Abstract
Background
Severe Acute Respiratory Syndrome coronavirus-2 (SARS-CoV-2) has challenged public health agencies globally. In order to effectively target government responses, it is critical to identify the individuals most at risk of coronavirus disease-19 (COVID-19), developing severe clinical signs, and mortality. We undertook a systematic review of the literature to present the current status of scientific knowledge in these areas and describe the need for unified global approaches, moving forwards, as well as lessons learnt for future pandemics.
Methods
Medline, Embase and Global Health were searched to the end of April 2020, as well as the Web of Science. Search terms were specific to the SARS-CoV-2 virus and COVID-19. Comparative studies of risk factors from any setting, population group and in any language were included. Titles, abstracts and full texts were screened by two reviewers and extracted in duplicate into a standardised form. Data were extracted on risk factors for COVID-19 disease, severe disease, or death and were narratively and descriptively synthesised.
Results
One thousand two hundred and thirty-eight papers were identified post-deduplication. Thirty-three met our inclusion criteria, of which 26 were from China. Six assessed the risk of contracting the disease, 20 the risk of having severe disease and ten the risk of dying. Age, gender and co-morbidities were commonly assessed as risk factors. The weight of evidence showed increasing age to be associated with severe disease and mortality, and general comorbidities with mortality. Only seven studies presented multivariable analyses and power was generally limited. A wide range of definitions were used for disease severity.
Conclusions
The volume of literature generated in the short time since the appearance of SARS-CoV-2 has been considerable. Many studies have sought to document the risk factors for COVID-19 disease, disease severity and mortality; age was the only risk factor based on robust studies and with a consistent body of evidence. Mechanistic studies are required to understand why age is such an important risk factor. At the start of pandemics, large, standardised, studies that use multivariable analyses are urgently needed so that the populations most at risk can be rapidly protected.
Registration
This review was registered on PROSPERO as CRD42020177714.
Keywords: Coronavirus, COVID-19, Systematic review, Review, Risk factors, Morbidity, Mortality
Introduction
The world is currently experiencing a pandemic of coronavirus disease (COVID-19) caused by the Severe Acute Respiratory Syndrome coronavirus-2 (SARS-CoV-2) [1]. The risk of morbidity and mortality from the virus is strongly stratified, with poor clinical outcomes considered more likely in certain vulnerable groups. For example, studies from different countries have established that older age groups are at increased risk of death [2, 3].
The ability to identity the population groups most at risk from the virus has manifold public health purposes. Using such data, stratified vaccination policies for governmental delivery can be designed, similar to those for influenza [4]. It may also be possible to prioritise more active monitoring of groups more at risk of clinical deterioration, and facilitate access to healthcare facilities by early identification of the individuals most likely to progress to severe disease who would thus be in need of intensive care and ventilation. Official advice can be issued to vulnerable groups to let them know that they are more at risk from SARS-CoV-2 virus, to promote behaviour modification [5, 6]. Such population groups can also be the target of more formalised ‘segment and shield’ approaches: having divided the population into groups that present with similar health care concerns and needs (segmenting) it is possible to determine which groups require extra protection by reducing interaction with other groups (shielding), whilst relaxing restrictions for the rest of the population [7]. Potential public health policies along this route have been critiqued, however, on an inclusivity basis, particularly due to the unintended harmful consequences to already marginalised groups [8].
In the UK, vulnerable people were stratified into two tiers early on- 30th March 2020 (Table 1); those at risk of severe illness, who were advised to be particularly stringent with social distancing measures, and those within that group at further risk – described as ‘shielded’ individuals – who were advised to self-isolate and were provided with additional advice [9–12]. The former categorisation was based on the groups targeted for National Health Service programmes on influenza vaccination and the latter on clinical consensus. These strata were deliberately broad, to maximise the number of individuals protected. As the evidence evolves – e.g. regarding whether the development of lesions in the cardiovascular system contributes meaningfully to disease pathogenesis in patients with and without pre-existing cardiovascular conditions [13] – there is the opportunity for the categorisation of risk of COVID-19 and serious outcomes from COVID-19 to become more evidence-based.
Table 1.
UK risk groupings for COVID-19 disease on 30th March 2020
| At risk of severe illness | Shielding | |
|---|---|---|
| Aged 70 or older (regardless of medical conditions) | ||
| Aged under 70 anda | ||
| Chronic (long-term) mild to moderate respiratory diseases, such as asthma, COPD, emphysema or bronchitis | People with severe chest conditions such as cystic fibrosis or severe asthma (requiring hospital admissions or courses of steroid tablets) | |
| Chronic heart disease, such as heart failure | ||
| Chronic kidney disease | People with severe diseases of body systems, such as severe kidney disease (dialysis) | |
| Chronic liver disease, such as hepatitis | ||
| Chronic neurological conditions, such as Parkinson’s disease, motor neurone disease, MS, a learning disability or cerebral palsy | ||
| Diabetes | ||
| A weakened immune system as the result of conditions such as HIV and AIDS, or medicines such as steroid tablets | People who have received an organ transplant and remain on ongoing immunosuppression medication | |
| People with cancer who are undergoing active chemotherapy or radiotherapy | ||
| People with cancers of the blood or bone marrow such as leukaemia who are at any stage of treatment | ||
| Being seriously overweight (a BMI of 40 or above) | ||
| Those who are pregnant | ||
During epidemics and pandemics of emerging infectious diseases, it is critical to rapidly and accurately identify the populations most at risk. In the case of COVID-19, we undertook a systematic review and quality assessment of the rapidly-evolving global literature in this area, looking at three key outcomes: COVID-19 disease, disease severity, and mortality from the condition. Any potential risk factors, populations, and study designs were included. Arising from our findings, we highlight key knowledge gaps in the current literature and the need for unified global approaches moving forwards, particularly for the next pandemic.
Materials and methods
Literature search
We systematically searched Medline, Embase, and Global Health (all via the Ovid platform), in addition to the Web of Science, for published literature between 1st November 2019 and 26th March 2020; then subsequently updated this search for a later period to 29th April. In order to avoid missing publications on risk factors, only terms specific to the virus and the disease were used, which were combined with ‘or’:
‘coronavirus’
‘covid-19’
‘severe acute respiratory syndrome coronavirus 2’
‘2019-nCoV-2’
‘SARS-CoV-2’
‘acute respiratory syndrome’
No limits or filters were applied to the search. The same search terms were used across all databases.
Reference lists of included papers and review articles were also searched, as was the grey literature of public health reports for the 26 countries with the highest numbers of reported patients with COVID-19 at the end of April 2020, for other countries it was assumed there would be insufficient numbers of cases to yield relevant data.
Eligibility criteria and study selection
The following inclusion and exclusion criteria were applied to the search results.
Inclusion criteria:
Studies had to provide comparative data on risk factors of any kind for disease (versus no disease), severe disease (versus milder disease) or mortality (versus survival),
Studies were eligible if they presented data on patients with polymerase chain reaction (PCR)-confirmed SARS-CoV-2 infections. There was considerable variation in case definitions between studies, but PCR testing was the gold standard test for active disease at the start of the pandemic [14], and other testing methods such as Loop-Mediated Isothermal Amplification or serological tests were not included,
Any study design,
Any population group,
Any language of publication.
Exclusion criteria:
No comparator group included in the study,
Publication concerned other viruses and diseases,
Work conducted in animals or in vitro,
Study population was less than 20 individuals.
Two reviewers independently screened all titles, abstracts and full texts for both literature searches. Discrepancies were resolved by consensus. In all cases where studies were published in any language other than English, with no translations available, these were screened by at least one additional reviewer, with further quality control by another member of the reviewing team.
Data extraction
Three reviewers independently double-extracted the studies into a pre-designed spreadsheet that collected:
First author,
Paper title,
Journal,
Type of study,
Country,
Study population,
Overall number in study,
Number with PCR confirmed SARS-CoV-2,
Median age of participants/age range,
Sex ratio,
Analytical method used,
Factors adjusted for during the analysis,
Whether disease, disease severity, or death (or a combination of these) was the outcome of interest,
The definition of disease severity used, if applicable,
The risk factors analysed and the direction of effect.
Results were compared and discrepancies resolved by discussion. Data from studies published in languages other than English, at this stage only the Chinese language, were extracted by two additional reviewers, with further quality control by another member of the reviewing team.
Quality assessment
Two reviewers independently assessed the quality of included studies. Studies published in languages other than English were quality assessed by two additional reviewers, with further quality control by another member of the reviewing team. Assessments were undertaken from the perspective of the objectives of this review, which were not necessarily identical to the objectives of the underlying studies. The quality of included studies was assessed using a checklist adapted from Downs and Black [15], as per the guidance issued by Deeks et al. [16] When assessing the power of studies, the minimum sample size required to detect a relative increase in risk of 10% from a statistically conservative baseline of 50% among the unexposed was calculated at different powers using the Kelsey method within Epi Info, software made available by the United States Center for Disease Control [17]. This 10% value was based on governmental discussions taking place in the UK at the time the review took place. An alpha of 5% was set as the standard. Pragmatically, we assumed only two strata and a ratio of 1:1 between exposure strata. Different thresholds were used for case-control studies and for cohort or cross-sectional studies. These criteria were scored from 0 (< 70% power) to 5 (> 99% power). We considered results sufficient adjusted for confounding if they adjusted for at least the minimal variable set of age, sex, ethnicity and any measure of comorbidities. For ethnically homogenous populations, the need for adjustment for ethnicity was discounted. If two analyses were presented within a single paper with different quality scores, the most conservative score was retained. Studies were not excluded on the basis of the quality assessment.
Analysis and synthesis
Studies were grouped on the basis of the outcome examined (disease, disease severity, mortality) and then the risk factors examined. Results were classified on the basis of whether they presented evidence as to the exposure under study being a risk factor, taking into account the number of individuals exposed. Where studies focussed on a single risk factor of interest with adjustment for confounding, we extracted all data on potential risks in order to maximise the value of our dataset (whilst accepting that such mutually adjusted estimates for covariates may remain confounded even if that for the primary exposure does not) [18]. As there was substantial heterogeneity in study design, reporting, and the risk factors examined, we present a detailed descriptive summary and narrative synthesis of our findings, rather than a meta-analysis.
Registration and reporting
This review was registered on PROSPERO as CRD42020177714 and is reported according to the PRISMA guidelines.
Results
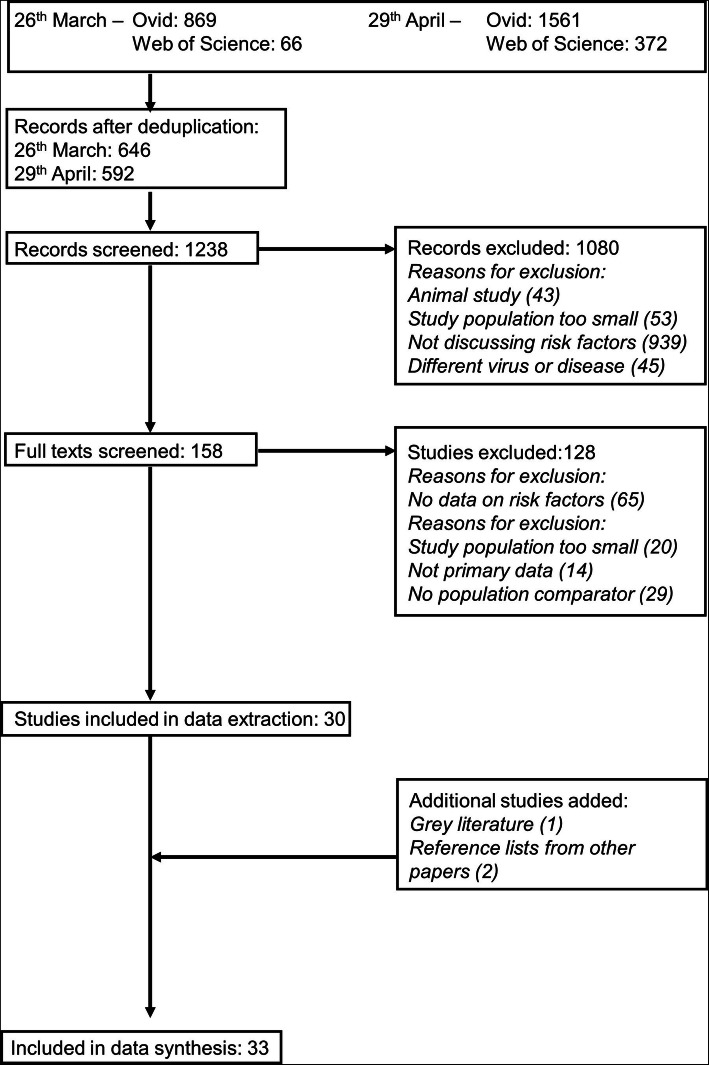
Two thousand eight hundred and sixty-eight hits were obtained by the searches across the two dates (Fig. 1). After de-duplication across the different databases, this was reduced to 1238. Thirty studies were included at the extraction stage; the main reasons for exclusion were small numbers of participants and studies not having a comparator population. From the grey literature an additional report was included and two studies were identified from reference lists.
Fig. 1.
PRISMA flow chart of selection
Included studies are presented in Table 2. Twenty-nine of the 33 studies were conducted in China, with one each from France, Italy, Singapore and a combined study from England, Wales and Northern Ireland. Six were studies with COVID-19 disease as the outcome, 20 of disease severity and ten of mortality. One additional study looked at a combined outcome of disease severity and mortality.
Table 2.
Included studies
| Author | Type of study | Country | Study population | Overall number of patients | Analytical method used | Factors included in multivariable model | Disease, disease severity, or death | Definition of severity |
|---|---|---|---|---|---|---|---|---|
| Cai [19] | Retrospective cohort | China | Hospitalised patients, single hospital | 298 | Univariable; logistic regression | N/A; age, sex, comorbidities, clinical markers, travel history | Disease severity | As per the international guidelines for community-acquired pneumonia, with a scoring system based on demographics, comorbid illness, physical examination findings, and laboratory and radiographic findings |
| Chen [20] | Retrospective cohort | China | Hospitalised patients, single hospital | 249 | Logistic regression | Age, sex, comorbidities and a range of clinical measures | Disease severity | ICU admission |
| Chen [21] | Retrospective cohort with a nested case-control study | China | Hospitalised patients (moderately, severely or critically ill), single hospital | 274 | Univariable | N/A | Death | N/A |
| Chen [22] | Case-control | China | COVID-19 vs. pneumonia negative for SARS-CoV-2 | 104 (78 COVID-19) | Univariable | N/A | Disease | N/A |
| Chen [23] | Retrospective cohort | China | Hospitalised patients, single hospital | 21 | Univariable | N/A | Disease severity | Severe meets any of the following criteria- respiratory distress, RR ≥ 30 breaths/min; SpO2 ≤ 93% at rest; and PaO2/FIO2 ≤ 300. Patients with greater than 50% lesion progression within 24 to 48 h in pulmonary imaging. |
| Cheng [24] | Case-control | China | Patients presenting with fever diagnosed with pneumonia by specialists with chest CT scans | 38 (11 COVID-19) | Univariable | N/A | Disease | N/A |
| Chinese CDC [25] | Retrospective cohort | China | Nationwide surveillance | 72,314 | Univariable | N/A | Death | N/A |
| Fan [26] | Retrospective cohort | Singapore | Hospitalised patients, National Centre for Infectious Diseases | 67 | Univariable | N/A | Disease severity | ICU admission |
| Grasselli [27] | Prospective cohort with a nested case-control study | Italy | Hospitalised in ICU across 72 hospitals | 1591 | Univariable | N/A | Death | N/A |
| Han [28] | Retrospective cohort | China | Hospitalised patients, single hospital | 273 | Univariable | N/A | Disease severity | Severe met at least one of the following conditions: a) shortness of breath, RR ≥ 30 times/min, b) oxygen saturation (resting state) ≤93%, or c) PaO2/FiO2 ≤ 300 mmHg., in addition to positive SARS-CoV-2 RNA nucleic acid test by Reverse transcription polymerase chain reaction, fever, or other respiratory symptoms (the typical CT image abnormities of viral pneumonia were optional). Critical patients also needed to meet at least one of the extra following conditions: a) respiratory failure that needs to receive mechanical ventilation; b) shock; and c) multiple organ failure that need to be transferred to the ICU. |
| Huang [29] | Retrospective cohort | China | Hospitalised patients, single hospital | 41 | Univariable | N/A | Disease severity | ICU admission |
| ICNARC [30] | Case-control (disease) / Prospective cohort (disease severity) | England, Northern Ireland and Wales | Hospitalised patients in ICU across a network of hospitals | 12,502 (6720 COVID-19) | Univariable | N/A | Disease; Disease severity | Receiving advanced, as opposed to basic, respiratory support |
| Liang [31] | Prospective cohort | China | Hospitalised patients across 575 hospitals | 1590 | Logistic regression; Cox regression | Logistic model adjusted for age, sex, smoking history, comorbidities, cancer | Disease severity, Death | Invasive ventilation, ICU admission, death |
| Liu [32] | Retrospective cohort | China | Hospitalised patients, single hospital | 119 | Univariable | N/A | Disease severity | Severe- dyspnoea accompanied by hypoxemia, sometimes acute respiratory distress syndrome, septic shock and multiple organ failure |
| Liu [33] | Retrospective cohort | China | Hospitalised patients, single hospital | 4880 | Logistic regression | Age, sex | Disease | N/A |
| Qiu [34] | Retrospective cohort | China | Hospitalised paediatric patients, three hospitals | 36 | Univariable | N/A | Disease severity | Moderate disease (mild was baseline)- mild pneumonia; symptoms such as fever, cough, fatigue, headache, and myalgia; no complications and manifestations related to severe conditions |
| Ruan [35] | Retrospective cohort with a nested case-control study | China | Hospitalised patients across two hospitals with a definitive outcome | 150 | Univariable | N/A | Death | N/A |
| Shi [36] | Retrospective cohort | China | Hospitalised patients across a province | 487 | Univariable; logistic regression | N/A; age, sex, hypertension; full list unknown | Disease severity | Severe pneumonia, characterised by fever, cough, dyspnoea, bilateral pulmonary infiltrates, and acute respiratory injury |
| Simmonet [37] | Retrospective cohort | France | Hospitalised patients in ICU, single hospital | 124 | Logistic regression | Age, sex, diabetes, hypertension, BMI | Disease severity | Receiving invasive mechanical ventilation, determined when oxygen therapy (≥ 10 L/min) with target SpO2 (90–94%) was ineffective, and when RR was above 25/min, with signs of acute respiratory failure, despite maximal oxygen therapy |
| Tian [38] | Retrospective cohort | China | Hospitalised individuals transferred from the hospitals of Beijing to the designated hospitals for specialist treatment of infectious diseases by Beijing Emergency Medical Service | 262 | Univariable | N/A | Disease severity | Severe- dyspnoea or respiratory failure in addition to fever, respiratory symptoms and radiographic evidence of pneumonia |
| Wan [39] | Prospective cohort | China | Hospitalised patients, single hospital | 135 | Univariable | N/A | Disease severity | Severe group- respiratory distress, RR ≥ 30 breaths/minute in a resting state, a mean oxygen saturation of ≤93%, and an PaO2/FiO2 ≤ 300 mmHg |
| Wang [40] | Case-control | China | Country-wide data compared to historic SARS/MERS | 11,425 (835 COVID-19) | Univariable | N/A | Disease | N/A |
| Wang [41] | Retrospective cohort | China | Hospitalised patients, single hospital | 138 | Univariable | N/A | Disease severity | ICU admission |
| Wang [42] | Prospective cohort | China | Hospitalised patients, single hospital | 116 | Univariable | N/A | Disease severity | Severe- fever or suspected respiratory infection, plus one of the following: RR > 30 breaths/min; severe respiratory distress; or SpO2 ≤ 93% on room air |
| Wang [43] | Cross-sectional | China | Hospitalised patients, single hospital | 69 | Univariable | N/A | Disease severity | SpO2 < 90% |
| Wu [44] | Retrospective cohort | China | Hospitalised patients, single hospital | 201 | Univariable; Cox regression | N/A | Disease severity, Death | ARDS, mechanical ventilation |
| Wu [45] | Prospective cohort | China | Country-wide data | 44,672 | Univariable | N/A | Death | N/A |
| Yang [46] | Retrospective cohort | China | Hospitalised patients, single hospital, admitted to ICU | 52 | Univariable; Cox regression | N/A | Death | N/A |
| Yuan [47] | Retrospective cohort | China | Hospitalised patients, single hospital | 27 | Univariable | N/A | Death | N/A |
| Zhang [48] | Retrospective cohort | China | Hospitalised patients, single hospital | 140 | Univariable | N/A | Disease severity | Severe COVID-19 was designated when the patients had one of the following criteria: (a) respiratory distress with respiratory frequency ≥ 30/min; (b) pulse oximeter oxygen saturation ≤ 93% at rest; and (c) oxygenation index (PaO2/FiO2) ≤ 300 mmHg. |
| Zhang [49] | Retrospective cohort | China | Hospitalised patients (patients with no severe underlying diseases), single hospital | 95 | Univariable | N/A | Disease severity | Severe- RR ≥ 30 times / min; at rest, oxygen saturation ≤ 93%; PaO2/FiO2 ≤ 300 mmHg |
| Zhang [50] | Retrospective cohort | China | Hospitalised patients, single hospital | 120 | Univariable | N/A | Disease severity | Severe- when patients met one of the following criteria: (1) respiratory distress with a breathing rate ≥ 30/min; (2) pulse oximeter oxygen saturation ≤ 93% at rest; (3) oxygenation index (PaO2/FiO2) ≤300 mmHg; (4) respiratory failure requiring mechanical ventilation; (5) shock; and (6) combined with other organ failure requiring ICU monitoring and treatment |
| Zhou [51] | Retrospective cohort with a nested case-control study | China | Hospitalised patients across two hospitals with a definitive outcome | 191 | Univariable; logistic regression | N/A; age, coronary heart disease, Sequential Organ Failure Assessment score, lymphocyte count, D-dimer | Death | N/A |
ARDS acute respiratory distress syndrome, BMI body mass index, CDC Center for Disease Control and Prevention, CT computed tomography, FiO2 inspired oxygen fraction, ICNARC intensive care national audit and research centre, ICU intensive care unit, MERS Middle Eastern Respiratory Syndrome, N/A not applicable, PaO2 arterial partial pressure of oxygen, RR respiratory rate, SARS severe acute respiratory syndrome, SpO2 oxygen saturation
Quality assessment
Included studies were generally too small to detect a 10% increase in risk of disease, disease severity, or mortality (Table 3). One study among the 33 was assessed to have 95% power and two others 99%; all were large, national, investigations. As 26 studies were purely descriptive or presented univariable analysis only, there was no adjustment for confounding. Remaining studies with a regression component did not adjust for our minimal confounder set. Only nine studies provided estimates of the random variability of effect estimates. The majority of studies ascertained exposure information from clinical records, which would have collected data prospectively and thus with limited recall bias. Blinding of outcome and exposure recording by investigators was not documented. In the case of certain disease severity outcomes, such as admittance to intensive care units (ICU), variability in thresholds for reaching these outcomes is likely to exist between settings and clinicians.
Table 3.
Quality assessment
| Author | Is the study design clearly reported? | Is the hypothesis/aim/objective of the study clearly described? | Are the main outcomes to be measured clearly described in the Introduction or Methods section? | Are the characteristics of the patients included in the study clearly described? | Are the distributions of principal confounders in each group of subjects to be compared clearly described? | Are the main findings of the study clearly described? | Does the study provide estimates of the random variability in the data for the main outcomes? | Have actual probability values been reported for the main outcomes except where the probability value is < 0.001? | Was there potential for recall bias in the ascertainment of the exposure? | Was there potential for differential or non-differential misclassification of the exposure? | Was there potential for observer bias in ascertainment of the outcome? | Was there potential for differential or non-differential misclassification of the outcome? | If any of the results of the study were based on ‘data dredging’ was this made clear? | Do the analyses adjust for different lengths of follow-up of patients, or in case-control studies, is the time period between the intervention and outcome the same for cases and controls? | Were the statistical tests used to assess the main outcomes appropriate? | Were the main outcome measures used accurate (valid and reliable)? | Were the patients in different intervention groups (trials and cohort studies) or were the cases and controls (case-control studies) recruited from the same population? | Were study subjects in different intervention groups (trials and cohort studies) or were the cases and controls (case-control studies) recruited over the same period of time? | Was there adequate adjustment for confounding in the analyses of interest? | Were losses of patients to follow-up taken into account? | Are the study results appropriately interpreted e.g. in terms of the strength of the evidence, its application/implications, causality? | Did the study have sufficient power to detect a clinically important effect where the probability value for a difference being due to chance is less than 5%?a |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cai [19] | Y | Y | Y | Y | Y | Y | Y | Y | N | N | Y | Y | N/A | N | Y | Y | Y | Y | Y | U | Y | 0 |
| Chen [20] | Y | Y | Y | Y | N | Y | Y | Y | N | U | N | Y | N/A | N | Y | Y | Y | Y | N | U | Y | 0 |
| Chen [21] | Y | Y | Y | Y | N | Y | N | N | Y | Y | N | N | N/A | N | N/A | Y | Y | Y | N | U | N | 0 |
| Chen [22] | Y | Y | Y | Y | N | Y | N | Y | N | N | N | N | N/A | N | Y | Y | Y | Y | N | U | Y | 0 |
| Chen [23] | Y | Y | Y | Y | N | Y | N | Y | N | U | Y | Y | N/A | N | Y | U | Y | Y | N | U | Y | 0 |
| Cheng [24] | Y | Y | Y | Y | N | Y | N | Y | N | N | Y | Y | N/A | N | Y | Y | Y | Y | N | U | N | 0 |
| Chinese CDC [25] | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | N | N/A | Y | Y | Y | Y | Y | N | U | Y | 5 |
| Fan [26] | Y | Y | Y | Y | N | Y | N | Y | N | N | Y | Y | N/A | N | Y | Y | Y | Y | N | U | Y | 0 |
| Grasselli [27] | Y | Y | Y | Y | N | Y | Y | Y | N | N | Y | Y | N/A | N | Y | Y | Y | Y | N | U | Y | 0 |
| Han [28] | Y | Y | Y | Y | N | Y | N | N | N | N | N | N | N/A | N | N/A | U | Y | Y | N | U | Y | 0 |
| Huang [29] | Y | Y | Y | Y | N | Y | N | Y | Y | Y | Y | Y | N/A | N | Y | Y | Y | Y | N | U | Y | 0 |
| ICNARC [30] | Y | Y | N | Y | Y | Y | N | N | U | U | U | U | N/A | N | N/A | U | N | N | N | U | Y | 4 |
| Liang [31] | Y | Y | Y | Y | N | Y | Y | Y | N | Y | Y | Y | N/A | Y | Y | Y | Y | Y | Y | Y | Y | 0 |
| Liu [32] | Y | Y | Y | Y | N | Y | N | N | N | N | Y | Y | N/A | N | Y | U | Y | Y | N | U | Y | 0 |
| Liu [33] | Y | Y | Y | Y | N | Y | Y | Y | U | U | N | N | N/A | N | Y | Y | Y | Y | N | U | Y | 3 |
| Qiu [34] | Y | Y | Y | Y | N | Y | N | Y | N | N | Y | Y | N/A | N | Y | U | Y | Y | N | U | Y | 0 |
| Ruan [35] | Y | Y | Y | Y | N | Y | N | Y | N | N | Y | Y | N/A | N | Y | Y | Y | Y | N | U | Y | 0 |
| Shi [36] | Y | Y | Y | Y | N | N | Y | Y | N | U | U | U | N/A | N | Y | U | Y | Y | N | U | Y | 0 |
| Simmonet [37] | Y | Y | Y | Y | N | Y | Y | Y | N | N | Y | Y | N/A | N | Y | Y | Y | Y | N | U | Y | 0 |
| Tian [38] | Y | Y | Y | Y | N | Y | N | Y | N | N | Y | Y | N/A | N | Y | U | Y | Y | N | U | Y | 0 |
| Wan [39] | Y | Y | Y | Y | N | Y | N | Y | N | N | Y | Y | N/A | N | Y | Y | Y | Y | N | U | Y | 0 |
| Wang [40] | Y | Y | Y | Y | N | Y | N | N | N | N | N | N | N/A | N | N/A | Y | N | N | N | U | Y | 0 |
| Wang [41] | Y | Y | Y | Y | N | Y | N | Y | N | U | Y | Y | N/A | N | Y | Y | Y | Y | N | U | Y | 0 |
| Wang [42] | Y | Y | Y | Y | N | Y | N | Y | N | U | Y | Y | N/A | N | Y | U | Y | Y | N | U | Y | 0 |
| Wang [43] | Y | Y | Y | Y | N | Y | N | Y | N | U | N | Y | N/A | N | Y | Y | Y | Y | N | U | Y | 0 |
| Wu [44] | Y | Y | Y | Y | N | Y | Y | Y | N | U | N | Y | N/A | Y | Y | U | Y | Y | N | Y | Y | 0 |
| Wu [45] | Y | Y | Y | Y | N | Y | N | N | U | U | Y | Y | N/A | N | N/A | Y | Y | Y | N | U | Y | 5 |
| Yang [46] | Y | Y | Y | Y | N | Y | N | N | N | U | N | Y | N/A | N | N/A | U | Y | Y | N | U | Y | 0 |
| Yuan [47] | Y | Y | Y | Y | N | Y | N | Y | N | U | N | N | N/A | N | Y | Y | Y | Y | N | U | Y | 0 |
| Zhang [48] | Y | Y | Y | Y | N | Y | N | Y | N | U | N | N | N/A | N | Y | Y | Y | Y | N | U | Y | 0 |
| Zhang [49] | Y | Y | Y | Y | N | Y | N | Y | N | N | N | N | N/A | N | Y | Y | Y | Y | N | U | N | 0 |
| Zhang [50] | Y | Y | Y | Y | N | Y | N | Y | U | U | N | Y | N/A | N | Y | U | Y | Y | N | U | Y | 0 |
| Zhou [51] | Y | Y | Y | Y | N | Y | Y | Y | N | U | N | N | N/A | N | Y | Y | Y | Y | N | U | Y | 0 |
a0 = < 70% power, 1 = 70%, 2 = 80%, 3 = 90%, 4 = 95%, 5 = 99%. CDC Center for Disease Control Prevention, ICNARC intensive care national audit and research centre, N/A not applicable
Risk factors for disease
Six studies compared the likelihood of having COVID-19 to other infectious conditions (Table 4). Of note, as testing strategies were largely focussed on hospitalised individuals i.e. those displaying noticeable symptoms, studies were of the likelihood of COVID-19 disease, rather than more broadly of SARS-CoV-2 infection (and particularly of severe disease, although patients with mild and symptomatic infection were also reported to be hospitalised in some studies for the purposes of isolation or observation). Age and sex were key foci as potential risk factors, comparing patients with COVID-19 to either: a) SARS-CoV or Middle Eastern Respiratory Syndrome (MERS), or b) other forms of pneumonia. Generally, sex ratios were skewed such that men were over-represented among those with disease. In England, Northern Ireland, and Wales, Asian and Black individuals were found to be at increased risk of COVID-19 in descriptive analyses, with 15.4 and 10.7% of patients falling into these groupings, respectively, versus 5.8 and 2.8% of individuals with other viral pneumonia [30]. Higher body mass index (BMI) was also suggested to be a risk factor with two descriptive analyses, for example in the Intensive Care National Audit and Research Centre (ICNARC) report 31.2% of COVID-19 patients had a BMI of 30- < 40, versus 23.5% of people with other viral pneumonia [30, 37]. Given the large, national, scope of the ICNARC dataset, results from it are particularly likely to be reliable.
Table 4.
Potential risk factors for disease
| Potential risk factor | Study supports risk | Study does not support risk or is neutral |
|---|---|---|
| Age |
Patients older than those with COVID-19 and younger than those with MERS (descriptive) [40] Younger ages (median 45 years) in patients with COVID-19 than other pneumonia (median 61) (statistical test) [22] Increasing risk of positivity for SARS-CoV-2 among COVID-19 suspects (on the basis of symptoms/contact tracing) with age (odds ratio 1.02) but unclear categorisation of age (multivariable regression) [33] |
Median age 60 years in those with COVID-19 and 61 in those with other viral pneumonia (descriptive) [30] Mean age 50 years in COVID-19 patients vs. 44 in individuals with other pneumonia (statistical test) [24] |
| Sex |
Sex ratio skewed towards men for COVID-19, akin to MERS but not SARS (descriptive) [40] Sex ratio skewed towards men for COVID-19 versus other viral pneumonia (descriptive) [30] Greater proportion male in COVID-19 versus other pneumonia, although small sample size and thus low statistical certainty (statistical test) [24] Increasing risk of positivity for SARS-CoV-2 among COVID-19 suspects (on the basis of symptoms/contact tracing) among males versus female (odds ratio 1.16) (logistic regression) [33] |
Sex distribution similar amongst patients with COVID-19 and other pneumonia (statistical test) [22] |
| Ethnicity | Higher percentage of Black and Asian individuals amongst COVID-19 patients than patients with other viral pneumonias (descriptive) [30] | |
| Index of multiple deprivation | Distribution of deprivation similar across COVID-19 and other viral pneumonia (descriptive) [30] | |
| Body mass index |
Greater proportion of COVID-19 patients had higher body mass index than individuals with other pneumonia (descriptive) [37] Greater proportion of COVID-19 patients had higher body mass index than individuals with other viral pneumonia (descriptive) [30] |
|
| Pregnancy | Percentage of women who were pregnant similar across COVID-19 and other viral pneumonia (descriptive) [30] |
MERS middle eastern respiratory syndrome, SARS severe acute respiratory syndrome
Risk factors for severe disease
Among the 20 studies of risk factors for severe versus milder disease and one of a mixed outcome (severe disease and death), a wide array of definitions of severity were used, such as ICU admission, the need for mechanical ventilation, and various measures of respiration and oxygenation (Table 2). Many risk factors were examined (Table 5). As well as potential demographic risks (age, sex, ethnicity), behavioural traits (smoking) and broad clinical factors (BMI, infectious diseases) were analysed. Large numbers of papers sought to explore the implications of different comorbidities on the risk of severe COVID-19, particularly respiratory and cardiovascular conditions.
Table 5.
Potential risk factors for disease severity
| Potential risk factor | Study supports risk | Study does not support risk or is neutral |
|---|---|---|
| Sex |
Odds ratio for severe disease 3.68 for men compared to women (multivariable regression) [36] Odds ratio for invasive mechanical ventilation 2.83 for men compared to women (multivariable regression) [37] Females less likely to be admitted to ICU, require mechanical ventilation, or die; odds ratio 0.61 (multivariable logistic regression) [31] |
Sex distribution similar in severe and non-severe disease (descriptive) [30, 49] Sex distribution similar in severe and non-severe disease (statistical test) [23, 26, 28, 29, 32, 34, 38, 39, 41–44, 48–50] Sex distribution similar in severe and non-severe disease (multivariable regression) [19, 20] |
| Age |
Averagea 61 years severe disease, 45 otherwise (statistical test) [38] Averagea 61 years severe disease, 52 years moderate disease (statistical test) [23] Averagea 56 years severe disease, 44 years mild disease (statistical test) [39] Median 67 years acute respiratory distress syndrome, 52 severe, 45 mild (statistical test) [42] Median 64 years severe patients, 52 years otherwise (statistical test) [48] Mean 61 years severe, otherwise 40 (statistical test) [50] Median 71 years SpO2 < 90%, 37 years SpO2 ≥ 90% (statistical test) [43] Median 66 years patients in ICU, 51 otherwise (statistical test) [41] Median 54 years patients in ICU, 41 otherwise (statistical test) [26] 65 years and over 3.26 times the hazard rate of ARDS than those under 65 (univariable regression) [44] Age associated with ICU admission, odds ratio 1.06 but unclear for what categorisation of age (multivariable regression) [20] |
Distribution of age did not differ by disease severity (descriptive) [30, 34, 49] Distribution of age did not differ by disease severity (statistical test) [28, 29, 32] Confidence interval for effect of age (categorisation unclear) crosses the null (multivariable regression) [37] |
|
Mean 56 years severe disease, 45 years mild disease; odds ratio 1.06 but unclear categorisation of age (multivariable regression) [36] Mean 63 years severe disease, 41 years mild disease; odds ratio 1.08 but unclear categorisation of age (multivariable regression) [19] Older individuals more likely to be admitted to ICU, require mechanical ventilation, or die; odds ratio 1.05, categories of age unclear (multivariable logistic regression) [31] |
||
| Ethnicity | 76.3% of individuals receiving basic respiratory support were White versus 65.6% receiving advanced respiratory support; Asian and Black ethnicities appear most at risk of severe disease (England, Northern Ireland and Wales; descriptive) [30] | Distribution of disease severity similar across ethnic groups (Chinese, Malay, Indian, other – with small numbers in groups other than Chinese; study in Singapore; descriptive) [26] |
| Deprivation | Distribution across deprivation categories similar (descriptive) [30] | |
| Pregnancy | Distribution in pregnant and non-pregnant individuals similar across disease severity (descriptive) [30] | |
| Smoking | 100% of current smokers had severe disease, but only six individuals smoked [50] |
Distribution in current and non-current smokers similar across disease severity (descriptive), only three individuals smoked [39] Distribution in current and non-current smokers similar across disease severity (statistical test); small numbers who smoked [29] Distribution in historical/current and non-smokers similar across disease severity (statistical test) [36, 48] |
| Body mass index |
≥35 kg/m2 risk factor versus < 25 kg/m2 for invasive mechanical ventilation; odds ratio 7.36. Results for other strata cross the null (multivariable regression) [37] Increasing body mass index increased risk; odds ratio 1.17 (categorisation unclear) [19] |
Distribution of disease severity similar across body mass index categories (descriptive) [30] |
| Any/other comorbidity | Presence of comorbidity more common among those with severe disease (statistical test) [39, 41, 48, 50] |
Distribution with and without condition similar across disease severity (statistical test) [23, 29, 36] Distribution with and without comorbidities not otherwise considered in the study similar across disease severity (statistical test) [50] Distribution with and without condition similar across disease severity (multivariable regression) [20] |
| Cardiovascular disease/chronic heart disease/coronary heart disease |
Presence of comorbidity more common among those with severe disease (descriptive) [39] Presence of comorbidity more common among those with severe disease (statistical test) [19, 36, 41, 43, 50] |
Distribution with and without condition similar across disease severity (descriptive) [30, 44] Distribution with and without condition similar across disease severity (statistical test) [29, 48] |
| Hypertension |
Presence of comorbidity more common among those with severe disease (statistical test) [41, 43, 50] Hazard ratio of ARDS 1.82 in those with the condition versus those without (univariable regression) [44] Odds ratio of severe disease 2.71 in those with the condition versus those without (multivariable regression) [36] Odds ratio of being admitted to ICU, require mechanical ventilation, or die 1.89 in those with the condition versus those without (multivariable regression) [31] |
Distribution with and without condition similar across disease severity (descriptive) [39] Distribution with and without condition similar across disease severity (statistical test); one study with small numbers with the condition [23, 29, 42, 48] Confidence interval in presence and absence of condition crosses the null (multivariable regression) [19] Confidence interval in presence and absence of condition crosses the null (multivariable regression, result borderline) [37] |
| Diabetes |
Presence of comorbidity more common among those with severe disease (descriptive) [39] Presence of comorbidity more common among those with severe disease (statistical test) [19, 36, 41, 43, 50] Hazard ratio of ARDS 2.34 in those with the condition versus those without (univariable regression) [44] Odds ratio of being admitted to ICU, require mechanical ventilation, or die 2.21 in those with the condition versus those without (multivariable regression) [31] |
Distribution with and without condition similar across disease severity (statistical test); small numbers with condition [23] Distribution with and without condition similar across disease severity (statistical test) [29, 48] Distribution with and without condition similar across disease severity (statistical test, borderline result) [42] Confidence interval in presence and absence of condition crosses the null (multivariable regression) [37] |
| Respiratory/pulmonary disease | Distribution with and without condition similar across disease severity (descriptive) [30, 39] | |
| Asthma | Distribution with and without condition similar across disease severity (statistical test); small numbers with condition [43] | |
| Chronic obstructive pulmonary disease (COPD) |
Presence of comorbidity more common among those with severe disease (descriptive); small numbers with condition [39] Presence of comorbidity more common among those with severe disease (statistical test); both studies have small numbers with the condition [41, 50] Odds ratio of being admitted to ICU, require mechanical ventilation, or die 3.40 in those with the condition versus those without (multivariable regression) [31] |
Distribution with and without condition similar across disease severity (statistical test); small numbers with condition [29, 43, 48] |
| Pulmonary tuberculosis | Distribution with and without condition similar across disease severity (statistical test); small numbers with condition [48] | |
| Malignancy |
Presence of comorbidity more common among those with severe disease (statistical test); small numbers with condition [39] Presence of comorbidity more common among those with severe disease (statistical test) [36, 42, 50] Presence of comorbidity more common among those with severe disease (multivariable analysis) [31] |
Distribution with and without condition similar across disease severity (descriptive) [30] Distribution with and without condition similar across disease severity (statistical test) [41] Distribution with and without condition similar across disease severity (statistical test); small numbers with condition [19, 29, 43] |
| Cerebrovascular disease | Presence of comorbidity more common among those with severe disease (statistical test) [41] | |
| Arrhythmia | Distribution with and without condition similar across disease severity (statistical test); small numbers with the condition [48] | |
| Cerebral infarction | Distribution with and without condition similar across disease severity (statistical test) [42] | |
| Stroke | Distribution with and without condition similar across disease severity (statistical test); small numbers with condition [48] | |
| Aorta sclerosis | Distribution with and without condition similar across disease severity (statistical test); small numbers with condition [48] | |
| Chronic kidney disease/renal issues | Presence of comorbidity more common among those with severe disease (statistical test) [42] |
Distribution with and without condition similar across disease severity (descriptive) [30] Distribution with and without condition similar across disease severity (statistical test); small numbers with the condition [41] |
| Chronic renal disease/insufficiency | Distribution with and without condition similar across disease severity (statistical test); one study has small numbers of patients with the condition [36, 48] | |
| Chronic liver disease |
Distribution with and without condition similar across disease severity (descriptive), sometimes small numbers with condition [19, 30, 39] Distribution with and without condition similar across disease severity (statistical test) [36, 41] Distribution with and without condition similar across disease severity (statistical test); small numbers with condition [29, 50] |
|
| Fatty liver and abnormal liver function | Distribution with and without condition similar across disease severity (statistical test) [48] | |
| Hyperlipidaemia | Distribution with and without condition similar across disease severity (statistical test) [48] | |
| Dyslipidemia | Confidence interval in presence and absence of condition crosses the null (multivariable regression) [37] | |
| Chronic gastritis/gastric ulcer | Distribution with and without condition similar across disease severity (statistical test) [48] | |
| Cholelithiasis | Distribution with and without condition similar across disease severity (statistical test) [48] | |
| Urolithiasis | Distribution with and without condition similar across disease severity (statistical test); small numbers with condition [48] | |
| Thyroid diseases | Distribution with and without condition similar across disease severity (statistical test); small numbers with the condition [48] | |
| Electrolyte imbalance | Presence of comorbidity more common among those with severe disease (statistical test); small numbers with condition [48] | |
| Agglomerative disease | Distribution with and without condition similar across disease severity (descriptive); small numbers with the condition [39] | |
| Immunocompromised | Distribution with and without condition similar across disease severity (descriptive) [30] | |
| Chronic hepatitis | Distribution with and without condition similar across disease severity (statistical test); small numbers with condition [43] | |
| HIV | Distribution with and without condition similar across disease severity (statistical test); small numbers with condition [41] | |
| Living without assistance | Distribution with and without condition similar across disease severity (descriptive) [30] |
One study included death in a combined measure of disease severity [31]. aUnclear as to whether mean, median or mode. ARDS acute respiratory distress syndrome, ICU intensive care unit, SpO2 oxygen saturation
The least equivocal evidence was presented for age as a risk factor, including four studies where it was an independent risk in a multivariable regression model [19, 20, 31, 36]. The clearest analysis to present age data (i.e. which used different comparison groups) was a univariable regression model where individuals 65 years and over had 3.26 times the hazard rate of ARDS than those under 65 [44]. Eight studies suggested that diabetes could be a risk factor [19, 31, 36, 39, 41, 43, 44, 50], six hypertension [31, 36, 41, 43, 44, 50], and four the presence of unspecified comorbidities) [39, 41, 48, 50], but the balance of evidence for these co-morbidities being risk factors was generally inconclusive. Many other factors were examined by one study, often with small numbers of individuals with the condition. None of the included studies for disease severity were assessed to have been powered to detect a 10% increase in effect size.
Risk factors for mortality
Ten studies examined risk factors for mortality, often by nesting case-control studies within prospective or retrospective cohorts (Table 6). Among these studies, many included statistical testing, but none presented an adjusted regression model for the risk factors considered.
Table 6.
Potential risk factors for mortality
| Potential risk factor | Study supports risk | Study does not support risk or is neutral |
|---|---|---|
| Sex | Men more at risk (descriptive) [21] |
Sex distribution similar amongst patients who died and survived (descriptive) [25, 35, 46, 47] Confidence interval for males versus females crosses the null (univariable regression) [44] |
| Age |
Over 60 years particularly at risk (descriptive) [21] 8% case fatality ratio in 70–79 year olds and 14.8% in those over 80. Overall figure 2.3% (descriptive) [45] Median age in those who died 52 years, 65 years among survivors (descriptive) [46] Over 50 years of age particularly at risk- 1.3% died 50–59 years, 3.6% 60–69 years, 8.0% 70–79 years, 14.8% 80 years plus; less than 1% all other age groups (descriptive) [25] Risk begins to increase at approximately 50 years (statistical test, but graphical presentation) [35] Median age in those who died 68 years, among those who survived 55 (statistical test) [47] Over 61 years, increasing per 10 year age group (statistical test) [27] 65 years and older 6.17 the hazard rate of those under 65 (univariable regression) [44] |
|
| Smoking |
Proportion of smokers similar among those who died versus those who did not (descriptive); one study had small numbers of smokers [21, 46] Distribution of current smokers similar among survivors and non-survivors (univariable regression analysis, not included in multivariable model) [51] |
|
| Pregnancy | Proportion of women who were pregnant similar amongst patients who died versus survived (descriptive) [21] | |
| Any comorbidity | Presence of any comorbidity more common among those dying (descriptive) [21, 35, 46, 47, 51] | |
| Hypertension |
Presence of condition more common among those dying (descriptive) [3, 21, 25, 27] Presence of condition more common among those dying (statistical test) [47, 51] |
Confidence interval for individuals with and without the condition crosses the null (univariable regression) [44] |
| Cardiovascular disease/chronic heart disease |
Presence of condition more common among those dying (descriptive) [21, 25, 35, 45] Presence of condition more common among those dying (statistical test) [47, 51] |
Distribution dying in presence and absence of comorbidity similar (descriptive), sometimes small numbers with the condition [44, 46] |
| Diabetes |
Presence of condition more common among those dying (descriptive) [21, 25, 45, 46] Presence of condition more common among those dying (statistical test) [47, 51] |
Confidence interval for individuals with and without the condition crosses the null (univariable regression) [44] |
| Chronic respiratory/lung disease (chronic obstructive lung disease) |
Presence of condition more common among those dying (descriptive) [21, 45] Presence of condition more common among those dying (statistical test) [51] |
Distribution dying in presence and absence of comorbidity similar (descriptive) [46] |
| Respiratory infectious disease | Presence of condition more common among those dying (descriptive) [25] | |
| Malignancy | Presence of condition more common among those dying (descriptive) [3, 25] | Distribution dying in presence and absence of comorbidity similar (descriptive), sometimes small numbers with the condition [46, 47, 51] |
| Cerebral infarction/ cerebrovascular disease | Presence of condition more common among those dying (descriptive) [46] | Distribution dying in presence and absence of comorbidity similar (statistical test); small numbers with the condition [47] |
| Chronic gastritis | Distribution dying in presence and absence of comorbidity similar (statistical test); small numbers with the condition [47] | |
| Chronic kidney disease | Presence of condition more common among those dying (statistical test); small numbers with the condition [51] | |
| Dementia | Distribution dying in presence and absence of comorbidity similar (descriptive); small numbers with the condition [46] | |
| Malnutrition | Distribution dying in presence and absence of comorbidity similar (descriptive); small numbers with the condition [46] | |
| Hepatitis B virus infection | Distribution dying in presence and absence of comorbidity similar (descriptive) [21] |
Eight studies examined age and all provided evidence for it being a risk factor for mortality [21, 25, 27, 35, 44–47], although none adjusted for other factors, such as comorbidities. Age groups from 50 upwards were considered particularly at risk. In the single regression analysis, the hazard rate for death in those 65 years or over was estimated to be six times that of individuals under 65 [44]. The evidence was similarly consistent for general comorbidities (albeit all the studies were descriptive); among individuals who died, comorbidities were 1.5 to 2.8 times more common than among those who survived [21, 35, 46, 47, 51]. Specific comorbidities were discussed in several studies, generally under overarching classifications such as ‘cardiovascular disease’ or ‘diabetes’, with more specific definitions not provided. Evidence was more equivocal, but still in favour, of hypertension [3, 21, 25, 27, 47, 51], cardiovascular disease [21, 25, 35, 45, 47, 51], diabetes [21, 25, 45–47, 51], and chronic respiratory/lung diseases being risk factors (references presented for studies in support only) [21, 45, 51]. Of these studies, data from two well-powered, national-level studies from China supported cardiovascular disease and diabetes as risk factors for mortality from COVID-19 [25, 45].
Discussion
In this systematic review of risk factors for COVID-19 disease, disease severity and mortality, we document 33 comparative studies examining sociodemographic, behavioural and clinical exposures. Age and sex were very commonly examined; a wide array of comorbidities have also been considered.
Within the synthesised evidence, risk factors for mortality were the clearest, plausibly partly because this outcome is easy to define. Increasing age (different studies presented different thresholds, but being over 50 years of age was common) was an uncontested risk factor. Five studies also presented evidence for the presence of any comorbidities being a risk factor [21, 35, 46, 47, 51], with none demonstrating evidence against. Given the increasing prevalence of comorbidities with age, the lack of adjustment for confounding in these studies likely over-emphasises the effect size of each risk factor. We note that work subsequent to our literature search documents an independent effect of age on COVID-19 mortality from overall comorbidities, as measured by the Charlson Comorbidity Index Score, but not vice-versa [52]. Another study published outside of the time range of our search found both age and an array of comorbidities, each analysed separately (chronic cardiac disease, chronic pulmonary disease, chronic kidney disease, chronic neurological disease, dementia, malignancy, moderate/severe liver disease; and obesity), to be independent risk factors (as well as sex) [53].
Risk factors for severe disease were more complex to synthesise, likely due to the mixed array of outcome measures that can also be prone to observer bias. The impact of age was very commonly assessed, generally showing evidence in favour of this being a risk factor (with a similar age spectrum to the mortality data). Ethnicity was studied in two publications internationally [26, 30], with mixed results. We note that such findings are likely to be highly context-specific, given that ethnicity acts as a proxy for a series of sociodemographic factors that are highly relevant to the spread of an infectious condition (as well as, perhaps, some biological traits).
Studies of risk factors for COVID-19 disease have been complicated by testing strategies globally, which have largely been concentrated on severe disease. As our knowledge of the full symptom spectrum of the disease moves forward, it will be possible to have a broader case definition that does not solely focus on viral testing, and thus the ability for more generalised complementary studies. Additionally, serological surveys assessing the history of infection with SARS-CoV-2 in different population groups will allow the identification of risk factors for infection, whether symptomatic or not. Both ethnicity (Black and Asian individuals at higher risk; from a single study in England, Northern Ireland and Wales) [30] and higher BMI were found to be associated with disease severity within the included literature [30, 37], again from descriptive studies only. While these studies were not eligible for our review, we note a series of reports from non-comparative studies documenting the potential influence of ethnicity on the likelihood of getting COVID-19 e.g. the work of Price-Haywood from the US [52]. Male sex was reasonably consistently shown to be a risk factor for presence of COVID-19 but not with severity of disease or mortality [24, 30, 40]. As with ethnicity, socioeconomic and behavioural factors make this association likely to vary between settings.
In considering the role of comorbidities in COVID-19, it is important to consider the underlying pathology of the virus. Respiratory coronaviruses associated with the common cold in immunocompetent people generally affect only cells in the upper respiratory tract (URT), whereas the previously discovered highly pathogenic coronaviruses SARS-CoV and MERS-CoV affect cells in the URT and lower respiratory tract (LRT). SARS-CoV-2 has been shown to do the same [54], and one of the host cell receptors it targets is Angiotensin-Converting Enzyme 2 (ACE2), with a second major receptor being Transmembrane Serine Protease 2 (TMPRSS2) [55]. SARS-CoV-2 can infect all the major cell types in the respiratory tract – type I and type II pneumocytes, alveolar macrophages and endothelial cells [56, 57]. This infection leads to cell death, with significant leaking of fluid into the alveolar spaces (pulmonary oedema), which compromises gas exchange [58], eventually leading to ARDS. The inflammatory response adds aggregation of repair proteins such as fibrin, which can lead to creation of hyaline membranes which further reduces the surface available for gas exchange [58]. Subsequently, inflammatory cells are activated, recruited by release or exposure of cytokines such as the interleukins (IL) 1β and 6, monocyte chemoattractant protein-1 [56], and proteins of the extracellular matrix, as well as upregulation of the complement system. Inflammatory cells release cytokines which have systemic effects, eventually leading to disseminated intravascular coagulation (DIC), hypotensive shock and metabolic disturbances if not checked [58].
This pathogenesis therefore offers several points where co-morbidities may exacerbate the process. The target receptor TMPRSS2 is modulated in response to air pollution and in autoimmune conditions such as asthma [55], which may affect the number of receptors available for SARS-CoV-2 to target, and ACE2 is involved in the renin-angiotensin system (RAS) which controls blood pressure. Viral interference causes dysfunction, which leads to a pro-inflammatory state and increased vascular permeability in response to changes in vascular contraction and sodium homeostasis – exacerbating the effect from the physical damage to the affected cells [58]. Conditions causing hypertension – both primary and secondary to renal disease, endocrine dysfunctions such as hypothyroidism, cardiovascular dysfunction such as arteriosclerosis, or neurological dysfunctions such as acute stress – also affect the RAS [58], meaning that these conditions might be expected to exacerbate pathology caused by SARS-CoV-2. Any condition creating a pro-inflammatory state, such as type II diabetes or pre-existing infection, or involving autoimmunity, such as type I diabetes, might also be expected to contribute to increased pathology. There is also the direct effect of cell damage – if the target tissues are already damaged this reduces ‘spare’ capacity and therefore the leeway for adaptation to allow the host to continue to maintain homeostasis whilst still being able to eliminate the pathogen and repair the damage. The need for inflammatory cells to clear the infection is also a potential area of interface with comorbidities e.g. conditions such as unsuppressed HIV infection, or congenital deficiencies, or cancer malignancies; or the administration of immunosuppressant drugs such as chemotherapy for cancer or steroids.
The effect of ageing was particularly strong within our review, both in terms of the magnitude of effect estimates and the number of studies presenting evidence. As well as the above impact of comorbidities, we note that the host’s age may influence pathogenesis, both in terms of the likelihood of having various comorbidities, and also due to its effect on the immune system. Indeed, the immune system becomes less effective over time (immunosenescence), which affects the quality and number of immune system cells generated [59]. Given the scale of the impact of age documented within this review, it seems unlikely that its effect can be explained by a single or a small number of comorbidities which are yet to be detected. This opens up the need to explore biological markers, for example ACE2 [60], and markers of immunosenescence.
The strengths of our review include its systematic approach and broad use of search terms to avoid missing studies. We additionally present a quality assessment to aid the interpretation of the strength of the evidence. In some instances, included publications may have focussed on one specific outcome, whereas our quality assessment took the perspective of the outcomes extracted for this review. We were unable to detect instances where two publications used the same patient populations for their analyses, potentially over-emphasising certain findings. Given the global nature of the pandemic, our review includes studies from around the world, albeit with a large preponderance from China, including studies conducted early after the emergence of SARS-CoV-2 when the at-risk population was predominantly those who had contact with Huanan seafood market and their contacts, and not necessarily representative of the general population. We note a particular lack of studies from the African continent and the Americas, which may have implications for generalisability. Given the rapidly evolving literature on COVID-19, we also note our exclusion of studies published online after April 2020 (and the time period in which the surrounding text was written), for example the Dai report on cancer as a risk factor [61] and our exclusion of preprints (which was undertaken to ensure that all included studies had undergone an external quality assessment prior to inclusion).
Across the included publications, variability in study design, exposure and outcome measurement, and analyses made exact syntheses of effect sizes across different risk factors very difficult. Measures of disease severity varied, e.g. admission to ICUs or clinical parameters such as percentage oxygen saturation of the blood. Even measures such as admission to ICU can be subjective and may be time-, clinician-, and health systems-dependent. If severity is recorded at admission, risk factors may reflect issues associated with delayed access to healthcare, which may differ between settings and healthcare systems. It is also important to note that, in some studies of disease severity, mild disease included both people who were hospitalised with symptoms and asymptomatic individuals identified through contact tracing. Generally, analyses were descriptive or univariable and thus did not control for confounding. As documented above, this may be particularly problematic when it comes to separating the impact of age and the presence of comorbidities, as well as for identifying which comorbidities truly increase risk, given that many patients may have multi-morbidity.
The implications of our findings are two-fold for COVID-19, firstly for current public health practice and secondly for the design of future studies. We flag a number of factors of interest that should be considered by governments and public health agencies when designing shielding strategies and the targeting of future vaccines, as well as in mathematical modelling projecting the likely impact of the pandemic over time. We note, however, the need for sensitive handling of population groups deemed to be at higher risk, and how such labelling does not devolve responsibility from public bodies to these individuals for their own welfare [8]. Some public health agencies are now including reporting of potential risk factors in their routine outputs, including ICNARC (included in this review) [30] and the newer European Centre for Disease Prevention and Control reports, which were released after this review was conducted [62].
Our review demonstrates both the volume of literature that can be published within only a few months since the appearance of an emerging infectious disease, and the need for co-ordinated approaches to such pathogens. Global efforts using national datasets are hugely valuable in systematically determining the aetiology of a disease, particularly to detect smaller effect sizes. Determination of the exact threshold of important risk depends on public perceptions of the disease [63], as well as policy needs. Data collection should be standardised where possible, e.g. by using consistent definitions of outcomes and the treatment of exposures (for example for hypertension, given that blood pressure is continuous). (For COVID-19 we note both the valuable World Health Organization interim guidelines on its management in providing consistent approaches for testing and the definition of ARDS [14], and that platforms such as the International Severe Acute Respiratory and Emerging Infections Consortium (ISARIC) have aimed to facilitate such standardisation [64].) The choice of comparison groups should also merit careful consideration; comparison to other forms of the same condition (e.g. SARS and MERS for COVID-19), although interesting, provide little information about risk groups to be currently acted upon. Where key potential risk factors of interest, such as deprivation, are linked to both the disease of interest and the comparator condition, this limits the inferences possible. Saying this, studies of COVID-19 with the comparator group of other forms of viral pneumonia are a useful complement to studies using a general population comparator, as they show whether people with particular risk factors are at risk over and above what they might experience from ‘normal’ respiratory viruses, which might inform the level of additional precautions they could consider taking.
Finally, appropriately adjusted multivariable analyses should be prioritised, in order to separate the implications of different risk factors and to infer true causal relationships, for example exploring specific markers of comorbidity severity and control, such as the use of specific medications. We can then make the recommendations for shielding criteria more targeted, meaning that the public can be made more aware of the risk factors that are likely to have clinical significance and adapt their behaviour accordingly. Early clinical studies during pandemics are critically important and published rapidly under extremely difficult circumstances, but we would argue that high-quality epidemiological studies should also be seen as a priority, and that emergency response plans should include provision of appropriate epidemiological and statistical expertise.
Conclusions
The volume of literature generated in the short time since the appearance of SARS-CoV-2 has been considerable. Many studies have sought to document the risk factors for COVID-19 disease, disease severity and mortality. Age was the only risk factor based on robust studies and with a consistent body of evidence. Mechanistic studies are required to understand why age is such an important risk factor. At the start of pandemics, large, standardised, studies using multivariable analyses – e.g. using national surveillance data – are urgently needed in order to inform stratified approaches to rapidly protecting the population groups most at risk.
Acknowledgements
Not applicable.
Abbreviations
- ACE2
Angiotensin-converting enzyme 2
- ARDS
Acute respiratory distress syndrome
- BMI
Body mass index
- CDC
Center for Disease Control and Prevention
- COPD
Chronic obstructive pulmonary disease
- COVID-19
Coronavirus disease-19
- CT
Computed tomography
- DIC
Disseminated intravascular coagulation
- FiO2
Inspired oxygen fraction
- ICNARC
Intensive care national audit and research centre
- ICU
Intensive care unit
- IL
Interleukin
- ISARIC
International severe acute respiratory and emerging infections consortium
- LRT
Lower respiratory tract
- MERS
Middle Eastern Respiratory Syndrome
- MS
Multiple sclerosis
- N/A
Not applicable
- PaO2
Arterial partial pressure of oxygen
- PCR
Polymerase chain reaction
- RAS
Renin-angiotensin system
- RR
Respiratory rate
- SARS
Severe acute respiratory syndrome
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus-2
- SpO2
Oxygen saturation
- TMPRSS2
Transmembrane serine protease 2
- URT
Upper respiratory tract
Authors’ contributions
HRS, MF and AS conceived of the work. HRS, MF, CJ, EV and CS designed the work. MF, CJ, HRS, EV, MDM, YJ and XW acquired and interpreted the data. CRS, UA, CM, JLKM, LDR, CR, SJS, MEJW and AS also interpreted the data. HRS and MF drafted the work. CJ, EV, CRS, MDM, UA, CM, YJ, JLKM, LDR, CR, SJS, XW, MEJW and AS revised it critically for important intellectual content. All authors give final approval of the version to be published. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding
HRS and MF are supported by the Medical Research Council [MR/R008345/1]. CJ’s salary came through MRC core funding MC_UU_12023/26. SJS is funded by the Wellcome Trust [WT 209560/Z/17/Z]. CRS has received funding from the Medical Research Council [MR/R008345/1], the National Institute for Health Research [11/46/23] and the New Zealand Health Research Council [20/1018] and Ministry for Business, Innovation and Employment. EV is funded by the Medical Research Council [MR/R008345/1] through the EAVE II grant and supported by the Scottish Government. We also acknowledge the support of HDR UK. The views and opinions expressed here are those of the authors and do not necessarily reflect those of the Health Technology Assessment programme, NIHR, NHS, or the UK Department of Health. Our funders had no role in the design of the study and collection, analysis, and interpretation of data, and in writing the manuscript.
Availability of data and materials
All data generated or analysed during this study are included in this published article and the underlying papers.
Declarations
Ethics approval and consent to participate
This is a systematic review and therefore presents secondary data. No ethical approvals were required.
Consent for publication
Not applicable.
Competing interests
CR is a member of the Scottish Government’s Chief Medical Officer’s COVID-19 Advisory Group, a member of the UK SPI-M committee and the Commission Human Medicines COVID-19 Vaccine Safety Benefits and Risks Working Group. JLKM is Incident Director for COVID-19 at Public Health Scotland and reports no conflicts of interest. LDR serves on a number of Scottish Government Advisory Groups, including COVID-19. MEJW is a member of the SPI-M advisory committee for the UK Government and the Covid-19 Advisory Group for the Scottish Government. AS is a member of the Scottish Government’s Chief Medical Officer’s COVID-19 Advisory Group and the New and Emerging Respiratory Virus Threats (NERVTAG) Risk Stratification Subgroup. HRS is an advisor to the Scottish Parliament’s COVID committee. The views represented in this article do not represent the views of the UK or Scottish Government. All other authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
U. Agrawal, C. McCowan, Y. Jia, J. L. K. Murray, L. D. Ritchie, C. Robertson, S. J. Stock, X. Wang and M. E. J. Woolhouse contributed equally to this work.
References
- 1.World Health Organization. WHO Director-General's opening remarks at the media briefing on COVID-19 2020 [updated 11th March 2020. Available from: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19%2D%2D-11-march-2020. Accessed 20 Mar 2020.
- 2.CDC COVID-19 Response Team. Severe Outcomes Among Patients with Coronavirus Disease 2019 (COVID-19)- United States, February 12–March 16, 2020. Morb Mortal Wkly Rep. 2020;69(12):343–6. [DOI] [PMC free article] [PubMed]
- 3.Wu JT, Leung K, Bushman M, Kishore N, Niehus R, de Salazar PM, et al. Estimating clinical severity of COVID-19 from the transmission dynamics in Wuhan, China. Nat Med. 2020;26:506–10. [DOI] [PMC free article] [PubMed]
- 4.Ortiz JR, Perut M, Dumolard L, Wijesinghe PR, Jorgensen P, Ropero AM, Danovaro-Holliday MC, Heffelfinger JD, Tevi-Benissan C, Teleb NA, Lambach P, Hombach J. A global review of national influenza immunization policies: analysis of the 2014 WHO/UNICEF joint reporting form on immunization. Vaccine. 2016;34(45):5400–5405. doi: 10.1016/j.vaccine.2016.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.NHS Digital. Coronavirus (COVID-19): Shielded patients list 2020. Available from: https://digital.nhs.uk/coronavirus/shielded-patient-list. Accessed 27 May 2020.
- 6.Health Protection Surveillance Centre. Guidance for Vulnerable Groups 2020. Available from: https://www.hpsc.ie/a-z/respiratory/coronavirus/novelcoronavirus/guidance/vulnerablegroupsguidance/. Accessed 3 June 2020.
- 7.van Bunnik BAD, Morgan ALK, Bessell P, Calder-Gerver G, Zhang F, Haynes S, et al. Segmentation and shielding of the most vulnerable members of the population as elements of an exit strategy from COVID-19 lockdown. MedRxiv. 2020. 10.1101/2020.05.04.20090597. [DOI] [PMC free article] [PubMed]
- 8.Ganguli-Mitra A, Young I, Engelmann L, Harper I, McCormack D, Marsland R, et al. Segmenting communities as public health strategy: a view from the social sciences and humanities. Wellcome Open Res. 2020;5:104. 10.12688/wellcomeopenres.15975.1. [DOI] [PMC free article] [PubMed]
- 9.NHS Digital. Statement for people who think they have inaccurately been sent communication about being vulnerable to coronavirus 2020 [updated 24th March 2020. Available from: https://digital.nhs.uk/news-and-events/latest-news/statement-on-coronavirus-communications. Accessed 26 Mar 2020.
- 10.Public Health England. Guidance on social distancing for everyone in the UK and protecting older people and vulnerable adults 2020 [updated 30th March 2020. Available from: https://www.gov.uk/government/publications/covid-19-guidance-on-social-distancing-and-for-vulnerable-people/guidance-on-social-distancing-for-everyone-in-the-uk-and-protecting-older-people-and-vulnerable-adults. Accessed 27 Apr 2020.
- 11.Cabinet Office. Staying alert and safe (social distancing) 2020 [Available from: https://www.gov.uk/government/publications/staying-alert-and-safe-social-distancing/staying-alert-and-safe-social-distancing#protecting-different-groups-of-people. Accessed 28 May 2020.
- 12.Public Health Scotland. Search criteria for highest risk patients for shielding. Version 4.0 2020 [Available from: https://hpspubsrepo.blob.core.windows.net/hps-website/nss/3008/documents/1_covid-19-search-criteria-highest-risk-patients.pdf. Accessed 3 June 2020.
- 13.Zheng YY, Ma YT, Zhang JY, Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17(5):259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization. Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected Interim guidance 2020 [updated 13th March 2020. Available from: https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-. Accessed 27 May 2020. (ncov)-infection-is-suspected.
- 15.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377–384. doi: 10.1136/jech.52.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deeks JJ, Dinnes J, D'Amico R, Sowden AJ, Sakarovitch C, Song F, et al. Evaluating non-randomised intervention studies. Health Technol Assess. 2003;7(27):iii-x, 1–iii-x173. doi: 10.3310/hta7270. [DOI] [PubMed] [Google Scholar]
- 17.Arner TGS, G.G.; Friedman, R.; Lantinga, M.; Sangam, S.; Zubieta, J.C.; Sullivan, K.M.; Brendel, K.A.; Gao, Z.; Fontaine, N.; Shu, M.; Fuller, G.; Smith, D.C.; Nitschke, D.A.; Fagan, R.F.;. Epi info™, a database and statistics program for public health professionals. GA, USA: Centers for Disease Control and Prevention; 2011.
- 18.Westreich D, Greenland S. The table 2 fallacy: presenting and interpreting confounder and modifier coefficients. Am J Epidemiol. 2013;177(4):292–298. doi: 10.1093/aje/kws412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cai Q, Huang D, Ou P, Yu H, Zhu Z, Xia Z, et al. COVID-19 in a designated infectious diseases hospital outside Hubei Province, China. Allergy. 2020;75(7):1742–52. 10.1111/all.14309. [DOI] [PubMed]
- 20.Chen J, Qi T, Liu L, Ling Y, Qian Z, Li T, Li F, Xu Q, Zhang Y, Xu S, Song Z, Zeng Y, Shen Y, Shi Y, Zhu T, Lu H. Clinical progression of patients with COVID-19 in Shanghai, China. J Infect. 2020;80(5):e1–6. 10.1016/j.jinf.2020.03.004. [DOI] [PMC free article] [PubMed]
- 21.Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: Retrospective study. The BMJ. 2020;368(no pagination). [DOI] [PMC free article] [PubMed]
- 22.Chen X, Yang Y, Huang M, Liu L, Zhang X, Xu J, et al. Differences between COVID-19 and suspected then confirmed SARS-CoV-2-negative pneumonia: a retrospective study from a single center. J Med Virol. 2020;92(9):1572–79. 10.1002/jmv.25810. [DOI] [PubMed]
- 23.Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, Wang T, Zhang X, Chen H, Yu H, Zhang X, Zhang M, Wu S, Song J, Chen T, Han M, Li S, Luo X, Zhao J, Ning Q. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130(5):2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng Z, Lu Y, Cao Q, Qin L, Pan Z, Yan F, Yang W. Clinical features and chest CT manifestations of coronavirus disease 2019 (COVID-19) in a single-center study in Shanghai, China. AJR Am J Roentgenol. 2020;215(1):121–126. doi: 10.2214/AJR.20.22959. [DOI] [PubMed] [Google Scholar]
- 25.Epidemiology Working Group for Ncip Epidemic Response CCfDC, Prevention The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41(2):145–151. doi: 10.3760/cma.j.issn.0254-6450.2020.02.003. [DOI] [PubMed] [Google Scholar]
- 26.Fan BE, Chong VCL, Chan SSW, Lim GH, Lim KGE, Tan GB, et al. Hematologic parameters in patients with COVID-19 infection. Am J Hematol. 2020;95(6):E131–E134. 10.1002/ajh.25774. [DOI] [PubMed]
- 27.Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, Cereda D, Coluccello A, Foti G, Fumagalli R, Iotti G, Latronico N, Lorini L, Merler S, Natalini G, Piatti A, Ranieri MV, Scandroglio AM, Storti E, Cecconi M, Pesenti A, for the COVID-19 Lombardy ICU Network Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020;323(16):1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han H, Xie L, Liu R, Yang J, Liu F, Wu K, et al. Analysis of heart injury laboratory parameters in 273 COVID-19 patients in one hospital in Wuhan, China. J Med Virol. 2020;92(7):819–23. 10.1002/jmv.25809. [DOI] [PMC free article] [PubMed]
- 29.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Intensive Care National Audit and Research Centre. ICNARC report on COVID-19 in critical care 2020 [Available from: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&cad=rja&uact=8&ved=2ahUKEwjS4MK86NbpAhVPQ0EAHQ3dD5gQFjAAegQIBhAB&url=https%3A%2F%2Fwww.icnarc.org%2FDataServices%2FAttachments%2FDownload%2Fc5a62b13-6486-ea11-9125-00505601089b&usg=AOvVaw3iovn0oEBhP6xPnV27l9HR. Accessed 21 May 2020.
- 31.Liang W, Guan W, Chen R, Wang W, Li J, Xu K, Li C, Ai Q, Lu W, Liang H, Li S, He J. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21(3):335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu R, Ma Q, Han H, Su H, Liu F, Wu K, et al. The value of urine biochemical parameters in the prediction of the severity of coronavirus disease 2019. Clin Chem Lab Med. 2020;58(7):1121–24. 10.1515/cclm-2020-0220. [DOI] [PubMed]
- 33.Liu R, Han H, Liu F, Lv Z, Wu K, Liu Y, Feng Y, Zhu C. Positive rate of RT-PCR detection of SARS-CoV-2 infection in 4880 cases from one hospital in Wuhan, China, from Jan to Feb 2020. Clin Chim Acta. 2020;505:172–175. doi: 10.1016/j.cca.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qiu H, Wu J, Hong L, Luo Y, Song Q, Chen D. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID-19) in Zhejiang, China: an observational cohort study. Lancet Infect Dis. 2020;20(6):689–696. doi: 10.1016/S1473-3099(20)30198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46(5):846–48. 10.1007/s00134-020-05991-x [DOI] [PMC free article] [PubMed]
- 36.Shi Y, Yu X, Zhao H, Wang H, Zhao R, Sheng J. Host susceptibility to severe COVID-19 and establishment of a host risk score: findings of 487 cases outside Wuhan. Crit Care. 2020;24(1):108. doi: 10.1186/s13054-020-2833-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simonnet A, Chetboun M, Poissy J, Raverdy V, Noulette J, Duhamel A, et al. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity. 2020;28(7):1195–99. 10.1002/oby.22831. [DOI] [PMC free article] [PubMed]
- 38.Tian S, Hu N, Lou J, Chen K, Kang X, Xiang Z, Chen H, Wang D, Liu N, Liu D, Chen G, Zhang Y, Li D, Li J, Lian H, Niu S, Zhang L, Zhang J. Characteristics of COVID-19 infection in Beijing. J Infect. 2020;80(4):401–406. doi: 10.1016/j.jinf.2020.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wan S, Xiang Y, Fang W, Zheng Y, Li B, Hu Y, Lang C, Huang D, Sun Q, Xiong Y, Huang X, Lv J, Luo Y, Shen L, Yang H, Huang G, Yang R. Clinical features and treatment of COVID-19 patients in Northeast Chongqing. J Med Virol. 2020;92(7):797–806. doi: 10.1002/jmv.25783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang C, Horby PW, Hayden FG, Gao GF. A novel coronavirus outbreak of global health concern. Lancet. 2020;395(10223):470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 1061;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang L, Li X, Chen H, Yan S, Li D, Li Y, Gong Z. Coronavirus disease 19 infection does not result in acute kidney injury: an analysis of 116 hospitalized patients from Wuhan, China. Am J Nephrol. 2020;51(5):343–348. doi: 10.1159/000507471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Z, Yang B, Li Q, Wen L, Zhang R. Clinical features of 69 cases with coronavirus disease 2019 in Wuhan, China. Clin Infect Dis. 2020;71(15):769–77. 10.1093/cid/ciaa272. [DOI] [PMC free article] [PubMed]
- 44.Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934–43. 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed]
- 45.Wu Z, JM MG. Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72314 Cases from the Chinese Center for Disease Control and Prevention. JAMA. 1239;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 46.Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yuan M, Yin W, Tao Z, Tan W, Hu Y. Association of radiologic findings with mortality of patients infected with 2019 novel coronavirus in Wuhan, China. Plos One. 2020;15(3):e0230548. 10.1371/journal.pone.0230548. [DOI] [PMC free article] [PubMed]
- 48.Zhang JJ, Dong X, Cao YY, Yuan YD, Yang YB, Yan YQ, et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy: Eur J Allergy Clin Immunol. 2020;75(7):1730–41. 10.1111/all.14238. [DOI] [PubMed]
- 49.Zhang G, Zhang J, Wang B, Zhu X, Wang Q, Qiu S. Analysis of clinical characteristics and laboratory findings of 95 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a retrospective analysis. Respir Res. 2020;21(1):74. doi: 10.1186/s12931-020-01338-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang R, Ouyang H, Fu L, Wang S, Han J, Huang K, et al. CT features of SARS-CoV-2 pneumonia according to clinical presentation: a retrospective analysis of 120 consecutive patients from Wuhan city. Eur Radiol. 2020. epub ahead of print. 10.1007/s00330-020-06854-1. [DOI] [PMC free article] [PubMed]
- 51.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 1054;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Price-Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and mortality among Black patients and white patients with Covid-19. N Engl J Med. 2020;382(26):2534–2543. doi: 10.1056/NEJMsa2011686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Docherty AB, Harrison EM, Green CA, Hardwick HE, Pius R, Norman L, et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO clinical characterisation protocol: prospective observational cohort study. BMJ. 2020;369:m1985. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen J. Pathogenicity and transmissibility of 2019-nCoV-A quick overview and comparison with other emerging viruses. Microbes Infect. 2020;22(2):69–71. doi: 10.1016/j.micinf.2020.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gengler IW, J.C.; Speth, M.M.; Sedaghat A.R.;. Sinonasal pathophysiology of SARS-CoV-2 and COVID-19: a systematic review of the current evidence. Laryngoscope Invest Otolaryngol. 2020;5(3):354–9. 10.1002/lio2.384. [DOI] [PMC free article] [PubMed]
- 56.Chu H, Chan JF, Wang Y, Yuen TT, Chai Y, Hou Y, et al. Comparative replication and immune activation profiles of SARS-CoV-2 and SARS-CoV in human lungs: an ex vivo study with implications for the pathogenesis of COVID-19. Clin Infect Dis. 2020;71(6):1400–1409. doi: 10.1093/cid/ciaa410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fu Y, Cheng Y, Wu Y. Understanding SARS-CoV-2-mediated inflammatory responses: from mechanisms to potential therapeutic tools. Virol Sin. 2020;35(3):266–271. doi: 10.1007/s12250-020-00207-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kumar VA, A.K.; Aster, J.C.; . Robbins & Cotran pathologic basis of disease, 9e (Robbins pathology) 2014. 1408 p.
- 59.Pangrazzi L, Meryk A, Naismith E, Koziel R, Lair J, Krismer M, Trieb K, Grubeck-Loebenstein B. “Inflamm-aging” influences immune cell survival factors in human bone marrow. Eur J Immunol. 2017;47(3):481–492. doi: 10.1002/eji.201646570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bunyavanich S, Do A, Vicencio A. Nasal gene expression of angiotensin-converting enzyme 2 in children and adults. JAMA. 2020;323(23):2427–2429. doi: 10.1001/jama.2020.8707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dai M, Liu D, Liu M, Zhou F, Li G, Chen Z, Zhang Z, You H, Wu M, Zheng Q, Xiong Y, Xiong H, Wang C, Chen C, Xiong F, Zhang Y, Peng Y, Ge S, Zhen B, Yu T, Wang L, Wang H, Liu Y, Chen Y, Mei J, Gao X, Li Z, Gan L, He C, Li Z, Shi Y, Qi Y, Yang J, Tenen DG, Chai L, Mucci LA, Santillana M, Cai H. Patients with Cancer appear more vulnerable to SARS-CoV-2: a multicenter study during the COVID-19 outbreak. Cancer Discov. 2020;10(6):783–791. doi: 10.1158/2159-8290.CD-20-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.European Centre for Disease Prevention and Control. ECDC launches new weekly COVID-19 surveillance report 2020 [Available from: https://www.ecdc.europa.eu/en/news-events/ecdc-launches-new-weekly-covid-19-surveillance-report. Accessed 27 May 2020.
- 63.McGlothlin AE, Lewis RJ. Minimal clinically important difference: defining what really matters to patients. JAMA. 2014;312(13):1342–1343. doi: 10.1001/jama.2014.13128. [DOI] [PubMed] [Google Scholar]
- 64.International Severe Acute Respiratory and Emerging Infection Consortium. COVID-19 Clinical Research Resources 2020 [Available from: https://isaric.tghn.org/covid-19-clinical-research-resources/. Accessed 30 May 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article and the underlying papers.