Abstract
Purpose of review
Although thoracentesis is generally considered safe, procedural complications are associated with increased morbidity, mortality, and healthcare costs. In this article, we review the risk factors and prevention of the most common complications of thoracentesis including pneumothorax, bleeding (chest wall hematoma and hemothorax), and re-expansion pulmonary edema.
Recent findings
Recent data support the importance of operator expertise and the use of ultrasound in reducing the risk of iatrogenic pneumothorax. Although coagulopathy or thrombocytopenia and the use of anticoagulant or antiplatelet medications have traditionally been viewed as contraindications to thoracentesis, new evidence suggests that patients may be able to safely undergo thoracentesis without treating their bleeding risk. Re-expansion pulmonary edema, a rare complication of thoracentesis, is felt to result in part from the generation of excessively negative pleural pressure. When and how to monitor changes in pleural pressure during thoracentesis remains a focus of ongoing study.
Summary
Major complications of thoracentesis are uncommon. Clinician awareness of risk factors for procedural complications and familiarity with strategies that improve outcomes are essential components for safely performing thoracentesis.
Keywords: hemothorax, iatrogenic pneumothorax, re-expansion pulmonary edema, thoracentesis, ultrasound
INTRODUCTION
Thoracentesis is a common procedure performed by a wide range of healthcare providers in both the inpatient and outpatient settings [1]. Although generally considered a low-risk intervention, complications of thoracentesis, including pneumothorax, bleeding (puncture site bleeding, chest wall hematoma, and hemothorax), and re-expansion pulmonary edema (REPE), can lead to increased morbidity, mortality, and healthcare cost [2,3]. For example, an iatrogenic pneumothorax can add 4.4 days to hospital length of stay and $18000 in additional healthcare expenditures [4].
Research into the incidence and risk factors of procedural complications is limited by a lack of randomized trials and publication bias with subsequent underreporting [5■■,6]. Specifically, this has led to barriers in the study of the true incidence of therapeutic complications, that is complications not present prior to a procedural intervention, in surgical laparoscopy, and in left ventricular assist device placement [7–9]. Nevertheless, in the last several years more evidence has emerged allowing prediction and prevention of thoracentesis complications [6,10].
The goal of this article is to review the latest evidence from the past 3 years, supplemented by historical articles, describing the risk factors and prevention of pneumothorax, bleeding, and REPE (Table 1). Infrequent complications, such as tumor seeding of the catheter tract [15], catheter fracture [16,17], injury to abdominal viscera [17], and vasovagal syncope [18] are out of the scope of this article and will not be discussed.
Table 1.
Summary of studies evaluating the major thoracentesis-related complications over the past 3 years
| Author | Study type | Main finding |
|---|---|---|
| Pneumothorax (PTX) | ||
| [3] | Retrospective cohort | Decreased risk of PTX with ultrasound guidance (2.26 vs. 3.09%); decreased mortality with ultrasound guidance (7.4 vs. 4.2%); decreased hospital cost ($2075) and length of stay (1.5 days) |
| [5■■] | Systematic review and meta-analysis | PTX rate 6.0%; no technical aspects of procedure were associated with increased risk of PTX |
| [11■■] | Prospective cohort | 0.61% PTX rate; PTX associated with greater than 1500 cm3 removed, unilateral procedure, more than one pass through skin, BMI < 18 kg/m2 |
| Bleeding complications | ||
| [5■■] | Systematic review and meta-analysis | Bleeding complication rate of 1.0% |
| [11■■] | Prospective cohort | 0.05% rate of hemothorax without correction of bleeding risk; no association of bleeding risk with patient or laboratory parameters |
| [12] | Case control | No decrease in bleeding complications with correction of coagulopathy and thrombocytopenia |
| [13] | Prospective cohort | No increased risk of bleeding complications in patients with laboratory evidence of increased bleeding risk (coagulopathy or thrombocytopenia) or on medications that increase bleeding risk (clopidogrel, low molecular weight heparin, heparin) |
| [14■] | Prospective cohort | 4% (95% confidence interval 0–10%) incidence of bleeding complications when performing thoracentesis in patients on clopidogrel |
| Re-expansion pulmonary edema (REPE) | ||
| [5■■] | Systematic review and meta-analysis | Only two studies included with range of incidence from 0 to 16% |
| [11■■] | Prospective cohort | 0.01% incidence of REPE; Association with volume of fluid removed and non-inflammatory conditions |
PNEUMOTHORAX
Pneumothorax is the most common complication of thoracentesis, with historical incidence rates as high as 19% [19]. Iatrogenic pneumothorax significantly impacts patient outcomes. A recent meta-analysis found that up to one-third of cases require chest tube drainage [2]. Furthermore, iatrogenic pneumothorax has been linked to increased healthcare costs and mortality [3,4,20].
It is important to note that the appearance of air within the pleural space on chest radiography following thoracentesis does not necessarily indicate a true procedural complication (injury to the visceral pleura). During drainage of the pleural space, atmospheric air can inadvertently enter the pleural space creating a small pneumothorax that requires no intervention. A unique form of a pneumothorax, termed pneumothorax ex vacuo, may also occur when excessively negative pleural pressures are generated in the setting of a lung unable to fully re-expand as seen in endobronchial obstruction, atelectasis, and visceral pleural thickening [21]. The increased intrapleural gradient can entrain air into the pleural space, creating a pneumothorax located in the position of the pleural fluid that was just removed (Fig. 1). A pneumothorax ex vacuo rarely requires aggressive treatment, and therefore, we do not believe pneumothorax ex vacuo should be viewed as a procedural complication of thoracentesis [22].
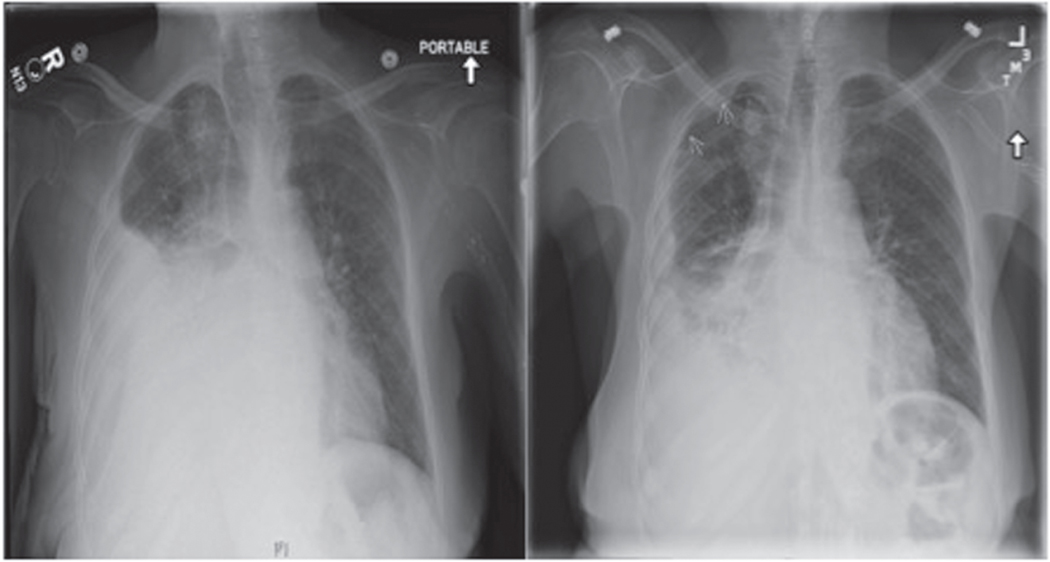
FIGURE 1.
Pneumothorax ex vacuo. This anterior-posterior chest radiograph (left) demonstrates a large pleural effusion in a patient with a chronic exudative right-sided effusion. After thoracentesis, repeat posterior-anterior chest radiography (right) showed a partially re-expanded lung with persistent effusion and evidence of apical pneumothorax, as indicated by arrows, consistent with pneumothorax ex vacuo.
Incidence
Although older studies report high rates of procedure-related pneumothorax [19], recent literature, which has emphasized operator experience and the use of ultrasound, has found pneumothorax to be an uncommon complication of thoracentesis.
Ault et al. [11■■], in a review of 9320 thoracenteses performed by an experienced operator, found a pneumothorax rate of 0.6%. Similarly, Heidecker et al. [21] identified only one pneumothorax from inadvertent lung puncture in a series of 367 procedures. Although it is important to note that studies over the past 3 years have varied in their use of postprocedural radiography, rates of iatrogenic pneumothorax rates have ranged from 0 to 3% [3,23–25]. Two recent meta-analyses reported an overall pneumothorax rate of 6% [2,5■■]. We believe the overestimation of pneumothorax rates results from inappropriate chest radiography after thoracentesis identifying clinically insignificant pneumothorax from atmospheric air, not visceral pleural puncture.
Use of ultrasound
The biggest contributor to the reduced incidence of pneumothorax has been the use of ultrasound. Ultrasound allows the operator to visualize the characteristics of a pleural effusion [26], identify the most accessible area of pleural fluid, measure the exact distance a needle must travel to enter the pleural effusion, avoid important surrounding structures, and localize intercostal vessels. There are extensive data supporting the use of preprocedural ultrasound in marking the location of the fluid to decrease the rate of complications from thoracentesis [27,28].
In an observational cohort study of over 61000 thoracentesis (45% of which were performed under ultrasound guidance), Mercaldi and Lanes [3] found that the use of ultrasound reduced the risk of pneumothorax by 19% [odds ratio (OR) 0.81; 95% CI, 0.74–0.90] and reduced healthcare cost and length of stay. Similarly, Cavanna et al. [23], in a study of 445 patients undergoing thoracentesis for malignant pleural effusion, showed that the use of ultrasound was associated with a statistically significant decrease in pneumothorax rate (1 vs. 9%, P < 0.0001). Numerous other studies have found that the use of ultrasound is associated with a decreased rate of pneumothorax [27,29,30]. In one of the most thorough evaluations to date, Gordon et al. [2] performed a systematic review of pneumothorax in thoracentesis. The investigators found that the use of ultrasound significantly reduced the rate of pneumothorax (OR 0.3; 95% CI, 0.2–0.7).
Although not all studies have demonstrated a reduced risk of pneumothorax with the use of ultrasound [5■■], the reduced complication rates reported throughout the literature and the minimal risks associated with ultrasound use have led many consensus guidelines and expert reviews to endorse the routine use of ultrasound [31,32].
Whether the use of real-time ultrasound guidance (where the procedure is performed under continuous ultrasound visualization) offers additional benefit beyond that afforded by preprocedural ultrasound use has not been rigorously studied. However, when accessing small or loculated effusions in a challenging anatomic location, we believe clinicians should consider the use of real-time ultrasound guidance.
Additional risk factors
Clinicians should be aware of several patientspecific and procedural factors, which increase the risk of iatrogenic pneumothorax [6]. Ault et al. [11■■] found that underweight patients (defined as a BMI less than 18kg/m2) were three times more likely to experience a pneumothorax. The authors attributed this risk to several factors: a smaller distance between the lung and chest wall, underestimation of the distance the needle has entered the skin, and the use of underweight patients as a surrogate for poor overall health. Although mechanical ventilation is frequently cited as a risk for pneumothorax, results from several studies failed to show an association [2,11■■,33]. Additionally, multiple needle passes through the skin have consistently been associated with higher rates of pneumothorax [2,11■■]. The study by Ault et al. also showed that sampling a small pleural effusion may raise the risk of pneumothorax [6]. Conversely, multiple studies suggest that the risk of pneumothorax rises with drainage volumes greater than 1.5 liter in contrast to draining less than 1.5 liter [6,11■■,34]. However, there are no clear data on the safest amount of fluid drained [35]. There are also no convincing data that the type of needle or catheter used impacts pneumothorax rates [5■■].
Finally, operator experience plays a significant role in procedural complication rates including pneumothorax. Gordon et al. [2] found a pneumothorax rate of 8.5% in procedures performed by less-experienced operators compared with a rate of 3.9% for experienced providers (P = 0.04). Additionally, Ault et al. [11■■] also supported this conclusion as they demonstrated a pneumothorax rate of 0.6% when an expert provider performed or directly supervised all procedures. Studies that have examined the impact of simulation and competency-based testing on complication rates further highlight the importance of instituting systems that ensure provider experience and comfort with thoracentesis [36,37].
BLEEDING COMPLICATIONS
A spectrum of bleeding complications, including puncture site bleeding, chest wall hematoma, and hemothorax, can be seen with thoracentesis. Although rare, a hemothorax should be suspected when a patient develops vital sign instability, a drop in hematocrit, or rapid pleural fluid reaccumulation following thoracentesis.
Incidence
The incidence of hemothorax following thoracentesis remains low despite operators increasingly performing procedures on patients with known bleeding risk factors [10]. Ault et al. identified only 17 (0.18%) bleeding complications post-thoracentesis procedures, only five of which were hemothoraces (0.01%). Data from Ault et al. [11■■] included a number of procedures performed on patients with uncorrected coagulopathy or thrombocytopenia. Similarly, one case series of procedures performed on patients with hematologic malignancies and following stem cell transplant (a majority of which had no evidence of thrombocytopenia or coagulopathy), showed hemothorax rates ranging from 0 to 2% [38,39]. Additionally, a recent systematic review of 48 studies found a similar overall risk of significant hemorrhage of 1% [5■■].
Correction of bleeding risk
Traditionally, the use of anticoagulant or antiplatelet medications and laboratory markers of abnormal hemostasis, including an elevated international normalized ratio (INR) or partial thromboplastin time (PTT) and low platelet count, were felt to be the best predictors of bleeding complications during thoracentesis. As a result, consensus guidelines, including those from the British Thoracic Society and the Society of Interventional Radiology, recommend not performing a thoracentesis until a patient’s INR is less than 1.5, platelet count is greater than 50000/μl, and antiplatelet medications such as clopidogrel have been held for 5 days. This was graded as, ‘C level’, evidence indicating this was derived from well-conducted case–control or cohort studies by the British Thoracic Society and expert opinion by the Society of Interventional Radiology [31,40].
Several recent studies cast doubt on these recommendations. In a retrospective case–control study, Hibbert et al. [12] compared the rate of thoracentesis-related bleeding complications in patients who received blood product transfusions for platelet counts less than 50000/μl and INRs greater than 1.6 to patients with similar abnormal laboratory values who did not receive preprocedural blood products. All four hemorrhagic complications identified in this study occurred in patients who received transfusions prior to their procedure. Puchalski et al. [13] prospectively evaluated bleeding complications in 312 patients undergoing thoracentesis, including 130 patients (42%) at increased risk of bleeding (INR>1.5, platelet count <50000/μl, creatinine>1.5mg/dl, use of clopidogrel or use of low molecular weight heparin). No cases of hemothorax were identified in this series of patients and there was no significant difference in the postprocedure hematocrit levels between patient groups. In another prospective cohort study, Mahmood et al. [14■] identified only one hemothorax in 25 patients undergoing thoracentesis while on clopidogrel. Similarly, the study by Ault et al. [11■■] found no association between INR, PTT, or platelet count with the incidence of bleeding complications.
The above studies represent a growing body of evidence that suggests patients can safely undergo thoracentesis procedures while avoiding the risks associated with blood product transfusions or stopping anticoagulant or antiplatelet medications. Randomized trials are needed to better stratify procedural bleeding risk, especially for those patients with multiple risk factors for bleeding. At this time, we do not believe there is enough definitive evidence to guide practice. Therefore, we recommend proceduralists use their clinical judgment and experience in making decisions on bleeding risk and blood product administration before thoracentesis procedures.
Use of ultrasound and defining vascular anatomy
Studies evaluating the use of ultrasound to prevent bleeding complications are confounded by whether abnormal markers of hemostasis were corrected by blood transfusion and an overall low incidence of bleeding events [5■■,10]. This confounding makes it difficult to measure the full effect of ultrasound alone on the reduction of bleeding complications.
Ultrasound allows the operator to clearly identify the structures commonly implicated in hemorrhagic complications of thoracentesis, including the abdominal viscera and posterior intercostal arteries (ICAs), the latter of which is the most common cause of hemothorax. The ICA is part of the neurovascular bundle, which courses along the subcostal groove below the inferior border of the rib. Although the insertion of a thoracentesis catheter along the superior border of the rib is frequently performed to prevent arterial injury, the degree to which the ICA is exposed within the intercostal space varies significantly [41]. Helm et al. [42] described this heterogeneity by measuring the exposure of the ICA within the intercostal space with the use of computed tomography vascular reconstructions of the ICA. Only 17% of ICAs were completely shielded by the superior rib in the subcostal groove 3cm lateral to the spine, while 97% were shielded in the subcostal groove at a distance of 6cm lateral to the spine. ICA exposure also increased in more caudal intercostal spaces and were found to be more tortuous in the elderly.
The above data support performing thoracentesis within the ‘triangle of safety’, a recommendation supported by guidelines and recent reviews (Fig. 2) [10,31]. However, numerous patient factors including the location of pleural fluid, body habitus, and patient positioning may limit the feasibility of this approach. When performing a thoracentesis close to the spine or along a more caudal rib, clinicians should consider using a vascular ultrasound probe with color flow to identify the ICA and therefore, prevent inadvertent arterial laceration. Limited data suggest that providers can reliably identify the ICA with the use of vascular ultrasound supplemented with color flow, although the impact of this technique on procedural complications has yet to be evaluated [43].
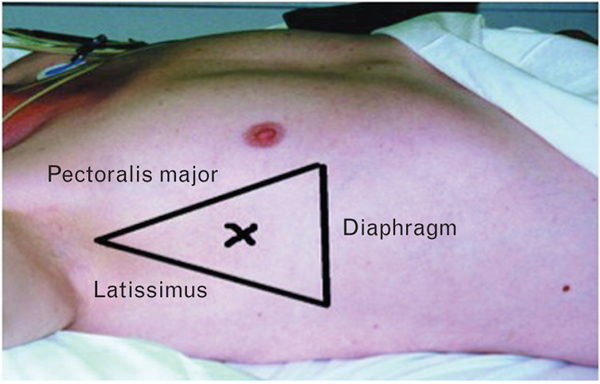
FIGURE 2.
Triangle of safety. The triangle of safety is bordered by the lateral edge of the pectoralis major, the lateral edge of the latissimus dorsi, the fifth intercostal space, and the base of the axilla. The British Thoracic Society recommends that pleural aspiration should occur within the triangle of safety. Reprinted with permission of the British Medical Journal [62].
We recommend attempting to localize the ICA with a vascular probe and color flow before thoracentesis procedures, especially for procedures performed in close proximity to the spine and in the elderly.
RE-EXPANSION PULMONARY EDEMA
Symptomatic REPE is an uncommon complication of thoracentesis and is characterized by the development of hypoxemia and new alveolar infiltrates within 24h of pleural fluid drainage (Fig. 3) [44]. Although radiographic evidence of re-expansion edema is most commonly seen in the re-expanded lung, involvement of the contralateral lung has also been reported [45,46].
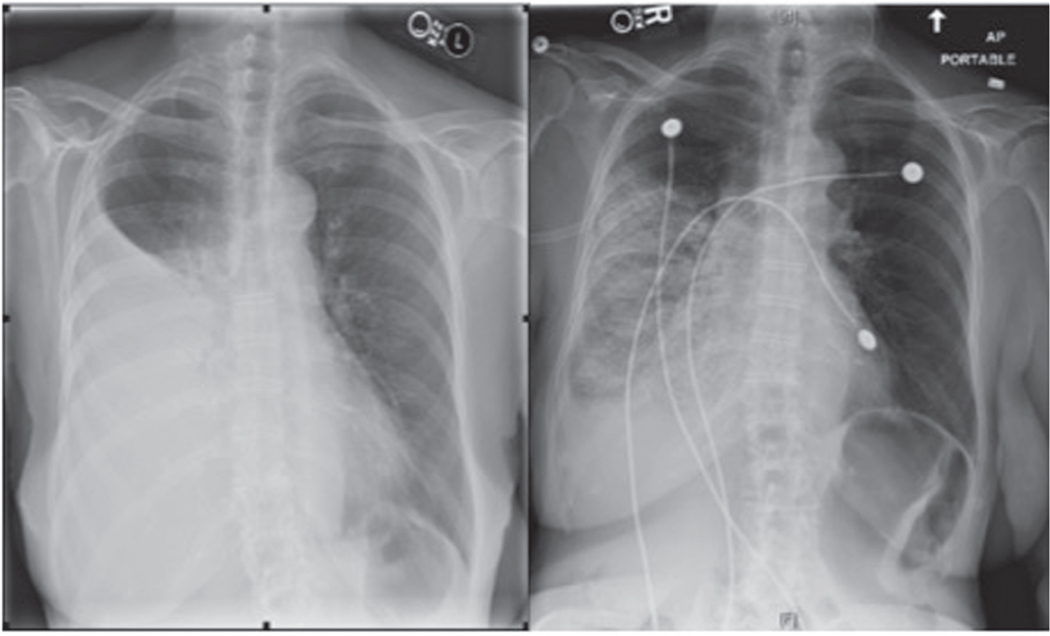
FIGURE 3.
Re-expansion pulmonary edema. The image on the left is an anterior-posterior (AP) chest radiograph showing a large right-sided pleural effusion. Immediately after large volume thoracentesis, the patient developed hypoxemic respiratory failure and post thoracentesis AP chest radiography (right) demonstrated a right-sided alveolar infiltrate consistent with the diagnosis of re-expansion pulmonary edema. Reprinted with permission of the American Thoracic Society. Copyright © 2016 American Thoracic Society [63].
The exact pathophysiology of REPE remains poorly understood. Nevertheless, evidence from animal models [47,48] and studies of edema fluid [49] in patients with REPE suggest that increased hydrostatic forces in the re-expanding lung and direct injury to the alveolar–capillary barrier may contribute to REPE pathogenesis.
Incidence
Determining the true incidence of symptomatic REPE is challenging as studies have frequently identified cases of REPE based solely on chest imaging without the requirement for concurrent clinical symptoms [27,50]. Asymptomatic REPE has little clinical significance because many patients with radiographic REPE have improved dyspnea following thoracentesis [51]. Numerous studies have also cast doubt on the utility of obtaining routine chest radiographs following thoracentesis in the absence of clinical symptoms [52–54].
Although REPE rates up to 16% have been reported in the literature, several large prospective case series have confirmed that symptomatic REPE is a rare complication of thoracentesis [5■■]. In a review of 185 thoracentesis patients, Feller-Kopman et al. [51] identified only one patient (0.5%) who developed symptomatic REPE, whereas the study by Ault et al. [11■■] found only 10 (0.01%)cases of REPE.
Pleural pressure monitoring
Identifying risk factors for the development of symptomatic REPE is challenging given the low incidence rates in publications with the largest series of patients. Because studies have shown that removal of large volumes of fluid are associated with the risk of REPE, guidelines and reviews frequently advocate for the cessation of fluid removal after drainage of 1–1.5 liters [31,51,55]. However, current evidence suggests REPE is related to intrapleural pressure rather than volume of fluid removed [51,56].
Several strategies are available to assess changes in pleural pressure during a thoracentesis. Pleural pressure can be directly measured during fluid removal with the aid of manometry [57]. Although data linking pleural elastance and drops in measured pleural pressure to procedural complications are limited, many experts advocate direct pleural pressure monitoring and recommend halting further fluid removal if pleural pressure drops below −20 cm H2O [51,56,58,59]. However, it is unknown if this cutoff is influenced by patient clinical factors (e.g., serum albumin). Data also suggest that operators should avoid rapid drops in pleural pressure, which has led to recommendation of avoidance of vacuum containers for pleural drainage [51,60]. Alternatively, clinicians may choose to use patient symptoms as a surrogate for pleural pressure, as data suggest that the development of chest discomfort correlates with marked drops in pleural pressure [56,61]. In their recent study, Ault et al. [11■■] used both symptoms, including chest tightness, cough, and pain referred to the upper chest or neck, and assessment of resistance to fluid aspiration using a 10cm3 syringe as a signal to halt fluid removal and found a 0.01% rate of REPE. This supports the efficacy of monitoring noninvasive surrogates for excessive negative pleural pressure.
CONCLUSION
Although thoracentesis is a well-tolerated procedure, clinicians need to be familiar with potential procedural complications and strategies that reduce the risk of patient harm. Pneumothorax is the most common complication of thoracentesis although the use of ultrasound and an emphasis on operator expertise have significantly lowered complication rates. Bleeding complications such as hemothorax are uncommon and there is increasing evidence that thoracentesis can often be performed without correction of bleeding diatheses. REPE is also a rare complication of thoracentesis. The avoidance of excessively negative pleural pressures either through direct measurement of pleural pressure or attention to patient symptoms may reduce the risk of REPE.
KEY POINTS.
Thoracentesis is a well-tolerated procedure, although complications can lead to increased morbidity, mortality, and healthcare cost.
Clinicians must distinguish between pneumothorax ex vacuo, which occurs when excessively negative pleural pressures are generated in the setting of a lung unable to fully re-expand, and mechanical puncture of the visceral pleura, a true procedural complication of thoracentesis.
Pneumothorax is the most common complication of thoracentesis. Operator expertise, the use of ultrasound, and recognition of patient-specific and procedural risk factors for pneumothorax can reduce this complication.
Although further randomized trials are needed, current evidence suggests that patients may not require correction of abnormal hemostasis before thoracentesis.
Strategies that limit excessively negative pleural pressure may reduce the risk of REPE.
Acknowledgements
The authors would like to thank education librarian, Linda O’Dwyer, at Northwestern University’s Galter Health Sciences Library for her help conducting our literature search. We also acknowledge Dr Douglas Vaughan, Chair Department of Medicine, for support of this work.
Financial support and sponsorship
None.
Footnotes
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
■ of special interest
■■ of outstanding interest
- 1.Owings MF, Kozak LJ. Ambulatory and inpatient procedures in the United States, 1996. Vital Health Stat 13 1998; 139:1–119. [PubMed] [Google Scholar]
- 2.Gordon CE, Feller-Kopman D, Balk EM, et al. Pneumothorax following thoracentesis: a systematic review and meta-analysis. Arch Intern Med 2010; 170:332–339. [DOI] [PubMed] [Google Scholar]
- 3.Mercaldi CJ, Lanes SF. Ultrasound guidance decreases complications and improves the cost of care among patients undergoing thoracentesis and paracentesis. Chest 2013; 143:532–538. [DOI] [PubMed] [Google Scholar]
- 4.Zhan C, Smith M, Stryer D. Accidental iatrogenic pneumothorax in hospitalized patients. Med Care 2006; 44:182–186. [DOI] [PubMed] [Google Scholar]
- 5.Wilcox ME, Chong CA, Stanbrook MB, et al. Does this patient have an exudative pleural effusion? The Rational Clinical Examination systematic review. JAMA 2014; 311:2422–2431.■■In this meta-analysis and systematic review, 37 studies were analysed to evaluate the specific aspects of thoracentesis procedures associated with complication risks.
- 6.Daniels CE, Ryu JH. Improving the safety of thoracentesis. Curr Opin Pulm Med 2011; 17:232–236. [DOI] [PubMed] [Google Scholar]
- 7.Gill JR, Ely SF, Toriello A, et al. Adverse medical complications: an under-reported contributory cause of death in New York City. Public Health 2014; 128:325–331. [DOI] [PubMed] [Google Scholar]
- 8.Arnold FW. Laparoscopy complications. Understating under-reporting. BMJ 2011; 342:d793. [DOI] [PubMed] [Google Scholar]
- 9.Tchantchaleishvili V, Umakanthan R, Karp S, et al. General surgical complications associated with the use of long-term mechanical circulatory support devices: are we ‘under-reporting’ problems? Expert Rev Med Devices 2013; 10:379–387. [DOI] [PubMed] [Google Scholar]
- 10.Puchalski J. Thoracentesis and the risks for bleeding: a new era. Curr Opin Pulm Med 2014; 20:377–384. [DOI] [PubMed] [Google Scholar]
- 11.Ault MJ, Rosen BT, Scher J, et al. Thoracentesis outcomes: a 12-year experience. Thorax 2015; 70:127–132.■■In a review of 9320 thoracentesis procedures, this study highlights the importance of operator experience, as well as several other patient and procedural factors that are associated with complications of thoracentesis.
- 12.Hibbert RM, Atwell TD, Lekah A, et al. Safety of ultrasound-guided thoracentesis in patients with abnormal preprocedural coagulation parameters. Chest 2013; 144:456–463. [DOI] [PubMed] [Google Scholar]
- 13.Puchalski JT, Argento AC, Murphy TE, et al. The safety of thoracentesis in patients with uncorrected bleeding risk. Ann Am Thorac Soc 2013; 10:336–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mahmood K, Shofer SL, Moser BK, et al. Hemorrhagic complications of thoracentesis and small-bore chest tube placement in patients taking clopidogrel. Ann Am Thorac Soc 2014; 11:73–79.■This small prospective cohort study suggested that thoracentesis may be safely performed in patients taking clopidogrel.
- 15.Aguilar-Torres FG, Schlueter DP, Perlman L, et al. Subcutaneous implantation of an adenocarcinoma following thoracentesis. Wis Med J 1977; 76:S19–S21. [PubMed] [Google Scholar]
- 16.Sue DY, Lam K. Retention of catheter fragment after thoracentesis: report of two cases. Postgrad Med 1982; 72:101–102; 105–6. [DOI] [PubMed] [Google Scholar]
- 17.Seneff MG, Corwin RW, Gold LH, et al. Complications associated with thoracocentesis. Chest 1986; 90:97–100. [DOI] [PubMed] [Google Scholar]
- 18.Collins TR, Sahn SA. Thoracocentesis. Clinical value, complications, technical problems, and patient experience. Chest 1987; 91:817–822. [DOI] [PubMed] [Google Scholar]
- 19.Grogan DR, Irwin RS, Channick R, et al. Complications associated with thoracentesis. A prospective, randomized study comparing three different methods. Arch Intern Med 1990; 150:873–877. [DOI] [PubMed] [Google Scholar]
- 20.Despars JA, Sassoon CS, Light RW. Significance of iatrogenic pneumothoraces. Chest 1994; 105:1147–1150. [DOI] [PubMed] [Google Scholar]
- 21.Heidecker J, Huggins JT, Sahn SA, et al. Pathophysiology of pneumothorax following ultrasound-guided thoracentesis. Chest 2006; 130:1173–1184. [DOI] [PubMed] [Google Scholar]
- 22.Ponrartana S, Laberge JM, Kerlan RK, et al. Management of patients with “ex vacuo” pneumothorax after thoracentesis. Acad Radiol 2005; 12:980–986. [DOI] [PubMed] [Google Scholar]
- 23.Cavanna L, Mordenti P, Berte R, et al. Ultrasound guidance reduces pneumothorax rate and improves safety of thoracentesis in malignant pleural effusion: report on 445 consecutive patients with advanced cancer. World J Surg Oncol 2014; 12:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perazzo A, Gatto P, Barlascini C, et al. Can ultrasound guidance reduce the risk of pneumothorax following thoracentesis? J Bras Pneumol 2014; 40:6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zanforlin A, Gavelli G, Oboldi D, et al. Ultrasound-guided thoracenthesis: the V-point as a site for optimal drainage positioning. Eur Rev Med Pharmacol Sci 2013; 17:25–28. [PubMed] [Google Scholar]
- 26.Yang PC, Luh KT, Chang DB, et al. Value of sonography in determining the nature of pleural effusion: analysis of 320 cases. AJR Am J Roentgenol 1992; 159:29–33. [DOI] [PubMed] [Google Scholar]
- 27.Jones PW, Moyers JP, Rogers JT, et al. Ultrasound-guided thoracentesis: is it a safer method? Chest 2003; 123:418–423. [DOI] [PubMed] [Google Scholar]
- 28.Diacon AH, Brutsche MH, Solèr M. Accuracy of pleural puncture sites: a prospective comparison of clinical examination with ultrasound. Chest 2003; 123:436–441. [DOI] [PubMed] [Google Scholar]
- 29.Barnes TW, Morgenthaler TI, Olson EJ, et al. Sonographically guided thoracentesis and rate of pneumothorax. J Clin Ultrasound 2005; 33:442–446. [DOI] [PubMed] [Google Scholar]
- 30.Raptopoulos V, Davis LM, Lee G, et al. Factors affecting the development of pneumothorax associated with thoracentesis. AJR Am J Roentgenol 1991; 156:917–920. [DOI] [PubMed] [Google Scholar]
- 31.Havelock T, Teoh R, Laws D, et al. Pleural procedures and thoracic ultrasound: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010; 65 (Suppl 2):ii61–ii76. [DOI] [PubMed] [Google Scholar]
- 32.Frankel HL, Kirkpatrick AW, Elbarbary M, et al. Guidelines for the appropriate use of bedside general and cardiac ultrasonography in the evaluation of critically ill patients-Part I: General ultrasonography. Crit Care Med 2015; 43:2479–2502. [DOI] [PubMed] [Google Scholar]
- 33.Mayo PH, Goltz HR, Tafreshi M, et al. Safety of ultrasound-guided thoracentesis in patients receiving mechanical ventilation. Chest 2004; 125:1059–1062. [DOI] [PubMed] [Google Scholar]
- 34.Josephson T, Nordenskjold CA, Larsson J, et al. Amount drained at ultrasound-guided thoracentesis and risk of pneumothorax. Acta Radiol 2009; 50:42–47. [DOI] [PubMed] [Google Scholar]
- 35.Feller-Kopman D, Maldonado F, Mullon JJ. Point/counterpoint: should pleural manometry be performed routinely during thoracentesis? Yes/no. Chest 2012; 141:844–850. [DOI] [PubMed] [Google Scholar]
- 36.Duncan DR, Morgenthaler TI, Ryu JH, et al. Reducing iatrogenic risk in thoracentesis: establishing best practice via experiential training in a zero-risk environment. Chest 2009; 135:1315–1320. [DOI] [PubMed] [Google Scholar]
- 37.Wayne DB, Barsuk JH, O’Leary KJ, et al. Mastery learning of thoracentesis skills by internal medicine residents using simulation technology and deliberate practice. J Hosp Med 2008; 3:48–54. [DOI] [PubMed] [Google Scholar]
- 38.Bass J, White DA. Thoracentesis in patients with hematologic malignancy: yield and safety. Chest 2005; 127:2101–2105. [DOI] [PubMed] [Google Scholar]
- 39.Adam AK, Zamlut M, Soubani AO. The yield and safety of thoracentesis in hematopoietic stem cell transplantation recipients. Lung 2007; 185:257–262. [DOI] [PubMed] [Google Scholar]
- 40.Patel IJ, Davidson JC, Nikolic B, et al. Consensus guidelines for periprocedural management of coagulation status and hemostasis risk in percutaneous image-guided interventions. J Vasc Interv Radiol 2012; 23:727–736. [DOI] [PubMed] [Google Scholar]
- 41.Wraight WM, Tweedie DJ, Parkin IG. Neurovascular anatomy and variation in the fourth, fifth, and sixth intercostal spaces in the mid-axillary line: a cadaveric study in respect of chest drain insertion. Clin Anat 2005; 18:346–349. [DOI] [PubMed] [Google Scholar]
- 42.Helm EJ, Rahman NM, Talakoub O, et al. Course and variation of the intercostal artery by CT scan. Chest 2013; 143:634–639. [DOI] [PubMed] [Google Scholar]
- 43.Salamonsen M, Dobeli K, McGrath D, et al. Physician-performed ultrasound can accurately screen for a vulnerable intercostal artery prior to chest drainage procedures. Respirology (Carlton, Vic) 2013; 18:942–947. [DOI] [PubMed] [Google Scholar]
- 44.Light R. Pleural disease. 5th ed. Philadelphia: Lippincott Williams & Wilkins; 2007. [Google Scholar]
- 45.Ragozzino MW, Greene R. Bilateral reexpansion pulmonary edema following unilateral pleurocentesis. Chest 1991; 99:506–508. [DOI] [PubMed] [Google Scholar]
- 46.Tarver RD, Broderick LS, Conces DJ Jr. Reexpansion pulmonary edema. J Thorac Imaging 1996; 11:198–209. [PubMed] [Google Scholar]
- 47.Suzuki S, Tanita T, Koike K, et al. Evidence of acute inflammatory response in reexpansion pulmonary edema. Chest 1992; 101:275–276. [DOI] [PubMed] [Google Scholar]
- 48.Jackson RM, Veal CF, Alexander CB, et al. Re-expansion pulmonary edema. A potential role for free radicals in its pathogenesis. Am Rev Respir Dis 1988; 137:1165–1171. [DOI] [PubMed] [Google Scholar]
- 49.Sue RD, Matthay MA, Ware LB. Hydrostatic mechanisms may contribute to the pathogenesis of human re-expansion pulmonary edema. Intensive Care Med 2004; 30:1921–1926. [DOI] [PubMed] [Google Scholar]
- 50.Mahfood S, Hix WR, Aaron BL, et al. Reexpansion pulmonary edema. Ann Thorac Surg 1988; 45:340–345. [DOI] [PubMed] [Google Scholar]
- 51.Feller-Kopman D, Berkowitz D, Boiselle P, et al. Large-volume thoracentesis and the risk of reexpansion pulmonary edema. Ann Thorac Surg 2007; 84:1656–1661. [DOI] [PubMed] [Google Scholar]
- 52.Doyle JJ, Hnatiuk OW, Torrington KG, et al. Necessity of routine chest roentgenography after thoracentesis. Ann Intern Med 1996; 124:816–820. [DOI] [PubMed] [Google Scholar]
- 53.Capizzi SA, Prakash UB. Chest roentgenography after outpatient thoracentesis. Mayo Clin Proc 1998; 73:948–950. [DOI] [PubMed] [Google Scholar]
- 54.Aleman C, Alegre J, Armadans L, et al. The value of chest roentgenography in the diagnosis of pneumothorax after thoracentesis. Am J Med 1999; 107:340–343. [DOI] [PubMed] [Google Scholar]
- 55.Thomsen TW, DeLaPena J, Setnik GS. Videos in clinical medicine. Thoracentesis. N Engl J Med 2006; 355:e16. [DOI] [PubMed] [Google Scholar]
- 56.Light RW, Jenkinson SG, Minh VD, et al. Observations on pleural fluid pressures as fluid is withdrawn during thoracentesis. Am Rev Respir Dis 1980; 121:799–804. [DOI] [PubMed] [Google Scholar]
- 57.Doelken P, Huggins JT, Pastis NJ, et al. Pleural manometry: technique and clinical implications. Chest 2004; 126:1764–1769. [DOI] [PubMed] [Google Scholar]
- 58.Villena V, Lopez-Encuentra A, Pozo F, et al. Measurement of pleural pressure during therapeutic thoracentesis. Am J Respir Crit Care Med 2000; 162:1534–1538. [DOI] [PubMed] [Google Scholar]
- 59.Feller-Kopman D. Therapeutic thoracentesis: the role of ultrasound and pleural manometry. Curr Opin Pulm Med 2007; 13:312–318. [DOI] [PubMed] [Google Scholar]
- 60.Pavlin J, Cheney FW Jr. Unilateral pulmonary edema in rabbits after reexpansion of collapsed lung. J Appl Physiol Respir Environ Exerc Physiol 1979; 46:31–35. [DOI] [PubMed] [Google Scholar]
- 61.Feller-Kopman D, Walkey A, Berkowitz D, et al. The relationship of pleural pressure to symptom development during therapeutic thoracentesis. Chest 2006; 129:1556–1560. [DOI] [PubMed] [Google Scholar]
- 62.Peek GJ, Morcos S, Cooper G. The pleural cavity. BMJ 2000; 320:1318–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Walter JM, Matthay MA, Gillespie CT, et al. Acute hypoxemic respiratory failure after large-volume thoracentesis. Ann Am Thorac Soc 2016; 13:438–443. [DOI] [PMC free article] [PubMed] [Google Scholar]