Abstract
The Cepheid Xpert Xpress SARS-CoV-2/Flu/RSV combination test received emergency use authorization approval by the United States Food and Drug Administration in December 2020, and Health Canada approval in January 2021. The performance characteristics of the GeneXpert Xpert Xpress SARS-CoV-2/Flu/RSV combination test were assessed at Lakeridge Health Oshawa and the National Microbiology Laboratory of Canada. The combination test was compared to the Xpert SARS-CoV-2 and Xpert Flu/RSV assays, and the BioFire FilmArray Respiratory Panel 2.1 (RP2.1) test kit. Materials evaluated were serial dilutions of chemically-inactivated SARS-CoV-2 and remnant clinical specimens (nasal or nasopharyngeal swabs) collected from patients. The limit of detection (LOD) for the SARS-CoV-2 component of the Xpert SARS-CoV-2/Flu/RSV combination test was determined to be <100 viral copies/mL when using chemically-inactivated SARS-CoV-2. In total, 86 clinical positive and 51 clinical negative samples were used for this study, with mixtures of clinical positives being used to mimic coinfection and screen for competitive inhibition. The combination test showed a high percent agreement with the Xpert SARS-CoV-2 and Xpert Flu/RSV tests, as well as the BioFire FilmArray RP2.1. Based on the findings from this study and a growing body of research, the Xpert SARS-CoV-2/Flu/RSV combination test will serve as an effective replacement for the Xpert SARS-CoV-2 and Xpert Flu/RSV assays.
1. Introduction
The coronavirus disease 2019 (COVID-19) pandemic has created an unprecedented demand for diagnostic testing. In response, many SARS-CoV-2 rapid tests have been approved by the United States Food and Drug Administration (FDA) and Health Canada in 2020, including the Cepheid® GeneXpert® Xpert® Xpress SARS-CoV-2 Assay. The GeneXpert performs rapid, fully-automated, and self-contained multiplex RT-qPCR tests with run times of <50 min. The Xpert SARS-CoV-2 assay targets two SARS-CoV-2 genome regions, the envelope (E) and the nucleocapsid (N), and returns a cycle threshold (Ct) value if the target is detected within 45 amplification cycles.
In response to the COVID-19 pandemic, existing platforms that support multiplex respiratory assays, such as the BioMérieux BioFire® FilmArray®, have expanded their panels to include SARS-CoV-2. Consistent with this trend, Cepheid has developed a new formulation of their Xpert Flu/RSV (Respiratory Syncytial Virus) assay that includes targets for SARS-CoV-2. This new multiplex Xpert® SARS-CoV-2/Flu/RSV test will effectively replace existing Xpert SARS-CoV-2 and Xpert Flu/RSV assays that have been widely implemented across North America.
The current Xpert Xpress SARS-CoV-2 and Xpert Xpress Flu/RSV assays have been demonstrated to be highly-sensitive tests in numerous analytical and clinical studies. Cepheid reports 100% sensitivity (n = 35) at 250 copies (cp)/mL for its SARS-CoV-2 assay, while independent studies have reported a limit of detection (LOD) ranging from 8.3 to 60 cp/mL [1], [2], [3]. The assay has shown excellent agreement with the Roche Cobas 6800 system [4], [5], [6], [7], [8] and laboratory-developed RT-qPCR tests [3,6]. Similarly, the GeneXpert Flu/RSV assay has shown a high level of agreement (~97%) with established laboratory-developed tests [9,10] and the BioFire FilmArray [11].
As a rapid near-point-of-care device, the GeneXpert is currently used as a testing solution to improve turnaround times in major health centers. As an example of its utility in urban health centers, one hospital study found the GeneXpert Flu/RSV test significantly improved turnaround time and decreased the time until isolation from an average of 21 to 4 h in comparison with their standard laboratory-developed Influenza test [12]. The GeneXpert has also been utilized in Northern, Remote, and Isolated (NRI) communities throughout Canada during the COVID-19 pandemic to support rapid on-site testing and to significantly decrease the test turnaround time. As the GeneXpert is being widely used in a variety of settings, evaluation of the newly-developed SARS-CoV-2/Flu/RSV combination assay is essential.
In this evaluation study, remnant clinical samples were used to evaluate the SARS-CoV-2/Flu/RSV combination test and compare it with the Xpert Xpress SARS-CoV-2 and Xpert Xpress Flu/RSV assays. The LOD of the SARS-CoV-2 component of the SARS-CoV-2/Flu/RSV combination test was characterized using commercially available, chemically-inactivated virus. In addition, the Xpert Xpress SARS-CoV-2/Flu/RSV assay was compared to the BioFire FilmArray RP2.1 panel, another rapid multiplex assay that tests for 22 pathogens including SARS-CoV-2, Influenza A/B, and RSV. This evaluation was performed across two independent laboratories, the National Microbiology Laboratory [NML (Winnipeg, MB)] and Lakeridge Health (Oshawa, Canada).
2. Materials and methods
2.1. Clinical specimens
Remnant universal transport media (UTM) from nasopharyngeal or nasal clinical swabs collected at the Cadham Provincial Laboratory (Winnipeg, Canada), Lakeridge Health Oshawa (Oshawa, Canada), and the Public Health Ontario Laboratory (Toronto, Canada). Study samples included 51 clinical specimens negative for SARS-Cov-2, Influenza and RSV, and an additional 76 clinical positive specimens containing either SARS-CoV-2, Influenza A, Influenza B, or RSV. Of these, 51 were obtained from prospective sampling and the remainder were previously characterized by laboratory-developed RT-qPCR tests or the Cepheid GeneXpert. Selected samples were chosen to cover a wide range of Ct values from approximately 15–40. To simulate patient coinfection with multiple target viruses, remnant transport media from an additional 24 clinical specimens was mixed in various combinations to create 10 contrived samples, each containing two or three target viral pathogens. All samples used for this study were ethics-exempt, anonymized, diagnostic samples.
2.2. Dilution series and limit of detection experiments with chemically-inactivated SARS-CoV-2
Nasal swabs from healthy SARS-CoV-2 negative donors were collected and placed in UTM at a ratio of one swab per two mL of UTM to form a nasal matrix. ZeptoMetrix (Buffalo, United States) NATrol chemically-inactivated SARS-CoV-2 virus (Cat: NATSARS-ST), at a stock concentration 1.2 × 106 cp/mL, was serially-diluted in this nasal matrix to concentrations ranging from 16 cp/mL to 1000 cp/mL. For each dilution, 300 µL was tested on the GeneXpert using either the Xpert SARS-CoV-2 assay or the Xpert SARS-CoV-2/Flu/RSV assay as per the manufacturer's instructions. To conserve test cartridges required for the pandemic response, the dilution series was performed only for SARS-CoV-2 in duplicate at 16, 32, 64, 125, and 250 cp/mL, while single dilutions were prepared at 500 and 1000 cp/mL.
2.3. Evaluation of the Xpert SARS-CoV-2/flu/RSV assay using clinical specimens
To investigate the percent positive agreement of the Xpert SARS-CoV-2/Flu/RSV combination test, 300 µL of transport media from nasal or nasopharyngeal swabs was tested in parallel using the Xpert SARS-CoV-2, Xpert Flu/RSV, and Xpert SARS-CoV-2/Flu/RSV assays. To mimic coinfection, clinical samples containing SARS-CoV-2, Influenza, or RSV were combined in equal volumes to represent coinfection with two or three viruses, and tested across all three Xpert assays. For a subset of these clinical specimens, including the combined samples used to mimic coinfection, 300 µL of transport media was also tested on the BioFire FilmArray as per the manufacturer's instructions. In addition, GeneXpert tests performed at Lakeridge Health Oshawa were timed beginning from when the cartridge was loaded onto the device and ending with cartridge ejection.
3. Results
Commercially available, chemically-inactivated SARS-CoV-2 was used to compare the LOD performance of the Xpert SARS-CoV-2/Flu/RSV assay and the Xpert SARS-CoV-2 assay (Table 1 ). Both assays detected SARS-CoV-2 at an input concentration of 64 cp/mL but only the Xpert SARS-CoV-2 assay detected the virus at 32 cp/mL; however, the sample size for determining LOD was limited. Note that only one Ct value is provided for SARS-CoV-2 on the SARS-CoV-2/Flu/RSV assay, as both E and N2 targets are detected on the same channel and cannot be read separately.
Table 1.
SARS-CoV-2 limit of detection with the Xpert SARS-CoV-2/Flu/RSV combination assay versus the Xpert SARS-CoV-2 assay. Dilutions at 1000 and 500 viral copies/mL were tested in singlicate, and the remaining dilutions in duplicate. Ct values shown are the average across all replicates.
| Input concentration (copies/mL) | Xpert SCV2/Flu/RSV | Xpert SARS-CoV-2 | |
|---|---|---|---|
| Ct | E Ct | N2 Ct | |
| 1000 | 34.5 | 34.4 | 37.1 |
| 500 | 35.6 | 35.2 | 39.2 |
| 250 | 36.1 | 35.7 | 40.0 |
| 125 | 37.2 | 37.2 | 40.0 |
| 64 | 38.5 | 38.4 | 41.2 |
| 32 | ND | 39.8 | 41.4 |
| 16 | ND | ND | 43.0 (1/2) |
| Negative | ND | ND | ND |
SCV2 = SARS-CoV-2; ND = Not detected.
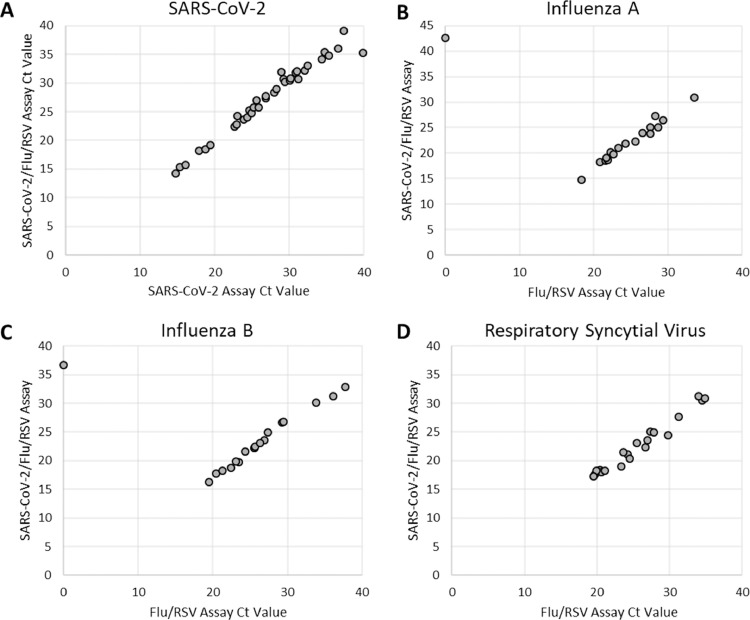
In a side-by-side comparison between 40 SARS-CoV-2 positive clinical samples and 86 negative specimens, there was 100% agreement between the Xpert SARS-CoV-2 assay and SARS-CoV-2/Flu/RSV assay (Table 2 ; Fig. 1 A). Ct values of clinical positives ranged from 14.8 to 43.1, and reported Ct values were similar across both assays (Fig. 1 A). Note: two previous SARS-CoV-2 positive clinical samples with high Ct values (40.1 and 43.1), were not detected by either assay in the side-by-side comparison. The presence of multiple viral targets did not have a notable effect on test performance or agreement between assays; as SARS-CoV-2 Ct values remained similar across both assays, regardless of the presence of Influenza A, Influenza B or RSV targets (Supplemental Table 1).
Table 2.
Concordance of the Xpert SARS-CoV-2 Assay with the Xpert SARS-CoV-2/Flu/RSV Assay for the detection of SARS-CoV-2.
| Result with Xpert SARS-CoV-2 Assay | Result with Xpert SARS-CoV-2/Flu/RSV Combination Test | |
|---|---|---|
| SARS-CoV-2 | ||
| Positive | Negative | |
| Positive | 40 | 0 |
| Negative | 0 | 86 |
Fig. 1.
Ct value comparisons between different Xpert assays using clinical specimens. Overall, reported Ct values are lower on the Xpert SARS-CoV-2/Flu/RSV combination test as compared to others. The Xpert SARS-CoV-2/Flu/RSV assay detected one high Ct Influenza A clinical sample and one high Ct Influenza B clinical that were not detected by the Flu/RSV test.
Similar side-by-side comparisons of the Xpert Flu/RSV and Xpert SARS-CoV-2/Flu/RSV assays were performed (Table 3 ; Fig. 1 B–D). Overall, there was a high level of concordance between assays, with 99.0% agreement for Influenza A and Influenza B, and 100% agreement for RSV (Table 3). One high-Ct (Ct=42.6) Influenza A clinical sample and one high-Ct (36.7) Influenza B clinical sample were not detected by the Xpert Flu/RSV assay but were detected with the Xpert SARS-CoV-2/Flu/RSV combination test. Overall, reported Ct values for Influenza A, Influenza B, and RSV were notably lower for the Xpert SARS-CoV-2/Flu/RSV assay, with targets reporting an average of 2.8, 3.3, and 3.1 cycles earlier, respectively (Fig. 1 B–D).
Table 3.
Concordance of the Xpert Flu/RSV Assay with the SARS-CoV-2/Flu/RSV Assay for the detection of Influenza A, Influenza B and RSV.
| Result with Xpert Flu/RSV Assay | Result with Xpert SARS-CoV-2/Flu/RSV Combination Test | |||||
|---|---|---|---|---|---|---|
| Influenza A | Influenza B | RSV | ||||
| Positive | Negative | Positive | Negative | Positive | Negative | |
| Positive | 17 | 0 | 18 | 0 | 20 | 0 |
| Negative | 1 | 87 | 1 | 86 | 0 | 85 |
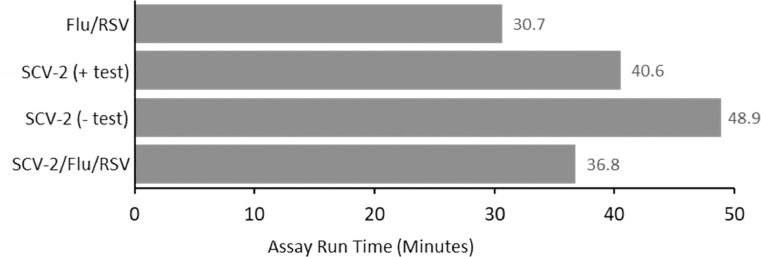
The test run time was compared across all of the Xpert assays investigated in this study (Fig. 2 ). On average, the shortest run time was the Xpert Flu/RSV assay with a run time of 30.7 min, followed by the Xpert SARS-CoV-2/Flu/RSV assay at 36.8 min. The Xpert SARS-CoV-2 assay had the longest run time, with an average of 48.9 min for negative test runs and 40.6 min for positive tests (the Xpert SARS-CoV-2 assay has an early termination option for positive test runs).
Fig. 2.
Average assay run time across the different GeneXpert Xpress assays evaluated in this study. Because the SARS-CoV-2 (SCV-2) assay can terminate early during positive tests, average time is displayed as both the run time for either positive (+) or negative (-) tests.
Finally, this study compared the Xpert SARS-CoV-2/Flu/RSV Assay with the BioFire FilmArray RP2.1 panel (Table 4 ). Overall, the two assays were highly concordant, with 100% agreement for SARS-CoV-2 and RSV targets, and 97% agreement for Influenza A and Influenza B. Two samples were discordant between the platforms – one high Ct (Ct = 43.2) Influenza A clinical sample was detected exclusively by the Xpert SARS-CoV-2/Flu/RSV assay, and one high Ct (Ct = 34.8) Influenza B clinical sample was detected exclusively with the BioFire RP2.1 Panel. As the RP2.1 Panel is a strictly qualitative test, comparisons of Ct values are not possible between these platforms. The RP2.1 Panel has a total of 22 targets and detected additional pathogens within these clinical samples including Adenovirus, Rhinovirus/Enterovirus, Bordetella pertussis, and ParaInfluenza (Supplemental Table 1). Two high Ct value Influenza A specimens (Ct values 40.5 and 40.6) detected by the laboratory-based test failed to be detected by any assays performed on either the GeneXpert or Biofire FilmArray.
Table 4.
Concordance of the BioFire FilmArray RP2.1 panel with the Xpert SARS-CoV-2/Flu/RSV assay for the detection of Influenza A, Influenza B, RSV and SARS-CoV-2.
| Result with BioFire FilmArray RP2.1 | Result with Xpert SARS-CoV-2/Flu/RSV Combination Test | |||||||
|---|---|---|---|---|---|---|---|---|
| SARS-CoV-2 | Influenza A | Influenza B | RSV | |||||
| Positive | Negative | Positive | Negative | Positive | Negative | Positive | Negative | |
| Positive | 16 | 0 | 7 | 0 | 8 | 1 | 10 | 0 |
| Negative | 0 | 17 | 1 | 25 | 0 | 24 | 0 | 23 |
4. Discussion and conclusion
A laboratory and clinical evaluation of the Xpert SARS-CoV-2/Flu/RSV combination test was performed by comparing it to existing Xpert assays for SARS-CoV-2 and Flu/RSV as well as the BioFire FilmArray RP2.1 Panel. This study required a total of 151 clinical samples (including mixtures) that were processed at two independent institutions.
When used as a multiplex assay, the new Xpert SARS CoV-2/Flu/RSV combination assay has a run time of approximately 36.8 min as opposed to run times of 40.6 min for a positive and 48.9 min for a negative SARS-CoV-2 result when utilizing the Xpert SARS-CoV-2 assay. Cepheid reports that when the combination test is run as a single assay for only SARS-CoV-2, an early termination of the test can be applied which can reduce the test run time to as short as 25 min (as per the package insert). BioMérieux reports a run time of approximately 45 min, consistent with our observations during this study.
The combination test reports on a single channel for SARS-CoV-2, but otherwise utilizes the same test architecture as the Xpert SARS-CoV-2 and Xpert Flu/RSV assays. This evaluation has shown that the Xpert SARS-CoV-2/Flu/RSV combination test is highly concordant with existing Xpert assays and the BioFire RP2.1 Panel. Mixtures of clinical samples were used to identify potential issues that are known to affect multiplex assays such as competitive inhibition. The presence of multiple targets did not have a notable effect on agreement between assays nor reported Ct values.
These findings are consistent with other recent research comparing the Xpert SARS-CoV-2/Flu/RSV to other Xpert assays [13,14] or the BioFire FilmArray RP2.1 panel [13] where a high percent agreement (>98%) was also observed. Together, these studies provide strong evidence that the Xpert SARS-CoV-2/Flu/RSV combination test can effectively replace the Xpert SARS-CoV-2 and Xpert Flu/RSV assays currently in use across North America.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jcvp.2021.100014.
Appendix. Supplementary materials
References
- 1.Zhen W., Smith E., Manji R., Schron D., Berry G.J. Clinical evaluation of three sample-to-answer platforms for the detection of SARS-CoV-2. J. Clin. Microbiol. 2020 doi: 10.1128/JCM.00783-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Becker M.G., Taylor T., Kiazyk S., Cabiles D.R., Meyers A.F.A., Sandstrom P.A. Recommendations for sample pooling on the Cepheid GeneXpert® system using the Cepheid Xpert® Xpress SARS-CoV-2 assay. PLoS ONE. 2020;15 doi: 10.1371/journal.pone.0241959. Darlix J-LE, editor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolters F., van de Bovenkamp J., van den Bosch B., van den Brink S., Broeders M., Chung N.H., et al. Multi-center evaluation of Cepheid Xpert® Xpress SARS-CoV-2 point-of-care test during the SARS-CoV-2 pandemic. J. Clin. Virol. 2020;128 doi: 10.1016/j.jcv.2020.104426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moran A., Beavis K.G., Matushek S.M., Ciaglia C., Francois N., Tesic V., et al. The detection of SARS-CoV-2 using the Cepheid Xpert Xpress SARS-CoV-2 and Roche cobas SARS-CoV-2 assays. J. Clin. Microbiol. 2020 doi: 10.1128/JCM.00772-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smithgall M.C., Scherberkova I., Whittier S., Green D.A. Comparison of Cepheid Xpert Xpress and Abbott ID Now to Roche cobas for the rapid detection of SARS-CoV-2. bioRxiv. 2020; 2020.04.22.055327. doi:10.1101/2020.04.22.055327 [DOI] [PMC free article] [PubMed]
- 6.Lieberman J., Pepper G., Naccache S.N., Huang M., Jerome K.R., Greninger A.L. Comparison of commercially available and laboratory developed assays for in vitro detection of SARS-CoV-2 in clinical laboratories. medRxiv. 2020; 2020.04.24.20074559. doi:10.1101/2020.04.24.20074559 [DOI] [PMC free article] [PubMed]
- 7.Broder K., Babiker A., Myers C., White T., Jones H., Cardella J., et al. Test agreement between Roche cobas 6800 and Cepheid Genexpert Xpress SARS-CoV-2 assays at high cycle threshold ranges. J. Clin. Microbiol. 2020;58 doi: 10.1128/JCM.01187-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldenberger D., Leuzinger K., Sogaard K.K., Gosert R., Roloff T., Naegele K., et al. Brief validation of the novel GeneXpert Xpress SARS-CoV-2 PCR assay. J. Virol. Methods. 2020;284:8–10. doi: 10.1016/j.jviromet.2020.113925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ho Y.I.I., Wong A.H., Lai R.W.M. Comparison of the Cepheid Xpert Xpress Flu/RSV assay to in house Flu/RSV triplex real-time RT-PCR for rapid molecular detection of Influenza A, Influenza B and respiratory syncytial virus in respiratory specimens. J. Med. Microbiol. 2018;67:1576–1580. doi: 10.1099/jmm.0.000841. [DOI] [PubMed] [Google Scholar]
- 10.Salez N., Nougairede A., Ninove L., Zandotti C., de Lamballerie X., Charrel R.N. Prospective and retrospective evaluation of the Cepheid Xpert® Flu/RSV XC assay for rapid detection of Influenza A, Influenza B, and respiratory syncytial virus. Diagn Microbiol Infect Dis. 2015;81:256–258. doi: 10.1016/j.diagmicrobio.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wahrenbrock M.G., Matushek S., Boonlayangoor S., Tesic V., Beavis K.G., Charnot-Katsikas A. Comparison of cepheid xpert Flu/RSV XC and biofire filmarray for detection of Influenza a, Influenza b, and respiratory syncytial virus. J. Clin. Microbiol. 2016;54:1902–1903. doi: 10.1128/JCM.00084-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berry L., Lansbury L., Gale L., Carroll A.M., Lim W.S. Point of care testing of Influenza A/B and RSV in an adult respiratory assessment unit is associated with improvement in isolation practices and reduction in hospital length of stay. J. Med. Microbiol. 2020;69:697–704. doi: 10.1099/jmm.0.001187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mostafa H.H., Carroll K.C., Hicken R., Berry G.J., Manji R., Smith E., et al. Multi-center evaluation of the Cepheid Xpert® Xpress SARS-CoV-2/Flu/RSV test. J. Clin. Microbio.l. 2020;59 doi: 10.1128/JCM.02955-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leung E.C., Chow V.C., Lee M.K., Tang K.P., Li D.K., Lai R.W. Evaluation of the Xpert Xpress SARS-CoV-2/Flu/RSV assay for simultaneous detection of SARS-CoV-2, Influenza A/B and respiratory syncytial viruses in nasopharyngeal specimens. J. Clin. Microbiol. 2021;59(4) doi: 10.1128/JCM.02965-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.