Highlights
-
•
Huge angiomyofibroblastoma of the Vulva.
-
•
Differential diagnosis between vulvar angiomyofibroblastoma and vulvar aggressive angiomyxoma.
-
•
Comprehensive approach diagnosing and therapy of vulvar angiomyofibroblastoma.
Keywords: Angiomyofibroblastoma, Aggressive Angiomyxoma, Vulva tumour
Abstract
Mesenchymal tumours of the vulva are rare and consist of two types, difficult to distinguish but with different prognoses. Angiomyofibroblastoma (AMFB) is a benign tumour, whereas Aggressive Angiomyxoma (AA) is an infiltrating tumour. We describe a 22-year-old nulliparous patient with a vulvar mass sized 19 cm in diameter. After preoperative assessment by ultrasound, chest X-ray, and MRI, wide excision on the tumour was done and diagnosed as AMFB. Differentiation from AA is being discussed.
1. Introduction
Mesenchymal tumours are defined by expanded connective tissue tumour growth. In the mesenchymal tumours of the vulva, there are two rare types, angiomyofibroblastoma (AMFB) and aggressive angiomyxoma (AA). In daily practice, these two types of mesenchymal tumours of the vulva are very difficult to distinguish (Sultanoglu and Demirbakan, 2019, Wang et al., 2018). Thus, a comprehensive understanding of these two types of mesenchymal tumours is very important, especially to determine the therapeutic action taken including operation technique and follow-up. In this report, we describe an AMFB mimicking AA. We will discuss the differential diagnosis of AMFB and AA.
2. Case presentation
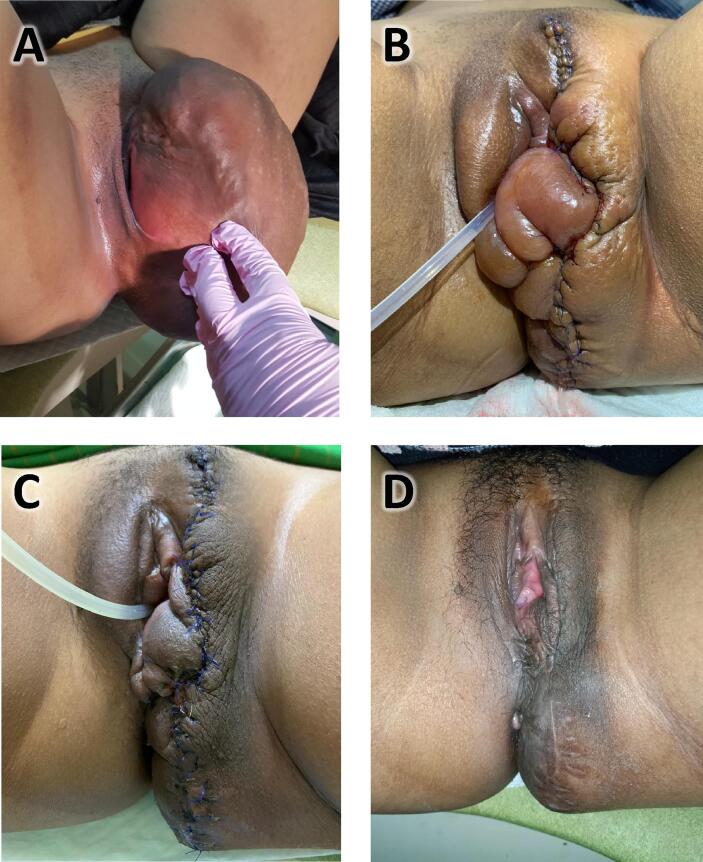
A 22-year-old nulliparous woman presented with a huge genital mass, 19 cm in diameter, having developed over 10 years period of time. The mass did not cause pain, nor bleeding, nor discharge. The menstrual cycle was normal. The patient nor her family had a history of malignancy nor similar conditions. On physical examination, her general condition was within normal limits. On gynecological examination there was a mass on the left vulva sized 19 × 14 × 10 cm with elastic to firm consistency without signs of inflammation (Fig. 1A). Visual inspection of the vagina was not possible, but on pelvic examination, the portio was difficult to reach, the vagina was smooth, and there were no signs of tumour infiltration into the vagina.
Fig. 1.
(A) Left vulvar mass sized 19 × 14 × 10 cm and not growing from the vagina; (B) After surgery and reconstruction of the vulva; (C) 1 month after surgery; (D) 6 month after surgery.
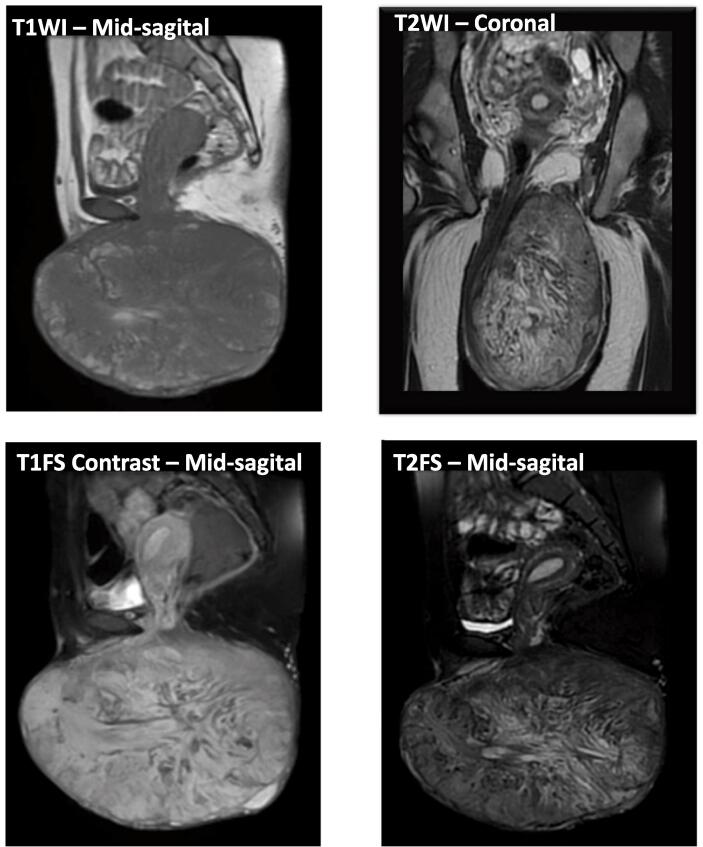
On ultrasound examination, the internal genitalia was within normal limits. A chest X-ray was equally within normal limits without signs of lung metastases. Pelvic Magnetic Resonance Imaging (MRI) revealed a mass with heterogeneous intensity and enhancement, sized 18.9 × 14.1 × 10.5 cm involving the left vulvo-perineal region without the involvement of external urethral orifice, vaginal wall, or rectum. No pelvic lymphadenopathy was found and the uterus, bladder, and rectum appeared to be normal (Fig. 2).
Fig. 2.
MR images show bulky mass sized 18.9 × 10.5 × 14.1 cm under the skin layer on left vulva and perineal region. There is no involvement of the external urethral orifice, vaginal, anal, and rectum. No infiltration into the pelvic cavity. No sign of pelvic lymph node enlargement. Hypointense with hyperintense foci on T1WI; hypo-hyperintense on T2WI; Strong heterogenous enhancement on T1FS contrast and lipid foci on T2FS.
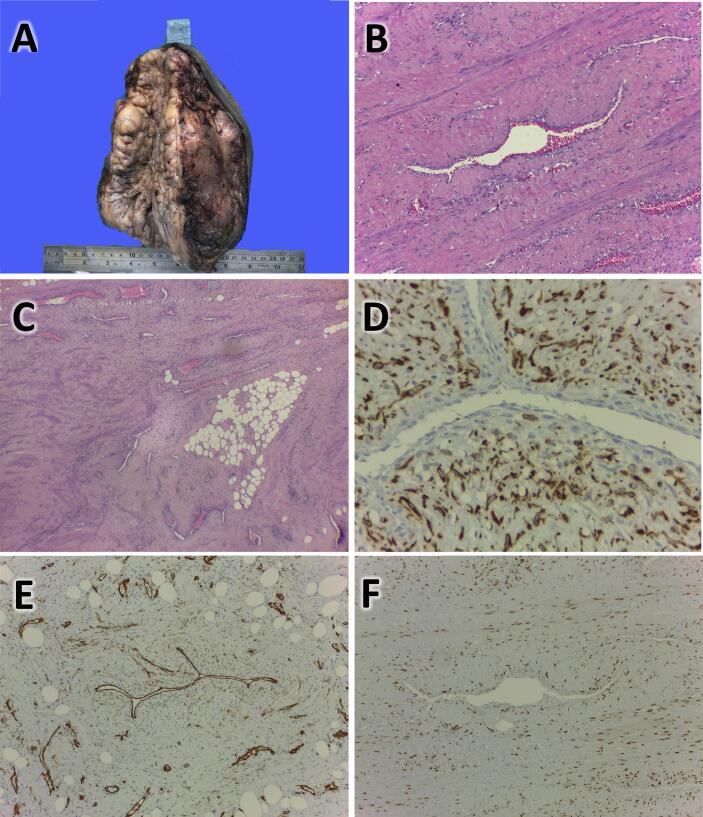
Wide tumour excision and vulvar reconstruction was performed by Division of Gynecologic Oncology only (Fig. 1, B–D). Histopathologic examination revealed a well differentiated tumour without rupture of its capsule. The tumour had thin-walled blood vessels surrounded by structurally arranged stromal cells. The stroma was arranged in an edematous to collagenous matrix with alternating zone cellularity. Stromal cells with an elongated nucleus, fine chromatin, eosinophilic cytoplasm, or mitosis were rare. The tumour contained fatty tissue (Fig. 3, A–C).
Fig. 3.
(A) Macroscopic appearance after surgery, bumpy white-greyish mass with size 19 × 14 × 10.5 cm; (B) Histopathologic finding: Abundant vessels with stromal backgrounds are seen, and increase cellularity around the vessels; (C) Histopathologic finding: Proliferation of vessels and lipomatous area; (D) Desmin Immunohistochemistry (IHC) is positive on cells surrounding the vessel (brown color); (E) CD34 IHC is positive on the vessels wall; (F) ER IHC is positive on nuclei of cells surrounding vessels (brown).
On the suspicion of AMFB, a immunohistochemical examination was done. On immunohistochemistry, staining for Smooth Muscle Actin (SMA), Estrogen Receptor (ER), and Progesterone Receptor (PR) was found to be positive. The tumour was partially positive for desmin, positive for Ki67 (<10% Nuclei) and positive for CD34 (Fig. 3, D–F). The patient was followed for the next two years and no signs or symptoms of recurrence were found.
3. Discussion
AMFB is a rare benign tumour, categorized as a mesenchymal tumour originating from especially the external female genital organs, such as the vulva or vagina (Eckhardt et al., 2018, Sultanoglu and Demirbakan, 2019, Wang et al., 2018). However, in several publications it has been suggested that AMFB might occur also in males in various areas like glans of penis, intravesically, and even inguinally, mimicking a hernia (Deka et al., 2017, Monib and Ibrahim, 2019). AMFB was first introduced in 1992 by Fletcher et al. who described the tumour as a slowly growing, painless connective tissue tumour with an average size of <5 cm, in patients with an average age of 45 years, and low rate of recurrence. Clinically, these tumour are often mistaken for Bartholin’s gland-enlargement, leiomyoma, inguinal hernia, or AA (Fletcher et al., 1992).
Among various diagnoses, AA resembles AMFB, but it has a much higher 2-year recurrence rate. With this prognosis it needs more aggressive therapy, which would be overtreatment for the benign AMFB (Table 1) (Wang et al., 2018, Fletcher et al., 1992, Nagai et al., 2010). AA was introduced first by Steeper and Rosai (1983). It was described as an infiltrating tumour with an average size of >5 cm, typically occurring at an even younger age than AMFB and with faster growth in weeks to months rather than years (Wang et al., 2018, Nagai et al., 2010, Steeper and Rosai, 1983, Brzezinska et al., 2018).
Table 1.
Differences of Angiomyofibroblastoma (AMFB), Aggressive Angiomyxoma (AA) and Current Case.
| AMFB | CASE | AA | |
|---|---|---|---|
| Clinical Findings | |||
| Diameter | 1–30 cm (mean: 4.7 cm) | 19 cm | >10 cm |
| MRI | |||
| Gd-T1 weighted | Strong heterogeneous enhancement | Strong heterogenous enhancement | Swirled intense pattern |
| Border of the mass | Well Circumscribed | Well Circumscribed | Infiltrative |
| Histopathology | |||
| Blood Vessel | Small capillary, Thin wall-walled blood vessels | Thin-walled blood vessels | Larger capillary, Thick-walled blood vessels |
| Stroma |
|
|
|
| Immunohistochemistry | |||
| Desmin | +/− | + | +/− |
| SMA | +/− | + | +/− |
| ER | + | + | + |
| PR | + | + | + |
| CD34 | +/− | + | +/− |
| Ki67 | +/− | + (<10% nuclei) | NA |
| Prognosis | |||
| Recurrence rate | Low recurrence rate | No Recurrence after 2 years follow up | High recurrence rate |
Gd-C: Gadolinium Chelate; SMA: Smooth Muscle Actin; ER: Estrogen Receptor; PR: Progesterone Receptor; NA: Not Available.
In this case, the mass was found on the vulva and sized 19 × 14 × 10 cm, has developed slowly over 10 years in a young patient (Fig. 1A). These clinical data are ambiguous, as the slow tumour growth would fit the diagnosis of AMFB, whereas the size of the tumour as well the age of the patient would be more in line with AA (Wang et al., 2018, Steeper and Rosai, 1983).
On MRI, AMFB is typically well circumscribed. AMFB is most often described as hypointense on T1 weighted (T1W) images, similar to that of skeletal muscle, hyperintense on T2 weighted (T2W) images, and with hyperenhancement on gadolinium chelate (Gd-C) enhanced images. Areas of hyperenhancement correspond to areas of hypercellularity and vascularity, with little collagenous stroma and water content. Intermediate intensity on T2W and less avid enhancement are thought to represent areas of intermediate cellularity and collagenous stroma. AMFB does not typically show infiltrative patterns but it can grow around structures. Conversely, if infiltration and swirled or layered patterns are observed, AA is much more likely than AMFB (Wang et al., 2018, Brzezinska et al., 2018, Shoji et al., 2017).
Our patient has a lipid-containing mass and heterogenous hyperenhancement (some part of the tumour did not show enhancement) with size 18.9 × 14.1 × 10.5 cm. The mass did not infiltrate nor did it show invasion into adjacent organs (urethra, vagina, anorectal) (Fig. 2). These findings are in concordance with the characteristic of AMFB, despite the considerable size of the tumour (Fletcher et al., 1992, Nagai et al., 2010).
Wide excision with free margin and vulvar reconstruction was done on our patient by Division of Gynecologic Oncology (Fig. 1B–D). Thus we intended to prevent recurrence in case of misdiagnosis (Wang et al., 2018). Further histopathology study on the tissue was made. The main microscopic characteristics of AMFB are small and thin-walled blood vessels, round to oval stromal cells, and edematous to collagenous matrix. While in AA the main characters are medium to big thick-walled blood vessels, stromal cells that are short spindle or stellate shaped, and a stroma rich in hyaluronic acid (Fletcher et al., 1992, Steeper and Rosai, 1983).
Patient's histopathologic examination tends to support the diagnosis of AMFB because of the thin-walled blood vessels, a stromal cell with an oval nucleus, and edematous to collagenous matrix was found (Fig. 3, B–C).
On immunohistochemistry, the result supports the diagnosis of AMFB as according to Wang et al., Desmin test might be positive in 1–5% of patients, while in that study the Ki67 was negative. However, Eckhardt et al. reported tumours to be positive for Desmin in 50–60% of cases, like our patient. Also, Phanindra et al. mention that the immunohistochemical pattern of both AMFB and AA may be very similar. Thus the diagnosis should consider the whole examination comprehensively starting from anamnesis, physical examination, imaging study, histopathology study, immunohistochemical study, to patient follow up of recurrence (Wang et al., 2018, Eckhardt et al., 2018, Deka et al., 2017).
4. Conclusion
Angiomyofibroblastoma and aggressive angiomyxoma are two rare mesenchymal benign tumours that are very similar. To differentiate, a comprehensive review of the clinical manifestations, imaging study, histopathology, and immunohistochemical pathologic examination is needed. The best therapeutic choice seems to be wide excision with free margins, even though AMFB only needs simple excision.
Informed consent
Written informed consent was obtained from the patient for anonymized publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in- Chief of this journal on request.
CRediT authorship contribution statement
Tricia Dewi Anggraeni: Conceptualization, Methodology, Investigation, Resources, Supervision. Laila Nuranna: Validation, Writing - review & editing. Muhammad Luthfiyanto: Writing - original draft, Visualization. Nuryati Chairani Siregar: Resources. Tantri Hellyanti: Resources. Trifonia Pingkan Siregar: Resources. Leonardo Alexandra: Visualization.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors thank Prof. dr. R.H.M Verheijen in Department of Gynecological Oncology, University Medical Center Utrecht, The Netherlands for advice in the writing of this manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gore.2021.100751.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- Brzezinska B.N., Clements A.E., Rath K.S., Reid G.C. A persistent mass: A case of aggressive Angiomyxoma of the vulva. Gynecol. Oncol. Rep. 2018;24:15–17. doi: 10.1016/j.gore.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deka P.M., Bagawade J.A., Deka P., Baruah R., Shah N. A rare case of intravesical angiomyofibroblastoma. Urology. 2017;106:e15–e18. doi: 10.1016/j.urology.2017.05.008. [DOI] [PubMed] [Google Scholar]
- Eckhardt S., Rolston R., Palmer S., Ozel B. Vaginal angiomyofibroblastoma: A case report and review of diagnostic imaging. Case Rep. Obstet. Gynecol. 2018;2018:1–8. doi: 10.1155/2018/7397121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher C.D.M., Tsang W.Y.W., Fisher C., Lee K.C., Chan J.K.C. Angiomyofibroblastoma of the vulva: A benign neoplasm distinct from aggressive angiomyxoma. Am. J. Surg. Pathol. 1992;16:373–382. doi: 10.1097/00000478-199204000-00006. [DOI] [PubMed] [Google Scholar]
- Monib S., Ibrahim M. Massive angiomyofibroblastoma of the glans penis. BMJ Case Rep. 2019;12:10–13. doi: 10.1136/bcr-2019-229486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai K., Aadachi K., Saito H. Huge pedunculated angiomyofibroblastoma of the vulva. Int. J. Clin. Oncol. 2010;15:201–205. doi: 10.1007/s10147-010-0026-0. [DOI] [PubMed] [Google Scholar]
- Shoji T., Takeshita R., Mukaida R., Sato T., Taguchi M., Sasou S. Angiomyofibroblastoma of the vulva diagnosed preoperatively: A case report. Mol. Clin. Oncol. 2017;7:407–411. doi: 10.3892/mco.2017.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steeper T.A., Rosai J. Aggressive angiomyxoma of the female pelvis and perineum. Report of nine cases of a distinctive type of gynecologic soft-tissue neoplasm. Am. J. Surg. Pathol. 1983;7:463–475. doi: 10.1097/00000478-198307000-00009. [DOI] [PubMed] [Google Scholar]
- Sultanoglu B., Demirbakan K. Angiomyofibroblastoma of the Vulva: A Case Report. World J Surg Surgical Res. 2019;2:1091. [Google Scholar]
- Wang Y., Zhang Y., Lv B., Feng Y. Large-sized pedunculated and polypoidal angiomyofibroblastoma of the vulva: A case report and literature review. J. Obstet. Gynaecol. Res. 2018;44:1492–1497. doi: 10.1111/jog.13692. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.