Abstract
By the beginning of 2021, the battle against coronavirus disease 2019 (COVID-19) remains ongoing. Investigating the adaptive immune response against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which causes COVID-19, in patients who have recovered from this disease could contribute to our understanding of the natural host immune response. We enrolled 38 participants in this study. 7 healthy participants and 31 COVID-19 patients who had recovered from COVID-19 and categorized them into 3 groups according to their previous clinical presentations: 10 moderate, 9 mild, and 12 asymptomatic. Flow cytometry analysis of peripheral lymphocyte counts in recovered patients showed significantly increased levels of CD4+ T cells in patients with a history of mild and moderate COVID-19 symptoms compared with those healthy individuals (p < 0.05 and p < 0.0001 respectively). whereas no significant difference was observed in the CD8+ T cell percentage in COVID-19-recovered patients compared with healthy individuals. Our study demonstrated that antibodies against the SARS-CoV-2 spike protein (anti-S) IgG antibody production could be observed in all recovered COVID-19 patients, regardless of whether they were asymptomatic (p < 0.05)or presented with mild (p < 0.0001) or moderate symptoms (p < 0.01). Anti-S IgG antibodies could be detected in participants up to 90 days post-infection. In conclusion, the lymphocyte levels in recovered patients were associated with the clinical presentation of the disease, and further analysis is required to investigate relationships between different clinical presentations and lymphocyte activation and function.
Keywords: Cellular immunity, Humoral immunity, COVID-19
1. Introduction
Coronavirus disease 2019 (COVID-19) was classified as a pandemic, spreading worldwide and causing mild to severe respiratory infections. This disease is caused by a virus in the Coronaviridae subfamily, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), resulting in a respiratory infection with a variable clinical presentation that can be classified as asymptomatic, mild, moderate, or severe (Jacob, 2020). The majority of cases are mild, requiring 2 to 3 weeks for recovery. In contrast, severe clinical presentations are associated with inflammation in other systems, including the gastrointestinal, neurological, and cardiovascular systems. The disease outcome can be affected by several factors, such as age, sex, obesity, presence of chronic disease and other factors, knowing the immunological differences associated with these factors can help in revealing the immunological mechanisms behind the severity of COVID-19 infection (Brodin, 2021).
Several studies have shown an association between disease severity and the cellular immune response. Cytokine storm (Chen et al., 2020, Huang et al., 2020), lymphopenia (Liu et al., 2020), and T cell exhaustion (Zheng et al., 2020) are immune response markers that have been correlated with COVID-19 disease pathogenesis (Chen et al., 2020, Tan et al., 2020, Wang et al., 2020, Zheng et al., 2020). Reductions in the total numbers of T cells, CD4+ T cell, CD8+ T cell, and natural killer (NK) cells have been reported in COVID-19 patients, especially in severe cases (Diao et al., 2020, Henry et al., 2020, Liu et al., 2020). During recovery, T cell levels are restored and cytokine levels decrease, including interleukin (IL)-6, IL-10, and tumor necrosis factor-α (TNFα), which suggests a negative association between T lymphocyte counts and cytokine production (Diao et al., 2020). In addition to T lymphocyte counts, another study demonstrated T cell exhaustion among COVID-19 patients. The expression levels of the T cell exhaustion markers programmed death 1 (PD-1) and T cell immunoglobulin and mucin domain-3 (Tim-3) have been shown to increase during the progression of the COVID-19 symptomatic stage, suggesting an adaptive immune evasion (Diao et al., 2020). Further studies examining lymphocyte behavior after recovery will facilitate our understanding of the roles played by T cells in disease progression.
Several clinical vaccine trials are currently ongoing, and most of the tested vaccines are designed to enhance the production of neutralizing antibodies against the SARS-CoV-2 spike protein (Folegatti et al., 2020, Yu et al., 2020, Zhu et al., 2020). However, whether these vaccines will generate long-term or full immunity against COVID-19 remains unclear (WHO, 2020). Increasing our understanding of the natural host immune responses and the development of long-term protective immunity will improve the design of vaccine interventions. A case study report describing a family that recovered from COVID-19 suggested that the initial SARS-Cov-2 infection introduced protection at the humoral level against recurrent infection with the virus (Mahallawi, 2020). Antibodies against SARS-CoV-2 protein can be detected within 7–14 days after infection, which is associated with increased plasma cells (Hashem et al., 2020). SARS-CoV-2 anti-IgG and anti-IgM and neutralizing antibodies have been detected in the serum of patients with different COVID-19 clinical presentations (Hashem et al., 2020, Lin et al., 2020, Mahallawi, 2020).
We aimed to investigate the immune response following COVID-19 recovery among patients with different clinical presentations, including asymptomatic (12 patients), mild (9 patients), and moderate (10 patients) disease progression, and due to the limited access to severe cases they were not included in this study. We examined whether differences in the clinical presentation were associated with changes in T lymphocyte, B lymphocyte, and NK cell counts after recovery and correlated the cell count with the humoral response to obtain insight into the long-term protective immunity that develops in response to COVID-19.
2. Materials and methods
2.1. RNA extraction
Nasopharyngeal (NP) and oropharyngeal (OP) swabs were collected from all participants, placed in a transport medium in synthetic tip flocked swabs (BD, USA), and stored 2–8 °C for a maximum of 72 h. Nucleic acids were extracted using Roche Magna Pure LC (RNA Viral Isolation Kit, USA) by first adding 200 µL of each sample to a MagNA pure LC 96-well plate. Specific reagents were then added, according to the manufacturer’s instructions for nucleic acid extraction. To control for the presence of PCR inhibitors, 1 µL of internal control, provided with the Altona diagnostic RealStar® SARS-Cov-2 RT-PCR kit 1.0, was added to each tube.
2.2. One-step, real-time, RT-PCR amplification
After viral RNA extraction was performed, a reverse transcriptase-polymerase chain reaction (RT-PCR) mixture was prepared using the Altona diagnostic RealStar® SARS-Cov-2 RT-PCR kit 1.0, which targets the sequences for the SARS-CoV-2 envelope (E gene) and spike (S gene) proteins, using a one-step, real-time, RT-PCR method as indicated by the kit manual. The preparation of the one-step, real-time, RT-PCR mixture was done as instructed Altona diagnostic RealStar® SARS-Cov-2 RT-PCR kit 1.0, 5 µL of Master A mix and 15 µL of Master B mix were added. Then, 10 µL extracted RNA was added for a final volume of 30 µL. A non-template negative control (water) and positive control were included in every RT-PCR run.
All RT-PCR tubes were sealed, carefully transferred to a Real-Time LC 480 (Roche, USA), and subjected to the following program: a single cycle of 55 °C for 20 min, a single cycle of 95 °C for 2 min, and 45 cycles of 95 °C for 15 s, 55 °C for 45 s, and 72 °C for 15 s. All RT-PCR steps were performed aseptically in a DNA-free extraction room while wearing disposable gloves and protective clothing to prevent contamination.
2.3. Flow cytometry assay
Flow cytometry (FCM) was performed to count peripheral blood mononuclear (PBMN) cell subsets in all 38 participant samples, which were prepared from anticoagulated whole blood according to the BD Bioscience procedure manual (Phillip Ruiz, 2007). Prepared PBMN cells were stained with the following antibodies: anti-CD45 (PerCP-Cy5.5-A), anti-CD3 (FITC-A), anti-CD4 (PE-Cy7-A), anti-CD8 (APC-Cy7-A), anti-CD19 (APC-A), and anti-CD16 + 56 (PE-A). Stained samples were loaded onto a BD FACSCanto flow cytometer (BD Biosciences). BD Multitest 6-color system enumerates absolute counts (cells/μL) as well as lymphocyte percentages of mature T helper/inducer (CD3+CD4+), T suppressor/cytotoxic (CD3+CD8+), total T (CD3+) cells, B (CD3–CD19+), and NK (CD3–CD16+/CD56+) subsets. The gating strategy was applied according to procedure manual and analyzed using the BD FACSCanto 3.1 software (Phillip Ruiz (2007)).
2.4. Enzyme-Linked immunosorbent assay (ELISA)
An enzyme-linked immunosorbent assay (ELISA) was used to detect anti-S IgG in the participants’ serum samples, according to a previously published protocol (Mahallawi, 2021). In brief, a 96-well ELISA plate (Costar; Corning, Corning, NY, USA) was coated with 100 µL/well (concentration 2 µg/mL) SARS-CoV-2 recombinant S protein (Sino Biological, Beijing, China). The plates were then covered and incubated overnight at 4 °C in the dark. The plates were then washed with washing buffer [phosphate buffered saline (PBS) containing 0.05% Tween-20; Sigma-Aldrich, St. Louis, MO, USA] five times, using an automated microplate washer. Blocking buffer [PBS containing 0.05% fetal bovine serum (FBS) that was heat-inactivated at 56 °C for 60 min; Sigma-Aldrich] was added at 150 µL/well. Serum samples were diluted 1:100 in blocking buffer, 100 µL diluted serum was added to each well, and the plate was incubated for 30 min at room temperature. After washing the plates five times with washing buffer, alkaline phosphatase-conjugated goat anti-human IgG secondary antibody (1:1,000 in blocking buffer, Sigma-Aldrich) was added at 100 µL/well. Plates were incubated at room temperature for 30 min, then washed 5 times with washing buffer. Substrate P-nitrophenyl phosphate (p-NPP, Sigma-Aldrich) was added at 100 µL/well and incubated in the dark for 30 min. Finally, 100 µL stopping solution (1.2 N sodium hydroxide, Reagecon, UK) was added, and the reaction was measured using a microplate reader (ELX800; BioTek) at an optical density of 405 nm (OD405). The cut-off OD405 was calculated as below and was 0.29.
2.5. Patient samples and controls
A total of 38 participants were involved in this study. 31 COVID-19 post recovered patients, who were hospitalized at Madinah Hospital, Saudi Arabia, participated in this study after providing written informed consent. and were categorized as follows: 10 moderate, 9 mild, and 12 asymptomatic. We obtained the medical records and collected data for all hospitalized patients and asymptomatic contacts with PCR-confirmed COVID-19 diagnoses, as reported to the Ministry of Health (MOH) in the Madinah region between June 15, 2020, and August 1, 2020. When the data were extracted from the patients file at the hospital, there were no other diagnosed infections for all the patients enrolled in the study. In addition, 7 healthy participants were recruited from a blood bank as negative controls and informed written consent also obtained from them. All healthy participants were eligible blood donors, they were free from Hepatitis (B and C virus), Human immunodeficiency virus (1 and 2), Syphilis, Malaria, Human T-cell lymphotropic virus type 1, and all of them were not exposed to SARS-Cov-2 before. This study was approved by the research ethics committee of the General Directorate of Health Affairs in Al Madinah (IRB number: 496).
2.6. Statistical analysis
Statistical analysis was conducted using GraphPad Prism, version 8.0 (Graph-Pad Software, Inc., CA, USA), and R (version 4.0.0) software. Differences in lymphocyte percentages between groups were analyzed using the Kruskal-Wallis test with Dunn’s post test for multiple comparisons. Values are presented as the mean ± standard deviation (SD), and significance is reported as *P ≤ 0.05, **P ≤ 0.01; ***P ≤ 0.001, and ****P ≤ 0.0001.
3. Results
3.1. Demographic data and real time-PCR verification
A total of 38 participants were involved in this study, with ages ranging from 20 to 63 years and a median age of 33 years. Among these participants, 31 had past exposure to SARS-CoV-2 and were categorized according to their disease severity. Based on medical history, 10 patients experienced moderate symptoms, 9 patients presented with mild symptoms, and 12 patients were asymptomatic (Table 1). As negative controls, 7 participants were enrolled in this study who had not previously been exposed to SARS-CoV-2, according to a declaration form. The anti-S IgG levels, as assessed by ELISA, were negative for all control participants.
Table 1.
Characteristic of participants involved in the study.
| No. of participants | Age (mean) | Sex | Days post infection (mean) | Clinical presentation | |
|---|---|---|---|---|---|
| Control | 7 | 20–43 (32.1) | 4 (Male) 3 (Female) |
– | Healthy individuals |
| Asymptomatic | 12 | 22–63 (37.9) | 8 (Male) 4 (Female) |
40–97 (65.5) | Patients showing no symptoms |
| Mild | 9 | 23–53 (36) | 8 (Male) 1 (Female) |
33–69 (52.4) | Patients with mild symptoms and no oxygen requirements or pneumonia on chest X-ray |
| Moderate | 10 | 23–44 (32.2) | 7 (Male) 3 (Female) |
37–80 (60.3) | Patients with respiratory symptoms and lung infiltrates in less than 50% of the lung field |
*The disease severity was categorized based on Saudi MOH guidelines.
Long-term SARS-CoV-2 infection has been reported, with some patients reported as having positive SARS-CoV-2 PCR tests for longer than 6 weeks (Lin et al., 2020). To confirm that all tested cohorts were SARS-CoV-2-negative at the time of the study, RT-PCR was performed for all 38 participants. The negative results confirmed that all participants were SARS-CoV-2-negative at the time of this study (these data were obtained from the Saudi (MOH).
3.2. Previous COVID-19 exposure influences T lymphocyte levels in recovered patients
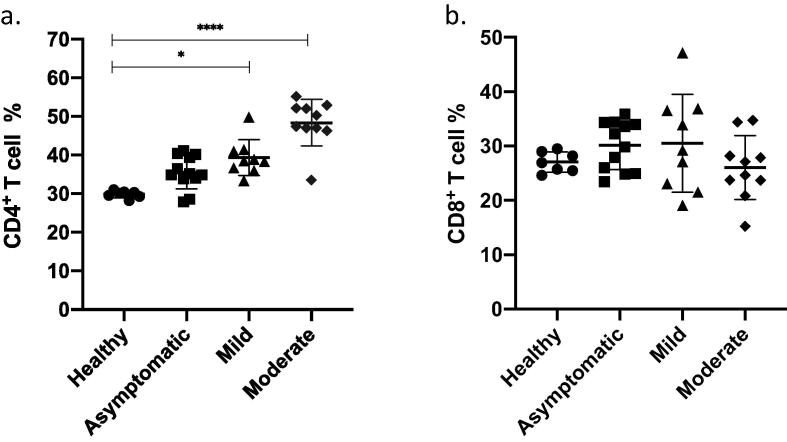
Most patients present with lymphocytopenia during COVID-19, which can serve as an early predictor of disease severity in patients (Tan et al., 2020); thus, lymphocyte counts can reflect the level of cellular immunity in patients. Flow cytometry analysis of PBMNs collected from recovered patients showed significantly increased levels of CD4+ T cells in patients with a history of mild and moderate COVID-19 symptoms compared with those healthy individuals (Fig. 1a, p < 0.05 and p < 0.0001 respectively). These results indicated that the CD4+ T cell percentage increased after recovery from COVID-19 and that the degree of increase was proportional to the severity of the clinical manifestation. In contrast, the CD8+ T cell analysis did not show any significant differences between the 3 groups of patients and healthy controls (Fig. 1b, p > 0.05). Thus, suggesting that SARS-CoV-2 infection does not appear to have a long-term effect on the CD8+ T cell percentage.
Fig. 1.
T lymphocytes percentage. Peripheral blood was collected from healthy donors (n = 7) and from different group of participants who recovered from COVID-19 infection (n = 31). Participants were grouped based on the clinical symptoms they had during the infection and samples were collected after recovery from the infection. Using flow cytometry analysis, T cells were identified and the percentage of CD3+ CD4+ (a) and the percentage of CD3+ CD8+ cells (b) were calculated and shown in graphs (mean ± SD) *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001(Kruskal-Wallis test with Dunn’s post test for multiple comparisons).
3.3. Past exposure to COVID-19 influences B lymphocyte numbers and IgG production in recovered patients
Cell-mediated immune responses have been shown to play an important role in response to SARS-CoV-2 infection, as supported by the production of SARS-CoV-2-specific antibodies post-infection, indicating the induction of humoral immunity following recovery (Hashem et al., 2020). Flow cytometry analysis of PBMNs from recovered patients who presented with mild symptoms demonstrated a significantly increased percentage of B cells compared with healthy individuals (Fig. 2a, p < 0.0001). A similar increase was observed for the production of specific anti-S IgG (Fig. 2b, p < 0.0001), and moreover, anti-S IgG production was observed in all COVID-19 patients, regardless of whether they presented as asymptomatic or with mild or moderate symptoms. The levels of anti-S IgG antibodies remained high for up to 90 days post-infection in some participants, which reflected long-term and persistent antibody levels after recovery. Interestingly, when we compared the specific anti-S IgG levels between total males and females from all COVID-19 recovered patients’ subset, a significant increase in anti-S IgG antibody levels was observed for males compared with females (Fig. 2c, p < 0.05). Similar findings of a sex-dependent humoral immune response against SARS CoV-2 have been reported previously (Robbiani et al., 2020).
Fig. 2.
B lymphocytes percentage and IgG concentration. Peripheral blood was collected from healthy donors (n = 7) and from different group of participants who recovered from COVID-19 infection (n = 31). Participants were grouped based on the clinical symptoms they had during the infection and samples were collected after the recovery from the infection. Using Flowcytometry analysis B cells were identified as CD3- CD19+ and the percentage of B cells are shown in graphs (mean ± SD) (a). Serum samples were collected from all groups and concentration of IgG was measured using ELISA for different group of participants and shown in graphs (mean ± SD) (b), IgG level was compared between male and female (c). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 (Kruskal-Wallis test with Dunn’s post test for multiple comparisons).
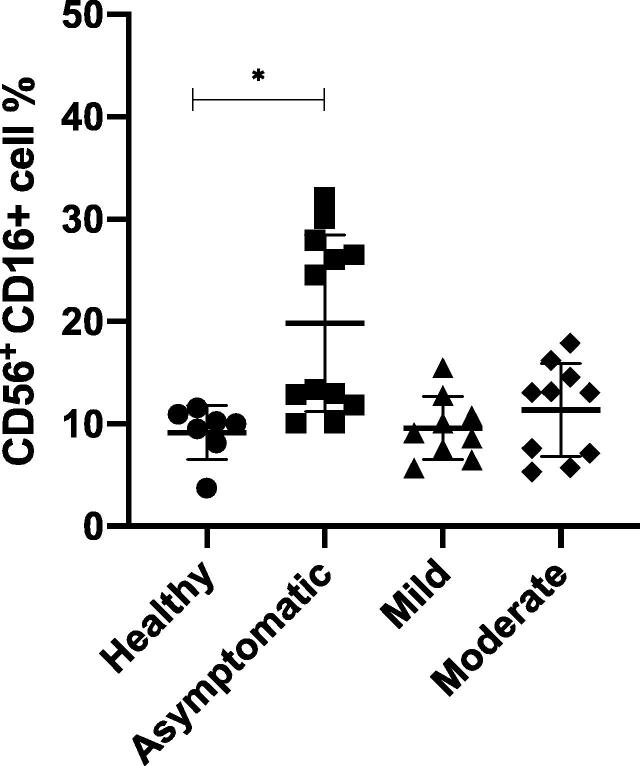
3.4. Past COVID-19 exposure influences natural killer (NK) cells in recovered patients
Lymphocytopenia is a prevalent COVID-19 marker, with reduced NK cell counts reported during infection (Yang et al., 2020). The NK cell numbers were analyzed to determine whether any long-term decrease in NK cell numbers occurs as a result of SARS-CoV-2 infection and whether post-infection changes in NK cell numbers are associated with the severity of the clinical disease manifestation. In this study, we identified no significant differences in the percentages of NK cells after recovery among symptomatic patients compared with that in healthy individuals (Fig. 3, p > 0.05). However, patients with asymptomatic infections showed a significantly increased NK cell percentage compared with that in the healthy group (p < 0.05), suggesting a possible role for NK cells in the manifestation of asymptomatic infections.
Fig. 3.
Natural Killer (NK) cells percentage. Peripheral blood was collected from healthy donors (n = 7) and from different group of participants who recovered from COVID-19 infection (n = 31). Participants were grouped based on the clinical symptoms they had during the infection and samples were collected after recovery from infection. Using flow cytometry analysis, NK cells were identified as CD16+ CD56+ cells and the percentages were calculated and are shown (mean ± SD). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001(Kruskal-Wallis test with Dunn’s post test for multiple comparisons).
4. Discussion
The SARS-CoV-2 infection has had a global impact and can cause respiratory infections with varying degrees of severity, including asymptomatic manifestations in some patients. The World Health Organization has classified COVID-19 as a pandemic, and scientists have been attempting to understand differences in the immune responses of patients with various disease manifestations. Understanding the natural host immune response to this virus and the role played by this response in the determination of disease progression can greatly impact both treatment and prevention strategies. We categorized the participants in the present study according to the COVID-19 coronavirus disease guidelines established by the Saudi ministry of health (MOH). The Saudi MOH has established the following categories for disease severity: asymptomatic, mild, moderate, and severe (MOH, 2020). However, due to the limited access and difficulty in following up with severe cases, they were difficult to obtain and include in this study; therefore, we opted not to include this category of patient.
The present study aimed to examine whether variations in COVID-19 disease severity affects the long-term immune status after recovery. In this study, we examined lymphocyte counts after COVID-19 recovery in patients with various clinical presentations. We analyzed lymphocyte counts and anti-S IgG antibody levels to evaluate both the memory humoral and cellular immune responses following recovery. Our results provided insights into the longevity of natural host immunity to SARS-CoV-2 infections.
We have shown in the current study that the CD4+ T cell percentage increased in all recovered COVID-19 patients compared with those in healthy individuals. The severity of the COVID-19 disease presentation was positively associated with the level of CD4+ T cells after recovery. This finding was similar to those of another study, which reported an increase in CD4+ T cell numbers in discharged patients (Lin et al., 2020). Another study investigated whether these increased CD4+ T cells are SARS-CoV-2-specific memory T cells by examining the T cell response to peptides spanning the SARS-CoV-2 proteome. It has been found in other study that isolated CD4+ T cells from all recovered patients were determined to represent SARS-CoV-2-specific memory T cells (Peng et al., 2020). Similarly, a study presented by Grifoni et al. demonstrated the presence of a CD4+ T cell response in all recovered patients and CD8+ T cell responses in 70% of recovered patients (Grifoni et al., 2020). Moreover, the level of SARS-CoV-2-specific memory T cells increased in patients who had severe disease presentation compared with that in patients who presented with mild cases (Peng et al., 2020), which is similar to the relationship between CD4+ T cells and disease severity that was detected in our study. This association could reflect the high viral load that has been reported for severe cases and the resulting T cell exhaustion or could be due to virus-induced immunopathology and a poor innate immune response. In summary, while our data indicated that CD4+ T cells increased with disease severity, other studies have reported that increased CD4+ T cells in COVID-19-recovered patients are possibly SARS-CoV-2-specific memory T cells.
The cellular invasion of SARS-CoV-2 requires the receptor-binding domain (RBD) of the S protein to bind with the angiotensin-converting enzyme 2 (ACE2) receptor on the cell membrane. Antibodies against S protein and against the viral nucleocapsid protein can be detected starting one week after disease onset and remain detectable for at last three months (Iyer et al., 2020, Lou et al., 2020, Xu et al., 2020). We detected an increase in antibody levels (IgG) in all recovered patients compared to healthy individual, regardless of disease severity, with the highest levels detected in patients who presented with mild symptoms, that was also reflected in B cell percentage. Other reports have detected anti-RBD and anti-S IgG antibodies in plasma samples from recovered individuals (Juno et al., 2020, Robbiani et al., 2020), although anti-RBD and anti-S IgM antibody responses were only detected in 15% and 34% of recovered individuals, respectively (Robbiani et al., 2020). Expanded clones of the viral antigen-specific B cells were detected in all recovered individuals, with anti-RBD IgG memory cells detected in recurrent and clonally expanded antibody responses (Robbiani et al., 2020). However, which clinical presentations were associated with these observations was not clear. In this study, we found that mild symptomatic infections were associated with the highest levels of B cells and IgG antibodies. However, the variation in the results between individual in the mild and moderate groups were observed in this study, no correlation detected in demographic data that could explain this variation (age, gender or days post infection), therefore, larger cohort should be analyzed to further examine these findings.
In this study, we detected a significant difference in the anti-S IgG antibody levels between men and women. Other studies have also reported the increased activity of neutralizing antibodies in men compared with women, and this difference was not found to be associated with age, symptom severity, or symptom duration (Robbiani et al., 2020). These findings could suggest a difference in the humoral response to SARS-CoV-2 between males and females and require further investigation using larger cohorts as well.
NK cells are lymphocytes that play a cytotoxic role during the innate immune response to viral infections. The role played by NK cells during COVID-19 disease is not yet well studied. SARS-CoV-2 patients with moderate to severe disease have been reported to present low levels of peripheral NK cells that are highly activated (Maucourant et al., 2020). In the current study, after recovery, the percentages of CD56+ CD16+ NK cells in moderate and severe patients were not significantly different from that in healthy individuals; however, the asymptomatic patients displayed an increase in the NK percentage. This finding suggests a potential role for the NK cell response in asymptomatic patients and requires further investigation. Although a clear variation between the asymptomatic individuals was observed. Yet this variation was not correlated with any demographic data (age, gender or days post infection), further study should involve a larger cohort to confirm this finding and analyze the role of NK cells in COVID-19 asymptomatic infection. This upregulation of NK cells may indicate a stronger role for this arm of the innate immune response in asymptomatic patients, which may represent the first line of defense that is sustained after recovery.
In conclusion, the lymphocyte levels appear to be affected by different clinical presentations of COVID-19 among patients. Many studies have analyzed immune cells in recovered patients; however, to the best of our knowledge, this is the first study performed in Saudi Arabia to investigate lymphocyte counts and humoral responses in recovered patients in different clinical disease manifestation. The differences in the NK and B cells percentages among the groups with varying clinical manifestations might reflect the different roles these cells play during disease progression. The small cohort size, resulting in insufficient samples, represents the major limitation of this study. Additionally, collecting samples from recovered patients >3 months post infection can provide an insight into immune restoration in COVID-19 patients. More studies should investigate the functional differences among these immune cell types between groups with different disease manifestations, which may provide additional insights into the roles played by these cell types during natural host immunity. CD4+ T cell can differentiate into different effector cells (T helper 1, T helper 2, T helper 17, follicular helper T cell and regulatory T cell) all of which have different roles in viral infection. Additional analysis of CD4+ T cell populations may facilitate the identification of which CD4+ T cell subsets are related to this increase. Likewise, measuring cytokine levels could also demonstrate the functional effects of these increased CD4+ T cell percentages. Thus, additional studies examining the functional roles of CD4+ T cells and B cells in response to SARS-CoV-2 would further our understanding of the cellular immune response during and after COVID-19.
Ethical approval
This study was approved by the ethical committee institutional review board, general directorate of health affairs in Madinah – Ministry of health (MOH), all participants provided informed written consent.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Authors acknowledge the Regional lab in AL-Madinah city – Ministry of health (MOH), and all participants in this study.
Footnotes
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Peer review under responsibility of King Saud University.
References
- Brodin P. Immune determinants of COVID-19 disease presentation and severity. Nat. Med. 2021;27(1):28–33. doi: 10.1038/s41591-020-01202-8. [DOI] [PubMed] [Google Scholar]
- Chen G., Wu D., Guo W., Cao Y., Huang D., Wang H., Ning Q. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Invest. 2020;130(5):2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diao B., Wang C., Tan Y., Chen X., Liu Y., Ning L., Chen Y. Reduction and Functional Exhaustion of T Cells in Patients With Coronavirus Disease 2019 (COVID-19) Front. Immunol. 2020;11:827. doi: 10.3389/fimmu.2020.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folegatti P.M., Ewer K.J., Aley P.K., Angus B., Becker S., Belij-Rammerstorfer S., Oxford C.V.T.G. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396(10249):467–478. doi: 10.1016/S0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grifoni A., Weiskopf D., Ramirez S.I., Mateus J., Dan J.M., Moderbacher C.R., Sette A. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell. 2020;181(7):1489–1501 e1415. doi: 10.1016/j.cell.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashem A.M., Algaissi A., Almahboub S.A., Alfaleh M.A., Abujamel T.S., Alamri S.S., Li X. Early Humoral Response Correlates with Disease Severity and Outcomes in COVID-19 Patients. Viruses. 2020;12(12) doi: 10.3390/v12121390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry B.M., de Oliveira M.H.S., Benoit S., Plebani M., Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin. Chem. Lab. Med. 2020;58(7):1021–1028. doi: 10.1515/cclm-2020-0369. [DOI] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer A.S., Jones F.K., Nodoushani A., Kelly M., Becker M., Slater D., Charles R.C. Dynamics and significance of the antibody response to SARS-CoV-2 infection. medRxiv. 2020 doi: 10.1101/2020.07.18.20155374. [DOI] [Google Scholar]
- Jacob C.O. On the genetics and immunopathogenesis of COVID-19. Clin. Immunol. 2020;220 doi: 10.1016/j.clim.2020.108591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juno J.A., Tan H.X., Lee W.S., Reynaldi A., Kelly H.G., Wragg K., Wheatley A.K. Humoral and circulating follicular helper T cell responses in recovered patients with COVID-19. Nat. Med. 2020;26(9):1428–1434. doi: 10.1038/s41591-020-0995-0. [DOI] [PubMed] [Google Scholar]
- Lin L., Luo S., Qin R., Yang M., Wang X., Yang Q., Hu D. Long-term infection of SARS-CoV-2 changed the body's immune status. Clin. Immunol. 2020;218 doi: 10.1016/j.clim.2020.108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R., Wang Y., Li J., Han H., Xia Z., Liu F., Zhu C. Decreased T cell populations contribute to the increased severity of COVID-19. Clin. Chim. Acta. 2020;508:110–114. doi: 10.1016/j.cca.2020.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou B., Li T.D., Zheng S.F., Su Y.Y., Li Z.Y., Liu W., Chen Y. Serology characteristics of SARS-CoV-2 infection after exposure and post-symptom onset. Eur. Respir. J. 2020;56(2) doi: 10.1183/13993003.00763-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahallawi W. Case Report: A recovered SARS CoV-2 patient protected from reinfection. Front. Med. (Lausanne) 2020;7 doi: 10.3389/fmed.2020.564264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahallawi Waleed. A serological assay to detect human SARS-CoV-2 antibodies. J. Taibah Univ. Medical Sci. 2021;16:57–62. doi: 10.1016/j.jtumed.2020.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maucourant C., Filipovic I., Ponzetta A., Aleman S., Cornillet M., Hertwig L., Karolinska C.-S.G. Natural killer cell immunotypes related to COVID-19 disease severity. Sci. Immunol. 2020;5(50) doi: 10.1126/sciimmunol.abd6832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOH, 2020. Saudi MoH Protocol for Patients Suspected of/Confirmed with COVID-19. Version 2.2. Retrieved from https://www.moh.gov.sa/Ministry/MediaCenter/Publications/Documents/MOH-therapeutic-protocol-for-COVID-19.pdf.
- Peng Y., Mentzer A.J., Liu G., Yao X., Yin Z., Dong D., Dong T. Broad and strong memory CD4(+) and CD8(+) T cells induced by SARS-CoV-2 in UK convalescent individuals following COVID-19. Nat. Immunol. 2020;21(11):1336–1345. doi: 10.1038/s41590-020-0782-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillip Ruiz, M., Michael J. Borowitz, Henok Tilahun, T.C., Liz Keyer, & Coxey, A., 2007. Productivity and Efficiency of 6-Color BD Multitest and BD Trucount Technologies.
- Robbiani D.F., Gaebler C., Muecksch F., Lorenzi J.C.C., Wang Z., Cho A., Nussenzweig M.C. Convergent antibody responses to SARS-CoV-2 in convalescent individuals. Nature. 2020;584(7821):437–442. doi: 10.1038/s41586-020-2456-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan L., Wang Q., Zhang D., Ding J., Huang Q., Tang Y.Q., Miao H. Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. Signal Transduct Target Ther. 2020;5(1):33. doi: 10.1038/s41392-020-0148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Nie J., Wang H., Zhao Q., Xiong Y., Deng L., Zhang Y. Characteristics of Peripheral Lymphocyte Subset Alteration in COVID-19 Pneumonia. J. Infect. Dis. 2020;221(11):1762–1769. doi: 10.1093/infdis/jiaa150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO, 2020. Draft landscape of COVID-19 candidate vaccines. Retrieved from https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines.
- Xu Y., Xiao M., Liu X., Xu S., Du T., Xu J., Wang M. Significance of serology testing to assist timely diagnosis of SARS-CoV-2 infections: implication from a family cluster. Emerg Microbes Infect. 2020;9(1):924–927. doi: 10.1080/22221751.2020.1752610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Gou J., Gao J., Huang L., Zhu Z., Ji S., Zhang Y. Immune characteristics of severe and critical COVID-19 patients. Signal Transduct Target Ther. 2020;5(1):179. doi: 10.1038/s41392-020-00296-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Tostanoski L.H., Peter L., Mercado N.B., McMahan K., Mahrokhian S.H., Barouch D.H. DNA vaccine protection against SARS-CoV-2 in rhesus macaques. Science. 2020;369(6505):806–811. doi: 10.1126/science.abc6284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng M., Gao Y., Wang G., Song G., Liu S., Sun D., Tian Z. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell. Mol. Immunol. 2020;17(5):533–535. doi: 10.1038/s41423-020-0402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu F.C., Li Y.H., Guan X.H., Hou L.H., Wang W.J., Li J.X., Chen W. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. Lancet. 2020;395(10240):1845–1854. doi: 10.1016/S0140-6736(20)31208-3. [DOI] [PMC free article] [PubMed] [Google Scholar]