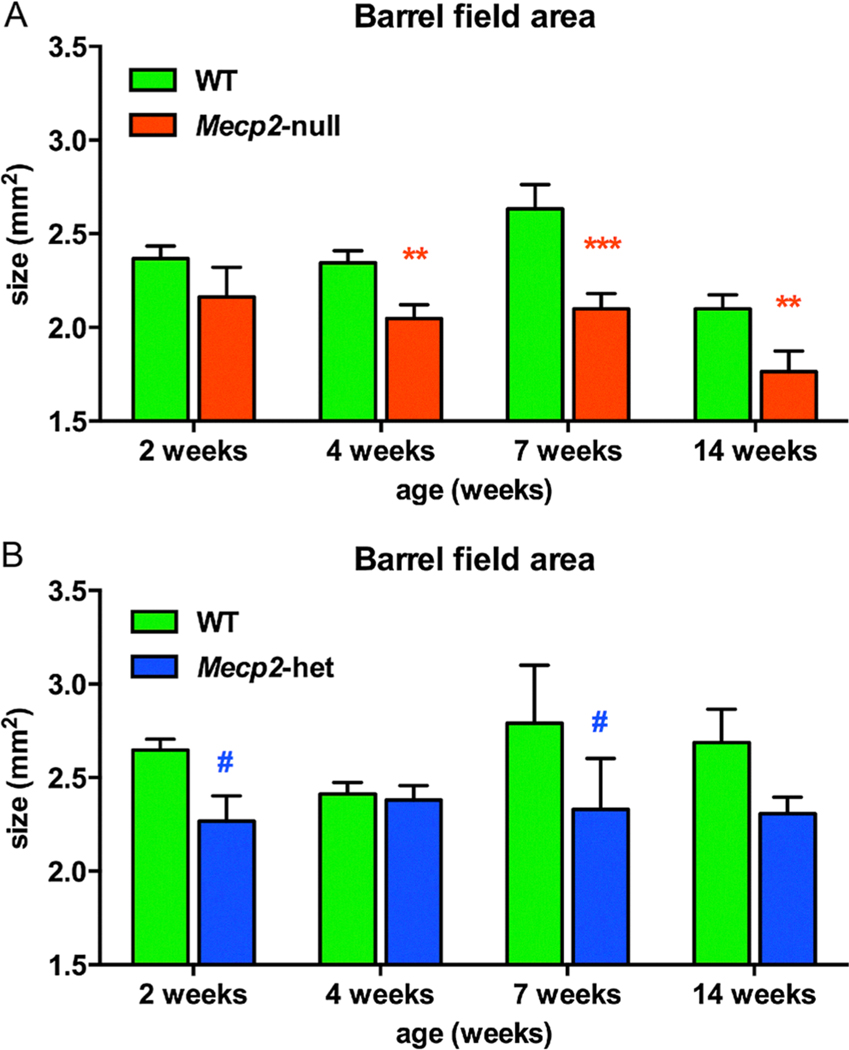
The barrel field model for cortical development and activity dependent plasticity in rodents has been used to better understand somatosensory deficits that have been demonstrated in patients with RTT (Badr, Witt-Engerström, & Hagberg, 1987; Yoshikawa, Kaga, Suzuki, Sakuragawa, & Arima, 1991). Mice use their whiskers to navigate through their environment and their brain has a remarkable structural and functional map where each whisker has a corresponding barrel in primary somatosensory cortex. Previous studies have shown that the barrel field is plastic and its size and map is modified by activity (Crair & Malenka, 1995; Inan & Crair, 2007). In our study we performed littermate pair comparisons between WT and Mecp2-null mice and between WT and Mecp2-heterozygous mice at 2, 4, 7 and 14 weeks of age and found a significant decrease in the cross-sectional area barrel field of Mecp2-null mice, at 4 weeks of age and beyond (Fig. 6A). This finding is similar to another report of decreased barrel field size in P10 and P40 Mecp2-null mice (Moroto et al., 2013). Paired comparisons between Mecp2-heterozygous and WT mice did not show significant differences at any age but there was a trend for the barrel field area to be smaller in Mecp2-heterozygous than in WT mice at 2 and 7 weeks of age (p = 0.06, 0.10, respectively) (Fig. 6B). The decreases in barrel field area are consistent with recent reports showing decreased dendritic complexity and organization of neurons within the barrel field of MeCP2-deficient mice (Lee, Tsytsarev, & Erzurumlu, 2017).
Fig. 6.
Barrel field cross sectional area was reduced in Mecp2-null mice by fours weeks of age. (A) Littermate comparisons showed that cross-sectional area of the barrel field area was significantly smaller in Mecp2-null than compared to WT mice at 4 weeks of age (n = 11; red**) and the defect persisted through the lifespan of these mice at 7 weeks (n = 9; red***) and at 14 weeks of age (n = 4; red**). Mecp2-het mice (n = 5–10) showed a trend for decreased barrel field size at 2 weeks (blue#-p = 0.06) and 7 weeks (#p = 0.10) as compared to WT mice.
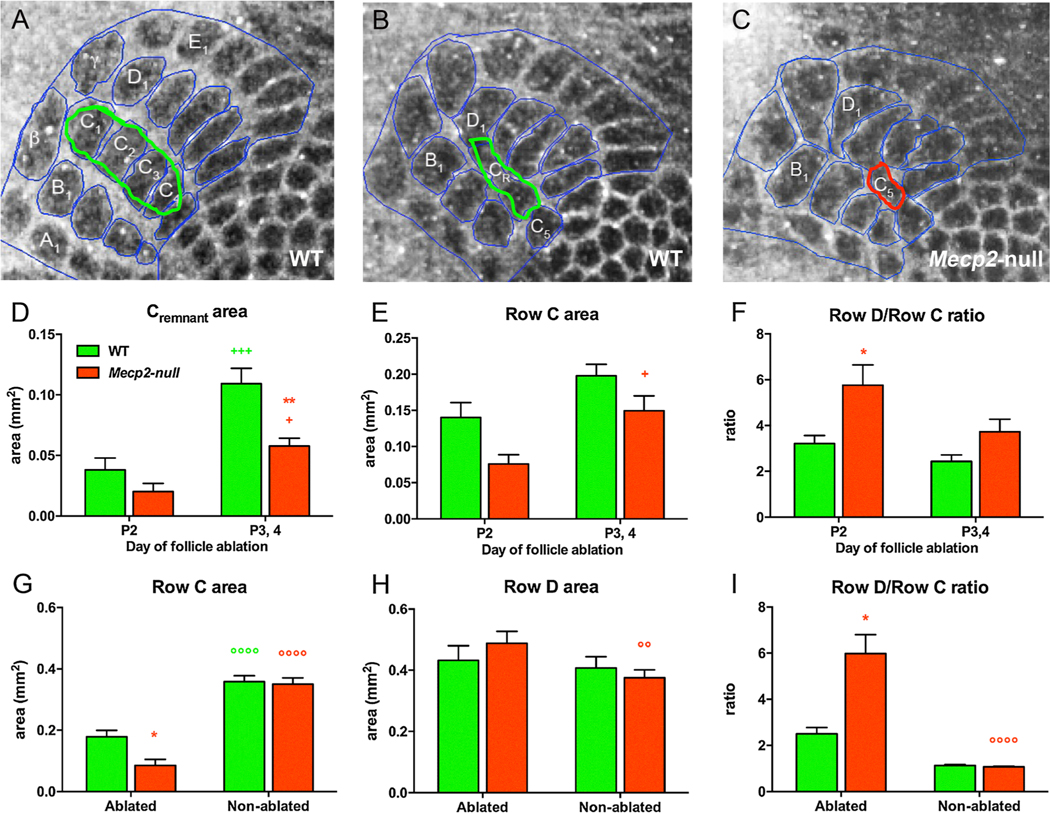
To determine whether structural plasticity was altered in Mecp2-null mice, follicle ablations (FA) of the middle row (Row C1-C4) whiskers on the right side of the snout were performed a day after birth on P2, when plasticity is maximal and on P3 and P4 when the critical window for this plasticity is closing. Barrel cortex sections were processed for visualization with cytochrome oxidase staining and area measurements of individual barrels, of Rows B, C and D and of the whole barrel field (outlined in blue in Fig. 7A). Representative images of the barrel field highlight the remnant of the Row C lesions (CR) for WT (green outline) and Mecp2-null (red outline) mice are shown in Fig. 7B and 7C respectively. The cross-sectional area of the whole barrel field did not differ in Mecp2--null versus WT mice and for the left (ablated) versus right (non-ablated control) side of the cortex. As expected when FA were performed at P3, 4, CR did not shrink as much as when lesions were performed at P2 (Fig. 7D). However, the change in CR size was less marked in the Mecp2-null mice than WT. As a result, post-hoc comparisons showed that CR area was significantly smaller in Mecp2-null compared to WT mice that were lesioned at P3, 4 (Fig. 7D). Likewise, two-way ANOVA found significant effects of age and genotype on Row C area (Fig. 7E). Effects on adjacent Row B and D were less dramatic than for Row C (data not shown). The ratio of Row D/Row C is used as an index of plasticity and in Mecp2-null mice Row D/Row C ratio was significantly increased compared to WT mice when lesions were performed at P2 (Fig. 7F).
Fig. 7.
Follicle ablation plasticity is altered in Mecp2-null mice. Barrel field organization post-follicle ablation on P2 is depicted by cytochrome oxidase staining in (A)–(C). (A) is from the right (non-ablated) side and (B) is from the left (ablated) side of a WT mouse. (C) is the barrel field on the ablated side of a Mecp2-null mouse. (D) When FA was performed at P3, 4, Row C remnant (CR) did not shrink as much as when lesions were performed at P2 in both WT (n = 5) and Mecp2-null mice (n = 8) (+++, + respectively). The area of CR was significantly decreased in Mecp2-null compared to WT mice after P3/4 post-ablation (**). (E) The area of Row C was larger in Mecp2-null mice when FA was performed at P3,4 versus P2 (+). (F) The ratio of Row D to Row C was used as a measure of experience-induced plasticity. After FA on P2, the Row D/Row C ratio on the ablated side was significantly larger in Mecp2-null than in WT mice (*). (G) Regardless of genotype, the area of Row C was larger on the intact, non-ablated side (°°°°for WT and for Mecp2-null). On the ablated side, Row C area was significantly smaller in Mecp2-null mice than in the WT (*). (H) Neighboring Row D area was not statistically different on the ablated or intact side of WT mice but was smaller on the intact side than on the ablated side in Mecp2-null mice (°°). (I) The Row D/Row C ratio was significantly smaller on the intact side in Mecp2-null mice (°°°°) and as a result was larger in Mecp2-null mice than in WT mice on the ablated side (*). Genotype differences are red* for WT versus Mecp2-null mice; differences between day of follicle ablation are denoted with green+ for WT mice and red+ for Mecp2-null mice; green° denote differences between the ablated versus the non-ablated side for WT mice and red° denote differences between the ablated versus the non-ablated side for Mecp2-null mice. The numbers of symbols refer to p values (+ or *p < 0.05, °°p < 0.01, +++ p < 0.001, °°°°p < 0.0001).
In order to assess any interaction effect of genotype X ablation, cross sectional areas on the right side of the brains (non-ablated) was compared to that on the left side (ablated) in the same animal. Since no differences were observed when FA occurred at P2 versus at P 3, 4, we collapsed the data across time point for further analyses. As expected, when Row C on the ablated side was compared to that on the non-ablated side, the area was much smaller than on the non-ablated side in both WT and Mecp2-null mice but Row C was significantly smaller in Mecp2-null versus WT mice (Fig. 7G). We also observed ablation effects on adjacent Rows B in both WT and Mecp2-null mice and Row D in Mecp2-null mice such that Row D was on the ablated side was larger than on the non-ablated side (Fig. 7H). As a result of these ablation effects the Row D/Row C ratio was significantly larger in Mecp2-null mice than in WT mice (Fig. 7I). The results indicate that the barrel field of Mecp2-null mice exhibits altered structural plasticity after FA, with the main effect being increased shrinkage in the lesioned row of barrels.
5.2. The critical period for visual cortical plasticity is altered by MeCP2 deficiency
Loss of MeCP2 in the visual cortex leads to decreases in visual acuity and visual evoked potentials in mice and patients with RTT (Banerjee et al., 2016; Durand et al., 2012; von Tetzchner et al., 1996). The visual cortex is known for its well-defined critical window necessary for proper development. Recently there has been a great deal of work to investigate the role of MeCP2 in visual cortex function and development. In addition to the morphological changes seen in other cortical areas, the visual cortex also experiences an abnormal critical period. Mecp2tm1.1Bird-null mice show an accelerated maturation of V1 neurons and premature closure of the window leading to deficits in visual acuity in these mice. Changes in this window of plasticity seem to be due to both GABAergic and glutamatergic changes within the visual cortex. This accelerated maturation is linked to an increase in GABA presence within parvalbumin interneurons (Krishnan et al., 2015). Mecp2 deletion in parvalbumin-expressing interneurons disrupts the critical period and perturbs the development of ocular dominance columns. Mecp2 deletion in GABAergic neurons in V1 abolishes the critical period (He et al., 2014). NMDA receptor maturation (NR2B to NR2A subunit switch) is also faster in parvalbumin interneurons and slower in pyramidal cells within the visual cortex (as compared to age-matched WT mice) (Mierau, Patrizi, Hensch, & Fagiolini, 2015). Mecp2lox-Stop/y mice with heterozygous NR2A receptor gene expression show a reduction in NR2A density in parvalbumin cells at P30 and an increase in NR2B density relative to Mecp2lox-Stop/y mice resulting in a lengthening of the maturation window and the prevention of visual deficits in these mice (Durand et al., 2012; Mierau et al., 2015) (Table 1, Fig. 1).
5.3. MeCP2-dependent structural changes leads to disruptions in long-range forebrain circuit connectivity
It is well established that structural changes in MeCP2-deficient neurons result in local circuit disruptions (e.g. long-term plasticity mechanisms in the hippocampus). Recently we’ve begun to understand how these structural changes impact long-range circuitry within the MeCP2-deficient brain as well. The most studied circuit thus far is the forebrain/default mode network, a network comprised of various forebrain cortical regions implicated in higher cognitive processing (Anticevic et al., 2012; Raichle, 2015). Forebrain neurons show decreased spine densities, imbalances in excitatory/inhibitory tone, and impaired mechanisms of plasticity (Belichenko et al., 2008, 2009; Blue et al., 2011; Dani & Nelson, 2009; Dani et al., 2005; Lo, Blue, & Erzurumlu, 2015; Sceniak et al., 2016). Recent studies have revealed that local disruptions in the forebrain such as striatal specific Mecp2-deletion can result in circuit-level disruption, namely decreased dopaminergic input into the striatum (Su et al., 2015). Kron et al., demonstrated decreased expression of the immediate early gene Fos in various cortical regions including the prelimbic cortex, infralimbic cortex, retrosplenial cortex, motor cortex, and nucleus accumbens that is able to be recovered with systemic ketamine administration (8–100 mg/kg), however no corresponding behavioral assessments were conducted to determine whether recovery from functional or behavioral dysfunction related to the default mode network (Kron et al., 2012). However, in a more recent study utilizing DREADD technology, Howell and colleagues demonstrated that activation of the medial prefrontal cortex improved neurobehavioral outcomes including conditioned fear responses and respiratory phenotype (Howell et al., 2017). Further, the authors demonstrated that mPFC activation was able to recover Fos expression in the nucleus of the solitary tract, a brainstem nuclei integral to the respiratory circuit. Future studies broadening our knowledge of how MeCP2-dependent neural dysfunction leads to circuit-wide disruptions holds promise for furthering our understanding of the complex nature of cognitive dysfunction seen in RTT.
6. The role of glia in neuronal structural and functional deficits of neurons in models of RTT
6.1. Morphological changes in glia
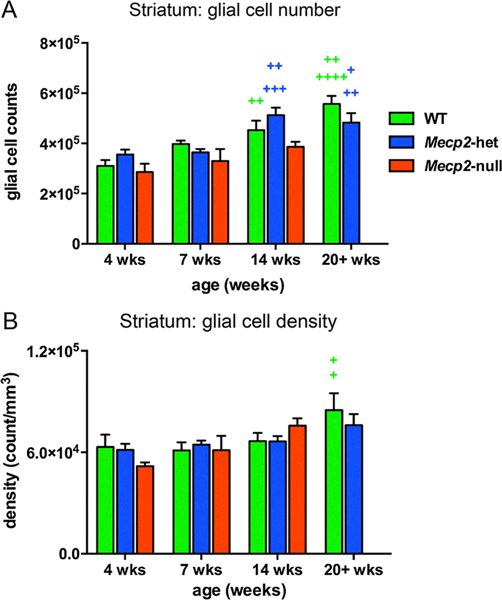
Loss of MeCP2 does not result in overt changes in glial presence or structure as evidenced by studies showing no changes in glial number, morphology or surface markers at phenotype onset in Mecp2-null mice (Cronk et al., 2015; Schafer et al., 2016). In our own study we also didn’t observe marked genotypic differences in glial cell number but did find differences in the trajectory of development. We used unbiased stereological methods to assess the number of glial cells and the density of glial cells in the striatum of 4, 7 and 14 week old WT, Mecp2-heterozygous and Mecp2-null mice and also 20 + weeks old Mecp2-heterozygous and WT mice. For both Mecp2-heterozygous and WT mice, the number of glial cells increased from 4 to 14 weeks and from 4 to 20+ weeks of age of age (Supplemental Table 5, Fig. 8A). In contrast, glial cell number did not change over age in Mecp2-null mice. Developmental changes in glial cell density were less striking than those for glial cell number (Supplemental Table 5, Fig. 8B).
Fig. 8.
Glial cell number increased in WT mice and Mecp2-heterozygous mice but not in Mecp2-null mice over the lifespan. No genotype differences were found for glial cell number (A) or glial cell density (B). Glial cell count increased with age in WT and Mecp2-het mice (age difference depicts with green+ for WT mice and blue+ for Mecp2-het mice) but not in Mecp2-null mice. (A) In WT mice, glial cell counts were higher at 14 weeks of age than at 4 weeks of age (+ +); at 20+ weeks cell count values were higher than at 4 or 7 weeks of age (+ +++, ++ respectively). In Mecp2-het mice glial cell counts at 14 weeks of age were higher than at 4 or 7 weeks (+++, ++ respectively); at 20+ weeks counts were higher than at 4 and 7 weeks (++, + respectively). (B) Glial cell density increased slightly with age, especially in the oldest WT and Mecp2-het mice. In WT mice, the density of glial cells at 20+ weeks of age was significantly higher than at 4 and 7 weeks of age. The numbers of symbols refer to p values (+ for both comparisons. +p0.05, ++p < 0.01, +++p < 0.001, ++++p < 0.0001).
While overall glial cell number and density in Mecp2-null mice were not different in comparison to WT mice, morphological differences in microglia provide more insight into the impact of MeCP2 deficiency on glia. Microglia and astrocyte morphology has been tied to functional state. Microglia with small somas and long, thin, complex processes are prototypical ‘quiescent’ or ‘surveilling’ microglia whereas microglia with large rounded somas and few or no processes are indicative of ‘activated’ microglia involved in inflammatory processes (Boche, Perry, & Nicoll, 2013; Colton & Wilcock, 2010; Kozlowski & Weimer, 2012). Morphological changes in microglia have been observed at the beginning and even prior to phenotype onset. Microglia soma size is decreased in early or pre-phenotypic Mecp2tm1.1Bird-null mice compared to age-matched WT mice but later at 8–12 weeks of age, when Mecp2tm1.1Bird-null mice showed strong phenotypic features, microglia somas in the cortex, hippocampus, and cerebellum were larger than and had reduced branching complexity compared to the WT (Cronk et al., 2015).
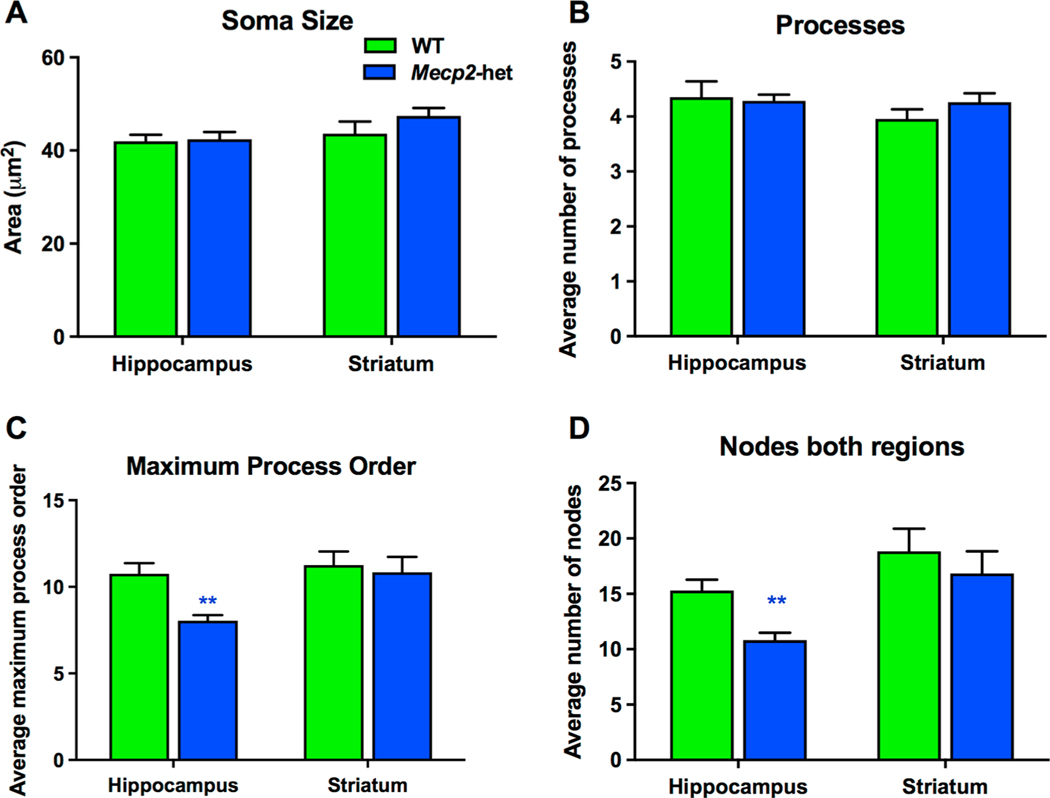
We investigated the morphological differences in phenotypic Mecp2-heterozygous mice in the hippocampus and the striatum. Using IBA-1 immunohistochemistry to visualize microglia morphology and Neurolucida cell tracing software, we found region-dependent changes in microglia morphology of 6–12 month old Mecp2-heterozygous mice. No significant differences were detected in cell size, as quantified by soma area and number of processes, in either hippocampus or striatum, (Fig. 9A, B, Supplementary Table 6). However, we did observe significant reductions in the number of nodes/bifurcations in these processes and the maximum order number in hippocampal microglia indicating that microglia process complexity was diminished in systems with MeCP2 deficiency (Supplemental Table 6, Fig. 9C–D). No such alterations in microglial process complexity were observed in the striatum (Supplemental Table 6, Fig. 9C–D). These findings indicate a subtle change in microglia phenotype towards an ‘activated’ state within the hippocampus. Mecp2-null microglia have been noted to have a particularly ‘activated’ morphology in late-phenotype universally across cortex, hippocampus, and cerebellum. In Mecp2-heterozygous mice, the activation may be subtler due to some MeCP2 presence. Further, morphological changes only being present in the hippocampus suggest potential region-specific influences on microglia morphology. Other literature from healthy mice has demonstrated regional differences in microglia in young and young-adult mice (De Biase et al., 2017; Lawson, Perry, Dri, & Gordon, 1990) that was potentially programmed or due to regional microenvironments. This gives us further impetus to understand the relationship between glia and neurons in these various regions and their contributions to each other’s structure and function.
Fig. 9.
Microglia morphology in the hippocampus of Mecp2-heterozygous mice is altered compared to WT mice. Microglia morphology in the hippocampus and striatum of Mecp2-het mice was quantified by tracing Iba1 + cells in photo-micrographs using Neurolucida software. Soma area and the number of primary processes extending from the soma were no different between Mecp2-het (n = 13) and WT female mice (n = 13) (A, B). Compared to WT mice Mecp2-het microglia did show a decrease in process complexity within the hippocampus as measured by the average maximum process order and average number of nodes or bifurcations of these processes (C, D, blue** p < 0.01). In contrast, no such differences were observed for the striatum.
6.2. MeCP2-related glial dysfunction and its impact on neurons
Glia play an integral role in supporting neuronal function and synaptic connectivity with oligodendrocytes ensheathing axons with myelin, astrocytes maintaining blood brain barrier function and synaptic cleft clearance mechanisms, and microglia modifying synapses and removing debris. All classes of glia are functionally altered by MeCP2 loss or deficiency and in turn have negative impacts on neuronal structure and function. MeCP2-deficient astrocytes and microglia have known toxic effects on neurons via glutamatergic mechanisms. It was first demonstrated that WT neurons exposed to MeCP2-deficient astrocyte conditioned media had shorter dendritic processes similar to those in MeCP2-deficient neuronal cultures (Ballas, Lioy, Grunseich, & Mandel, 2009). Subsequent work demonstrated that astrocytes contributed to pathologically high extracellular glutamate levels as a result of glutamine synthetase up-regulation, dysregulated excitatory amino acid transporters and decreased rates of glutamate and glutamine clearance (Okabe et al., 2012). Mecp2-null microglia also contributed to these pathologically high extracellular glutamate levels by upregulation of glutaminase and the glutamine transporter SNAT1 (Jin et al., 2015; Maezawa & Jin, 2010). While these changes in glutamate production, release and clearance by glia are toxic in vitro, these effects have not been studied in vivo. This is an important next step as glutamatergic neurons also have abnormal structure and function within the cortex and forebrain, which may alter the impact of the MeCP2-deficient glia.
In addition to increased glutamate production, MeCP2-deficient microglia also show changes in immune regulation and extracellular maintenance prior to phenotype onset. RNA-sequencing of microglia from young and adult Mecp2-heterozygous mice found distinct patterns of differentially expressed genes (Zhao et al., 2017). Most notably, at 5 weeks of age, Mecp2tm1.1Bird-hetereozygous mice exhibited increased expression of MHC class II associated genes while the expression of genes associated with extracellular matrix organization, circulatory system development and heat shock proteins were reduced (Zhao et al., 2017). At 30 weeks of age, when the Mecp2-heterozygous mice are symptomatic, there was a general increase in genes regulated by NF-kB and a decrease in cytoskeleton-related genes and in NADH dehydrogenase (Zhao et al., 2017). There also is ample evidence for enhanced levels of oxidative stress and immune dysregulation in patients and in several mouse models of RTT (Cortelazzo et al., 2014; De Filippis et al., 2015; Filosa et al., 2015; Großer et al., 2012; Leoncini et al., 2015; Nance et al., 2017; Pecorelli, Cervellati, Hayek, & Valacchi, 2016; Theoharides, Athanassiou, Panagiotidou, & Doyle, 2015). In the neonatal period, which is well prior to phenotype onset, Mecp2-null mice show cytokine dysregulation including increased expression of tumor necrosis factor alpha (TNFα) and reductions in anti-inflammatory cytokines interleukin 4 and 10 (IL-4, IL-10) (Nance et al., 2017). Furthermore, our group and others have demonstrated that Mecp2-null and Mecp2-heterozygous mice show a perturbed response to immune challenge (Cronk et al., 2015; Nance et al., 2017; Zhao et al., 2017). Immune dysregulation and oxidative stress have been directly correlated to phenotypic severity in mice suggesting that these mechanism of cell injury could be good therapeutic targets (De Felice et al., 2014; Großer et al., 2012). Free radical scavengers such as Trolox have been shown to improve neuronal function and synaptic plasticity in Mecp2tm1.1Bird-null (Janc & Müller, 2014). We also have demonstrated that targeting the antioxidant/anti-inflammatory compound N-acetyl-cysteine (NAC) directly to activated microglia and astrocytes using PAMAM dendrimers resulted in an attenuation of the abnormal immune response in vitro and delayed the progression of overall neurobehavioral phenotype in vivo, indicating that glial dysfunction and immune dysregulation have a role in phenotype progression in MeCP2-deficient mice (Nance et al., 2017).
Glia also have a primary role in neural circuit development by regulating synapse development and pruning (Hoshiko, Arnoux, Avignone, Yamamoto, & Audinat, 2012; Paolicelli et al., 2011a; Parkhurst et al., 2013; Schafer et al., 2012; Ueno & Yamashita, 2014; Ueno et al., 2013). One mechanism by which this occurs is through CX3CL1 fractalkine signaling. This ligand is released from neurons and the corresponding receptor, CX3CR1 is found constitutively expressed on microglia. CX3CR1 is overexpressed in Mecp2-null mice and ablation of this receptor improves weight, overall phenotype, respiration, and motor function (Horiuchi, Smith, Maezawa, & Jin, 2016). Microglia morphology normalizes and insulin growth factor (IGF) expression increases in Mecp2-null mice. Ablating this receptor may lead to decreased synaptic pruning however these outcome measures have yet to be evaluated (Horiuchi et al., 2016).
While there is ample evidence that glial dysfunction is present and contributes to structure/functional deficits of neurons and resulting behavioral phenotypes, there is speculation as to the weight of importance of dysfunctional glia on the neural system as a whole. Derecki and colleagues rescued Mecp2-null phenotype with bone marrow derived transplantation of WT microglia but in contrast, Wang and colleagues were unable to replicate these findings and further showed that conditional expression of MeCP2 in microglia had no impact on survival or neurobehavioral phenotype (Derecki et al., 2012; Wang et al., 2015). While this discrepancy has yet to be understood, it (along with research since) demonstrates that we have much yet to learn about glia and their interaction with the rest of the neural environment in RTT.
7. Looking forward: Understanding the MeCP2-related structural deficits in the context of postnatal brain development
Through the use of genetic mouse models, we have learned a great deal about the nature of structural deficits in RTT and their relationship with behavioral phenotype. Silencing of hippocampal Mecp2 leads to long-term memory deficits and in some cases changes in dendritic spine densities (Chapleau et al., 2009; Gulmez Karaca et al., 2018). Global postnatal loss of MeCP2 beginning either at 5 or 10 weeks of age not only recapitulates the behavioral phenotype in male and female mice but also resulted in decreased dendritic arborization and spine density in the hippocampus and in decreased CA1 thickness, as well as decreased brain size 10 weeks after the beginning of Mecp2 inactivation via Tamoxifen administration (Nguyen et al., 2012). Mecp2-deletion specifically in GABA-ergic forebrain neurons results in altered striatal structure and function including subregion-depedent changes in DA TH, and D2 receptors (i.e. decreased DA in rostral dorsal striatum but increased relative to WT in the caudal rostral striatum) and coinciding deficits in open field and rotarod performance (Su et al., 2015). Further, recovery of MeCP2 levels within the forebrain alone improves mobility and locomotion but not rotarod performance. We have begun to see that with recovery of MeCP2 and structural and functional changes comes an improvement in short- and long-range connectivity. However, while the gene therapy and Mecp2 re-expression data are promising, there are indications that AAV transfection of Mecp2 does not necessarily ‘cure’ mice and may have the most beneficial effects in younger mice (Garg et al., 2013; Matagne et al., 2017). Delayed reactivation in Mecp2tm2Bird-null mice 7–10 weeks of age and in Mecp2tm2Bird-heterozygous 9–10 months of age mice plateau in neurobehavioral phenotype score (i.e. do not continue to increase in severity but also do not decrease in severity) (Lang et al., 2014). Given this information, it seems that MeCP2 loss in juvenile mice has long-term impacts even if MeCP2 levels are recovered later in life. Postnatally, the brain continues to grow and mature well into late adolescence in a complex series of events, some of which are extremely time sensitive. Experience-dependent plasticity and neural organization such as what has been seen in barrel cortex and visual cortex are two examples of these mechanisms that are perturbed by MeCP2 loss (Krishnan et al., 2015; Lee et al., 2017; Moroto et al., 2013). These as well as other mechanisms by which long-term connections in the brain are established must be evaluated in the context of gene therapy. We have yet to examine the effects of MeCP2 re-expression or restoration on these types of structural deficits after these developmental windows have passed. Arguably understanding not only the relationship of these structural deficits with MeCP2 expression but also in the context of development is critical for fully understanding how we may be able to approach and treat RTT patients with gene therapy or other future therapeutic options.
8. Conclusion
In conclusion, regional structural changes in genetic models of RTT show great similarity to the alterations in brain structure of patients with RTT. These region-specific modifications also coincide with phenotype onset and contribute to larger issues of circuit connectivity, progression, and severity. While many of these changes are due to neuronal cell-autonomous effects as demonstrated by successful rescue with gene therapy or conditional Mecp2 expression, there may be non-cell autonomous mechanisms of neuronal dysfunction including those caused by MeCP2-deficient glia that negatively impact healthy neuronal function. Collectively, this body of work has provided a solid foundation on which to continue to build our understanding of the role of MeCP2 on neuronal and glial structure and function, its greater impact on brain development, and potential new therapeutic avenues.
Supplementary Material
Acknowledgement
This work was supported by NIH grants R21NS10085 and 1U54 HD079123 (SK and MEB) and the Hartwell Foundation (ESS).
Footnotes
Appendix A. Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nlm.2018.11.007.
References
- Aldridge JW, Berridge KC, & Rosen AR (2004). Basal ganglia neural mechanisms of natural movement sequences. Canadian Journal of Physiology and Pharmacology, 82(8–9), 732–739. 10.1139/y04-061. [DOI] [PubMed] [Google Scholar]
- Allemang-Grand R, Ellegood J, Spencer Noakes L, Ruston J, Justice M, Nieman BJ, & Lerch JP (2017). Neuroanatomy in mouse models of Rett syndrome is related to the severity of Mecp2 mutation and behavioral phenotypes. Molecular Autism, 8(1), 1–13. 10.1186/s13229-017-0138-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Saavedra M, Sáez MA, Kang D, Zoghbi HY, & Young JI (2007). Cell-specific expression of wild-type MeCP2 in mouse models of Rett syndrome yields insight about pathogenesis. Human Molecular Genetics, 16(19), 2315–2325. 10.1093/hmg/ddm185. [DOI] [PubMed] [Google Scholar]
- Anderson A, Wong K, Jacoby P, Downs J, & Leonard H (2014). Twenty years of surveillance in Rett syndrome: What does this tell us? Orphanet Journal of Rare Diseases, 9(1), 10.1186/1750-1172-9-87 no pagination. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A, Cole MW, Murray JD, Corlett PR, Wang XJ, & Krystal JH (2012). The role of default network deactivation in cognition and disease. Trends in Cognitive Sciences, 16(12), 584–592. 10.1016/j.tics.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asaka Y, Jugloff DGM, Zhang L, Eubanks JH, & Fitzsimonds RM (2006). Hippocampal synaptic plasticity is impaired in the Mecp2-null mouse model of Rett syndrome. Neurobiology of Disease, 21(1), 217–227. 10.1016/j.nbd.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Badr GG, Witt-Engerström I, & Hagberg B (1987). Brain stem and spinal cord impairment in Rett syndrome: Somatosensory and auditory evoked responses investigations. Brain and Development, 9(5), 517–522. 10.1016/S0387-7604(87)80076-1. [DOI] [PubMed] [Google Scholar]
- Ballas N, Lioy DT, Grunseich C, & Mandel G (2009). Non-cell autonomous influence of MeCP2-deficient glia on neuronal dendritic morphology. Nature Neuroscience, 12(3), 311–317. 10.1038/nn.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A, Rikhye RV, Breton-Provencher V, Tang X, Li C, Li K, ... Sur M (2016). Jointly reduced inhibition and excitation underlies circuit-wide changes in cortical processing in Rett syndrome. Proceedings of the National Academy of Sciences, 113(46), E7287–E7296. 10.1073/pnas.1615330113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belichenko NP, Belichenko PV, Li HH, Mobley WC, & Francke U (2008). Comparative study of brain morphology in Mecp2 mutant mouse models of Rett syndrome. The Journal of Comparative Neurology, 508(1), 184–195. 10.1002/cne.21673. [DOI] [PubMed] [Google Scholar]
- Belichenko PV, Wright EE, Belichenko NP, Masliah E, Li HH, Mobley WC, & Francke U (2009). Widespread changes in dendritic and axonal morphology in Mecp2-mutant mouse models of Rett syndrome: Evidence for disruption of neuronal networks. Journal of Comparative Neurology, 514(3), 240–258. 10.1002/cne.22009. [DOI] [PubMed] [Google Scholar]
- Berger-Sweeney J (2011). Cognitive deficits in Rett syndrome: What we know and what we need to know to treat them. Neurobiology of Learning and Memory, 96(4), 637–646. 10.1016/j.nlm.2011.05.006. [DOI] [PubMed] [Google Scholar]
- Bhattacherjee A, Winter MK, Eggimann LS, Mu Y, Gunewardena S, Liao Z, ... Smith PG (2018). Motor, somatosensory, viscerosensory and metabolic impairments in a heterozygous female rat model of Rett syndrome. International Journal of Molecular Sciences, 19(1), 10.3390/ijms19010097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blue ME, Naidu S, & Johnston MV (1999). Development of amino acid receptors in frontal cortex from girls with rett syndrome. Annals of Neurology, 45(4), 541–544. . [DOI] [PubMed] [Google Scholar]
- Blue ME, Kaufmann WE, Bressler J, Eyring C, O’Driscoll C, Naidu S, & Johnston MV (2011). Temporal and regional alterations in NMDA receptor expression in Mecp2-null mice. Anatomical Record (Hoboken, N.J. : 2007), 294(10), 1624–1634. 10.1002/ar.21380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boche D, Perry VH, & Nicoll JAR (2013). Review: Activation patterns of microglia and their identification in the human brain. Neuropathology and Applied Neurobiology, 39(1), 3–18. 10.1111/nan.12011. [DOI] [PubMed] [Google Scholar]
- Brodkin J, Frank D, Grippo R, Hausfater M, Gulinello M, Achterholt N, & Gutzen C (2014). Validation and implementation of a novel high-throughput behavioral phenotyping instrument for mice. Journal of Neuroscience Methods, 224, 48–57. 10.1016/j.jneumeth.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter JC, Lanham DC, Pham D, Bibat G, Naidu S, & Kaufmann WE (2008). Selective cerebral volume reduction in Rett syndrome: A multiple-approach MR imaging study. American Journal of Neuroradiology, 29(3), 436–441. 10.3174/ajnr.A0857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahrour M, & Zoghbi HY (2007). The story of Rett syndrome: From clinic to neurobiology. Neuron, 56(3), 422–437. 10.1016/j.neuron.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Chao HT, Zoghbi HY, & Rosenmund C (2007). MeCP2 controls excitatory synaptic strength by regulating glutamatergic synapse number. Neuron, 56(1), 58–65. 10.1016/j.neuron.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapleau CA, Calfa GD, Lane MC, Albertson AJ, Larimore JL, Kudo S, ... Pozzo-Miller L (2009). Dendritic spine pathologies in hippocampal pyramidal neurons from Rett syndrome brain and after expression of Rett-associated MECP2 mutations. Neurobiology of Disease, 35(2), 219–233. 10.1016/j.nbd.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiron C, Bulteau C, Loc’h C, Raynaud C, Garreau B, Syrota A, & Maziere B (1993). Dopaminergic D2 receptor SPECT imaging in Rett syndrome: increase of specific binding in striatum. Journal of Nuclear Medicine, 34(10), 1717–1721. [PubMed] [Google Scholar]
- Colton CA, & Wilcock DM (2010). Assessing activation states in microglia. CNS & Neurological Disorders - Drug Targets, 9(2), 174–191. 10.2174/187152710791012053. [DOI] [PubMed] [Google Scholar]
- Cortelazzo A, De Felice C, Guerranti R, Signorini C, Leoncini S, Pecorelli A, ...Hayek J (2014). Subclinical inflammatory status in Rett syndrome. Mediators of Inflammation, 2014. 10.1155/2014/480980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crair MC, & Malenka RC (1995). A critical period for long-term potentiation at thalamocortical synapses. Nature. 10.1038/375325a0. [DOI] [PubMed] [Google Scholar]
- Cronk JC, Derecki NC, Ji E, Xu Y, Lampano AE, Smirnov I, ... Kipnis J (2015). Methyl-CpG binding protein 2 regulates microglia and macrophage gene expression in response to inflammatory stimuli. Immunity, 42(4), 679–691. 10.1016/j.immuni.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani VS, Chang Q, Maffei A, Turrigiano GG, Jaenisch R, & Nelson SB (2005). Reduced cortical activity due to a shift in the balance between excitation and inhibition in a mouse model of Rett syndrome. Proceedings of the National Academy of Sciences, 102(35), 12560–12565. 10.1073/pnas.0506071102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani VS, & Nelson SB (2009). Intact long-term potentiation but reduced connectivity between neocortical layer 5 pyramidal neurons in a mouse model of Rett syndrome. Journal of Neuroscience, 29(36), 11263–11270. 10.1523/JNEUROSCI.1019-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Biase LM, Schuebel KE, Fusfeld ZH, Jair K, Hawes IA, Cimbro R, ... Bonci A (2017). Local cues establish and maintain region-specific phenotypes of basal ganglia microglia. Neuron, 95(2), 341–356.e6. 10.1016/j.neuron.2017.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Felice C, Della Ragione F, Signorini C, Leoncini S, Pecorelli A, Ciccoli L, ... D’Esposito M (2014). Oxidative brain damage in Mecp2-mutant murine models of Rett syndrome. Neurobiology of Disease, 68, 66–77. 10.1016/j.nbd.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Filippis B, Valenti D, de Bari L, De Rasmo D, Musto M, Fabbri A, ... Vacca RA (2015). Mitochondrial free radical overproduction due to respiratory chain impairment in the brain of a mouse model of Rett syndrome: Protective effect of CNF1. Free Radical Biology & Medicine, 83, 167–177. 10.1016/j.freeradbiomed.2015.02.014. [DOI] [PubMed] [Google Scholar]
- Derecki NC, Cronk JC, Lu Z, Xu E, Abbott SBG, Guyenet PG, & Kipnis J (2012). Wild-type microglia arrest pathology in a mouse model of Rett syndrome. Nature, 484(7392), 105–109. 10.1038/nature10907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand S, Patrizi A, Quast KBB, Hachigian L, Pavlyuk R, Saxena A, ... Fagiolini M (2012). NMDA receptor regulation prevents regression of visual cortical function in the absence of Mecp2. Neuron, 76(6), 1078–1090. 10.1016/j.neuron.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filosa S, Pecorelli A, D’Esposito M, Valacchi G, Hajek J, D’Esposito M, & Hajek J (2015). Exploring the possible link between MeCP2 and oxidative stress in Rett syndrome. Free Radical Biology & Medicine, 88, 81–90. 10.1016/j.freeradbiomed.2015.04.019. [DOI] [PubMed] [Google Scholar]
- Fukuda T, Itoh M, Ichikawa T, Washiyama K, & Goto YI (2005). Delayed maturation of neuronal architecture and synaptogenesis in cerebral cortex of Mecp2-deficient mice. Journal of Neuropathology and Experimental Neurology, 64(6), 537–544. 10.1093/jnen/64.6.537. [DOI] [PubMed] [Google Scholar]
- Gantz SC, Ford CP, Neve KA, & Williams JT (2011). Loss of Mecp2 in substantia nigra dopamine neurons compromises the nigrostriatal pathway. Journal of Neuroscience, 31(35), 12629–12637. 10.1523/JNEUROSCI.0684-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg SK, Lioy DT, Cheval H, McGann JC, Bissonnette JM, Murtha MJ, ... Mandel G (2013). Systemic delivery of MeCP2 rescues behavioral and cellular deficits in female mouse models of Rett syndrome. Journal of Neuroscience, 33(34), 13612–13620. 10.1523/JNEUROSCI.1854-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogliotti RG, Senter RK, Fisher NM, Adams J, Zamorano R, Walker AG, ... Niswender CM (2017). mGlu 7 potentiation rescues cognitive, social, and respiratory phenotypes in a mouse model of Rett syndrome. Science Translational Medicine, 9(403), eaai7459. 10.1126/scitranslmed.aai7459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogliotti RG, Senter RK, Rook JM, Ghoshal A, Zamorano R, Malosh C, ... Niswender CM (2016). MGlu5 positive allosteric modulation normalizes synaptic plasticity defects and motor phenotypes in a mouse model of Rett syndrome. Human Molecular Genetics, 25(10), 1990–2004. 10.1093/hmg/ddw074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybiel AM (2008). Habits, rituals, and the evaluative brain. Annual Review of Neuroscience, 31(1), 359–387. 10.1146/annurev.neuro.29.051605.112851. [DOI] [PubMed] [Google Scholar]
- Graybiel AM, Grafton ST, 2015. The Striatum: Where Skills and Habits Meet, 1–13. 10.1101/cshperspect.a021691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybiel AM, & Saka E (2002). A genetic basis for obsessive grooming. Neuron, 33(1), 1–2. 10.1016/S0896-6273(01)00575-X. [DOI] [PubMed] [Google Scholar]
- Großer E, Hirt U, Janc OA, Menzfeld C, Fischer M, Kempkes B, ... Müller M (2012). Oxidative burden and mitochondrial dysfunction in a mouse model of Rett syndrome. Neurobiology of Disease, 48(1), 102–114. 10.1016/j.nbd.2012.06.007. [DOI] [PubMed] [Google Scholar]
- Gulmez Karaca K, Brito DVC, Zeuch B, & Oliveira AMM (2018). Adult hippocampal MeCP2 preserves the genomic responsiveness to learning required for long-term memory formation. Neurobiology of Learning and Memory, 149(November 2017), 84–97. 10.1016/j.nlm.2018.02.010. [DOI] [PubMed] [Google Scholar]
- Guy J, Hendrich B, Holmes M, Martin JE, & Bird A (2001). A mouse Mecp2-null mutation causes neurological symptoms that mimic Rett syndrome. Nature Genetics, 27(3), 322–326. 10.1038/85899. [DOI] [PubMed] [Google Scholar]
- Hao S, Tang B, Wu Z, Ure K, Sun Y, Tao H, ... Tang J (2015). Forniceal deep brain stimulation rescues hippocampal memory in Rett syndrome mice. Nature, 526(7573), 430–434. 10.1038/nature15694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He LJ, Liu N, Cheng TL, Chen XJ, Li YD, Shu YS, ... Zhang XH (2014). Conditional deletion of Mecp2 in parvalbumin-expressing GABAergic cells results in the absence of critical period plasticity. Nature Communications, 5, 1–15. 10.1038/ncomms6036. [DOI] [PubMed] [Google Scholar]
- Horiuchi M, Smith L, Maezawa I, & Jin L-W (2016). CX3CR1 ablation ameliorates motor and respiratory dysfunctions and improves survival of a Rett syndrome mouse model. Brain, Behavior, and Immunity, 60, 106–116. 10.1016/j.bbi.2016.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshiko M, Arnoux I, Avignone E, Yamamoto N, & Audinat E (2012). Deficiency of the microglial receptor CX3CR1 impairs postnatal functional development of thalamocortical synapses in the barrel cortex. Journal of Neuroscience, 32(43), 15106–15111. 10.1523/JNEUROSCI.1167-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell CJ, Sceniak MP, Lang M, Krakowiecki W, Abouelsoud FE, Lad SU, ... Katz DM (2017). Activation of the medial prefrontal cortex reverses cognitive and respiratory symptoms in a mouse model of Rett syndrome. ENEURO.0277–17.2017 Eneuro, 4(6), 10.1523/ENEURO.0277-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocher PR (1999). Dendritic and synaptic development in human cerebral cortex: Time course and critical periods. Developmental Neuropsychology, 16(3), 347–349. 10.1207/S15326942DN1603. [DOI] [Google Scholar]
- Inan M, & Crair MC (2007). Development of cortical maps: Perspectives from the barrel cortex. Neuroscientist, 13(1), 49–61. 10.1177/1073858406296257. [DOI] [PubMed] [Google Scholar]
- Janc OA, & Müller M (2014). The free radical scavenger Trolox dampens neuronal hyperexcitability, reinstates synaptic plasticity, and improves hypoxia tolerance in a mouse model of Rett syndrome. Frontiers in Cellular Neuroscience, 8(February), 56. 10.3389/fncel.2014.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin L-W, Horiuchi M, Wulff H, Liu X-B, Cortopassi GA, Erickson JD, & Maezawa I (2015). Dysregulation of glutamine transporter SNAT1 in Rett syndrome microglia: A mechanism for mitochondrial dysfunction and neurotoxicity. Journal of Neuroscience, 35(6), 2516–2529. 10.1523/JNEUROSCI.2778-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RA, Lam M, Punzo AM, Li H, Lin BR, Ye K, ... Chang Q (2012). 7,8-dihydroxyflavone exhibits therapeutic efficacy in a mouse model of Rett syndrome. Journal of Applied Physiology, 112(5), 704–710. 10.1152/japplphysiol.01361.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jugloff DGM, Vandamme K, Logan R, Visanji NP, Brotchie JM, & Eubanks JH (2008). Targeted delivery of an Mecp2 transgene to forebrain neurons improves the behavior of female Mecp2-deficient mice. Human Molecular Genetics, 17(10), 1386–1396. 10.1093/hmg/ddn026. [DOI] [PubMed] [Google Scholar]
- Kao FC, Su SH, Carlson GC, & Liao W (2015). MeCP2-mediated alterations of striatal features accompany psychomotor deficits in a mouse model of Rett syndrome. Brain Structure & Function, 220(1), 419–434. 10.1007/s00429-013-0664-x. [DOI] [PubMed] [Google Scholar]
- Kaufmann WE, Stallworth JL, Everman DB, & Skinner SA (2016). Neurobiologically-based treatments in Rett syndrome: Opportunities and challenges. Expert Opinion on Orphan Drugs, 4(10), 1043–1055. 10.1080/21678707.2016.1229181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr B, Alvarez-Saavedra M, Sáez MA, Saona A, & Young JI (2008). Defective body-weight regulation, motor control and abnormal social interactions in Mecp2 hypomorphic mice. Human Molecular Genetics, 17(12), 1707–1717. 10.1093/hmg/ddn061. [DOI] [PubMed] [Google Scholar]
- Kondo M, Gray LJ, Pelka GJ, Christodoulou J, Tam PPL, & Hannan AJ (2008). Environmental enrichment ameliorates a motor coordination deficit in a mouse model of Rett syndrome - Mecp2 gene dosage effects and BDNF expression. European Journal of Neuroscience, 27(12), 3342–3350. 10.1111/j.1460-9568.2008.06305.x. [DOI] [PubMed] [Google Scholar]
- Kozlowski C, & Weimer RM (2012). An automated method to quantify microglia morphology and application to monitor activation state longitudinally in vivo. PLoS One, 7(2), 1–9. 10.1371/journal.pone.0031814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan K, Wang B-S, Lu J, Wang L, Maffei A, Cang J, & Huang ZJ (2015). MeCP2 regulates the timing of critical period plasticity that shapes functional connectivity in primary visual cortex. Proceedings of the National Academy of Sciences, 112(34), E4782–E4791. 10.1073/pnas.1506499112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kron M, Howell CJ, Adams IT, Ransbottom M, Christian D, Ogier M, & Katz DM (2012). Brain activity mapping in Mecp2 mutant mice reveals functional deficits in forebrain circuits, including key nodes in the default mode network, that are reversed with ketamine treatment. The Journal of Neuroscience, 32(40), 13860–13872. 10.1523/JNEUROSCI.2159-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang M, Wither RG, Colic S, Wu C, Monnier PP, Bardakjian BL, ... Eubanks JH (2014). Rescue of behavioral and EEG deficits in male and female Mecp2-deficient mice by delayed Mecp2 gene reactivation. Human Molecular Genetics, 23(2), 303–318. 10.1093/hmg/ddt421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson LJ, Perry VH, Dri P, & Gordon S (1990). Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience, 39(1), 151–170. 10.1016/0306-4522(90)90229-W. [DOI] [PubMed] [Google Scholar]
- Lee L-J, Tsytsarev V, & Erzurumlu RS (2017). Structural and functional differences in the barrel cortex of Mecp2 null mice. Journal of Comparative Neurology, 3951–3961. 10.1002/cne.24315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leoncini S, De Felice C, Signorini C, Zollo G, Cortelazzo A, Durand T, ... Hayek J (2015). Cytokine dysregulation in MECP2- and CDKL5-related Rett syndrome: Relationships with aberrant redox homeostasis, inflammation, and ω−3 PUFAs. 421624 Oxidative Medicine and Cellular Longevity, 2015. 10.1155/2015/421624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Xu X, & Pozzo-Miller L (2016). Excitatory synapses are stronger in the hippocampus of Rett syndrome mice due to altered synaptic trafficking of AMPA-type glutamate receptors. Proceedings of the National Academy of Sciences, 113(11), E1575–E1584. 10.1073/pnas.1517244113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo F-S, Blue ME, & Erzurumlu RS (2015). Enhancement of postsynaptic GABA A and extrasynaptic NMDA receptor-mediated responses in the barrel cortex of Mecp2-null mice. Journal of Neurophysiology. 10.1152/jn.00944.2015 jn.00944.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonetti G, Angelucci A, Morando L, Boggio EM, Giustetto M, & Pizzorusso T (2010). Early environmental enrichment moderates the behavioral and synaptic phenotype of MeCP2 null mice. Biological Psychiatry, 67(7), 657–665. 10.1016/j.biopsych.2009.12.022. [DOI] [PubMed] [Google Scholar]
- Lu H, Ash RT, He L, Kee SE, Wang W, Yu D, ... Zoghbi HY (2016). Loss and gain of MeCP2 cause similar hippocampal circuit dysfunction that is rescued by deep brain stimulation in a Rett syndrome mouse model. Neuron, 91(4), 739–747. 10.1016/j.neuron.2016.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maezawa I, & Jin LW (2010). Rett syndrome microglia damage dendrites and synapses by the elevated release of glutamate. Journal of Neuroscience, 30(15), 5346–5356. 10.1523/JNEUROSCI.5966-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matagne V, Ehinger Y, Saidi L, Borges-Correia A, Barkats M, Bartoli M, ... Roux JC (2017). A codon-optimized Mecp2 transgene corrects breathing deficits and improves survival in a mouse model of Rett syndrome. Neurobiology of Disease, 99, 1–11. 10.1016/j.nbd.2016.12.009. [DOI] [PubMed] [Google Scholar]
- Metcalf BM, Mullaney BC, Johnston MV, & Blue ME (2006). Temporal shift in methyl-CpG binding protein 2 expression in a mouse model of Rett syndrome. Neuroscience, 139(4), 1449–1460. 10.1016/j.neuroscience.2006.01.060. [DOI] [PubMed] [Google Scholar]
- Mierau SB, Patrizi A, Hensch TK, & Fagiolini M (2015). Cell-specific regulation of N-methyl-D-aspartate receptor maturation by Mecp2 in cortical circuits. Biological Psychiatry. 10.1016/j.biopsych.2015.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretti P (2006). Learning and memory and synaptic plasticity are impaired in a mouse model of Rett syndrome. Journal of Neuroscience, 26(1), 319–327. 10.1523/JNEUROSCI.2623-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroto M, Nishimura A, Morimoto M, Isoda K, Morita T, Yoshida M, ... Hosoi H (2013). Altered somatosensory barrel cortex refinement in the developing brain of Mecp2-null mice. Brain Research, 1537, 319–326. 10.1016/j.brainres.2013.09.017. [DOI] [PubMed] [Google Scholar]
- Mullaney BC, Johnston MV, & Blue ME (2004). Developmental expression of methyl-CpG binding protein 2 is dynamically regulated in the rodent brain. Neuroscience, 123(4), 939–949. 10.1016/j.neuroscience.2003.11.025. [DOI] [PubMed] [Google Scholar]
- Murakami JW, Courchesne E, Haas RH, Press GA, & Yeung-Courchesne R (1992). Cerebellar and cerebral abnormalities in Rett syndrome: A quantitative MR analysis. American Journal of Roentgenology, 159(1), 177–183. 10.2214/ajr.159.1.1609693. [DOI] [PubMed] [Google Scholar]
- Naidu S, Kaufmann WE, Abrams MT, Pearlson GD, Lanham DC, Fredericksen K, & Johnston MV (2001). Neuroimaging studies in Rett syndrome. Brain and Development, 23, S62–S71. 10.1016/S0387-7604(01)00381-3. [DOI] [PubMed] [Google Scholar]
- Nance E, Kambhampati SP, Smith ES, Zhang Z, Zhang F, Singh S, ... Kannan S (2017). Dendrimer-mediated delivery of N-acetyl cysteine to microglia in a mouse model of Rett syndrome. Journal of Neuroinflammation, 14(1), 1–19. 10.1186/s12974-017-1004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson ED, Kavalali ET, & Monteggia LM (2006). MeCP2-dependent transcriptional repression regulates excitatory neurotransmission. Current Biology, 16(7), 710–716. 10.1016/j.cub.2006.02.062. [DOI] [PubMed] [Google Scholar]
- Neul JL, Kaufmann WE, Glaze DG, Christodoulou J, Clarke AJ, Bahi-Buisson N, ... Percy AK (2010). Rett syndrome: Revised diagnostic criteria and nomenclature. Annals of Neurology, 68(6), 944–950. 10.1002/ana.22124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen MVC, Du F, Felice CA, Shan X, Nigam A, Mandel G, ... Ballas N (2012). MeCP2 is critical for maintaining mature neuronal networks and global brain anatomy during late stages of postnatal brain development and in the mature adult brain. Journal of Neuroscience, 32(29), 10021–10034. 10.1523/JNEUROSCI.1316-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe Y, Takahashi T, Mitsumasu C, Kosai K, Tanaka E, & Matsuishi T (2012). Alterations of gene expression and glutamate clearance in astrocytes derived from an MeCP2-null mouse model of Rett syndrome. e35354 PloS One, 7(4), 10.1371/journal.pone.0035354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panayotis N, Pratte M, Borges-Correia A, Ghata A, Villard L, & Roux JC (2011). Morphological and functional alterations in the substantia nigra pars compacta of the Mecp2-null mouse. Neurobiology of Disease, 41(2), 385–397. 10.1016/j.nbd.2010.10.006. [DOI] [PubMed] [Google Scholar]
- Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, ... Gross CT (2011a). Development synaptic pruning by microglia is necessary for normal brain synaptic pruning by microglia is necessary for normal. Brain Development, 333(September), 1456–1459. 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, ... Gross CT (2011b). Synaptic pruning by microglia is necessary for normal brain synaptic pruning by microglia is necessary for normal brain development. Science, 333(September), 1456–1459. 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- Parkhurst CN, Yang G, Ninan I, Savas JN, Yates JR, Lafaille JJ, ... Gan WB (2013). Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell, 155(7), 1596–1609. 10.1016/j.cell.2013.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrizi A, Picard N, Simon AJ, Gunner G, Centofante E, Andrews NA, & Fagiolini M (2016). Chronic administration of the N-methyl-D-aspartate receptor antagonist ketamine improves Rett syndrome phenotype. Biological Psychiatry, 79(9), 755–764. 10.1016/j.biopsych.2015.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecorelli A, Cervellati C, Hayek J, & Valacchi G (2016). OxInflammation in Rett syndrome. International Journal of Biochemistry and Cell Biology, 81, 246–253. 10.1016/j.biocel.2016.07.015. [DOI] [PubMed] [Google Scholar]
- Pelka GJ, Watson CM, Radziewic T, Hayward M, Lahooti H, Christodoulou J, & Tam PPL (2006). Mecp2 deficiency is associated with learning and cognitive deficits and altered gene activity in the hippocampal region of mice. Brain, 129(4), 887–898. 10.1093/brain/awl022. [DOI] [PubMed] [Google Scholar]
- Pohodich AE, Yalamanchili H, Raman AT, Wan YW, Gundry M, Hao S, ... Zoghbi HY (2018). Forniceal deep brain stimulation induces gene expression and splicing changes that promote neurogenesis and plasticity. ELife, 7, 1–31. 10.7554/eLife.34031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzo-Miller L, Pati S, & Percy AK (2015). Rett syndrome: Reaching for clinical trials. Neurotherapeutics, 12(3), 631–640. 10.1007/s13311-015-0353-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratte M, Panayotis N, Ghata A, Villard L, & Roux JC (2011). Progressive motor and respiratory metabolism deficits in post-weaning Mecp2-null male mice. Behavioural Brain Research, 216(1), 313–320. 10.1016/j.bbr.2010.08.011. [DOI] [PubMed] [Google Scholar]
- Qiu Z, Sylwestrak EL, Lieberman DN, Zhang Y, Liu X-Y, & Ghosh A (2012). The Rett syndrome protein MeCP2 regulates synaptic scaling. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 32(3), 989–994. 10.1523/JNEUROSCI.0175-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME (2015). The brain’s default mode network. Annual Review of Neuroscience, 38(1), 433–447. 10.1146/annurev-neuro-071013-014030. [DOI] [PubMed] [Google Scholar]
- Reiss AL, Faruque F, Naidu S, Abrams M, Beaty T, Bryan RN, & Moser H (1993). Neuroanatomy of Rett syndrome: A volumetiric imagmg study. Annals of Neurology, 34, 227–234. [DOI] [PubMed] [Google Scholar]
- Rietveld L, Stuss DP, McPhee D, & Delaney KR (2015). Genotype-specific effects of Mecp2 loss-of-function on morphology of layer V pyramidal neurons in heterozygous female Rett syndrome model mice. Frontiers in Cellular Neuroscience, 9(April), 1–17. 10.3389/fncel.2015.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaco RC, Fryer JD, Ren J, Fyffe S, Chao HT, Sun Y, ... Neul JL (2008). A partial loss of function allele of methyl-CpG-binding protein 2 predicts a human neurodevelopmental syndrome. Human Molecular Genetics, 17(12), 1718–1727. 10.1093/hmg/ddn062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaco RC, Mandel-Brehm C, Chao H-T, Ward CS, Fyffe-Maricich SL, Ren J, ... Neul JL (2009). Loss of MeCP2 in aminergic neurons causes cell-autonomous defects in neurotransmitter synthesis and specific behavioral abnormalities. Proceedings of the National Academy of Sciences of the United States of America, 106(51), 21966–21971. 10.1073/pnas.0912257106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaco RC, Mcgraw CM, Ward CS, Sun Y, Neul JL, & Zoghbi HY (2013). Female Mecp2+/− mice display robust behavioral deficits on two different genetic backgrounds providing a framework for pre-clinical studies. Human Molecular Genetics, 22(1), 96–109. 10.1093/hmg/dds406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sceniak MP, Lang M, Enomoto AC, James Howell C, Hermes DJ, & Katz DM (2016). Mechanisms of functional hypoconnectivity in the medial prefrontal cortex of Mecp2 null mice. Cerebral Cortex, 26(5), 1938–1956. 10.1093/cercor/bhv002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaevitz LR, Gómez NB, Zhen DP, & Berger-Sweeney JE (2013). MeCP2 R168X male and female mutant mice exhibit Rett-like behavioral deficits. Genes, Brain, and Behavior, 12(7), 732–740. 10.1111/gbb.12070. [DOI] [PubMed] [Google Scholar]
- Schaevitz LR, Moriuchi JM, Nag N, Mellot TJ, & Berger-Sweeney J (2010). Cognitive and social functions and growth factors in a mouse model of Rett syndrome. Physiology and Behavior, 100(3), 255–263. 10.1016/j.physbeh.2009.12.025. [DOI] [PubMed] [Google Scholar]
- Schafer DP, Heller CT, Gunner G, Heller M, Gordon C, Hammond T, ... Stevens B (2016). Microglia contribute to circuit defects in Mecp2 null mice independent of microglia-specific loss of Mecp2 expression. ELife, 5(2016. JULY), 1–19. 10.7554/eLife.15224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, ... Stevens B (2012). Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron, 74(4), 691–705. 10.1016/j.neuron.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid DA, Yang T, Ogier M, Adams I, Mirakhur Y, Wang Q, ... Katz DM (2012). A TrkB small molecule partial agonist rescues TrkB phosphorylation deficits and improves respiratory function in a mouse model of Rett syndrome. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 32(5), 1803–1810. 10.1523/JNEUROSCI.0865-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahbazian MD, Antalffy B, Armstrong DL, & Zoghbi HY (2002). Insight into Rett syndrome: MeCP2 levels display tissue-and cell-specific differences and correlate with neuronal maturation. Human Molecular Genetics, 11(2), 115–124. 10.1093/hmg/11.2.115. [DOI] [PubMed] [Google Scholar]
- Stearns NA, Schaevitz LR, Bowling H, Nag N, Berger UV, & Berger-Sweeney J (2007). Behavioral and anatomical abnormalities in Mecp2 mutant mice: A model for Rett syndrome. Neuroscience, 146(3), 907–921. 10.1016/j.neuroscience.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Stuss DP, Boyd JD, Levin DB, & Delaney KR (2012). MeCP2 mutation results in compartment-specific reductions in dendritic branching and spine density in layer 5 motor cortical neurons of YFP-H mice. PLoS ONE, 7(3), 1–11. 10.1371/journal.pone.0031896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su S-H, Kao F-C, Huang Y-B, & Liao W (2015). MeCP2 in the rostral striatum maintains local dopamine content critical for psychomotor control. Journal of Neuroscience, 35(15), 6209–6220. 10.1523/JNEUROSCI.4624-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam B, Naidu S, & Reiss AL (1997). Neuroanatomy in Rett syndrome, i, 487–489. [DOI] [PubMed] [Google Scholar]
- Szczesna K, De La Caridad O, Petazzi P, Soler M, Roa L, Saez MA, ... Esteller M (2014). Improvement of the rett syndrome phenotype in a Mecp2 mouse model upon treatment with levodopa and a dopa-decarboxylase inhibitor. Neuropsychopharmacology, 39(12), 2846–2856. 10.1038/npp.2014.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagawa Y, Kanold PO, Majdan M, & Shatz CJ (2005). Multiple periods of functional ocular dominance plasticity in mouse visual cortex. Nature Neuroscience, 8(3), 380–388. 10.1038/nn1410. [DOI] [PubMed] [Google Scholar]
- Tao XJ, Wu H, Coronado AA, De Laittre E, Osterweil XK, Zhang Y, ... Bear F (2016). Negative allosteric modulation of mGluR5 partially corrects pathophysiology in a mouse model of Rett syndrome. Neurobiology of Disease, 36(47), 11946–11958. 10.1523/JNEUROSCI.0672-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tau GZ, & Peterson BS (2010). Normal development of brain circuits. Neuropsychopharmacology, 35(1), 147–168. 10.1038/npp.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theoharides TC, Athanassiou M, Panagiotidou S, & Doyle R (2015). Dysregulated brain immunity and neurotrophin signaling in Rett syndrome and autism spectrum disorders. Journal of Neuroimmunology, 279, 33–38. 10.1016/j.jneuroim.2014.12.003. [DOI] [PubMed] [Google Scholar]
- Tropea D, Giacometti E, Wilson NR, Beard C, McCurry C, Fu DD, ... Sur M (2009). Partial reversal of Rett syndrome-like symptoms in MeCP2 mutant mice. Proceedings of the National Academy of Sciences, 106(6), 2029–2034. 10.1073/pnas.0812394106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno M, Fujita Y, Tanaka T, Nakamura Y, Kikuta J, Ishii M, & Yamashita T (2013). Layer v cortical neurons require microglial support for survival during postnatal development. Nature Neuroscience, 16(5), 543–551. 10.1038/nn.3358. [DOI] [PubMed] [Google Scholar]
- Ueno M, & Yamashita T (2014). Bidirectional tuning of microglia in the developing brain: From neurogenesis to neural circuit formation. Current Opinion in Neurobiology, 27, 8–15. 10.1016/j.conb.2014.02.004. [DOI] [PubMed] [Google Scholar]
- Veeraragavan S, Wan YW, Connolly DR, Hamilton SM, Ward CS, Soriano S, ... Samaco RC (2015). Loss of MeCP2 in the rat models regression, impaired sociability and transcriptional deficits of Rett syndrome. Human Molecular Genetics, 25(15), 3284–3302. 10.1093/hmg/ddw178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Tetzchner S, Jacobsen KH, Smith L, Skjeldal OH, Heiberg a, & Fagan JF (1990). Vision, cognition and developmental characteristics of girls and women with Rett syndrome. Developmental Medicine and Child Neurology, 38, 212–225. 10.1097/00001577-199801030-00013. [DOI] [PubMed] [Google Scholar]
- Wang J, Wegener JE, Huang T-W, Sripathy S, De Jesus-Cortes H, Xu P, ... Pieper AA (2015). Wild-type microglia do not reverse pathology in a mouse model of Rett syndrome. Nature, 521, E1–E4. 10.1038/nature10907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward BC, Kolodny NH, Nag N, & Berger-Sweeney JE (2009). Neurochemical changes in a mouse model of Rett syndrome: Changes over time and in response to perinatal choline nutritional supplementation. Journal of Neurochemistry, 108(2), 361–371. 10.1111/j.1471-4159.2008.05768.x. [DOI] [PubMed] [Google Scholar]
- Wegener E, Brendel C, Fischer A, Hülsmann S, Gärtner J, & Huppke P (2014). Characterization of the MeCP2R168X knockin mouse model for Rett syndrome. e115444 PLoS One, 9(12), 10.1371/journal.pone.0115444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng S-M, McLeod F, Bailey MES, & Cobb SR (2011). Synaptic plasticity deficits in an experimental model of Rett syndrome: Long-term potentiation saturation and its pharmacological reversal. Neuroscience, 180, 314–321. 10.1016/j.neuroscience.2011.01.061. [DOI] [PubMed] [Google Scholar]
- Wong DF, Ricaurte G, Gründer G, Rothman R, Naidu S, Singer H, ... & Gjedde (1998). Dopamine transporter changes in neuropsychiatric disorders. In: Advances in Pharmacology (Vol. 42, pp. 219–223). Academic Press. [DOI] [PubMed] [Google Scholar]
- Wong DF, Blue ME, Brašić JR, Nandi A, Valentine H, Stansfield KH, ... Naidu SB (2018). Are dopamine receptor and transporter changes in Rett syndrome reflected in Mecp2-deficient mice? Experimental Neurology, 307(October 2017), 74–81. 10.1016/j.expneurol.2018.05.019. [DOI] [PubMed] [Google Scholar]
- Wood L, & Shepherd GMG (2010). Synaptic circuit abnormalities of motor-frontal layer 2/3 pyramidal neurons in a mutant mouse model of Rett syndrome. Neurobiology of Disease, 38(2), 281–287. 10.1016/j.nbd.2010.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Miller EC, & Pozzo-Miller L (2014). Dendritic spine dysgenesis in Rett syndrome. Frontiers in Neuroanatomy, 8(September), 1–8. 10.3389/fnana.2014.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa H, Kaga M, Suzuki H, Sakuragawa N, & Arima M (1991). Giant somatosensory evoked potentials in the Rett syndrome. Brain and Development, 13(1), 36–39. 10.1016/S0387-7604(12)80295-6. [DOI] [PubMed] [Google Scholar]
- Zhao D, Mokhtari R, Pedrosa E, Birnbaum R, Zheng D, & Lachman HM (2017). Transcriptome analysis of microglia in a mouse model of Rett syndrome: Differential expression of genes associated with microglia/macrophage activation and cellular stress. Molecular Autism, 8(1), 17. 10.1186/s13229-017-0134-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.