Abstract
BACKGROUND:
PCV13 (conjugated polysaccharide) and PPSV23 (polysaccharide only) are two licensed vaccines targeting S. pneumoniae. The role of CD4 T-cell responses in pneumococcal vaccines among healthy participants and their impact on antibodies is not yet known.
METHODS:
Ten adults (5 old and 5 young) received PCV13 (prime) and a year later PPSV23 (boost). Blood samples were collected prior to and multiple time points after vaccination. CD4 T cells responding to CRM197, polysaccharide (PS), CRM197 conjugated polysaccharide (CPS), PCV13 and PPSV23 vaccines were measured by flow cytometry. Serum antibodies were analyzed via multiplex opsonophagocytosis (MOPA) and pneumococcal IgG assays.
RESULTS:
Vaccine-specific CD4 T cells were induced in all ten vaccinees post PCV13. Older vaccinees mounted higher peak responses and those specific for PCV13 and conjugated PS-1 were more polyfunctional compared to the younger group. Vaccine-elicited peripheral T follicular helper (Tfh) cells were only detected in the younger group who also exhibited a higher fold change in OPA titers post both vaccines. Importantly, Tfh cells following PCV13 correlated only with PCV13 serotype specific OPA titers after PPSV23 vaccination.
CONCLUSIONS:
These findings demonstrate age related differences in immune response and the potential importance of Tfh in modulating functional antibody responses following pneumococcal vaccination.
Keywords: PCV13, PPSV23, CD4 T cells, pneumococcal antibodies, opsonophagocytic antibodies, T follicular helper cells, proliferation, polysaccharide, glycoconjugate and CRM197
INTRODUCTION
Streptococcus pneumoniae is a major human pathogen that causes invasive pneumococcal disease (bacteremia and meningitis) as well as non-invasive infections such as pneumonia and otitis [1]. Immunocompromised and individuals at both extremes of age (infants/older adults) are especially vulnerable and currently two approved inactivated vaccines are routinely used [2–5] to prevent infection. Capsular polysaccharides are one of the major virulence factors and antibodies elicited against it are protective against infection with S. pneumoniae. However, T-cell independent or polysaccharide (PS) alone based vaccines [6, 7] are not immunogenic in infants/very young children and generate poor immunological memory despite repeated vaccinations. Tetanus or diphtheria toxoids are commonly used as carrier protein to make T cell dependent PS specific IgG production, promote isotype switching, induce T cell activation, and aid in the generation of long lived T and B cell immune memory [8]. Conjugated vaccines targeting extracellular polysaccharides from encapsulated pathogenic bacteria such as S. pneumoniae, N. meningitides and H. influenzae have been successful in preventing infections by these virulent pathogens in children [9–11].
Pneumovax®23 (PPSV23) is a 23 valent polysaccharide only based vaccine and is considered to be a T cell independent vaccine, as HLA-II is not considered capable of presenting glycan moieties. In order to recruit CD4 T cell responses, PCV13 was engineered with 13 different pneumococcal-specific polysaccharides (PS) that are independently covalently coupled to a carrier protein (diphtheria CRM197). Although both vaccines induce humoral immunity, PCV13 has been shown to elicit a higher magnitude antibody response [4]. A prevailing paradigm is that PCV13 stimulates a robust T cell response that aids in the maturation and development of memory B cell responses. Current advisory committee on immunization practices (ACIP) guidelines of the CDC recommend that PCV13 to be given to all individuals aged ≥ 65 yrs., followed by PPSV23 one year later [2]. Both vaccines are associated with fewer hospitalizations and while PCV13 has been shown to reduce the rate of vaccine-type pneumococcal pneumonia among older adults, their efficacy and maintenance of adaptive immunity in the aged is considered suboptimal [12, 13].
Opsonophagocytosis assays (OPA) measure serum antibodies’ ability to kill bacteria. Emerging consensus indicates that an ability to induce antibodies with opsonophagocytic activity (OPA) is a better indicator of pneumococcal vaccine efficacy in adults as compared to quantity of pneumococcal IgG antibodies [14, 15, 16 ]; yet how the CD4 T cell immune responses impact the generation of functional pneumococcal antibodies is not known. As such, it is assumed that PCV13 recruits help from CD4 T cells in the production of antibodies [17]. While a number of studies have evaluated pneumococcal-specific CD4 T cells induced by immunizing mice [18, 19], only one vaccination study has been performed in humans and the study subjects were infected with HIV [20]. Thus, the properties of T cells induced by both of these pneumococcal vaccines and their association with antibody responses are unclear.
In an attempt to determine the functionality of pneumococcal-specific CD4 T cells elicited by PCV13 prime and PPSV23 boost vaccinations administered to healthy adults (young vs. older adults) the following were investigated: a) kinetics and functional profile, as assessed by production of cytokines/effector molecules, of CD4 T cell responses; and b) examination of the relationship between the functionality of specific CD4 T cells in the blood and the levels of pneumococcal antibodies in the serum (based on measurement of quantitative pneumococcal IgG and functional pneumococcal activity by OPA).
MATERIALS AND METHODS
Cohort and study design:
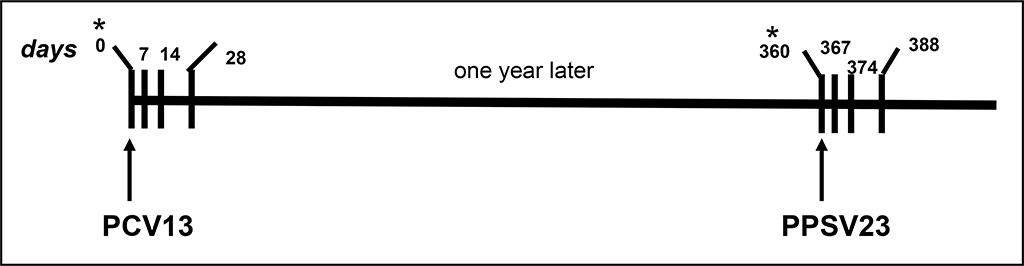
Ten healthy study participants, five aged 63–68 (older group) and five aged 24–27 years (younger group), received Prevnar-13 (PCV13) vaccine at the Alabama Vaccine Research Center at the University of Alabama at Birmingham (UAB), Table 1. Under an IRB approved study (X160205002), we obtained peripheral blood at baseline (prior to vaccination, d0) and following PCV13 vaccination (days 7, 14, and 28). Three individuals in each group remained in the follow up study and received PPSV23 boost at d360 and blood collection repeated, Figure 1. PBMC and serum were processed from blood drawn in ACD and SST tubes respectively and cryopreserved for use in immune assays.
Table 1.
Demographics of the study cohort.
| DONOR | AGE (yrs) | SEX | RACE |
|---|---|---|---|
| PVIR-001 | 67 | male | Caucasian |
| PVIR-002 | 65 | male | African-American |
| PVIR-003 | 65 | female | Caucasian |
| PVIR-004 | 68 | female | Caucasian |
| PVIR-005 | 63 | male | Caucasian |
| PVIR-006 | 27 | female | Caucasian |
| PVIR-007 | 24 | male | Mixed |
| PVIR-008 | 24 | female | African-American |
| PVIR-009 | 24 | female | African-American |
| PVIR-010 | 26 | female | Caucasian |
Figure 1.
An overall schema showing prime (PCV13) pneumococcal vaccination followed a year later by a boost (PPSV23) in healthy adults. Only six of the 10 individuals who received PCV13 also received the boost. The vertical lines show the dates of blood draws for both vaccines i.e. PCV13= days 0*, 7, 14 and 28; PPSV23= days 0 (360*), 7 (367), 14 (374) and 28 (388). *Baseline or pre-vaccine time point. PBMC and serum were processed and cryopreserved for use in immune assays.
Antigens:
Antigens used for PBMC stimulation were: CRM197; PS-serotypes 1, 6A and 11A; conjugated PS-serotype 1, 6A, and 11A; PCV13 vaccine; and PPSV23 vaccine. Serotype 1 is a zwitterionic polysaccharide whereas polysaccharides 6A and 11A are non-zwitterionic and unique to PCV13 and PPSV23, respectively. Pfizer provided all reagents except the two vaccines used as immunogens.
Flow cytometry based assays:
Multiparametric flow cytometry was used to evaluate vaccine-specific CD4 T cell responses as follows.
a). In-vitro CFSE based proliferation assay:
Using PBMC in a 5d assay, we measured the ability of proliferating CD4 T cells to upregulate expression of CD40L (CD154), IFN-γ, IL-2, IL-4 and granzyme B. Cryopreserved PBMC were thawed, labeled with CFSE (ThermoFisher), stimulated with PCV13 (2.5μg/ml), PPSV23 (5μg/ml), PS-1 (5μg/ml), PS-6A (5μg/ml), PS-11A (5μg/ml), conjugated PS-1 (5μg/ml), conjugated PS-6A (5μg/ml), conjugated PS-11A (5μg/ml), CRM197 (5μg/ml), or SEB (1μg/ml, Toxin Technology) and cultured at 37°C, 5% CO2. Antigen concentration used was based on prior studies [20, 21] and titration experiments performed in our lab. On d5, cells were restimulated for 12 hrs with antigen, anti-CD28, CD49d, and CD154 APC antibodies (BD Biosciences). After 1h, Golgi stop was added. Cells were stained with LIVE/DEAD fixable blue dye (ThermoFisher), washed, and surface stained with: CD3-APC eFluor 780 (ThermoFisher), CD14-PercpCy5.5, CD19-PercpCy5.5, CD4-V500 (all BD Biosciences). Cells were then washed, permeabilized with Cytofix/Cytoperm and stained intracellularly with Granzyme B-V450, IL2-PECy7, IL4-PE, and IFNγ- Alexa Fluor 700 (all BD Biosciences). Cells were fixed (4% paraformaldehyde), data collected on an LSRII flow cytometer (BD Biosciences) and analyzed using FlowJo software (version 9.7.6, Tree Star Inc.).
Polyfunctionality refers to the ability of antigen specific T cells to produce multiple effector molecules at the same time. A polyfunctionality index (PI) was used as a readout measure to determine CD4 T cell polyfunctionality and its variation between the two groups. This index is summed as a single number and allows statistical tests to be performed on multifunctional measures [22].
b). Ex-vivo based assay for measuring total antigen specific CD4 T cell responses using an activation induced marker (AIM) phenotype:
Antigen specific responses were analyzed ex-vivo by measuring the co-expression of the activation markers CD25/OX40 and CD25/PDL1 [23, 24] and T-follicular helper cell (Tfh) markers on CD4 T cells (CXCR5+PD1+). Cryopreserved PBMC from d0, 7, and 14 post PCV13 vaccination were thawed and stimulated with PCV13 (2.5μg/ml) or SEB (1μg/ml) in the absence of Golgi-inhibitors for 18–24hrs. Cells were stained with LIVE/DEAD fixable blue dye as above and surface stained with CD4-Qdot655 (ThermoFisher), CD3-APC eFluor 780 (ThermoFisher), OX40-PECy7 (Biolegend), CD8-AlexaFluor 700, CD14-AlexaFluor 700, CD19-AlexaFluor 700, CD45RA-450, CCR7-PercpCy5.5, CD25-FITC, PDL1-PE, CXCR5-Brilliant Violet 510, and PD1-APC (all BD Biosciences). Cells were fixed and analyzed on an LSRII as described above.
Antibody analysis:
Two different assays were utilized to determine pneumococcal antibody responses in serum at baseline (d0) and post prime and boost vaccinations.
a). Multiplex Opsonophagocytic assay (MOPA):
Serum from days 0 and 28 (post PCV13) and days 360 and 388 (days 0 and 28 post PPSV23), Figure 1 were analyzed in multiplex opsonophagocytosis assays (MOPAs). This assay was performed in the Pneumococcal Serology Reference Laboratory for the World Health Organization headed by Dr. Moon Nahm [25] following the protocol available at https://www.vaccine.uab.edu. Opsonization index (OI) titers are defined as the reciprocal of the interpolated dilution of serum that kills 50% of bacteria.
b). Multiplex bead array assay for pneumococcal IgG antibodies:
Pneumococcal IgG antibodies (ug/ml) at days 0 and 28 post each vaccine as described above were measured by ARUP Laboratories (Salt Lake City, UT) using their commercially available 23-serotype multiplex pneumococcal antibody assay.
Statistical analyses:
Statistical analyses were performed using Prism software (v 7, GraphPad). Data were compared between groups using nonparametric, two-tailed Mann-Whitney U test, and within groups using nonparametric, two-tailed Wilcoxon Signed Rank Test. Associations between variables were determined by using the nonparametric Spearman Rank correlation test. Longitudinal data were compared by calculating Area under the Curve. Proportions of nominal variables were compared using Fisher’s exact test.
RESULTS
a). Younger individuals have higher OPA antibody titers following vaccinations.
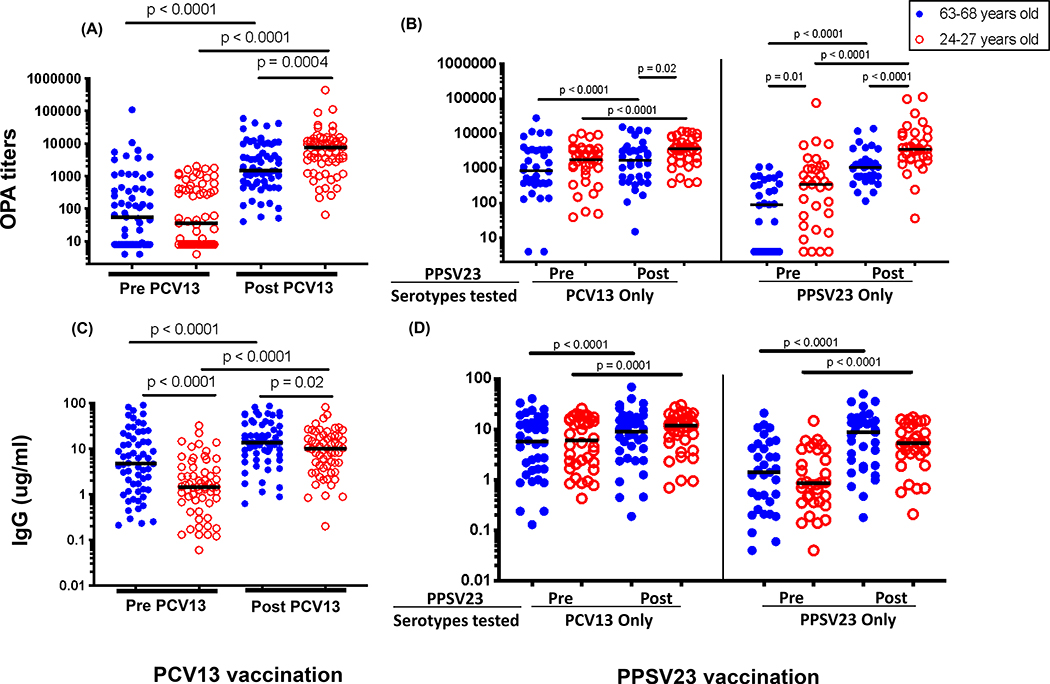
Serotype specific antibodies were measured in both a MOPA (Figure 2A, B) and a multiplex bead array assay (Figure 2C, D) using pre/post PCV13 vaccine samples (days 0 and 28, respectively) and pre/post PPSV23 boost samples (days 360 and 388, respectively). Irrespective of age, significant increase in functional OPA titers was observed following both PCV13 prime and PPSV23 boost although younger individuals boosted higher than older ones post prime vaccination (Figure 2A). Following PPSV23 boost, OPA titers to serotypes present in PCV13 were boosted in both groups, but to a higher degree in younger individuals (p=0.02). At the time of boost, OPA titers to PPSV23 exclusive serotypes were significantly higher in younger adults (p=0.01), and were boosted to higher levels post vaccination (p<0.0001, Figure 1B). Pneumococcal IgG levels also were increased after each vaccine irrespective of age (Figure 2C and D), however, antibody levels were lower in the younger group even before receiving their first vaccine and, while boosted over baseline, remained lower compared to the older group following PCV13 (Figure 2C). Each serotype specific antibody response at pre and post each vaccine is shown in Supplementary Figure 1.
Figure 2.
Serum antibody levels as measured in MOPA (A and B) and pneumococcal IgG (C and D) assays at pre (day 0 and 360) and post vaccination (day 28 and 388) time points for each vaccine are shown for young and old vaccinees. Each dot represents data for a single serotype. Data is shown for 13 (PCV13 MOPA, panel A), 12 (PCV13 IgG, panel C) and 23 (12 PCV13 and 11 PPSV23, panels B/D) serotypes specific for each vaccine. Panels A/C and B/D show data for 5 and 3 individuals in each age group respectively. Horizontal bars represent median value.
b). Younger individuals mount lower magnitude and less polyfunctional CD4 T cell responses following PCV13 vaccine.
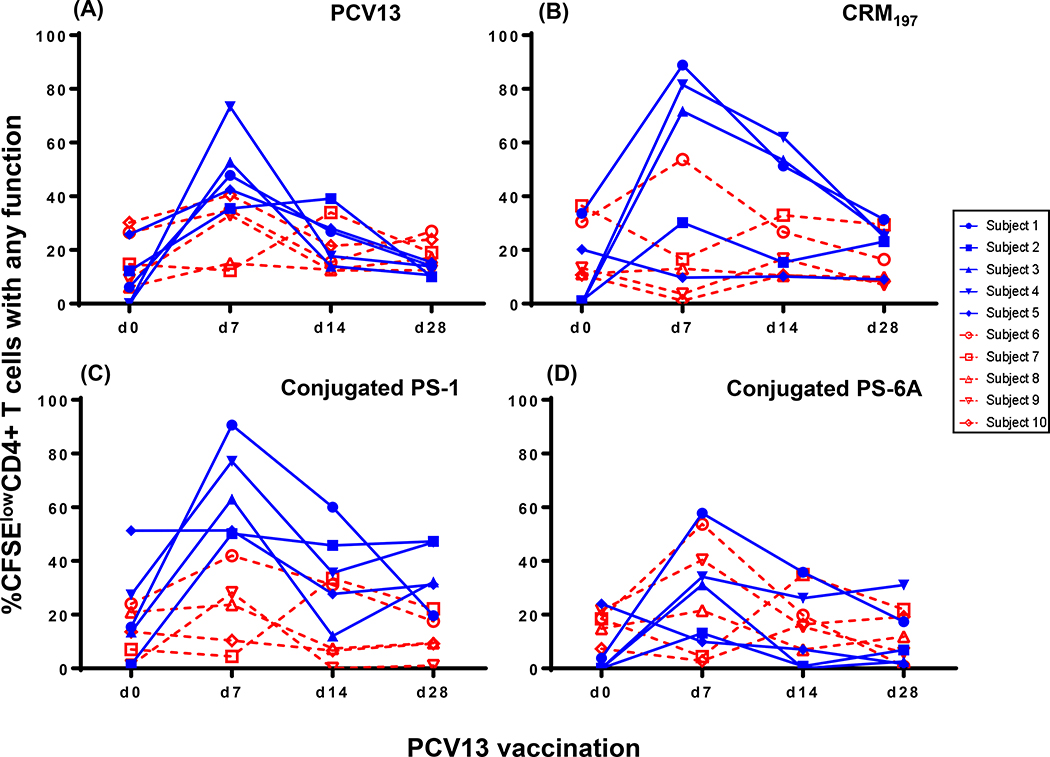
Following PCV13 vaccination, proliferative CD4 T cell responses producing 1 or more functions (IFN-γ, IL-2, IL-4 granzyme B and/or CD154) were detected in all ten vaccinees to at least one of the six antigens tested in both age groups (Figure 3A–D). These responses specific to the PCV13 vaccine, CRM197 carrier protein and conjugated polysaccharide (conjugated PS) specific (types 1 and 6A), peaked at day 7, and declined to baseline levels by 1-month post vaccination. In the older age group there was a trend for higher conjugated PS-1-specific CD4 T cells compared to CRM197 and conjugated PS-6A (p= 0.07, data not shown). Prior studies in mice suggested that zwitterionic polysaccharides such as serotype 1 can directly bind HLA-II molecules and elicit CD4 T cell responses [26, 27]. However, vaccine-induced responses to either unconjugated polysaccharides, PS-1 or PS-6A were not detected (Supplementary Figure 2).
Figure 3.
PBMC isolated at demonstrated time points (days 0, 7,14 and 28) following PCV13 vaccination were stimulated for five days and the kinetics of expression of IFN-γ, IL-2, IL-4, granzyme-B and/or CD154 by proliferating (CFSElow) CD4 T cells were measured. PCV13 (A), CRM197 (B), conjugated PS-1 (C) and conjugated PS-6A (D) were used as antigens. Data is shown for five older and five younger vaccinees. Blue= 63–68 years old and red= 24–27 years old.
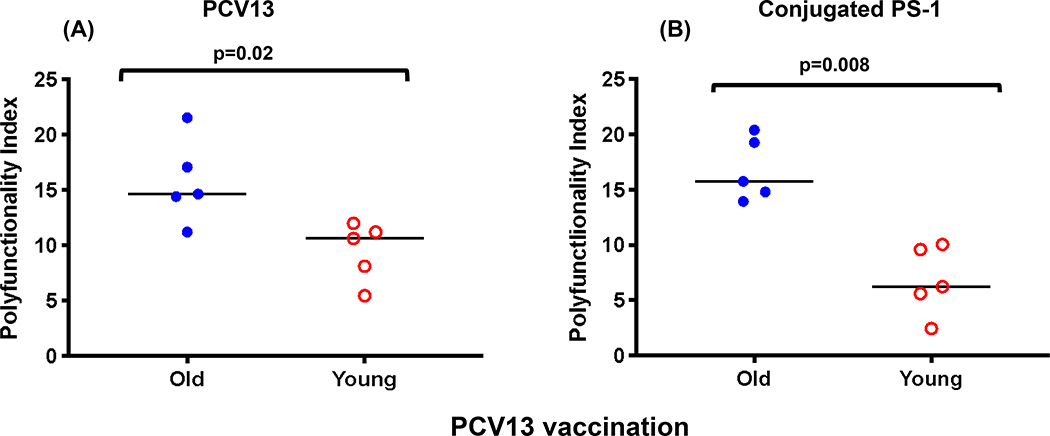
Antigen specific T cell polyfunctionality has been previously associated with better clinical disease outcomes [28, 29]. The polyfunctionality index [22], a statistical method to quantify polyfunctionality, was higher in the older group for both PCV13 and conjugated PS-1-specific CD4 T cells (p=0.02 and 0.008, Figure 4A and B). A trend for higher polyfunctionality index (PI) was seen in the older individuals for conjugated PS-1 specific responses compared to conjugated PS-6A (p=0.06, Supplementary Figure 3).
Figure 4.
Peak polyfunctionality index observed post PCV13 vaccination in the old and young vaccinees (N=five in each group) in response to PCV13 (A) and conjugated PS-1 (B) as antigens. Horizontal bar represents median value.
Since the kinetics, magnitude and the peak of the response varied between vaccinees, an area under the curve analysis (AUC) at days 0, 7, 14, and 28 following vaccination was performed. Similar to the proliferative responses, conjugated PS-1 specific CD4 T cells producing IFN-γ, IL-4, and granzyme B were significantly increased in the older compared to the younger group. PCV13-specific IL-4 producing CD4 T cells were also higher in the older group. Alternatively, CPS-6A specific proliferative CD4 T cells upregulating CD154 and IFN-γ were decreased in the older group (Supplementary Figure 4).
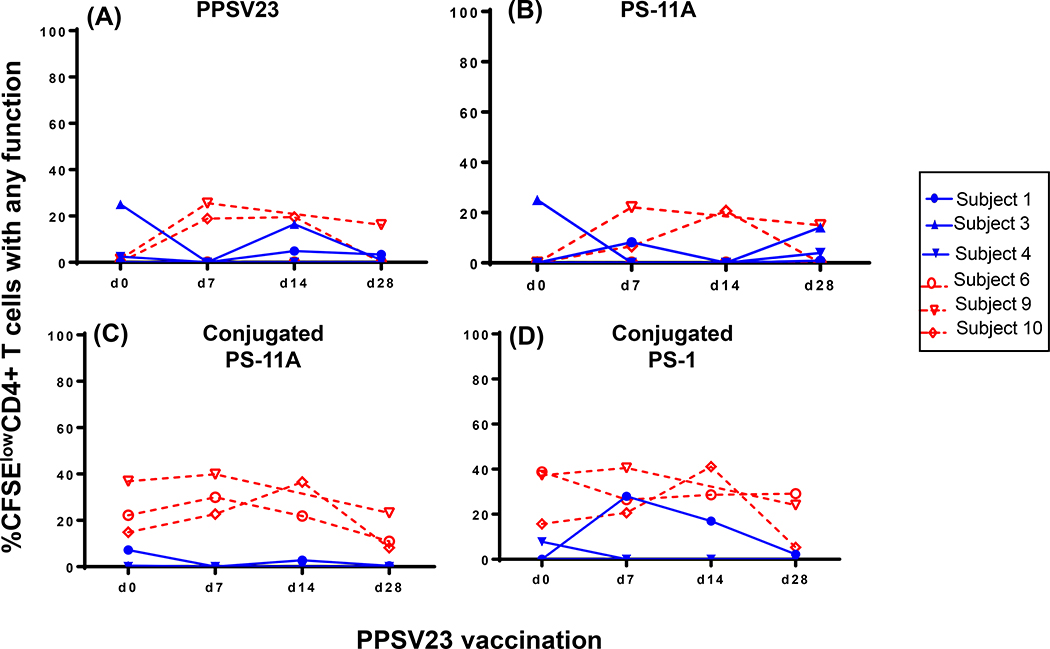
c). Low magnitude boosting of CD4 T cell responses following PPSV23.
Three of the five individuals in each age group received PPSV23 one-year post PCV13 vaccine. CD4 T cell responses post PPSV23 are shown in Figure 5 and Supplementary Figure 5. Overall, none (older age group) to low (younger age group) CD4 T cell responses to PPSV23 were observed. Specifically, two of the three younger vaccinees appeared to mount CD4 T cell response to PPSV23 and PS-11A with peak response observed 7 – 14 days post vaccination (Figure 5A and B, respectively). For other antigens either no response was seen (PS-1), the response was higher at baseline and did not boost with PPSV23 (conjugated PS-11A, PCV13 and CRM197) or low-level boosting was detected (CPS-1; Figure 5C, D and Supplementary Figure 5).
Figure 5.
PBMC isolated at various time points following PPSV23 vaccination (days 360, 367, 374 and 388) were stimulated for five days and the kinetics of expression of IFN-γ, IL-2, IL-4, granzyme-B and/or CD154 by proliferating (CFSElow) CD4 T cells were measured. PPSV23 (A), PS-11A (B), conjugated PS-11A (C) and conjugated PS-1 (D) were used as antigens. Data is shown for three each of older and younger vaccinees. These vaccinees had previously received PCV13 vaccine a year earlier. Blue= 63–68 years old and red= 24–27 years old.
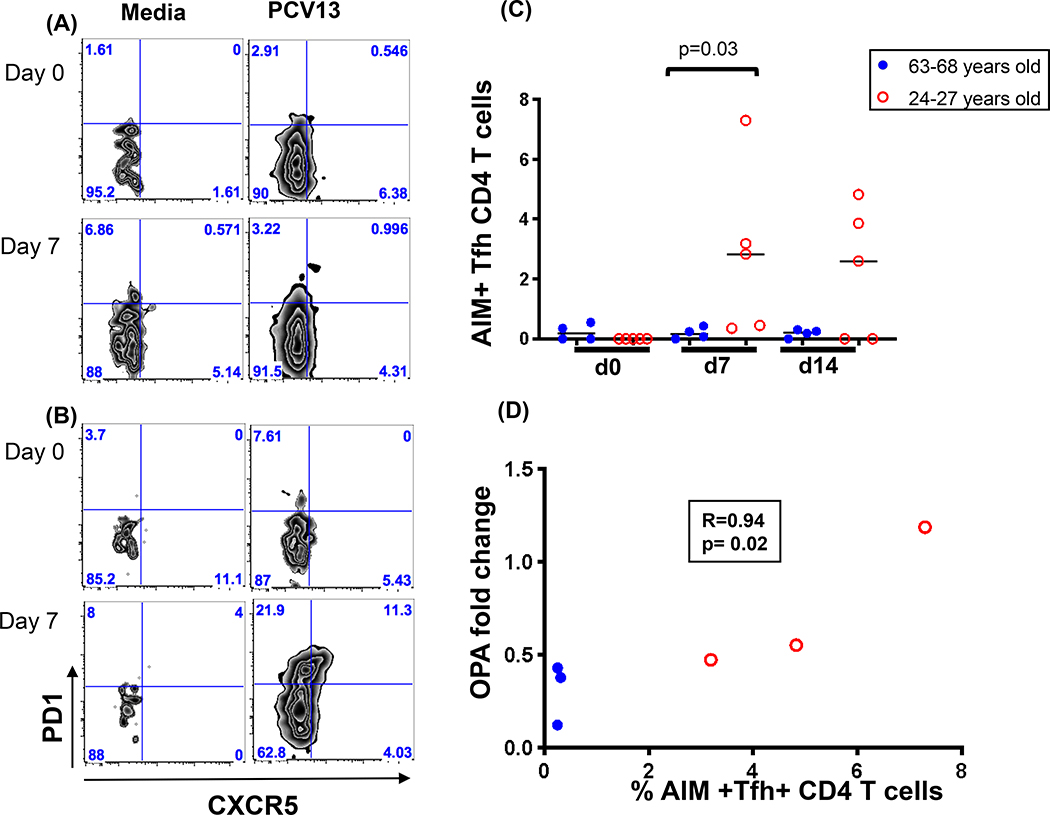
d). Peripheral antigen specific T helper follicular (Tfh) cells detected in younger individuals.
CD4 Tfh cells play an important role in the maturation of antibody responses [30]. To test whether this CD4 T cell subset was induced following vaccination and whether it differed based on the age of vaccinees, a previously described technique that utilizes activation induced markers (AIM) along with traditional Tfh markers to identify rare antigen specific CD4 Tfh cells was utilized. This phenotype was evaluated in response to stimulation with PCV13 in four individuals (based on sample availability) from each age group using day 7 and 14 PBMC samples post PCV13 administration. Representative examples of CD4+ CXCR5+ PD1+ peripheral Tfh populations are shown in Figure 6A and B. The cumulative antigen specific (AIM+) CD4 Tfh cell data is presented in Figure 6C and illustrates that these cells were only observed in the younger group (p=0.03).
Figure 6.
Antigen specific CD4 T follicular helper cells at 7 days post PCV13 vaccination in response to stimulation with PCV13 as antigen. Activation Induced Marker (AIM) expression (CD25+OX40+) was first used to identify antigen specific cells and the frequency of Tfh in these were determined using a CCR7+CD45RA-CXCR5+PD-1+ phenotype. Representative data in an old (A) and young vaccinee (B) is shown. Media only condition was used as a negative control. Cumulative data for four older and five younger vaccinees is shown in panel (C). Spearman correlation between fold changes in OPA antibody titers and AIM+Tfh+ cells specific for PCV 13 antigen is shown in panel (D).
e). CD4 Tfh cells post PCV13 vaccine correlate with fold changes in OPA titers.
As Tfh cells have been demonstrated to be essential for optimal antibody responses [31], it was anticipated that this subset of T cells may correlate with functional antibody responses. Therefore, we evaluated whether any T cell subset(s) correlated with antibody titers (functional and/or binding) and if so, whether they were specific to serotype immunogens included in the prime (PCV13) or boost (PPSV23) vaccine. A month after PPSV23 vaccination, the OPA fold change in serotypes only specific for PCV13 significantly correlated with peak PCV13 specific Tfh cells generated 7 days after receiving the first vaccine (p=0.02 and R=0.94, Figure 6D). No other CD4 T cell function(s) correlated with antibody titers (OPA or pneumococcal IgG levels) (data not shown). As such, these findings indicate that the peripheral Tfh phenotype generated after PCV13 vaccine assist in boosting the OPA antibody responses directed against PCV13 serotypes.
DISCUSSION
To the best of our knowledge, this is the first human data investigating age related differences in the functionality of CD4 T cells and quality of the antibody responses post PCV13 vaccination in both younger (24–27 year old) and older individuals (63–68 years old). Following PCV13 vaccination, older individuals mounted higher magnitude and breadth of CD4 T cell responses. Within these responses, PCV13 and conjugated PS-1 specific CD4 T cell responses had higher polyfunctionality compared to younger vaccinees. In the older age group, vaccine-induced CD4 T cells declined to baseline levels by one-month post PCV13 administration and were not boosted by PPSV23. Interestingly, none of these higher CD4 T cell responses correlated with vaccine induced antibody titers. Overall, the younger age vaccinees had consistently higher antibody titers and tended to have a lower magnitude of total CD4 T cell responses as measured by multiple functional parameters. The notable exception was the induction of peripheral antigen specific CD4 Tfh cells, which were detected exclusively in this age group and correlated with vaccine induced PCV13-specific functional antibodies following PPSV23 boost.
Due to the anticipated low ex vivo frequency of antigen specific cells, we used CFSE based 5-day proliferation assay coupled with cytokine/effector production to measure CD4 T cell responses. In a prior study, which measured HIV-specific memory T cells in the context of HIV-infected patients, the percent of antigen specific CFSElow cells correlated well with ex vivo baseline CD4 T cell frequency [32]. In addition, a previous study [20] also determined CD4 T cells responses using a lymphoproliferative assay (LPA); however, this study was conducted in HIV infected adults, the study groups were different (PCV7/PPSV23 i.e. combo vs. PPSV23 alone) and the prime/boost were one month apart. The authors of this study observed CD4 proliferative responses to CRM197 and these cells produced Th1 cytokines (IFN-γ and IL-2) as measured in an ELISA at 1 and 6 months post boost vaccination.
In light of previous study by Rabian et al [20] that showed that higher Tfh (CD4+CXCR5+) frequency at baseline was associated with a better lymphoproliferative response to CRM197, we wanted to determine whether these cells also played a role in the response to pneumococcal vaccines in healthy individuals. High expression of CXCR5 and PD-1 on CD4T cells has been shown to be associated with peripheral memory Tfh cells [33] capable of promoting antibody responses and related to the bona fide Tfh in the germinal center. [34–37].
We used a sensitive assay to identify the total pool of antigen specific CD4 T cells based on dual expression of activation markers such as CD25/OX40 [23, 24, 38] followed by selection for Tfh cell phenotype. One of the major findings of our study is that functional antibodies to PCV13 serotypes measured a month after boost vaccination correlate with frequency of antigen specific CD4 Tfh cells observed a week after receiving PCV13. Our data may still be an underestimation and could be addressed in future studies aimed at evaluating these responses in the lymph node biopsies obtained from vaccinated individuals. Interestingly, the effect on OPA was largely driven by the Tfh cell responses in the younger vaccinees. In older vaccinees, it is possible that Tfh responses peak prior to or later than day 7; however, this explanation is not consistent with the lower functional antibodies observed in this group. In line with our findings, a prior study in HIV infected adults, showed that fold increase in IgG2 antibodies at d28, following PCV13 vaccination, correlated with non-antigen specific ICOS expressing Tfh cells at day 7 [39]. Future experiments using coculture of sorted antigen specific Tfh cells and B cells will show the direct relevance of the former in pneumococcal vaccines.
A subset of polysaccharides such as those derived from Streptococcus pneumonia (type 1), Bacteroides fragilis and Staphylococcus aureus (type 5 and 8) have been shown to activate CD4 T cells in the presence of antigen presenting cells (APC) due to a zwitterion charge motif present within each repeating unit in the PS [40] i.e. zwitterion polysaccharide (ZPS) [41]. A prior study in mice showed that T cell receptor usage of CD4 T cells stimulated with polysaccharide (PS) or protein alone were different [26]. In S. pneumoniae, a gram-positive pathogen with over 90 capsular polysaccharides, non-ZPS are in the majority. Although several studies from a single group conducting studies in mice have shown that carbohydrate specific CD4 T cells exist [18, 19, 42], no studies in humans have evaluated the elicitation and role of polysaccharide specific CD4 T cells. We analyzed a ZPS serotype, PS-1, and two non–ZPS serotypes, 6A and 11A, that are specific to PCV13 and PPSV23, respectively. We did not observe any significant PS specific CD4 T cells even to the PS-1, which is a ZPS. Interestingly, we did observe functional differences in the CD4 T cells targeting carrier protein and glycoconjugate as antigens.
While several studies have shown high inter-lab variability in multiplexed pneumococcal IgG testing [14, 43, 44], we used the same laboratory for all IgG measurements and utilized between-group comparisons. Additionally, the presence of IgG antibodies produced in response to vaccine does not indicate their functional ability to kill bacteria [16, 45] and adults may develop pneumococcal infections despite high levels of circulating IgG antibodies.[46, 47]. In contrast, multiple studies have shown that OPA titers are a much better indicator of an effective immune response post infection with S. pneumoniae or in those vaccinated against this pathogen [15, 16, 45, 48–50]. While older individuals had significantly higher baseline pneumococcal IgG, OPA titers at baseline were same for the two age groups and these were boosted by prime vaccination reaching higher levels in the younger group, consistent with prior data from our group [16]. Tfh cells may be important in the induction of functional opsonophagocytic but not pneumococcal binding antibodies. Alternatively, the molecular nature of the anti-PPS antibodies may have changed in the older adults, perhaps due to an accumulation of somatic mutations or derivation from different B cell subsets.
A significant caveat with respect to our findings is the small number of studied individuals. Nevertheless, we were still able to demonstrate significant differences in both antibody and T cell immune responses when comparing the two age groups. The main reason we were able to see differences was probably due to the inclusion of a young healthy vaccine group where pneumococcal vaccine is not currently recommended. Interestingly, there will be few future studies that will allow such comparisons to be performed due to the universal application of the PCV13 vaccine in US preschool children since 2010. Hence, there will be a limited window in which young unvaccinated adults can be studied.
Understanding T cell activation mechanisms is important in guiding improved new generation vaccines for encapsulated bacteria that exploit the polysaccharides in their virulence arsenal. The lack of antigen-specific peripheral Tfh cells in older adults is an important finding as these immune subsets are known to be key players in optimizing antibody responses [30]. Hence future vaccine optimization approaches need to consider elicitation and boosting of Tfh for generation of durable antibody responses among the elderly population.
Supplementary Material
Supplemental Figure 1. Serotype-specific pneumococcal responses measured by MOPA and multiplex IgG assays with respect to PCV13 vaccine (A) and PPSV23 vaccine (B). Comparisons of pre-vaccine (x-axis) and post-vaccine (y-axis) results from multiplex opsonophagocytosis assay (MOPA; reported as opsonization index, or OI; represented by open red circles) and a multiplex IgG assay (in μg/ml; represented by solid black circles). An identity line is included for reference. Responses for PCV13 serotypes (pre-vaccine measurement at day0; post-vaccine at day 28) in n=10 participants are shown (A). Serotype 6A is not included, as it is unique to PCV13 and therefore not contained in the 23-serotype multiplex IgG assay containing PPSV23 serotypes. Responses for PPSV23 serotypes (pre-vaccine measurement at day 360; post-vaccine at day 388) in n = 6 participants are also shown (B).
Supplementary Figure 2. The kinetics of expression of IFN-γ, IL-2, IL-4, granzyme-B and/or CD154 by proliferating (CFSElow) CD4 T cells post PCV13 in response to 5 day stimulation with PS-1 (A) and PS-6A (B) as antigens. Data is shown for five older and five younger vaccinees post prevnar vaccination. Blue= 63-68 years old and red= 24-27 years old.
Supplementary Figure 3. Polyfunctionality of CD4 T cell responses to conjugate 1 (CPS-1) and conjugate 6A (CPS-6A) in the old (A) and young (B) vaccinees post PCV13 vaccination. Responses are compared using Wilcoxon Signed Rank Test. Peak polyfunctionality index data was used in this analysis.
Supplementary Figure 4. Area under the curve (AUC) data for antigen specific response in each individual. PS= polysaccharide. For each antigen, responses in the old and young groups are compared using Mann-Whitney U test, p-values are shown as *(0.05) and ** (0.01). Vertical dotted lines show comparison of responses in old and young vaccinees for each measure.
Supplementary Figure 5. The kinetics of expression of IFN-γ, IL-2, IL-4, granzyme-B and/or CD154 by proliferating (CFSElow) CD4 T cells post PPSV23 in response to 5 day stimulation with PCV13 (A), CRM197 (B) and PS-1 (C). Data is shown older and younger vaccinees (N=3 each) and these vaccinees had previously received PCV13 vaccine. Blue= 63-68 years old and red= 24-27 years old.
ACKNOWLEDGEMENTS
We thank the study participants for providing us with samples for this study. In addition, we thank the nurses and staff at the Alabama Vaccine Research Clinic (AVRC) for their immense help in the conduct of this work. We also wish to extend our thanks to the UAB CFAR Flow Cytometry Core/Joint UAB Flow Cytometry Core, which is funded by NIH/NIAID P30 AI027767.
Funding: Research funding from UAB-CCTS grant UL1TR001417 (PAG), 5T32HL105346–09 (DCL) and Pfizer supported this work. ASA, SuS and MP are employed by Pfizer and as such receive salaries and stocks from Pfizer.
Abbreviations:
- PCV13
Prevnar 13
- PPSV23
Pneumovax 23
- PS
capsular polysaccharide
- CRM197
Diphtheria toxoid carrier protein
- CPS
CRM197 conjugated capsular polysaccharide
Footnotes
COI statement: The authors have a conflict of interest in the conduct of this study. Pfizer provided funding and the reagents used in this study. The University of Alabama at Birmingham (UAB) has intellectual property rights to several opsonophagocytosis assay reagents developed in M. H. Nahm’s laboratory.
REFERENCES
- 1.Said MA, Johnson HL, Nonyane BA, et al. Estimating the burden of pneumococcal pneumonia among adults: a systematic review and meta-analysis of diagnostic techniques. PloS one 2013; 8:e60273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pallotta A, Rehm SJ. Navigating pneumococcal vaccination in adults. Cleve Clin J Med 2016; 83:427–33. [DOI] [PubMed] [Google Scholar]
- 3.Tomczyk S, Bennett NM, Stoecker C, et al. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged >/=65 years: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morbidity and mortality weekly report 2014; 63:822–5. [PMC free article] [PubMed] [Google Scholar]
- 4.Jackson LA, Gurtman A, van Cleeff M, et al. Immunogenicity and safety of a 13-valent pneumococcal conjugate vaccine compared to a 23-valent pneumococcal polysaccharide vaccine in pneumococcal vaccine-naive adults. Vaccine 2013; 31:3577–84. [DOI] [PubMed] [Google Scholar]
- 5.Fedson DS. Preventing non bacteremic pneumococcal pneumonia in older adults: historical background and considerations for choosing between PCV13 and PPV23. Hum Vaccin Immunother 2014; 10:1322–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vos Q, Lees A, Wu ZQ, Snapper CM, Mond JJ. B-cell activation by T-cell-independent type 2 antigens as an integral part of the humoral immune response to pathogenic microorganisms. Immunol Rev 2000; 176:154–70. [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez-Fernandez A, Faro J, Fernandez C. Immune responses to polysaccharides: lessons from humans and mice. Vaccine 2008; 26:292–300. [DOI] [PubMed] [Google Scholar]
- 8.Pichichero ME. Protein carriers of conjugate vaccines: characteristics, development, and clinical trials. Hum Vaccin Immunother 2013; 9:2505–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cabaj JL, Nettel-Aguirre A, MacDonald J, Vanderkooi OG, Kellner JD. Influence of Childhood Pneumococcal Conjugate Vaccines on Invasive Pneumococcal Disease in Adults With Underlying Comorbidities in Calgary, Alberta (2000–2013). Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2016; 62:1521–6. [DOI] [PubMed] [Google Scholar]
- 10.Vu DM, Welsch JA, Zuno-Mitchell P, Dela Cruz JV, Granoff DM. Antibody persistence 3 years after immunization of adolescents with quadrivalent meningococcal conjugate vaccine. J Infect Dis 2006; 193:821–8. [DOI] [PubMed] [Google Scholar]
- 11.Kelly DF, Moxon ER, Pollard AJ. Haemophilus influenzae type b conjugate vaccines. Immunology 2004; 113:163–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonten MJ, Huijts SM, Bolkenbaas M, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med 2015; 372:1114–25. [DOI] [PubMed] [Google Scholar]
- 13.Jackson LA, Neuzil KM, Yu O, et al. Effectiveness of pneumococcal polysaccharide vaccine in older adults. N Engl J Med 2003; 348:1747–55. [DOI] [PubMed] [Google Scholar]
- 14.LaFon DC, Nahm MH. Measuring immune responses to pneumococcal vaccines. J Immunol Methods 2018; 461:37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daniels CC, Kim KH, Burton RL, et al. Modified opsonization, phagocytosis, and killing assays to measure potentially protective antibodies against pneumococcal surface protein A. Clinical and vaccine immunology : CVI 2013; 20:1549–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park S, Nahm MH. Older adults have a low capacity to opsonize pneumococci due to low IgM antibody response to pneumococcal vaccinations. Infection and immunity 2011; 79:314–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jha V, Janoff EN. Complementary Role of CD4+ T Cells in Response to Pneumococcal Polysaccharide Vaccines in Humans. Vaccines (Basel) 2019; 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Avci FY, Li X, Tsuji M, Kasper DL. Isolation of carbohydrate-specific CD4(+) T cell clones from mice after stimulation by two model glycoconjugate vaccines. Nature protocols 2012; 7:2180–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Middleton DR, Sun L, Paschall AV, Avci FY. T Cell-Mediated Humoral Immune Responses to Type 3 Capsular Polysaccharide of Streptococcus pneumoniae. Journal of immunology 2017; 199:598–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rabian C, Tschope I, Lesprit P, et al. Cellular CD4 T cell responses to the diphtheria-derived carrier protein of conjugated pneumococcal vaccine and antibody response to pneumococcal vaccination in HIV-infected adults. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2010; 50:1174–83. [DOI] [PubMed] [Google Scholar]
- 21.Fuery A, Richmond PC, Currie AJ. Human Infant Memory B Cell and CD4+ T Cell Responses to HibMenCY-TT Glyco-Conjugate Vaccine. PloS one 2015; 10:e0133126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Larsen M, Sauce D, Arnaud L, Fastenackels S, Appay V, Gorochov G. Evaluating cellular polyfunctionality with a novel polyfunctionality index. PloS one 2012; 7:e42403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dan JM, Lindestam Arlehamn CS, Weiskopf D, et al. A Cytokine-Independent Approach To Identify Antigen-Specific Human Germinal Center T Follicular Helper Cells and Rare Antigen-Specific CD4+ T Cells in Blood. Journal of immunology 2016; 197:983–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reiss S, Baxter AE, Cirelli KM, et al. Comparative analysis of activation induced marker (AIM) assays for sensitive identification of antigen-specific CD4 T cells. PloS one 2017; 12:e0186998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burton RL, Nahm MH. Development and validation of a fourfold multiplexed opsonization assay (MOPA4) for pneumococcal antibodies. Clinical and vaccine immunology : CVI 2006; 13:1004–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson JL, Jones MB, Cobb BA. Polysaccharide A from the capsule of Bacteroides fragilis induces clonal CD4+ T cell expansion. The Journal of biological chemistry 2015; 290:5007–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stephen TL, Niemeyer M, Tzianabos AO, Kroenke M, Kasper DL, Kalka-Moll WM. Effect of B7–2 and CD40 signals from activated antigen-presenting cells on the ability of zwitterionic polysaccharides to induce T-Cell stimulation. Infection and immunity 2005; 73:2184–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duvall MG, Precopio ML, Ambrozak DA, et al. Polyfunctional T cell responses are a hallmark of HIV-2 infection. Eur J Immunol 2008; 38:350–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Braeckel E, Desombere I, Clement F, et al. Polyfunctional CD4(+) T cell responses in HIV-1-infected viral controllers compared with those in healthy recipients of an adjuvanted polyprotein HIV-1 vaccine. Vaccine 2013; 31:3739–46. [DOI] [PubMed] [Google Scholar]
- 30.Crotty S T follicular helper cell differentiation, function, and roles in disease. Immunity 2014; 41:529–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bentebibel SE, Khurana S, Schmitt N, et al. ICOS(+)PD-1(+)CXCR3(+) T follicular helper cells contribute to the generation of high-avidity antibodies following influenza vaccination. Sci Rep 2016; 6:26494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Younes SA, Yassine-Diab B, Dumont AR, et al. HIV-1 viremia prevents the establishment of interleukin 2-producing HIV-specific memory CD4+ T cells endowed with proliferative capacity. The Journal of experimental medicine 2003; 198:1909–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chevalier N, Jarrossay D, Ho E, et al. CXCR5 expressing human central memory CD4 T cells and their relevance for humoral immune responses. Journal of immunology 2011; 186:5556–68. [DOI] [PubMed] [Google Scholar]
- 34.Bentebibel SE, Schmitt N, Banchereau J, Ueno H. Human tonsil B-cell lymphoma 6 (BCL6)-expressing CD4+ T-cell subset specialized for B-cell help outside germinal centers. Proc Natl Acad Sci U S A 2011; 108:E488–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.He J, Tsai LM, Leong YA, et al. Circulating precursor CCR7(lo)PD-1(hi) CXCR5(+) CD4(+) T cells indicate Tfh cell activity and promote antibody responses upon antigen reexposure. Immunity 2013; 39:770–81. [DOI] [PubMed] [Google Scholar]
- 36.Locci M, Havenar-Daughton C, Landais E, et al. Human circulating PD-1+CXCR3-CXCR5+ memory Tfh cells are highly functional and correlate with broadly neutralizing HIV antibody responses. Immunity 2013; 39:758–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morita R, Schmitt N, Bentebibel SE, et al. Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity 2011; 34:108–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.da Silva Antunes R, Paul S, Sidney J, et al. Definition of Human Epitopes Recognized in Tetanus Toxoid and Development of an Assay Strategy to Detect Ex Vivo Tetanus CD4+ T Cell Responses. PloS one 2017; 12:e0169086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abudulai LN, Fernandez S, Corscadden K, et al. Production of IgG antibodies to pneumococcal polysaccharides is associated with expansion of ICOS+ circulating memory T follicular-helper cells which is impaired by HIV infection. PloS one 2017; 12:e0176641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stephen TL, Fabri M, Groneck L, et al. Transport of Streptococcus pneumoniae capsular polysaccharide in MHC Class II tubules. PLoS pathogens 2007; 3:e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kalka-Moll WM, Tzianabos AO, Wang Y, et al. Effect of molecular size on the ability of zwitterionic polysaccharides to stimulate cellular immunity. Journal of immunology 2000; 164:719–24. [DOI] [PubMed] [Google Scholar]
- 42.Avci FY, Li X, Tsuji M, Kasper DL. A mechanism for glycoconjugate vaccine activation of the adaptive immune system and its implications for vaccine design. Nature medicine 2011; 17:1602–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.LaFon DC, Nahm MH. Interlaboratory variability in multiplexed pneumococcal antibody testing. The Journal of allergy and clinical immunology 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang X, Simmerman K, Yen-Lieberman B, Daly TM. Impact of analytical variability on clinical interpretation of multiplex pneumococcal serology assays. Clinical and vaccine immunology : CVI 2013; 20:957–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schenkein JG, Park S, Nahm MH. Pneumococcal vaccination in older adults induces antibodies with low opsonic capacity and reduced antibody potency. Vaccine 2008; 26:5521–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beck SC. Making sense of serotype-specific pneumococcal antibody measurements. Ann Clin Biochem 2013; 50:517–9. [DOI] [PubMed] [Google Scholar]
- 47.Hajjar J, Al-Kaabi A, Kutac C, Dunn J, Shearer WT, Orange JS. Questioning the accuracy of currently available pneumococcal antibody testing. The Journal of allergy and clinical immunology 2018; 142:1358–60. [DOI] [PubMed] [Google Scholar]
- 48.Nahm MH, Briles DE, Yu X. Development of a multi-specificity opsonophagocytic killing assay. Vaccine 2000; 18:2768–71. [DOI] [PubMed] [Google Scholar]
- 49.Wang D, Burton RL, Nahm MH, Soong SJ. A four-parameter logistic model for estimating titers of functional multiplexed pneumococcal opsonophagocytic killing assay. Journal of biopharmaceutical statistics 2008; 18:307–25. [DOI] [PubMed] [Google Scholar]
- 50.Lee H, Cha JH, Nahm MH, Burton RL, Kim KH. The 7-valent pneumococcal conjugate vaccine elicits cross-functional opsonophagocytic killing responses to Streptococcus pneumoniae serotype 6D in children. BMC infectious diseases 2013; 13:474. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Serotype-specific pneumococcal responses measured by MOPA and multiplex IgG assays with respect to PCV13 vaccine (A) and PPSV23 vaccine (B). Comparisons of pre-vaccine (x-axis) and post-vaccine (y-axis) results from multiplex opsonophagocytosis assay (MOPA; reported as opsonization index, or OI; represented by open red circles) and a multiplex IgG assay (in μg/ml; represented by solid black circles). An identity line is included for reference. Responses for PCV13 serotypes (pre-vaccine measurement at day0; post-vaccine at day 28) in n=10 participants are shown (A). Serotype 6A is not included, as it is unique to PCV13 and therefore not contained in the 23-serotype multiplex IgG assay containing PPSV23 serotypes. Responses for PPSV23 serotypes (pre-vaccine measurement at day 360; post-vaccine at day 388) in n = 6 participants are also shown (B).
Supplementary Figure 2. The kinetics of expression of IFN-γ, IL-2, IL-4, granzyme-B and/or CD154 by proliferating (CFSElow) CD4 T cells post PCV13 in response to 5 day stimulation with PS-1 (A) and PS-6A (B) as antigens. Data is shown for five older and five younger vaccinees post prevnar vaccination. Blue= 63-68 years old and red= 24-27 years old.
Supplementary Figure 3. Polyfunctionality of CD4 T cell responses to conjugate 1 (CPS-1) and conjugate 6A (CPS-6A) in the old (A) and young (B) vaccinees post PCV13 vaccination. Responses are compared using Wilcoxon Signed Rank Test. Peak polyfunctionality index data was used in this analysis.
Supplementary Figure 4. Area under the curve (AUC) data for antigen specific response in each individual. PS= polysaccharide. For each antigen, responses in the old and young groups are compared using Mann-Whitney U test, p-values are shown as *(0.05) and ** (0.01). Vertical dotted lines show comparison of responses in old and young vaccinees for each measure.
Supplementary Figure 5. The kinetics of expression of IFN-γ, IL-2, IL-4, granzyme-B and/or CD154 by proliferating (CFSElow) CD4 T cells post PPSV23 in response to 5 day stimulation with PCV13 (A), CRM197 (B) and PS-1 (C). Data is shown older and younger vaccinees (N=3 each) and these vaccinees had previously received PCV13 vaccine. Blue= 63-68 years old and red= 24-27 years old.