Abstract
Purpose:
This study was conducted to define the maximum tolerated dose (MTD), recommended phase two dose (RPTD), and toxicities of gemcitabine + dasatinib (GD) and gemcitabine + dasatinib + cetuximab (GDC) in advanced solid tumor patients.
Methods:
This study was a standard phase I 3+3 dose escalation study evaluating two combination regimens, GD and GDC. Patients with advanced solid tumors were enrolled in cohorts of 3–6 to either GD or GDC. Gemcitabine was dosed at 1000 mg/m2 weekly for 3 of 4 weeks, dasatinib was dosed in mg PO BID, and cetuximab was dosed at 250 mg/m2 weekly after a loading dose of cetuximab of 400 mg/m2. There were two dose levels for dasatinib: 1) gemcitabine + dasatinib 50 mg +/- cetuximab, and 2) gemcitabine + dasatinib 70 mg +/- cetuximab. Cycle length was 28 days. Standard cycle 1 dose-limiting toxicity (DLT) definitions were used. Eligible patients had advanced solid tumors, adequate organ and marrow function, and no co-morbidities that would increase risk of toxicity. Serum, plasma, and skin biopsy biomarkers were obtained pre- and on-treatment.
Results:
Twenty-five patients were enrolled, including 21 with pancreatic adenocarcinoma. Three patients received prior gemcitabine. Twenty-one patients were evaluable for toxicity and 16 for response. Four DLTs were observed: Grade (Gr) 3 neutropenia (GDC1, n=1), Gr 3 ALT (GD2, n=2), and Gr 5 pneumonitis (GDC2, n=1). Possible treatment-emergent adverse events (TEAEs) in later cycles included: Gr 3–4 neutropenia (n=7), Gr 4 colitis (n=1), Gr 3 bilirubin (n=2), Gr 3 anemia (n=2), Gr 3 thrombocytopenia (n=2), Gr 3 edema/fluid retention (n=1), and Gr 3 vomiting (n=3). Six of 16 patients (3 of whom were gemcitabine-refractory) had stable disease (SD) as best response, median duration = 5 mo (range 1–7). One gemcitabine-refractory patient had a partial response (PR). Median PFS was 2.9 mo (95% CI: 2.1, 5.8). Median OS was 5.8 mo (95% CI: 4.1, 11.8). Dermal wound biopsies demonstrated that dasatinib resulted in a decrease of total and phospho-Src levels, and cetuximab resulted in a decrease of EGFR and ERBB2 levels.
Conclusions:
The MTD/RPTD of GD is gemcitabine 1000mg/m2 weekly for 3 of 4 weeks and dasatinib 50 mg PO BID. The clinical activity of GD seen in this study was modest, and does not support its further investigation in pancreatic cancer.
Keywords: Gemcitabine, Dasatinib, Cetuximab, Pancreatic Cancer, Phase I
INTRODUCTION
Adenocarcinoma of the pancreas is the fourth leading cause of cancer-related death in the United States, with an estimated 55,440 new cases in 2018 and a mortality rate greater than 95% [1, 2]. Gemcitabine, a nucleoside analogue of deoxycytidine, is approved for the treatment of metastatic pancreatic cancer, based upon modest improvements in 1 year survival and median time to progression as compared to 5-FU [3, 4]. Src is a non-receptor tyrosine kinase that is overexpressed in 74% of pancreatic cancer tumors and activated in 60% of tumors. It has been associated with pancreatic cancer cell growth and tumorigenesis [5, 6], increased metastatic potential [7], increased angiogenic potential via upregulation of VEGF and interleukin-8 [8, 9], reduced survival particularly when combined with higher tumor grade [10–13], and gemcitabine-resistance in pancreatic cancer cell lines [14].
Dasatinib is a potent competitive inhibitor of critical oncogenic tyrosine kinase/kinase families including Src, BCR-ABL, c-KIT, PDGFRβ, and EPHA2. Dasatinib is FDA-approved for the treatment of newly diagnosed Philadelphia chromosome–positive (Ph+) chronic myeloid leukemia (CML) in chronic phase and Ph+ acute lymphoblastic leukemia. Dasatinib inhibits migration and invasion of pancreatic cancer cell lines, and inhibits the development of metastases in a mouse model of pancreatic cancer [13]. Inhibition of Src activity in pancreatic cancer cell lines causes decreased cellular invasiveness [15] and the combination of dasatinib and gemcitabine induced anti-proliferative effects and induced apoptotic cell death in gemcitabine-resistant pancreatic cancer cells [16]. In pancreatic cancer nude mouse models, treatment with dasatinib led to significant anti-tumor and anti-metastatic effects [17, 18]. Inhibition of Src activation increases gemcitabine-mediated cytotoxicity in vitro, and limits tumor growth in combination with gemcitabine in orthotopic models in vivo.
Beyond its direct effects, Src interacts with G-protein coupled receptors and receptor tyrosine kinases including the epidermal growth factor receptor (EGFR) [19]. Cetuximab is a monoclonal antibody that binds specifically to EGFR on both normal and tumor cells, and competitively inhibits the binding of epidermal growth factor and other ligands. This blocks phosphorylation and activation of receptor-associated kinases, resulting in inhibition of tumor cell growth, induction of apoptosis, and decreased matrix metalloproteinase and vascular endothelial growth factor production. Src modulates the function of EGFR through phosphorylation of tyrosine residues on EGFR that allow for coupling to downstream signaling events [20, 21]. In pancreatic cancer cells, the combination of dasatinib, erlotinib (small molecule EGFR inhibitor), and gemcitabine resulted in cooperative inhibition of cell migration and invasion, as well as cooperative inhibition of multiple signaling pathways [22]. The combined inhibition of Src and EGFR with gemcitabine inhibited constitutively activated STAT3 in vitro and in vivo. These preclinical data suggest that gemcitabine in combination with Src and EGFR inhibition may be a new therapeutic strategy to treat metastatic pancreatic cancer.
The goals of this study were to determine the MTD/RPTD and to evaluate the toxicity of the gemcitabine + dasatinib (GD) and gemcitabine + dasatinib + cetuximab (GDC) combinations in patients with solid tumors. While the current study was ongoing, data from SWOG S0205 failed to demonstrate benefit from gemcitabine + cetuximab treatment; therefore, the GDC treatment arm was not continued in the current study, and only the GD arm was expanded.
MATERIALS AND METHODS
Patients
General inclusion criteria for all patients were age >18 years, KPS >70%, life expectancy ≥3 mo, adequate organ and marrow function, capable of taking oral medications, willingness to use effective contraception, and negative pregnancy test. Specific inclusion criteria for the dose escalation phase include histologically confirmed solid tumor malignancy that is metastatic or unresectable and for which standard therapy would include gemcitabine or for which standard curative or palliative measures do not exist or are no longer effective. Specific inclusion criteria for the dose expansion phase included histologically or cytologically documented adenocarcinoma of the pancreas, radiologic or surgical proof of metastatic disease, no prior chemotherapy for metastatic pancreatic disease (prior 5-fluorouracil or capecitabine or other agents used as radiosensitizers with concurrent radiation therapy was permitted). Gemcitabine was only permitted if administered in the adjuvant setting with >6 mo between last gemcitabine treatment and diagnosis of metastatic disease.
General exclusion criteria included any anti-cancer treatments (except for hormonal therapy for metastatic breast or prostate cancer) or major surgeries within 28 days of day 1; pregnancy or breastfeeding; impairment of gastrointestinal function that could alter drug absorption; significant cardiac disease or stroke; bleeding diathesis or medications that inhibit platelet function; fluid retention; known HIV, hepatitis C, acute or chronic active hepatitis B; serious chronic infection requiring ongoing treatment; drugs that increase the risk of Torsades de Pointes; CYP3A4 inhibitors or inducers; proton pump inhibitors or H2 antagonists; or prior severe infusion reaction to a monoclonal antibody.
Study Design
The primary objectives of this study were to determine the MTD/RPTD of gemcitabine + dasatinib (GD) and of gemcitabine + dasatinib + cetuximab (GDC) in patients with advanced solid tumors. The secondary objectives of this study were to describe any DLTs for these regimens, describe any non-DLTs for these regimens, describe markers of efficacy for GD in patients with previously untreated metastatic pancreatic cancer (RR, PFS, OS), describe markers of efficacy for GDC for patients with previously untreated metastatic pancreatic cancer (RR, PFS, OS), and to describe changes in blood-based biomarkers for patients with previously untreated metastatic pancreatic cancer in the expanded cohort at the MTD/RPTD. Dermal wound biopsies were employed to evaluate pharmacodynamic changes in Src, EGFR, and ERBB2 activation after treatment.
This trial was an open-label, single center phase I trial at Duke University Medical Center (DUMC) of GD and GDC to assess the safety, tolerability, and MTD/RPTD of each combination. Additionally, preliminary efficacy of these combinations in adult patients with advanced solid tumors and with previously untreated metastatic pancreatic cancer was assessed. Study drugs were gemcitabine (G) was dosed at 1000 mg/m2 over 30 minutes on days 1, 8, and 15, dasatinib (D) in mg PO BID continuously, +/- cetuximab (C) 250 mg/m2 on days 1, 8, 15, and 22, after a loading dose of 400 mg/m2 on cycle 1 day 1. There were two dose levels for dasatinib: 1) gemcitabine + dasatinib 50 mg +/- cetuximab, and 2) gemcitabine + dasatinib 70 mg +/- cetuximab. Cycle length was 28 days. Due to the negative results of SWOG 0205, the GDC treatment arm did not complete enrollment, and only the GD arm was expanded.
Clinical and Radiographic Assessments
All baseline evaluations were conducted within 14 days prior to first dose of study drugs. Tumor assessment (radiographic and/or blood tumor markers) must have been completed within 28 days prior to first dose of study drug. At baseline, all patients had a history, physical examination, vital signs, baseline symptom assessment, height, weight, KPS, assessment of concurrent medications, tumor assessment, with prospective identification of target and non-target lesions that were followed throughout the study. Toxicity was assessed every visit, and as clinically indicated. DLTs were assessed during cycle 1. Patients also underwent dermal wound biopsies. Restaging studies were performed every 2 cycles (8 weeks), and as clinically indicated. Patients were followed for adverse events for 30 days after the last dose of treatment or until resolution of toxicity or start of another treatment regimen.
Safety
DLT was defined as follows, graded according to National Cancer Institute Common Toxicity Criteria for Adverse Events, version 3.05: hematologic toxicity ≥ grade 4 neutropenia or thrombocytopenia for >7 days duration, febrile neutropenia where ANC <500 and temperature >101°F, nausea/vomiting or diarrhea ≥ grade 3 and lasting ≥4 days despite adequate supportive measures, other non-hematologic toxicity ≥ grade 3 (excluding alopecia, anorexia, hyperbilirubinemia due to biliary obstruction or progressive disease, hypersensitivity reaction, and acneiform rash), any treatment-related death or treatment-related hospitalization (except for allergic reaction to cetuximab), inability to deliver >85% of scheduled gemcitabine, dasatinib, and cetuximab (in case of intra-cycle dose modifications) due to treatment-related toxicity, delay in recovery of toxicity that is considered treatment-related that results in 14 day delay in starting cycle 2, other severe or otherwise intolerable toxicities considered by the study Principal Investigator (PI) to be dose-limiting. Dose escalation proceeded according to the standard 3+3 design. Each patient in a given cohort was monitored for at least one full cycle before beginning enrollment of a cohort at the next dose level. The regimen at the dose level immediately below the one with unacceptable toxicity was the RPTD. If no unacceptable toxicity was seen at the highest dose level for either GD or GDC, the corresponding dosing regimen was considered the MTD/RPTD.
The duration of therapy was defined as treatment in the absence of treatment delays until one of the following: death, disease progression, noncompliance by patient or treating physician, persistent (≥4 weeks) NCI-CTCAE v3.0 Grade 3 or 4 adverse event or any significant adverse event that compromises the subject’s ability to participate in the study, investigator determination that it is not in the subject’s best interest to continue participation, or pregnancy. Patients felt to be clinically benefiting from treatment could be continued on treatment with the agreement of the treating physician and study PI.
Dermal Wound Biopsy
Skin Biopsy Procedures and Analysis of Granulation Tissue Biopsies
Skin biopsies were obtained using previously reported methods [23, 24]. Briefly, prior to treatment, a 4-millimeter (mm) skin punch biopsy (Fray Products Corp., Buffalo, NY) was created to stimulate granulation tissue formation followed by a 5mm biopsy of the healing wound (granulation tissue) collected seven days later. Tissue biopsies were embedded in Optimal Cutting Temperature (OCT) compound and then immediately snap frozen in liquid nitrogen and stored at -80°C. The granulation tissue biopsies (5 mm) were harvested at baseline (prior to any treatment) and after at least one week of treatment with the respective targeted agent or agents. The time points for each biopsy are listed in Figure 1.
Figure 1. Progression-Free Survival in Months from Consent Date.
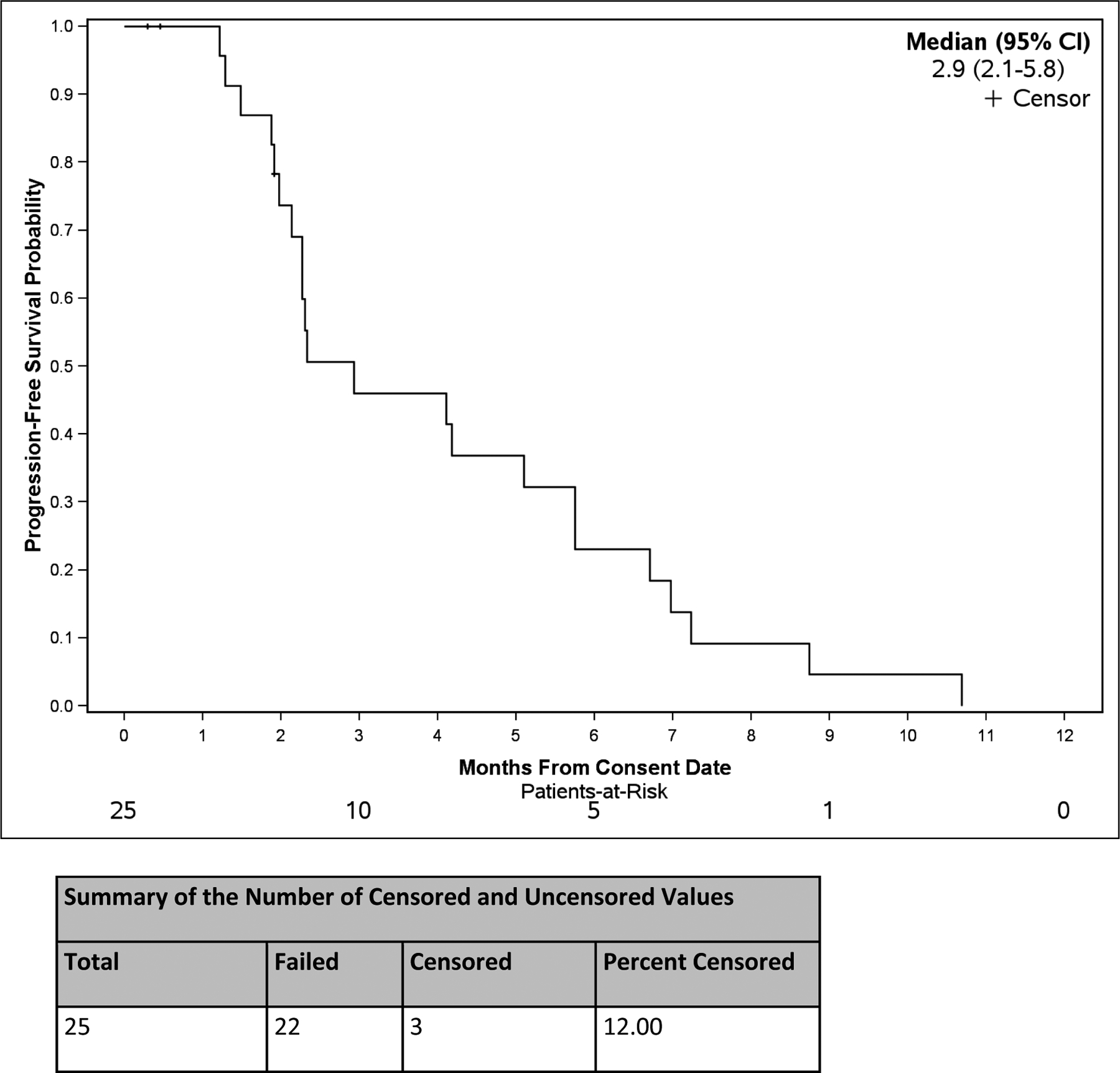
Progression-free survival probability from the date consent was obtained. The median progression-free survival and the 95% confidence interval are noted. The number censored and uncensored values are noted in the table below the graph.
At the time of analysis, tissue samples were thawed at room temperature, washed in ice-cold PBS and homogenized as previously described [24]. Protein lysate were analyzed using the Meso Scale Discovery platform (MSD, Gaithersburg, MD). Phospho (Tyr1173)/Total EGFR Whole Cell Lysate kit (Cat# K15104D) and Phospho (Tyr1248)/Total ErbB2 Assay Whole Cell Lysate Kit (Cat# K15125D) were used to evaluate EGFR and ErbB2 pathways. Src pathway was evaluated using assays developed in our laboratory using MSD ELISA platform. Briefly, Anti-tSrc antibody (Cell Signaling Technology, Inc., Danvers, MA, Cat#2108), anti-pSrc pY418antibody (Invitrogen, Carlsbad, CA, Cat# 44660G), were added at 1ug/ml to goat anti-mouse plates (MSD, Gaithersburg MD), and incubated at room temperature (R.T.) for 1 hour. The plates were washed with TBS/ 0.05% Tween-20 three times and protein lysate from granulation tissue protein lysate were added and incubated for 2 hours at R.T. Sulfo-TAG (MSD, Cat#R91AN-1) labeled anti-Src antibody (R&D, Cat# AF3389) were then added to the plates and incubated for 1 hour at R.T. after plates were washed. The electroluminescence value was normalized to each control and plotted as a percent of control. All assays were performed in duplicate using 50–100 ug of total protein according to manufacturer’s recommendations and read on MSD sector imager 2400 instrument (MSD, Cat# R92TC-2).
Statistical Analysis
This trial employs a phase I dose escalation design, in which 25 evaluable patients with advanced solid tumors were accrued to either the GD or GDC arm in alternating fashion. Descriptive statistics are provided for patient demographics and baseline characteristics. A summary is provided for the incidence and severity of toxicity that arises from treatment as well as other outcome variables (RR, PFS, OS). PFS is measured from consent date until documented progression of disease or death from any cause. OS is measured from consent date until death from any cause. Adverse events (non-DLTs) are summarized overall by counts and percentages. Wilcoxon signed rank tests were used to analyze change in total Src, phospho-Src, total EGFR, phospho(Tyr1173) EGFR, total ErbB2, and phospho(Tyr1248) ErbB2 expression and p-values are provided. Secondary endpoints are exploratory and p-values are not adjusted for multiple testing.
RESULTS
Patient Demographics
A total of 25 patients were enrolled, treated, and 21 were evaluable for toxicity. Patient demographics and tumor types are summarized in Table 1. The median age was 60 (range 34–73). There were 10 (40%) female patients and 15 (60%) male patients. Patients with pancreatic adenocarcinoma accounted for 21 patients (84%). There was one patient each with peritoneal mesothelioma, cervical carcinoma, ampullary adenocarcinoma, and cancer of unknown primary (4% each). Most patients had a Karnofsky performance status of 90 or 100 and an ECOG score of 0 (23 patients). Seventeen patients (68%) were previously untreated, 3 patients (12%) had 1 prior line of chemotherapy, 1 patient (4%) had 2 prior lines of chemotherapy, and 4 patients (16%) had 3 prior lines of chemotherapy. One patient (4%) had prior radiation therapy alone, while 7 patients (28%) had prior chemoradiation.
Table 1: Patient Demographics and Endpoints.
Demographics, prior treatments, duration on treatment, and reasons for coming off treatment for all study patients.
| Characteristic | Patients n (%) |
|---|---|
| Median age, years (range) | 60 (34–73) |
| Gender | |
| Female, no. (%) | 10 (40) |
| Male, no. (%) | 15 (60) |
| Ethnicity, no. (%) | |
| White | 24 (96) |
| Black | 1 (4) |
| Type of primary tumor, no. (%) | |
| Pancreatic adenocarcinoma | 21 (84) |
| Peritoneal mesothelioma | 1 (4) |
| Cervical carcinoma | 1 (4) |
| Ampullary adenocarcinoma | 1 (4) |
| Cancer of unknown primary | 1 (4) |
| Karnofsky performance status, no. (%) | |
| 100 | 5 (20) |
| 90 | 18 (72) |
| 80 | 2 (8) |
| ECOG Score | |
| 0 | 23 (92) |
| 1 | 2 (8) |
| # of Prior Chemotherapy Treatments | |
| 0 | 17 (68) |
| 1 | 3 (12) |
| 2 | 1 (4) |
| 3 | 4 (16) |
| Prior Radiation Therapy Without Concurrent Chemotherapy | |
| 0 | 24 (96) |
| 1 | 1 (4) |
| Number of Prior Combination Chemoradiation | |
| 0 | 17 (68) |
| 1 | 7 (28) |
| 2 | 1 (4) |
| Total # of Prior Treatments | |
| 0 | 12 (48) |
| 1 | 6 (24) |
| 2 | 2 (8) |
| 3 | 3 (12) |
| 4 | 2 (8) |
| Median time on therapy in days (range) | 56 (1, 273) |
| Mean number of cycles on therapy (range) | 2.9 (0.04–9.7) |
| Reason Off Treatment | |
| Intercurrent illness | 5 (21) |
| Noncompliance | 1 (4) |
| Progression of disease | 12 (50) |
| Patient choice | 1 (4) |
| Toxicity | 5 (21) |
Dose Escalation and MTD Determination
The dose escalation schema for GD and GDC are noted in Table 2. Dose findings were based on overall safety and tolerability of the investigational drug combination in other clinical studies. Four patients had treatment related DLTs. In the GD arm, 2 of 3 patients had Gr 3 ALT at GD2. In the 6 patients treated at GD1, none experienced a DLT. Therefore, GD1 (gemcitabine 1000 mg/m2 and dasatinib 50 mg PO BID) was identified as the MTD/RPTD. In the GDC arm, there were 2 DLTs: one patient had Gr 3 ANC with infection in GDC1, and 1 patient had a Gr 5 pneumonitis in GDC2. The final cohort in GDC2 was not completed due to difficulty with enrollment.
Table 2. Gemcitabine/Dasatinib and Gemcitabine/Dasatinib/Cetuximab Dosing.
Shown here are the dose levels for GD and GDC cohorts. The dose and frequency of gemcitabine (GD and GDC arms), dasatinib (GD and GDC arms), and cetuximab (GDC arm) are listed. The number of evaluable patients treated at each level (n treated) and number of DLTs (n DLTs) are listed.
| Dose Level | Gemcitabine (mg/m2) IV over 30 minutes, days 1, 8, 15 | Dasatinib (mg) PO BID continuous | Cetuximab IV days 1, 8, 15, 22a (mg/m2) | n treated | n DLTs |
|---|---|---|---|---|---|
| GD-1 | 1000 | 40 | 0 | 0 | 0 |
| GDC-1 | 250 | 0 | 0 | ||
| GD1 | 1000 | 50 | 0 | 6 | 0 |
| GDC1 | 250 | 6 | 1 | ||
| GD2 | 1000 | 70 | 0 | 3 | 2 |
| GDC2 | 250 | 5b | 1 | ||
| GD3 | 1000 | 100 | 0 | 0 | 0 |
| GDC3 | 250 | 0 | 0 |
GDC arms only. First dose of cetuximab will be a loading dose of 400 mg/m2 IV day 1 cycle 1 only. The remainder of the doses will be 250 mg/m2 IV.
Three of five patients on GDC2 came off treatment due to toxicity.
Safety
Of the 25 enrolled subjects, 21 subjects were evaluable for toxicity. Most adverse events were mild to moderate and resolved with supportive clinical care and protocol-specific dose holdings and reductions. Gr 3 through 5 adverse events, and the percentage of patients with treatment-related toxicities across all cycles is summarized in Table 3. Overall, there were 36 Gr 3 events in 16 subjects, and 9 of these events were considered to be possibly related to treatment. There were 3 Gr 4 events in 3 subjects; all were thought to be possibly related to treatment (colitis, dyspnea, and neutropenia). Overall, the most common treatment-related Gr ≥3 adverse events were neutropenia (n=7, 33%), lymphopenia (n=3, 14%), thrombocytopenia (n=2, 10%), and ALT elevation (n=2, 10%). All other treatment-related Gr ≥3 adverse events that were noted occurred at a frequency of 1 patient each (n=5% each). There was one treatment-related death in GDC2 due to a Gr 5 pneumonitis.
Table 3. Summary of Grade 3–5 Adverse Events across Dose Levels.
Included in this table are adverse events experienced in at least 2 patients, or adverse events that were classified as treatment-related in at least 1 patient.
| All AEs n(%) | Treatment-Related n(%) | |||
|---|---|---|---|---|
| Adverse Event Name | Grade 3 | Grade 4 | Grade 5 | Grades 3–5 |
| Alk phos | 1 (5) | 0 | 0 | 1 (5) |
| ALT elevation | 2* (10) | 0 | 0 | 2 (10) |
| Neutropenia | 6* (29) | 1 (5) | 0 | 7 (33) |
| Bilirubin elevation | 2 (10) | 0 | 0 | 0 |
| Colitis | 0 | 1 (5) | 0 | 1 (5) |
| Dyspnea | 0 | 1 (5) | 0 | 1 (5) |
| Edema | 1 (5) | 0 | 0 | 1 (5) |
| Fatigue | 2 (10) | 0 | 0 | 1 (5) |
| Hemoglobin | 2 (10) | 0 | 0 | 0 |
| Hyperglycemia | 2 (10) | 0 | 0 | 0 |
| Hypophosphatemia | 1 (5) | 0 | 0 | 1 (5) |
| Lymphopenia | 3 (14) | 0 | 0 | 3 (14) |
| Thrombocytopenia | 2 (10) | 0 | 0 | 2 (10) |
| Pneumonitis | 0 | 0 | 1* (5) | 1 (5) |
| Skin rash | 1 (5) | 0 | 0 | 1 (5) |
| Ulcer | 1 (5) | 0 | 0 | 1 (5) |
| Vomiting | 3 (14) | 0 | 0 | 0 |
Includes at least one DLT.
Efficacy
Patients were considered evaluable for tumor response if they underwent the first planned tumor assessment scan without clinical progression prior to the scan. Of the 25 subjects enrolled, 16 subjects were evaluable for response. For assessment of best response across all patients, 1 had a PR, 6 patients had SD, and 9 patients had progressive disease (PD). The median duration of SD was 6.4 mo (range 2.9–8.7). Median PFS (22 PFS events and 3 censored observations) was 2.9 mo (95% CI: 2.1, 5.8) (Fig 1). Median OS (15 deaths and 10 censored observations) was 5.8 mo (95% CI: 4.1, 11.8) (Fig 2).
Figure 2. Overall Survival in Months from Consent Date.
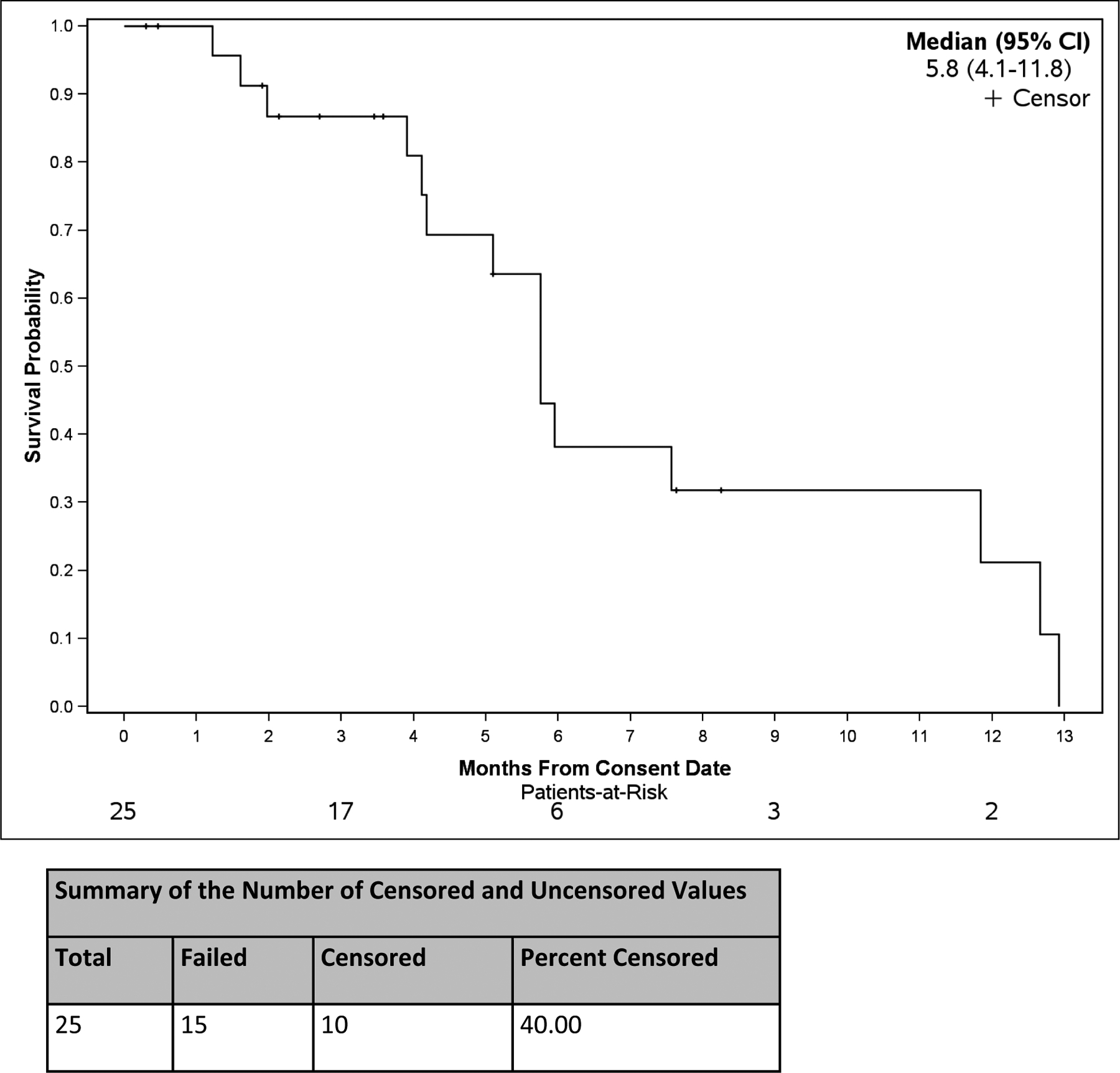
Survival probability from the date consent was obtained. The median overall survival and the 95% confidence interval are noted. The number censored and uncensored values are noted in the table below the graph.
Dermal Wound Biopsy Analysis
Levels of total and phosphorylated proteins from dermal wound biopsies, collected at baseline and on-treatment, were assessed in order to determine on-target effect of therapy. Eight patients treated with GD and eleven patients treated with GDC arm had granulation tissue biopsies available for analysis at both baseline (off-treatment) and day 8 after dosing treatment (on-treatment). Compared to baseline, total Src protein expression was decreased in both GD- and GDC-treated patients with the exception of 1 GD patient (Fig. 3 A, B). The reduction of total Src protein levels upon dasatinib treatment was statistically significant in GDC arm, but not the GD arm. Phospho-Src protein levels were significantly reduced with both GD and GDC treatment (Fig. 3 C, D). This result is consistent with the inhibitory effect on Src phosphorylation by dasatinib. Similar to total SRC expression, total and phosphorylated EGFR and ErbB2 protein expression were decreased in patients receiving cetuximab (GDC arm), while no changes were observed in patients only receiving GD treatment without cetuximab (Fig. 3 E–L). This result demonstrates the specific inhibition of EGFR and ErbB2 by cetuximab. In summary, these findings demonstrate direct pharmacodynamic inhibition of Src by dasatinib and EGFR pathway members by cetuximab.
Figure 3. Changes in total and phospho- Src (A-D), total and phospho-EGFR (E-H) and total and phosphor-ErbB2 (I-L) protein levels from baseline.
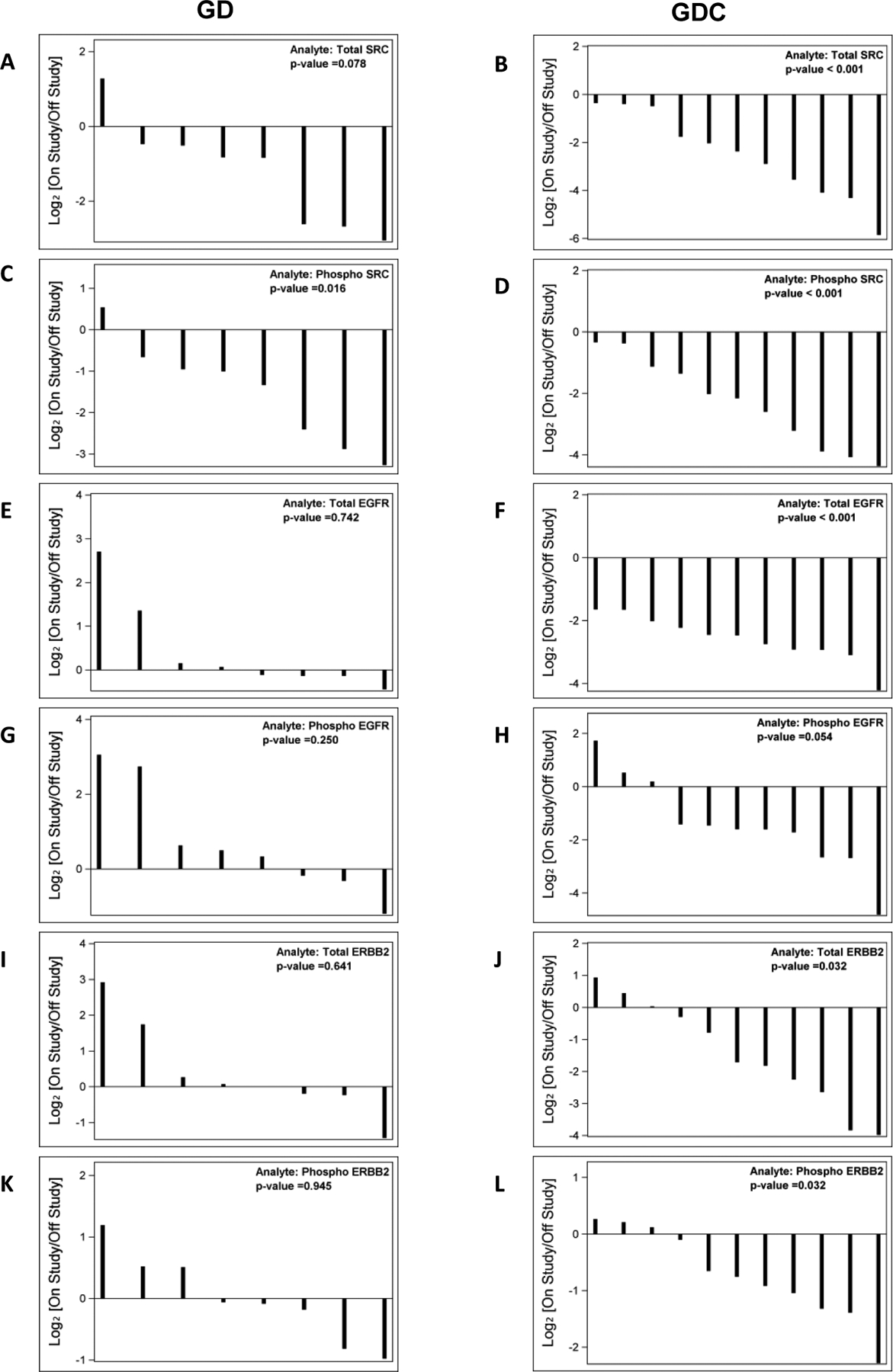
Waterfall plots on the left represent GD treatment arm and waterfall plots on the right represent GDC treatment arm.
DISCUSSION
In this phase I study, we evaluated GD and GDC in patients with solid tumors, mostly pancreatic cancer. Both combinations were reasonably well-tolerated, but due to data from SWOG 0205 which failed to demonstrate any clinical activity of gemcitabine + cetuximab, accrual to the GDC arm was not completed in the current study. The MTD/RPTD of GD was gemcitabine 1000 mg/m2 d1, d8, and d15 every 28 days and dasatinib 50 mg PO BID continuous. The median time on therapy was 61 days (range 1–361). One patient experienced a PR and six had SD with a median duration of 6.4 months. The median PFS was 2.9 mo (95% CI, 2.1–5.8). The median OS was 5.8 mo (95% CI, 4.1–11.8). There were 11 patients that had dose reductions.
Our results are consistent with other studies evaluating the efficacy of dasatinib in other solid tumors including pancreatic cancer. A single-arm, phase II study of dasatinib as first-line therapy in 51 patients with metastatic pancreatic cancer reported a median PFS of 2.1 mo and median OS of 4.7 mo [25]. Of the 34 evaluable patients, the best response achieved was SD in 10 patients (29.4%), though one patient had SD for 20 mo while on treatment. While single-agent dasatinib does not have much clinical activity in metastatic pancreatic cancer, a phase I study of gemcitabine/dasatinib showed SD ≥6 mo in 2 of 8 patients with pancreatic cancer, both of whom were gemcitabine-refractory, supporting our finding that dasatinib may overcome resistance to gemcitabine [26]. The combination of dasatinib/cetuximab had no objective responses and 10 of 23 evaluable patients had only SD as the best response, with a median duration of 4.3 mo [27]. Minimal clinical activity and toxicity may limit EGFR inhibition with gemcitabine and Src inhibition.
Many of the molecular events and mechanisms involved in wound repair and tumor angiogenesis and growth are similar [28]. Previously, we established a dermal wound model to detect the antiangiogenic effects of multiple antiangiogenic agents using granulation tissue as a surrogate tissue for tumor [23, 24, 29]. We were able to demonstrate Src pathway inhibition by dasatinib based on the reduction of phospho-Src protein levels in granulation tissue. In fact, similar reduction was seen in IGROV1 cells in vitro using our assay platform [30]. In the analysis presented, targeted EFGR pathway inhibition was evidenced by decreased levels of total and phospho-EGFR in patients only receiving cetuximab treatment. The reduction in total EGFR protein expression is consistent with the fact that cetuximab competitively inhibits EGFR ligand binding and promotes receptor internalization and degradation without stimulating tyrosine kinase phosphorylation [31]. Interestingly, cetuximab treatment also led to decreased levels of total and phospho-ErbB2 protein. ErbB2 functions primarily as a dimerization partner of EGFR but does not bind to ligands directly [32]. Therefore, this finding could be another confirmation of EGFR pathway inhibition.
Other Src inhibitors have also been studied in pancreatic cancer. SKLB261 is a multikinase inhibitor that potently inhibits Src, EGFR, and VEGFR2 kinases [33]. In vitro in human pancreatic cells, it inhibits cell proliferation, migration, invasion, and induces apoptosis, and in vivo in pancreatic cancer xenografts, it had more potent antitumor activities than dasatinib, erlotinib, or gemcitabine alone. KX2–391, a small molecule Src inhibitor and inhibitor of tubulin polymerization, targets the peptide substrate rather than the ATP binding site. The phase I trial of KX2–391 in 44 patients with refractory solid tumors, including 6 patients with pancreatic cancer resulted in 11 patients with SD ≥4 mo [34]. A phase I/II study of the Src inhibitor saracatinib in combination with gemcitabine in 22 patients with advanced or metastatic pancreatic cancer resulted in 6 patients (27.3%) with SD <4 mo, 5 patients (22.7%) with SD >4 mo, and 3 patients (9.1%) with PR [35]. A phase I study of the Src/Abl tyrosine kinase inhibitor bosutinib plus capecitabine in 31 evaluable patients, 5 with pancreatic cancer, resulted in a PR >24 weeks in 6% and SD >24 wks in 13% of all tumor types, but no patients with pancreatic cancer achieved a CR or PR.
Since the completion of the current study, dasatinib was studied in combination with gemcitabine in a large phase 2 placebo-controlled, double-blind study in locally-advanced pancreatic cancer [36]. In this study, 202 patients received gemcitabine plus dasatinib vs. placebo, with the option for radiotherapy. There was no statistically significant difference in OS or PFS, and there was more toxicity in the dasatinib arm, resulting in fewer patients who completed dosing cycles and more treatment discontinuation. If administered at doses and schedules with improved tolerability, this combination may have demonstrated greater clinical activity. A phase II study of emcitabine +/- dasatinib administered adjuvantly in patients previously treated with surgery completed accrual in November 2017, though results are not available to date.
To augment the clinical activity seen in this study, other therapeutic combinations may warrant exploration. Preclinical data suggests that concomitant targeting of EGFR in addition to TGF-β and Src may represent a novel therapeutic approach in pancreatic cancer [37]. Furthermore, simultaneously targeting SHP-2 and Src in pancreatic cancer has been shown to disrupt pancreatic cancer signaling and biology in vitro and in vivo in an orthotopic nude mouse model [38]. Dually targeting both SHP-2 and Src induced an additive loss of phosphorylation of Akt and ERK-1/2 and corresponding increases in expression of apoptotic markers and significantly reduced viability, adhesion, migration, and invasion of pancreatic cancer cells in vitro and tumor formation in vivo. It is possible that these and other combinations may demonstrate enhanced clinical activity than seen in the current study.
The ability to prospectively identify patients who are likely to benefit from this targeted therapy combination may also result in improved clinical activity in a subset of patients. In a phase I study of dasatinib with capecitabine, oxaliplatin, and bevacizumab in patients with metastatic colorectal cancer, high levels of Src activation were associated with improved response [39]. However, this study was limited by difficulties in assessing correlations between biomarker levels with response given small number of treatment responders. A Src biomarker-driven trial was not able to conclude if saracatinib could improve 6-mo survival in patients with previously treated pancreatic cancer, due to limited numbers [40].
In conclusion, we have identified that the MTD/RPTD of GD is gemcitabine 1000mg/m2 on d1, d8, and d15 every 28 days and dasatinib 50mg PO BID continuously. The combination of gemcitabine 1000 mg/m2 on d1, d8, and d15, dasatinib 100 mg PO BID, with or without cetuximab 250 mg/m2 every 21 days was limited by early hematologic and hepatic toxicities. Furthermore, later toxicities, including hematologic toxicities, pneumonitis and fluid retention were occasionally significant with the GDC combination. The GD regimen demonstrated some clinical benefit, as prolonged SD was noted. Wound biopsy analyses indicated direct target inhibition of Src as well as EGFR pathway members in response to the combinations tested. Evaluation of this combination in a more rigorous, appropriately powered, randomized study based on Src inhibitor biomarkers may result in improved treatment response in pancreatic cancer.
ACKNOWLEDGEMENTS
We gratefully acknowledge the participation of the patients and their caregivers in this study. We would like to acknowledge the Duke University GI Oncology Clinical Trials Team.
Funding Information: This was an investigator-initiated study supported by Bristol Myers Squib, Inc. The study was independently managed and analyzed. The final responsibility for the manuscript and the decision to submit for publication was made by the investigators. This work was also supported by National Institute of Health Grant 5K24-CA113755-05 (HH).
Footnotes
CONFLICT OF INTEREST
NBM is a clinical investigator on clinical trials supported by Incyte, ARMO, OncoMed, and Genentech/Roche. HU is a clinical investigator on clinical trials supported by Macrogenics, Merck, Genentech/Roche. ABN reports honoraria for consulting and advisory boards for Eli Lilly, Pfizer, and Kanghong Pharma. He has received grant support from Acceleron Pharma, Amgen, AstraZeneca/MedImmune, Eureka Therapeutics, Genentech, Leadiant Biosciences, MedPacto Inc, Novartis, Seattle Genetics, and Tracon Pharma. HH is employed by Genentech/Roche. The remaining authors have no conflicts of interest.
REFERENCES
- 1.Siegel RL et al. (2018) Cancer statistics, 2018. CA Cancer J Clin 68 (1), 7–30. [DOI] [PubMed] [Google Scholar]
- 2.Ries LAG (2007) SEER Cancer Statistics Review, 1975–2005, National Cancer Institute. Bethesda, MD. . http://seer.cancer.gov/csr/1975_2005/, (accessed). [Google Scholar]
- 3.Plunkett W et al. (1995) Gemcitabine: metabolism, mechanisms of action, and self-potentiation. Semin Oncol 22 (4 Suppl 11), 3–10. [PubMed] [Google Scholar]
- 4.Burris HA 3rd et al. (1997) Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 15 (6), 2403–13. [DOI] [PubMed] [Google Scholar]
- 5.Flossmann-Kast BB et al. (1998) Src stimulates insulin-like growth factor I (IGF-I)-dependent cell proliferation by increasing IGF-I receptor number in human pancreatic carcinoma cells. Cancer Res 58 (16), 3551–4. [PubMed] [Google Scholar]
- 6.Tanno S et al. (2001) AKT activation up-regulates insulin-like growth factor I receptor expression and promotes invasiveness of human pancreatic cancer cells. Cancer Res 61 (2), 589–93. [PubMed] [Google Scholar]
- 7.Menke A et al. (2001) Down-regulation of E-cadherin gene expression by collagen type I and type III in pancreatic cancer cell lines. Cancer Res 61 (8), 3508–17. [PubMed] [Google Scholar]
- 8.Trevino JG et al. (2005) Expression and activity of SRC regulate interleukin-8 expression in pancreatic adenocarcinoma cells: implications for angiogenesis. Cancer Res 65 (16), 7214–22. [DOI] [PubMed] [Google Scholar]
- 9.Summy JM et al. (2005) c-Src regulates constitutive and EGF-mediated VEGF expression in pancreatic tumor cells through activation of phosphatidyl inositol-3 kinase and p38 MAPK. Pancreas 31 (3), 263–74. [DOI] [PubMed] [Google Scholar]
- 10.Frame MC (2002) Src in cancer: deregulation and consequences for cell behaviour. Biochim Biophys Acta 1602 (2), 114–30. [DOI] [PubMed] [Google Scholar]
- 11.Hakam A et al. (2003) Coexpression of IGF-1R and c-Src proteins in human pancreatic ductal adenocarcinoma. Dig Dis Sci 48 (10), 1972–8. [DOI] [PubMed] [Google Scholar]
- 12.Lutz MP et al. (1998) Overexpression and activation of the tyrosine kinase Src in human pancreatic carcinoma. Biochem Biophys Res Commun 243 (2), 503–8. [DOI] [PubMed] [Google Scholar]
- 13.Morton JP et al. (2010) Dasatinib inhibits the development of metastases in a mouse model of pancreatic ductal adenocarcinoma. Gastroenterology 139 (1), 292–303. [DOI] [PubMed] [Google Scholar]
- 14.Duxbury MS et al. (2004) Inhibition of SRC tyrosine kinase impairs inherent and acquired gemcitabine resistance in human pancreatic adenocarcinoma cells. Clin Cancer Res 10 (7), 2307–18. [DOI] [PubMed] [Google Scholar]
- 15.Ito H et al. (2003) Inhibition of tyrosine kinase Src suppresses pancreatic cancer invasiveness. Surgery 134 (2), 221–6. [DOI] [PubMed] [Google Scholar]
- 16.Duong HQ et al. (2014) Combination of dasatinib and gemcitabine reduces the ALDH1A1 expression and the proliferation of gemcitabine-resistant pancreatic cancer MIA PaCa-2 cells. Int J Oncol 44 (6), 2132–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yezhelyev MV et al. (2004) Inhibition of SRC tyrosine kinase as treatment for human pancreatic cancer growing orthotopically in nude mice. Clin Cancer Res 10 (23), 8028–36. [DOI] [PubMed] [Google Scholar]
- 18.Trevino JG et al. (2006) Inhibition of SRC expression and activity inhibits tumor progression and metastasis of human pancreatic adenocarcinoma cells in an orthotopic nude mouse model. Am J Pathol 168 (3), 962–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bromann PA et al. (2004) The interplay between Src family kinases and receptor tyrosine kinases. Oncogene 23 (48), 7957–68. [DOI] [PubMed] [Google Scholar]
- 20.Ishizawar R and Parsons SJ (2004) c-Src and cooperating partners in human cancer. Cancer Cell 6 (3), 209–14. [DOI] [PubMed] [Google Scholar]
- 21.Biscardi JS et al. (2000) Tyrosine kinase signalling in breast cancer: epidermal growth factor receptor and c-Src interactions in breast cancer. Breast Cancer Res 2 (3), 203–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagaraj NS et al. (2011) Combined blockade of Src kinase and epidermal growth factor receptor with gemcitabine overcomes STAT3-mediated resistance of inhibition of pancreatic tumor growth. Clin Cancer Res 17 (3), 483–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lockhart AC et al. (2003) A clinical model of dermal wound angiogenesis. Wound Repair Regen 11 (4), 306–13. [DOI] [PubMed] [Google Scholar]
- 24.Jia J et al. (2015) Direct Evidence of Target Inhibition with Anti-VEGF, EGFR, and mTOR Therapies in a Clinical Model of Wound Healing. Clin Cancer Res 21 (15), 3442–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chee CE et al. (2013) Phase II study of dasatinib (BMS-354825) in patients with metastatic adenocarcinoma of the pancreas. Oncologist 18 (10), 1091–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hong DS et al. (2013) A phase 1 study of gemcitabine combined with dasatinib in patients with advanced solid tumors. Invest New Drugs 31 (4), 918–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Argiris A et al. (2012) Phase I and pharmacokinetic study of dasatinib and cetuximab in patients with advanced solid malignancies. Invest New Drugs 30 (4), 1575–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dvorak HF (1986) Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med 315 (26), 1650–9. [DOI] [PubMed] [Google Scholar]
- 29.Lockhart AC et al. (2003) Reduction of wound angiogenesis in patients treated with BMS-275291, a broad spectrum matrix metalloproteinase inhibitor. Clin Cancer Res 9 (2), 586–93. [PubMed] [Google Scholar]
- 30.Secord AA et al. (2014) Dasatinib (BMS-35482) interacts synergistically with docetaxel, gemcitabine, topotecan, and doxorubicin in ovarian cancer cells with high SRC pathway activation and protein expression. Int J Gynecol Cancer 24 (2), 218–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sunada H et al. (1990) Modulation of tyrosine, serine, and threonine phosphorylation and intracellular processing of the epidermal growth factor receptor by antireceptor monoclonal antibody. J Cell Physiol 142 (2), 284–92. [DOI] [PubMed] [Google Scholar]
- 32.Garrett TP et al. (2003) The crystal structure of a truncated ErbB2 ectodomain reveals an active conformation, poised to interact with other ErbB receptors. Mol Cell 11 (2), 495–505. [DOI] [PubMed] [Google Scholar]
- 33.Pan Y et al. (2015) A preclinical evaluation of SKLB261, a multikinase inhibitor of EGFR/Src/VEGFR2, as a therapeutic agent against pancreatic cancer. Mol Cancer Ther 14 (2), 407–18. [DOI] [PubMed] [Google Scholar]
- 34.Naing A et al. (2013) A phase I trial of KX2–391, a novel non-ATP competitive substrate-pocket- directed SRC inhibitor, in patients with advanced malignancies. Invest New Drugs 31 (4), 967–73. [DOI] [PubMed] [Google Scholar]
- 35.Renouf DJ et al. (2012) A phase I/II study of the Src inhibitor saracatinib (AZD0530) in combination with gemcitabine in advanced pancreatic cancer. Invest New Drugs 30 (2), 779–86. [DOI] [PubMed] [Google Scholar]
- 36.Evans TR et al. (2016) Phase 2 Placebo-Controlled, Double-Blind Trial of Dasatinib Added to Gemcitabine for Patients with Locally-Advanced Pancreatic Cancer. Ann Oncol. [DOI] [PubMed] [Google Scholar]
- 37.Deharvengt S et al. (2012) Concomitant targeting of EGF receptor, TGF-beta and SRC points to a novel therapeutic approach in pancreatic cancer. PLoS One 7 (6), e39684. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38.Gomes EG et al. (2013) Targeting the yin and the yang: combined inhibition of the tyrosine kinase c-Src and the tyrosine phosphatase SHP-2 disrupts pancreatic cancer signaling and biology in vitro and tumor formation in vivo. Pancreas 42 (5), 795–806. [DOI] [PubMed] [Google Scholar]
- 39.Strickler JH et al. (2014) Phase I study of dasatinib in combination with capecitabine, oxaliplatin and bevacizumab followed by an expanded cohort in previously untreated metastatic colorectal cancer. Invest New Drugs 32 (2), 330–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arcaroli J et al. (2012) Biomarker-driven trial in metastatic pancreas cancer: feasibility in a multicenter study of saracatinib, an oral Src inhibitor, in previously treated pancreatic cancer. Cancer Med 1 (2), 207–17. [DOI] [PMC free article] [PubMed] [Google Scholar]


