Abstract
Purpose
We explored the anti-inflammatory role of the DPP-4 inhibitor teneligliptin, using sitagliptin as comparator, in different in vitro models of low-grade inflammation (LGI), evaluating the hyperglycemia-induced endothelial inflammation, the macrophage polarization, and the endothelium–macrophage interaction.
Methods
The effects of DPP-4 and its inhibitors on macrophage polarization were evaluated in THP-1 cells by measuring mRNA expression of M1-M2 markers. HUVEC cells were used to analyze the effects of DPP-4 inhibitors on endothelial inflammation under normal and high glucose conditions. To evaluate the link between eNO and M1-M2 polarization, HUVECs were transfected with eNOS siRNA and co-cultured with THP-1 cells. The effects of DPP-4 inhibitors on macrophage polarization and eNO content were evaluated in a co-culture model of differentiated THP-1 cells + HUVECs under normal glucose (NG), high glucose (HG) and high metabolic memory (HM) conditions.
Results
DPP-4 regulated M1/M2 macrophage polarization. Teneligliptin reduced M1 and enhanced M2 macrophage phenotype under DPP-4 stimulation, and attenuated hyperglycemia-induced endothelial inflammation. In THP-1 cells co-cultured with eNOS depleted HUVECs, M1 markers were enhanced, while M2 reduced, indicating an important role of eNO in polarization to M2 phenotype. In the co-culture model with HUVECs exposed to HG and HM, teneligliptin reduced M1 and enhanced M2 population, by increasing eNO levels. The anti-inflammatory effects of sitagliptin were not observed in these LGI models.
Conclusion
Teneligliptin, but not sitagliptin, has anti-inflammatory effects in the various LGI models, by promoting a switch from M1 toward M2 phenotype and by decreasing hyperglycaemia-induced endothelial inflammation, suggesting that effects for LGI are different among DPP-4 inhibitors.
Keywords: DPP-4 inhibitors, low-grade inflammation, teneligliptin, macrophage polarization, endothelial inflammation, endothelial NO, high glucose, antioxidant defense
Introduction
Chronological age and overnutrition are the two main risk factors for the development of type 2 diabetes mellitus (T2DM) and for its cardiovascular (CV) complications. They promote a chronic-state of sustained, low-grade inflammation (LGI), a phenomenon termed either “inflammaging” or “metaflammation”, a critical component of T2DM.1–3 LGI involves the same cellular and molecular mediators of the acute inflammatory responses, despite being chronic in nature.2 Different in vitro models can be used to study the effect of specific substances on LGI. Among them, hyperglycemia-induced inflammation and cytokine-induced macrophages polarization represent useful tools to explore the anti-inflammatory potential of glucose-lowering drugs.3
Macrophages have a primary role in inflammation and host defense, and also participate in a variety of other homeostatic functions, including wound healing and tissue repair. Such pleiotropic effects are mediated by their response to diverse environmental signals; these give rise to distinct functional phenotypes that can range from a pro-inflammatory – classic M1 activation – to a more anti-inflammatory phenotype – alternative M2 activation.3,4 The M1-M2 polarization plays a role in LGI, which leads to insulin resistance and micro- and macrovascular diabetic complications.3–5 A key regulator of macrophage polarization is endothelial nitric oxide (eNO), which polarizes macrophages from the M1 to the M2 phenotype, suggesting an implication of endothelial cell–macrophage interaction in inflammation.6,7 However, its specific role under hyperglycemic conditions has not been fully evaluated.
Various glucose-lowering drugs are accompanied by different effects on LGI.8–10 In this sense, promising evidence is emerging for dipeptidyl peptidase-4 inhibitors. The use of DPP-4 inhibitors in the treatment of T2DM has increased since their introduction,11,12 given the favorable effects in terms of low risk of hypoglycemia and neutral effects on body weight and cardiovascular outcomes.13 The DPP-4 enzyme is found in serum and it has been associated with several different cellular functions;14 moreover, it is expressed on the surface of a variety of cell types, including those involved in immune response.15 In addition, DPP-4 has several non-incretin substrates and physiologically cleaves cytokines and chemokines, thus playing a major role in LGI.16
The effects of selected DPP-4 inhibitors on LGI are clearly emerging.17 Moreover, the role on macrophage polarization for some drugs of this glucose-lowering class has been demonstrated; in this sense, a recent paper showed that the DPP-4 itself enhances macrophage M1 polarization and inhibits M2 polarization, while DPP-4 inhibitor linagliptin attenuates these effects in vitro.5 However, the drug-intrinsic differences among the various DPP-4 inhibitors18 and their mechanisms for anti-inflammatory response remain to be unknown. It appears to be crucial, therefore, to determine the fundamental pharmacological mechanism of these inhibitors and the possible differences among them, in order to use the appropriate DPP-4 inhibitor for suitable patients.
Teneligliptin is a DPP-4 inhibitor with peculiar pharmacological and pharmacokinetics properties.19 It is characterized by a long-lasting effect on DPP-4, while is metabolized through multiple routes, thus not requiring dose adjustments in patients with renal impairment.19 It is accompanied by anti-oxidative and vaso-protective properties, as shown in vitro and in patients with T2DM.20,21 However, less information is available regarding the effects of teneligliptin on LGI.
Here, we explored the anti-inflammatory potential of teneligliptin with 3 commonly used in vitro models of LGI, ie cytokine-induced macrophage polarization, hyperglycemia-induced inflammation, and endothelium–macrophage interaction in the hyperglycemic environment, along with the possible mediators of such effect.
Methods
Cell Culture
Human THP-1 cells were purchased from ATCC and maintained in RPMI-1640 medium supplemented with 10% FBS, 1% penicillin/streptomycin, and 1% L-glutamine (Euroclone).
Human umbilical vein endothelial primary cells (HUVECs) were purchased by Lonza and cultured with EGM™-2 Bulletkit™ (Lonza) at 37°C in a humidified atmosphere with 5% CO2. Cells were used between 4 and 6 passages. HUVECs were seeded and allowed to attach overnight.
Teneligliptin hydrobromide hydrate (3-[(2S,4S)-4- [4-(3-methyl-1-phenyl-1H-pyrazol-5-yl)piperazin-1-yl]pyrrolidin-2-yl-carbonyl]thiazolidinehemipentahydrogenbromide hydrate) was provided by Mitsubishi Tanabe Pharma Corporation (Osaka, Japan).
Sitagliptin phosphate monohydrate (7-[(3R)-3-amino-1-oxo-4-(2,4,5-trifluorophenyl)butyl]-5,6,7,8-tetrahydro-3-(trifluoromethyl)-1,2,4-triazolo[4,3-a]pyrazine phosphate) was provided by BioVision.
Experimental designs are detailed in Supplementary Materials and Methods.
ROS Measurement
2ʹ,7ʹ-Dichlorofluorescein diacetate (H2DCFDA) was used to measure intracellular ROS production. And, 5×103 differentiated THP-1 cells were grown on clear flat bottom treated 96-well plates under the experimental conditions. At the end of the experiment, cells were stained with 20 mM H2DCFDA for 30 minutes at 37°C. H2DCFDA was kinetically measured at an excitation and emission wavelength of 485 nm and 530 nm using a fluorescent microplate reader (Synergy HT, BioTek Instruments, Inc., Winooski, Vermont, USA).
RNA Isolation and qRT-PCR
RNA was isolated from cells using Total RNA isolation kit (Norgen Biotek Corp, Thorold, Ontario, Canada) following the manufacturer’s instructions. Briefly, first-strand cDNA was prepared using 1–2ug of total RNA, the Superscript III RT kit and random hexamer primers (Invitrogen, Carlsbad, CA, USA) in a total volume of 25 µL. Reverse transcription reaction was carried out for 90 minutes at 50°C and an additional 10 minutes at 55°C. Quantitative real-time polymerase chain reaction (qRT-PCR) was performed on an ABI Prism 7900 sequence detection system using SybrGreen reagents (Takara Bio Company, Clontech, Mountain View, CA, USA) and TaqMan® Gene Expression Master Mix (Life Technologies, Madrid, Spain). Primers used were previously described.20,22,23
Protein Extraction
Cells were harvested and lysates were prepared using RIPA buffer (Sigma-Aldrich) with the addition of a protease and phosphatase inhibitor cocktail. Protein content of the lysates was determined using the Bradford reagent (Sigma-Aldrich).
Western Blot Analysis
Protein lysates (30μg) were resolved by SDS-polyacrylamide gel electrophoresis (PAGE-R Gold gels 4–20%, Lonza) and transferred to a polyvinylidene fluoride (PVDF) membrane. After blocking with 5% non-fat dry milk (NFDM) in 20 mM Tris–HCl (pH 7.5), 135 mM NaCl and 0.1% Tween-20, blots were incubated with monoclonal antibodies against human eNOS (Cell Signaling Technology) (1:1000). Human beta-Actin (1:1000) (Sigma-Aldrich) was used as a loaded control. Detection was performed using a secondary peroxidase-linked anti-mouse/rabbit antibody (1:3000) (GE Healthcare Europe GmbH) and an enhanced chemiluminescence system (Pierce Chemical Co) according to the manufacturer’s instructions. Proteins were revealed in a CCD camera (ImageQuantLAS4000, GE Healthcare, UK). Protein quantification was performed using computer-assisted densitometry (www.imagej.nih.gov, ImageJ Software, NIH).
NO Measurements
Assessment of NOx levels was performed according to a commercial assay kit, as described by the manufacturer (Abcam). Briefly, the cells were plated in 96-well plates at a density of 1×104 cells/well and cultured according to the different experimental conditions. At the end of the experiments, NO assay colorimetric kit was used to measure the total nitrate/nitrite in a two-step process: nitrate reductase was used to convert nitrate to nitrite; then, Griess reagent was used to convert nitrite to a deep purple azo-compound. The amount of the azochromophorereflected nitric oxide amount in the samples. Optical density was measured at 540 nm using a microplate reader (Synergy HT, BioTek Instruments, Inc.).
Statistical Analysis
In all experiments, measurements were normalized to controls, and the fold increase above the control condition was calculated. Numerical data were expressed as the mean ± SEM, and P values of <0.05 were considered statistically significant. Differences between the mean values of two groups were assessed using a two-tailed Student’s t-test. Differences in mean values between more than two groups were determined using one-way ANOVA. Turkey’s test for multiple comparisons was used to determine which pairs of means were different.
Results
DPP-4 Enhances M1 Polarization and Inhibits M2 Polarization in a ROS-Dependent Manner
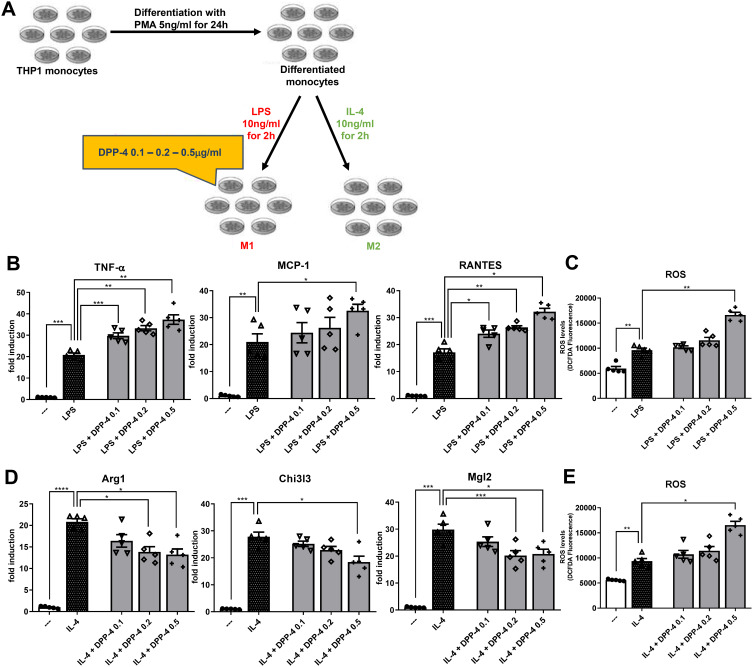
We determined whether DPP-4 directly regulates macrophage activation and/or polarization (experimental design in Figure 1A). Exposure of stably differentiated THP-1 cells to DPP-4 significantly increased mRNA expression of M1 markers, Tnf-α and Rantes upon stimulating with LPS, in a dose-dependent manner (Figure 1B). A significant up-regulation was observed for Mcp-1 (Figure 1B) and for the intracellular ROS production (Figure 1C) when DPP-4 was used at the highest concentration of 0.5μg/mL.
Figure 1.
DPP-4 enhances M1 polarization and inhibits M2 polarization in a ROS-dependent manner. (A) Experimental design. Fold changes in expression levels of (B) M1 markers (Tnf-α, Mcp-1 and Rantes) and (D) M2 markers (Arg1, Chi3l3 and Mgl2). Intracellular ROS production upon stimulation with (C) LPS for M1 polarization and (E) IL-4 for M2 polarization. For all diagrams, error bars are ± SEM and * ANOVA p < 0.05, ** ANOVA p < 0.01, *** ANOVA p < 0.001, **** ANOVA p < 0.0001; n = 5.
Exposure of macrophages to DPP-4 at 0.2 and 0.5μg/mL upon stimulation with IL-4, significantly reduced mRNA expression of M2 markers, Arg1 and Mgl2, in a dose-dependent manner. DPP-4 at the highest concentration also significantly decreased Chi3l3 (Figure 1D) and increased intracellular ROS production (Figure 1E).
Taken together, these results suggest that DPP-4 directly regulates M1-M2 macrophage polarization following LPS or IL-4 stimulation, by increasing M1 polarization and inhibiting M2 polarization possibly in a ROS-dependent manner.
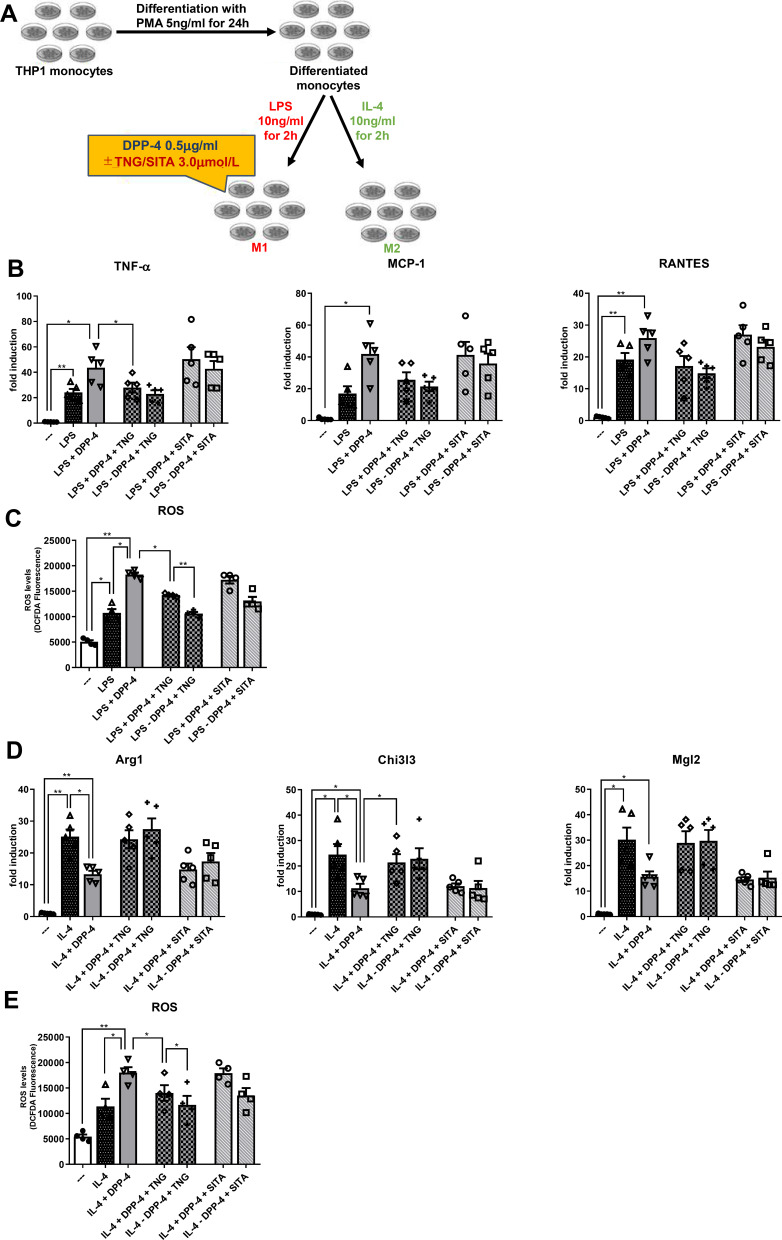
Teneligliptin, but Not Sitagliptin, Reduces M1 Polarization and Enhances M2 Polarization Under DPP-4 Stimulation
To test the effects of teneligliptin, we repeated the experiment, adding to the medium the DPP-4 inhibitor at three different concentrations20 (see Supplementary Figure 1). Once established the effective dose of teneligliptin in counteracting the pro-inflammatory effects of DPP-4 in stably differentiated THP-1 cells, we compared this property of teneligliptin with another DPP-4 inhibitor that is largely used in the clinical practice, sitagliptin (experimental design in Figure 2A). In macrophages stimulated by LPS and DPP-4 at the highest concentration of 0.5μg/mL, mRNA levels of M1 maker Tnf-α were significantly diminished in the presence of teneligliptin 3.0μmol/L, but not of sitagliptin at the same dose (Figure 2B). For all the M1 markers analyzed, a tendency to a decrease in their mRNA levels was observed with the addition of teneligliptin, but not sitagliptin, to the exposure of macrophages with LPS + DPP-4. Moreover, a decrease in ROS production was observed after administration of teneligliptin, but not sitagliptin (Figure 2C). On the other hand, the exposure to teneligliptin, but not sitagliptin, significantly increased the expression of M2 macrophage marker Chi3l3 (Figure 2D), and decreased ROS generation (Figure 2E). For the M2 markers analyzed, a tendency to an up-regulation in their mRNA levels was observed with the addition of teneligliptin, but not sitagliptin, to the exposure of macrophages with IL-4 + DPP-4 (Figure 2D).
Figure 2.
Teneligliptin, but not sitagliptin, reduces M1 polarization and enhances M2 polarization. (A) Experimental design. Fold changes in expression levels of (B) M1 markers (Tnf-α, Mcp-1 and Rantes) and (D) M2 markers (Arg1, Chi3l3 and Mgl2). Intracellular ROS production upon stimulation with (C) LPS for M1 polarization and (E) IL-4 for M2 polarization. For all diagrams, error bars are ± SEM and * ANOVA p < 0.05, ** ANOVA p < 0.01,; n = 5. Abbreviations: SITA, sitagliptin; TNG, teneligliptin.
Taken together, these results suggest that teneligliptin, but not sitagliptin, can suppress M1-polarized activation and induce M2-polarized activation under DPP-4 stimulation, possibly regulating intracellular ROS generation.
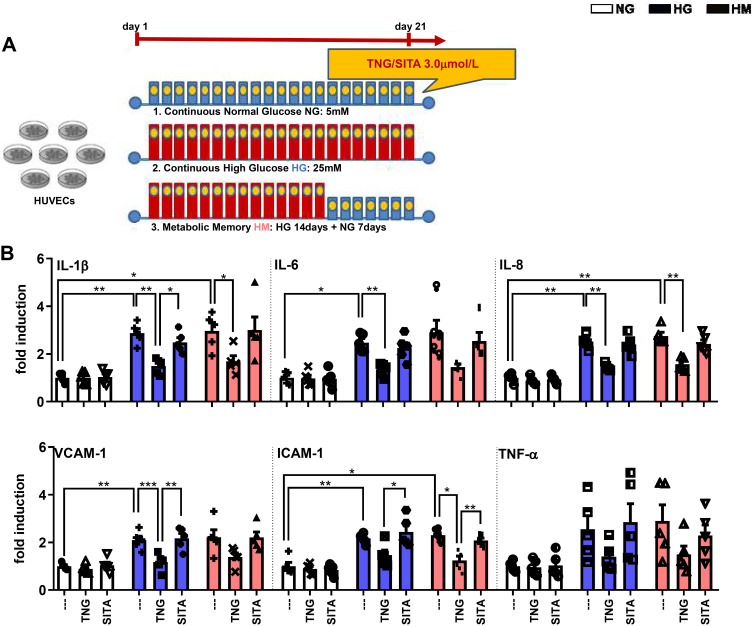
Teneligliptin, but Not Sitagliptin, Attenuates HG-Induced Endothelial Inflammation
mRNA expression of inflammatory cytokines derived from M1 macrophages, including Tnf-α, IL-1β, IL-6, and IL-8, as well as Vcam-1 and Icam-1 that mediate the adhesion of lymphocytes, monocytes, eosinophils, and basophils to vascular endothelium, was analyzed in order to determine the effects of teneligliptin, in comparison with sitagliptin, on endothelial inflammation under hyperglycemic conditions (experimental design in Figure 3A). As expected, HG and HM in general increased gene expression of all the markers analyzed, compared with the control NG condition. Such increase was significantly relevant for IL-1β, IL-6, IL-8, Vcam-1 and Icam-1 under HG conditions, and for IL-1β, IL-8 and Icam-1 under HM conditions. HUVECs exposed to HG and treated with teneligliptin showed a decrease in the mRNA expression of IL-1β, IL-6, IL-8 and Vcam-1, compared with the conditions of HG without DPP-4 treatment. Moreover, addition of teneligliptin decreased mRNA expression of IL-1β, IL-8 and Icam-1, compared with the condition of HM without DPP-4 treatment. Sitagliptin was not able to exert the same positive effects (Figure 3B).
Figure 3.
Teneligliptin, but not sitagliptin, attenuates HG-induced endothelial inflammation. (A) Experimental design. (B) Fold changes in expression levels of endothelial inflammation (Il-1β, Il-6, Il-8, Vcam-1, Icam-1 and Tnf-α). For all diagrams, error bars are ± SEM and * ANOVA p < 0.05, ** ANOVA p < 0.01, *** ANOVA p < 0.001; n = 5. Abbreviations: SITA, sitagliptin; TNG, teneligliptin.
These results demonstrate that the DPP-4 inhibitor teneligliptin, but not sitagliptin, can attenuate hyperglycemia-induced endothelial inflammation in HUVECs exposed to both conditions of HG and HM.
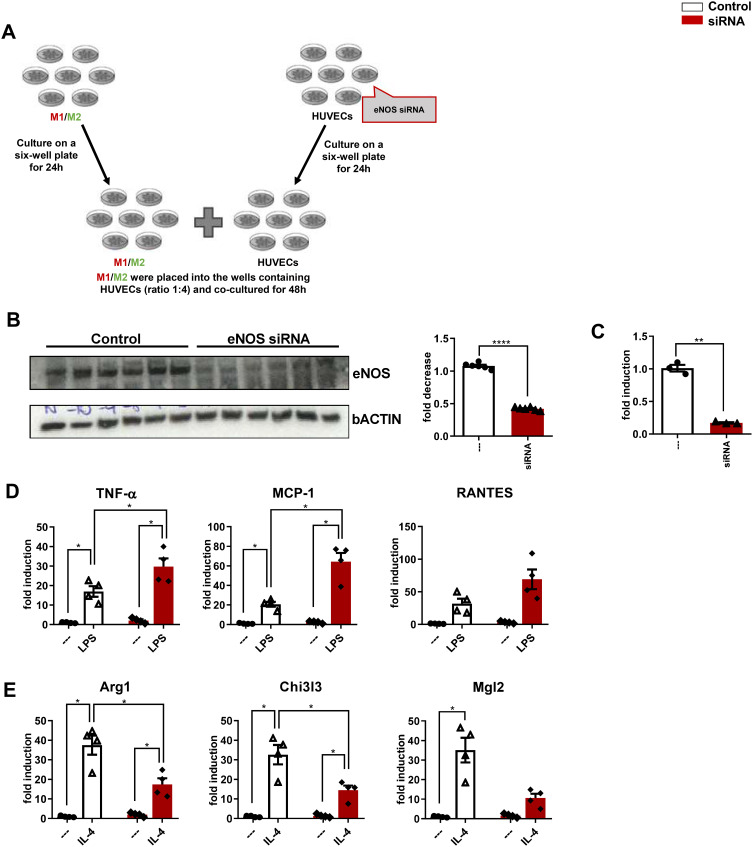
eNOS Has a Role in Polarization from M1 to M2 Population
To further test the hypothesis that endothelial NO signaling polarizes macrophages away from the M1 and toward the anti-inflammatory M2 phenotype, we used a co-culture system combining HUVECs with differentiated THP-1 cells. As a specific control, the HUVECs used for this study were either intact or had a reduced capacity to generate NO, by prior incubation with scrambled control siRNA or eNOS siRNA, respectively (experimental design in Figure 4A). As expected, eNOS protein was markedly reduced in HUVECs receiving eNOS versus scrambled siRNA (Figure 4B), and NO measurements demonstrated a proportionate reduction of NO content in eNOS depleted cells (Figure 4C).
Figure 4.
eNO is required for the polarization from M1 to M2 population. (A) Experimental design. (B) Western blot analysis of eNOS, and fold decrease. (C) NO levels. Fold changes in expression levels of (D) M1 markers (Tnf-α, Mcp-1 and Rantes) and (E) M2 markers (Arg1, Chi3l3 and Mgl2). For all diagrams, error bars are ± SEM and (B and C) ** t-test p < 0.01, **** t-test p < 0.0001; n = 6; (D and E) * ANOVA p < 0.05.
To determine whether decreased endothelial NO enhances M1 activation of differentiated THP-1 cells, these were stimulated with LPS. Compared with stably differentiated THP-1 co-cultured with control HUVECs, the induction of M1 markers, TNF-α and Mcp-1, was significantly enhanced in differentiated THP-1 cells co-cultured with HUVECs receiving eNOS siRNA (Figure 4D).
To determine whether decreased endothelial NO signaling had the opposite effect on macrophage M2 activation, the co-culture study was repeated except that THP-1 cells were incubated with IL-4, rather than LPS. As expected, IL-4 treatment significantly increased the mRNA expression of all M2 markers analyzed in differentiated THP-1 co-cultured with control HUVECs, and this effect was significantly blunted for Arg1 and Chi3l3 in THP-1 cells co-cultured with HUVECs receiving eNOS siRNA (Figure 4E).
These results confirm that eNOS signaling plays a role in polarization from M1 to M2 macrophage population, and that increased eNO levels attenuate the pro-inflammatory M1, increasing the anti-inflammatory M2 phenotype.
Teneligliptin, but Not Sitagliptin, Reduces M1 and Enhances M2 Polarization by Enhancing eNO Under HG-HM Conditions
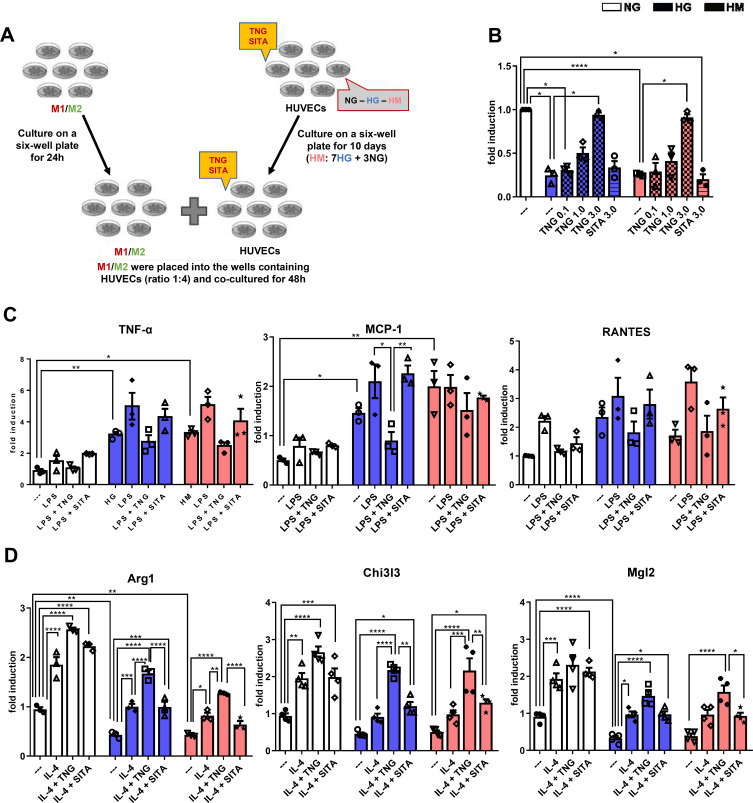
To test the hypothesis that teneligliptin can shift macrophage polarization from M1 to M2 phenotype by increasing eNOS that is blunted under hyperglycemic conditions, we repeated the experimental design based on the co-culture system combining differentiated THP-1 cells with HUVECs exposed to NG, HG and HM (experimental design in Figure 5A).
Figure 5.
Teneligliptin, but not sitagliptin, reduces M1 and enhances M2 population by enhancing eNO under HG-HM conditions. (A) Experimental design. (B) NO levels. Fold changes in expression levels of (C) M1 markers (Tnf-α, Mcp-1 and Rantes) and (D) M2 markers (Arg1, Chi3l3 and Mgl2). For all diagrams, error bars are ± SEM and * ANOVA p < 0.05, ** ANOVA p < 0.01, *** ANOVA p < 0.001, **** ANOVA p < 0.0001; n = 5. Abbreviations: SITA, sitagliptin; TNG, teneligliptin.
Firstly, we observed that eNO levels were significantly decreased when HUVECs were exposed to both conditions of HG and HM, compared to NG. Teneligliptin at the highest concentration used, but not sitagliptin, was able to revert eNO measurements, significantly increasing them in both HG and HM conditions (Figure 5B).
To determine whether decreased eNO upon HG and HM treatment, enhances M1 activation of differentiated THP-1 cells, these were stimulated with LPS and IL-4.
Compared with stably differentiated THP-1 co-cultured with HUVECs exposed to NG, the induction of M1 markers, TNF-α and Mcp-1, was enhanced in differentiated THP-1 cells co-cultured with HUVECs exposed to HG and HM. Moreover, we observed a tendency for teneligliptin, but not sitagliptin, to decrease the gene expression levels of all M1 markers analyzed for both hyperglycemic conditions (Figure 5C).
To determine whether teneligliptin has the opposite effect on macrophage M2 activation, the co-culture study was repeated except that THP-1 cells were incubated with IL-4, rather than LPS. As expected, both high glucose conditions decreased the mRNA expression of the M2 markers analyzed in differentiated THP-1 co-cultured with HUVECs exposed to HG and HM, compared to control NG-HUVECs; such decrease was statistically significant for Arg1 and Mgl2 in HG, and for Arg1 in HM. This effect was significantly reverted after the administration of teneligliptin; moreover, teneligliptin, but not sitagliptin, was able to enhance the positive effects of IL-4, and such increase was statistically significant for Arg1 and Chi3l3 in both conditions of HG and HM (Figure 5D).
These results confirm that eNOS has an important role in polarization from M1 to M2 macrophage population. Moreover, they demonstrate that eNO, blunted under hyperglycemic conditions and increased after teneligliptin treatment, is able to attenuate the pro-inflammatory M1, increasing the anti-inflammatory M2 macrophage phenotype.
Discussion
We investigated the anti-inflammatory effects of the DPP-4 inhibitor teneligliptin in different in vitro models of LGI, using sitagliptin, another member of the same glucose-lowering drug class, as a comparator. Here, we show that
DPP-4 protein dose-dependently promotes LPS-induced M1 polarization, while attenuating IL-4-induced M2 polarization;
teneligliptin is able to revert this phenomenon;
teneligliptin attenuates hyperglycemia-induced endothelial inflammation;
teneligliptin attenuates the high glucose-induced down-modulation of eNOS, leading to the regulation of M1-M2 polarization in the endothelium–macrophage interaction;
these anti-inflammatory effects are not observed with the administration of sitagliptin.
To our knowledge, this is the first report showing a marked anti-inflammatory effect of teneligliptin in three different in vitro models relevant for LGI and T2DM. Moreover, with the macrophage-endothelial co-culture model under high glucose conditions, we provide a new experimental method to deep insight into the effects of DPP-4 inhibitors on the M1-M2 polarization and the LGI.
Previous data showed marked anti-atherogenic and anti-inflammatory activities of teneligliptin in animal models. Indeed, teneligliptin significantly inhibited the development of atherosclerotic lesions in the aortic arch of normoglycemic ApoE mice, an effect accompanied by a decreased local expression of TNF-α, MCP-1, and IL-6.24 Interestingly, teneligliptin treatment in vitro was able to ameliorate the conversion of macrophages in foam cells using both macrophages from diabetic mice or patients with T2DM.25 Concerning its anti-inflammatory effects, it has been reported that teneligliptin inhibits the LPS-induced inflammation signal in human and mouse macrophages.26 Results of our work are compatible with these observations and support a pleiotropic, non-glycemia-linked, beneficial role of teneligliptin on LGI and on the atherosclerotic process. In addition, we found an improvement in eNOS expression with teneligliptin treatment, a result obtained also in a rat model of metabolic syndrome.27
Of note, treatment with teneligliptin, but not sitagliptin, significantly improved endothelial function and oxidative status in T2DM patients with chronic kidney disease (CKD), although HbA1c or renal function markers, like eGFR and urinary albumin, were not different between teneligliptin and sitagliptin group.21 Furthermore, teneligliptin reduced plasma levels of soluble P-selectin, platelet-derived microparticles, and plasminogen activator inhibitor-1 (PAI-1) in T2DM patients.28
The observation that the results obtained with teneligliptin were not reproduced with sitagliptin reinforces the notion that the DPP-4 inhibitors are accompanied by non-overlapping secondary effects,29 and drug intrinsic differences among diverse DPP-4 inhibitors exist.18,30 To explain these discrepancies, different hypotheses have been formulated, including their intrinsic structural differences, which may support their distinct effects beyond the pharmacological action on DPP-4.18,30 For example, teneligliptin shows five-fold higher activity than sitagliptin, and this difference in potency could be explained by the unique structure of teneligliptin, composed of five rings, leading to a small entropy loss upon binding to DPP-418. The novel chemical structure of teneligliptin may be also responsible for its hydroxyl radical (·OH) scavenging properties; in contrast, the DPP-4 inhibitor alogliptin and linagliptin do not exhibit scavenging effects.31 Moreover, the chemical properties of the various DPP-4 inhibitors may influence their tissue distribution and, as a consequence, their drug permeability profile.32 These variations might be related to the differences in vascular protective effects among DPP-4 inhibitors.33 In this sense, teneligliptin and linagliptin have notable tissue distribution properties in the kidney, which has the highest level of DPP-4 activity,34 followed by the lung, adrenal gland, jejunum and liver.32 Drug concentrations used in this study (teneligliptin 0.1, 1.0 and 3.0μmol/L, sitagliptin 3.0μmol/L) are about 0.25 to 8 times higher compared to the peak plasma concentration (Cmax) of each drug in the clinical practice dose; Cmax of teneligliptin (20mg) and sitagliptin (50mg, 100mg) are about 0.4μmol/L, 0.4μmol/L and 0.9μmol/L, respectively.19,35 However, tissue concentration of DPP-4 inhibitor is higher than plasma concentration; tissue-to-plasma ratio in rat is about 2–90 (teneligliptin) and 2–20 (sitagliptin).32 As the intracellular/intercellular concentration is important for our study, we supposed that the drug concentration in this study is achievable in the clinical practice.
Regarding the effects on endothelium exposed to high glucose conditions, our previous studies showed the different potential protective actions of the DPP-4 inhibitors teneligliptin and sitagliptin.20,22 In these two works, we demonstrated that teneligliptin, but not sitagliptin, had antioxidant properties by reducing ROS levels, the major triggers and promoters of the inflammatory cascades,36 and by initiating the transcriptional cascade of antioxidant genes at cellular level. Of note, the efficacy of teneligliptin 0.1μmol/L in reducing ROS was similar to that observed with sitagliptin 0.5μmol/L, suggesting that teneligliptin has more potent antioxidant properties.20 Moreover, teneligliptin, but not sitagliptin, enhanced proliferation and endoplasmic reticulum homeostasis, reduced apoptosis in HG conditions, and overcame the metabolic memory effect.20
The implications of the various DPP-4 inhibitors in the development of LGI are clearly emerging,10,16,17 but their effects on cardiovascular outcomes are considered neutral.13 In this sense it has been shown the strikingly strong correlation between drug exposure and cardiovascular risk reduction in diabetic subjects37; therefore, the duration of the current cardiovascular outcome studies on DPP-4 inhibitors might be too short to detect their potential cardiovascular protective effects. Teneligliptin appears to have multifaceted effects on endothelial function, via its antioxidant capabilities, anti-inflammatory properties, and anti-platelet activity. It has been largely demonstrated that oxidative stress induced by elevated glucose levels facilitates cardiovascular endothelial damage,36 leading to the perpetuation of vascular damage despite the achievement of improved glycemic control, the well-known metabolic memory effect.38 The present work and our previous studies20,22 showed that teneligliptin exerts an effective inhibition of inflammatory pathways, especially when the inflammatory triggers are applied for long periods, as demonstrated not only under high glucose conditions but also in the presence of metabolic memory conditions. This evidence suggests that early treatment with teneligliptin could prevent micro- and macrovascular complications or attenuate the progression of such complications in diabetic subjects.
The various DPP-4 inhibitors can differ in terms of potency, selectivity, kinetics and their pleiotropic effects, with the action of teneligliptin being more long-lasting compared to other drugs of the same class.19 These aspects could be useful for the selection of the appropriate therapy for patients with T2DM, making teneligliptin, with its unique characteristics, a particularly viable consideration for a diverse range of diabetic subjects.
Our results demonstrated that effects for LGI are different between teneligliptin and sitagliptin, and suggested that different DPP-4 inhibitors have different properties in terms of LGI. We evaluated the effects for LGI by using only two DPP-4 inhibitors (teneligliptin and sitagliptin). It is needed to evaluate the effects of other DPP-4 inhibitors for LGI with the detailed mechanisms of action. Moreover, additional experiments are required to assess the specificity of the observed reduction in LGI produced by teneligliptin, and to explore the potential underpinnings of the non-overlapping effects among diverse DPP-4 inhibitors. These studies may be useful for selection of specific DPP-4 inhibitors according to patient background.
Overall, we showed a pro-inflammatory role for DPP-4 protein in the hyperglycemic environment. In addition, we demonstrated a marked anti-inflammatory effect of a specific DPP-4 inhibitor, teneligliptin, in multiple models, an observation not reproduced with another member of the same class, ie, sitagliptin.
Acknowledgments
This work was supported by Mitsubishi Tanabe Pharma Corporation (Osaka, Japan) and by the Italian Ministry of Health - Ricerca Corrente to IRCCS MultiMedica.
Abbreviations
Arg1, arginase 1; Chi3l3, chitinase 3-like-3; eNO, endothelial nitric oxide; eNOS, endothelial nitric oxide synthetase; H2DCFDA, 2ʹ,7ʹ-dichlorofluorescein diacetate; HG, high glucose; HM, high metabolic memory; HUVECs, human umbilical vein endothelial cells; ICAM-1, intercellular adhesion molecule-1; IL, interleukin; LGI, low-grade inflammation; LPS, lipopolysaccharide; MCP-1, monocyte chemoattractant protein-1; Mgl2, macrophage galactose-type C-type lectin 2; NFDM, non-fat dried milk; NG, normal glucose; NO, nitric oxide; PMA, phorbol 12-myristate 13-acetate; PVDF, polyvinylidene fluoride; RANTES, regulated upon activation, normal T cell expressed; ROS, reactive oxygen species; SITA, sitagliptin; TNG, teneligliptin; T2DM, type 2 diabetes mellitus; TNF-α, tumor necrosis factor-alpha; VCAM-1, vascular cell adhesion molecule-1.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author, VDN, upon reasonable request.
Ethics Statement
The study was approved by Ethical committee of Hospital Clínic i Provincial de Barcelona.
Author Contributions
V.D.N. made substantial contributions to conception, design of the study, acquisition of data and interpretation of the results. F.P., H.I. and A.C. made substantial contributions to interpretation of the results. V.D.N. drafted the manuscript and F.P., H.I. and A.C. revised the paper for intellectual content. All the authors agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
VDN reports research fees from Mitsubishi Tanabe Pharma Corporation during the conduct of the study. FP reports grants from the Italian Ministry of Health - Ricerca Corrente. HI is the employee of Mitsubishi Tanabe Pharma Corporation. AC reports personal fees from BD, Eli Lilly, Mundipharma, Astra Zeneca, Roche Diagnostics, Theras, Berlin Chemie, Boehringer Ingelheim, Novo Nordisk; grants from the Italian Ministry of Health - Ricerca Corrente and Mitsubishi, outside the submitted work. The authors report no other conflicts of interest in this work.
References
- 1.Olivieri F, Prattichizzo F, Grillari J, Balistreri CR. Cellular senescence and inflammaging in age-related diseases. Mediators Inflamm. 2018;2018:9076485. doi: 10.1155/2018/9076485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hotamisligil GS. Inflammation, metaflammation and immunometabolic disorders. Nature. 2017;542(7640):177–185. [DOI] [PubMed] [Google Scholar]
- 3.Prattichizzo F, De Nigris V, Spiga R, et al. Inflammageing and metaflammation: the yin and yang of type 2 diabetes. Ageing Res Rev . 2018;41:1–17. [DOI] [PubMed] [Google Scholar]
- 4.Akira S, Misawa T, Satoh T, Saitoh T. Macrophages control innate inflammation. Diabetes, Obesity & Metabolism. 2013;15(Suppl 3):10–18. [DOI] [PubMed] [Google Scholar]
- 5.Zhuge F, Ni Y, Nagashimada M, et al. DPP-4 Inhibition by linagliptin attenuates obesity-related inflammation and insulin resistance by regulating M1/M2 macrophage polarization. Diabetes. 2016;65(10):2966–2979. [DOI] [PubMed] [Google Scholar]
- 6.Lee WJ, Tateya S, Cheng AM, et al. M2 macrophage polarization mediates anti-inflammatory effects of endothelial nitric oxide signaling. Diabetes. 2015;64(8):2836–2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tateya S, Rizzo NO, Handa P, et al. Endothelial NO/cGMP/VASP signaling attenuates Kupffer cell activation and hepatic insulin resistance induced by high-fat feeding. Diabetes. 2011;60(11):2792–2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prattichizzo F, Giuliani A, Mensà E, et al. Pleiotropic effects of metformin: shaping the microbiome to manage type 2 diabetes and postpone ageing. Ageing Res Rev. 2018;48:87–98. [DOI] [PubMed] [Google Scholar]
- 9.Prattichizzo F, De Nigris V, Micheloni S, La Sala L, Ceriello A. Increases in circulating levels of ketone bodies and cardiovascular protection with SGLT2 inhibitors: is low-grade inflammation the neglected component? Diabetes. Obesity Metabol. 2018;20(11):2515–2522. [DOI] [PubMed] [Google Scholar]
- 10.Yaribeygi H, Maleki M, Sathyapalan T, Jamialahmadi T, Sahebkar A. Anti-inflammatory potentials of incretin-based therapies used in the management of diabetes. Life Sci. 2020;241:117152. [DOI] [PubMed] [Google Scholar]
- 11.Christensen DH, Rungby J, Thomsen RW. Nationwide trends in glucose-lowering drug use, Denmark, 1999-2014. Clinical Epidemiology. 2016;8:381–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamamoto-Honda R, Takahashi Y, Mori Y, et al. Changes in antidiabetic drug prescription and glycemic control trends in elderly patients with Type 2 diabetes mellitus from 2005-2013: an analysis of the National Center Diabetes Database (NCDD-03). Internal Med. 2018;57(9):1229–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gallwitz B. Clinical Use of DPP-4 Inhibitors. Front Endocrinol. 2019;10:389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aso Y, Terasawa T, Kato K, et al. The serum level of soluble CD26/dipeptidyl peptidase 4 increases in response to acute hyperglycemia after an oral glucose load in healthy subjects: association with high-molecular weight adiponectin and hepatic enzymes. Transl Res. 2013;162(5):309–316. [DOI] [PubMed] [Google Scholar]
- 15.Ohnuma K, Hosono O, Dang NH, Morimoto C. Dipeptidyl peptidase in autoimmune pathophysiology. Advan Clin Chem. 2011;53:51–84.A. [DOI] [PubMed] [Google Scholar]
- 16.Avogaro A, Fadini GP. The pleiotropic cardiovascular effects of dipeptidyl peptidase-4 inhibitors. Br J Clin Pharmacol. 2018;84(8):1686–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aroor AR, Sowers JR, Jia G, DeMarco VG. Pleiotropic effects of the dipeptidylpeptidase-4 inhibitors on the cardiovascular system. Am J Physiol Heart Circulatory Physiol. 2014;307(4):H477–492.M. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nabeno M, Akahoshi F, Kishida H, et al. A comparative study of the binding modes of recently launched dipeptidyl peptidase IV inhibitors in the active site. Biochem Biophys Res Commun. 2013;434(2):191–196. [DOI] [PubMed] [Google Scholar]
- 19.Ceriello A, De Nigris V, Iijima H, Matsui T, Gouda M. The unique pharmacological and pharmacokinetic profile of teneligliptin: implications for clinical practice. Drugs. 2019;79(7):733–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pujadas G, De Nigris V, Prattichizzo F, La Sala L, Testa R, Ceriello A. The dipeptidyl peptidase-4 (DPP-4) inhibitor teneligliptin functions as antioxidant on human endothelial cells exposed to chronic hyperglycemia and metabolic high-glucose memory. Endocrine. 2017;56(3):509–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sagara M, Suzuki K, Aoki C, et al. Impact of teneligliptin on oxidative stress and endothelial function in type 2 diabetes patients with chronic kidney disease: a case-control study. Cardiovasc Diabetol. 2016;15:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Nigris V, Prattichizzo F, Mancuso E, Spiga R, Pujadas G, Ceriello A. Teneligliptin enhances the beneficial effects of GLP-1 in endothelial cells exposed to hyperglycemic conditions. Oncotarget. 2018;9(10):8898–8910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prattichizzo F, De Nigris V, Mancuso E, et al. Short-term sustained hyperglycaemia fosters an archetypal senescence-associated secretory phenotype in endothelial cells and macrophages. Redox Biol. 2018;15:170–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salim HM, Fukuda D, Higashikuni Y, et al. Teneligliptin, a dipeptidyl peptidase-4 inhibitor, attenuated pro-inflammatory phenotype of perivascular adipose tissue and inhibited atherogenesis in normoglycemic apolipoprotein-E-deficient mice. Vasc Pharmacol. 2017;96-98:19–25. [DOI] [PubMed] [Google Scholar]
- 25.Terasaki M, Hiromura M, Mori Y, et al. A dipeptidyl peptidase-4 inhibitor suppresses macrophage foam cell formation in diabetic db/db mice and type 2 diabetes patients. Int J Endocrinol. 2018;2018:8458304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hiromura M, Nohtomi K, Mori Y, et al. Caveolin-1, a binding protein of CD26, is essential for the anti-inflammatory effects of dipeptidyl peptidase-4 inhibitors on human and mouse macrophages. Biochem Biophys Res Commun. 2018;495(1):223–229. [DOI] [PubMed] [Google Scholar]
- 27.Nakagami H, Pang Z, Shimosato T, et al. The dipeptidyl peptidase-4 inhibitor teneligliptin improved endothelial dysfunction and insulin resistance in the SHR/NDmcr-cp rat model of metabolic syndrome. Hypertension Res. 2014;37(7):629–635. [DOI] [PubMed] [Google Scholar]
- 28.Okuda Y, Omoto S, Taniura T, Shouzu A, Nomura S. Effects of teneligliptin on PDMPs and PAI-1 in patients with diabetes on hemodialysis. Int J General Med. 2016;9:65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kröller-Schön S, Knorr M, Hausding M, et al. Glucose-independent improvement of vascular dysfunction in experimental sepsis by dipeptidyl-peptidase 4 inhibition. Cardiovasc Res. 2012;96(1):140–149. [DOI] [PubMed] [Google Scholar]
- 30.Luconi M, Cantini G, Ceriello A, Mannucci E. Perspectives on cardiovascular effects of incretin-based drugs: from bedside to bench, return trip. Int J Cardiol. 2017;241:302–310. [DOI] [PubMed] [Google Scholar]
- 31.Kimura S, Inoguchi T, Yamasaki T, et al. A novel DPP-4 inhibitor teneligliptin scavenges hydroxyl radicals: in vitro study evaluated by electron spin resonance spectroscopy and in vivo study using DPP-4 deficient rats. Metabolism. 2016;65(3):138–145. [DOI] [PubMed] [Google Scholar]
- 32.Nakamaru Y, Akahoshi F, Iijima H, Hisanaga N, Kume T. Tissue distribution of teneligliptin in rats and comparisons with data reported for other dipeptidyl peptidase-4 inhibitors. Biopharm Drug Disposition. 2016;37(3):142–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takai S, Sakonjo H, Jin D. Significance of vascular dipeptidyl peptidase-4 inhibition on vascular protection in Zucker diabetic fatty rats. J Pharmacol Sci. 2014;125(4):386–393. [DOI] [PubMed] [Google Scholar]
- 34.Mentlein R. Dipeptidyl-peptidase IV (CD26)–role in the inactivation of regulatory peptides. Regulatory Peptides. 1999;85(1):9–24. [DOI] [PubMed] [Google Scholar]
- 35.Bergman AJ, Stevens C, Zhou Y, et al. Pharmacokinetic and pharmacodynamic properties of multiple oral doses of sitagliptin, a dipeptidyl peptidase-IV inhibitor: a double-blind, randomized, placebo-controlled study in healthy male volunteers. Clin Ther. 2006;28(1):55–72. [DOI] [PubMed] [Google Scholar]
- 36.Kayama Y, Raaz U, Jagger A, et al. Diabetic cardiovascular disease induced by oxidative stress. Int J Mol Sci. 2015;16(10):25234–25263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roussel R, Steg PG, Mohammedi K, Marre M, Potier L. Prevention of cardiovascular disease through reduction of glycaemic exposure in type 2 diabetes: a perspective on glucose-lowering interventions. Diabetes Obesity Metabol. 2018;20(2):238–244. [DOI] [PubMed] [Google Scholar]
- 38.Testa R, Bonfigli AR, Prattichizzo F, La Sala L, De Nigris V, Ceriello A. The “metabolic memory” theory and the early treatment of hyperglycemia in prevention of diabetic complications. Nutrients. 2017;9:5. [DOI] [PMC free article] [PubMed] [Google Scholar]