Abstract
Recent reports identify sensory subtypes in ASD based on shared patterns of responses to daily sensory stimuli [Ausderau et al., 2014; Lane, Molloy, & Bishop, 2014]. Lane et al. propose that two broad sensory dimensions, sensory reactivity and multisensory integration, best explain the differences between subtypes, however this has yet to be tested. The present study tests this hypothesis by examining the latent constructs underlying Lane’s sensory subtypes. Participants for this study were caregivers of children with autism spectrum disorder (ASD) aged 2–12 years. Caregiver responses on the Short Sensory Profile (SSP), used to establish Lane’s sensory subtypes, were extracted from two existing datasets (total n 5 287). Independent component analyses were conducted to test the fit and interpretability of a two-construct structure underlying the SSP, and therefore, the sensory subtypes. The first construct was largely comprised of the taste/smell sensitivity domain, which describes hyper-reactivity to taste and smell stimuli. The second construct had a significant contribution from the low energy/weak domain, which describes behaviors that may be indicative of difficulties with multisensory integration. Findings provide initial support for our hypothesis that sensory reactivity and multisensory integration underlie Lane’s sensory subtypes in ASD.
Introduction
Sensory features are reported in up to 96% of individuals with autism spectrum disorder (ASD) and are now included in the diagnostic criteria for ASD [American Psychiatric Association, 2013; Ben-Sasson et al., 2008; Leekam, Nieto, Libby, Wing, & Gould, 2006; Schaaf & Lane, 2015]. Sensory features refer to atypical perceptual and behavioral responses to daily sensory stimuli. The presentation of sensory features in ASD can take a number of forms including: hyper-reactivity (i.e., an intense and usually negative behavioral response to a nonthreatening stimulus); hypo-reactivity (i.e., a reduced or absent response to a sensory stimulus), unusual sensory interests (i.e., behaviors that suggest craving or seeking of sensory input); inaccurate perception of sensory stimuli (i.e., misinterpretation of perceptual elements of sensory stimuli, e.g., size, volume, pitch), and/or difficulties integrating multiple concurrent sensory stimuli (e.g., distinguishing speech sounds in a visually distracting background). Anecdotal and caregiver reports indicate that individuals with ASD can experience varying combinations of sensory features across a range of modalities and at different levels of severity (Koenig & Kinnealy, 2008). Many functional difficulties in ASD including: transitioning to new environments, maintaining attention in a busy background, and interpreting nonverbal social cues have been attributed to sensory features [Koenig & Kinnealy, 2008; Schaaf & Lane, 2015] and therapies to address these issues are highly popular [Interactive Autism Network, 2014]. Further advances in our understanding of the role of sensory features in the clinical presentation of ASD, however, are limited by their imprecise characterization.
Recently, several research groups have identified subsets of individuals with ASD based on shared sensory features [Ausderau et al., 2014; Lane, Molloy, & Bishop, 2014; Tomchek, Huebner, & Dunn, 2014]. These subsets within ASD have been labeled “sensory subtypes.” The sensory subtyping approach signals a deviation from previous descriptions of sensory symptoms which focused on discrete sensory behaviors (e.g., hyperreactivity, hyporeactivity, etc.; Dunn, 1997) rather than patterns of behavior coincident within individuals. Identifying subsets of individuals with ASD through the more precise characterization of symptom profiles, such as sensory subtypes, has important implications for the field [IACC, 2013]. Linkage of sensory subtypes with specific performance limitations in ASD may guide clinical decision-making such that it is more evident “what will work for whom” [Trembath & Vivanti, 2014]. Subsequently, intervention effectiveness is likely to be optimized [Ashburner, Rodger, Ziviani, & Jones, 2014]. While various sensory subtyping models have been documented in the literature, there is promising convergence among models. Below, we discuss the two models that have focused exclusively on patterns of sensory features without inclusion of other, non-sensory, characteristics.
The first published sensory subtype model by Lane and colleagues proposed that individuals with ASD varied in the presentation of sensory features based on their frequency of sensory features and sensory modality affected [i.e., taste, smell, tactile, auditory, etc.; Lane, Dennis, & Geraghty, 2011; Lane, Molloy et al., 2014; Lane, Young, Baker, & Angley, 2010]. In the most recent version of this model, individuals with ASD were classified into one of four subtypes using parent responses to the Short Sensory Profile [SSP; Lane, Molloy et al., 2014; McIntosh, Miller, Shyu, & Dunn, 1999]. The four subtypes were characterized as follows:
Sensory Adaptive—sensory features are low in frequency and not substantive enough to result in functional performance limitations;
Taste/Smell Sensitive—sensory features are extreme in the area of taste/smell sensitivity;
Postural Inattentive—sensory features are extreme in the area of proprioceptive functioning as manifested through postural and force modulation issues; and
Generalized Sensory Difference—sensory features are clinically significant across all sensory modalities.
Preliminary work indicates that there are distinct non-sensory characteristics associated with each subtype. For example, children in the Taste/Smell Sensitive subtype have more severe communication impairments and are pickier eaters, while children in the Generalized Sensory Difference subtype demonstrate more maladaptive behaviors [Lane, Geraghty, Young, & Rostorfer, 2014; Lane et al., 2010]. Additionally, there are differences between the subtypes on daily living skills, emotional regulation, and attention [Tanner & Lane, unpublished manuscript]. Despite these differences, membership in the four sensory subtypes is not well explained by systematic variation in age, non-verbal IQ, or severity of non-sensory ASD symptoms such as social interaction, communication, and restricted and repetitive behaviors [Lane, Molloy et al., 2014].
In an alternate sensory subtype model, Ausderau and colleagues [2014] proposed four subtypes based on the parent report measure, the Sensory Experiences Questionnaire [SEQ; Baranek, 2009]. Ausderau et al.’s four subtypes were characterized as follows:
Mild—minimal sensory features;
Sensitive-Distressed—hyper-reactivity and enhanced sensory perception;
Attenuated-Preoccupied—hyporeactivity and sensory interests, repetitions, and seeking behaviors;
Extreme-Mixed—difficulty across all sensory areas.
Despite being derived from different tools, there are clear parallels between this subtyping model and that proposed by Lane and colleagues; both models consist of four subtypes where one has mild sensory features and another has extreme sensory features. The congruence between these two subtyping methodologies lends support to the validity of sensory subtypes in ASD and suggests a common theoretical structure underpinning the heterogeneity in sensory features in ASD. Elucidating the theoretical constructs underlying the observed subtypes would further our understanding of the manner in which sensory features influence and interact with non-sensory ASD signs and symptoms, thus creating pathways to optimize assessment and treatment prescription. In this preliminary study, we attempt to reveal the core theoretical or latent constructs that underpin the sensory subtype model described by Lane, Molloy et al. [2014].
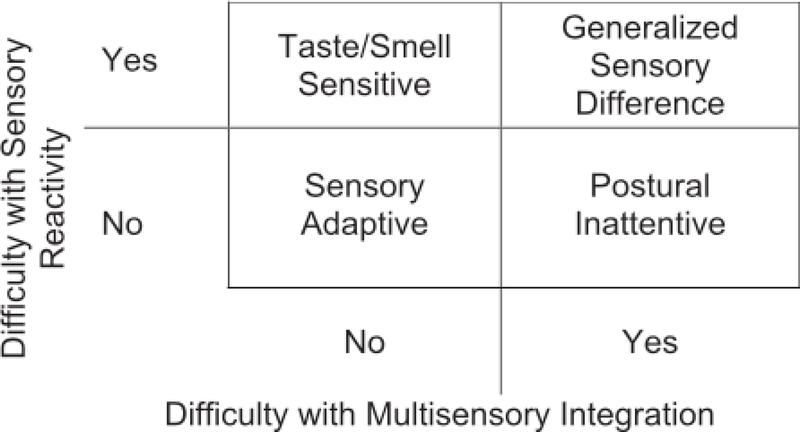
Based on the pattern of sensory features associated with each of the four subtypes, Lane and colleagues hypothesized that two latent constructs form the foundation for their sensory subtype schema. The two constructs are: (1) sensory reactivity, which refers to the intensity (too high, too low, or just right) of a response to a stimulus, and (2) multisensory integration, which is the ability to process multiple concurrent stimuli [Lane, Molloy et al., 2014]. The proposed relationship of the sensory subtypes to sensory reactivity and multisensory integration is depicted in Figure 1. Children with difficulties with sensory reactivity may demonstrate hyperreactivity or hyporeactivity, indicating impaired ability to adaptively modulate their response to a sensory stimulus. This construct directly aligns with Dunn’s [1997] conceptualization of sensory modulation difficulties whereby behavioral responses to sensory stimuli are classified according to the nature of the response (passive or active) and the neurological threshold for the response (too high or too low). In Lane et al.’s subtype schema, children who display hyper-reactivity to tastes and smells are classified into either Taste/Smell Sensitive or Generalized Sensory Difference subtypes. Those who also display hyper-reactivity more broadly in movement, tactile, visual, and auditory areas are classified as Generalized Sensory Difference [Lane, Molloy et al., 2014]. Similarly, children who exhibit extreme hypo-reactivity are assigned to the Generalized Sensory Difference subtype. Important to note is that, in direct contrast to Dunn’s model, under Lane et al.’s subtype schema, individual children may present with both hyper- and hyporeactivity concurrently. The clustering algorithm underlying this schema is based on the grouping of similarities between children rather than behaviors; therefore, a single theoretical construct of ‘sensory reactivity’ has been proposed representing the spectrum of these behaviors [Lane, Molloy et al., 2014]
Figure 1.
Relationship of sensory subtypes to sensory reactivity and multisensory integration.
Further, children with difficulties with multisensory integration may demonstrate behaviors that indicate inefficient or absent integration of multiple concurrent sensory stimuli. Multisensory integration difficulties are increasingly described in the ASD literature and have been recorded using both behavioral and imaging techniques [Brandwein et al., 2013; Cascio, Foss-Feig, Burnette, Heacock, & Cosby, 2012; Marco, Hinkley, Hill, & Nagarajan, 2011]. For example, difficulties with simultaneously processing visual, proprioceptive, and vestibular stimuli may result in an impaired ability to hold body positions or perform coordinated movements [Izawa et al., 2012; MacNeil & Mostofsky, 2012]. These behaviors are characteristic of children classified as either Postural Inattentive or Generalized Sensory Difference. As shown in Figure 1, Lane, Molloy et al.’s [2014] theory proposes that children with ASD experience core sensory impairment in: (1) sensory reactivity only (Taste Smell Sensitive); (2) multisensory integration only (Postural Inattentive) or (3) both sensory reactivity AND multisensory integration (Generalized Sensory Difference). This theory also suggests that a subset of children with ASD do not experience a core sensory impairment (Sensory Adaptive).
While Lane, Molloy et al. [2014] provide a plausible argument for the role of sensory reactivity and multisensory integration in the expression of sensory features in children with ASD, this theory is yet to be empirically tested. The overall objective of this study is to empirically assess the validity of the theory proposed by Lane, Molloy et al. [2014] whereby variance in sensory subtype classification can be explained by variance across the underlying constructs of sensory reactivity and multisensory integration. To do this, we test this theory in the original sample used by Lane, Molloy et al. [2014], Sample A, as well as in a second, independent sample, Sample B. Assessment of the latent constructs of a sensory subtypes model rather than a discrete sensory symptoms model [e.g., Dunn, 1997] is likely to reveal mechanisms by which intervention can be customized to individuals rather than specific behaviors.
Method
Participants
This study was a secondary analysis of sensory data from two independent datasets. The first dataset, Sample A (n 5 228), consisted of individuals presenting to a major, Midwestern US autism specialist center for diagnostic assessment of ASD between 2008 and 2010. Children were included in the study if: (a) they were ultimately diagnosed with an ASD by the evaluation team; and (b) they completed the comprehensive battery of diagnostic evaluations provided by the center, including the SSP. Children were 2–10 years of age (Table I).
Table 1:
Child Age and Gender
| Sample | n | Mean age (months) | Standard Deviation | Range | |
|---|---|---|---|---|---|
| Sample A | All children | 228 | 61 | 26.86 | 24–130 |
| Male | 203 | 60 | 26.15 | 24–130 | |
| Female | 25 | 66 | 32.01 | 26–130 | |
| Sample B | All children | 59 | 76 | 43.75 | 26–152 |
| Male | 49 | 75 | 44.28 | 26–152 | |
| Female | 10 | 79 | 43.13 | 29–148 |
The second dataset, Sample B (n559), consisted of data from the National Database for Autism Research [National Database for Autism Research, 2016]. Children were included in the present if: (a) there was item level SSP or Sensory Profile data available; (b) they were 2–12 years of age; and (c) they had an ASD diagnosis according to their Autism Diagnostic Observation Schedule (ADOS) score. Where only Sensory Profile data was available (n 5 19), relevant item-level data was extracted and a set of SSP scores were calculated prior to analysis. Table I provides age and gender information for both samples.
Measures
The SSP is a 38-item parent questionnaire designed to measure sensory processing in children [McIntosh, Miller, Shyu, & Dunn, 1999]. Scores are derived for seven sensory domains (tactile; taste/smell; movement; visual/ auditory sensitivity; under-responsive/seeks sensation; auditory filtering; and low energy/weak). Internal consistency of overall and sensory domain sections of the SSP reportedly ranges between r 5 0.70 and 0.90, and discriminative validity is acceptable [McIntosh et al., 1999]. The seven sensory domain structure is supported by a principal components factor analysis [Dunn & Brown, 1997].
Analysis
In the present study, we performed an independent component analysis (ICA) on SSP domain z-scores to examine the hypothesis that two constructs underpin the SSP, and therefore Lane, Molloy et al.’s [2014] sensory subtypes. ICA is commonly used as a data reduction technique that extracts underlying constructs from observed data. We used ICA to determine the nature of constructs underlying the seven domains of the SSP. The rationale for ICA analysis was that, because the sensory subtyping schema was derived from the SSP, by testing the latent constructs of the SSP, we also test the latent constructs of the subtyping schema. ICA was selected over principal components analysis as it is often more effective at reconstructing the original sources from which the data were constructed [e.g., Bugli & Lambert, 2007]. We conducted an ICA with the number of components specified as two, using Sample A. Next, we conducted an identical ICA using Sample B to determine the level of convergence of findings from the two independent samples. We examined the estimated mixing matrices (A) obtained from the ICA, which can be interpreted similarly to factor loadings in a factor analysis, where larger absolute values indicate a stronger relationship [Bell & Sejnowski, 1995]. In factor analysis, clear guidelines exist regarding what constitutes a significant factor loading. In contrast, ICA loadings are interpreted more relatively—that is, which loadings, relative to other loadings in the same analysis, are large and which are small. Analyses were performed using the statistical software R [R Development Core Team, 2011] and the supplemental packages “fastICA” [Marchini, Heaton, Ripley, & Ripley, 2007] and “icapca” [Woods, 2014].
Results
Loadings of the domains onto the two components ranged from an absolute value of 0.214 to 2.795 in Sample A (Table II). The SSP domain that most substantially contributed to Component 1 was the taste/smell sensitivity domain (–2.795), followed by tactile sensitivity (−0.892) and under-responsive/seeks sensation (–0.828). The SSP domain most that substantially contributed to Component 2 was low energy/weak (1.645). Component 2 also had noteworthy contributions from under-responsive/seeks sensation (1.307), tactile sensitivity (1.079), and auditory filtering (1.074).
Table 2:
Two-component solution from sample A depicting the loadings of short sensory profile domains
| Low energy/Weak | Taste smell | Movement | Tactile | Visual/Auditory sensitivity | Under-responsive/Seeks sensation | Auditory Filtering | |
|---|---|---|---|---|---|---|---|
| 1 | −0.316 | −2.795 | −0.393 | −0.892 | −0.704 | −0.828 | −0.601 |
| 2 | 1.645 | −0.214 | 0.714 | 1.079 | 0.938 | 1.307 | 1.074 |
Domain loadings in Sample B ranged from an absolute value of 0.039 to 2.064. Results of the twoconstruct solution in Sample B (Table III) indicated convergence between the two samples in that Component 1 had the most substantial contribution from the taste/ smell sensitivity domain (2.045), followed by tactile sensitivity (1.182) and under-responsive/seeks sensation (1.375). Moreover, Component 2 had the most substantial contribution from the low energy/weak domain (2.064), with moderate contributions by auditory filtering (0.926) and tactile sensitivity (0.898). However, one difference between the samples was that the underresponsive/seeks sensation domain did not have a strong loading on Component 2 (0.653) in Sample B.
Table 3:
Two-component solution from sample B depicting the loadings of short sensory profile domains
| Low energy/Weak | Taste smell | Movement | Tactile | Visual/Auditory sensitivity | Under-responsive/Seeks sensation | Auditory Filtering | |
|---|---|---|---|---|---|---|---|
| 1 | −0.647 | 2.045 | 0.039 | 1.182 | 0.393 | 1.375 | 0.895 |
| 2 | 2.064 | 0.808 | 0.836 | 0.898 | 0.582 | 0.653 | 0.926 |
Discussion
The overall objective of this study was to examine preliminary empirical evidence for the hypothesis that two latent constructs, sensory reactivity and multisensory integration, underlie Lane, Molloy et al.’s [2014] sensory subtypes in ASD. The findings from this study provide initial support for this hypothesis. Using an ICA with a confirmatory approach on two independent samples, we found evidence for a sensory reactivity component (Component 1) and a multisensory integration component (Component 2). We discuss the findings in more detail below and consider the implications for future research.
The primary contribution to Component 1 in both samples was the taste/smell sensitivity domain of the SSP. The taste/smell sensitivity domain consists of items pertaining to behaviors that are arguably associated with sensory reactivity such as hyper-reactivity to certain tastes, smells, or food textures. Tactile sensitivity also made a moderate contribution to this component in both samples. Tactile sensitivity on the SSP consists of items indicative of tactile hyper-reactivity including distress with grooming activities and physical contact with others. Interestingly, the SSP domain of underresponsive/seeks sensation was also a moderate contributor to Component 1 in both samples. This result provides some support for a single latent construct of sensory reactivity, as proposed by Lane, Molloy et al. [2014], underlying sensory subtypes in ASD that includes both hyperand hypo-reactive dimensions. This strength of this conclusion needs to be tempered, however, on consideration of the additional moderate contribution of the under-responsive/seeks sensation domain to Component 2 in Sample A. This result suggests that under-responsivity/seeking may also be theoretically linked to the construct of multisensory integration, which was not predicted by Lane, Molloy et al. [2014]. We offer two possible explanations for this result. First, under-responsivity/seeking may in fact be a separate, unique, but more minor latent construct that was not revealed in this study due to its focus on confirming a two-component solution. This possibility would need to be examined using a more exploratory approach to ICA. Under this scenario, the moderate inter-relationship of under-responsive/seeks sensation with the other two constructs may indicate a third “orthogonal” construct that pertains to all children with ASD and represents sensory symptom severity. Evidence for this hypothesis can be found in Lane et al.’s original subtyping studies, which found that subtypes differed from one another in terms of the frequency of sensory features reported by caregivers [Lane et al., 2010, 2011, Lane, Molloy et al., 2014]. Members of the Sensory Adaptive subtype reported relatively few sensory features while members of the Generalized Sensory Difference subtype reported high numbers of sensory features. These differences were most apparent when considering performance on the under-responsive/seeks sensation and auditory filtering domains of the SSP. The frequency of reported sensory symptoms was also identified in a recent study of adolescents with ASD as the most salient factor distinguishing sensory subtypes in this older group [Uljarevic, Lane, Kelly, & Leekam, 2016].
Second, the co-contribution of under-responsive/seeks sensation to both Component 1 and 2 may speak to a limitation in the organization of items on this domain of the SSP such that some items within this domain may represent sensory reactivity while others represent multisensory integration. Since the publication of the SSP, it has been generally acknowledged that underresponsivity and seeking most likely represent two distinct behavioral patterns. In more recent publications, including the updated version of the SSP measure [Dunn, 2014], these concepts are considered separately. Additionally, the subtype model proposed by Ausderau et al. [2014] more strongly delineates between hyperreactivity, hyporeactivity, and seeking dimensions. This is likely due, in part, to the factor structure of the SEQ, where items are assigned as either sensory hyperreactivity, hyporeactivity, or seeking [Baranek, 2009]. In our analysis, we examined loadings from SSP domains rather than item-level scores. Subsequently, we were unable to distill the relative contribution of each of the items representing either under-responsivity or seeking to each component. This is a limitation of the current study and future research should attempt the analysis on item-level, rather than domain-level, data for a more nuanced result.
The primary contribution to the second component in both samples was made by the SSP domain of low energy/weak. Items on the low energy/weak domain of the SSP describe behaviors that may be indicative of difficulties with multisensory integration—particularly postural function and force modulation, for example, “tires easily when holding a body position” or “seems to have a weak grasp.” Difficulties with postural function and force modulation have been linked in the literature with the integration of multiple sensory inputs from proprioceptive, visual, and vestibular systems [Izawa et al., 2012; MacNeil & Mostofsky, 2012]. Supporting the linkage of this component to multisensory integration, is the moderate contribution of the auditory filtering domain to Component 2 in both samples. Items from the auditory filtering domain assess behaviors such as being able to concentrate in the context of background noise and attend to others when they are speaking. One of the primary functions of multisensory integration is to be able to filter and prioritize multiple incoming sensory stimuli for the purposes of executive function, sustained attention, and attention shifting [Brock, Brown, Boucher, & Rippon, 2002]. Parentreported difficulties in these behaviors, therefore, may be indicative of a core impairment in multisensory integration. Future research is warranted, however, to confirm this conclusion. The SSP does not purport to measure multisensory integration function, as it is based on Dunn’s Model of Sensory Processing [Dunn, 1997]. Therefore, the present analysis likely lacks a complete set of items needed to fully capture the construct of multisensory integration. We do assert, however, that the findings of the present study support the possibility of multisensory integration as a key construct underlying the sensory subtypes that warrants further exploration.
The notion that sensory difficulties in ASD can be linked to discrete sensory functions, such as sensory reactivity and multisensory integration, is not novel. Marco et al. [2011] in their review of the neural underpinnings of sensory function in ASD found studies investigating sensory sensitivities, sensory perceptual deficits, and multisensory difficulties. A more recent review of sensory-related research in ASD summarized both behavioral and neurophysiologic studies within the categories of: (1) sensory reactivity and unusual sensory interests, (2) sensory perception, and (3) sensory integration [Schaaf & Lane, 2015]. While classifications such as these are easily justifiable within the context of research seeking to isolate the mechanism of a specific deficit, what is less clear is how these classifications relate to the clinical presentation of an individual child with ASD. Our study begins to close this gap by providing preliminary evidence supporting the validity of distinct patterns of sensory features based on defined latent constructs. This knowledge may further refine our processes for customized assessment and intervention selection. The findings from this preliminary study would be further strengthened if supported by additional studies that link specific behavioral and neural patterns to each sensory construct.
Limitations
There are a few limitations to the present study. First, Sample A is the same sample used in the original analysis by Lane Molloy et al. [2014] to identify sensory subtypes in ASD and our independent sample, Sample B, consisted of relatively few participants (n 5 59). It is known that results of ICAs conducted on small sample sizes should be interpreted with caution [Tichavsky, Koldovsky, & Oja, 2006]. As such, it will be necessary to further substantiate the findings of the present study using a larger independent dataset. Second, we conducted the ICA in a confirmatory way to test our hypothesis; conducting the ICA using a more exploratory approach would allow for the consideration of more or less than two components. Model fit indices could then be used to determine which number of components optimally explains the patterns in the data. Third, it is important to acknowledge that the children included in this analysis were predominantly male, with a male to female ratio of 7:1. This ratio is slightly higher than the ratio of males to females with ASD diagnoses, which is at least 4:1 according to epidemiological studies [Christensen, 2016]. Thus, it is possible that study findings may not be generalizable to females with ASD. Last, due to the nature of ICA, we were unable to test hypotheses about the relationships between the constructs underlying the sensory subtypes. For example, the present analysis offers no way to directly test our hypothesis that the under-responsive/seeks sensation domain is a severity classifier that spans across sensory reactivity and multisensory integration, although findings suggest that this may be the case. While the present analysis provides preliminary support for our hypothesis, the relationship between sensory reactivity and multisensory integration remains untested. Despite these limitations, the present study offers important novel findings, as this is the first study to examine the theoretical underpinnings of Lane’s sensory subtypes.
Summary and Future Research
The present study provides preliminary support for the hypothesis that the latent constructs of sensory reactivity and multisensory integration underlie the sensory subtypes of Sensory Adaptive, Taste/Smell Sensitive, Postural Inattentive, and Generalized Sensory Difference in children with ASD. An understanding of these constructs may prove useful for the development of much-needed clinical reasoning guidelines based on sensory subtype classification. Future studies should conduct a more exploratory ICA across a broader range of sensory assessments that include items related to multisensory integration to validate the findings of the present study and explore the existence of additional underlying constructs. Additionally, the relationships between sensory reactivity and multisensory integration should be explored via structural equation modeling. Last, if future work continues to support our findings, it will be important to consider findings in light of existing theoretical frameworks of sensory processing.
Acknowledgments
The authors wish to thank Elysa Marco, MD for her contributions to this project. This project was supported in part by Autism Speaks and cooperative agreement UA3 MC11054 through the U.S. Department of Health and Human Services, Health Resources and Services Administration, Maternal and Child Health Research Program to the Massachusetts General Hospital and the Autism Speaks Autism Treatment Network. Data used in the preparation of this article (Sample B) reside in the NIH-supported NIMH Data Repositories. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Washington, DC: American Psychiatric Association. [Google Scholar]
- Ashburner J, Rodger S, Ziviani J, & Jones J (2014). Occupational therapy services for people with autism spectrum disorders: Current state of play, use of evidence and future learning priorities. Australian Occupational Therapy Journal, 61, 110–120. doi: 10.1111/1440-1630.12083 [DOI] [PubMed] [Google Scholar]
- Ausderau KK, Furlong M, Sideris J, Bulluck J, Little LM, Watson LR, … Baranek GT (2014). Sensory subtypes in children with autism spectrum disorder: Latent profile transition analysis using a national survey of sensory features. Journal of Child Psychology and Psychiatry, 55, 935–944. doi: 10.1111/jcpp.12219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranek GT (2009). Sensory experiences questionnaire version 3.0. Unpublished manuscript. [Google Scholar]
- Bell AJ, & Sejnowski TJ (1995). An information-maximization approach to blind separation and blind deconvolution. Neural Computation, 7, 1129–1159. doi: 10.1162/neco.1995.7.6.1129 [DOI] [PubMed] [Google Scholar]
- Ben-Sasson A, Cermak SA, Orsmond GI, Tager-Flusberg H, Kadlec MB, & Carter AS (2008). Sensory clusters of toddlers with autism spectrum disorders: Differences in affective symptoms. Journal of Child Psychology and Psychiatry, 49, 817–825. doi: 10.1111/j.1469-7610.2008.01899.x [DOI] [PubMed] [Google Scholar]
- Brandwein AB, Foxe JJ, Butler JS, Russo NN, Altschuler TS, Gomes H, & Molholm S (2013). The development of multisensory integration in high-functioning autism: High- density electrical mapping and psychophysical measures reveal impairments in the processing of audiovisual inputs. Cerebral Cortex, 23, 1329–1341. doi: 10.1093/cercor/bhs109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock J, Brown CC, Boucher J, & Rippon G (2002). The temporal binding deficit hypothesis of autism. Development and Psychopathology, 14, 209–224. doi: 10.1017/S0954579402002018 [DOI] [PubMed] [Google Scholar]
- Bugli C, & Lambert P (2007). Comparison between principal component analysis and independent component analysis in electroencephalograms modelling. Biometrical Journal, 49, 312–327. doi: 10.1002/bimj.200510285 [DOI] [PubMed] [Google Scholar]
- Cascio CJ, Foss-Feig JH, Burnette CP, Heacock JL, & Cosby AA (2012). The rubber hand illusion in children with autism spectrum disorders: Delayed influence of combined tactile and visual input on proprioception. Autism, 16, 406–419. doi: 10.1177/1362361311430404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen DL (2016). Prevalence and characteristics of autism spectrum disorder among children aged 8 years— autism and developmental disabilities monitoring network, 11 sites, United States, 2012. MMWR. Surveillance Summaries, 65, 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn W (1997). The impact of sensory processing abilities on the daily lives of young children and their families: A conceptual model. Infants & Young Children. LWW, 9, 23–35. [Google Scholar]
- Dunn W (2014). Sensory Profile 2 manual. San Antonio, TX: Pearson. [Google Scholar]
- Dunn W, & Brown C (1997). Factor analysis on the Sensory Profile from a national sample of children without disabilities. American Journal of Occupational Therapy, 51, 490–495. doi: 10.5014/ajot.51.7.490 [DOI] [PubMed] [Google Scholar]
- Interagency Autism (IACC) Coordinating Committee. (2013). Strategic plan for autism spectrum disorder (ASD) research: 2013 update. Retrieved from the U.S. Department of Health and Human Services Interagency Autism Coordinating Committee website: http://iacc.hhs.gov/strategicplan/2013/index.shtml [Google Scholar]
- Interactive Autism Network. (2014). Autism treatment: What do we really know? [Internet]. Retrieved from http://www.iancommunity.org/cs/what_do_we_know/overview
- Izawa J, Pekny SE, Marko MK, Haswell CC, Shadmehr R, & Mostofsky SH (2012). Motor learning relies on integrated sensory inputs in ADHD, but over-selectively on proprioception in autism spectrum conditions. Autism Research, 5, 124–136. doi: 10.1002/aur.1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig KP, & Kinnealey M (2008). Research brief: Sensory, motor, and communication challenges for persons with autism spectrum disorders. Special Interest Section Quarterly: Sensory Integration, 31, 3–4. [Google Scholar]
- Lane AE, Dennis SJ, & Geraghty ME (2011). Brief report: Further evidence of sensory subtypes in autism. Journal of Autism and Developmental Disorders, 41, 826–831. doi: 10.1007/s10803-010-1103-y [DOI] [PubMed] [Google Scholar]
- Lane AE, Geraghty ME, Young GS, & Rostorfer JL (2014). Problem eating behaviors in autism spectrum disorder are associated with suboptimal daily nutrient intake and taste/smell sensitivity. ICAN: Infant, Child, & Adolescent Nutrition, 6, 172–180. [Google Scholar]
- Lane AE, Molloy CA, & Bishop SL (2014). Classification of children with autism spectrum disorder by sensory subtype: A case for sensory-based phenotypes: Sensory phenotypes in autism. Autism Research, 7, 322–333. doi: 10.1002/aur.1368 [DOI] [PubMed] [Google Scholar]
- Lane AE, Young RL, Baker AEZ, & Angley MT (2010). Sensory processing subtypes in autism: Association with adaptive behavior. Journal of Autism and Developmental Disorders, 40, 112–122. doi: 10.1007/s10803-009-0840-2 [DOI] [PubMed] [Google Scholar]
- Leekam SR, Nieto C, Libby SJ, Wing L, & Gould J (2006). Describing the sensory abnormalities of children and adults with autism. Journal of Autism and Developmental Disorders, 37, 894–910. doi: 10.1007/s10803-006-0218-7 [DOI] [PubMed] [Google Scholar]
- MacNeil LK, & Mostofsky SH (2012). Specificity of dyspraxia in children with autism. Neuropsychology, 26, 165–171. doi: 10.1037/a0026955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchini JL, Heaton C, Ripley BD, & Ripley MB (2007). The fastICA package. Retrieved from ftp://ftp.uni-bayreuth.de/pub/math/statlib/R/CRAN/doc/packages/fastICA.pdf [Google Scholar]
- Marco EJ, Hinkley LBN, Hill SS, & Nagarajan SS (2011). Sensory processing in autism: A review of neurophysiologic findings. Pediatric Research, 69, 48R–54R. doi: 10.1203/PDR.0b013e3182130c54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh DN, Miller LJ, Shyu V, & Dunn W (1999). Overview of the Short Sensory Profile (SSP). In The Sensory Profile: Examiner’s manual (pp. 59–73). San Antonio, TX: Psychological Corporation. [Google Scholar]
- R Development Core Team. (2011). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. Retrieved from http://www.R-project.org [Google Scholar]
- Schaaf RC, & Lane AE (2015). Toward a best-practice protocol for assessment of sensory features in ASD. Journal of Autism and Developmental Disorders, 45, 1380–1395. doi: 10.1007/s10803-014-2299-z [DOI] [PubMed] [Google Scholar]
- Tichavsky P, Koldovsky Z, & Oja E (2006). Performance analysis of the FastICA algorithm and Crame r-rao bounds for linear independent component analysis. IEEE Transactions on Signal Processing, 54, 1189–1203. [DOI] [PubMed] [Google Scholar]
- Tomchek SD, Huebner RA, & Dunn W (2014). Patterns of sensory processing in children with an autism spectrum disorder. Research in Autism Spectrum Disorders, 8, 1214–1224. doi: 10.1016/j.rasd.2014.06.006 [DOI] [Google Scholar]
- Trembath D, & Vivanti G (2014). Problematic but predictive: Individual differences in children with autism spectrum disorder. International Journal of Speech-Language Pathology, 16, 57–60. [DOI] [PubMed] [Google Scholar]
- Uljarevic M, Lane A, Kelly A, &., & Leekam S (2016). Sensory subtypes and anxiety in older children and adolescents with autism spectrum disorder. Autism Research, 9, 1073–1078. doi: 10.1002/aur.1602 [DOI] [PubMed] [Google Scholar]
- Woods R (2014). Package “icapca.” Retrieved from https://cran.rproject.org/web/packages/icapca/icapca.pdf.