Abstract
Diabetic macular edema (DME) is the most common cause of vision loss in diabetic retinopathy, affecting 1 in 15 patients with diabetes mellitus (DM). The disruption of the inner blood-retina barrier (BRB) has been largely investigated and attributed the primary role in the pathogenesis and progression in DME, but there is increasing evidence regarding the role of outer BRB, separating the RPE from the underlying choriocapillaris, in the occurrence and evolution of DME. The development of novel imaging technologies has led to major improvement in the field of in vivo structural analysis of the macula allowing us to delve deeper into the pathogenesis of DME and expanding our vision regarding this condition. In this review we gathered the results of studies that investigated specific outer BRB optical coherence tomography parameters in patients with DM with the aim to outline the current status of its role in the pathogenesis and progression of DME and identify new research pathways contributing to the advancement of knowledge in the understanding of this condition.
Keywords: Diabetic macular edema, External limiting membrane, Hyperreflective foci, Inner segment/outer segment line, Optical coherence tomography, Outer retinal barrier
Core Tip: Progress in optical coherence tomography technology allowed the identification of new pathogenic pathways in diabetic macular edema (DME) involving the outer retina and underlying choroid. The presence of fluid in the subretinal space is suggestive for the alteration of the outer blood retinal barrier and responds better to intravitreal triamcinolone as compared to anti- vascular endothelial growth factor. The disruption of external limiting membrane (ELM) is associated with visual impairment being a predictor of poor outcomes following the treatment with triamcinolone. The integrity of ELM and of the inner segment/outer segment line was found to correlate positively with best corrected visual acuity in DME.
INTRODUCTION
Diabetic macular edema (DME) is the most common cause of vision loss in diabetic retinopathy (DR)[1], affecting 1 in 15 patients with diabetes mellitus (DM)[2]. DME is the first cause of visual impairment within the group of working-age population in the developed countries[3]. The retina is one of the most metabolically active tissues in the organism, having high energetic demands. The complexity of the retinal activity requires a homeostatic microenvironment which is achieved by the functioning of two distinct blood-retina barriers, inner and outer. The disruption of the inner blood-retina barrier (BRB) has been largely investigated and attributed the primary role in the pathogenesis and progression in DME, but there is increasing evidence regarding the role of outer BRB, separating the retinal pigmented epithelium (RPE) from the underlying choriocapillaris, in the occurrence and evolution of DME[4]. The normal functioning of the RPE is crucial for the retina, as it removes the waste resulting from the phagocytosis of the photoreceptors’ outer segments, it provides nutrients for the photoreceptors and it substitutes the lymphatics by pumping the fluid from the inner retina to the choriocapillaris[5]. In the diabetic retina, the highly hypoxic microenvironment leads to the over-expression of vascular endothelial growth factor (VEGF) with subsequent depletion of occludin and damage of the tight junctions between the RPE cells[6]. The RPE alteration in DME was demonstrated both morphologically and functionally. Thus, electron microscopy studies performed on the retinas with DME induced in animal models found shrank nuclei, reduced endoplasmic reticulum, in-folding cell membrane, altered melanosome and loss of RPE cells[5]. Electroretinography on a mouse model showed the early decrease of c wave before the occurrence of any photoreceptor dysfunction[7]. The impact of VEGF on the RPE function was demonstrated on cell cultures: increase of VEGF led to the increase of transepithelial resistance (TER) which is a marker of RPE barrier’s function. Following VEGF neutralization with an antibody, the RPE barrier’s function recovered partially[8]. Following the analysis of the RPE cells’ proteome, 62% of the proteins involved in the retinoic metabolism were found to be altered in diabetic eyes without retinopathy. Interestingly, these proteins were modified also in nonretinal tissue, proving that the alteration of RPE is part of the systemic effect of diabetes[9].
The development of novel imaging technologies has led to major improvement in the field of in vivo structural analysis of the macula allowing us to delve deeper into the pathogenesis of DME and expanding our vision regarding this condition[10]. Within the last decades through the implementation of specialized computer software systems and modern mathematical tools (fractal/multifractal and lacunarity analysis)[11,12] non-invasive predictive complementary tools were developed for an early diagnosis of patients with DME[13].
In this review we gathered the results of studies that investigated specific outer BRB optical coherence tomography (OCT) parameters in patients with DM with the aim to outline the current status of its role in the pathogenesis and progression of DME and identify new research pathways contributing to the advancement of knowledge in the understanding of this condition.
OUTER RETINAL BARRIER AND THE CHOROID IN THE PATHOGENESIS OF DME
The normal functioning of the retina is ensured by the blood-retinal barrier (BRB) which regulates the entry and exit of fluid and molecules, maintaining the retina transparent and dehydrated[14]. BRB is affected early in diabetic retinopathy (DR) which translates into increased vascular permeability and retinal edema[15]. BRB has two components, inner and outer.
Inner BRB is constituted by the tight junctions (zonula occludens) between the endothelial cells within the retinal vessel walls which allow interactions with pericytes and smooth muscle cells[16]. Pericytes have a critical role in maintaining of BRB, by liberating a lipid mediator which modulates it[17]. Retinal Müller cells and astrocytes stabilize the tight junctions between the endothelial cells[18], whereas microglia produces soluble factors which are important for vesicular communication[15].
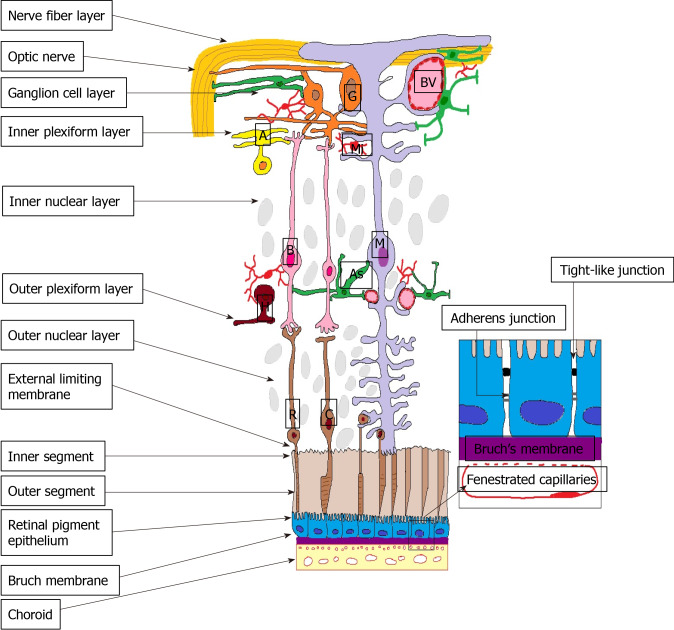
Outer BRB is formed by the intercellular junction complex of the RPE. More specifically, the basolateral membrane of the RPE faces the Bruch’s membrane, separating the RPE from the fenestrated endothelium of the choriocapillaris[19] (Figure 1).
Figure 1.
Outer blood-retina barrier. M: Müller cells; A: Amacrine cells; G: Ganglion cells; BV: Blood vessels; As: Astrocyte; B: Bipolar cells; R: Rods; C: Cones; H: Horizontal cells; Mi: Microglia; ON: Optic nerve; NFL: Nerve fibre layer; GCL: Ganglion cell layer; IPL: Inner plexiform layer; INL: Inner nuclear layer; OPL: Outer plexiform layer; ONL: Outer nuclear layer; ELM: External limiting membrane; IS: Inner segment photoreceptors; OS: Outer segment photoreceptors; RPE: Retinal pigment epithelium; BM: Bruch’s Membrane; Ch: Choroid.
These tight, adherens and gap junctions control the transport of fluids and solutes between the choroidal capillaries and the photoreceptor layers, thus maintaining the integrity of the retina[18]. It was proved that the RPE cells express major histocompatibility complex molecules, adhesion molecules and cytokines, thus playing an important role in immune processes[20]. Healthy RPE also regulates the retinal oxidative stress, therefore its malfunctioning reduces the level of antioxidants[19].
Even if the TER is much lower than the resistance of the inter-endothelial junctions at the inner retinal barrier, it efficiently prevents proteins and water from the choroid to enter the subretinal space and it allows water to exit towards the choroid following an osmotic gradient[14]. RPE dysfunction leads to the disruption of fluid transportation from the subretinal space towards the choriocapillaris which is translated into DME.
Hyperglycemia leads to the alteration of the junctional complexes at the level of the outer blood-retinal barrier subsequently to the activation of metalloproteinases by oxidative and nitrosative stress[14]. Since RPE is a highly polarized epithelium, any cytoskeletal alteration damages not only the junctions, but also the adequate distribution of membrane transporters leading to subretinal fluid accumulation[14].
Serous detachment of the macula which is very suggestive for the breakdown of the RPE barrier is observed in approximately one third of the DME cases[20,21].
Decanini et al[9] analyzed the RPE proteome in preretinopathic diabetic human donor eyes and identified significant biochemical changes preceding the clinically evident diabetic retinopathy. Some of the RPE altered proteins overlap with findings from other tissues affected by DM, but others were identified as novel biomarkers, such as proteins involved in retinoid metabolism, membrane dynamics and protein transport, proving that DM affects multiple cellular processes. Given the importance of RPE for the retinal functioning, these alterations may play a major role in the early pathogenesis of DR[9].
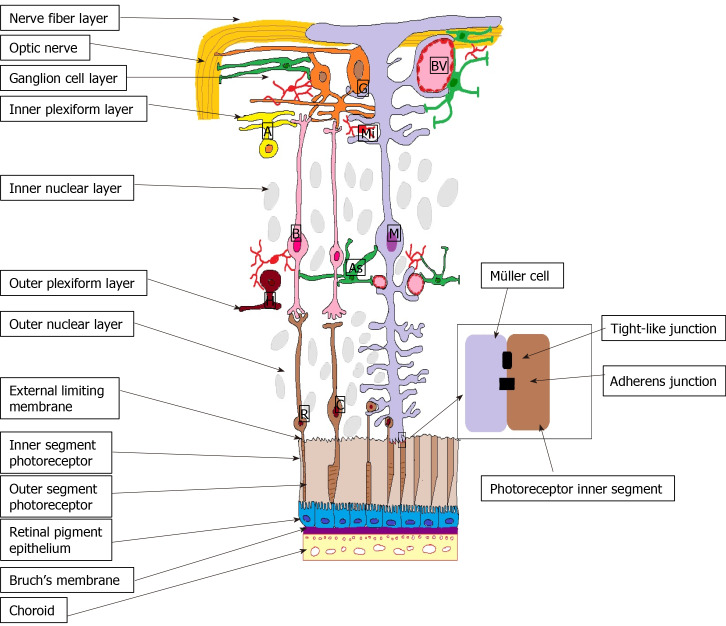
The external limiting membrane (ELM) is formed by tight-like and adherens junctions located at the interface between the retinal Müller glial cells and photoreceptor inner segments (Figure 2). Even if its role in the coordination of fluid movement around the macula is not fully understood, earlier studies showed that ELM serves as an important barrier to free protein diffusion across the retina[14]. Hyperglycemia alters the ELM by disrupting the tight junctions by the activation of PKCζ[22]. In DR the disruption of the junction protein complexes from the OLM translates clinically by lower visual acuity[23] and poor response to anti-VEGF therapy in DME[24,25].
Figure 2.
External limiting membrane. M: Müller cells; A: Amacrine cells; G: Ganglion cells; BV: Blood vessels; As: Astrocyte; B: Bipolar cells; R: Rods; C: Cones; H: Horizontal cells; Mi: Microglia; ON: Optic nerve; NFL: Nerve fibre layer; GCL: Ganglion cell layer; IPL: Inner plexiform layer; INL: Inner nuclear layer; OPL: Outer plexiform layer; ONL: Outer nuclear layer; ELM: External limiting membrane; IS: Inner segment photoreceptors; OS: Outer segment photoreceptors; RPE: Retinal pigment epithelium; BM: Bruch’s Membrane; Ch: Choroid.
Despite the information presented above, experimental studies proved that the RPE junction barrier is highly resistant to acute hypoxia/ischemia[26]. Besides, it is not known in which extent the RPE barrier is affected by retinal ischemia and the in vivo mechanisms involved in RPE dysfunction are not fully understood. For these reasons, the alterations of the outer retinal barrier drew less attention to the pathogenesis of DME compared to the ones of the inner retinal barrier[14].
The choroid is a highly vascularized and pigmented structure whose main role is to provide oxygen and nutrients to the intensely metabolic active outer retinal layers, namely the central avascular fovea and the prelaminary portion of the optic nerve[27]. Although the pathogenesis of DR is mainly attributed to the dysregulation of the retinal vasculature, there is evidence pointing to diabetic choroidopathy[28]. Histological studies of the choroid revealed atrophy of the choriocapillary endothelium, laminar deposits and narrowing of the luminal area in diabetic patients without DR[29,30], as well as basement membrane thickening, capillary dropout and choroidal neovascularization[28].
OPTICAL COHERENCE TOMOGRAPHY BIOMARKERS OF THE OUTER RETINA IN DME
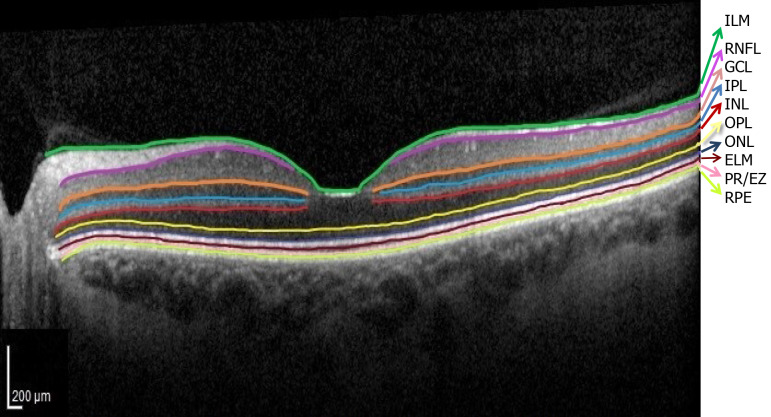
Currently, precision retinology is in-conceivable without the use of OCT which allows early diagnosis, monitoring and individualization of treatment in patients with DM. The normal OCT aspect of the retinal layers is illustrated in Figure 3.
Figure 3.
Normal optical coherence tomography aspect of the retinal layers. Segmentation software automatically marked the 10 retinal layers. (ILM: Internal limiting membrane; RNFL: Retinal nerve fiber layer; GCL: Ganglion cell layer; IPL: Inner plexiform layer; INL: Inner nuclear layer; OPL: Outer plexiform layer; ONL: Outer nuclear layer; ELM: External limiting membrane; PR/EZ: Photoreceptor layer/ellipsoid zone (inner and outer photoreceptor segment junction; RPE: Retinal pigment epithelium).
Serous retinal detachment
Based on the OCT appearance, three major types of DME were individualized: diffuse sponge-like thickening type, cystoid type (thickening of the fovea with intraretinal cysts) and serous retinal detachment (SRD) type (thickening of the fovea with subretinal fluid)[18,31,32].
Each of these lesions occurs in individual retinal layers, as follows: cystoid spaces are located mainly in the inner nuclear layer (INL) and outer plexiform layer (OPL); in SRD the extracellular fluid pools between the photoreceptors outer segments (PROS) and RPE; sponge-like retinal swelling at the fovea is identified in the OPL[33]. Whereas the sponge-like retinal swelling is frequently accompanied by abnormalities of the vitreo-macular interface causing the thickening of the retinal parenchyma at the level of the OPL[33], the presence of fluid in the subretinal space suggests the alteration of the outer BRB being caused by the migration of fluid from the retina through a weakened and permeable ELM or from the hyperpermeable vessels in the choriocapillaris through a dysfunctional RPE[18,32]. In the early stage of the disease the subretinal fluid originates in the hyperpermeable choriocapillaris through a dysfunctional RPE and as the disease progresses in the breakdown of the outer BRB through a permeable ELM[34,35]. The clinical significance of SRD derives from the observation that its presence is associated with poor visual prognosis, probably due to the disruption of ELM[36].
Several studies compared the effectiveness of anti-VEGF treatment according to the OCT appearance of DME and found that the SRD type which associated ELM and RPE impairment did not respond well[37,38].
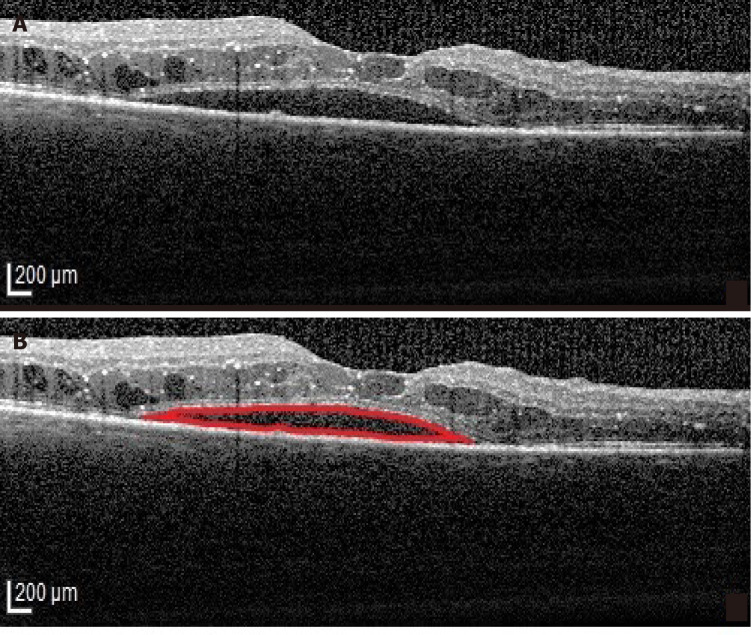
Intravitreal triamcinolone was more effective than anti-VEGF therapy in reducing macular thickness and improving vision in eyes with the SRD type of DME (Figure 4) in a prospective case series[39]. However, the relatively short follow-up period (24 mo) requires careful interpretation of these results, especially since long term steroid related complications (cataract, glaucoma) are well known[40,41]. Recently, good results with a dexamethasone implant in SRD were reported and OCT factors associated with better outcomes were identified as the absence of HF and a continuous ellipsoid zone (EZ) at the fovea[42]. The better outcome of SRD following intravitreal steroids as compared to intravitreal anti-VEGF is explained by the role of inflammation in its pathogenesis. In the SRD type of DME increased concentrations of inflammatory cytokines and higher levels of IL-6 were found in the aqueous humor and the vitreous[36]. It is believed that the source of IL-6 is represented by the scavenger cells attracted by the ELM damage[20].
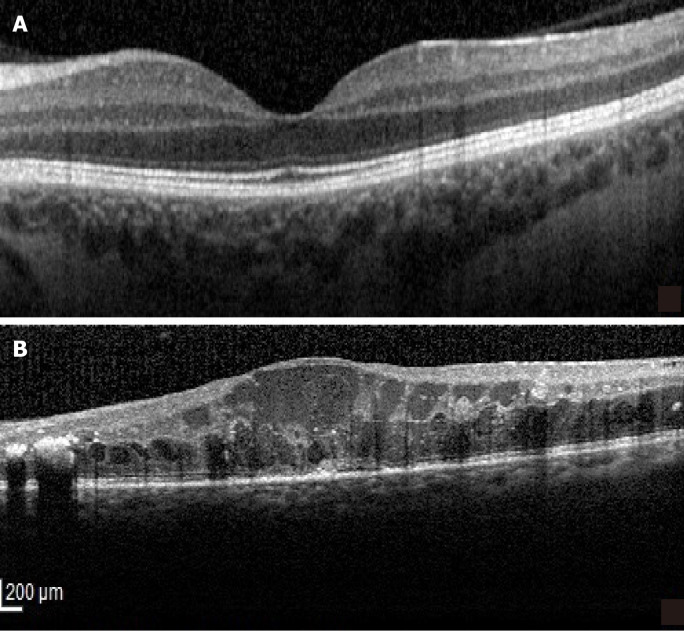
Figure 4.
Serous retinal detachment type of diabetic macular edema. A: Optical coherence tomography (OCT)-Retinal neurosensory detachment; B: Highlighted OCT image showing the neurosensory detachment (red).
Outer retinal layers
OCT studies offered insights into the outer retina, proving that the disruption of the EZ occurs subsequently to the disruption of ELM[43]. The base of this observation is that ELM has tight junctions between the Müller cells and photoreceptor cells which are similar to those between the RPE cells. As such, ELM is functioning like a third retinal barrier against macromolecules[44] whose malfunctioning leads to the accumulation of fluid in DME. As a result, surrogate biomarkers of the outer retina were proposed to determine the progression of DR[45]. A grading of the ultrastructural changes in correlation with the severity of the disease was elaborated, grade 0 meaning no disruption of ELM and EZ, grade 1 meaning ELM disruption with intact EZ and grade 2 meaning disruption of ELM and EZ[46]. The disruption of ELM allows blood components to reach and potentially damage the photoreceptor layer. The damage of ELM in DME could be explained by the extension of the cystoid spaces from the INL to the OPL[47], or by the occurrence of a tear in the outer retinal layers in eyes with SRD[34]. Several studies have shown that ELM disruption is associated with visual impairment in DME[47-49] being a predictor of poor outcomes following the treatment with triamcinolone[50]. The integrity of ELM and inner segment/outer segment (IS/OS) line was found to be positively correlated with best corrected visual acuity (BCVA) in multiple studies[50-54]. ELM and IS/OS are useful hallmarks for the evaluation of photoreceptor layer whose integrity is closely related to the final BCVA[25].
Murakami et al[55] proved that whereas the thickness of the inner retinal layers is positively correlated with the visual impairment, the outer retinal thickness is correlated negatively with poor visual prognosis following vitrectomy for DME. Thus, thinning of the outer retina and photoreceptor degeneration contributes at least partly[33] to the apparently paradoxical changes of visual acuity that were reported by the Diabetic Retinopathy Clinical Research Network[56].
Many studies proved the importance of the inner IS/OS line in DME[47,48,50,51,53,57-60]. It was showed that the transverse length of the disrupted IS/OS line is correlated with the visual acuity[50] and that the length of PROS is associated with the visual function in DME[49]. PROS correlated better with visual acuity than macular thickness, suggesting it as a reliable biomarker of visual acuity in patients with DME[49].
One issue is to point out without a doubt that the IS/OS line that we see on the OCT images corresponds to the actual histological junction between the outer and inner photoreceptors segments. Correlating the microstructures seen on the OCT images with the histological findings, it was speculated that this hyperreflective band was located at the EZ in the inner segments[61]. One important observation is that reflectivity around this band increased after the exposure to light, suggesting that the line is a marker of the photoreceptor function per se[62,63].
Decreased thickness of the PR layer was reported in patients with proliferative diabetic retinopathy-diabetic macular oedema (PDR-DMO) and nonproliferative diabetic retinopathy (NPDR)-DMO[2] and attributed to the reduced values of PROS length in a relatively hypoxic environment at the level of the outer retina[64].
When correlating the OCT parameters of the outer retina with the visual function, Damian et al[2] found a low positive correlation between the outer retina and BCVA in the PDR-DME group and a low negative correlation between the RPE thickness and BCVA in the NPDR-DME group. The authors argue that the results are limited by the analyzing of cell thickness not morphology and therefore thickness within normal range is compatible with altered cellular anatomy.
RPE-PR complex
RPE layer is crucial for the survival of PR cells, the two layers being considered as a functional unit due to their interdependence.
In a recent study it was proved that the RPE thickness was decreased in all quadrants in patients with PDR-DME and in some quadrants in the ones with NPDR-DME[2]. This finding may be subsequent to the disruption of the RPE-PR complex possibly due to ischemia[65,66]. Kaarniranta et al[67] proved that constant oxidative stress which is a feature of DR leads to the impairment of autophagy and heterophagy in the RPE cells. However, the same authors found occasionally increased RPE thickness in patients with PDR-DME and NPDR-DME[2] which are explained either by the growing of new cells over the RPE cells in order to compensate the fluid leakage within the retina[5] or by the accumulation of shed PROS that are not timely engulfed by the RPE cells due to the alteration of their phagocytosis capacity[68]. The findings of a thickened RPE in diabetic patients may also be a consequence of impaired glycogen metabolism ant its accumulation inside the RPE. It has been shown that glycogen content is increased in the RPE from diabetic donors, as well as in RPE cells grown in hyperglycemic conditions, as consequence of an increase in glycogen synthase activity, whereas the glycogen phosphorylase was normal[69].
Tavares Ferreira et al[70] found a thicker RPE layer and thinner PR layer in patients with DM without DR as compared to nondiabetic controls. Xia et al[68] reported an increased thickness of the RPE-PR complex measured as a whole, but no changes in the thickness of retinal nerve fiber layer (RNFL) and ganglion cell layer (GCL) in type 2 diabetic patients without retinopathy and concluded that the modifications of the RPE-PR complex preceded the loss of ganglion cells in the diabetic retina without microvascular abnormalities. This finding is consistent with evidence from electrophysiology[71] and color vision which are impaired in patients without clinical DR[72-75].
“Parallelism” of the retinal layers
SD-OCT made it possible to define a new parameter, “parallelism”, referring to the integrity of the retinal layers and serving as a potential biomarker to prognosticate visual outcome in DME. The orientation of the photoreceptor status layer at the fovea was categorized including the continuity of ELM, inner segment EZ and presence of HF in the outer retinal layers. Parallelism was found to be significantly lower in eyes with DME as compared to normal eyes and positively correlated with visual acuity. The absence of HF in the outer retinal layers was associated significantly with higher parallelism and better visual acuity[76].
HF
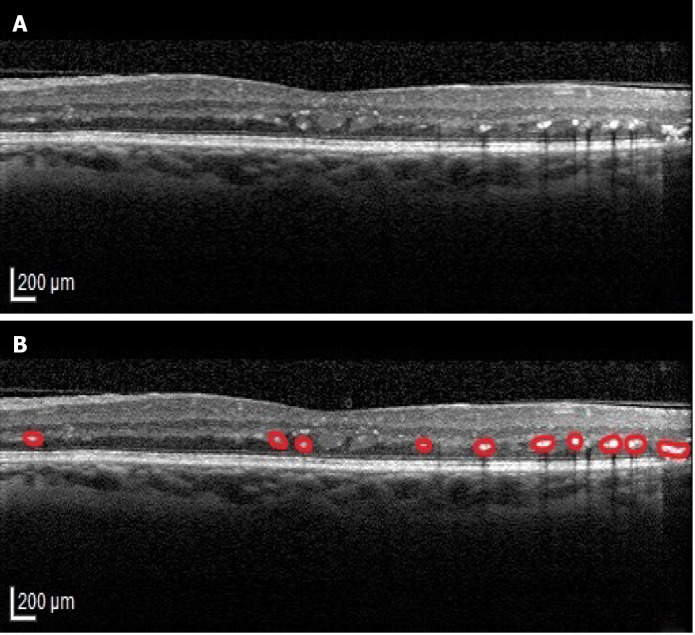
HF were described as dot like lesions on the OCT images[77] (Figure 5). When identified in the external retinal layers, HF were associated with poor visual outcomes in patients with DME and foveal SRD[34], but also in the ones with DME without SRD[51]. Bolz et al[77] postulated that HF in eyes with DME are lipid-laden macrophages and represent the precursors of hard exudates. The observation that the disruption of the ELM and IS/OS line is associated with HF stands for the theory that these lesions reciprocally promote the degeneration of photoreceptor (PR) cells in DME[33].
Figure 5.
Hyperreflective foci. A: Original optical coherence tomography (OCT) image; B: Highlighted OCT image revealing hyperreflective foci (red).
Correlations between the inner and outer retina
When correlating the inner and outer retinal barriers, Das et al[78] found that there is a strong association of disorganization of the inner retinal layers (DRIL) (Figure 6) with the disruption of ELM and EZ in DME, advancing the hypothesis that DRIL could be responsible for the disorganization of the outer retinal architecture. Another study found a highly positive correlation between the thickness of the inner retina and of the central RPE in the NPDR-DME group and a low negative one in the PDR-DME group, stressing the importance of the retinopathy grade on the DME and pointing out that whereas in NPDR the edema involves the entire retina and is mostly vasogenic, in PDR it is driven mainly by ischemia[2].
Figure 6.
Disorganization of the inner retinal layers. A: Normal optical coherence tomography aspect of the macula; B: Disorganization of the inner retinal layers.
OPTIAL COHERENCE TOMOGRAPHY (ANGIOGRAPHY) BIOMARKERS OF THE CHOROID IN DME
Enhanced depth imaging OCT and swept source OCT (SS-OCT) allowed to examine the choroid. Vascular changes and thickness alterations of the choroid were reported, outlining the diabetic choroidopathy[79,80].
Choroidal thickness in patients with DM
Several studies have found that DM is associated with decreased choroidal thickness (CT)[80-83]. Since the choroid is the main source of oxygen and nutrients for the outer retina and RPE, this may lead to increased retinal vulnerability to diabetes related hypoxia and ischemia. Moreover, a trend towards choroidal thinning paralleling the increasing severity of DR has been proved[84].
Even in the absence of any clinical retinopathy, some authors reported the significant decrease of CT in patients with DM, suggesting that the decreased choroidal blood flow might be the primary event[80,81] . Choroidal thinning was particularly obvious in the subfoveal and inferior regions in a study conducted by Esmaeelpour et al[81]. The same study group noted the perimacular retinal thinning probably caused by the optic nerve fiber layer atrophy[81].
Other studies have shown opposite results, in the sense that thicker choroid was identified in patients with DR[32,80,85]. Tavares Ferreira et al[86] measured CT in diabetic patients without DR and found significantly increased CT superiorly to the fovea, proposing it as a possible early preclinical change in diabetes. Using SS-OCT, the same authors identified vascular choroidal remodeling in diabetic patients without retinopathy and choroidal small vessel loss in the areas of previous laser photocoagulation in patients with proliferative diabetic retinopathy[87]. Choroidal thickening increased with the severity of DR, significantly[27] or not significantly[88].
At present there is no consensus on the temporal relationship between the choroidal changes and retinopathy. Some authors claim that the onset of choroidopathy precedes retinopathy, while others argue that the two events are independent[32,80,85,87,89]. Several studies offered evidence of choroidopathy occurring only in the most severe stages of DR[82,83,90] or worsening with the increasing severity of DR[32,89].
CT and DME
Patients with DME have clinically significant thinner subfoveal choroid compared to healthy controls, but when compared to other grades of DR, NPDR and PDR, their choroid is thicker[84]. Kim et al[32] reported significantly thicker choroids in DME patients as compared to non-DME patients. When the type of DME was further evaluated, CT was significantly greater in the SRD group than in the cystoid type DME[32]. However, other authors reported thinner choroid in case of clinically significant macular edema, explaining this finding as an artifact due to the attenuation of signal transmission and reflection by the edema itself[79,91]. Despite this, Gerendas et al[91] reported that in the fellow non-affected eye the choroid was also thinner, suggesting that systemic factors are involved in the pathogenesis of this finding. Esmaeelpour et al[81] reported no choroidal thinning below the lesions in patients with DME.
CT after treatment for DR
It was shown that panretinal photocoagulation (PRP) is followed initially by choroidal swelling within one week, which is explained by the shifting of vessels from the peripheral retina to the foveal area[92,93], followed by the thinning of the choroid, possibly by downregulation of VEGF[27]. Cho et al[93] found at 1 and 3 mo after PRP an increased subfoveal CT concomitant with a significant CT decrease in the photocoagulated area.
Regarding anti-VEGF treatment, several studies reported choroidal thinning over the first 6 mo[94,95]. Rayess et al[96] showed that subfoveal choroidal thickness (SFCT) is a predictor of response to anti-VEGF therapy in the sense that a greater SFCT at baseline is associated with better outcomes. One explanation is that greater choroidal thickness stands for intact choriocapillaris, less ischemic outer retina and better preservation of photoreceptors[96].
Systemic factors, like blood hemoglobin, arterial blood pressure and hypercho-lesterolemia, influence CT[84].
Future developments of CT as biomarker in DME
CT in patients with DR is a highly unreliable parameter and multiple studies show different results because there is a poor control of variables, a wide range of collecting data and different devices are used. There is evidence that the choroid thins with progressing DME as well as following PRP and anti-VEGF injections. It was reported that longer standing DME is associated with worse anatomical and functional outcomes following anti-VEGF treatments. Therefore, a thicker choroid prior to treatment would probably indicate a shorter duration DME and be associated with better outcomes after treatment. Thus, CT may be also attributed the role of prognostic biomarker able to predict the response to treatment in DME. However, in order to get reliable data on CT, future studies should accomplish certain requirements: to define clearly the scleral-choroidal junction, to include local (refractive status), and general (age, diabetes duration, HbA1C) factors in the analysis, to make a longitudinal approach and use longer follow up intervals[28].
Choroidal vascular index in patients with DM
Choroidal vascular index (CVI) is a term that means the ratio of choroidal luminal area to total choroidal area which was recently introduced as a novel biomarker to monitor the progression of DR[97]. CVI may be attributed the role of an early biomarker, because studies proved that while CT is unaltered in DR, CVI correlates with progressing DR[83]. CVI alteration before the onset of DR, supports the theory of choroidal primary damage in DR[98].
Decreased choroidal blood flow creates a hypoxic environment for the RPE and photoreceptor cells, disrupting the phagocytosis and rendering the RPE cells fragile[99,100]. The condition is aggravated by the subsequent production of superoxide and soluble inflammatory factors[68].
Whereas most of the studies focused on the CT showing its thinning in patients with DM, proportional with the severity of DR, a multicenter cross-sectional study used SS-OCT images to analyze choroidal vascularity in different stages of DR and introduced new quantitative parameters, such as choroidal vascular density (CVD) and choroidal vascular volume (CVV)[28]. According to this study, the eyes with DME and PDR had a reduced CVD and eyes with PDR had also a reduced CVV compared to controls, reflecting the notion that vascular abnormalities increase with the severity of DR. In eyes with NPDR without DME, the overall CVD was significantly reduced, but not at macula, suggesting that although diffuse choroidopathy may be present in early stages of DR, submacular choroidopathy only becomes present in later stages of DR. The same authors proved that in diabetic patients without DR, the choroidal vascular indices did not show significant differences compared to controls. Thus, it is suggested that the occurrence of diabetic choroidopathy does not precede that of retinopathy, although further studies are required to elucidate this important issue for understanding the diabetic eye disease[28].
A recent study analyzed CVI after intravitreal injection of ranibizumab in eyes with DME and found the significant reduction of CVI and choroidal blood flow only in the no-PRP group, but not in the PRP-treated group. Moreover, a significant correlation between the central retinal thickness and choroidal blood flow was found in the no-PRP group[101].
OCTA choroidal biomarkers in DME
In diabetic patients without DR, an OCTA study showed that the choroidal foveal flow area was significantly decreased compared to controls suggesting that the compromise of the circulation starts in the foveal choroidal layer in DR, preceding the occurrence of mycroaneurysms. When DR develops, both the retinal and choroidal capillaries are significantly reduced[102].
INDOCYANINE GREEN ANGIOGRAPHY
Years before the recent studies with OCT, a paper using indocyanine green angiography (ICGA) in patients with NPDR disclosed microvascular findings in addition to those described with fluorescein angiography: lobular spotty hyperfluo-rescent and hypofluorescent areas in the very late phase, diffuse late-phase hyperfluorescence corresponding to retinal capillary non-perfusion areas on fluorescein angiography and retinal edema[103]. However, due to its invasiveness and lack of quantification, ICGA is limited in detecting ischemia in early DR[102].
CONCLUSION
Advances in OCT technology allow a more detailed investigation of the outer retina and choroid providing biomarkers that mediate the decoding of pathogenesis, monitoring and selection of the best treatment option for DME. By identifying new OCT biomarkers at the level of outer retina and choroid, paths for early diagnosis and identification of novel therapeutic targets in DME are opened.
ACKNOWLEDGEMENTS
The authors wish to thank Dr. Ioana Damian, MD, PhD student, Dr. Corina-Iuliana Suciu, MD, PhD student and Dr. Vlad-Ioan Suciu, MD, PhD student, for the contribution to the illustrations of this manuscript.
Footnotes
Conflict-of-interest statement: The authors declare no conflict of interest regarding the publication of this paper.
Manuscript source: Invited manuscript
Peer-review started: January 13, 2021
First decision: February 12, 2021
Article in press: March 24, 2021
Specialty type: Ophthalmology
Country/Territory of origin: Romania
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Marques JH S-Editor: Zhang H L-Editor: A P-Editor: Wang LL
Contributor Information
Ştefan Ţălu, Directorate of Research, Development and Innovation Management (DMCDI), Technical University of Cluj-Napoca, Cluj-Napoca 400020, Romania.
Simona Delia Nicoara, Department of Ophthalmology, Iuliu Haţieganu University of Medicine and Pharmacy, Cluj-Napoca 400012, Romania. simonanicoara1@gmail.com.
References
- 1.Kang K, Lee H, Jang M, Kim HC, Chung H. Diabetic macular edema with pachychoroid features. BMC Ophthalmol. 2020;20:392. doi: 10.1186/s12886-020-01663-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Damian I, Nicoara SD. Optical Coherence Tomography Biomarkers of the Outer Blood-Retina Barrier in Patients with Diabetic Macular Oedema. J Diabetes Res. 2020;2020:8880586. doi: 10.1155/2020/8880586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wong WM, Chee C, Bhargava M, Chai C, Lin H, Zhao P, Ariadarma Mangunkusumo E, Naing T, Yuen YS, Wong TY, Su X, Lingam G. Systemic Factors Associated with Treatment Response in Diabetic Macular Edema. J Ophthalmol. 2020;2020:1875860. doi: 10.1155/2020/1875860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu HZ, Le YZ. Significance of outer blood-retina barrier breakdown in diabetes and ischemia. Invest Ophthalmol Vis Sci. 2011;52:2160–2164. doi: 10.1167/iovs.10-6518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xia T, Rizzolo LJ. Effects of diabetic retinopathy on the barrier functions of the retinal pigment epithelium. Vision Res. 2017;139:72–81. doi: 10.1016/j.visres.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 6.Hartnett ME, Lappas A, Darland D, McColm JR, Lovejoy S, D'Amore PA. Retinal pigment epithelium and endothelial cell interaction causes retinal pigment epithelial barrier dysfunction via a soluble VEGF-dependent mechanism. Exp Eye Res. 2003;77:593–599. doi: 10.1016/s0014-4835(03)00189-1. [DOI] [PubMed] [Google Scholar]
- 7.Samuels IS, Lee CA, Petrash JM, Peachey NS, Kern TS. Exclusion of aldose reductase as a mediator of ERG deficits in a mouse model of diabetic eye disease. Vis Neurosci. 2012;29:267–274. doi: 10.1017/S0952523812000326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miyamoto N, de Kozak Y, Jeanny JC, Glotin A, Mascarelli F, Massin P, BenEzra D, Behar-Cohen F. Placental growth factor-1 and epithelial haemato-retinal barrier breakdown: potential implication in the pathogenesis of diabetic retinopathy. Diabetologia. 2007;50:461–470. doi: 10.1007/s00125-006-0539-2. [DOI] [PubMed] [Google Scholar]
- 9.Decanini A, Karunadharma PR, Nordgaard CL, Feng X, Olsen TW, Ferrington DA. Human retinal pigment epithelium proteome changes in early diabetes. Diabetologia. 2008;51:1051–1061. doi: 10.1007/s00125-008-0991-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Țălu Ș, Baltă F, Ţălu SD, Merticariu A, Ţălu M. Fourier Domain-Optical Coherence Tomography in diagnosing and monitoring of retinal diseases. In: Vlad S, Ciupa RV, Nicu AI. International Conference on Advancements of Medicine and Health Care through Technology. Berlin, Heidelberg: Springer, 2009: 26. [Google Scholar]
- 11.Ţălu Ş, Stach S, Călugăru DM, Lupaşcu CA, Nicoară SD. Analysis of normal human retinal vascular network architecture using multifractal geometry. Int J Ophthalmol. 2017;10:434–438. doi: 10.18240/ijo.2017.03.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Țălu Ș. Characterization of retinal vessel networks in human retinal imagery using quantitative descriptors. HVM Bioflux. 2013;5:52–57. [Google Scholar]
- 13.Tălu S. Multifractal geometry in analysis and processing of digital retinal photographs for early diagnosis of human diabetic macular edema. Curr Eye Res. 2013;38:781–792. doi: 10.3109/02713683.2013.779722. [DOI] [PubMed] [Google Scholar]
- 14.Daruich A, Matet A, Moulin A, Kowalczuk L, Nicolas M, Sellam A, Rothschild PR, Omri S, Gélizé E, Jonet L, Delaunay K, De Kozak Y, Berdugo M, Zhao M, Crisanti P, Behar-Cohen F. Mechanisms of macular edema: Beyond the surface. Prog Retin Eye Res. 2018;63:20–68. doi: 10.1016/j.preteyeres.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 15.Duh EJ, Sun JK, Stitt AW. Diabetic retinopathy: current understanding, mechanisms, and treatment strategies. JCI Insight. 2017;2 doi: 10.1172/jci.insight.93751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klaassen I, Van Noorden CJ, Schlingemann RO. Molecular basis of the inner blood-retinal barrier and its breakdown in diabetic macular edema and other pathological conditions. Prog Retin Eye Res. 2013;34:19–48. doi: 10.1016/j.preteyeres.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 17.McGuire PG, Rangasamy S, Maestas J, Das A. Pericyte-derived sphingosine 1-phosphate induces the expression of adhesion proteins and modulates the retinal endothelial cell barrier. Arterioscler Thromb Vasc Biol. 2011;31:e107–e115. doi: 10.1161/ATVBAHA.111.235408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chung YR, Kim YH, Ha SJ, Byeon HE, Cho CH, Kim JH, Lee K. Role of Inflammation in Classification of Diabetic Macular Edema by Optical Coherence Tomography. J Diabetes Res. 2019;2019:8164250. doi: 10.1155/2019/8164250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simó R, Villarroel M, Corraliza L, Hernández C, Garcia-Ramírez M. The retinal pigment epithelium: something more than a constituent of the blood-retinal barrier--implications for the pathogenesis of diabetic retinopathy. J Biomed Biotechnol. 2010;2010:190724. doi: 10.1155/2010/190724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vujosevic S, Torresin T, Berton M, Bini S, Convento E, Midena E. Diabetic Macular Edema With and Without Subfoveal Neuroretinal Detachment: Two Different Morphologic and Functional Entities. Am J Ophthalmol. 2017;181:149–155. doi: 10.1016/j.ajo.2017.06.026. [DOI] [PubMed] [Google Scholar]
- 21.Shereef H, Comyn O, Sivaprasad S, Hykin P, Cheung G, Narendran N, Yang YC. Differences in the topographic profiles of retinal thickening in eyes with and without serous macular detachment associated with diabetic macular oedema. Br J Ophthalmol. 2014;98:182–187. doi: 10.1136/bjophthalmol-2013-303095. [DOI] [PubMed] [Google Scholar]
- 22.Omri S, Behar-Cohen F, Rothschild PR, Gélizé E, Jonet L, Jeanny JC, Omri B, Crisanti P. PKCζ mediates breakdown of outer blood-retinal barriers in diabetic retinopathy. PLoS One. 2013;8:e81600. doi: 10.1371/journal.pone.0081600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ito S, Miyamoto N, Ishida K, Kurimoto Y. Association between external limiting membrane status and visual acuity in diabetic macular oedema. Br J Ophthalmol. 2013;97:228–232. doi: 10.1136/bjophthalmol-2011-301418. [DOI] [PubMed] [Google Scholar]
- 24.Ashraf M, Souka A, Adelman R. Predicting outcomes to anti-vascular endothelial growth factor (VEGF) therapy in diabetic macular oedema: a review of the literature. Br J Ophthalmol. 2016;100:1596–1604. doi: 10.1136/bjophthalmol-2016-308388. [DOI] [PubMed] [Google Scholar]
- 25.Muftuoglu IK, Mendoza N, Gaber R, Alam M, You Q, Freeman WR. Integrity of outer retinal layers after resolution of central involved diabetic macular edema. Retina. 2017;37:2015–2024. doi: 10.1097/IAE.0000000000001459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaur C, Foulds WS, Ling EA. Blood-retinal barrier in hypoxic ischaemic conditions: basic concepts, clinical features and management. Prog Retin Eye Res. 2008;27:622–647. doi: 10.1016/j.preteyeres.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 27.Campos A, Campos EJ, Martins J, Ambrósio AF, Silva R. Viewing the choroid: where we stand, challenges and contradictions in diabetic retinopathy and diabetic macular oedema. Acta Ophthalmol. 2017;95:446–459. doi: 10.1111/aos.13210. [DOI] [PubMed] [Google Scholar]
- 28.Wang JC, Laíns I, Providência J, Armstrong GW, Santos AR, Gil P, Gil J, Talcott KE, Marques JH, Figueira J, Vavvas DG, Kim IK, Miller JW, Husain D, Silva R, Miller JB. Diabetic Choroidopathy: Choroidal Vascular Density and Volume in Diabetic Retinopathy With Swept-Source Optical Coherence Tomography. Am J Ophthalmol. 2017;184:75–83. doi: 10.1016/j.ajo.2017.09.030. [DOI] [PubMed] [Google Scholar]
- 29.McLeod DS, Lutty GA. High-resolution histologic analysis of the human choroidal vasculature. Invest Ophthalmol Vis Sci. 1994;35:3799–3811. [PubMed] [Google Scholar]
- 30.Cao J, McLeod S, Merges CA, Lutty GA. Choriocapillaris degeneration and related pathologic changes in human diabetic eyes. Arch Ophthalmol. 1998;116:589–597. doi: 10.1001/archopht.116.5.589. [DOI] [PubMed] [Google Scholar]
- 31.Otani T, Kishi S, Maruyama Y. Patterns of diabetic macular edema with optical coherence tomography. Am J Ophthalmol. 1999;127:688–693. doi: 10.1016/s0002-9394(99)00033-1. [DOI] [PubMed] [Google Scholar]
- 32.Kim JT, Lee DH, Joe SG, Kim JG, Yoon YH. Changes in choroidal thickness in relation to the severity of retinopathy and macular edema in type 2 diabetic patients. Invest Ophthalmol Vis Sci. 2013;54:3378–3384. doi: 10.1167/iovs.12-11503. [DOI] [PubMed] [Google Scholar]
- 33.Murakami T, Yoshimura N. Structural changes in individual retinal layers in diabetic macular edema. J Diabetes Res. 2013;2013:920713. doi: 10.1155/2013/920713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ota M, Nishijima K, Sakamoto A, Murakami T, Takayama K, Horii T, Yoshimura N. Optical coherence tomographic evaluation of foveal hard exudates in patients with diabetic maculopathy accompanying macular detachment. Ophthalmology. 2010;117:1996–2002. doi: 10.1016/j.ophtha.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 35.Giocanti-Aurégan A, Hrarat L, Qu LM, Sarda V, Boubaya M, Levy V, Chaine G, Fajnkuchen F. Functional and Anatomical Outcomes in Patients With Serous Retinal Detachment in Diabetic Macular Edema Treated With Ranibizumab. Invest Ophthalmol Vis Sci. 2017;58:797–800. doi: 10.1167/iovs.16-20855. [DOI] [PubMed] [Google Scholar]
- 36.Sonoda S, Sakamoto T, Yamashita T, Shirasawa M, Otsuka H, Sonoda Y. Retinal morphologic changes and concentrations of cytokines in eyes with diabetic macular edema. Retina. 2014;34:741–748. doi: 10.1097/IAE.0b013e3182a48917. [DOI] [PubMed] [Google Scholar]
- 37.Shimura M, Yasuda K, Yasuda M, Nakazawa T. Visual outcome after intravitreal bevacizumab depends on the optical coherence tomographic patterns of patients with diffuse diabetic macular edema. Retina. 2013;33:740–747. doi: 10.1097/IAE.0b013e31826b6763. [DOI] [PubMed] [Google Scholar]
- 38.Seo KH, Yu SY, Kim M, Kwak HW. Visual and morphologic outcomes of intravitreal ranibizumab for diabetic macular edema based on optical coherence tomography patterns. Retina. 2016;36:588–595. doi: 10.1097/IAE.0000000000000770. [DOI] [PubMed] [Google Scholar]
- 39.Liu Q, Hu Y, Yu H, Yuan L, Hu J, Atik A, Guan M, Li D, Li X, Tang S. Comparison of intravitreal triamcinolone acetonide versus intravitreal bevacizumab as the primary treatment of clinically significant macular edema. Retina. 2015;35:272–279. doi: 10.1097/IAE.0000000000000300. [DOI] [PubMed] [Google Scholar]
- 40.Yang Y, Bailey C, Loewenstein A, Massin P. Intravitreal corticosteroids in diabetic macular edema: pharmacokinetic considerations. Retina. 2015;35:2440–2449. doi: 10.1097/IAE.0000000000000726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Diabetic Diabetic Retinopathy Clinical Research Network (DRCR.net), Beck RW. Edwards AR, Aiello LP, Bressler NM, Ferris F, Glassman AR, Hartnett E, Ip MS, Kim JE, Kollman C. Three-year follow-up of a randomized trial comparing focal/grid photocoagulation and intravitreal triamcinolone for diabetic macular edema. Arch Ophthalmol. 2009;127:245–251. doi: 10.1001/archophthalmol.2008.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zur D, Iglicki M, Busch C, Invernizzi A, Mariussi M, Loewenstein A International Retina Group. OCT Biomarkers as Functional Outcome Predictors in Diabetic Macular Edema Treated with Dexamethasone Implant. Ophthalmology. 2018;125:267–275. doi: 10.1016/j.ophtha.2017.08.031. [DOI] [PubMed] [Google Scholar]
- 43.Saxena S, Ruia S, Prasad S, Jain A, Mishra N, Natu SM, Meyer CH, Gilhotra JS, Kruzliak P, Akduman L. Increased serum levels of urea and creatinine are surrogate markers for disruption of retinal photoreceptor external limiting membrane and inner segment ellipsoid zone in type 2 diabetes mellitus. Retina. 2017;37:344–349. doi: 10.1097/IAE.0000000000001163. [DOI] [PubMed] [Google Scholar]
- 44.Bunt-Milam AH, Saari JC, Klock IB, Garwin GG. Zonulae adherentes pore size in the external limiting membrane of the rabbit retina. Invest Ophthalmol Vis Sci. 1985;26:1377–1380. [PubMed] [Google Scholar]
- 45.Phadikar P, Saxena S, Ruia S, Lai TY, Meyer CH, Eliott D. The potential of spectral domain optical coherence tomography imaging based retinal biomarkers. Int J Retina Vitreous. 2017;3:1. doi: 10.1186/s40942-016-0054-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jain A, Saxena S, Khanna VK, Shukla RK, Meyer CH. Status of serum VEGF and ICAM-1 and its association with external limiting membrane and inner segment-outer segment junction disruption in type 2 diabetes mellitus. Mol Vis. 2013;19:1760–1768. [PMC free article] [PubMed] [Google Scholar]
- 47.Murakami T, Nishijima K, Akagi T, Uji A, Horii T, Ueda-Arakawa N, Muraoka Y, Yoshimura N. Optical coherence tomographic reflectivity of photoreceptors beneath cystoid spaces in diabetic macular edema. Invest Ophthalmol Vis Sci. 2012;53:1506–1511. doi: 10.1167/iovs.11-9231. [DOI] [PubMed] [Google Scholar]
- 48.Shin HJ, Lee SH, Chung H, Kim HC. Association between photoreceptor integrity and visual outcome in diabetic macular edema. Graefes Arch Clin Exp Ophthalmol. 2012;250:61–70. doi: 10.1007/s00417-011-1774-x. [DOI] [PubMed] [Google Scholar]
- 49.Murakami T, Nishijima K, Sakamoto A, Ota M, Horii T, Yoshimura N. Association of pathomorphology, photoreceptor status, and retinal thickness with visual acuity in diabetic retinopathy. Am J Ophthalmol. 2011;151:310–317. doi: 10.1016/j.ajo.2010.08.022. [DOI] [PubMed] [Google Scholar]
- 50.Forooghian F, Stetson PF, Meyer SA, Chew EY, Wong WT, Cukras C, Meyerle CB, Ferris FL 3rd. Relationship between photoreceptor outer segment length and visual acuity in diabetic macular edema. Retina. 2010;30:63–70. doi: 10.1097/IAE.0b013e3181bd2c5a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maheshwary AS, Oster SF, Yuson RM, Cheng L, Mojana F, Freeman WR. The association between percent disruption of the photoreceptor inner segment-outer segment junction and visual acuity in diabetic macular edema. Am J Ophthalmol 2010; 150: 63-67. :e1. doi: 10.1016/j.ajo.2010.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Uji A, Murakami T, Nishijima K, Akagi T, Horii T, Arakawa N, Muraoka Y, Ellabban AA, Yoshimura N. Association between hyperreflective foci in the outer retina, status of photoreceptor layer, and visual acuity in diabetic macular edema. Am J Ophthalmol 2012; 153: 710-717, 717. :e1. doi: 10.1016/j.ajo.2011.08.041. [DOI] [PubMed] [Google Scholar]
- 53.Yanyali A, Bozkurt KT, Macin A, Horozoglu F, Nohutcu AF. Quantitative assessment of photoreceptor layer in eyes with resolved edema after pars plana vitrectomy with internal limiting membrane removal for diabetic macular edema. Ophthalmologica. 2011;226:57–63. doi: 10.1159/000327597. [DOI] [PubMed] [Google Scholar]
- 54.Wong RL, Lee JW, Yau GS, Wong IY. Relationship between Outer Retinal Layers Thickness and Visual Acuity in Diabetic Macular Edema. Biomed Res Int. 2015;2015:981471. doi: 10.1155/2015/981471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Murakami T, Nishijima K, Akagi T, Uji A, Horii T, Ueda-Arakawa N, Muraoka Y, Yoshimura N. Segmentational analysis of retinal thickness after vitrectomy in diabetic macular edema. Invest Ophthalmol Vis Sci. 2012;53:6668–6674. doi: 10.1167/iovs.12-9934. [DOI] [PubMed] [Google Scholar]
- 56.Diabetic Retinopathy Clinical Research Network, Browning DJ, Glassman AR, Aiello LP, Beck RW, Brown DM, Fong DS, Bressler NM, Danis RP, Kinyoun JL, Nguyen QD, Bhavsar AR, Gottlieb J, Pieramici DJ, Rauser ME, Apte RS, Lim JI, Miskala PH. Relationship between optical coherence tomography-measured central retinal thickness and visual acuity in diabetic macular edema. Ophthalmology. 2007;114:525–536. doi: 10.1016/j.ophtha.2006.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Querques G, Bux AV, Martinelli D, Iaculli C, Noci ND. Intravitreal pegaptanib sodium (Macugen) for diabetic macular oedema. Acta Ophthalmol. 2009;87:623–630. doi: 10.1111/j.1755-3768.2009.01580.x. [DOI] [PubMed] [Google Scholar]
- 58.Sakamoto A, Nishijima K, Kita M, Oh H, Tsujikawa A, Yoshimura N. Association between foveal photoreceptor status and visual acuity after resolution of diabetic macular edema by pars plana vitrectomy. Graefes Arch Clin Exp Ophthalmol. 2009;247:1325–1330. doi: 10.1007/s00417-009-1107-5. [DOI] [PubMed] [Google Scholar]
- 59.Alasil T, Keane PA, Updike JF, Dustin L, Ouyang Y, Walsh AC, Sadda SR. Relationship between optical coherence tomography retinal parameters and visual acuity in diabetic macular edema. Ophthalmology. 2010;117:2379–2386. doi: 10.1016/j.ophtha.2010.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Otani T, Yamaguchi Y, Kishi S. Correlation between visual acuity and foveal microstructural changes in diabetic macular edema. Retina. 2010;30:774–780. doi: 10.1097/IAE.0b013e3181c2e0d6. [DOI] [PubMed] [Google Scholar]
- 61.Spaide RF, Curcio CA. Anatomical correlates to the bands seen in the outer retina by optical coherence tomography: literature review and model. Retina. 2011;31:1609–1619. doi: 10.1097/IAE.0b013e3182247535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bizheva K, Pflug R, Hermann B, Povazay B, Sattmann H, Qiu P, Anger E, Reitsamer H, Popov S, Taylor JR, Unterhuber A, Ahnelt P, Drexler W. Optophysiology: depth-resolved probing of retinal physiology with functional ultrahigh-resolution optical coherence tomography. Proc Natl Acad Sci USA. 2006;103:5066–5071. doi: 10.1073/pnas.0506997103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Srinivasan VJ, Wojtkowski M, Fujimoto JG, Duker JS. In vivo measurement of retinal physiology with high-speed ultrahigh-resolution optical coherence tomography. Opt Lett. 2006;31:2308–2310. doi: 10.1364/ol.31.002308. [DOI] [PubMed] [Google Scholar]
- 64.Verma A, Rani PK, Raman R, Pal SS, Laxmi G, Gupta M, Sahu C, Vaitheeswaran K, Sharma T. Is neuronal dysfunction an early sign of diabetic retinopathy? Eye (Lond) 2009;23:1824–1830. doi: 10.1038/eye.2009.184. [DOI] [PubMed] [Google Scholar]
- 65.Boynton GE, Stem MS, Kwark L, Jackson GR, Farsiu S, Gardner TW. Multimodal characterization of proliferative diabetic retinopathy reveals alterations in outer retinal function and structure. Ophthalmology. 2015;122:957–967. doi: 10.1016/j.ophtha.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Reznicek L, Kernt M, Haritoglou C, Kampik A, Ulbig M, Neubauer AS. In vivo characterization of ischemic retina in diabetic retinopathy. Clin Ophthalmol. 2010;5:31–35. doi: 10.2147/OPTH.S13850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kaarniranta K, Sinha D, Blasiak J, Kauppinen A, Veréb Z, Salminen A, Boulton ME, Petrovski G. Autophagy and heterophagy dysregulation leads to retinal pigment epithelium dysfunction and development of age-related macular degeneration. Autophagy. 2013;9:973–984. doi: 10.4161/auto.24546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xia Z, Chen H, Zheng S. Alterations of Retinal Pigment Epithelium-Photoreceptor Complex in Patients with Type 2 Diabetes Mellitus without Diabetic Retinopathy: A Cross-Sectional Study. J Diabetes Res. 2020;2020:9232157. doi: 10.1155/2020/9232157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hernández C, Garcia-Ramírez M, García-Rocha M, Saez-López C, Valverde ÁM, Guinovart JJ, Simó R. Glycogen storage in the human retinal pigment epithelium: a comparative study of diabetic and non-diabetic donors. Acta Diabetol. 2014;51:543–552. doi: 10.1007/s00592-013-0549-8. [DOI] [PubMed] [Google Scholar]
- 70.Tavares Ferreira J, Alves M, Dias-Santos A, Costa L, Santos BO, Cunha JP, Papoila AL, Abegão Pinto L. Retinal Neurodegeneration in Diabetic Patients Without Diabetic Retinopathy. Invest Ophthalmol Vis Sci. 2016;57:6455–6460. doi: 10.1167/iovs.16-20215. [DOI] [PubMed] [Google Scholar]
- 71.Schneck ME, Shupenko L, Adams AJ. The fast oscillation of the EOG in diabetes with and without mild retinopathy. Doc Ophthalmol. 2008;116:231–236. doi: 10.1007/s10633-007-9088-3. [DOI] [PubMed] [Google Scholar]
- 72.Feitosa-Santana C, Paramei GV, Nishi M, Gualtieri M, Costa MF, Ventura DF. Color vision impairment in type 2 diabetes assessed by the D-15d test and the Cambridge Colour Test. Ophthalmic Physiol Opt. 2010;30:717–723. doi: 10.1111/j.1475-1313.2010.00776.x. [DOI] [PubMed] [Google Scholar]
- 73.Gella L, Raman R, Kulothungan V, Pal SS, Ganesan S, Sharma T. Impairment of Colour Vision in Diabetes with No Retinopathy: Sankara Nethralaya Diabetic Retinopathy Epidemiology and Molecular Genetics Study (SNDREAMS- II, Report 3) PLoS One. 2015;10:e0129391. doi: 10.1371/journal.pone.0129391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wolff BE, Bearse MA Jr, Schneck ME, Dhamdhere K, Harrison WW, Barez S, Adams AJ. Color vision and neuroretinal function in diabetes. Doc Ophthalmol. 2015;130:131–139. doi: 10.1007/s10633-014-9476-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tan NC, Yip WF, Kallakuri S, Sankari U, Koh YLE. Factors associated with impaired color vision without retinopathy amongst people with type 2 diabetes mellitus: a cross-sectional study. BMC Endocr Disord. 2017;17:29. doi: 10.1186/s12902-017-0181-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Uji A, Murakami T, Unoki N, Ogino K, Horii T, Yoshitake S, Dodo Y, Yoshimura N. Parallelism for quantitative image analysis of photoreceptor-retinal pigment epithelium complex alterations in diabetic macular edema. Invest Ophthalmol Vis Sci. 2014;55:3361–3367. doi: 10.1167/iovs.14-13948. [DOI] [PubMed] [Google Scholar]
- 77.Bolz M, Schmidt-Erfurth U, Deak G, Mylonas G, Kriechbaum K, Scholda C Diabetic Retinopathy Research Group Vienna. Optical coherence tomographic hyperreflective foci: a morphologic sign of lipid extravasation in diabetic macular edema. Ophthalmology . 2009;116:914–920. doi: 10.1016/j.ophtha.2008.12.039. [DOI] [PubMed] [Google Scholar]
- 78.Das R, Spence G, Hogg RE, Stevenson M, Chakravarthy U. Disorganization of Inner Retina and Outer Retinal Morphology in Diabetic Macular Edema. JAMA Ophthalmol. 2018;136:202–208. doi: 10.1001/jamaophthalmol.2017.6256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Querques G, Lattanzio R, Querques L, Del Turco C, Forte R, Pierro L, Souied EH, Bandello F. Enhanced depth imaging optical coherence tomography in type 2 diabetes. Invest Ophthalmol Vis Sci. 2012;53:6017–6024. doi: 10.1167/iovs.12-9692. [DOI] [PubMed] [Google Scholar]
- 80.Vujosevic S, Martini F, Cavarzeran F, Pilotto E, Midena E. Macular and peripapillary choroidal thickness in diabetic patients. Retina. 2012;32:1781–1790. doi: 10.1097/IAE.0b013e31825db73d. [DOI] [PubMed] [Google Scholar]
- 81.Esmaeelpour M, Považay B, Hermann B, Hofer B, Kajic V, Hale SL, North RV, Drexler W, Sheen NJ. Mapping choroidal and retinal thickness variation in type 2 diabetes using three-dimensional 1060-nm optical coherence tomography. Invest Ophthalmol Vis Sci. 2011;52:5311–5316. doi: 10.1167/iovs.10-6875. [DOI] [PubMed] [Google Scholar]
- 82.Regatieri CV, Branchini L, Carmody J, Fujimoto JG, Duker JS. Choroidal thickness in patients with diabetic retinopathy analyzed by spectral-domain optical coherence tomography. Retina. 2012;32:563–568. doi: 10.1097/IAE.0b013e31822f5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Unsal E, Eltutar K, Zirtiloğlu S, Dinçer N, Ozdoğan Erkul S, Güngel H. Choroidal thickness in patients with diabetic retinopathy. Clin Ophthalmol. 2014;8:637–642. doi: 10.2147/OPTH.S59395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Melancia D, Vicente A, Cunha JP, Abegão Pinto L, Ferreira J. Diabetic choroidopathy: a review of the current literature. Graefes Arch Clin Exp Ophthalmol. 2016;254:1453–1461. doi: 10.1007/s00417-016-3360-8. [DOI] [PubMed] [Google Scholar]
- 85.Lee HK, Lim JW, Shin MC. Comparison of choroidal thickness in patients with diabetes by spectral-domain optical coherence tomography. Korean J Ophthalmol. 2013;27:433–439. doi: 10.3341/kjo.2013.27.6.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tavares Ferreira J, Vicente A, Proença R, Santos BO, Cunha JP, Alves M, Papoila AL, Abegão Pinto L. Choroidal thickness in diabetic patients without diabetic retinopathy. Retina. 2018;38:795–804. doi: 10.1097/IAE.0000000000001582. [DOI] [PubMed] [Google Scholar]
- 87.Ferrara D, Waheed NK, Duker JS. Investigating the choriocapillaris and choroidal vasculature with new optical coherence tomography technologies. Prog Retin Eye Res. 2016;52:130–155. doi: 10.1016/j.preteyeres.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 88.Kase S, Endo H, Yokoi M, Kotani M, Katsuta S, Takahashi M, Kase M. Choroidal thickness in diabetic retinopathy in relation to long-term systemic treatments for diabetes mellitus. Eur J Ophthalmol. 2016;26:158–162. doi: 10.5301/ejo.5000676. [DOI] [PubMed] [Google Scholar]
- 89.Xu J, Xu L, Du KF, Shao L, Chen CX, Zhou JQ, Wang YX, You QS, Jonas JB, Wei WB. Subfoveal choroidal thickness in diabetes and diabetic retinopathy. Ophthalmology . 2013;120:2023–2028. doi: 10.1016/j.ophtha.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 90.Adhi M, Brewer E, Waheed NK, Duker JS. Analysis of morphological features and vascular layers of choroid in diabetic retinopathy using spectral-domain optical coherence tomography. JAMA Ophthalmol. 2013;131:1267–1274. doi: 10.1001/jamaophthalmol.2013.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gerendas BS, Waldstein SM, Simader C, Deak G, Hajnajeeb B, Zhang L, Bogunovic H, Abramoff MD, Kundi M, Sonka M, Schmidt-Erfurth U. Three-dimensional automated choroidal volume assessment on standard spectral-domain optical coherence tomography and correlation with the level of diabetic macular edema. Am J Ophthalmol. 2014;158:1039–1048. doi: 10.1016/j.ajo.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang Z, Meng X, Wu Z, Zou W, Zhang J, Zhu D, Chen T, Zhang Q. Changes in Choroidal Thickness After Panretinal Photocoagulation for Diabetic Retinopathy: A 12-Week Longitudinal Study. Invest Ophthalmol Vis Sci. 2015;56:2631–2638. doi: 10.1167/iovs.14-16226. [DOI] [PubMed] [Google Scholar]
- 93.Cho GE, Cho HY, Kim YT. Change in subfoveal choroidal thickness after argon laser panretinal photocoagulation. Int J Ophthalmol. 2013;6:505–509. doi: 10.3980/j.issn.2222-3959.2013.04.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Laíns I, Figueira J, Santos AR, Baltar A, Costa M, Nunes S, Farinha C, Pinto R, Henriques J, Silva R. Choroidal thickness in diabetic retinopathy: the influence of antiangiogenic therapy. Retina. 2014;34:1199–1207. doi: 10.1097/IAE.0000000000000053. [DOI] [PubMed] [Google Scholar]
- 95.Yiu G, Manjunath V, Chiu SJ, Farsiu S, Mahmoud TH. Effect of anti-vascular endothelial growth factor therapy on choroidal thickness in diabetic macular edema. Am J Ophthalmol 2014; 158: 745-751. :e2. doi: 10.1016/j.ajo.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rayess N, Rahimy E, Ying GS, Bagheri N, Ho AC, Regillo CD, Vander JF, Hsu J. Baseline choroidal thickness as a predictor for response to anti-vascular endothelial growth factor therapy in diabetic macular edema. Am J Ophthalmol 2015; 159: 85-91. :e1–3. doi: 10.1016/j.ajo.2014.09.033. [DOI] [PubMed] [Google Scholar]
- 97.Agrawal R, Gupta P, Tan KA, Cheung CM, Wong TY, Cheng CY. Choroidal vascularity index as a measure of vascular status of the choroid: Measurements in healthy eyes from a population-based study. Sci Rep. 2016;6:21090. doi: 10.1038/srep21090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Markan A, Agarwal A, Arora A, Bazgain K, Rana V, Gupta V. Novel imaging biomarkers in diabetic retinopathy and diabetic macular edema. Ther Adv Ophthalmol . 2020;12:2515841420950513. doi: 10.1177/2515841420950513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nesper PL, Roberts PK, Onishi AC, Chai H, Liu L, Jampol LM, Fawzi AA. Quantifying Microvascular Abnormalities With Increasing Severity of Diabetic Retinopathy Using Optical Coherence Tomography Angiography. Invest Ophthalmol Vis Sci. 2017;58:BIO307–BIO315. doi: 10.1167/iovs.17-21787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Muir ER, Rentería RC, Duong TQ. Reduced ocular blood flow as an early indicator of diabetic retinopathy in a mouse model of diabetes. Invest Ophthalmol Vis Sci. 2012;53:6488–6494. doi: 10.1167/iovs.12-9758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Okamoto M, Yamashita M, Ogata N. Effects of intravitreal injection of ranibizumab on choroidal structure and blood flow in eyes with diabetic macular edema. Graefes Arch Clin Exp Ophthalmol. 2018;256:885–892. doi: 10.1007/s00417-018-3939-3. [DOI] [PubMed] [Google Scholar]
- 102.Li L, Almansoob S, Zhang P, Zhou YD, Tan Y, Gao L. Quantitative analysis of retinal and choroid capillary ischaemia using optical coherence tomography angiography in type 2 diabetes. Acta Ophthalmol. 2019;97:240–246. doi: 10.1111/aos.14076. [DOI] [PubMed] [Google Scholar]
- 103.Weinberger D, Kramer M, Priel E, Gaton DD, Axer-Siegel R, Yassur Y. Indocyanine green angiographic findings in nonproliferative diabetic retinopathy. Am J Ophthalmol. 1998;126:238–247. doi: 10.1016/s0002-9394(98)00148-2. [DOI] [PubMed] [Google Scholar]