Abstract
Background
Clinical studies show that children exposed to anaesthetics for short times at young age perform normally on intelligence tests, but display altered social behaviours. In non-human primates (NHPs), infant anaesthesia exposure for several hours causes neurobehavioural impairments, including delayed motor reflex development and increased anxiety-related behaviours assessed by provoked response testing. However, the effects of anaesthesia on spontaneous social behaviours in juvenile NHPs have not been investigated. We hypothesised that multiple, but not single, 5 h isoflurane exposures in infant NHPs are associated with impairments in specific cognitive domains and altered social behaviours at juvenile age.
Methods
Eight Rhesus macaques per group were anaesthetised for 5 h using isoflurane one (1×) or three (3×) times between postnatal days 6 and 12 or were exposed to room air (control). Cognitive testing, behavioural assessments in the home environment, and provoked response testing were performed during the first 2 yr of life.
Results
The cognitive functions tested did not differ amongst groups. However, compared to controls, NHPs in the 3× group showed less close social behaviour (P=0.016), and NHPs in the 1× group displayed increased anxiety-related behaviours (P=0.038) and were more inhibited towards novel objects (P<0.001).
Conclusions
5 h exposures of NHPs to isoflurane during infancy are associated with decreased close social behaviour after multiple exposures and more anxiety-related behaviours and increased behavioural inhibition after single exposure, but they do not affect the cognitive domains tested. Our findings are consistent with behavioural alterations in social settings reported in clinical studies, which may guide future research.
Keywords: anaesthesia, behaviour, brain development, cognitive testing, neurotoxicity, non-human primate, social behaviour
Editor's key points.
-
•
Retrospective clinical studies reveal ambiguous findings on whether anaesthesia exposure of young children is associated with impaired neurobehavioural development.
-
•
The effects of single or multiple exposures of infant non-human primates (NHPs) on cognitive and behavioural performance were studied in juvenile animals at 2 yr of age corresponding to human children aged 6–8 yr.
-
•
The cognitive functions tested did not differ compared with controls, but NHPs in the triple-exposure group showed less close social behaviour and in the single-exposure group displayed increased anxiety-related behaviours and were more inhibited towards novel objects.
-
•
These findings are consistent with behavioural alterations in social settings reported in clinical studies.
Retrospective clinical studies reveal ambiguous findings on whether anaesthesia exposure of young children is associated with impaired neurobehavioural development.1,2 Some of these studies indicate that a single anaesthesia exposure may not affect cognition later in life, but multiple anaesthesia exposures affect future cognitive performance.3 More recently, three clinical studies (Pediatric Anesthesia Neurodevelopment Assessment [PANDA], Mayo Anesthesia Safety in Kids [MASK], and General Anesthesia compared to Spinal anesthesia [GAS]) that investigated anaesthesia neurotoxicity in children found no difference for their primary outcome of general intelligence (intelligence quotient [IQ]) after 1–2 h of anaesthesia exposure during infancy.4, 5, 6 However, secondary outcomes based on reports from parents and caregivers using behavioural questionnaires and checklists revealed alterations in executive functions or behaviours in school-aged children. For example, the MASK study found alterations in executive functions that were substantial in the multiple- and subtle in the single-exposure group.
Preclinical studies in non-human primates (NHPs) provide robust evidence that a single exposure to general anaesthesia early in life results in acute widespread apoptosis of neurones and oligodendrocytes in the developing brain. Affected brain areas include those involved in social behaviour, such as the amygdala and prefrontal and cingulate cortices.7, 8, 9, 10 Long-term observations in NHPs revealed that anaesthesia exposure during infancy resulted in impaired neurobehavioural development later in life. NHPs exposed to volatile anaesthetics three times showed increased anxiety-related behaviours at 6 months,11 which were still evident at 1 and 2 yr of age.12 Another study found that NHPs exposed to sevoflurane for 4 h on three subsequent occasions had impaired visual recognition memory, first detected at 1 yr of life.13 On the other hand, a battery of cognitive tests in 3.5-yr-old NHPs showed no effects in most cognitive domains for animals exposed one time to a mixture of isoflurane and nitrous oxide for 8 h,14 but found several cognitive deficiencies in animals exposed to ketamine for a total of 24 h.15
To investigate functional alterations associated with neurotoxic effects of infant anaesthesia, we exposed 24 NHPs (three groups of eight) to isoflurane for 5 h, either one (1×) or three (3×) times during infancy, and evaluated alterations in several behaviours and cognitive domains. Animals in the control group (Ctr) were handled and evaluated similarly, but were exposed only to room air. In a previous report, we evaluated NHPs at 3 and 12 months of age, and we found that animals in the 3× group displayed delayed motor reflexes at 3 months of age and more anxiety-related behaviours together with an increased frequency of appeasement-related behaviours at 1 yr of age.16 In the present study, we re-evaluated the same animals during the second year of life and after being housed with their peers in social groups.
In previous studies of anaesthesia-exposed NHPs, most behavioural findings were derived from provoked response testing, but none assessed natural or spontaneous social behaviours in the home environment of the animals. Importantly, results from recent clinical studies evaluating parental ratings of children's natural behaviours suggest that both single (median exposure time of 45–80 min) and multiple (median total exposure time of 187 min) exposures to anaesthesia during infancy were associated with behavioural alterations at home and at school.4, 5, 6 In the present study, we investigated potential behavioural alterations in 2-yr-old juvenile NHPs after single or multiple 5 h isoflurane exposure during the first two weeks of life by observing individual animals in their home environment. In addition, we assessed behaviours using provoked response testing and investigated potential effects of single or multiple exposures on specific cognitive domains. We hypothesised that multiple, but not single, 5 h isoflurane exposures during infancy are associated with cognitive impairments and altered social behaviours in juvenile NHPs.
Methods
Ethical approval
This study was approved by the Oregon National Primate Research Center (ONPRC) Institutional Animal Care and Use Committee and was in compliance with all federal regulations and the guidelines set forth in the Guide for the Care and Use of Laboratory Animals.17 The ONPRC is accredited by AAALAC International.
Animal housing
Animal housing conditions have been described for the same cohort of animals during the first year of life.16 Briefly, rhesus macaques (Macaca mulatta; 12 male and 12 female animals) weighing 0.4–0.5 kg at birth were raised and housed at the ONPRC (Beaverton, OR, USA). In their first year of life, infant NHPs were housed in outdoor enclosures (∼130 m2) containing 30–50 individuals. At about 1 yr of age, the animals were weaned from their dams and moved into one of four new social groups containing five to six study subjects. The animals were put into one of these social groups as they approached 1 yr of age, assuring that each group contained a mixture of subjects from each exposure group, sex, and hierarchical dominance rank. The composition of the social groups for sex, hierarchical dominance, and exposure group is shown in Supplementary Table 1. These social groups were housed in indoor pens or ‘home cages’ measuring ∼16 m3. These home cages were located in different animal rooms that housed up to 32 monkeys in total, including additional non-experimental monkeys. For physical examinations during pregnancy, most of the dams received low doses of sedative (ketamine hydrochloride [HCl], 5–10 mg kg−1, one to two times) as part of the normal husbandry practice at the ONPRC, but none of them was exposed to isoflurane or other anaesthetics during that period. The animals were fed commercial monkey chow (LabDiet Monkey Diet, St Louis, MO, USA) twice a day, supplemented with grain or produce daily, and had access to water ad libitum. The animals were exposed to a 12:12 h light:dark cycle, with lights on at 7:00 a.m. After completing all cognitive and behavioural assessments at the age of 2 yr, the animals were euthanised using sodium pentobarbital (120 mg kg−1, i.v.) and perfused to collect their brains for future histological studies.
General anaesthesia
Anaesthesia exposure has been described.16 Briefly, the day before exposure, neonates together with their dams were transferred from their breeding group into an appropriately sized single cage; they were removed from the breeding group within 15 min with minimal distress. On the next day, the dam was lightly sedated with ketamine 5 mg kg−1 i.m. before transfer of the neonate to the operating theatre. After baseline measurements (HR, respiration, temperature, BP, weight, blood gases, and metabolic parameters), the neonate was carefully hand restrained and, under spontaneous ventilation, exposed to the anaesthetic agent (isoflurane) via face mask. This induction technique mimics the method used routinely in young children. After reaching a state of tolerance, the animal was tracheally intubated and mechanically ventilated. Anaesthesia was maintained within an end-tidal isoflurane concentration of 0.7–1.5 vol% in 30% oxygen for 5 h. This exposure provided a surgical plane of anaesthesia that was repeatedly monitored by verification of the absence of any movement and <10% increase in HR or BP in response to a mechanical stimulus. This anaesthesia regimen was analogous to that used in a human operating theatre.
The animals were controlled using full physiological monitoring continuously with parameters recorded every 15 min and received interventions, including warming, i.v. fluids, glucose supplementation, and BP support, as needed. The animals did not receive any drugs to control pulmonary secretions. A 5-h exposure with isoflurane was chosen because it induces a prominent acute increase in brain cell apoptosis in this animal model.18 At the end of the exposure period, isoflurane was discontinued and, once appropriate reflexes were recovered, the animals were extubated, typically within 10 min. After another 1–2 h in a NHP incubator, the fully recovered neonates were transferred back to their dams. The animals were randomised to undergo this procedure either one time (1×), on postnatal day 6 (P6), or on three separate occasions (on P6, P9, and P12; 3×). The animals in the Ctr group underwent the same procedures at the three indicated time points, including removal from the dam, i.v. catheter placement, physiological measurements, and handling, but were exposed to room air. The animals in the 1× group were exposed to isoflurane on P6 and handled like the Ctr group on P9 and P12.
The exposure to isoflurane was well tolerated and infant NHPs remained physiologically stable throughout exposure and recovery.16 Sex distribution was equal in all three groups, and there were initially eight animals per group. In the absence of enough information about the cognitive and behavioural outcomes evaluated in this study to calculate group size with a statistical power analysis, group size was determined based on previous work with NHPs that used similar tests.15,19,20 Because of chronic diarrhoea that did not resolve with treatment, one female NHP in the 3× group was removed from the study before 1 yr of age. Thus, at the time of the assessments, there were eight animals in the Ctr and 1× groups and seven in the 3× group, all of which were healthy and had normal growth parameters.
Assessment of cognitive function
Multiple tests of cognitive development were conducted between 1 and 2 yr of age. Tests were selected to assess frontal lobe and basal ganglia functions, including object permanence, spatial working memory, executive function, and stimulus–response learning.21,22 These tests were chosen because the cognitive domains that they measure involve brain areas known to undergo extensive and acute neuronal and glial apoptosis in NHPs exposed to anaesthetics during infancy.7, 8, 9 These tests also assess relatively late-maturing abilities that are still developing at 1 yr of age and have been shown to detect differences in cognitive development in NHPs.23 All testing was done by trained personnel blinded to treatment groups. Cognitive testing took place in a mobile cage adjacent to the home cages of the animals. All cognitive and motor tests were performed in a modified version of the Wisconsin General Test Apparatus (WGTA) that was attached to the cage. It had a testing board with two wells placed symmetrically to the left and right of the centre where food treats (raisin, peanut, or candy) could be placed. The side of the cage facing the WGTA had widely spaced vertical bars that allowed the monkey to reach the covered wells and retrieve the food treats. An opaque horizontal sliding screen could be closed between the monkey and the testing board, and a one-way-vision screen could be lowered between the testing board and the tester.
Delayed response task
Spatial working memory was measured using the delayed response task.24 This task is functionally equivalent to the Piaget Stage IV object permanence task (the ‘A-not-B’ task) used to assess cognitive development in human infants.23 Specifically, these tests measure the ability to guide responses based on representational knowledge and memory. The age of NHPs in our study was suitable for performing in the delayed response task, as previous studies have shown that NHPs younger than 1 yr of age are already capable of performing this task, although their performance increases gradually over the first 3 yr.25, 26, 27, 28 NHPs performed test trials consisting of three phases: in the cue phase, the infant NHP watched as a food treat was placed in one of two wells, which were then covered simultaneously with two solid 7 cm square black plaques so that the food was no longer visible. In the delay phase, an opaque screen was closed between the monkey and the wells for a variable time period (delay) between 1 and 30 s. In the response phase, the screen was opened and the animal was allowed to choose one well and remove the plaque to retrieve the treat. If they removed the wrong plaque, the screen quickly closed to prevent them from retrieving the treat. The correct/incorrect response was recorded by a tester, who was not visible to the NHP. There was a training phase, where the delay successively increased in 1 s steps from 1 to 5 s. At each delay, the NHPs were required to give 80% correct responses for 2 days before progressing to the next step. Those completing the training requirement progressed to the testing phase, in which delays of 1, 5, 10, 20, and 30 s were intermixed in each session, with five trials at each delay for a total of 25 trials per session. Testing continued for a total of 10 sessions. There was no difference in the acquisition rate (i.e. the number of trials and sessions) between treatment groups. The right–left position of the treat randomly varied between trials. Performance was measured as the percentage of correct responses at each delay, and this value was expected to decrease progressively with longer delays.
Reversal learning and set-shifting tasks
Stimulus–response learning, executive function, and cognitive flexibility were measured with a series of visual discrimination tasks. These discrimination learning and reversal tasks have been used successfully in NHPs younger than 1 yr of age.29,30 The stimuli were solid objects (∼3 cm3) affixed to solid 7 cm square black plaques covering the two wells of the testing board of the WGTA. For each task, the well under the correct object was baited with food treats, while the opaque screen was closed to prevent the monkey from seeing where the food was placed. The screen was then opened to allow the NHP to choose one of the objects and, if correct, retrieve the treat. Testing proceeded through the following sequence of tasks: (i) NHPs first learned to select between objects with two shapes: a triangle (Tri) and a rectangle (Rec), with the Tri being the correct response. The objects were randomly either red or green, but at this stage, the colour was irrelevant to performing the task. (ii) Once the animals reached criterion performance (80% correct responses) on this task, they were then tested on a reversal learning task, in which the previously incorrect shape, the Rec, became the correct selection; two additional reversals of the shape discrimination task were administered. (iii) The next stage was a ‘set-shifting’ task, in which colour became the relevant dimension of the stimulus, with green being the correct response while ignoring the shape of the objects. Finally, the correct colour was reversed to red. In summary, the correct stimuli for the six tasks, in order, were Tri, Rec, Tri 2, Rec 2, green, and red. The NHPs were tested on each task for 40 trials per day until criterion performance was reached, and then proceeded to the next task. The measured dependent variables were the number of (i) days and (ii) errors (choice of the incorrect stimulus) before reaching criterion performance on each task. The set-shifting task is similar to the Dimensional Change Card Sort task used in young children31 and has been adapted to test NHPs.32 The ability to perform this task matures at about 4 yr of age in children, which is approximately equivalent to 1 yr of age in rhesus monkeys.
Life Savers retrieval test
Fine motor skills and visuospatial ability were assessed using the Life Savers retrieval test.24,33 In an initial training phase, NHPs were trained to remove a doughnut-shaped hard candy (Life Savers™) from a straight metal rod. For testing, the animals were required to thread the Life Savers from rod configurations of successively increasing difficulties with zero, one, two, and three bends of 90°. The rods with one or two bends were each presented in two orientations, with the bends facing either towards the NHP's front (the easier alternative) or to its left, making for a total of six rod configurations. The time limit for retrieval was 45 s for easy routes (zero or one bend) and 120 s for more difficult routes (two or three bends). The NHPs were required to complete 24 trials with each configuration before proceeding to the next level of difficulty. However, some NHPs (distributed across the three groups) failed to complete the full 24 trials for the more difficult configurations within the defined time limits. Therefore, the number of trials used for analysis was reduced to 15 at each level of difficulty, except for four animals that only completed 12–13 trials for one or more of the more difficult configurations. The distribution of those animals was one in the Ctr, one in the 1×, and two in the 3× groups. The dependent variable measured in this test was the mean latency to retrieve the Life Savers for each rod configuration.
Behavioural testing
At 2 yr of age, we assessed behaviours using both home-cage assessments (i.e. the assessed behaviours are spontaneous) and provoked response testing (i.e. the behaviours are triggered by exposure to a stimulus) paradigms (human intruder test and novel object test). These were performed very similarly at 1 yr of age, as reported.16 All behavioural observations and testing were performed by an investigator blinded to the treatment groups.
Home-cage behavioural assessments
Behavioural assessments in the home cage were performed to examine the juvenile NHPs' responses to everyday events within their social group. Over a period of 2–3 weeks, 10 min focal observations were conducted two to three times per week by a trained and highly experienced observer, resulting in a total of 60 min of observation per animal. The observer was familiar to the NHPs, but blinded to their treatments. After entering the room and standing next to the home cage for 10 min of acclimation, the observer recorded the behaviour of each individual directly to a laptop computer using a software for behavioural research (The Observer; Noldus Information Technology, Wageningen, the Netherlands). These observations were conducted between noon and 3:00 p.m. to avoid the confounding effects of the time of day. Behaviours coded (ethogram; Supplementary Table 2) were focused on social behaviours, aggression, anxiety, and submissive behaviour.
Human intruder test
We used the human intruder test (HIT) to assess the NHPs' temperament.34,35 Behavioural responses to the following conditions were tested: being alone, being with a human that was unknown to the NHP (human intruder) with a diverted gaze (potential threat), and being with the human intruder making direct eye contact with the NHP (an explicit threat). For testing, subjects were removed from their social groups and brought, one at a time, to a novel cage in a dedicated testing room (2.4 × 3.0 m). A one-way window allowed observation and videotaping. After an initial 10 min phase to acclimate to the novel cage without the human intruder, behaviours were recorded for 2 min (alone phase). After this assessment, the human intruder entered the room and stood in ∼0.3 m distance to the cage for 2.0 (0.1) min, presenting the facial profile only (profile phase). After another 2 min alone period (alone 2), the intruder re-entered and made direct eye contact for 2.0 (0.1) min (stare phase). To avoid confounding factors related to varying appearance, the intruder was consistently a woman and wearing the same protective clothing. The test ended with another 2 min alone phase. Behavioural data are reported as percentage of time of each behaviour to correct for small variations (within 6 s) in recording time of both the profile and stare phases.
Novel object test
The novel object test (NOT) was used to assess the NHPs' propensity to explore novel objects (food or toys) that were either non-threatening or potentially threatening.16 This test was performed immediately after the HIT and in the same cage with the NHP alone while testing. Immediately after the NOT, the NHP was transferred back to the social group. The investigator sequentially placed various novel objects in the cage, each one remaining for 5 min. Except for the novel food, all items were removed before the introduction of a new object. In the order presented, the novel objects were a piece of kiwi (novel food), a hanging toy, Mr. Potato Head™ (a potentially threatening stimulus because of large eyes), a rubber snake with a piece of apple (highly desirable item) on top, and a black box that hung at the outside of the cage. These objects had been presented to the NHPs in previous NOTs performed at 3 months and 1 yr of age; therefore, these objects are not strictly novel. To evaluate inhibition towards novel objects, three behaviours were measured: inspecting, touching, or manipulating the novel objects (Supplementary Table 2). Each of the five novel objects remained in the cage for 5 min. An inhibition score was created based on whether or not the animal inspected, touched, or manipulated each object. A value of 1 was given each time an animal did not inspect, touch, or manipulate an object. An accumulative inhibition score for each animal was obtained by adding these values of each behaviour for all novel objects. These accumulative inhibition scores ranged from 0 (not inhibited) to 15 (fully inhibited). Additionally, we created two accumulative inhibition scores, one for non-threatening objects (kiwi, hanging toy, and black box) and another for potentially threatening objects (rubber snake and Mr. Potato Head) to analyse the data with regard to the type of objects.
Statistics
All data are presented as mean (standard deviation) or median [25th–75th percentiles] for variables with normal or non-normal distribution, respectively. We identified and excluded outliers using a method called the robust regression and outlier (ROUT) test36 with Q=1%. We report the number of outliers detected in each group and the final sample size for each test in the respective figure legend or table. We tested data for normal distribution using the Shapiro–Wilk normality test. For cognitive test analyses, we used a two-way mixed-design analysis of variance (anova) with the Geisser–Greenhouse correction for adjusting degrees of freedom of the F statistic, because of the use of repeated measurements. The between-subjects factor was the exposure groups and the within-subjects repeated measurements factor was either delay, task, or configuration, depending on the specific cognitive test. All anova test results are reported together with η2 a standardised measure of the effect size, which was considered small (0.01<η2<0.06), medium (0.06<η2<0.14), or large (η2>0.14).37 The degrees of freedom of the F statistic were corrected using the Geisser–Greenhouse epsilon and rounded to the nearest whole number. We used Tukey's multiple comparison test to compare between exposure groups (Ctr, 1×, and 3×) and to report the adjusted P-value and 95% confidence intervals. For the analysis of the home-cage assessment and the human intruder test, we used either one-way anova with Tukey's multiple comparison test or Kruskal–Wallis with Dunn's multiple comparison test to detect significant differences amongst groups for variables with normal or non-normal distribution, respectively. Tukey and Dunn's post hoc tests correct the level of significance for multiple comparisons across the exposure groups within each test. However, as stated in the report of the behaviours at 1 yr of age,1 because an individual's response in one test does not predict how it will respond in another test, we did not adjust the level of significance across tests or test families.35 For the NOT, we used univariate generalised linear model regression with a Poisson distribution, using inhibition score as the outcome variable and treatment group as the predictor variable. This test was used because our data were not normally distributed and to avoid the decreased power in the Kruskal–Wallis test. Univariate models (which consider a treatment effect) were compared with a null model (an intercept-only model, where there is no effect of treatment) using Akaike's information criteria (AIC) scores.38 A decrease in at least 2 AIC points is considered an improved model. For all statistical hypothesis testing, we used two-tailed tests, and P<0.05 was considered statistically significant. We used GraphPad Prism version 8.0 (GraphPad Software, San Diego, CA) and R software version 3.2.139 for statistical analyses.
Results
Assessment of cognitive function at 1–2 yr of age
Delayed response task
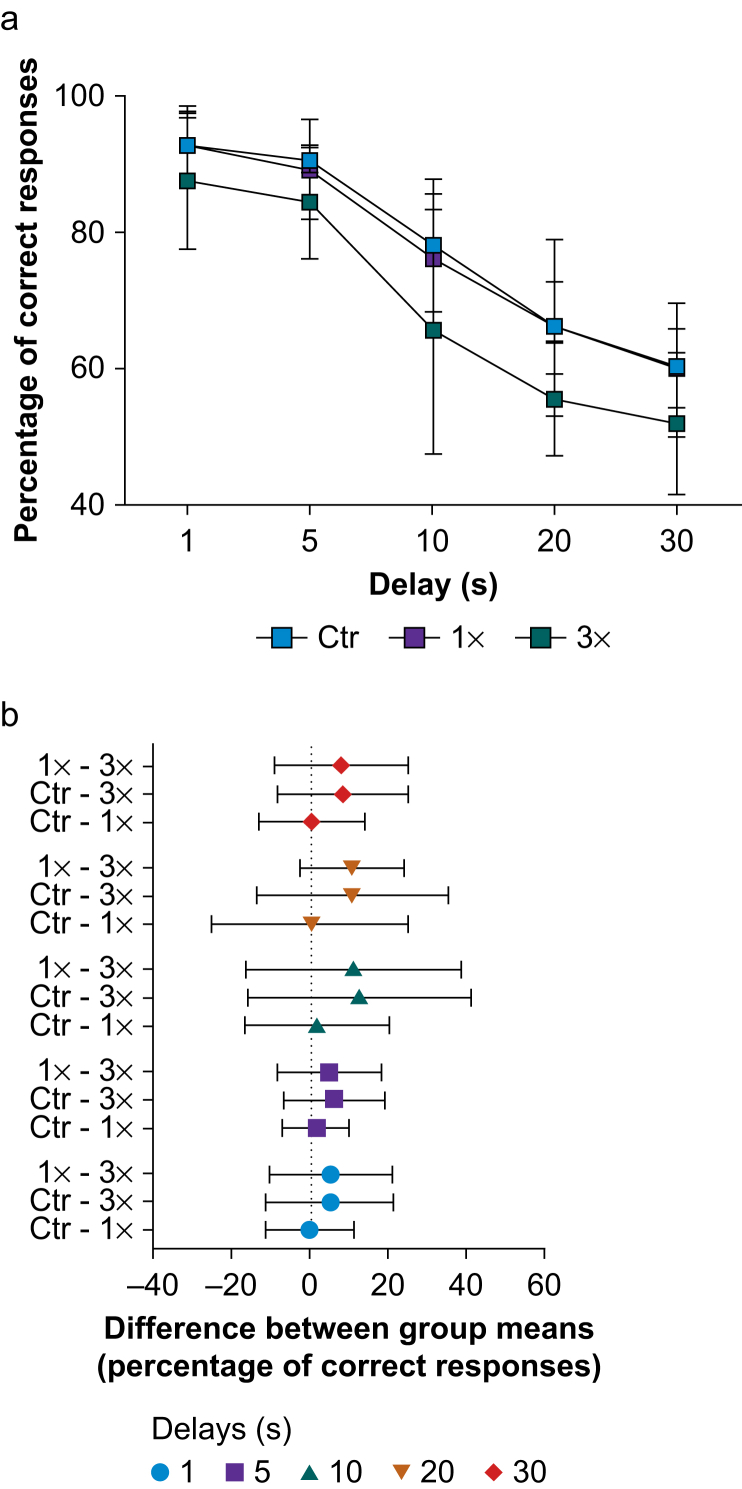
Performance on the delayed response task showed the expected decrease in the percentage of correct responses with increasing delays for all groups (Fig 1a; main effect of delay; F(3,33)=64.8; P<0.001; ƞ2=0.65; two-way mixed-design anova). The 3× group had the lowest mean percentage of correct responses, particularly for longer delays. However, there were no statistically significant differences in performance amongst groups (main effect of group; F(2,13)=2.63; P=0.110; ƞ2=0.05) and no significant interaction between group and delay (F(8,52)=0.30; P=0.962; ƞ2<0.01). The anova post hoc analysis confirmed that there were no statistically significant differences between group means at any delay (P>0.05 for all; Tukey's multiple comparison test), and the 95% confidence intervals of the mean differences overlapped (Fig 1b), supporting statistical equivalence amongst the exposure groups. Not all NHPs were able to achieve criterion performance on the training phase (four, one, and two NHPs in the Ctr, 1×, and 3× groups, respectively); therefore those failing NHPs did not perform in the testing phase, and the n for this test was reduced accordingly as shown in the figure legend (Fig 1).
Fig 1.
Delayed response task. (a) Percentage of correct responses in the delayed response task across five different delays in juvenile non-human primates (NHPs) exposed one (1×) or three (3×) times to isoflurane during infancy and in controls (Ctr). Data are means (standard deviation); n (NHPs): Ctr: 4; 1×: 7; 3×: 5. (b) 95% confidence intervals of the differences between exposure group means for all delays. Two-way mixed-design analysis of variance and Tukey's multiple comparison test.
Reversal learning and set-shifting tasks
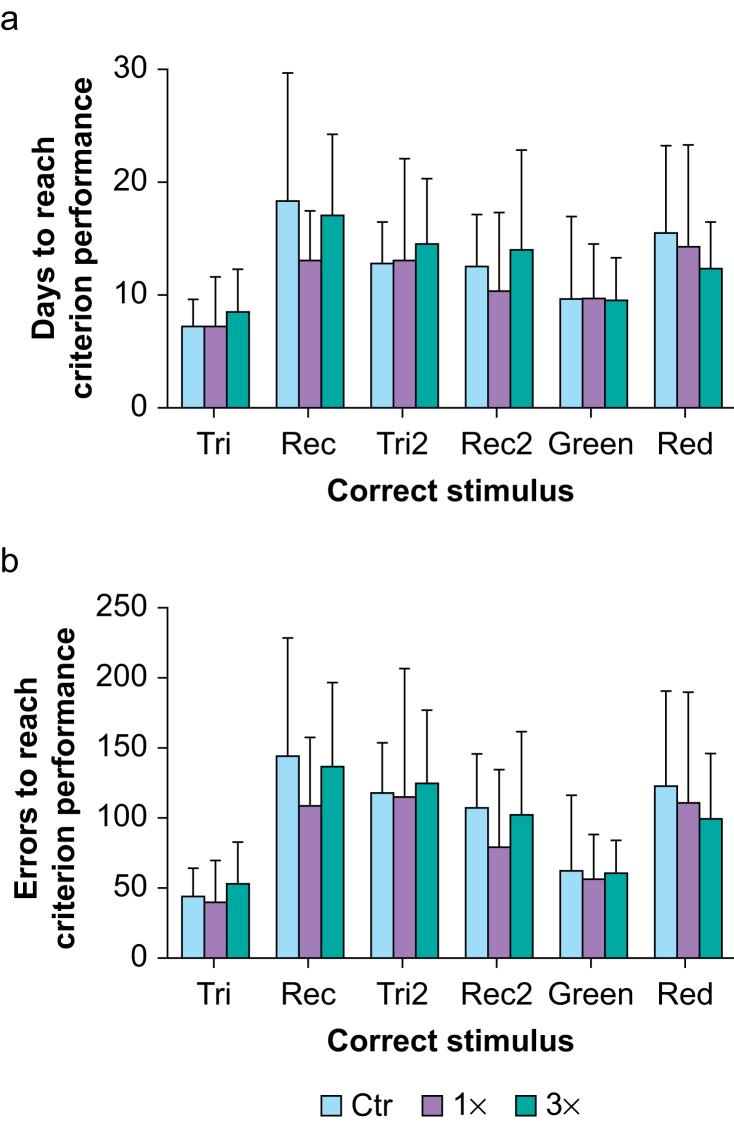
The number of days (Fig 2a) and errors (Fig 2b) to reach criterion performance (80% correct responses) varied across the visual discrimination tasks for all groups. The main effect of the task (two-way mixed-design anova) shows significant effects for both the number of days (F(3,49)=5.07; P=0.004; ƞ2=0.14) and errors (F(3,45)=9.00; P<0.001; ƞ2=0.20) to reach criterion performance. As expected, the first reversal of the shape discrimination was the most difficult task as the number of days and errors increased from Tri to Rec. Then, as the NHPs learned the reversal tasks, the number of days and errors to reach criterion performance decreased with subsequent reversals of the shape discrimination task. Unexpectedly, the set-shifting task (stimulus changed to colour) required relatively few days and errors to reach criterion performance, perhaps because of the saliency of the colour stimulus. However, there were no significant differences amongst groups in the number of days (main effect of group; F(2,16)=0.48; P=0.630; ƞ2=0.02) or errors (main effect of group; F(2,16)=0.49; P=0.620; ƞ2=0.02) to reach criterion performance across the series of tasks. There were no significant interactions between task and group for both the number of days (task × group; F(10,80)=0.24; P=0.992; ƞ2=0.01) and errors (task × group; F(10,80)=0.25; P=0.989; ƞ2=0.01) to reach criterion performance. There were one and three NHPs that did not complete all tasks in the Ctr and 3× groups, respectively; they were excluded from the entire analysis. However, similar results were obtained with a mixed-model analysis (not shown) when these animals were included. Furthermore, although there were more failing NHPs in the 3× group, a χ2 test for trend did not reveal a statistically significant difference between groups. The final n for this series of discrimination task is indicated in the figure legend.
Fig 2.
Reversal learning and set-shifting tasks. The (a) number of days and (b) errors to reach criterion performance (80% correct responses) in each of the sequential visual discrimination tasks in juvenile non-human primates (NHPs) exposed one (1×) or three (3×) times to isoflurane during infancy and in controls (Ctr). Tri = triangle (initial correct stimulus); Rec = rectangle (first reversal); Tri2 = second reversal, with triangle as correct stimulus; Rec2 = third reversal, with rectangle as correct stimulus; Green = set-shifting to colour, with green as correct stimulus; Red = colour reversal, with red as correct stimulus. Data are means (standard deviation); n (NHPs): Ctr: 7; 1×: 8; 3×: 4.
Fine motor skills and visuospatial ability
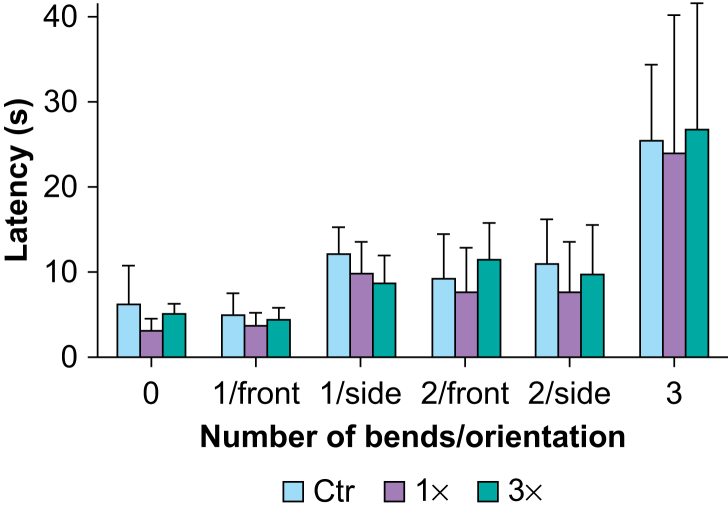
As expected, the latency to retrieve a Life Savers candy from a metal rod increased with the difficulty of the rod configuration (Fig 3; main effect of configuration; F(2,35)=44.62; P<0.001; ƞ2=0.60; two-way mixed-design anova). However, no significant differences were found among groups (main effect of group; F(2,20)=0.92; P=0.414; ƞ2=0.01) in retrieval latency or for interaction between configuration and group (configuration × group; F(10,100)=0.33; P=0.971; ƞ2=0.01).
Fig 3.
Fine motor skills. Latency to retrieve a Life Savers candy from a metal rod across six configurations of increasing difficulty based on the number and orientation (front or side) of right-angle bends in juvenile non-human primates (NHPs) exposed one (1×) or three (3×) times to isoflurane during infancy and in controls (Ctr). Data are means (standard deviation); n (NHPs): Ctr: 8; 1×: 8; 3×: 7.
Behavioural assessment at 2 yr of age
Spontaneous behaviours
Home-cage assessment
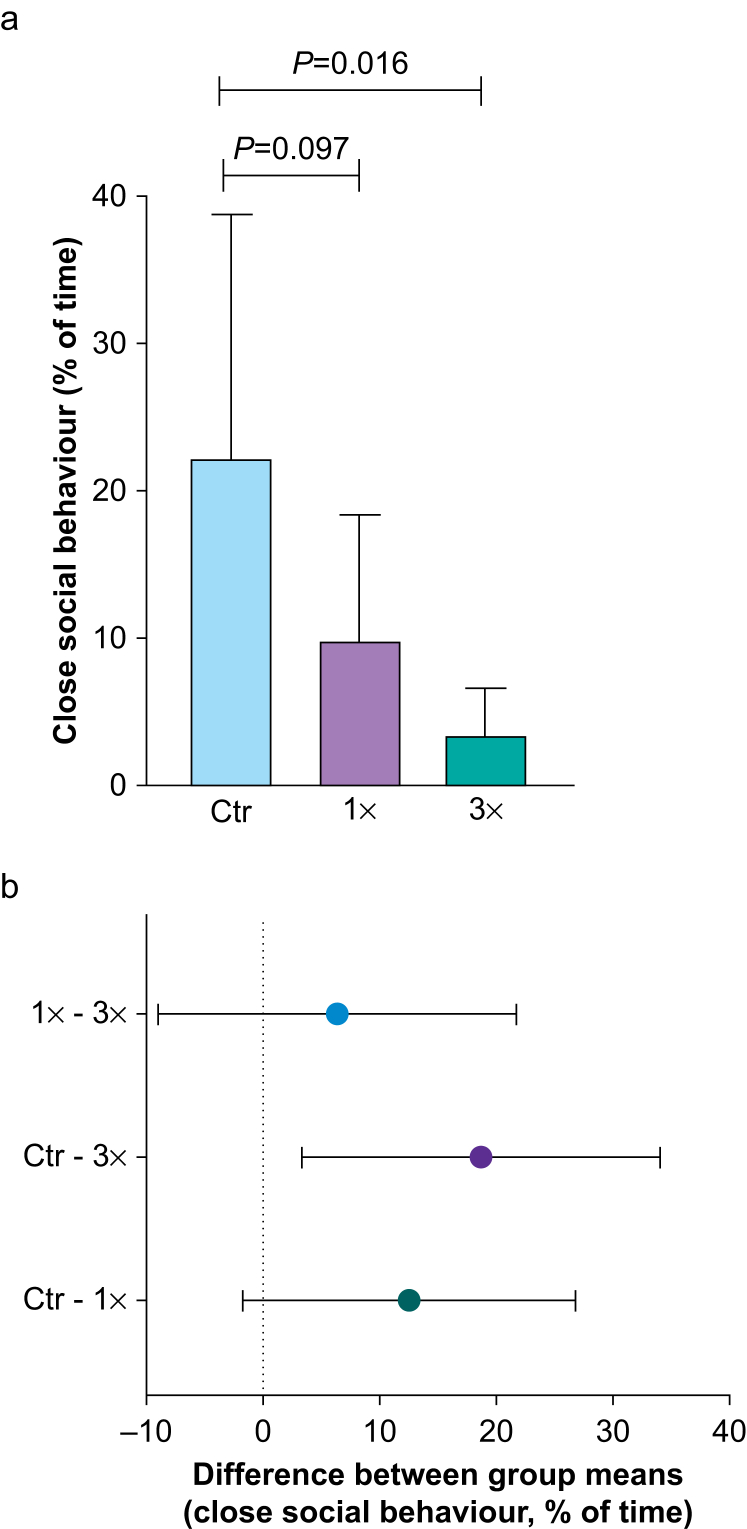
To assess whether anaesthesia exposure affects spontaneous behaviour of 2-yr-old NHPs in social groups, we performed focal observations on each individual in the home cage as described in the Methods. We evaluated the animals' interactions within social groups by assessing the time spent in close social behaviours (grooming, huddling, or touching) with other individuals. We found that juvenile NHPs in both exposed groups spent less time engaged in close social behaviour than those in the Ctr group (Fig 4a; one-way anova; F(2,19)=5.10; P=0.017; ƞ2=0.35). Post hoc analysis showed that the differences between the Ctr and exposed groups reached statistical significance for the 3× group, but not for the 1× group, and there were no differences between the exposed groups (Ctr vs 3× P=0.016; Ctr vs 1× P=0.097; 1× vs 3× P=0.565; Tukey's multiple comparison test). The 95% confidence intervals of the mean differences confirm and illustrate these findings (Fig 4b). On average, the time NHPs spent in close social behaviour was ∼two-fold and six-fold lower in the 1× and 3× groups than in the Ctr group (Ctr: 22.3 [16.4]%; 1×: 9.9 [8.3]%; 3×: 3.6 [3.0]%), respectively. Using the ROUT test, one outlier was identified in the 3× group and excluded from analysis. When the outlier was included, the 3× group was 5.6 (5.9)%, whereas the multiple comparison analysis of the data showed a similar P-value between the Ctr and 3× groups (P=0.041). In addition, we evaluated the aggressive, submissive, and anxiety behaviours described in the ethogram (Supplementary Table 2), but found no statistically significant differences in the frequencies of these behaviours among the three groups (Supplementary Table 3).
Fig 4.
Home-cage assessment of social behaviour. Duration of close social behaviour in juvenile non-human primates (NHPs) exposed one (1×) or three (3×) times to isoflurane during infancy and in controls (Ctr). Data are mean (standard deviation); n (NHPs): Ctr: 8; 1×: 8; 3×: 6; one-way analysis of variance and Tukey's multiple comparison test. One outlier was identified and excluded in the 3× group.
Provoked response testing
Human intruder test
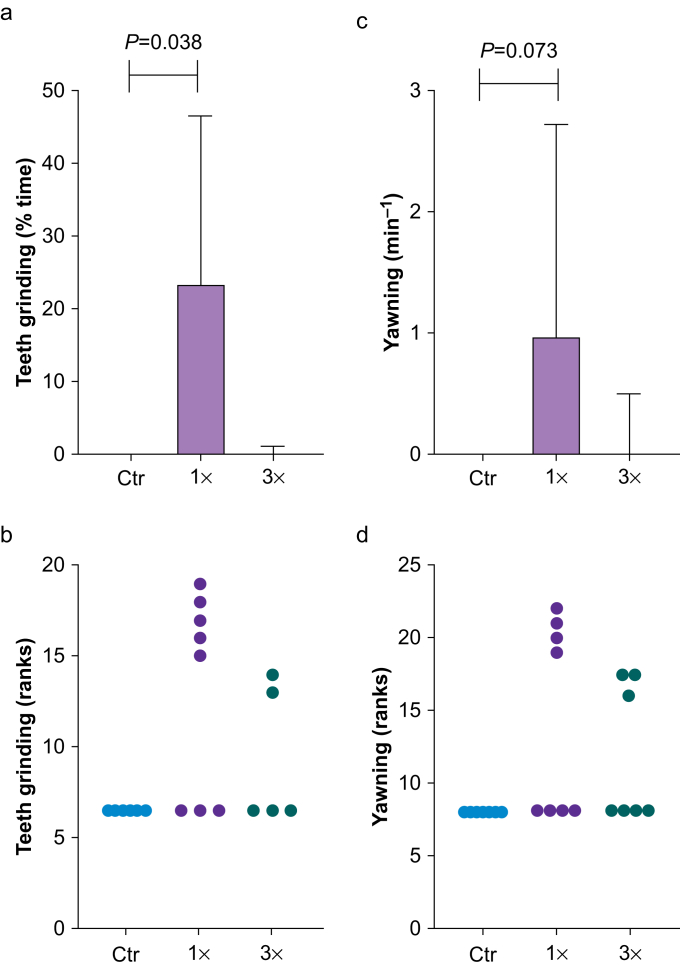
We used the human intruder test to assess behaviours indicative of anxious temperament and behavioural inhibition, as described in the ethogram (Supplementary Table 2), during the profile and stare phases. After the animals were acclimatised and before the human intruder entered the room (alone phase), locomotive behaviour did not differ between exposure groups (Supplementary Table 3). During the profile phase, juvenile NHPs decreased locomotive behaviour and, in general, spent most of the time frozen in place with no differences among groups (Supplementary Table 3). In the stare phase, we found that NHPs exposed to isoflurane one time showed longer duration of teeth grinding (Ctr: 0.0 [0.0, 0.0]; 1×: 23.4 [0.0, 46.4]; 3×: 0.0 [0.0, 1.1]) and higher frequencies of yawning (Ctr: 0.0 [0.0, 0.0]; 1×: 1.0 [0.0, 2.7]; 3×: 0.0 [0.0, 0.5]), both behaviours indicative of anxiety (Fig 5). Statistical analysis showed that these differences were significant for teeth grinding (Fig 5a and b; H(2)=6.37; P=0.031; Kruskal–Wallis test), but not for yawning (Fig 5c and d; H(2)=5.02; P=0.075; Kruskal–Wallis test). Dunn's multiple comparison test revealed statistically significant differences for teeth grinding between the Ctr and 1× groups (P=0.038), but not between the Ctr and 3× groups (P>0.999), whereas for yawning, the differences between the Ctr and 1× groups were not significant (P=0.073), and there were no differences between the Ctr and 3× groups (P=0.536). We found two outliers in the teeth grinding data of the Ctr and 3× groups and one outlier in the yawning data of the Ctr group. Including these outliers in the analysis, the statistics for teeth grinding were Ctr: 0.0 [0.0, 4.2] and 3×: 0.7 [0.0, 22.5] and for yawning were Ctr: 0.0 [0.0, 4.2]. In addition, we did not detect significant differences between groups for teeth grinding (H(2)=3.72; P=0.155) or for yawning (H(2)=3.65; P=0.162), when we included these outliers in the analysis. Additionally, compared with controls, exposed NHPs showed less lip smacking, indicative of appeasement behaviour, but differences were not statistically significant (Supplementary Table 3).
Fig 5.
Human intruder test (HIT) for anxiety-related behaviours. (a) Duration of teeth grinding and (b) frequency of yawning in the stare phase of the HIT in juvenile non-human primates (NHPs) exposed one (1×) or three (3×) times to isoflurane during infancy and in controls (Ctr). Data are median [25th–75th percentiles]; n (NHPs; teeth grinding): Ctr: 6; 1×: 8; 3×: 5; n (NHPs; yawning): Ctr: 7; 1×: 8; 3×: 7; Kruskal–Wallis and Dunn's multiple comparison test. Two outliers for teeth grinding data of the Ctr and 3× groups and one outlier for yawning data of the Ctr group were identified and excluded.
Novel object test (NOT)
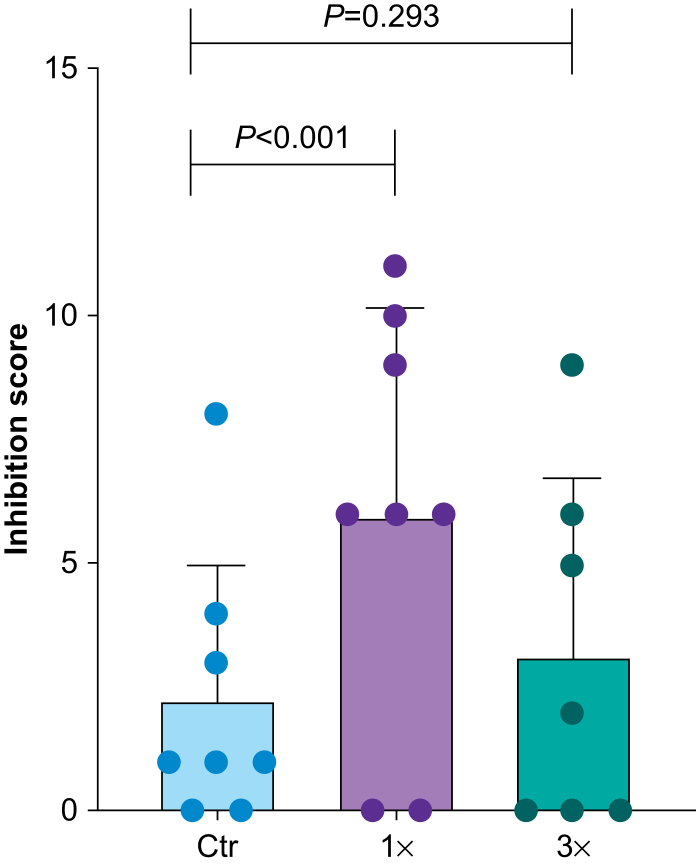
We evaluated the NHPs' propensity to explore novel objects by creating inhibition scores based on the assessed latencies of the animal's actions during presentation of novel objects, as described in Supplementary Table 2 and Methods. Compared with the Ctr group, NHPs in the 1× group were significantly more inhibited (less likely to approach novel objects), whereas the inhibition scores in the 3× group were not significantly different (Fig 6; Ctr vs 1× P<0.001; Ctr vs 3× P=0.293; Poisson regression; null model AIC=80.0; treatment model AIC=71.7). In addition, we explored whether inhibition scores differed based on whether the object was non-threatening or potentially threatening, and whether there was an interaction between object type and treatment. We compared four models with a null model (where AIC=193.0):
-
(i)
Treatment only: NHPs in the 1× group showed more inhibition than the Ctr group (P<0.001; AIC=181.0).
-
(ii)
Object type only: there was no difference in inhibition scores between non-threatening and potential threatening objects (P>0.1; AIC=193.0).
-
(iii)
Additive model with treatment and object type (treatment + object type): the effect of object type was not significant (P>0.1; AIC=182.0), but the 1× group was significantly more inhibited than the other groups (P<0.001).
-
(iv)
Interactive model with treatment and object type (treatment × object type): the main effect of object type was not significant (P>0.1), and the interactions between treatment and object type were not significant (P>0.1; AIC=186.0), but the 1× group was significantly more inhibited than the other groups (P<0.001).
Fig 6.
Novel object test (NOT). Inhibition scores in the NOT for juvenile non-human primates (NHPs) exposed one (1×) or three (3×) times to isoflurane during infancy and for controls (Ctr). Data are mean (standard deviation); n (NHPs): Ctr: 8; 1×: 8; 3×: 7; univariate Poisson regression (delta Akaike's information criteria=8.3).
Discussion
We report behavioural alterations in 2-yr-old juvenile NHPs after one or three consecutive 5 h isoflurane exposures under fully controlled physiological conditions within the first 2 weeks of life. Previous studies showed that even a single 5 h exposure caused a prominent acute increase in apoptosis of neurones and oligodendrocytes in this animal model,7,10 but the functional alterations that accompany such structural changes later in life are less well characterised. We observed NHPs in their home environment (social group) to examine the effects of infant anaesthesia exposure on spontaneous social behaviours, and we used provoked response testing to identify anxiety-related and inhibition behaviours at 2 yr of age. We also tested potential consequences of these exposures on cognitive development during their second year of life.
We found that infant exposure to isoflurane for 5 h, either one or three times, did not affect the cognitive domains tested, but did result in alterations in behavioural development. The major effects were observed by assessing spontaneous behaviours in social groups using focal observations. Close social behaviour was decreased in both exposed groups with respect to the Ctr group with an effect size considered large37 and with the animals in the 3× group reaching statistical significance (dose dependence). We also found differences in some of the behaviours assessed with provoked response testing. The 1× group showed increased anxiety-related behaviours and more inhibition when presented with novel objects with respect to the Ctr group, but surprisingly these behavioural alterations were not observed in the 3× group.
Our cognitive assessments in NHPs during the second year of life did not reveal statistically significant differences between groups in spatial working memory, as measured by the delayed response test, or in executive function and cognitive flexibility as measured by both reversal learning and set-shifting tasks. Our comprehensive assessment at this age also included a test of fine motor function and visuomotor ability, which similarly found no differences between groups. Even though we recognised high levels of intra-individual variability in each of the measures, our results suggest that single or multiple 5 h anaesthesia exposures during infancy have no major effects on the cognitive domains tested or on the fine motor skills when assessed in this animal model during the second year of life. Similarly, a previous study that used a cognitive test battery to evaluate NHPs at 3.5 yr of age found only minor impairments after a single 8 h exposure to an anaesthetic combination of isoflurane with nitrous oxide during infancy.14 The deficits found in the exposed animals were limited to decreased responses in the progressive ratio task, a test designed to measure motivation. Interestingly, in our study, some of the NHPs that did not complete the more difficult levels in the Life Savers retrieval test were the same that failed to complete the reversal learning and set-shifting tasks (n=1 in the Ctr group and n=2 in the 3× group). It is possible that these three animals may have had less motivation to participate; however, we did not specifically assess motivation in our study. In another study, infant ketamine exposure was associated with several deficits using the same battery of cognitive tests in similar-aged NHPs.15 However, the duration of exposure (24 h) and the choice of anaesthetic in that study differed significantly from the exposure conditions used in the previously mentioned study14 and in our study. In addition, that particular regimen (24 h ketamine infusion) does not represent a typical anaesthetic procedure in paediatric patients.
Another study that assessed cognitive function in NHPs found that three 4-h exposures to sevoflurane during infancy resulted in impairment in visual recognition memory starting at 1 yr of age.13 In NHPs at 24–30 months of age, there was a statistically significant decrease in performance at all delays in the group exposed to anaesthesia three times.13 However, in contrast to our study, this study assessed a different memory function (spatial working memory vs visual recognition memory) using a distinct approach (delay response task vs object recognition task). We recognise that our data show a trend towards a lower performance for NHPs in the 3× group, especially at longer delays (Fig 1a). Furthermore, the longest delay in our assessment was 30 s, and we therefore cannot reject more pronounced (or statistically significant) effects if longer delays were used in our assays. In this regard, Zeamer and colleagues40 found that neurotoxic hippocampal lesions in 10- to 12-day-old NHPs had a significant effect on animal performance at the age of 18 months in a visual recognition task at either very short (10 s) or very long (120 s) delays, but not at intermediate delays. Alvarado and colleagues13 found that the effects of infant anaesthesia exposure on NHP's performance at 12–18 months of age depended on the delay in the visual recognition task. In addition, in our study we cannot discard a potential effect of infant anaesthesia exposure on performance (especially those NHPs in the 3× group) if the assessment had taken place at a later age, considering that others have seen significant effects of infant anaesthesia or hippocampal lesions only at later ages.13,40
Motor dexterity (a measurement of fine motor skills) has been found to be impaired in children with multiple anaesthetic exposures during infancy.6 In contrast, we did not find statistically significant differences in the latency to retrieve the Life Savers among groups. However, we cannot ignore the possibility that dexterity or other fine motor skills were affected by isoflurane exposure during infancy, as these were not measured in our study. Taken together, although our data do not show statistically significant effects of infant anaesthesia exposure in the performance of NHPs in the specific cognitive tests we used, we cannot discard positive effects on these cognitive domains if NHPs were assessed at different ages or under different testing conditions. For example, although several studies have shown that the age of NHPs in our study is suitable for the specific tests used,25, 26, 27,29,30 the contribution of the prefrontal cortex activity in these tests at this age is still under debate.25
Decreased affiliative behaviour was the major finding in juvenile NHPs 2 yr after infant anaesthesia exposure in our study. Importantly, affiliative behaviour in NHPs develops progressively from early infancy to juvenile age (2.5 yr),41 and therefore, a decrease in this behaviour at this critical age may have long-term consequences for social development. Decreased affiliative behaviour in humans or reduced social interactions are behaviours that can be indicative of underlying social anxiety or depression.42 Children with anxiety disorders often withdraw from social company and avoid social interactions to reduce visceral arousal.43 On the other hand, social withdrawal can also accompany depression.44,45 Moreover, children that show decreased social interaction and are socially withdrawn are at risk for a range of difficulties later in life, including development of anxiety or depression disorders, and problems in social settings at school and at home. Although we did not continue to follow the NHPs until adulthood, the decreased affiliative behaviour observed in juveniles suggests that these animals may be at risk of developing anxiety- or depression-like behaviours later in life.
In a previous report, we evaluated the same NHPs at 1 yr of age, immediately after they had been weaned and transferred into a new social group with peers.16 We found that 3× exposed NHPs displayed increased anxiety-related behaviours and more appeasement behaviour,16 which has been associated with social anxiety in humans.46, 47, 48 This group of 3× exposed NHPs that appeared more anxious-like at 1 yr of age displayed decreased social interaction as they spent less time in close distance to their peers when they were re-evaluated at 2 yr of age. Therefore, the increased social withdrawal in exposed NHPs might resemble behaviours indicative of social anxiety in humans. Whilst decreased affiliative behaviour has also been associated with depression in humans,42 we have no data from our animal cohort that indicate that exposed NHPs developed depression-like behaviours.
Anxious temperament early in life is associated with increased risk of developing anxiety and depressive disorders.49 Anxious temperament has been assessed in NHPs using behavioural tests, including the HIT.50 Using this assessment tool at 2 yr of age, we found that 1× exposed NHPs showed increased anxious temperament as indicated by increased duration of teeth grinding during the stare phase of the HIT. Similarly, other studies in NHPs reported a persistent anxious phenotype after multiple exposures to sevoflurane during infancy.11,12 A field study with free-ranging colonies of NHPs found that anxious temperament was associated with social inhibition, as animals with high anxious temperament stayed more distant from their peers.50 Surprisingly, we did not find anxiety-related behaviours in NHPs in the 3× group. Future studies should consider using several different anxiety assessment tools to address this interesting observation.
Exposure to isoflurane during infancy also resulted in increased inhibition at 2 yr of age. Specifically, NHPs in the 1× group were more inhibited towards novel objects (less likely to explore) compared with the Ctr group. The 3× group also appeared more inhibited than the Ctr group, but this difference did not reach statistical significance. Other studies have shown that NHPs are more inhibited towards threatening objects and less inhibited towards novel/non-threatening/non-aversive objects.19,51, 52, 53, 54, 55, 56 However, in our study we did not find differences in inhibition depending on the type of object (non-threatening vs threatening). This discrepancy might be attributable to differences in testing conditions, including object characteristics, age at the time of testing, assessment tool, and testing duration. We cannot explain why single, but not multiple, exposed NHPs were more inhibited towards novel objects than controls, but we conclude that this was not attributable to combining non-threatening and threatening objects in our analysis, as separately analysing the inhibition scores for different object types gave the same results.
Behavioural inhibition represents a temperamental tendency that can be observed in toddlers in response to novel objects, people, or situations.57 Early studies suggested that behavioural inhibition indicated a proneness for general anxiety,58,59 and subsequent studies revealed that increased behavioural inhibition in early childhood could specifically predict social anxiety disorders.60, 61, 62, 63 Increased inhibition and anxiety-related behaviours in single-exposure NHPs and decreased affiliative behaviours in social settings in multiple-exposure animals may support the hypothesis that, in this animal model, isoflurane exposure increases the risk for behaviours resembling those in social anxiety in humans. Importantly, this phenotype of social-anxiety-like behaviour is based on different types of assessments consisting of observations of spontaneous behaviours and provoked response testing.
Our findings together with previous studies in NHPs11,12,14,16 suggest that exposure to volatile anaesthetics during infancy affects social and anxiety-related behaviours, but that the effects on cognitive functions are absent or rather small. These findings appear to be in line with most recent clinical studies addressing the effects of infant exposure to general anaesthetics. These clinical studies found small or no differences in IQ or in assessments of cognitive function, including academic achievement and teacher evaluations.4, 5, 6,64,65 A battery of cognitive tests in children, similar to those used in NHPs,14 revealed that anaesthesia exposure was not associated with deficits in cognitive domains.66 However, two recent clinical studies (MASK and PANDA) that compared exposed and unexposed study subjects suggest that anaesthesia exposures during infancy are associated with impairments in either executive function (MASK) or behaviours (PANDA) based on parental ratings.56 Impairments in executive functions were also found in parental ratings in a RCT comparing general vs spinal anaesthesia exposures during infancy (GAS trial).4 Of note, the median exposure times in these clinical studies varied between 45 and 80 min for single and 187 min for multiple exposures and were different from those in our study.
Our study has some limitations. The small number of NHPs used per group (seven or eight) may have limited our ability to detect small alterations in behavioural and cognitive assessments, and with an equal sex distribution in these small groups, we were unable to determine possible sex-dependent differences within treatment groups. It is important to address potential small alterations in behaviours, including those that are sex dependent, in future studies with a larger number of NHPs, as they become highly relevant when considering impact on child development and the large and increasing number of children exposed to anaesthesia.67 In our analysis of fine motor skills and visuospatial ability, we reduced the number of trials from 24 to 15 to obtain latency means from the same number of trials for every difficulty level. We also analysed the data by keeping 24 trials at all difficulty levels and giving maximum latencies for uncompleted trials, and by including all completed trials. These analyses revealed similar results and did not change the conclusions from our analysis based on 15 trials. Our statistical tests have been adjusted for multiple comparisons across the three animal groups (Ctr, 1×, and 3×). Whilst the comparison of multiple outcomes in behaviour and cognition in the same cohort of animals bears the potential risk of an increase in the experiment-wise Type I error rate, we did not adjust for multiplicity across families of tests because such adjustment (for independent and unrelated tests) would proceed at the expense of increasing false negatives, which constitutes a more serious problem.68 Our study is limited to behaviours and cognition that can be assessed in NHPs between 1 and 2 yr of age; whether anaesthesia exposure during infancy also affects neurobehavioural development at later stages was not investigated. In addition, whether our findings in NHPs exposed to isoflurane can be generalised to other anaesthetics remains unknown. However, the increased anxiety-related behaviours we found in the same NHPs at 116 and 2 yr after infant isoflurane exposure are consistent with the increase in these behaviours in NHPs after infant exposure to sevoflurane, reported in other studies.11,12 Importantly, we chose a length of 5 h for anaesthesia exposures because we had strong evidence that it causes robust structural changes in the brain of this animal model.7,10 However, we recognise that only few human neonatal procedures requiring anaesthesia extend to 5 h and even fewer would involve three episodes of such length. Whether the behavioural alterations we found can be reproduced with shorter and more clinically relevant exposures needs to be investigated.
Our approach of studying the effects of infant anaesthesia exposure in the NHP model also has several strengths. Compared with other available animal models, NHP brain development and complexity most closely resemble those in human brain. NHPs are highly social animals and exhibit a repertoire of behaviours of interest similar to those in humans, allowing us to study the effects of infant anaesthesia exposure on social behaviours in 2-yr-old juvenile NHPs. Importantly, we exposed the NHPs under conditions closely resembling those in the paediatric clinical setting, using continuous monitoring to maintain stable physiological conditions throughout the exposure. To our knowledge, this is the first study simultaneously investigating single and multiple exposures in the same animal cohort 2 yr after infant anaesthesia. In contrast to most human studies, our study design allowed evaluation of the effects of infant isoflurane exposure on social behaviour and cognition without potential confounding effects from concomitant surgery procedures or pre-existing medical conditions. Our findings may help reduce and refine subsequent animal studies in the area, specifically by focusing on the assessment of social behaviours after infant anaesthesia exposure.
Conclusions
Our study in 2-yr-old juvenile NHPs provides novel evidence for alterations in social behaviour assessed in the home environment after single and multiple isoflurane exposures during infancy. These NHPs model the developmental stage of children at 6–8 yr of age. Disturbances in social behaviour at this age could have serious long-term effects in children starting to attend school or other new environments, and could result in difficulties in adapting to new social settings. We found that neither single nor multiple 5 h exposures to isoflurane during infancy impaired the cognitive functions tested, but these exposures were associated with reduced close social behaviour. In addition, single exposed NHPs showed increased anxiety-related behaviours and inhibition towards novel objects. These findings in NHPs resemble the outcomes of most recent clinical studies that found no effects on general intelligence, but reported alterations in social behaviours in children exposed to anaesthesia during infancy.
Authors' contributions
Study design/planning: AMB, KC, MN, LDM, GAD
Study conduct: AMB, KC, MN, LDM, NR, AB, BG
Data analysis: AMB, VN, JFP-Z, KC, MN
Writing paper: AMB, VN, JFP-Z, KC, MN
Revising paper: all authors
Funding
International Anesthesia Research Society (Frontiers in Anesthesia Research Award 2012) to AMB; National Institutes of Health (P51OD011092) to the Oregon National Primate Research Center; International Anesthesia Research Society (Board of Trustees award) to AMB; Departments of Anesthesiology of Oregon Health & Science University and Columbia University to AMB; Emory University, the University of North Carolina, and NOUS Imaging, Inc. to DAF.
Declarations of interest
The authors declare that they have no conflicts of interest.
Acknowledgements
The authors thank Allison Heagerty for performing the statistical analysis of the novel object trial and Lisa A. Eicher for proofreading the manuscript.
Handling editor: Hugh C Hemmings Jr
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bja.2020.10.015.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Olsen E.A., Brambrink A.M. Anesthetic neurotoxicity in the newborn and infant. Curr Opin Anaesthesiol. 2013;26:535–542. doi: 10.1097/01.aco.0000433061.59939.b7. [DOI] [PubMed] [Google Scholar]
- 2.Disma N., O’Leary J.D., Loepke A.W. Anesthesia and the developing brain: a way forward for laboratory and clinical research. Paediatr Anaesth. 2018;28:758–763. doi: 10.1111/pan.13455. [DOI] [PubMed] [Google Scholar]
- 3.Flick R.P., Katusic S.K., Colligan R.C. Cognitive and behavioral outcomes after early exposure to anesthesia and surgery. Pediatrics. 2011;128:e1053–e1061. doi: 10.1542/peds.2011-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCann M.E., de Graaff J.C., Dorris L. Neurodevelopmental outcome at 5 years of age after general anaesthesia or awake-regional anaesthesia in infancy (GAS): an international, multicentre, randomised, controlled equivalence trial. Lancet. 2019;393:664–677. doi: 10.1016/S0140-6736(18)32485-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun L.S., Li G., Miller T.L. Association between a single general anesthesia exposure before age 36 months and neurocognitive outcomes in later childhood. JAMA. 2016;315:2312–2320. doi: 10.1001/jama.2016.6967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Warner D.O., Zaccariello M.J., Katusic S.K. Neuropsychological and behavioral outcomes after exposure of young children to procedures requiring general anesthesia: the Mayo Anesthesia Safety in Kids (MASK) study. Anesthesiology. 2018;129:89–105. doi: 10.1097/ALN.0000000000002232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brambrink A.M., Evers A.S., Avidan M.S. Ketamine-induced neuroapoptosis in the fetal and neonatal rhesus macaque brain. Anesthesiology. 2012;116:372–384. doi: 10.1097/ALN.0b013e318242b2cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Creeley C.E., Dikranian K.T., Dissen G.A., Back S.A., Olney J.W., Brambrink A.M. Isoflurane-induced apoptosis of neurons and oligodendrocytes in the fetal rhesus macaque brain. Anesthesiology. 2014;120:626–638. doi: 10.1097/ALN.0000000000000037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Noguchi K.K., Johnson S.A., Dissen G.A. Isoflurane exposure for three hours triggers apoptotic cell death in neonatal macaque brain. Br J Anaesth. 2017;119:524–531. doi: 10.1093/bja/aex123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schenning K.J., Noguchi K.K., Martin L.D. Isoflurane exposure leads to apoptosis of neurons and oligodendrocytes in 20- and 40-day old rhesus macaques. Neurotoxicol Teratol. 2017;60:63–68. doi: 10.1016/j.ntt.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raper J., Alvarado M.C., Murphy K.L., Baxter M.G. Multiple anesthetic exposure in infant monkeys alters emotional reactivity to an acute stressor. Anesthesiology. 2015;123:1084–1092. doi: 10.1097/ALN.0000000000000851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raper J., De Biasio J.C., Murphy K.L., Alvarado M.C., Baxter M.G. Persistent alteration in behavioural reactivity to a mild social stressor in rhesus monkeys repeatedly exposed to sevoflurane in infancy. Br J Anaesth. 2018;120:761–767. doi: 10.1016/j.bja.2018.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alvarado M.C., Murphy K.L., Baxter M.G. Visual recognition memory is impaired in rhesus monkeys repeatedly exposed to sevoflurane in infancy. Br J Anaesth. 2017;119:517–523. doi: 10.1093/bja/aew473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Talpos J.C., Chelonis J.J., Li M., Hanig J.P., Paule M.G. Early life exposure to extended general anesthesia with isoflurane and nitrous oxide reduces responsivity on a cognitive test battery in the nonhuman primate. Neurotoxicology. 2019;70:80–90. doi: 10.1016/j.neuro.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 15.Paule M.G., Li M., Allen R.R. Ketamine anesthesia during the first week of life can cause long-lasting cognitive deficits in rhesus monkeys. Neurotoxicol Teratol. 2011;33:220–230. doi: 10.1016/j.ntt.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coleman K., Robertson N.D., Dissen G.A. Isoflurane anesthesia has long-term consequences on motor and behavioral development in infant rhesus macaques. Anesthesiology. 2017;126:74–84. doi: 10.1097/ALN.0000000000001383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Committee for the update of the guide for the Care and use of laboratory animals. Guide for the Care and Use of laboratory animals. 8th Edn. National Academies Press; Washington, DC: 2011. [Google Scholar]
- 18.Brambrink A.M., Back S.A., Riddle A. Isoflurane-induced apoptosis of oligodendrocytes in the neonatal primate brain. Ann Neurol. 2012;72:525–535. doi: 10.1002/ana.23652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chudasama Y., Izquierdo A., Murray E.A. Distinct contributions of the amygdala and hippocampus to fear expression. Eur J Neurosci. 2009;30:2327–2337. doi: 10.1111/j.1460-9568.2009.07012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raper J., Wilson M., Sanchez M., Machado C.J., Bachevalier J. Pervasive alterations of emotional and neuroendocrine responses to an acute stressor after neonatal amygdala lesions in rhesus monkeys. Psychoneuroendocrinology. 2013;38:1021–1035. doi: 10.1016/j.psyneuen.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goldman-Rakic P.S. Development of cortical circuitry and cognitive function. Child Dev. 1987;58:601–622. [PubMed] [Google Scholar]
- 22.Packard M.G., Knowlton B.J. Learning and memory functions of the basal ganglia. Annu Rev Neurosci. 2002;25:563–593. doi: 10.1146/annurev.neuro.25.112701.142937. [DOI] [PubMed] [Google Scholar]
- 23.Diamond A., Goldman-Rakic P.S. Comparison of human infants and rhesus monkeys on Piaget’s AB task: evidence for dependence on dorsolateral prefrontal cortex. Exp Brain Res. 1989;74:24–40. doi: 10.1007/BF00248277. [DOI] [PubMed] [Google Scholar]
- 24.Bachevalier J., Landis L.S., Walker L.C. Aged monkeys exhibit behavioral deficits indicative of widespread cerebral dysfunction. Neurobiol Aging. 1991;12:99–111. doi: 10.1016/0197-4580(91)90048-o. [DOI] [PubMed] [Google Scholar]
- 25.Alexander G.E., Goldman P.S. Functional development of the dorsolateral prefrontal cortex: an analysis utilizing reversible cryogenic depression. Brain Res. 1978;143:233–249. doi: 10.1016/0006-8993(78)90566-8. [DOI] [PubMed] [Google Scholar]
- 26.Diamond A. Developmental time course in human infants and infant monkeys, and the neural bases of, inhibitory control in reaching. Ann N Y Acad Sci. 1990;608:637–669. doi: 10.1111/j.1749-6632.1990.tb48913.x. discussion 69–76. [DOI] [PubMed] [Google Scholar]
- 27.Kubota K. Learning of a hiding task and a delayed response task in infant rhesus monkeys. Neurosci Res. 1994;18:301–313. doi: 10.1016/0168-0102(94)90166-x. [DOI] [PubMed] [Google Scholar]
- 28.Zhou X., Zhu D., Qi X.L. Neural correlates of working memory development in adolescent primates. Nat Commun. 2016;7:13423. doi: 10.1038/ncomms13423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rose C., Luetjens C.M., Grote-Wessels S., Weinbauer G.F. Feasibility of repeated testing for learning ability in juvenile primates for pediatric safety assessment. Regul Toxicol Pharmacol. 2015;73:571–577. doi: 10.1016/j.yrtph.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 30.Makori N., Watson R.E., Hogrefe C.E., Lalayeva N., Oneda S. Object discrimination and reversal learning in infant and juvenile non-human primates in a non-clinical laboratory. J Med Primatol. 2013;42:147–157. doi: 10.1111/jmp.12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zelazo P.D. The dimensional change card sort (DCCS): a method of assessing executive function in children. Nat Protoc. 2006;1:297–301. doi: 10.1038/nprot.2006.46. [DOI] [PubMed] [Google Scholar]
- 32.Moore T.L., Killiany R.J., Herndon J.G., Rosene D.L., Moss M.B. A non-human primate test of abstraction and set shifting: an automated adaptation of the Wisconsin card sorting test. J Neurosci Methods. 2005;146:165–173. doi: 10.1016/j.jneumeth.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 33.Burbacher T.M., Grant K.S. Methods for studying nonhuman primates in neurobehavioral toxicology and teratology. Neurotoxicol Teratol. 2000;22:475–486. doi: 10.1016/s0892-0362(00)00073-8. [DOI] [PubMed] [Google Scholar]
- 34.Kalin N.H., Shelton S.E. Defensive behaviors in infant rhesus monkeys: environmental cues and neurochemical regulation. Science. 1989;243:1718–1721. doi: 10.1126/science.2564702. [DOI] [PubMed] [Google Scholar]
- 35.Coleman K., Pierre P.J. Assessing anxiety in nonhuman primates. ILAR J. 2014;55:333–346. doi: 10.1093/ilar/ilu019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Motulsky H.J., Brown R.E. Detecting outliers when fitting data with nonlinear regression—a new method based on robust nonlinear regression and the false discovery rate. BMC Bioinform. 2006;7:123. doi: 10.1186/1471-2105-7-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cohen J. Routledge Academic; New York, NY: 1988. Statistical power analysis for the behavioral sciences. [Google Scholar]
- 38.Akaike H. Factor analysis and AIC. Psychometrika. 1987;52:317–332. [Google Scholar]
- 39.R Development Core Team R. R Foundation for Statistical Computing; Vienna: 2011. A language and environment for statistical computing. [Google Scholar]
- 40.Zeamer A., Heuer E., Bachevalier J. Developmental trajectory of object recognition memory in infant rhesus macaques with and without neonatal hippocampal lesions. J Neurosci. 2010;30:9157–9165. doi: 10.1523/JNEUROSCI.0022-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hinde R.A., Spencer-Booth Y. The behaviour of socially living rhesus monkeys in their first two and a half years. Anim Behav. 1967;15:169–196. doi: 10.1016/s0003-3472(67)80029-0. [DOI] [PubMed] [Google Scholar]
- 42.Rubin K.H., Coplan R.J., Bowker J.C. Social withdrawal in childhood. Annu Rev Psychol. 2009;60:141–171. doi: 10.1146/annurev.psych.60.110707.163642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Crozier W.R., Alden L.E. John Wiley & Sons; New York, NY: 2005. Essentials of social anxiety for clinicians. [Google Scholar]
- 44.Harrist A.W., Zaia A.F., Bates J.E., Dodge K.A., Pettit G.S. Subtypes of social withdrawal in early childhood: sociometric status and social-cognitive differences across four years. Child Dev. 1997;68:278–294. [PubMed] [Google Scholar]
- 45.Strauss C.C., Forehand R., Smith K., Frame C.L. The association between social withdrawal and internalizing problems of children. J Abnorm Child Psychol. 1986;14:525–535. doi: 10.1007/BF01260521. [DOI] [PubMed] [Google Scholar]
- 46.Price J.S., Gardner R., Jr., Erickson M. Can depression, anxiety and somatization be understood as appeasement displays? J Affect Disord. 2004;79:1–11. doi: 10.1016/S0165-0327(02)00452-4. [DOI] [PubMed] [Google Scholar]
- 47.Trower P., Gilbert P. New theoretical conceptions of social anxiety and social phobia. Clin Psychol Rev. 1989;9:19–35. [Google Scholar]
- 48.Gilbert P. Evolution and social anxiety. The role of attraction, social competition, and social hierarchies. Psychiatr Clin North Am. 2001;24:723–751. doi: 10.1016/s0193-953x(05)70260-4. [DOI] [PubMed] [Google Scholar]
- 49.Fox A.S., Shelton S.E., Oakes T.R., Davidson R.J., Kalin N.H. Trait-like brain activity during adolescence predicts anxious temperament in primates. PLoS One. 2008;3 doi: 10.1371/journal.pone.0002570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fox A.S., Kalin N.H. A translational neuroscience approach to understanding the development of social anxiety disorder and its pathophysiology. Am J Psychiatry. 2014;171:1162–1173. doi: 10.1176/appi.ajp.2014.14040449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bliss-Moreau E., Moadab G., Bauman M.D., Amaral D.G. The impact of early amygdala damage on juvenile rhesus macaque social behavior. J Cogn Neurosci. 2013;25:2124–2140. doi: 10.1162/jocn_a_00483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Izquierdo A., Suda R.K., Murray E.A. Comparison of the effects of bilateral orbital prefrontal cortex lesions and amygdala lesions on emotional responses in rhesus monkeys. J Neurosci. 2005;25:8534–8542. doi: 10.1523/JNEUROSCI.1232-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kalin N.H., Shelton S.E., Davidson R.J. The role of the central nucleus of the amygdala in mediating fear and anxiety in the primate. J Neurosci. 2004;24:5506–5515. doi: 10.1523/JNEUROSCI.0292-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meunier M., Bachevalier J., Murray E.A., Malkova L., Mishkin M. Effects of aspiration versus neurotoxic lesions of the amygdala on emotional responses in monkeys. Eur J Neurosci. 1999;11:4403–4418. doi: 10.1046/j.1460-9568.1999.00854.x. [DOI] [PubMed] [Google Scholar]
- 55.Mineka S., Keir R., Price V. Fear of snakes in wild- and laboratory-reared rhesus monkeys (Macaca mulatta) Anim Learn Behav. 1980;8:653–663. [Google Scholar]
- 56.Machado C.J., Kazama A.M., Bachevalier J. Impact of amygdala, orbital frontal, or hippocampal lesions on threat avoidance and emotional reactivity in nonhuman primates. Emotion. 2009;9:147–163. doi: 10.1037/a0014539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang M.Q., Ji M.H., Zhao Q.S. Neurobehavioural abnormalities induced by repeated exposure of neonatal rats to sevoflurane can be aggravated by social isolation and enrichment deprivation initiated after exposure to the anaesthetic. Br J Anaesth. 2015;115:752–760. doi: 10.1093/bja/aev339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Biederman J., Rosenbaum J.F., Bolduc-Murphy E.A. A 3-year follow-up of children with and without behavioral inhibition. J Am Acad Child Adolesc Psychiatry. 1993;32:814–821. doi: 10.1097/00004583-199307000-00016. [DOI] [PubMed] [Google Scholar]
- 59.Biederman J., Rosenbaum J.F., Hirshfeld D.R. Psychiatric correlates of behavioral inhibition in young children of parents with and without psychiatric disorders. Arch Gen Psychiatry. 1990;47:21–26. doi: 10.1001/archpsyc.1990.01810130023004. [DOI] [PubMed] [Google Scholar]
- 60.Clauss J.A., Blackford J.U. Behavioral inhibition and risk for developing social anxiety disorder: a meta-analytic study. J Am Acad Child Adolesc Psychiatry. 2012;51:1066. doi: 10.1016/j.jaac.2012.08.002. –75.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hirshfeld-Becker D.R., Biederman J., Henin A. Behavioral inhibition in preschool children at risk is a specific predictor of middle childhood social anxiety: a five-year follow-up. J Dev Behav Pediatr. 2007;28:225–233. doi: 10.1097/01.DBP.0000268559.34463.d0. [DOI] [PubMed] [Google Scholar]
- 62.Schwartz C.E., Snidman N., Kagan J. Adolescent social anxiety as an outcome of inhibited temperament in childhood. J Am Acad Child Adolesc Psychiatry. 1999;38:1008–1015. doi: 10.1097/00004583-199908000-00017. [DOI] [PubMed] [Google Scholar]
- 63.Rosenbaum J.F., Biederman J., Bolduc-Murphy E.A. Behavioral inhibition in childhood: a risk factor for anxiety disorders. Harv Rev Psychiatry. 1993;1:2–16. doi: 10.3109/10673229309017052. [DOI] [PubMed] [Google Scholar]
- 64.O’Leary J.D., Janus M., Duku E. A population-based study evaluating the association between surgery in early life and child development at primary school entry. Anesthesiology. 2016;125:272–279. doi: 10.1097/ALN.0000000000001200. [DOI] [PubMed] [Google Scholar]
- 65.Ing C.H., DiMaggio C.J., Malacova E. Comparative analysis of outcome measures used in examining neurodevelopmental effects of early childhood anesthesia exposure. Anesthesiology. 2014;120:1319–1332. doi: 10.1097/ALN.0000000000000248. [DOI] [PubMed] [Google Scholar]
- 66.Warner D.O., Chelonis J.J., Paule M.G. Performance on the operant test battery in young children exposed to procedures requiring general anaesthesia: the MASK study. Br J Anaesth. 2019;122:470–479. doi: 10.1016/j.bja.2018.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sun L. Early childhood general anaesthesia exposure and neurocognitive development. Br J Anaesth. 2010;105:i61–i68. doi: 10.1093/bja/aeq302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fiedler K., Kutzner F., Krueger J.I. The long way from alpha-error control to validity proper: problems with a short-sighted false-positive debate. Perspect Psychol Sci. 2012;7:661–669. doi: 10.1177/1745691612462587. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.