Abstract
Adenocarcinoma of the lacrimal gland is an epithelial malignancy with an incidence according to the literature of 5–7%. It is clinically revealed by an upper palpebral mass often associated with an inflammatory exophthalmos. It is a high-grade malignancy and there are no pathognomonic clinical or radiological signs. The Core 14G needle biopsy technique is now the simplest procedure to remove tissue from the mass to be analyzed. This allows a precise histological and immunohistochemical study, to establish a diagnosis of certainty and to institute a rapid and adequate therapeutic management in order to improve the prognosis.
We report the case of a de novo lacrimal gland adenocarcinoma diagnosed in a 55-year-old patient. The patient presented with a subpalpebral mass, located in the superior-external angle of the globe, which was very inflammatory. The radiological work-up revealed a left intra-orbital tumor process, extra-conical, developed at the expense of the lacrimal gland, in contact with the external wall of the orbit with local cortical effractions. The anatomopathological examination of the specimen obtained by Core 14G needle biopsy, under local anesthesia and on an outpatient basis, was in favor of a moderately differentiated and infiltrating adenocarcinoma of the lacrimal gland. The extension workup did not reveal a metastatic focus. A total exenteration was performed with complementary postoperative radiotherapy. The patient was followed up after 2 years without recurrence.
Keywords: Exophthalmos, Lacrimal gland, Adenocarcinoma, Histology
Highlights
-
•
Adenocarcinoma has the particularity of occurring de novo or developing within a pleomorphic adenoma of the lacrimal gland.
-
•
It is a tumor with a high grade of malignancy.
-
•
The anatomo-pathological examination makes the diagnosis of certainty.
-
•
The core 14G needle biopsy is performed under local anesthesia.
-
•
It allows a more laborious histopathological study with immunohistochemical complement.
-
•
The aim of the treatment is an early locoregional control.
-
•
A complete excision with adjuvant radiotherapy is recommended.
1. Introduction
Adenocarcinoma is a tumor that develops in the epithelial cells of the lacrimal gland. It represents a separate entity among malignant tumors, with an incidence of 5–7% according to the literature [1,2]. Adenocarcinoma was first described in 1996 by Katz et al. [1]. Adenocarcinoma has the particularity of occurring de novo or developing in pleomorphic adenoma of the lacrimal gland [3]. It is a tumor with a high grade of malignancy, so it is essential to diagnose adenocarcinomas of the lacrimal gland at a very early stage and to adapt the treatment because they are very aggressive and have a poorer prognosis. However, the clinical-radiological and pathological characteristics of adenocarcinoma of the lacrimal gland are still unclear. It very often presents as a swelling of the superior-external angle of the eye, sometimes quite palpable and painful, with inflammatory exophthalmos of rapidly progressive evolution. A Core 14G needle biopsy is then very useful and can be performed in ambulatory, early. It will allow to establish the diagnosis of certainty on histological and immunohistochemical analysis of the tissue sample. After an assessment of the extent of the disease, mainly looking for involvement of the liver, brain, bone and lung, the treatment is radical by total exenteration with additional radiotherapy to improve the prognosis.
We would like to highlight the major interest of performing Core 14 G needle biopsy in order to have a precise and rapid diagnosis, which allows through a good management to improve the prognosis and the survival rate of patients.
2. Case presentation
Our work consists of a single case report and has been reported in accordance with SCARE 2020 criteria [4].
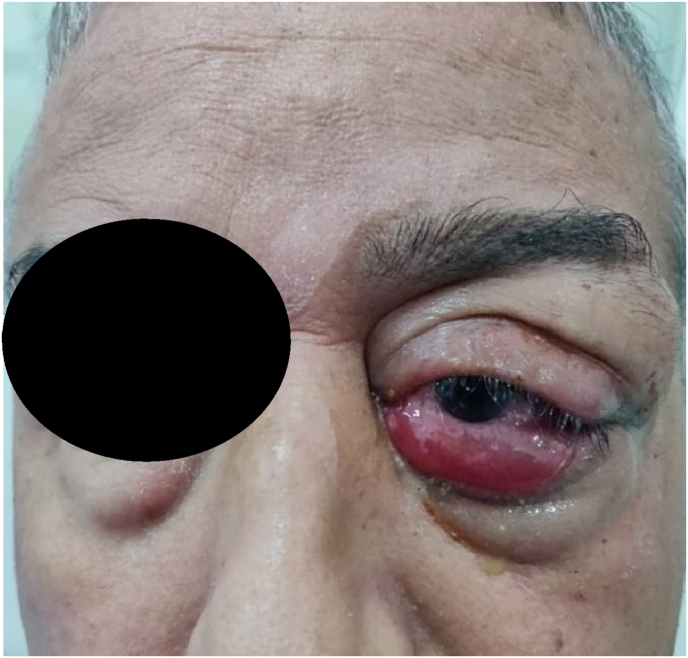
The patient is 55 years old, with no particular medical or surgical history, who is consulting for a palpebral tumefaction of the left eye, insidious but rapidly progressive, painful with a decrease in visual acuity, which has been evolving over the last 2 months. Objective clinical examination an indurated palpebral superolateral mass of the left eye, non-mobile in relation to the adjacent planes, of solid appearance, responsible for dystopia of the left eye with axial inflammatory exophthalmos, irreducible, without vascular thrill. Oculomotricity in adduction and elevation of the globe is limited and there is a lower chemosis [Fig. 1]. Corrected visual acuity was 2/10. At the slit lamp, the ocular tone was 24 mmHg, there was superficial punctate keratitis in the lower part, an opalescent lens and a normal fundus. The examination of the adelphe eye is without particularity, normal, with a corrected visual acuity at 10/10.
Fig. 1.
Swelling over the lacrimal gland with inflammatory exophthalmos.
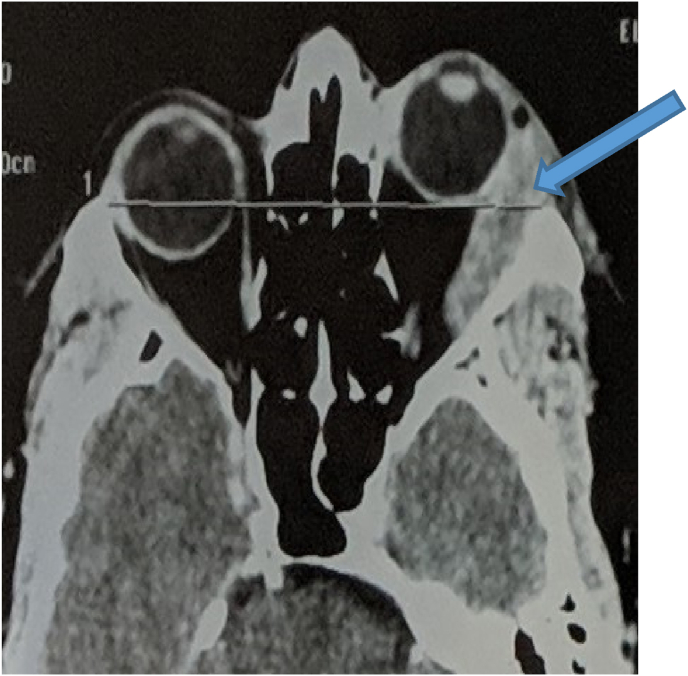
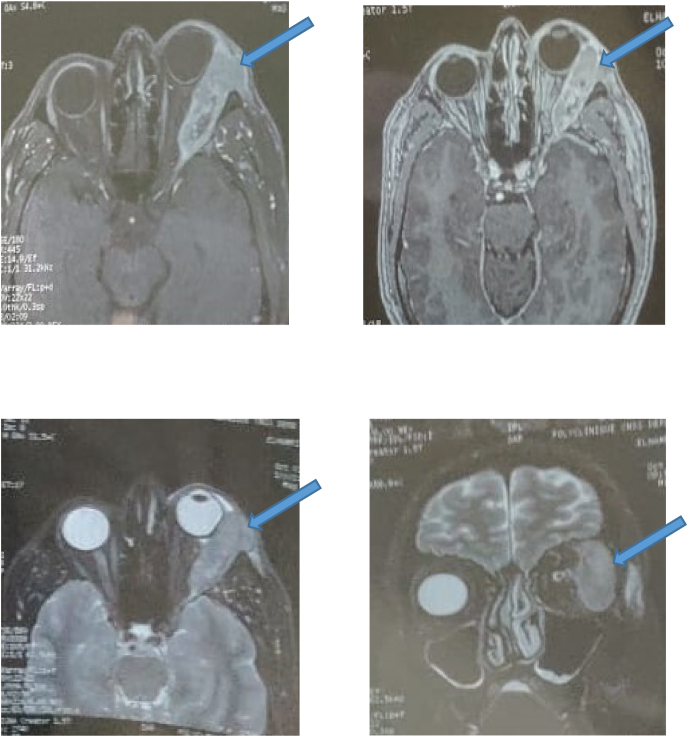
The orbital scanner shows the presence of a tumor process with a dense left extra-conical formation type infiltrating the lacrimal gland and the right lateral muscle with grade II exophthalmos [Fig. 2]. MRI finds a serpentine heterogeneous formation centered on the superolateral angle of the orbit, extra-conical in hyposignal T1, and intermediate signal T2, enhanced after injection of PDC, which measures 53 × 21 mm. This mass infiltrates the upper eyelid and comes into contact with the globe from the inside while respecting its sphericity and the insertion of the upper and external oculomotor muscles. It extends posteriorly to the middle zone of the orbit but remains extra-conical, and externally to the outer wall of the orbit with cortical effraction in places [Fig. 3]. The optic nerve and intra-conical fat are respected.
Fig. 2.
CT axial sections showing grade II exophthalmos with dense left extra-conical tumor process infiltrating the lacrimal gland and lateral right muscle.
Fig. 3.
MRI axial sections in T1, uninjected (A), injected (B); T2 in axial section C and coronal section showing a serpentine heterogeneous formation centered on the super-external angle of the orbit, extra-conical in hyposignal T1, and intermediate signal T2, enhanced after injection of PDC.
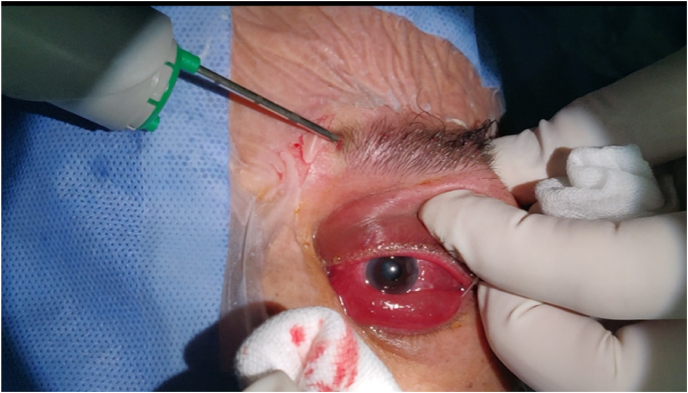
A biopsy of the tumor via the upper palpebral approach was performed with a Core 14 G needle, performed as an outpatient under local anesthesia [Fig. 4]. Five cores of tissue from the lacrimal gland mass were obtained. The anatomopathological examination with immunohistochemical study revealed a moderately differentiated and infiltrating adenocarcinoma of the left lacrimal gland.
Fig. 4.
Needle biopsy Core 14 G.
The extension work-up, consisting of a brain and thoraco-abdomino-pelvic scanner, a cervical ultrasound and a bone scan, was normal.
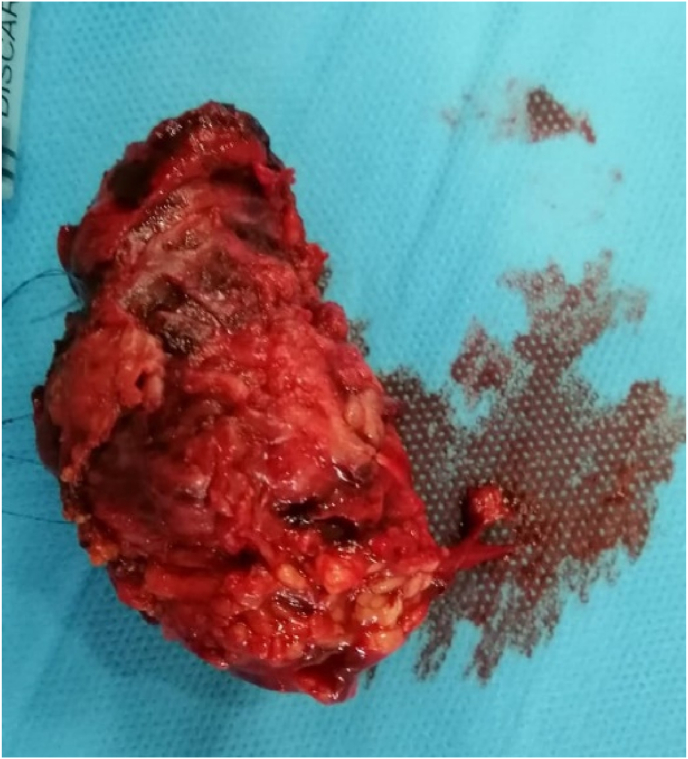
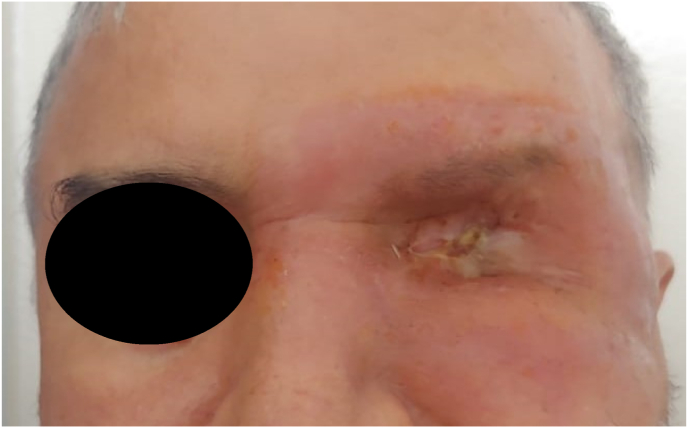
He underwent total orbital exenteration with analysis of the surgical specimen [Fig. 5]. A dressing of absorbable hemostatic gauze of natural origin was put in place to ensure good hemostasis and thus allow rapid healing. In the immediate postoperative period, treatment with antibiotics and local and general corticosteroids was initiated and continued for 15 days. The patient was followed up in consultation with local care every week for the first month, then every month for the first 6 months, then every 3 months for a year until complete healing [Fig. 6], then every year, with a 2-year follow-up, without recurrence. The histological study confirmed de novo, moderately differentiated adenocarcinoma, the periocular adipose tissue was invaded, the eyeball was intact, the upper and lower palpebral margins were adequate, and the periosteum of her right orbit was invaded in the superior temporal. Concomitantly with the oncology-radiotherapy department, the patient underwent additional postoperative adjuvant radiotherapy. In view of the facial aesthetic prejudice, an epithesis is planned.
Fig. 5.
Exentration operating specimen.
Fig. 6.
Appearance after one year of follow-up: good healing of the exenteration.
3. Clinical discussion
Tumors of the lacrimal gland make up less than 5% of all orbital lesions biopsied [5]. Epithelial malignancies of the lacrimal gland constitute 4% of all lacrimal gland tumors. Adenocarcinoma accounts for about 7% of all epithelial tumors of the lacrimal gland [6]. This tumor has a more aggressive behavior and shorter patient survival time than adenoid cystic carcinoma, although the most common epithelial malignancy is primary adenoid cystic carcinoma [7]. It affects older patients (18–80 years of age, mean 58 years of age) and is more common in the male population [7]. The tumor usually begins in the upper eyelid as a growth and is accompanied by clinical symptoms such as pseudoptosis of the eyelids, exophthalmos, dystopia, pain and decreased visual acuity [2,5].
CT and MRI scans are used to differentiate between different types of masses and determine the extent of lesions involving the tear gland and bone fossa. It is often very difficult to diagnose a specific disease based on imaging features alone [7]. The typical appearance of this tumor in orbital CT showed an irregularly shaped lesion with a focal destructive change in the lacrimal gland. Adjacent extraocular muscle or bone invasion was not uncommon. Some cases also showed calcifications in CT imaging [8].
The extension workup includes a brain CT scan, thoraco-abdominopelvic CT scan, a bone scan and a cervical ultrasound scan before any therapeutic procedure, since the most common metastatic sites are the lungs, bones, liver and brain [2].
Anatomical pathological examination establishes the diagnosis of certainty. An external orbitotomy with an upper bone flap allows access to the tumor and to perform a biopsy with extemporaneous anatomical-pathological examination which establishes the histological diagnosis. This procedure is often cumbersome and too invasive.
But another simpler procedure is the Core 14 G needle biopsy. This has the advantage of being much more practical, can be performed on an outpatient basis and is done under local anesthesia. It allows, without being too invasive, to obtain a tissue in the form of a core which would be used for a more laborious histo-pathological study with immunohistochemical complement. The latter allows staging of the tumor, but in the absence of a specific histo-pathological classification of lacrimal gland tumors, they are often classified according to the histological classification of tumors of the salivary glands with which they share many similarities [5,9,10].
Some studies go further in the search for specificity and suggest GCDFP-15, AR and Her-2 as biomarkers for primary ductal adenocarcinoma of the lacrimal gland [11]. But there is no true confirmation, awaiting further extensive and in-depth studies.
Primary ductal adenocarcinoma of the lacrimal gland is very aggressive, with a high grade of malignancy. The goal of treatment is early locoregional control. Complete excision with adjuvant radiotherapy is recommended [][5], [12][] [5,]] [[5], [12]]: Regional lymph node involvement should be assessed methodically; regional node dissection and/or radiotherapy should be undertaken even in the absence of palpable lymphadenopathy [1] Patients under this protocol have shown a better survival rate and fewer recurrences [7].
Mortality is associated with early lymphatic spread from the tumor cells to the lymph nodes and lungs [6]. The mortality rate of these tumors is about 70% and usually occurs 2–3 years after the initial appearance of the tumor [2].
4. Conclusion
Malignant tumors of the lacrimal gland pose a therapeutic management problem. CT and MRI are very useful to evaluate lacrimal gland lesions but histology makes the diagnosis of certainty. Core 14G needle biopsy seems to be the simplest, most convenient and even reproducible procedure to analyze a fragment of the tumor and make an early diagnosis. Primary ductal adenocarcinoma is generally considered an aggressive malignancy; however, the best treatment and overall prognosis, although unknown, are dependent on stage and early diagnosis. Further studies are needed to standardize the best treatment option.
Consent to publication
Written informed consent was obtained from the patients for publication of this cases report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Declaration of competing interest
No interest statement.
References
- 1.Katz S.E., Rootman J., Dolman P.J., White V.A., Berean K.W. Adénocarcinome canalaire primaire de la glande lacrymale. Ophtalmologie. 1996;103(1):157–162. doi: 10.1016/S0161-6420(96)30746-X. [DOI] [PubMed] [Google Scholar]
- 2.Civit T., Kleina O., Baylacb F. Tumeursépithéliales de la glandelacrymale. Neurochirurgie. 2010;56:152–157. doi: 10.1016/j.neuchi.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 3.O. Galatoire, M. Hamédani, M. Putterman, O. Berges, S. Morax: Adenocarcinome-Au-Sein-D-Un-Adenome-Pleomorphe-De-La Glande Lacrymale, doi:JFO-10-2005-28-8-0181-5512-101019-200507638]. [DOI] [PubMed]
- 4.Agha R.A., Franchi T., Sohrabi C., Mathew G., for the SCARE Group The SCARE 2020 guideline: updating consensus surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2020;84:226–230. doi: 10.1016/j.ijsu.2020.10.034. [DOI] [PubMed] [Google Scholar]
- 5.Milman T., Shields J.A., Husson M. Adénocarcinome canalaire primaire de la glande lacrymale. Ophtalmologie. 2005;112:2048–2051. doi: 10.1016/j.ophtha.2005.04.029. [DOI] [Google Scholar]
- 6.Police R.L., Gamel J.W. Aesculapius Publishing; Birmingham, Ala.: 1978. Tumeurs épithéliales de la glande lacrymale: une analyse de 265 cas. Dans: jacobiec FA, éd. Tumeurs oculaires et annexielles; pp. 787–805. [Google Scholar]
- 7.Jose L., Tovilla-Canales M.D., Sharon Ball M.D., Osiris Olvera M.D., et Fernando B., Martin M.D. 2013. Mexico: Diagnostic et traitement des néoplasies des glandes lacrymales, review of ophtalmology. avril. [Google Scholar]
- 8.Yang H.Y., Wu C.H., Tsai C.C., Yu W.K., Kao S.C., Liu C.J. Adénocarcinome canalaire primaire de la glande lacrymale: deux rapports de cas et revue de la littérature. Taiwan J Ophthalmol. 2018;8(1):42–48. doi: 10.4103/tjo.tjo_3_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clauser L., Galie M., Tieghi R., Cavazzini L. Adénocarcinome de la glande lacrymale: rapport d'un cas. Oral Maxillofac. Surg. 2002;60:318–321. doi: 10.1053/joms.2002.30595. [DOI] [PubMed] [Google Scholar]
- 10.Khalil M., Arthurs B. Adénocarcinome basocellulaire de la glande lacrymale. Ophtalmologie. 2000;107(1):164–168. doi: 10.1016/S0161-6420(99)00007-X. [DOI] [PubMed] [Google Scholar]
- 11.Zhu M.M., Cui H.G., Teng X.D. GCDFP-15, AR et Her-2 comme biomarqueurs pour l'adénocarcinome canalaire primaire de la glande lacrymale: un cas chinois et une revue de la littérature. Onco cible Ther. 2015;8:1017–1024. doi: 10.2147/OTT.S82168. Publié le 11 mai 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kurisua Y., Shibayamaa Y., Tsujib M. Un cas d'adénocarcinome canalaire primaire de la glande lacrymale: étude histopathologique et immunohistochimique. Pathol. Res. Pract. 2005;201:49–53. doi: 10.1016/j.prp.2004.11.003. [DOI] [PubMed] [Google Scholar]