Abstract
Background
Many patients with acute low back pain (LBP) first seek care from primary care physicians. Evidence is lacking for interventions to prevent transition to chronic LBP in this setting. We aimed to test if implementation of a risk-stratified approach to care would result in lower rates of chronic LBP and improved self-reported disability.
Methods
We conducted a pragmatic, cluster randomized trial using 77 primary care clinics in four health care systems across the United States. Practices were randomly assigned to a stratified approach to care (intervention) or usual care (control). Using the STarTBack screening tool, adults with acute LBP were screened low, medium, and high-risk. Patients screened as high-risk were eligible. The intervention included electronic best practice alerts triggering referrals for psychologically informed physical therapy (PIPT). PIPT education was targeted to community clinics geographically close to intervention primary care clinics. Primary outcomes were transition to chronic LBP and self-reported disability at six months. Trial Registry: ClinicalTrials.gov NCT02647658
Findings
Between May 2016 and June 2018, 1207 patients from 38 intervention and 1093 from 37 control practices were followed. In the intervention arm, around 50% of patients were referred for physical therapy (36% for PIPT) compared to 30% in the control. At 6 months, 47% of patients reported transition to chronic LBP in the intervention arm (38 practices, n = 658) versus 51% of patients in the control arm (35 practices, n = 635; OR=0.83 95% CI 0.64, 1.09; p = 0.18). No differences in disability were detected (difference -2·1, 95% CI -4.9–0.6; p = 0.12). Opioids and imaging were prescribed in 22%–25% and 23%–26% of initial visits, for intervention and control, respectively. Twelve-month LBP utilization was similar in the two groups.
Interpretation
There were no differences detected in transition to chronic LBP among patients presenting with acute LBP using a stratified approach to care. Opioid and imaging prescribing rates were non-concordant with clinical guidelines.
Funding
Patient-Centered Outcomes Research Institute (PCORI) contract # PCS-1402–10867
Research in context.
Evidence before this study
Before undertaking this trial, we used the most comprehensive systematic review and practice guidelines for low back pain to date, issued in 2007 by the American College of Physicians and the American Pain Society in Annals of Internal Medicine, which reported insufficient data to support any specific approach to prevent the transition from acute to chronic LBP. Our own literature search did not identify any definitive prospective clinical trials comparing different patient-oriented interventions with enough long-term follow-up to determine effectiveness for the prevention of the transition from acute to chronic LBP.
Added value of this study
Given differences in United States versus European health care delivery, we assessed whether the stratified approach: (1) was feasible to implement in the US; (2) had the potential to prevent high-risk patients with acute LBP from transitioning to chronic LBP; and (3) reduced patient exposure to unnecessary, expensive, and potentially harmful tests (e.g., MRIs), medications (e.g., opioids), and procedures (e.g., epidural steroid injections).
Implications of all the available evidence
By evaluating the stratified approach to LBP in a large pragmatic RCT conducted in the United States, we assessed implementation as well as effectiveness in US primary care settings. Primary care physicians in the United States will be better able to judge the potential effectiveness of the stratified approach in reducing the risk of acute LBP leading to persistent disability due to chronic LBP.
Alt-text: Unlabelled box
1. Introduction
Internationally, low back pain (LBP) is one of the most prevalent, potentially disabling, and costly conditions for which people seek health care [1], [2], [3]. In the United States (US), up to 80% of adults will experience at least one episode of LBP in their lifetime [4,5] with close to one-quarter reporting an acute episode of LBP (e.g., an incident within previous month) [6]. In terms of cost, spinal pain represents the most costly health care condition in the US with estimates exceeding $134 billion [7]. Approximately 70% of these costs come from the vast majority of patients receiving non-surgical care [8].
A large proportion of patients experiencing acute LBP initially seek care from primary care physicians (PCPs) [9], [10], [11]. The prognosis of acute LBP is generally purported to be good [12]. Systematic reviews of studies in primary care settings [13] and general patient populations [14], however, indicate that on average, only 33% of patients seen in primary care settings recover in three months, approximately 65 percent of patients still experience pain after 12 months, and 33 percent have relapses of work absence. These findings suggest that the general attitude of uncomplicated, spontaneous recovery is overestimated.
Recent studies report equivocal results using a LBP-specific, stratified approach in primary care, which entails using a nine-item prognostic screening tool (i.e., the STarTBack tool) [15] to assess risk factors for disabling chronic LBP, coupled with matched intervention pathways that include combined physical and biobehavioral interventions. The approach has shown promise in European-based studies [16,17] but was unsuccessful in a one-site US-based study [18]. Subsequent qualitative analyses suggested the negative result was attributable to providers (i.e., physicians and physical therapists) failing to implement the recommended matched treatments [19].
The Targeted Interventions to Prevent Chronic Low Back Pain in High-Risk Patients (TARGET) Trial was a cluster randomized large pragmatic trial designed to comprehensively assess whether the stratified approach to LBP was effective in US-based PCP clinics. Specifically, we targeted high risk patients with aLBP and sought to test if the stratified approach, defined as Usual Care (UC) supplemented with referral to Psychologically Informed Physical Therapy (UC+PIPT), is superior to UC alone as demonstrated by lower rates of transition to chronic LBP, lower self-reported disability, and lower healthcare utilization.
2. Methods
2.1. Study design and participants
TARGET was a multi-site, pragmatic cluster randomized trial with 1:1 allocation ratio. The protocol is included in Supplement 1 and has been previously published [20]. Clusters were primary care clinics in five health care systems (University of Pittsburgh Medical Center [UPMC], Boston Medical Center [BMC], Johns Hopkins Medicine [JHM], Intermountain Health [IH], Medical University of South Carolina[MUSC]) with eligibility criteria of willingness to participate and presence of a minimal number (N ≥ 12) of networked, busy primary care clinics working with a common electronic medical record (EMR). There were no changes in clinic or patient eligibility criteria after the trial began. A trial steering committee oversaw the study. Approval of the study protocol by all site institutional research ethics boards specified: (1) for processes conducted at the primary care clinics, the study was viewed as a quality improvement initiative, (2) for the follow-up assessments at six months, the study was viewed as research requiring patient informed consent for collection of patient-reported primary outcomes, and (3) for the 12-month EMR review patient consent or waiver of HIPAA authorization and patient consent was required.
Sites had varying approaches to obtaining consent for the six-month survey follow-up and 12-month EMR review: (1) BMC obtained consent at the time of the six-month follow-up and EMR data for the 12 months after the index visit were extracted only for those who consented. (2) JHM required consent at the index visit for 6-month follow-up but had a HIPAA waiver and waiver of consent for the 12-month limited data set from the EMR for all patients screened as acute. (3) The University of Pittsburgh was the IRB of record for UPMC and IH. UPMC and IH required consent for six-month follow-up but had a waiver of HIPAA authorization and consent for the 12-month EMR review, with both sites obtaining consent for the six-month follow-up at the time of the follow-up. During the implementation phase of the study, full protocol implementation and data collection were not successful at MUSC, therefore, they were excluded from primary analysis.
All adult patients (18 years or older) who presented at a participating primary care clinic with a primary complaint of LBP were eligible for screening. Patients who endorsed chronic symptoms based on a two-item LBP Questionnaire derived from the NIH Chronic LBP task force were ineligible [21]. The questions included: 1) How long has low back pain interfered with your ability to do regular daily activities, and 2) How often has low back pain interfered with your ability to do regular daily activities? A response of “more than three months” to question one, and a response of “half the days or more than half the days” to question two defined chronic LBP. All remaining patients completed the nine-item STarTBack Tool, which produces two scores: an overall score and a distress subscale score, the latter of which focusses on five psychological obstacles to recovery (e.g., fear, anxiety, catastrophizing, etc.). Scoring four of five defined the high-risk group and coupled with a confirmatory primary encounter or ordering diagnosis of back pain in the EMR completed eligibility. Patients were excluded retrospectively if they presented with specific non-musculoskeletal causes of LBP (examples: cauda equina syndrome, cancer-related back pain, epidural abscess, or vertebral fracture). There were no changes to the eligibility criteria for patients after the trial began.
2.2. Interventions
For all participating primary care clinics, prior to recruitment we used educational outreach through grand rounds, staff meetings, and online modules to review acute LBP guidelines without monitoring or incentivizing adherence. Education was based on the most up to date guidelines [22] and included recommendations for a focused history and physical examination, with diagnostic imaging and testing not indicated unless signs of severe or progressive neurologic deficits or serious non-musculoskeletal conditions were present/suspected [20].
Clinics randomized to the intervention received enhanced usual care with specific instructions for immediate referral of high-risk patients to a stratified approach to care [15], [16], [17]. In all study locations except one (BMC), referrals occurred through an automatic best practice alert (BPA) in the EMR triggered by scores from the STartBack tool. For two study sites (UPMC and IH), the order was electronically linked to an in-network, centralized physical therapy service or to an administrator who followed up with the patient to schedule the physical therapy. At the remaining sites, the referral was either printed in the patient's after visit summary to be given to the physical therapist or a patient navigator followed up with the patient to schedule physical therapy.
In keeping with the pragmatic nature of the trial, additional resources used for implementation included: (1) monetary support for PCP staff time to detect patients eligible for screening (both groups); (2) EMR programming for tablets and web portals (baseline data input; both groups); (3) EMR programming support for detection of high-risk patients and initiation of BPA in the EMR (intervention group); and (4) patient navigator support (both groups, BMC site only).
Once intervention clinics were established, we identified common physical therapy referral sites and specifically targeted sites through letters from PCPs that included their desire to implement the stratified approach and strong encouragement to attend the courses. A training program for PIPT [23] was delivered to physical therapists who commonly receive referrals from the primary care clinics randomized to the intervention. More information on the training program has been published elsewhere [20,23]. Briefly, the course content provided an overview of the theoretical rationale and supporting data for the approach, specific management principles and skills, with demonstration and practice, and treatment monitoring components. The approach focused on educating patients about their condition, reducing fear of movement, and improving coping skills, as well as addressing physical impairments, such as mobility deficits and pain. In all, we trained 329 physical therapists, 77 in Pittsburgh, 61 in Boston, 80 in Salt Lake City and 111 in Baltimore.
Due to the pragmatic nature of the trial, standardization of the PIPT delivery was not closely monitored. However, we developed a PIPT checklist to be included with the PT referral that included key components of PIPT [20].
2.3. Randomization and masking
Primary care clinics were the unit of allocation instead of patients due to: (1) the overwhelmingly negative PCP stakeholder feedback about burden of individual randomization in busy primary care settings and (2) our concerns about cross contamination of the PIPT intervention if PCPs were referring both intervention and control patients to the same physical therapy clinic, especially when considering the open layout of many physical therapy practices and the proximity of therapists when treating patients. Primary care clinics were randomized 1:1 to UC+PIPT (intervention) or UC (control) using a stratified permuted block randomization with block size of four. A study biostatistician blinded to clinic names generated the allocation list in Rx64 3.3.2 . The unidentified clinics were stratified by site (health care system), then sorted by the number of annual back pain encounters and payer mix (i.e., percentage of patients covered by Medicaid or self-pay). Once sorted, the allocation list was applied and sent to the lead coordinator who added the clinic names, then sent the lists to their respective site coordinator. The site coordinators were responsible for communicating with the clinics, the training teams, and the information technology specialists to ensure the correct training and referrals were available. After randomization was implemented, any patient presenting with LBP as the primary reason for the primary care visit was eligible for screening. After allocation implementation, site personnel, clinical staff at the clinics, and the TARGET study team were not blinded to intervention arms. Staff conducting the 6-month follow-ups and personnel retrieving the electronic medical record data were masked to intervention arms. Patients were not blinded to the intervention.
2.4. Outcomes
The primary outcomes were patient-reported (1) transition to chronic LBP and (2) back-related disability at six months. We chose six months follow-up duration given the NIH Task Force definition, the six months duration immediately after the portal of entry would best capture the transition to chronic that could most plausibly be linked to the initial acute LBP visit [24]. Chronicity was determined through the patients’ answers to a simple two-item questionnaire adapted from the NIH Task Force on research standards for chronic LBP [21]. Functional disability outcome was measured with the Oswestry Disability Index (ODI) [25], which is a reliable, well-validated patient reported legacy instrument that assesses the functional impact of the back pain on the patient. The primary outcomes were obtained by the University Center for Social and Urban Research (UCSUR) at the University of Pittsburgh for patients at the UPMC, BMC and JHM health systems, while the research team at IH obtained 6-month follow-up data using well-established internal protocols. Six-month data collection processes were standardized and collected by one of three methods: (1) trained interviewers using a computer-assisted telephone interview (CATI) version of the questionnaires, (2) web-based responses via a link sent in email, and (3) return-mail responses to questionnaires mailed at 6 months to the participant's home address.
The secondary outcomes of the study extracted from the EMR were LBP-related processes of care and medical utilization over 12 months from the index visit as documented in the EMR. Process of care measures were defined as LBP-related referral to physical therapy (PT) or PIPT, referral to medical specialists, diagnostic imaging, and orders for opioid prescriptions and other LBP-related pain medications. Medical utilization outcomes were defined as outpatient visits (primary care and specialists), receipt of diagnostic imaging, interventional pain procedures (e.g., epidural injections), electrodiagnostic tests (e.g., nerve conduction velocity), surgeries, hospitalizations, and ED visits for back pain. Twelve-month all source utilization data were extracted directly from the EMRs for BMC, IH, and JHM systems, while 12-month utilization data for UPMC were extracted from the PCORnet [26].
Due to the pragmatic nature of the trial (i.e., a quality improvement comparison of two standards of care, UC or UC+PIPT), and the low-risk nature of the intervention, no adverse event data were collected.
2.5. Statistical analysis
We assumed a 6-month transition rate from acute to chronic of 25–30% [27,28] and a priori hypothesized that the acute to chronic transition rate at 6 months for patients seen at stratified care clinics would be reduced by 40% (i.e., 10–12 absolute percent) compared to those seen at usual care clinics. The originally proposed sample size of N = 60 clinics and 48 patients per clinic was based on 90% power, 12.5% cluster size variation and 10% non-response rate at 6 months (two-sided α = 0.05, intraclass correlation coefficient = 0.05) [27,[29], [30], [31]]. During the third year of the trial, the funder requested an assessment of feasible sample size due to slow recruitment and higher than expected non-response rates. The number of required clinics had been enrolled at that time. With 60 PCP clinics and fixed m = 10–14 patient completers for the 6-month assessment, we were projected to have over 80% power to detect the original 40% relative reduction. We therefore modified the targeted enrollment to an average of m = 31 high-risk patients per clinic (60*31 = 1860) in the QI phase of the study to account for the high variation in cluster sizes (assuming coefficient of variation = 0.65) and a 40% non-response rate at 6 months [32].
Analysis was by intention to treat where group assignment is based on the clinic of the patient's index visit. The unit of analysis was the patient. We estimated and compared the rates of transition to chronic LBP at six months between patients from the UC and UC+PIPT clinics using a generalized linear mixed model with a logit link controlling for site, a fixed effect for intervention, and a random clinic effect to account for clustering. For the second primary outcome, we estimated and compared the average back-related functional disability (Oswestry) at six months using linear mixed models controlling for baseline Oswestry and site as fixed effects and random clinic effects to account for the cluster randomized design. Corresponding 95% confidence intervals were calculated for each estimate of the intervention effect (odds ratios for six-month transition, mean difference for Oswestry). Analyses for the primary outcomes of transition to chronic LBP and disability were initially conducted only in those individuals with six-month survey data. We conducted sensitivity analysis using multiple imputation under ignorable and non-ignorable assumptions. We explored the effect of referral to a stratified approach to care by conducting an ‘as treated’ analysis comparing those patients who received a referral to PIPT, referral to PT, or received no PIPT/PT referral using the same models. We used generalized linear mixed models with logit link controlling for the cluster randomized trial design with random effects for clinics and fixed effect for site to compare all explanatory and secondary measures of processes of care and utilization over 12 months. We conducted all analyses using SAS version 9.4 (SAS Institute). All tests were two-sided α = 0.05.
2.6. Role of the funding source
The sponsor of the study had no role in the design, in the collection, analysis, and interpretation of data in the writing of the report and in the decision to submit the paper for publication. Also, the corresponding author had full access to all the data in the study and final responsibility for the decision to submit for publication.
3. Results
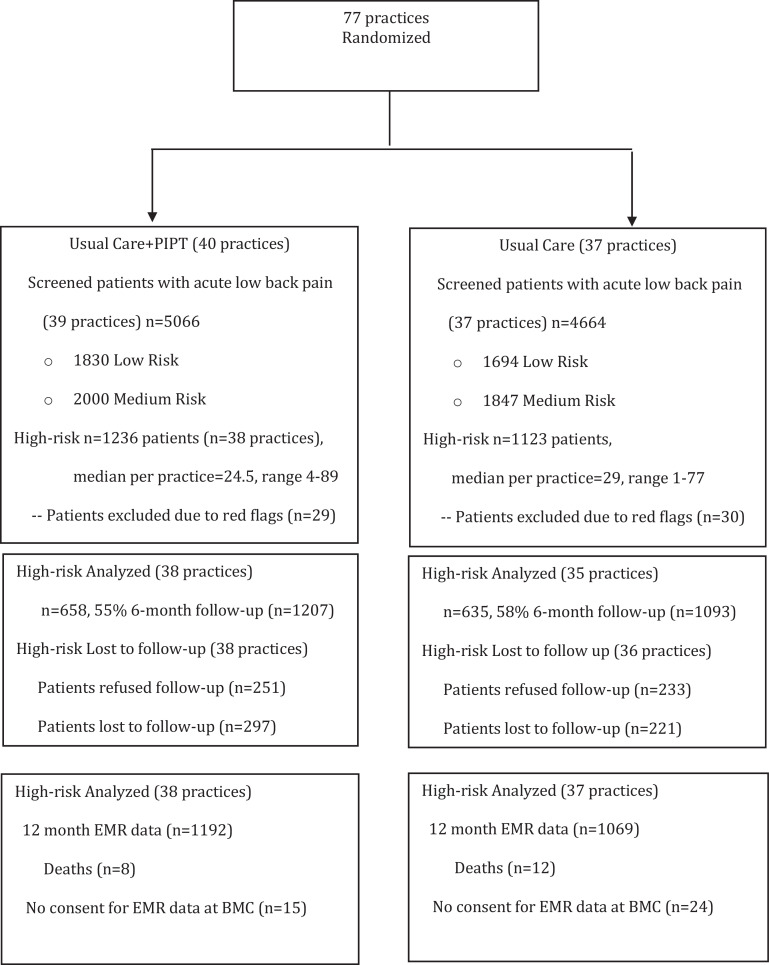
Seventy-seven clinics were randomized across four sites. Shortly after randomization and during the implementation phase for the UPMC sites, one clinic failed to successfully implement the approach and was eliminated prior to screening of patients. Patients were screened in 76 clinics from May 2016 until June 2018 with six-month follow-up ending in February 2019 and 12-month utilization outcomes retrieved through June 2019. Of interest, almost 50% of patients initially seen for LBP were already chronic, far exceeding epidemiological estimates for chronicity in PCP environments. Of the nearly 10,000 patients with acute LBP who were screened, 36% were classified as low risk, 40% as medium risk, and 24% as high-risk. For the high-risk group that were randomized, 55% had 6-month patient reported outcomes, of which we were able to capture 95–96% of 12-month healthcare utilization data in both intervention arms (Fig. 1).
Fig. 1.
Trial profile. PIPT=Psychologically Informed Physical Therapy. EMR=Electronic Medical Record. BMC=Boston Medical Center.
Volume of LBP in the past 12 months and payer mix were similar between clinics randomized to UC and UC+PIPT (Table 1). The baseline characteristics of the patients, including STarT Back total score and subscores, were also similar between intervention arms (Table 1).
Table 1.
Baseline characteristics by intervention group.
| Usual Care+PIPT (n = 1207) | Usual Care (n = 1093) | |
|---|---|---|
| Age (years) | 49·3 (16·2) | 50.6 (17.1) |
| Sex, female | 721 (60%) | 635 (58%) |
| Health Insurance | ||
| Private | 539 (45%) | 450 (41%) |
| Medicare | 212 (18%) | 208 (19%) |
| Medicaid (proxy for underserved) | 163 (14%) | 137 (13%) |
| Self-pay | 106 (9%) | 92 (8%) |
| Other | 52 (4%) | 58 (5%) |
| Missing/Unknown | 135 (11%) | 148 (14%) |
| Psychological Comorbidities | ||
| Depression | 51 (4%) | 34 (3%) |
| Anxiety | 66 (5%) | 36 (3%) |
| BMI, mean ± SD | 31·0 (7·3) | 31·0 (7·0) |
| n (%) with BMI data | 1067 (88%) | 954 (87%) |
| Obese | 516 (48%) | 476 (50%) |
| Smoking | 221 (18%) | 171 (16%) |
| Current smoker | 717 (59%) | 678 (62%) |
| Not current smoker | 269 (22%) | 244 (22%) |
| Missing/Unknown | ||
| Race | ||
| White | 949 (79) | 769 (70) |
| Black | 181 (15) | 224 (20) |
| Other | 25 (2) | 57 (5) |
| Missing/Unknown/Declined | 52 (4) | 43 (4) |
| Ethnicity | ||
| Hispanic | 75 (6) | 72 (7) |
| Non-Hispanic | 1087 (90) | 995 (91) |
| Missing/Unknown/Declined | 45 (4) | 26 (2) |
| Eligible Diagnosis | ||
| Axial back pain | 823 (68) | 737 (67) |
| Back pain and legs symptoms | 383 (32) | 356 (33) |
| Oswestry Disability Index | 48.0 (17·4) | 48.3 (17·5) |
| n (%) with ODI data | 1161 (96) | 1063 (97) |
| Chronic LBP Questionnaire Duration of LBP | ||
| Less than 1 month | 754 (62) | 644 (59) |
| 1 – 3 months | 342 (28) | 328 (30) |
| More than 3 months | 111 (9) | 121 (11) |
| STarT Back score (range 0–9) | 7·2 (1·1) | 7·2 (1·1) |
| STarT Back subscore (range 0–5) | 4·4 (0·5) | 4·3 (0·5) |
| Participating clinics | 38 | 37 |
| Regional site | ||
| UPMC | 17 | 16 |
| BMC | 6 | 6 |
| IH | 9 | 9 |
| JHM | 6 | 6 |
| Clinic volume LBP past 12 months | 535 (383, 690) | 485·0 (398·0, 832·0) |
| Clinic percent Medicaid and self-pay | 8.0 (6·6, 26·3) | 8.5 (6·7, 15·7) |
Data are mean (SD), median (p25, p75) or number (%). BMI=Body Mass Index. LBP=Low Back Pain. Depression includes any diagnosis of ICD-10 codes: F32.0, F32.1, F32.2, F32.4, F32.8, F32.89, F32.9, F33.0, F33.1, F33.2, F33.3, F33.4, F33.40, F33.41, F33.8, F33.9; Anxiety includes any diagnosis of ICD-10 codes: F06.4, F41.1, F41.3, F41.8, F41.9. STarT=Subgroups for Targeted Treatment. UPMC=University of Pittsburgh Medical Center. BMC=Boston Medical Center. IH=Intermountain Health. JHM=Johns Hopkins Medical.
At the index visit, 57% of patients in the intervention group received either a “general” PT or a specific PIPT order (Table 2). About 30% received a referral to PT in the usual care group. Very few patients were referred to a specialist in either group with 5% being referred to non-surgical medical specialists and 5%-6% to spine surgeons. Non-surgical medical specialists include physical medicine & rehabilitation, anesthesiology, primary care sports medicine, or pain medicine. About one in six received a referral for x-ray and only 6–7% for magnetic resonance/computerized tomography (MR/CT). With respect to medication, 22–25% of patients received a prescription for opioids, around 36–44% muscle relaxants, and 30% NSAIDs. Other medication prescriptions were rare (acetaminophen, benzodiazepines).
Table 2.
Processes of Care at the Index Visit up to 21 Days.
| Usual Care + PIPT (n = 1207) % (95% CI) | Usual Care (n = 1093) % (95% CI) | Odds Ratio (95% CI) | p-value | |
|---|---|---|---|---|
| PIPT Checklist in medical | ||||
| record | 7.8 (5.4, 10.4) | 0.0 (0.0, 0.0) | – | – |
| Referral to PIPT or PT | 56·8 (49·9, 63·5) | 29·3 (23·5, 35·8) | – | – |
| Referral to PIPT | 35.9 (26.2, 46.8) | 0.4 (0.2, 1.2) | – | – |
| Referral to Specialist | ||||
| Complementary and | 1.8 (0.9, 3.3) | 1.1 (0.5, 2.3) | 1.60 (0.59, 4.20) | 0.36 |
| Integrative Health | 1.2 (0.6, 2.1) | 0.9 (0.4, 1.8) | 1.34 (0.53, 3.42) | 0.53 |
| Behavioral Health/ | 5.0 (3.8, 6.6) | 5.0 (3.7, 6.6) | 1.00(0.66, 1.52) | 0.99 |
| Counseling$ | 5.0 (3.6, 7.0) | 6.4 (4.6, 8.9) | 0.76 (0.47, 1.25) | 0.28 |
| Non-Surgical Medical | ||||
| Specialist | ||||
| Spine Surgery | ||||
| Diagnostic Imaging Order | ||||
| X-ray MR/CT | 15.0 (11.5, 19.2) 7.2 (5.3, 9.7) | 18.1 (14.1, 23.0) 6.1 (4.4, 8.5) | 0.80 (0.53, 1.21) 1.19 (0.74, 1.91) | 0.28 0.47 |
| Opioid Prescription | 22.3 (18.3, 26.9) | 24.5 (20.1, 29.5) | 0.89 (0.62, 1.26) | 0.50 |
| Other Prescriptions | ||||
| Acetaminophen | 1.0 (0.44, 2.1) | 1.6 (0.8, 3.4) | 0.57 (0.20, 1.64) | 0.29 |
| Benzodiazepines | 2.5 (1.6, 3.8) | 2.6 (1.7, 4.1) | 0.95 (0.51, 1.79) | 0.87 |
| Muscle relaxants | 44.0 (39.3, 48.7) | 36.2 (31.6, 41.0) | 1.38 (1.05, 1.83) | 0.024 |
| NSAIDs | 29.0 (25.3, 32.9) | 31.2 (27.3, 35.5) | 0.90 (0.69, 1.17) | 0.41 |
PIPT=Psychologically informed physical therapy. PT=Physical therapy. MR=Magnetic resonance. CT=computerized tomography. NSAIDs=non-steroidal anti-inflammatory including topical.
All proportions, odds ratios and confidence intervals estimated using generalized linear mixed models with random effects for clinic adjusted for site.
did no adjust for site due to convergence issues
The six-month follow-up non-response rate (44%) did not differ between the intervention and usual care group but was higher than anticipated. Those with completed follow-up were younger and were slightly more likely to be women (61% versus 56%), have private health insurance (50% versus 44% among those with information), be obese (51% versus 46%) and white (77% versus 71%). There were no differences detected on the baseline ODI or STarTBack between those with and without six-month follow-up data and those with follow-up data were slightly less likely to report back pain duration < one month (58% versus 64%).
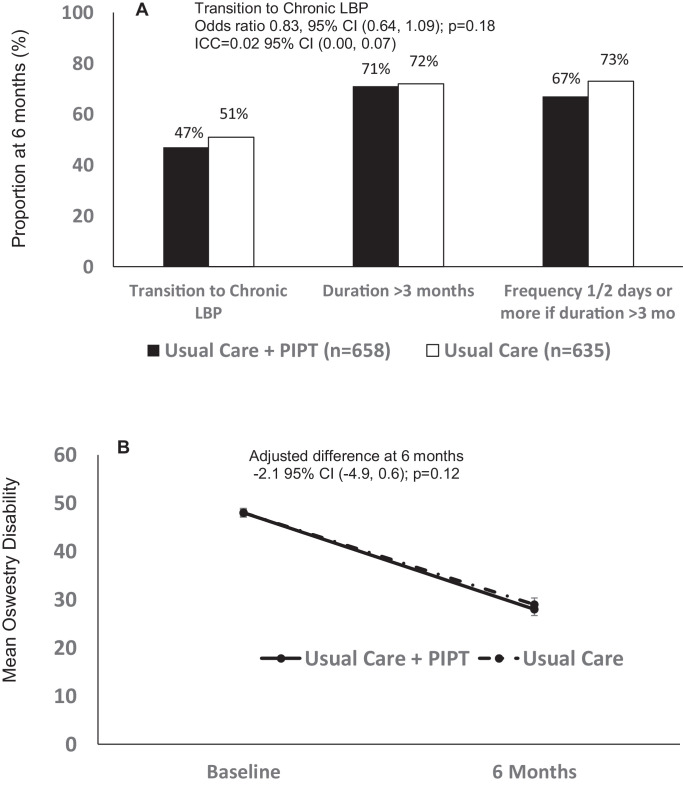
About half of patients in both groups reported transition to chronic LBP at 6 months (Fig. 2A). Just over 70% stated that LBP had interfered with their ability to do regular daily activities for more than 3 months. Among them about 70% responded the frequency of LBP interference was half or more than half the days in the previous six months. Both groups were lower on functional disability showing scores indicative of moderate disability rather than severe disability, which was the average at baseline (Fig. 2B). In the exploratory “as treated” analysis, rates of chronic LBP at 6 months for patients who received referral to PIPT, referral to PT, or no referral to either were 51%, 48%, and 48%, respectively, p = 0.92. The results and inference did not change using multiple imputation assuming missingness at random. Imputation under non-ignorable missingness showed no group differences except under extreme, highly unlikely scenarios where the proportion that transitioned to chronic among non-responders was double in the intervention compared to the control.
Fig. 2.
Primary outcomes of the TARGET Trial at 6 months. (A) Transition to chronic low back pain (B) Change in Oswestry Disability Index represented by mean ± standard error.
Up to 12 months after the index visit, less than 10% of patients received additional referral to PIPT or PT for LBP from any physician type (Table 3). Very few patients received referral to non-surgical or surgical specialists (2–4%) and roughly 7–8% received an opioid prescription, 6–8% a prescription for muscle relaxants, and 5–8% a prescription for NSAIDs (Table 3).
Table 3.
Secondary outcomes of processes of care and health care utilization over 12 months.
| Usual Care+PIPT (n = 1192) % (95% CI) | Usual Care (n = 1069) % (95% CI) | Odds Ratio (95% CI) | p-value | |
|---|---|---|---|---|
| Process of Care after Index Visit up to 12 Months | ||||
| Referral to PIPT or PT | 8.1 (6.4, 10.2) | 7.0 (5.4, 9.1) | 1.17 (0.81, 1.70) | 0.40 |
| Referral to PIPT | 3.4 (1.9, 4.8) | 0.0 (0.0, 0.0) | — | — |
| Referral to Specialist | ||||
| Complementary and | 0.4 (0.2, 1.02) | 0.6 (0.2, 1.3) | 0.75 (0.2, 0.46) | 0.63 |
| Integrative Health | 0.5 (0.2, 1.1) | 0.9 (0.4, 1.8) | 0.55 (0.17, 1.76) | 0.31 |
| Behavioral Health/ | 3.3 (2.4, 4.7) | 1.9 (1.3, 3.0) | 1.75 (1.02, 3.01) | 0.0425 |
| Counseling$ | 3·2 (2·1, 4·9) | 3·8 (2·5, 5·7) | 0·84 (0·46, 1·54) | 0·57 |
| Non-Surgical Medical | ||||
| Specialist | ||||
| Spine Surgery | ||||
| Diagnostic Imaging Order | ||||
| X-ray | 2.5 (1.7, 3.8) | 2.5 (1.7, 3.9) | 0.99 (0.60, 1.65) | 0.98 |
| MR/CT$ | 2.9 (1.9, 4.3) | 3.4 (2.3, 5.1) | 0.83 (0.46, 1.50) | 0.54 |
| Opioid Prescription | 7.0 (5.3, 9.1) | 7.5 (5.7, 9.9) | 0.92 (0.61, 1.40) | 0.69 |
| Other Prescriptions | ||||
| Acetaminophen$ | 0.4 (0.2, 1.1) | 0.7 (0.3, 1.6) | 0.58 (0.16, 2.12) | 0.41 |
| Benzodiazepines$ | 1·4 (0·8, 2·6) | 1·8 (1·0, 3·2) | 0·80 (0·55, 1·16) | 0·58 |
| Muscle relaxants | 6.7 (5.2, 8.5) | 8.2 (6.4, 10.4) | 0.78 (0.54, 1.12) | 0.23 |
| NSAIDs | 5.6 (4.1, 7.6) | 7.7 (5.7, 10.2) | 0.72 (0.46, 1.12) | 0.14 |
| 12 Month Utilization | ||||
| Primary Care Visit | 46.7 (42.3, 51.0) | 49.5 (45.0, 54.1) | 0.89 (0.69, 1.15) | 0.37 |
| Specialist Visits (Any) | 14.6 (11.9, 17.6) | 17.2 (14.02, 20.8) | 0.82 (0.59, 1.13) | 0.22 |
| 3.3 (2.2, 5.2) | 2.9 (1.8, 4.7) | 1.15 (0.59, 2.23) | 0.68 | |
| Orthopedic Surgery$ | 0.2 (0.04, 0.7) | 0.6 (0.2, 1.5) | 0.27 (0.05, 1.53) | 0.14 |
| Neurosurgery$ | 1.8 (1.0, 3.3) | 1.3 (0.6, 2.6) | 1.37 (0.53, 3.55) | 0.51 |
| Physical Medicine & | 1.9 (1.1, 3.2) | 2.4 (1.4, 4.1) | 0.79 (0.36, 1.75) | 0.56 |
| Rehabilitation$ | 7.7 (5.8, 10.1) | 11.2 (8.7, 14.4) | 0.66 (0.44, 0.99) | 0.044 |
| Anesthesiology/Pain | ||||
| Management$ | ||||
| Other | ||||
| Imaging (Any) | 15.3 (12.4, 18.7) | 17.1 (13.8, 20.9) | 0.88 (0.62, 1.24) | 0.45 |
| X-ray | 8.9 (6.7, 11.6) | 9.5 (7.1, 12.5) | 0.93 (0.61,1.44) | 0.75 |
| CT/MRI | 8.3 (6.6, 10.5) | 9.7 (7.6, 12.2) | 0.85 (0.61, 1.19) | 0.33 |
| Advanced studies# | 0.08 (0, 0.25) | 0 (–,–) | – | – |
| Interventional Pain Procedures$ | 2.3 (1.4, 3.7) | 3.3 (2.1, 5.1) | 0.70 (0.36, 1.38) | 0.30 |
| Electrodiagnostic Testing$ | 0.2 (0.1, 0.8) | 0.3 (0.1, 1.0) | 0.65 (0.11, 3.88) | 0.63 |
| Surgery (Any) | 1.7 (1.0, 2.8) | 1.2 (0.7, 2.2) | 1.37 (0.74, 2.53 | 0.31 |
| Fusion | 1.5 (0.0, 32.6) | 1.2 (0.0, 35.4) | 1.24 (0.02, 66.7) | 0.62 |
| Non-fusion# | 0·08 (0, 0·25) | 0·09 (0, 0·27) | — | – |
| Red flag# | 0.08 (0, 0.25) | 0 (–,–) | — | – |
| Other# | 0.17 (0, 0.40) | 0 (–,–) | — | – |
| Hospitalizations# | 0.17 (0, 0.41) | 0.19 (0, 0.44) | — | – |
| ED visits | 5.2 (3.7, 7.2) | 7.0 (5.1, 9.5) | 0.74 (0.45, 1.19) | 0.21 |
PIPT=Psychologically informed physical therapy. PT=Physical therapy. MR=Magnetic resonance. CT=computerized tomography. NSAIDs=non-steroidal anti-inflammatory including topical. ED=Emergency department. All proportions, odds ratios, and confidence intervals are estimated from generalized linear mixed models with random effect for clinic adjusting for site.
did not adjust for site due to convergence issues from small cell counts
estimates using survey sampling approach to adjust for clustering
4. Discussion
The stratified management approach in this pragmatic trial consisted of identifying high-risk patients using prognostic screening and coupling it with immediate referral for PIPT treatment. This intervention did not result in decreasing the rate of transitioning from acute to chronic LBP. Stratified care also failed to demonstrate effectiveness in self-reported disability, nor did it make a difference in process-based measures of utilization derived from the EMR. Ultimately, approximately half of high-risk patients reported transition to chronic LBP at six months. The “as treated” analyses demonstrated very similar results, with 51% and 48% of patients transitioning to chronic in the intervention and usual care groups, respectively.
Almost half the entire sample transitioned to chronic LBP, a figure that far exceeds most previous reports in primary care. We used the NIH Consensus Panel [21] definition of chronic LBP, which is new to the literature, thus difficult to gauge comparatively. However, the definition was created through a comprehensive consensus process with the goal of addressing previous gaps in literature, namely, that definitions used for chronic LBP were highly variable.
Patients with LBP commonly enter the health care system through primary care settings. Clinical guidelines for initial uncomplicated LBP management in primary care consistently include avoiding diagnostic imaging and minimizing opioid prescribing [33]. Systematic reviews of primary care management reflect low adherence to these guidelines [34,35]. Similarly, in our study we found lack of full adherence to clinical guidelines: one in four acute, high-risk patients received opioid prescriptions at the index visit. One in five patients received orders for diagnostic imaging.
Despite creating an automated process of identifying high-risk patients and generating referrals for matching PIPT, only half received a referral to PIPT in the stratified group compared to a third of patients receiving a referral to PT in the usual care group. Adherence to initiating PIPT referrals was far below expectations of the study team as well as our PCP stakeholder groups. We engaged with key stakeholders prior to and during the study, which included key participating primary care physicians and representative national primary care professional groups. The implementation strategies executed were mutually agreed upon prior to the study and included a twofold process: (1) automatically generating a BPA in the EMR for the medical assistants and/or PCPs for all high-risk patients, with specific instructions for immediate referral to PIPT, and (2) once the referrals were executed and processed by the PCP, expedited mechanisms to notify participating PT clinics.
The implementation shortfall in the BPA-PIPT referral linkage was a major limitation in the TARGET Trial and demonstrates significant challenges that remain in present primary care environments. Though the BPA approach was favored by PCP stakeholders, subsequent discussion with PCPs along with more recent reports describe “alert fatigue,” which is blamed for the high override rates that hamper decision-support systems [36], suggesting that BPA effectiveness may be overestimated. Causes of alert fatigue, which include cognitive overload from very busy EMR environments and desensitization from too many alerts, have been proposed. Regardless of the cause, overestimating the BPA effect may serve to decrease expectations, as evidenced by the interpretation of our result from our PCP stakeholders, characterized by the quote, “a 60% adherence to a BPA is ‘pretty good.’” Though we were able to almost double the rate of PT referral in the stratified approach over usual care, we still missed 40% of the high-risk cohort. There is existing evidence indicating this issue is not simply a reflection of participating TARGET trial sites. An analysis of 2.5 million individuals in the US with newly diagnosed LBP indicated that 55% with new episode of LBP received no treatment, and about 33% received care that was inconsistent with clinical guidelines [8].
Funding guidelines prohibiting funds to pay for physical therapy treatments led the study team to balance the pragmatic-explanatory continuum more toward pragmatic with regard to flexibility adherence and delivery of the intervention [37]. Our approach to PIPT delivery, though highly generalizable, was without additional resources to assure clinical implementation, particularly when considering the barriers in PIPT delivery. With more recent guidelines advocating for “non-pharmacologic-first” approaches to pain [38], it remains to be seen if payers will explore payment reform that includes reducing barriers to non-pharmacologic care such as physical therapy (whether it be psychologically informed or not).
One major limitation of this study was the limited treatment fidelity reflected in the low rate of PIPT referrals. We attempted to implement a new protocol in already busy primary care clinics. Despite its simplicity, the protocol required changes in workflow and staff commitment. Clearly, overcoming implementation barriers would be critical before another attempt at a PCP-based pragmatic trial of the stratified approach to LBP. A second major limitation was the 40% non-response of the primary outcome at 6 months. Standards for non-response rates have not been established for pragmatic trials, which often use different follow up procedures than explanatory trials [39]. In the TARGET trial “best practices” for capturing patient reported outcomes in pragmatic trials were incorporated, including options for phone, web-based, and mail follow up capture [40]. Even with this 40% non-response rate, there were similar rates between treatment arms and we captured follow-up on over 500 patients per treatment arm, which limits measurement error when modeled across different imputation approaches [41]. Indeed, in our own imputation models the primary analyses were robust to several different approaches and scenarios. Data on our secondary outcomes were much more complete as we were able to ascertain process of care measures and utilization in the EMR for over 90 percent of our sample. One limitation to these measures, however, is that they only capture healthcare use within the healthcare system. Any care sought outside the system would be missed. Additional analyses of our data will supplement the EMR data with claims data which may provide additional information for comparisons.
When considering LBP in general, numerous trials and RCTs have been performed so far, comparing dozens of interventions, including stratified approaches. These research findings have been synthesized for more than 25 years into LBP practice guidelines. Given the rate of non-concordance with LBP practice guidelines, there remains doubt as to whether available evidence can be effectively translated into everyday practice [8]. Pragmatic trials, such as TARGET, that combine research rigor with treatment delivery in real world settings will often clearly reveal this harsh reality [42]. Though disappointing, the results of the TARGET trial illustrate the implementation challenge in translating evidence to practice [43]. Some may respond to a negative pragmatic trial by calling for a more explanatory trial whereby treatment fidelity is emphasized and more thoroughly assessed. This may be necessary to isolate PIPT efficacy; however, we cannot lose sight of the fact that additional explanatory trials will not address the implementation challenges that come with establishing effectiveness in everyday practice settings.
Future work should focus on solving implementation challenges, perhaps by considering hybrid designs (e.g., Type III Implementation/Effectiveness), which would allow testing the feasibility and acceptance of more active approaches to implementing stratified care. Implementation strategies that could be compared include: (1) EMR “forced function” strategies (e.g., administrative approaches that automate choices or prevent further steps if a BPA is not considered) whereby selected patients with LBP are almost immediately directed to alternative care providers that can provide immediate access to evidence-based, non-pharmacologic interventions; (2) aligned payment models (e.g., shared savings) whereby evidence-based care is incentivized and non-guideline-concordant care (e.g., x-rays, opioid prescriptions) are dis-incentivized similar to other chronic conditions (e.g., diabetes or hypertension); (3) primary care co-management models with Advanced Practice Providers (nurse practitioners, physicians assistants) to support PCPs by using protocolized approaches such as stratified care for LBP; (4) integrated models of care where other health professionals (e.g., physiotherapists, behavioral health professionals) with specific training in musculoskeletal/mental health approaches are co-located in the PCP environment; (5) models of care that promote alternatives to primary care as the portal of entry for people with a primary complaint of LBP.
In a large pragmatic trial in acute LBP, we did not find reduction in transition to chronic LBP using a stratified approach to care. Overall, US primary care practices involved with this trial had difficulty implementing the stratified approach targeting high-risk patients in the intervention arm. In both treatment arms, opioid prescribing and diagnostic imaging rates were not concordant with clinical guidelines for acute LBP. Future investigations of the stratified approach should focus on the challenges of implementing the referral in primary care and in the delivery of appropriate care by providers such as physical therapists.
Funding
The trial was funded by the Patient Centered Outcomes research Institute (PCORI) contract number PCS-1402–10,867.
Contributors
AD and RS conceived the study and are the principal investigators. All authors contributed to the design and implementation of the study. JMS, SSK and JAF coordinated all data ascertainment. CGP was responsible for the statistical analysis. SZG, MJS, CMG, STW, and JMB provided expertise in psychologically informed physical therapy, and reviewed and revised earlier drafts of the manuscript.
JAF, JKF, GAS, ADW, GPB, STH, KIM, STW, and PLE contributed to the acquisition, analysis, and interpretation of the data. AD, CGP, and RS drafted the manuscript. All authors critically reviewed and revised the manuscript and have read and approved the final version.
TARGET trial investigators
Principal Investigators: Anthony Delitto, Robert B. Saper
Co-Investigators: Charity G. Patterson, Joel M. Stevans, Jennifer A. Freel, Janet K. Freburger, Samannaaz S. Khoja, Gwendolyn A. Sowa, Ajay D. Wasan, Carol M. Greco, Michael J. Schneider, Gerard P. Brennan, Stephen T. Hunter, Kate I. Minnick, Steven T. Wegener, Patti L. Ephraim, Steven Z. George, Jason M. Beneciuk, David C. Morrisette
Trial support: PCORI contract # PCS-1402–10,867
Trial Steering Committee: Delitto, Saper, Patterson, Brennan, Wegener, George, Beneciuk
Declaration of Competing Interest
Dr. Delitto reports grants from PCORI, grants from NIH, grants from DOD, outside the submitted work; Dr. Patterson reports grants from Patient Centered Outcomes Research Institute, during the conduct of the study; Dr. Stevans reports grants from PCORI, during the conduct of the study; Dr. Khoja reports grants from PCORI, during the conduct of the study; Dr. Freel has nothing to disclose; Dr. Schneider reports grants from Patient Centered Outcomes Research Institute, during the conduct of the study; Dr. Greco reports grants from Patient-Centered Outcomes Research Institute, during the conduct of the study; Dr. Sowa reports grants from PCORI, during the conduct of the study; grants from NIH, USAMRMC, VA, The Pittsburgh Foundation, outside the submitted work; Dr. Brennan reports other institutional support from Patient Centered Outcomes Research Institute (PCORI), during the conduct of the study; other institutional support from Patient Centered Outcomes Research Institute (PCORI), outside the submitted work; Stephen J. Hunter has nothing to disclose; Dr. Minick has nothing to disclose; Dr. Wasan has nothing to disclose; Dr. Wegener reports grants from PCORI, during the conduct of the study; Ms. Ephraim has nothing to disclose; Dr. Freburger reports other from American Physical Therapy Association, outside the submitted work; Dr. Beneciuk reports grants from Patient-Centered Outcomes Research Institute, during the conduct of the study; Dr. George reports personal fees from Rehab Essentials, Inc, personal fees from Med Risk, LLC, grants from NIH, other from Duke University, all outside the submitted work; Dr. Saper has nothing to disclose.
Acknowledgments
Acknowledgements
The TARGET trial investigative team would like to acknowledge the leadership teams at UPMC: Chief Scientific Officer, Steven Shapiro, MD, the President of Community Medicine Incorporated, Fran Solano, MD, and Chief Quality Officer, Tami Minnier, for their support throughout all phases of the TARGET trial. We would also like to acknowledge our patient and payer stakeholders for their valuable input into the design and interpretation of the TARGET trial. Patient stakeholders included Maria Tamasy (Pittsburgh, PA), Jewel Cash (Boston, MA), Scott Lake (Salt Lake City, UT), and Penny Cowan (American Chronic Pain Society). Payer stakeholders included Pamela Peele, PhD, and Michael Parkinson, MD (UPMC-Health Plan), and David Elton (OPTUM Health). Provider stakeholders included Russell Phillips, MD (Harvard Medical School Center for Primary Care) and William Boissonnault, DPT (American Physical Therapy Association). We also wish to acknowledge Clair Smith, MS, at the University of Pittsburgh for conducting reporting and data analysis for the study.
We would also like to acknowledge the team members from Boston Medical Center including clinic site champions Laura Goldman, MD, Stephanie Charles, MD, Jonathan Berz, MD, Stephen Tringale, MD, Jennifer Lo, MD, and Katherine Gergen Barnett, MD. We are grateful to those who designed and executed data extraction including Associate Chief Medical Information Officer Rebecca Mishuris, MD, Director of Clinical Research Informatics for Boston University William Adams, MD, Medical Director of the Boston HealthNet Charles Williams, MD, and Clinical Data Warehouse Research Manager Linda Rosen. We wish to also thank Karen Mattie, MSPT, Director of Rehabilitation Services, and Christopher Joyce, DPT who provided physical therapy leadership and research staff including Chelsea Lemaster, Dorothy Plumb, Salvatore D'Amico, Jessica Howard, Alex Femia, Samia Jaffar, Iniya Rajendran, Bhumi Patel, and Kristen Mikhail.
Data Sharing
The study is registered with ClinicalTrials.gov, number NCT02647658, and select data from the Figures and Table 1, Table 2, Table 3 have been added to the registration. In accordance with the protocol, no investigators will have access to participant data with identifiers. De-identified data will be shared with external investigators and the funder upon request. Requests for data Inter-university Consortium for Political and Social Research (ICPSR) at the University of Michigan – to provide access to de-identified data by no later than July 01, 2021. Requests for access to the data can be made via the ICPSR website: https://www.icpsr.umich.edu/.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2021.100795.
Appendix. Supplementary materials
References
- 1.The burden of musculoskeletal conditions at the start of the new millennium. World Health Organ Tech Rep Ser. 2003;919(i-x):1–218. back cover. [PubMed] [Google Scholar]
- 2.Buchbinder R, van Tulder M, Oberg B. Low back pain: a call for action. Lancet. 2018;391(10137):2384–2388. doi: 10.1016/S0140-6736(18)30488-4. [DOI] [PubMed] [Google Scholar]
- 3.Clark S, Horton R. Low back pain: a major global challenge. The Lancet. 2018;391(10137):2302. doi: 10.1016/S0140-6736(18)30725-6. [DOI] [PubMed] [Google Scholar]
- 4.Freburger JK, Holmes GM, Agans RP. The rising prevalence of chronic low back pain. Arch Intern Med. 2009;169(3):251–258. doi: 10.1001/archinternmed.2008.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rubin DI. Epidemiology and risk factors for spine pain. Neurol Clin. 2007;25(2):353–371. doi: 10.1016/j.ncl.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Hoy D, Bain C, Williams G. A systematic review of the global prevalence of low back pain. Arthritis Rheumat. 2012;64(6):2028–2037. doi: 10.1002/art.34347. [DOI] [PubMed] [Google Scholar]
- 7.Dieleman JL, Cao J, Chapin A. US health care spending by payer and health condition, 1996-2016. JAMA. 2020;323(9):863–884. doi: 10.1001/jama.2020.0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim LH, Vail D, Azad TD. Expenditures and health care utilization among adults with newly diagnosed low back and lower extremity pain. JAMA Netw Open. 2019;2(5) doi: 10.1001/jamanetworkopen.2019.3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Institute of Medicine (US) Committee on Advancing Pain Research C, and Education . National Academy of Sciences; Washington DC: 2011. Relieving pain in america: a blueprint for transforming prevention, care, education, and research. [PubMed] [Google Scholar]
- 10.Shekelle PG, Markovich M, Louie R. An epidemiologic study of episodes of back pain care. Spine (Phila Pa 1976) 1995;20(15):1668–1673. doi: 10.1097/00007632-199508000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Kosloff TM, Elton D, Shulman SA, Clarke JL, Skoufalos A, Solis A. Conservative spine care: opportunities to improve the quality and value of care. Population Health Management. 2013;16(6):390–396. doi: 10.1089/pop.2012.0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bigos S, Bower O, Braen G, al e. Acute Low Back Problems in Adults. Clinical Practice Guideline No. 14. Rockville, MD, 1994.
- 13.Itz CJ, Geurts JW, van Kleef M, Nelemans P. Clinical course of non-specific low back pain: a systematic review of prospective cohort studies set in primary care. Eur J Pain. 2013;17(1):5–15. doi: 10.1002/j.1532-2149.2012.00170.x. [DOI] [PubMed] [Google Scholar]
- 14.Hestbaek L, Leboeuf-Yde C, Manniche C. Low back pain: what is the long-term course? A review of studies of general patient populations. Eur Spine J. 2003;12(2):149–165. doi: 10.1007/s00586-002-0508-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hill JC, Dunn KM, Lewis M. A primary care back pain screening tool: identifying patient subgroups for initial treatment. Arthritis Rheum. 2008;59(5):632–641. doi: 10.1002/art.23563. [DOI] [PubMed] [Google Scholar]
- 16.Foster NE, Mullis R, Hill JC. Effect of stratified care for low back pain in family practice (IMPaCT Back): a prospective population-based sequential comparison. AnnFamMed. 2014;12(2):102–111. doi: 10.1370/afm.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hill JC, Whitehurst DG, Lewis M. Comparison of stratified primary care management for low back pain with current best practice (STarT Back): a randomised controlled trial. Lancet. 2011;378(9802):1560–1571. doi: 10.1016/S0140-6736(11)60937-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cherkin D, Balderson B, Wellman R. Effect of low back pain risk-stratification strategy on patient outcomes and care processes: the MATCH randomized trial in primary care. J Gen Intern Med. 2018;33(8):1324–1336. doi: 10.1007/s11606-018-4468-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsu C, Evers S, Balderson BH. Adaptation and implementation of the STarT back risk stratification strategy in a US health care organization: a process evaluation. Pain Medicine. 2018 doi: 10.1093/pm/pny170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Delitto A, Patterson CG, Stevans JM. Study protocol for targeted interventions to prevent chronic low back pain in high-risk patients: a multi-site pragmatic cluster randomized controlled trial (TARGET Trial) Contemp Clin Trials. 2019;82:66–76. doi: 10.1016/j.cct.2019.05.010. [DOI] [PubMed] [Google Scholar]
- 21.Deyo RA, Dworkin SF, Amtmann D. Report of the NIH task force on research standards for chronic low back pain. J Pain. 2014;15(6):569–585. doi: 10.1016/j.jpain.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chou R, Qaseem A, Snow V, et al. Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society. [summary for patients in Ann Intern Med. 2007 Oct 2;147(7):I45; PMID: 17909203 ]. Annals of Internal Medicine 147(7):478-91,2007; 147(7): 478–91. [DOI] [PubMed]
- 23.Beneciuk JM, George SZ, Greco CM. Targeted interventions to prevent transitioning from acute to chronic low back pain in high-risk patients: development and delivery of a pragmatic training course of psychologically informed physical therapy for the TARGET trial. Trials. 2019;20(1):256. doi: 10.1186/s13063-019-3350-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Treede RD, Rief W, Barke A. Chronic pain as a symptom or a disease: the IASP classification of chronic pain for the international classification of diseases (ICD-11) Pain. 2019;160(1):19–27. doi: 10.1097/j.pain.0000000000001384. [DOI] [PubMed] [Google Scholar]
- 25.Fairbank JC, Pynsent PB. The oswestry disability index. Spine (Phila Pa 1976) 2000;25(22):2940–2952. doi: 10.1097/00007632-200011150-00017. discussion 52. [DOI] [PubMed] [Google Scholar]
- 26.Liyanage H, Liaw ST, Jonnagaddala J, Hinton W, de Lusignan S. Common data models (CDMs) to enhance international big data analytics: a diabetes use case to compare three CDMs. Stud Health Technol Inform. 2018;255:60–64. [PubMed] [Google Scholar]
- 27.Becker A, Leonhardt C, Kochen MM. Effects of two guideline implementation strategies on patient outcomes in primary care: a cluster randomized controlled trial. Spine (Phila Pa 1976) 2008;33(5):473–480. doi: 10.1097/BRS.0b013e3181657e0d. [DOI] [PubMed] [Google Scholar]
- 28.Mehling WE, Gopisetty V, Bartmess E. The prognosis of acute low back pain in primary care in the United States: a 2-year prospective cohort study. Spine (Phila Pa 1976) 2012;37(8):678–684. doi: 10.1097/BRS.0b013e318230ab20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adams G, Gulliford MC, Ukoumunne OC, Eldridge S, Chinn S, Campbell MJ. Patterns of intra-cluster correlation from primary care research to inform study design and analysis. J Clin Epidemiol. 2004;57(8):785–794. doi: 10.1016/j.jclinepi.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 30.Lonsdale C, Hall AM, Williams GC. Communication style and exercise compliance in physiotherapy (CONNECT): a cluster randomized controlled trial to test a theory-based intervention to increase chronic low back pain patients' adherence to physiotherapists' recommendations: study rationale, design, and methods. BMC Musculoskelet Disord. 2012;13:104. doi: 10.1186/1471-2474-13-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmidt CO, Chenot JF, Pfingsten M. Assessing a risk tailored intervention to prevent disabling low back pain–protocol of a cluster randomized controlled trial. BMC Musculoskelet Disord. 2010;11:5. doi: 10.1186/1471-2474-11-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eldridge SM, Ashby D, Kerry S. Sample size for cluster randomized trials: effect of coefficient of variation of cluster size and analysis method. Int J Epidemiol. 2006;35(5):1292–1300. doi: 10.1093/ije/dyl129. [DOI] [PubMed] [Google Scholar]
- 33.Qaseem A, Wilt TJ, McLean RM, Forciea MA. Physicians ftCGCotACo. Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the american college of physicians. Ann Internal Med. 2017;166(7):514–530. doi: 10.7326/M16-2367. [DOI] [PubMed] [Google Scholar]
- 34.Downie A, Hancock M, Jenkins H. How common is imaging for low back pain in primary and emergency care? Systematic review and meta-analysis of over 4 million imaging requests across 21 years. Br J Sports Med. 2019 doi: 10.1136/bjsports-2018-100087. [DOI] [PubMed] [Google Scholar]
- 35.Deyo RA, Von Korff M, Duhrkoop D. Opioids for low back pain. Bmj. 2015;350:g6380. doi: 10.1136/bmj.g6380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ancker JS, Edwards A, Nosal S. Effects of workload, work complexity, and repeated alerts on alert fatigue in a clinical decision support system. BMC Med Inform Decis Mak. 2017;17(1):36. doi: 10.1186/s12911-017-0430-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Loudon K, Treweek S, Sullivan F, Donnan P, Thorpe KE, Zwarenstein M. The PRECIS-2 tool: designing trials that are fit for purpose. BMJ. 2015;350:h2147. doi: 10.1136/bmj.h2147. [DOI] [PubMed] [Google Scholar]
- 38.National Academies of Sciences E, and Medicine . In: The role of nonpharmacological approaches to pain management: proceedings of a workshop. Washington (DC): national academies press (US) PNS Stroud C, Bain L., editors. National Academies Press (US); Washington, DC: 2019 Apr 12. 2019 Apr 12. 2019. [PubMed] [Google Scholar]
- 39.Lentz TA, Curtis LH, Rockhold FW. Designing, conducting, monitoring, and analyzing data from pragmatic randomized clinical trials: proceedings from a multi-stakeholder think tank meeting. Ther Innov Regul Sci. 2020 doi: 10.1007/s43441-020-00175-7. [DOI] [PubMed] [Google Scholar]
- 40.Bennett AV, Jonsson M, Chen RC, Al -Khatib SM, Weinfurt KP, Curtis LH. Applying patient-reported outcome methodology to capture patient-reported health data: Report from an NIH Collaboratory roundtable. Healthcare. 2020;8(3) doi: 10.1016/j.hjdsi.2020.100442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rombach I, Gray AM, Jenkinson C, Murray DW, Rivero-Arias O. Multiple imputation for patient reported outcome measures in randomised controlled trials: advantages and disadvantages of imputing at the item, subscale or composite score level. BMC Med Res Methodol. 2018;18(1):87. doi: 10.1186/s12874-018-0542-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weinfurt KP, Hernandez AF, Coronado GD. Pragmatic clinical trials embedded in healthcare systems: generalizable lessons from the NIH collaboratory. BMC Med Res Methodol. 2017;17(1):144. doi: 10.1186/s12874-017-0420-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rubenstein LV. Finding joy in the practice of implementation science: what can we learn from a negative study? J General Internal Med. 2019;34(1):9–11. doi: 10.1007/s11606-018-4715-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.