Abstract
Baroreflex function is an integral component maintaining consistent blood pressure. Hypertension is often associated with baroreflex dysfunction, and environmental risk factors such as high salt diet exacerbate hypertension in subjects with baroreflex dysfunction. However, the interactions between high salt diet, baroreflex dysfunction, and hypertension are incompletely understood. The endothelin system is another potent mediator of blood pressure control especially in response to a high salt diet. We hypothesized that the endothelin B (ETB) receptor activation on adrenergic nerves decreases baroreflex sensitivity. We utilized male ETB receptor deficient (ETB-def) rats that express functional ETB receptors only on adrenergic nerves and transgenic (TG) controls to evaluate baroreflex function during normal (0.49% NaCl) and high (4.0% NaCl) salt diets. In conscious rats equipped with telemetry, ETB-def rats had an increased lability of systolic blood pressure (SBP) compared to TG controls as indicated by higher standard deviation (SD) of SBP under both normal (10.2±0.6 vs. 12.4±0.9 mmHg, respectively, p=0.0001) and high (11.7±0.6 vs. 16.1±1.0 mmHg, p=0.0001) salt diets. In anesthetized preparations, ETB-def rats displayed reduced heart rate (p genotype=0.0167) and renal sympathetic nerve (p genotype=0.0022) baroreflex sensitivity. We then gave male Sprague-Dawley rats the selective ETB receptor antagonist, A-192621 (10 mg/kg/day), to block ETB receptors. Following ETB receptor antagonism, even though SBP increased (131±7 before vs. 152±8 mmHg after, p<0.0001), the lability (standard deviation) of SBP decreased (9.3±2.0 vs. 7.1±1.1 mmHg, p=0.0155). These data support our hypothesis that ETB receptors on adrenergic nerves contribute to baroreflex dysfunction.
Keywords: baroreflex, endothelin, sympathetic nerves, salt, hypertension
1. Introduction:
Healthy baroreflex function is an integral part of maintaining stable blood pressure through reflexive adjustments to heart rate and sympathetic nervous system activity. Aberrations to baroreflex function occur in a number of cardiovascular diseases such as hypertension (Han et al., 2015; Iliescu et al., 2013; Sheng et al., 2020) and heart failure (Becker et al., 2016; Zucker et al., 2012). Although numerous factors can influence baroreflex function, there remains a large gap in our current understanding of how environmental factors such as dietary salt influence the baroreflex. High salt diet has been shown to increase baroreflex sensitivity in healthy adults (Babcock et al., 2018) and animal models (Huang et al., 1994; Simmonds et al., 2014). Conversely, in diseases such as salt-sensitive hypertension, baroreflex dysfunction is thought to play a causitive role in the progression of the hypertensive response to high salt diet (Bugenhagen et al., 2010; Coruzzi et al., 2005). Activation of the baroreflex may even be a therapeutic option in treatment resistant hypertensive patients by increasing sodium excretion (Lipphardt et al., 2019), which has also been demonstrated in animal models (Hildebrandt et al., 2014; Hildebrandt et al., 2016).
A previous study in rats demonstrated a robust salt-sensitive increase in blood pressure when the baroreflex was disrupted by sinoaortic denervation (Osborn et al., 1998). An interesting observation in these studies was that denervated animals on a high salt diet displayed an increased amplitude of the diurnal blood pressure rhythm (i.e. greater night/day difference) after being placed on high salt diet. A similar finding was also observed in Sprague-Dawley rats given a specific ETB receptor blocker and high salt diet (Pollock et al., 2001), and we have also reported robust high salt diet-induced increases in diurnal blood pressure amplitude in endothelin B (ETB) receptor deficient (ETB-def) rats (Becker et al., 2017a; Speed et al., 2018). These rats lack functional ETB receptors except where a transgene with functional endothelin B receptors are expressed under dopamine beta-hydroxylase promoter control (Gariepy et al., 1998), such as efferent sympathetic neurons and adrenals.
The endothelin system is an integral modulator of blood pressure through its actions on the vasculature, renal excretion of sodium, and autonomic nervous system function (Davenport et al., 2016; Kohan et al., 2011). Although the endothelin system is a likely mediator of baroreflex function through its modulation of these organ systems, very little is known about the contribution of the endothelin system to baroreflex control in normal physiology or disease. Both the endothelin A and B receptors appear to influence sympathetic norepinephrine release to the vasculature (Kita et al., 1998) and heart (Lehmann et al., 2014), especially following cardiac ischemia (Backs et al., 2005; Yamamoto et al., 2005), but the specific role of either receptor in baroreflex function is not well understood.
Given the similar phenotype of the ETB-def rat to sinoartic denervated rats in relation to salt-sensitive increases in blood pressure and diurnal blood pressure amplitude and the role of endothelin signaling in sympathetic activity, we hypothesized that the ETB receptor activation on sympathetic nerves decreases baroreflex sensitivity. Our approach will focus on the ETB-def rat where we and others have extensively characterized the salt-sensitivity of blood pressure (Becker et al., 2017a; Gariepy et al., 2000; Matsumura et al., 2000; Ohkita et al., 2005; Speed et al., 2018) and the potential role of ETB receptors on sympathetic nerve activity (Becker et al., 2017b; Dai et al., 2004; Lau et al., 2006). Herein, we attempted to further probe the contribution of endothelin B receptor activation specifically on baroreflex function utilizing both a conscious, telemetry-based approach measuring spontaneous baroreflex activation and an anesthetized, baroreflex curve generation approach in normal salt and high salt fed rats.
2. Materials and Methods:
2.1. Animals and Surgical Preparation:
ETB-def rats and transgenic (TG) controls were bred from an in-house colony. All procedures were approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham and conducted in accordance with the Guide for the Care and Use of Laboratory Animals (8th ed., National Academy of Sciences, 2011) by the NIH. Animals were housed in 12:12 hr light cycle with lights on at 7 am and lights off at 7 pm. We and others have previously described the ETB-def and associated TG control rats in depth (Becker et al., 2017a; Becker et al., 2017b; Gariepy et al., 2000; Gariepy et al., 1998). In brief, the ETB-def rat is derived from the spotting lethal rat, which has a spontaneous mutation in the EDNRB gene rendering ETB receptors nonfunctional. This results in the failure of enteric nerve development and causes a lethal megacolin in homozygous rats. This lethal megacolon was prevented by the insertion of a functional EDNRB gene under human dopamine-β-hydroxylase (DβH) promoter control (Gariepy et al., 1998). Thus, ETB-def rats have functional ETB receptor expression in DβH positive tissues, but lack functional receptors elsewhere. Our breeding scheme consists of mating rats homozygous for transgene (DβH-EDNRB) expression and heterozygous for the spotting lethal (sl) mutation. ETB-def offspring are homozygous for both the transgene and spotting lethal mutation (DβH-EDNRB+/+;sl+/+). The littermate TG controls, posess the transgene but are spotting lethal negative (DβH-EDNRB+/+;sl−/−) and therefore retain functional endogenous EDNRB expression. Offspring heterozygous for the spotting lethal mutation (DβH-EDNRB+/+;sl+/−) are euthanized or used as breeders. Tail snips are sent to Transnetyx (Transnetyx Inc, Cordova, TN) for genotyping and confirmation of presence of transgene.
Male ETB-def and TG rats of starting weights from 250–350g were instrumented with telemetry transmitters (HD-S10, Data Sciences International, Minneapolis, MN) implanted in the abdominal aorta as previously described (Becker et al., 2017a). Animals were allowed to recover for one week following surgery before baseline blood pressure recordings were completed. Following approximately one week of baseline recordings during which time animals were fed a 0.49% NaCl, normal salt diet (TD.96208; Envigo, Indianapolis, IN), rats were switched to a high salt (4.0% NaCl) diet (TD.92034) for two weeks. Figures 1–3 and Tables 1–2 are a further analysis of previously published telemetry data (Becker et al., 2017a). All other data and analyses are derived from newly instrumented animals specific to this study.
Figure 1: ETB-def rats have elevated blood pressure lability during normal salt and high salt diet.
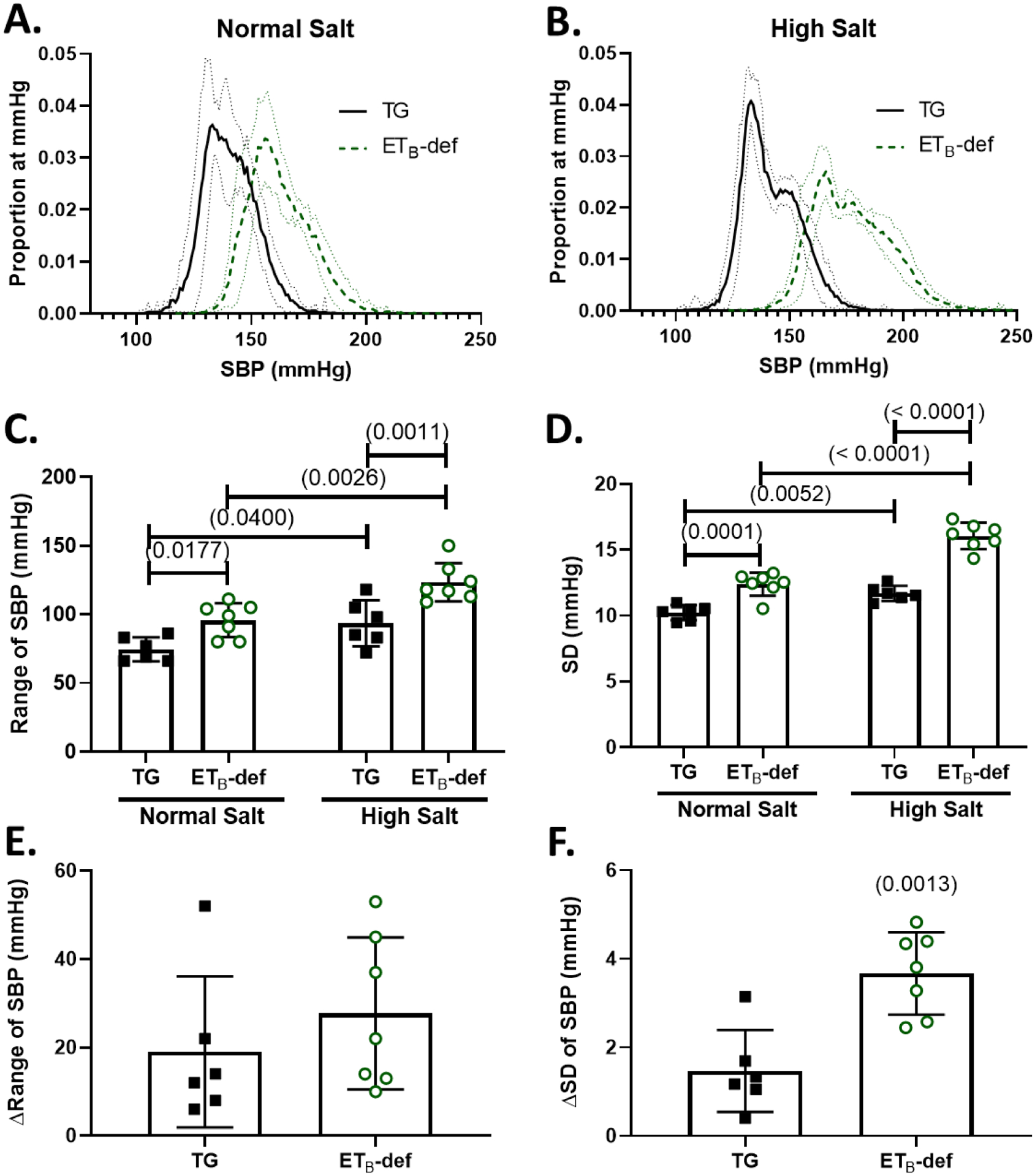
A/B: frequency distribution plots of proportion of instances at a given SBP (number of instances of 10-second bins at each mmHg / total number of bins) during normal salt diet (A) and 8–11 days of high salt diet (B) Solid line represents mean ± 95% confidence interval. C: Range of SBP during normal and high salt diet, 2-way repeated measures ANOVA Psalt = 0.0005; Pgenotype = 0.0009; Pinteraction = 0.3808, with Sidak’s test for multiple comparisons. D: Standard deviation during normal and high salt diet, 2-way repeated measures ANOVA Psalt < 0.0001; Pgenotype < 0.0001; Pinteraction = 0.0013, with Sidak’s test for multiple comparisons. E: Change in the SBP range between normal salt and high salt diets, unpaired Student’s t-test p = 0.3808, F: Change in the standard deviation of SBP between normal salt and high salt diets, unpaired Student’s t-test, SBP = systolic blood pressure, TG = transgenic, ETB-def = endothelin B deficient, SD = standard deviation.
Figure 3: ETB-def rats have fewer true:non-baroreflex sequences following high salt diet.
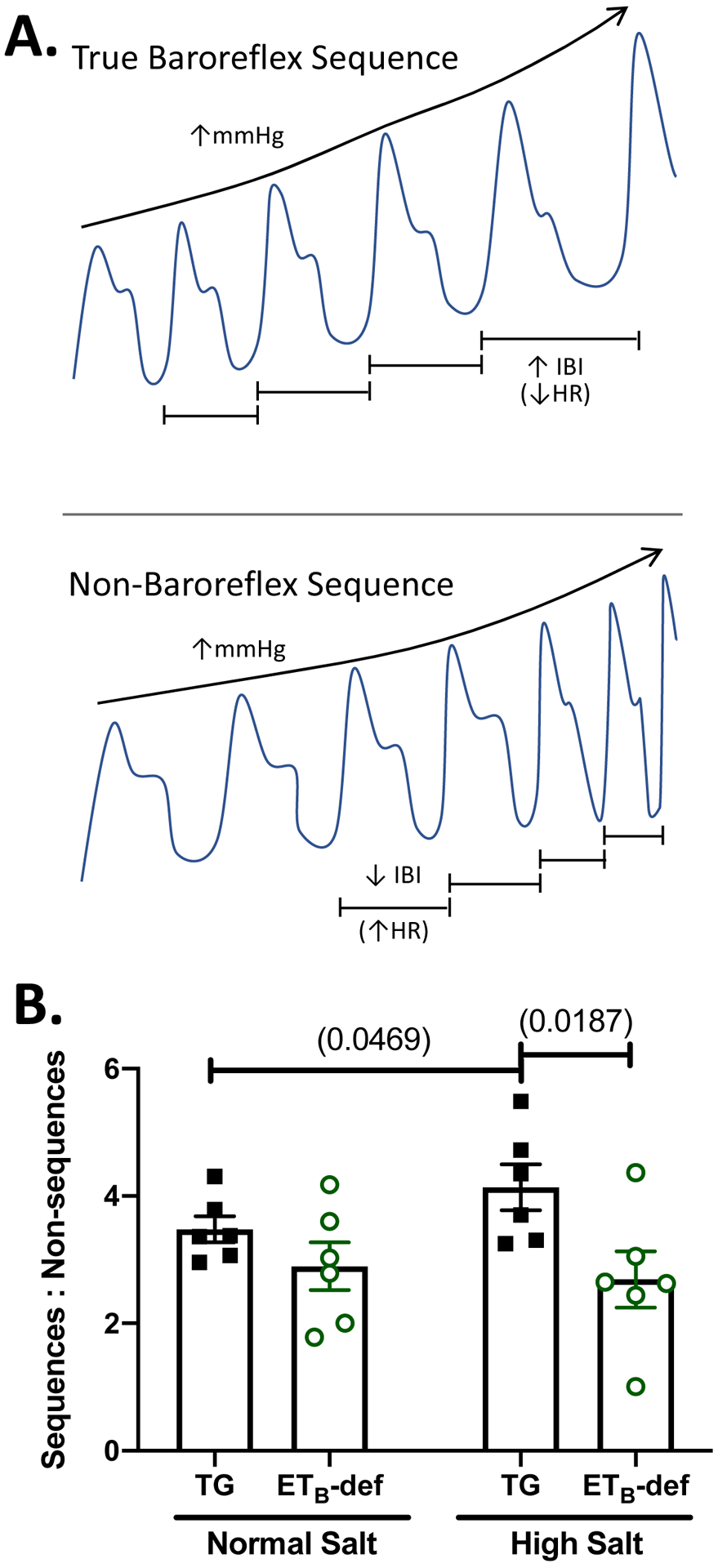
A: simplified graphical representation of a true baroreflex sequence (top panel: blood pressure and interbeat interval, or IBI, both increasing) vs. a non-baroreflex/feedforward sequence (bottom panel: blood pressure and IBI change in opposite directions). B: ratio of true baroreflex sequences to non-baroreflex sequences in transgenic TG controls and ETB-def rats during normal salt (0.49% NaCl) or on days 8–11 of high salt (4.0% NaCl) diet. 2-way repeated measures ANOVA, Psalt = 0.2233; Pgenotype = 0.0572; Pinteraction = 0.0332 with Sidak’s multiple comparison test. IBI = interbeat interval, HR = heart rate, TG = transgenic, ETB-def = endothelin B deficient.
Table 1 -.
Lability Parameters during Active and Inactive Periods
| TG Controls (n = 6) | ETB-def (n = 7) | |||||
|---|---|---|---|---|---|---|
| Normal Salt | High Salt | Normal Salt | High Salt | 3-way RM ANOVA (p) | ||
| SBP Range (mmHg) | S = 0.0006 | S*G = 0.6177 | ||||
| Active | 72.2 ± 3.1 | 84.2 ± 6.1 | 94.6 ± 5.0G | 113.0 ± 6.1GS | G = 0.0011 | S*TOD = 0.8037 |
| Inactive | 70.2 ± 3.8 | 84.3 ± 4.1 | 90.9 ± 5.0G | 105.0 ± 4.0G | TOD = 0.1404 | G*TOD = 0.2711 |
| S*G*TOD = 0.4544 | ||||||
| SBP SD (mmHg) | S < 0.0001 | S*G = 0.0444 | ||||
| Active | 10.6 ± 0.4 | 12.0 ± 0.2S | 12.7 ± 0.5G | 15.2 ± 0.4GS | G < 0.0001 | S*TOD = 0.0144 |
| Inactive | 9.6 ± 0.2 | 10.0 ± 0.2T | 11.8 ± 0.2G | 13.2 ± 0.4GST | TOD < 0.0001 | G*TOD = 0.9564 |
| S*G*TOD = 0.8546 | ||||||
| HR Range (bpm) | S = 0.0004 | S*G = 0.9134 | ||||
| Active | 255.8 ± 7.5 | 278.0 ± 7.6 | 265.3 ± 8.2 | 285.1 ± 6.2 | G = 0.0365 | S*TOD = 0.3688 |
| Inactive | 242.3 ± 4.3 | 273.7 ± 13.4 | 262.0 ± 8.1 | 293.3 ± 6.0 | TOD = 0.5893 | G*TOD = 0.3518 |
| S*G*TOD = 0.9199 | ||||||
| HR SD (bpm) | S = 0.0025 | S*G = 0.9481 | ||||
| Active | 48.7 ± 1.5 | 41.7 ± 1.2 | 57.9 ± 2.7 | 48.6 ± 1.5S | G = 0.0116 | S*TOD = 0.0378 |
| Inactive | 42.0 ± 3.5 | 36.0 ± 1.6 | 48.3 ± 3.6T | 44.2 ± 2.0 | TOD = 0.0005 | G*TOD = 0.7771 |
| S*G*TOD = 0.1363 | ||||||
SBP: systolic blood pressure, HR: heart rate, Normal salt (0.49% NaCl), High salt (4.0% NaCl), TOD: time of day, G: genotype, S: salt diet. 3-way Repeated Measures ANOVA with Sidak’s post-hoc test for multiple comparisons:
p < 0.05 genotype within salt diet and time of day;
p < 0.05 salt within genotype and time of day;
p < 0.05 time of day within salt and genotype. Mean ± SEM.
Group effect significance indicated by italics.
Table 2 -.
Cosinor Analysis
| TG Controls (n = 6) | ETB-def (n = 7) | ||||||
|---|---|---|---|---|---|---|---|
| Normal Salt | High Salt | Normal Salt | High Salt | 2-Way RM ANOVA (p) | |||
| SBP SD Amplitude (mmHg) | 1.0 ± 0.1 | 1.7 ± 0.2 | 1.2 ± 0.2 | 2.2 ± 0.2S | S = 0.0032 | G = 0.0492 | S*G = 0.4749 |
| HR SD Amplitude (bpm) | 7.5 ± 1.2 | 7.9 ± 0.5 | 8.8 ± 0.7 | 7.9 ± 1.5 | S = 0.7123 | G = 0.6319 | S*G = 0.3825 |
| SBP SD Acrophase (ZT) | 20.5 ± 0.7 | 19.8 ± 0.2 | 20.3 ± 0.7 | 20.0 ± 0.5 | S = 0.4140 | G = 0.9608 | S*G = 0.7426 |
| HR SD Acrophase (ZT) | 15.9 ± 0.9 | 15.9 ± 0.5 | 16.3 ± 0.2 | 16.3 ± 0.4 | S = 0.9235 | G = 0.4757 | S*G = 0.9685 |
SBP: systolic blood pressure, HR: heart rate, Normal salt (0.49% NaCl), High salt (4.0% NaCl), G: genotype, S: salt diet. 2-way Repeated Measures ANOVA with Sidak’s post-hoc test for multiple comparisons:
p < 0.05 vs. normal salt.Mean ± SEM.
Group effect significance indicated by italics.
2.2. Telemetry Analysis:
Telemetry recordings were taken in 10-minute sections every 20 minutes for 24 hrs per day throughout the experiment using Ponemah v6.0 (Data Sciences International). Data were analyzed offline in 10 second bins using Ponemah v6.5, and blood pressure and heart rate were rounded to the nearest whole integer in Microsoft Excel. Using pivot tables in Microsoft Excel, the number of instances for each given mmHg of blood pressure or bpm of heart rate were determined for 3 consecutive days of baseline, days 8–11 following high salt diet, and days 3–5 of A-192621 treatment. Because of differences between animals in the number of total bins due to telemetry dropouts or noise, the count of each mmHg of blood pressure or bpm of heart rate was divided by a count of the total number of bins to calculate the proportion of each mmHg or bpm to total number of bins. The standard deviation (SD) of blood pressure and HR was calculated from pivot tables in Microsoft Excel. To evaluate the impact of diurnal factors in SD and range of blood pressure and HR, data were separated into active (zeitgeber time 12–24; 7 pm to 7am) and inactive (zeitgeber time 0–12; 7 am to 7pm). Spontaneous baroreflex measurements were analyzed as previously described utilizing Hemolab software using the sequence method (Harald Stauss Scientific, Iowa City, IA) (Becker et al., 2017a; Bertinieri et al., 1985; Stauss et al., 2006). Spontaneous baroreflex measurements were completed during the same time frame as lability parameters, 3 consecutive days of baseline, days 8–11 following high salt diet, and days 3–5 of A-192621 treatment.
2.3. Acute Baroreflex Measurement:
A separate group of 26 male TG and ETB-def rats were fed normal salt (0.49% NaCl) or high salt (4.0% NaCl) diet for 7–11 days and subsequently prepared for acute, anesthetized baroreflex experiments. Rats were anesthetized with an IP injection of 100 mg/kg body weight Inactin® (thiobutabarbital sodium salt hydrate; Sigma-Aldrich, St. Louis, MO), a tracheostomy was performed to allow for free breathing, and catheters were placed in the right femoral artery and vein for measurement of blood pressure and administration of fluids respectively. Sterile saline was administered at a rate of 2 mL per hr via the femoral vein to maintain electrolye stability throughout the course of the surgical preparation and experiment. Electrodes were placed in the skin of the right arm and left leg, and the differential signal amplified by 5k and low-pass filtered at 30 Hz with a Brownlee Precision Model 200 amplifier (NeuroPhase, SantaClara, CA) for generation of a single lead electrocardiogram. The left kidney was exposed and deflected via a flank incision, and a left renal sympathetic nerve bundle carefully dissected from surrounding tissues by fine forceps. The nerve was placed on a pair of stainless-steel recording electrodes and covered in warmed mineral oil for measurement of bipotential extracellular electrophysiological activity, and a reference electrode was placed nearby in surrounding tissue. The electrodes were connected to an HZP high impedance probe (Natus Neurology, Oakville, Ontario), and the signal amplified with a Grass P511 AC preamplifier (Natus Neurology), amplified at 100k, low pass filtered at 100 Hz, and high pass filtered at 1 kHz. Renal sympathetic nerve activity (RSNA) was visualized on an LG OS-3020D oscilloscope, and the auditory signal output for monitoring. All signals were recorded using Powerlab 4/30 and Labchart v8 (AD Instruments, Colorado Springs, CO) at 1k samples/sec. Blood pressure was lowered by a bolus IV infusion of 25 μg sodium nitroprusside (Sigma-Aldrich, St. Louis, MO) and raised by an IV infusion of 10 μg phenylephrine (Sigma-Aldrich, St. Louis, MO) as previously described (Becker et al. 2016). Interbeat interval (IBI) was calculated as time between each R wave from the electrocardiogram in ms. Nerve activity was rectified and integrated (timed constant of 10 ms). To minimize animal to animal differences in renal sympathetic nerve activity, resting/baseline discharge was set as 100% and background (following proximal and distal sectioning of nerve) was set as 0%. Three rats (1 TG on normal salt diet, 1 ETB-def on normal salt diet, and 1 ETB-def on high salt diet) did not have high quality RSNA recordings (such as signal to noise ratio < 2 or lack of charachteristic cardiac synchronous bursting) and are only included in the HR data set. Voltages were calibrated using the Grass P511 AC preamplifier controls for each individual experiment.
2.4. Endothelin B Antagonism:
In a separate set of experiments, male Sprague-Dawley or TG and ETB-def rats (250–300g) were instrumented with telemetry transmitters as described above and allowed to recover for one week. Baseline telemetry collections were acquired and drinking water was switched to that containing the selective ETB antagonist, A-192621 (PepTech Corp., Bedford, MA) (von Geldern et al., 1999, Wessale JL et al., 2002), measured and adjusted daily to administer a dose of 10 mg/kg/day in four rats. In a different group of four rats, Alzet minipumps (Model 2ML1, DURECT Corporation, Cupertino, CA) were implanted IP to deliver 10 mg/kg/day of A-192621. Blood pressure was recorded for one week following drinking water or minipump administration of A-192621. Telemetry data were analyzed as in the ETB-def experiment. The sampling period of the rats implanted with minipumps was shorter than needed for adequate spontaneous baroreflex analysis, thus only the 4 rats receiving drug in drinking water are included in these analyses.
2.5. Statistical Analysis
Data are presented as means ± standard error of the mean (SEM) or as mean and 95% confidence interval. Comparisons are made using one-way analysis of variance (ANOVA) testing, two-way repeated measures ANOVA, three-way repeated measures ANOVA, unpaired Student’s t-test, or paired Student’s t-test as appropriate. The particular statistical comparison used is designated within the text or figure legends. P values < 0.05 were considered statistically significant.
3. Results:
3.1. Blood Pressure and Heart Rate Lability
ETB-def rats demonstrated an increased blood pressure lability compared to TG controls as demonstrated by a flattening and broadening of the 24-hr blood pressure frequency distribution plots during both normal (Figure 1 A) and high (Figure 1 B) salt. This was quantified by an elevated range (Figure 1 C) and SD (Figure 1 D) of SBP in ETB-def rats compared to TG controls. Both genotypes had an increase in lability both in range and SD of SBP following high salt diet (Figure 1 C–D). However, the change in range of SBP following high salt diet was not significantly different between genotypes (Figure 1E), although the change in SD of SBP was significantly greater in ETB-def rats compared to TG controls (Figure 1F). Average, 24-hr SBP was higher in ETB-def compared to TG controls during both normal salt (ETB-def 162 ± 2, TG 141 ± 2 mmHg P < 0.0001) and high salt (ETB-def 177 ± 2, TG 142 ± 1 mmHg P < 0.0001). SBP statistically increased following high salt diet only in ETB-def (ETB-def P < 0.0001; TG P = 0.7899) , PGenotype < 0.0001, PSalt < 0.0001, PGenotype*Salt = 0.0002, 2-way RM ANOVA.
The range of HR was not statistically different across genotypes and salt diet as shown in the 24-hour HR frequency distribution plots (Figure 2 A–B) and quantified HR range (Figure 2 C). ETB-def rats had a greater SD of HR during normal salt diet compared to TG controls, and this difference became non-significant during high salt diet (Figure 2 D). Only ETB-def rats displayed a significant decrease in SD of HR following high salt diet (Figure 2 D), and there were no significant differences between genotypes in the change in HR range or change in HR SD following high salt diet (Figure 2 E–F). Average, 24-hr HRs between genotypes were not significantly different and high salt diet significantly lowered HR only in ETB-def rats (Normal Salt: ETB-def 350 ± 4, TG 341 ± 3; High Salt: ETB-def 333 ± 5, TG 335 ± 4, P = 0.0019 vs Normal Salt) bpm; PGenotype = 0.5511, PSalt = 0.0013, PGenotype*Salt = 0.0962 2-way RM ANOVA.
Figure 2: Heart rate lability is similar between TG and ETB-def rats during normal salt and high salt diet.
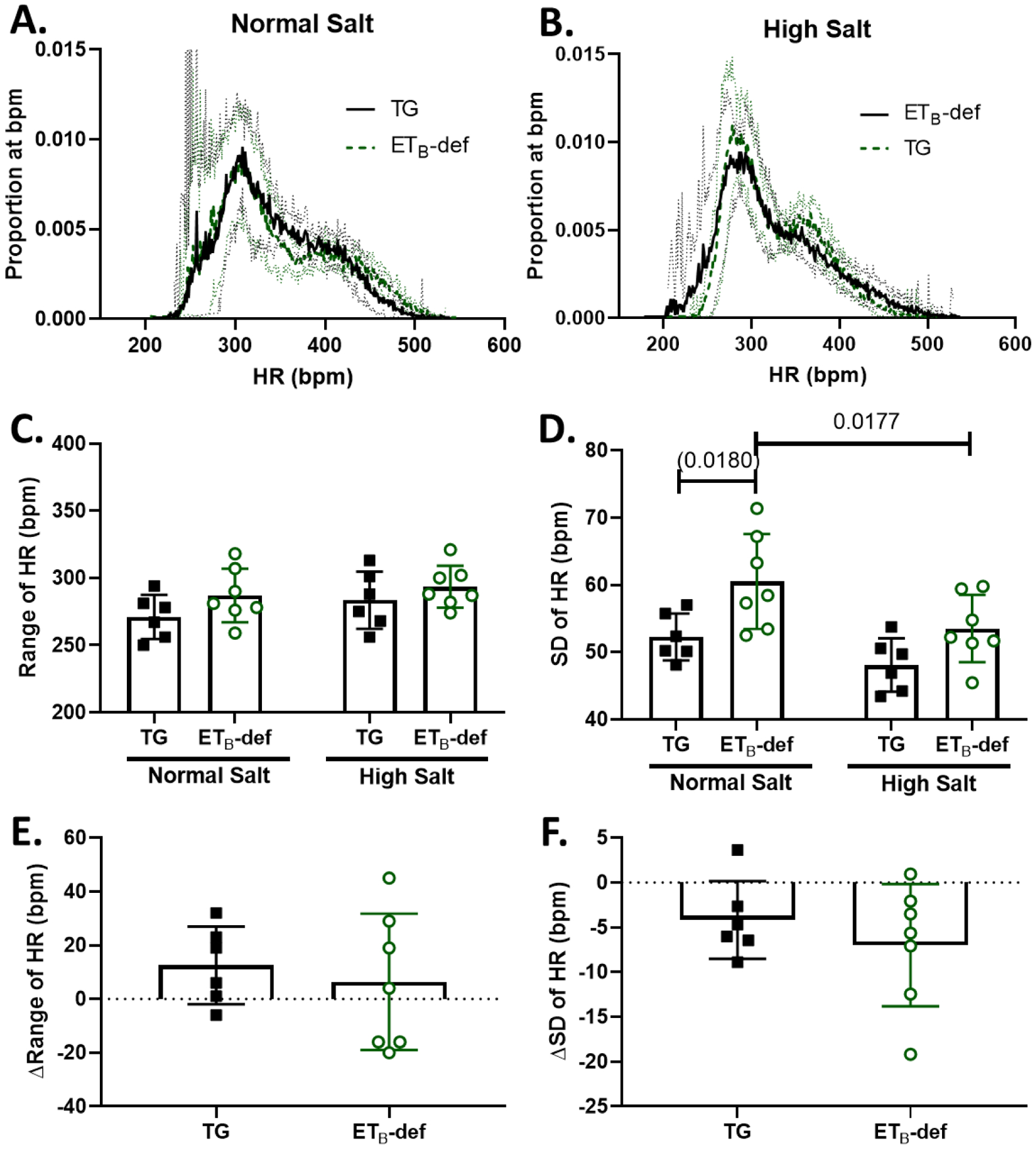
A/B: frequency distripution plots of proportion of instances at a given HR (number of instances of 10-second bins at each bpm / total number of bins) during normal salt diet (A) and 8–11 days of high salt diet (B) Solid line represents mean ± 95% confidence interval. C: Range of HR during normal and high salt diet, 2-way repeated measures ANOVA, Psalt = 0.1358; Pgenotype = 0.1510; Pinteraction = 0.6159, with Sidak’s test for multiple comparisons (no significant differences). D: Standard deviation of HR during normal and high salt diet, 2-way repeated measures ANOVA, Psalt = 0.0055; Pgenotype = 0.0151; Pinteraction = 0.4046, with Sidak’s test for multiple comparisons. E: Change in the HR range between normal salt and high salt diets, unpaired Student’s t-test p = 0.6159, F: Change in the standard deviation of HR between normal salt and high salt diets, unpaired Student’s t-test p = 0.4046. HR = heart rate, TG = transgenic, ETB-def = endothelin B deficient, SD = standard deviation.
The SD of SBP demonstrated a strong diurnal rhythm, being overall higher during rats’ active period; however, the range of SBP was not significantly affected by time of day (Table 1). Similarly, the range of HR was not affected by time of day and SD of HR demonstrated a significant influence from time of day, with higher SD during the active period (Table 1). Cosinor analysis further revealed that neither salt diet nor genotype had a significant effect on acrophase of any parameter, but that high salt diet caused a significant increase in SD of SBP amplitude only in ETB-def rats (Table 2).
3.2. Baroreflex Responses
We had previously reported no significant differences in spontaneous baroreflex sensitivity (i.e. the gain of the spontaneous baroreflex sequence) between ETB-def and TG controls (Becker et al., 2017a). One limitation of the sequence method of spontaneous baroreflex sensitivity; however, is the inclusion of only true baroreflex sequences in the analysis (i.e. only those with sequential increases or decreases in both SBP (ramps) and interbeat interval between heart beats are included in analysis of gain, Figure 3 A, top panel). We further probed the spontaneous baroreflex sequences of ETB-def and TG control rats on normal and high salt diets and evaluated the relative proportion of true baroreflex sequences to non-baroreflex sequences defined as sequential increases or decreases in SBP (ramps) without reciprocal changes in heart rate (Figure 3 A, bottom panel). We found that under high salt diet, the proportion of true baroreflex sequences to non-baroreflex sequences was significantly lower in ETB-def rats compared to TG controls (Figure 3 B). Also, TG rats had an increase in baroreflex to non-baroreflex sequences following high salt diet, an effect that was not significant in ETB-def rats.
We then further evaluated baroreflex function utilizing the modified Oxford method of baroreflex testing in anesthetized rats fed a normal salt diet or a high salt diet for 7–11 days by IV infusions of sodium nitroprusside and phenylephrine and plotting RSNA or IBI across mean arterial blood pressure (MAP). Representative traces are shown in Figure 4 and composite baroreflex curves and gain (first derivative of the 4-parameter baroreflex curve) in Figure 5 A–C. Overall, we did not observe any significant effects of genotype or salt diet on parameters of the RSNA curves as it relates to the bottom plateau, top plateau, or MAP BP50 (the MAP at halfway through curve). On a normal salt diet, there was a significant blunting of the max gain of RSNA response to changes in MAP in ETB-def rats compared to TG controls and this was not further affected by high salt diet (Figure 5 A,C; Table 3). Similarly, the bottom plateau and MAP50 of the HR response to changes in pressure were not significantly affected by genotype or salt diet; however, the top plateau and max gain were significantly lower in ETB-def rats compared to TG controls indicating a reduced ability to lower heart rate in response to increased MAP (Figure 5 B,D; Table 3).
Figure 4: Representative traces of modified Oxford baroreflex experiments.
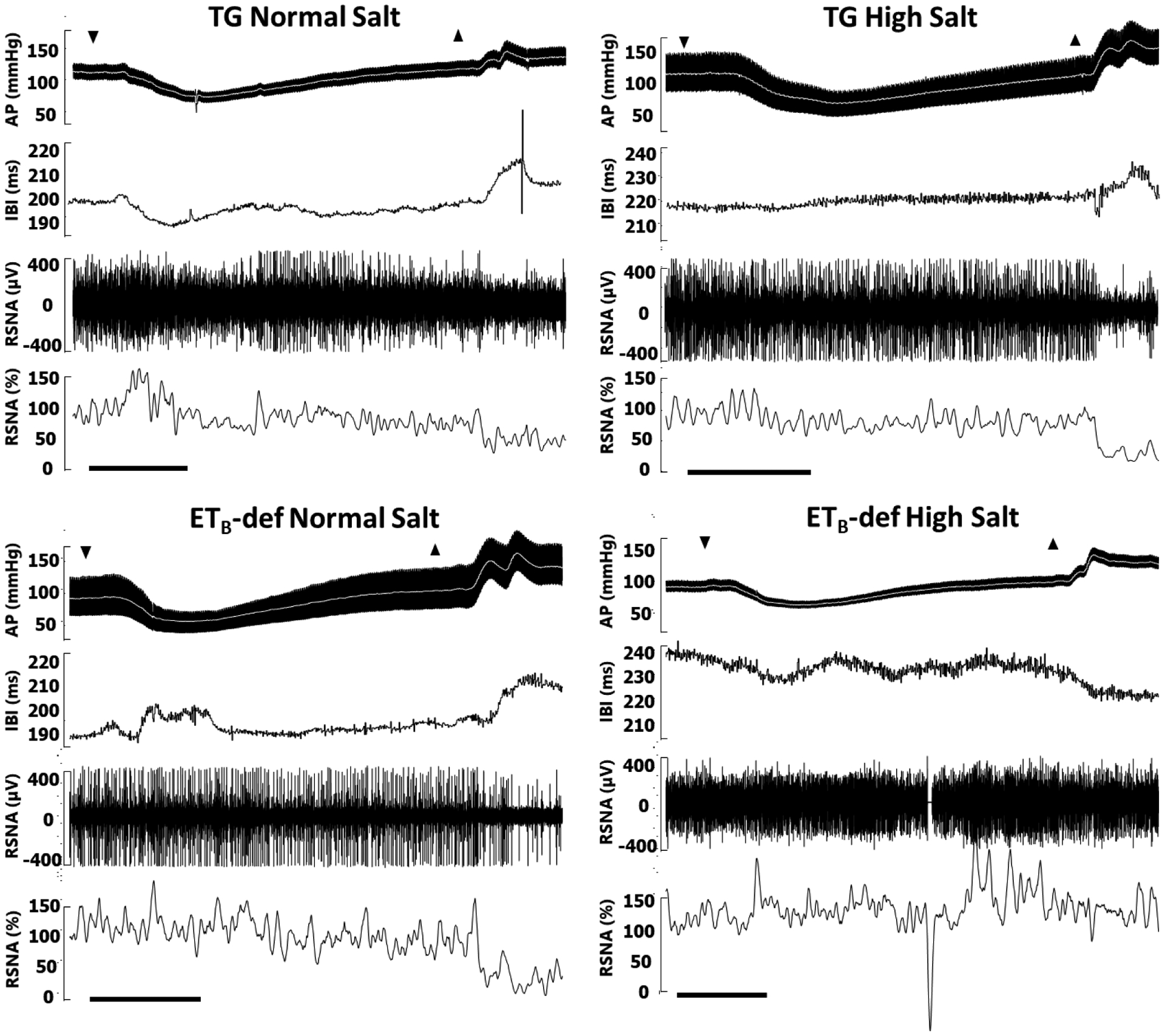
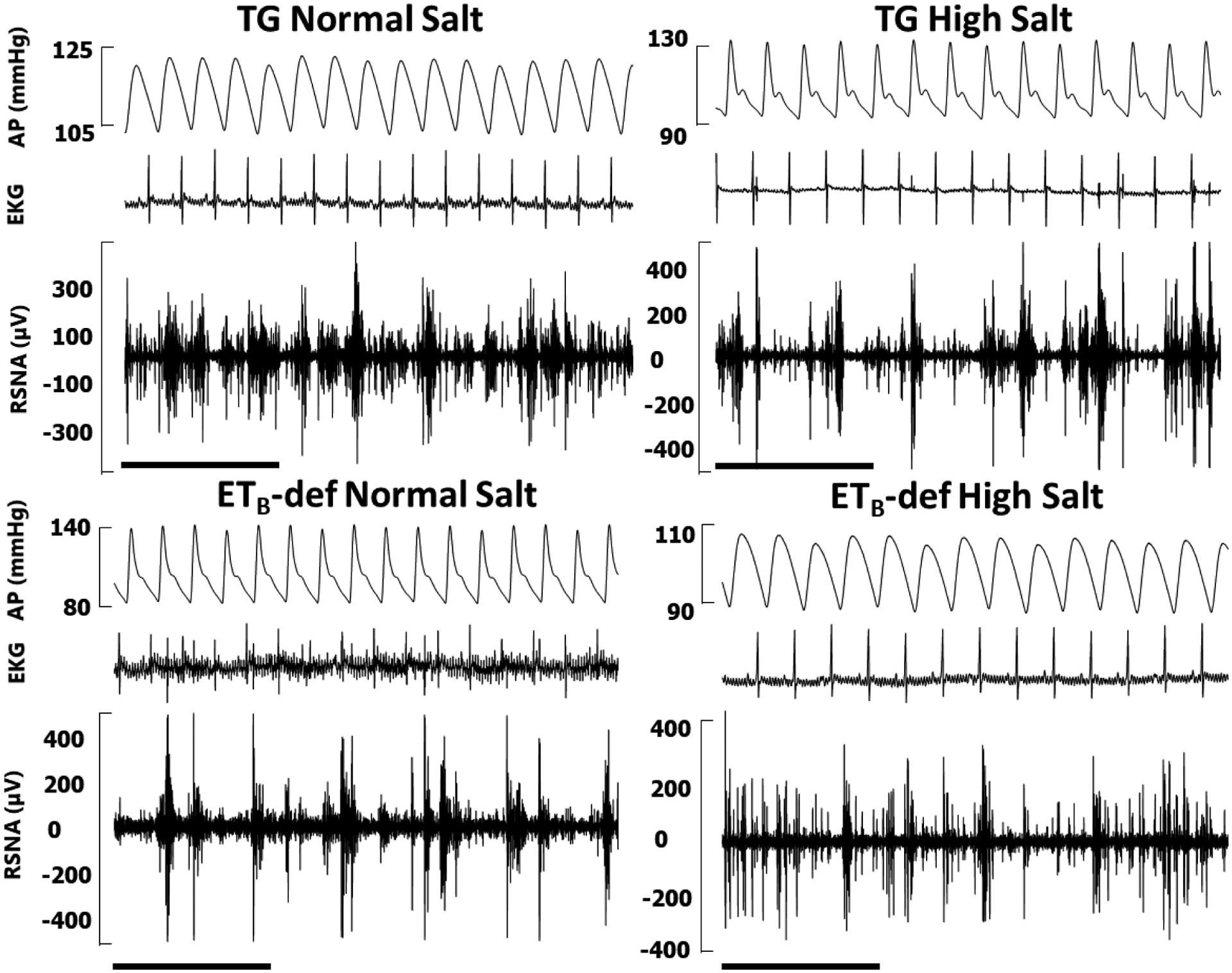
AP = arterial pressure, mean arterial pressure denoted by white line; IBI = interbeat interval derived from R-R of electrocardiogram (not pictured); RSNA = renal sympathetic nerve activity in raw voltage and as percent of baseline prior to sodium nitroprusside infusion. RSNA percent is smoothed in rolling 2 second intervals for clarity. A: Down arrows denote beginning of bolus infusion of sodium nitroprusside, up arrows denote beginning of bolus infustion of phenylephrine. Scale bars denote 30 seconds. B: Representative baseline segment from each of the four groups demonstrating signal fidelity, EKG, and pulse wave synchronicity of RSNA. Scale bars denote 1 second.
Figure 5: Heart rate baroreflex function is impaired in ETB-def rats compared to TG controls.
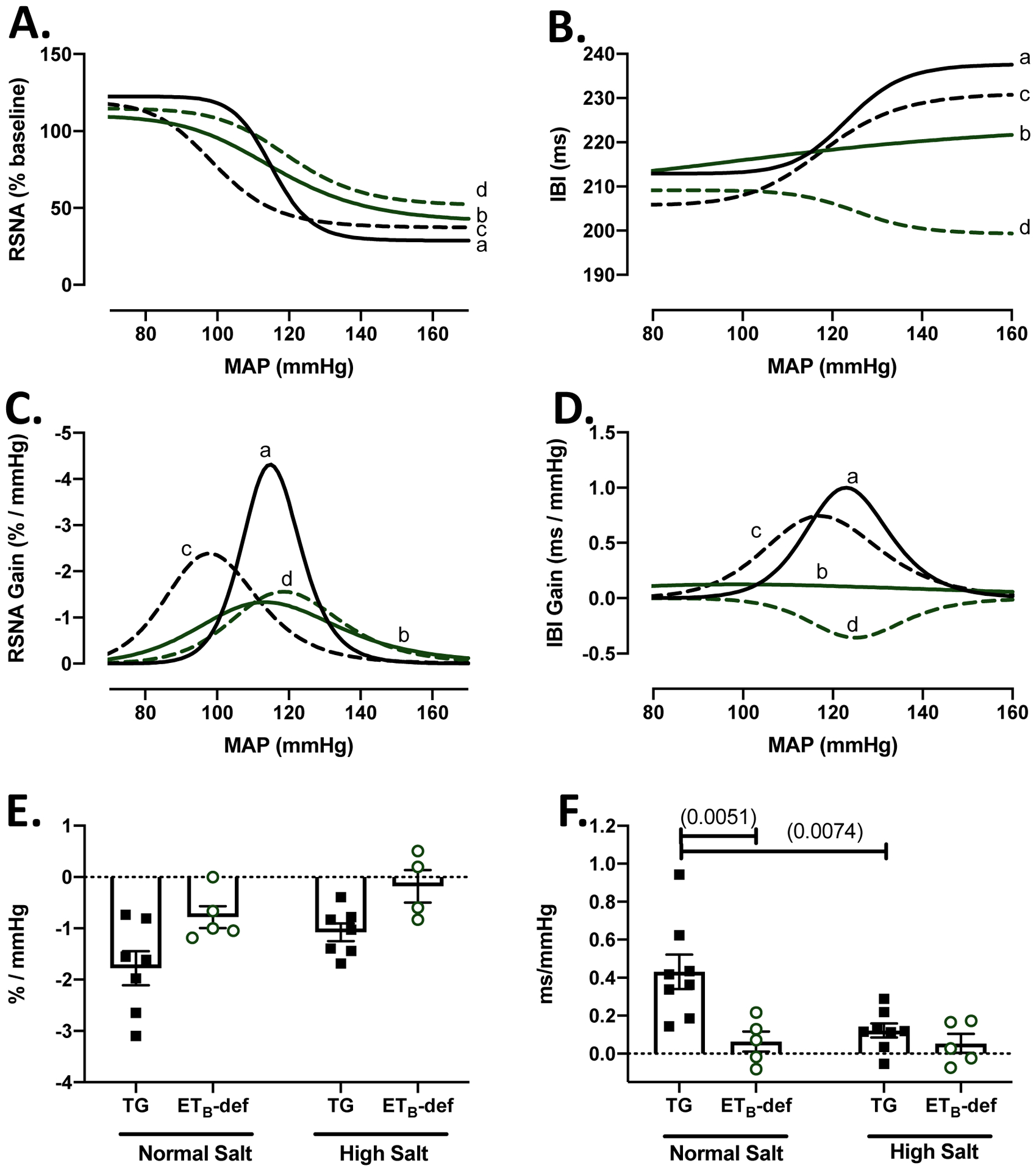
A: RSNA composite baroreflex curves. B: heart rate composite baroreflex curves. C: RSNA composite gain curves from first derivative of baroreflex curve. D. heart rate composite gain curves from first derivative of baroreflex curve. (A-D) a = TG NS; b = ETB-def NS; c = TG HS; d = ETB-def HS E. Linear regression of RSNA response to increase in pressure during phenylephrine, 2-way ANOVA Psalt = 0.0309; Pgenotype = 0.0030; Pinteraction = 0.8601, with Sidak’s test for multiple comparisons (no significant differences). F. Linear regression of heart rate response to increase in pressure during phenylephrine, 2-way ANOVA Psalt = 0.0298; Pgenotype = 0.0044; Pinteraction = 0.0400, with Sidak’s test for multiple comparisons. p values in (). RSNA = renal sympathetic nerve activity, MAP = mean arterial pressure, IBI = interbeat interval.
Table 3 –
4-Parameter Sigmoid Curve Baroreflex Components
| Heart Rate Baroreflex Response | |||||||
|---|---|---|---|---|---|---|---|
| TG Controls | ETB-def | ||||||
| Normal Salt (8) | High Salt (5) | Normal Salt (8) | High Salt (5) | 2-way ANOVA (p) | |||
| Bottom Plateau (ms) | 213.0 ± 7.5 | 213.5 ± 11.32 | 210.3 ± 8.0 | 191.3 ± 14.5 | S = 0.4028 | G = 0.2664 | S*G = 0.3746 |
| Top Plateau (ms) | 241.7 ± 10.3 | 239.2 ± 6.6 | 226.2 ± 13.3 | 204.5 ± 11.4 | S = 0.2799 | G = 0.0184 | S*G = 0.2145 |
| MAP BP50 (mmHg) | 140.6 ± 7.5 | 129.8 ± 9.6 | 146.1 ± 11.8 | 132.0 ± 13.72 | S = 0.7145 | G = 0.2514 | S*G = 0.3436 |
| Max Gain (ms / mmHg) | 0.99 ± 0.29 | 0.76 ± 0.21 | 0.42 ± 0.24 | −0.17 ± 0.38 | S = 0.1670 | G = 0.0167 | S*G = 0.5528 |
| Baseline IBI (ms) | 204.5 ± 9.4 | 235.2 ± 8.4 | 211.8 ± 9.3 | 197.2 ± 11.7 | S = 0.4273 | G = 0.1378 | S*G = 0.0327 |
| Baseline MAP (mmHg) | 101.1 ± 2.5 | 95.1 ± 3.1 | 96.5 ± 4.2 | 115.3 ± 3.8 | S = 0.0754 | G = 0.0348 | S*G = 0.0019 |
| RSNA Baroreflex Response | |||||||
| TG Controls | ETe-def | ||||||
| Normal Salt (8) | High Salt (5) | Normal Salt (8) | High Salt (5) | 2-way ANOVA (p) | |||
| Bottom Plateau (%) | 28.8 ± 9.3 | 37.22 ± 7.3 | 40.6 ± 6.9 | 51.3 ± 12.3 | S = 0.3097 | G = 0.1758 | S*G = 0.9008 |
| Top Plateau (%) | 122.5 ± 13.6 | 118.8 ± 7.8 | 109.9 ± 4.0 | 114.6 ± 8.2 | S = 0.9605 | G = 0.4363 | S*G = 0.7882 |
| MAP BP50 (mmHg) | 115.4 ± 3.8 | 99.4 ± 8.4 | 116.3 ± 5.0 | 120.2 ± 2.7 | S = 0.3540 | G = 0.1028 | S*G = 0.1345 |
| Max Gain (% / mmHg) | −3.8 ± 0.6 | −2.3 ± 0.4 | −1.4 ± 0.1G | −1.5 ± 0.1 | S = 0.1558 | G = 0.0022 | S*G = 0.0831 |
MAP: mean arterial pressure, IBI: inter beat interval, Normal salt (0.49% NaCl), High salt (4.0% NaCl), TG: transgenic controls; ETB-def: Endothelin B deficient; G: genotype, S: salt diet. 2-way ANOVA with Tukey’s post-hoc test for multiple comparisons:
p < 0.05 genotype;
p < 0.05 salt; Mean ± SEM.
Group effect significance indicated by italics.
Because ETB-def rats did not display a robust, typical sigmoidal HR curve during the baroreflex function testing (Figure 5B), we also calculated the slope of baroreflex sensitivity during the pressor response from IV infusion of phenylephrine and fit this portion with a linear regression model to isolate the sensitivity of HR and RSNA responses to increased MAP. Although not a complete picture of baroreflex function, this linear regression analysis removes the assumptions present in fitting the responses to a 4-parameter sigmoid curve. Similar to the sigmoid curve analysis, ETB-def rats had a lower RSNA gain compared to TG controls, and there was an overall trend of salt toward blunting the RSNA gain (Figure 5E). Heart rate responses to increases in MAP were significantly lower in ETB-def compared to TG controls and salt diet had a significant effect of lowering HR gain. Furthermore, ETB-def animals on normal salt diet had a significantly lower HR gain compared to normal salt TG controls, and high salt diet significantly lowered HR gain in TG animals compared to normal salt diet but did not significantly lower the already blunted gain of ETB-def (Figure 5F).
3.3. ETB Receptor Antagonism
Because ETB-def and TG control animals express ETB receptors via a transgene in DBH-positive tissues, the altered baroreflex activity could be due to the presence of ETB receptors in efferent sympathetic nerves. Therefore, we administered a selective ETB receptor antagonist, A-192621, in order to globally block ETB receptor activity. Following 4–6 days of ETB receptor antagonism, SBP increased in all animals (Figure 6 B), and this was accompanied by a decrease in variability measured by SD of SBP (Figure 6 C) along with an increase in the range of SBP (Figure 6 D). The route of administration of A-192621 (PO vs IP) did not have a significant effect on either SBP or SD of SBP as measured by two-way RM ANOVA (SBP: P Route*A-192621 = 0.1823; SD of SBP: P Route*A-192621 = 0.8430) thus these data were combined. The changes in blood pressure were accompanied by a decrease in heart rate; however, measures of heart rate variability were not significantly altered (Figure 7). There was no significant effect of ETB antagonism on the overall gain of the spontaneous baroreflex sensitivity (Figure 8 A), but there was a significant increase in the ratio of true baroreflex sequences to non-baroreflex sequences (Figure 8 B).
Figure 6: Blood pressure increases and lability decreases in Sprague-Dawley rats after ETB receptor antagonism.
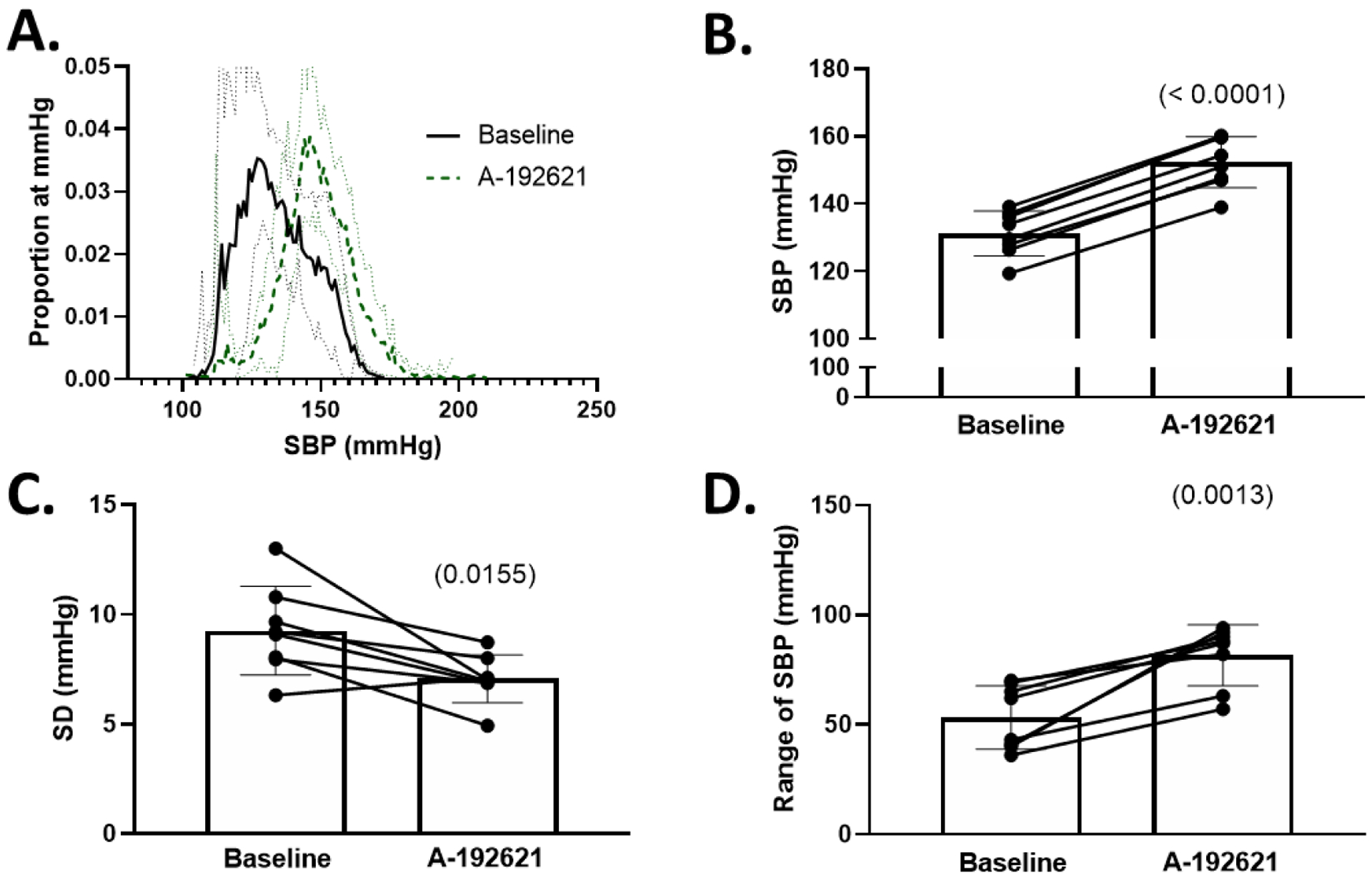
A. Histograms of proportion of instances at a given SBP (number of instances of 10-second bins at each mmHg / total number of bins) during baseline and following 3–5 days of selective endothelin B antagonism with A-192621. Solid line represents mean ± 95% confidence interval B. 24-hour average SBP at baseline and following A-192621. C. Standard deviation (SD) of SBP before and after A-192621. D. Range of SBP before and after A-192621. All comparisons made using paired Student’s t-test, p values in (). SBP = systolic blood pressure.
Figure 7: Heart rate decreases but heart rate lability is unchanged in Sprague-Dawley rats after ETB receptor antagonism.
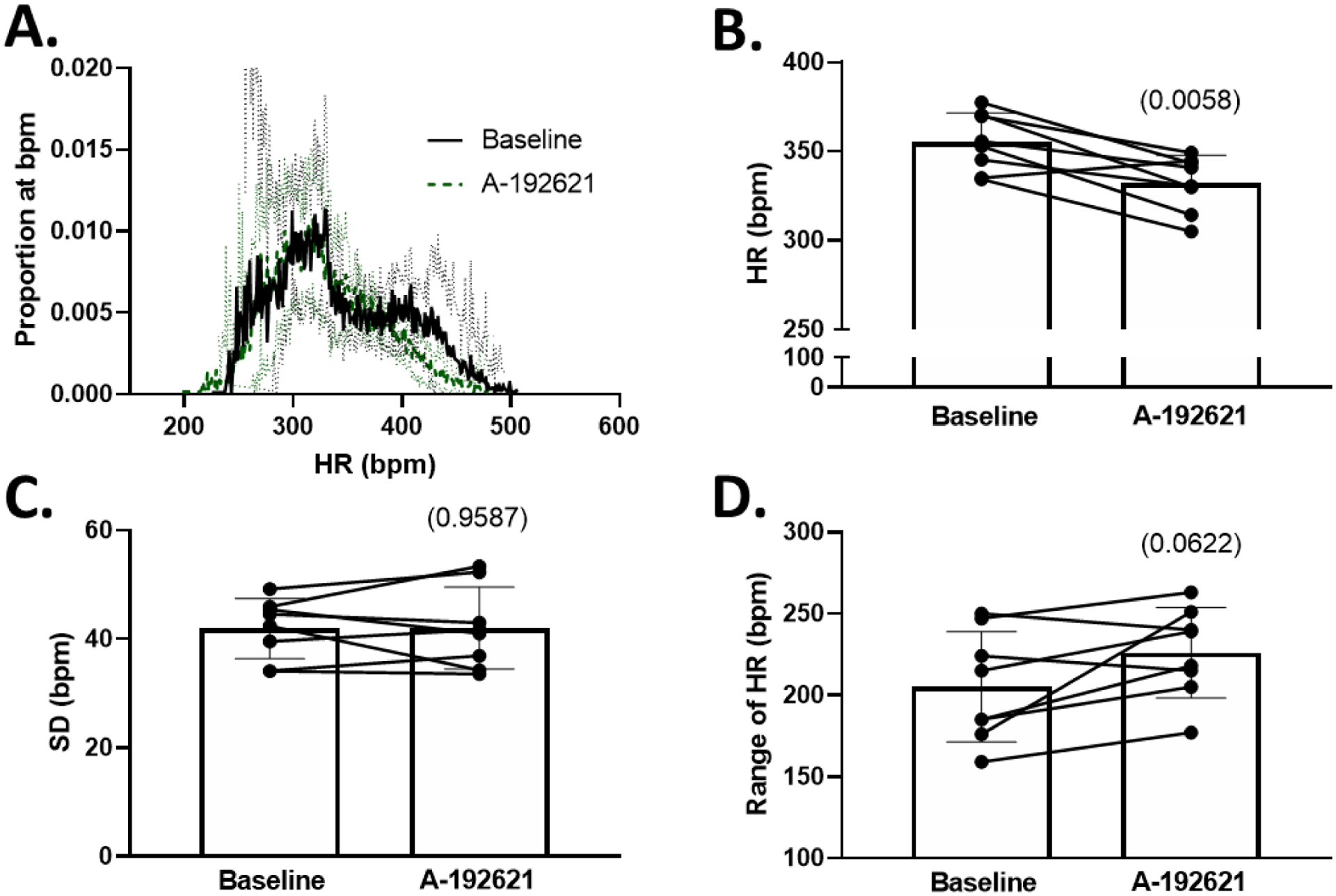
A. Histograms of proportion of instances at a given HR (number of instances of 10-second bins at each bpm / total number of bins) during baseline and following 3–5 days of endothelin B antagonism with A-192621. B. 24-hour average HR at baseline and following A-192621. C. Standard deviation (SD) of HR before and after A-192621. D. Range of HR before and after A-192621. All comparisons made using paired Student’s t-test, p values in (). HR = heart rate.
Figure 8: ETB receptor blockade increases the ratio of true:non-baroreflex sequences in Sprague-Dawley rats.
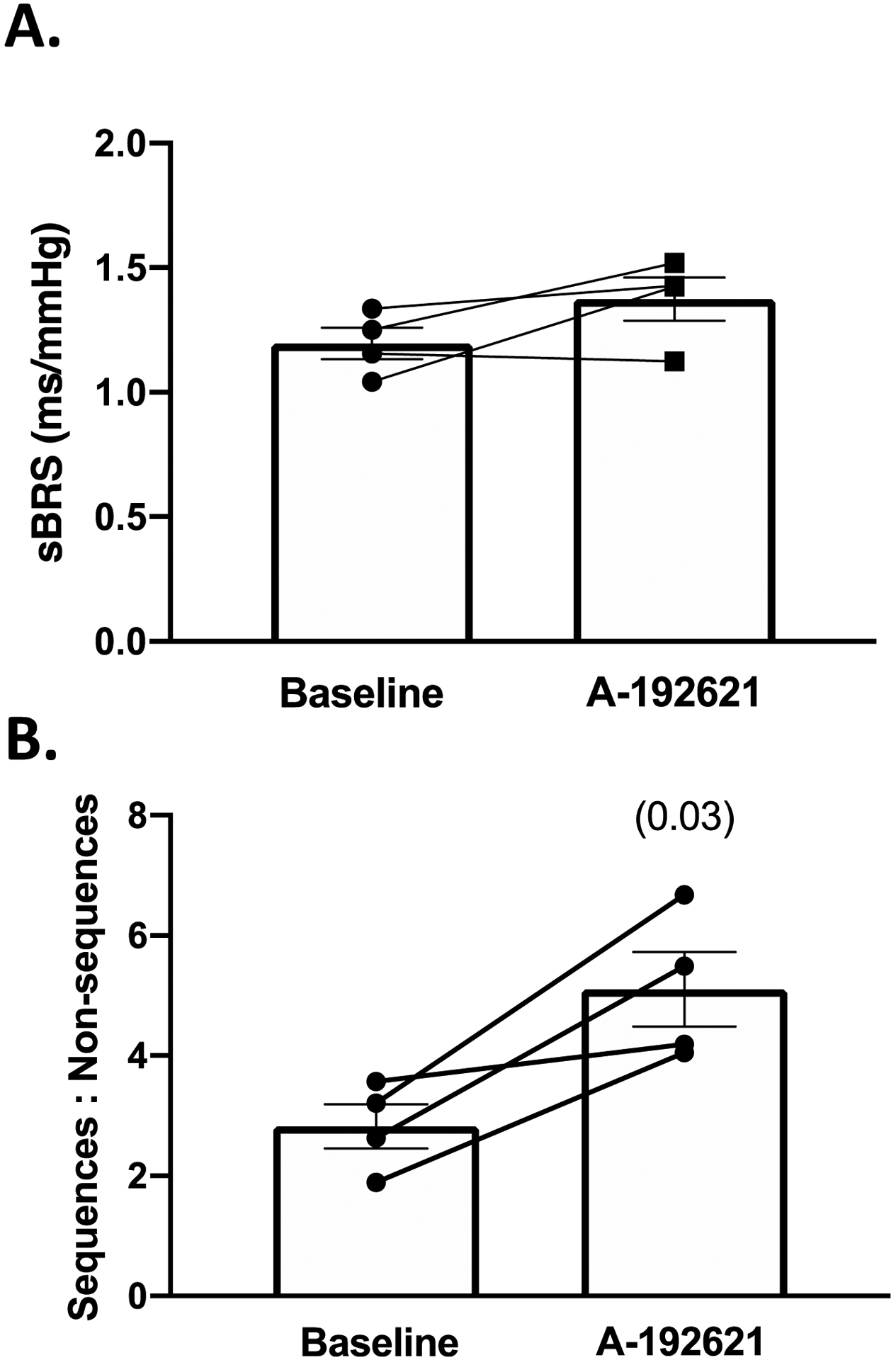
A: spontaneous baroreflex (sBRS) gain before and after 3–5 days of A-192621. B: ratio of true baroreflex sequences to non-baroreflex sequences during baseline and after 3–5 days of A-192621. All comparisons made using paired Student’s t-test, p values in ().
Antagonism of ETB receptors in ETB-def and TG controls resulted in a rise in blood pressure only in TG controls while ETB-def remained unaffected (Table 4). The range of SBP increased in both TG and ETB-def rats following A-192621 (Figure 9A–C); however, the lability of SBP as measured by SD of SBP was not significantly affected by A-192621 (Figure 9D). Spontaneous baroreflex sensitivity was not significantly different genotypes or following A-192621 (Figure 9E), nor where the ratio of true sequences:non-sequences although there was a trend towards reduction of true:non-sequences in TG controls following A-192621 (Figure 9F).
Table 4 –
24-hr Hemodynamic Parameters of TG and ETB-def before and after A-192621
| TG Controls (n = 7) | ETB-def (n = 6) | ||||||
|---|---|---|---|---|---|---|---|
| Baseline | A-192621 | Baseline | A-192621 | 2-Way RM ANOVA (p) | |||
| SBP (mmHg) | 130.5 ± 1.7 | 148.5 ± 2.3**** | 150.0 ± 2.5#### | 150.1 ± 2.7 | Tx = 0.0002 | G = 0.0030 | Tx*G = 0.0002 |
| MAP (mmHg) | 107.6 ± 1.5 | 122.8 ± 2.0**** | 125.3 ± 2.6#### | 125.9 ± 2.9 | Tx = 0.0006 | G = 0.0044 | Tx*G = 0.0003 |
| DBP (mmHg) | 88.5 ± 1.4 | 102.1 ± 1.9**** | 103.6 ± 2.7### | 103.0 ± 3.3 | Tx = 0.0010 | G = 0.0206 | Tx*G = 0.0005 |
| HR (bpm) | 362.9 ± 4.5 | 325.9 ± 4.8**** | 365.9 ± 6.8 | 359.9 ± 12.2## | Tx = 0.0001 | G = 0.0827 | Tx*G = 0.0015 |
SBP: systolic blood pressure, MAP: mean arterial pressure, DBP: diastolic blood pressure, HR: heart rate, G: genotype, Tx: treatment with A-192621. 2-way Repeated Measures ANOVA with Sidak’s post-hoc test for multiple comparisons:
p < 0.0001 vs. baseline;
p < 0.01 vs. TG,
p < 0.001 vs. TG
p < 0.0001 vs. TG Mean ± SEM.
Group effect significance indicated by italics.
Figure 9: ETB receptor blockade does not alter blood pressure lability or ratio of true:non-baroreflex sequences in ETB-def and TG rats.
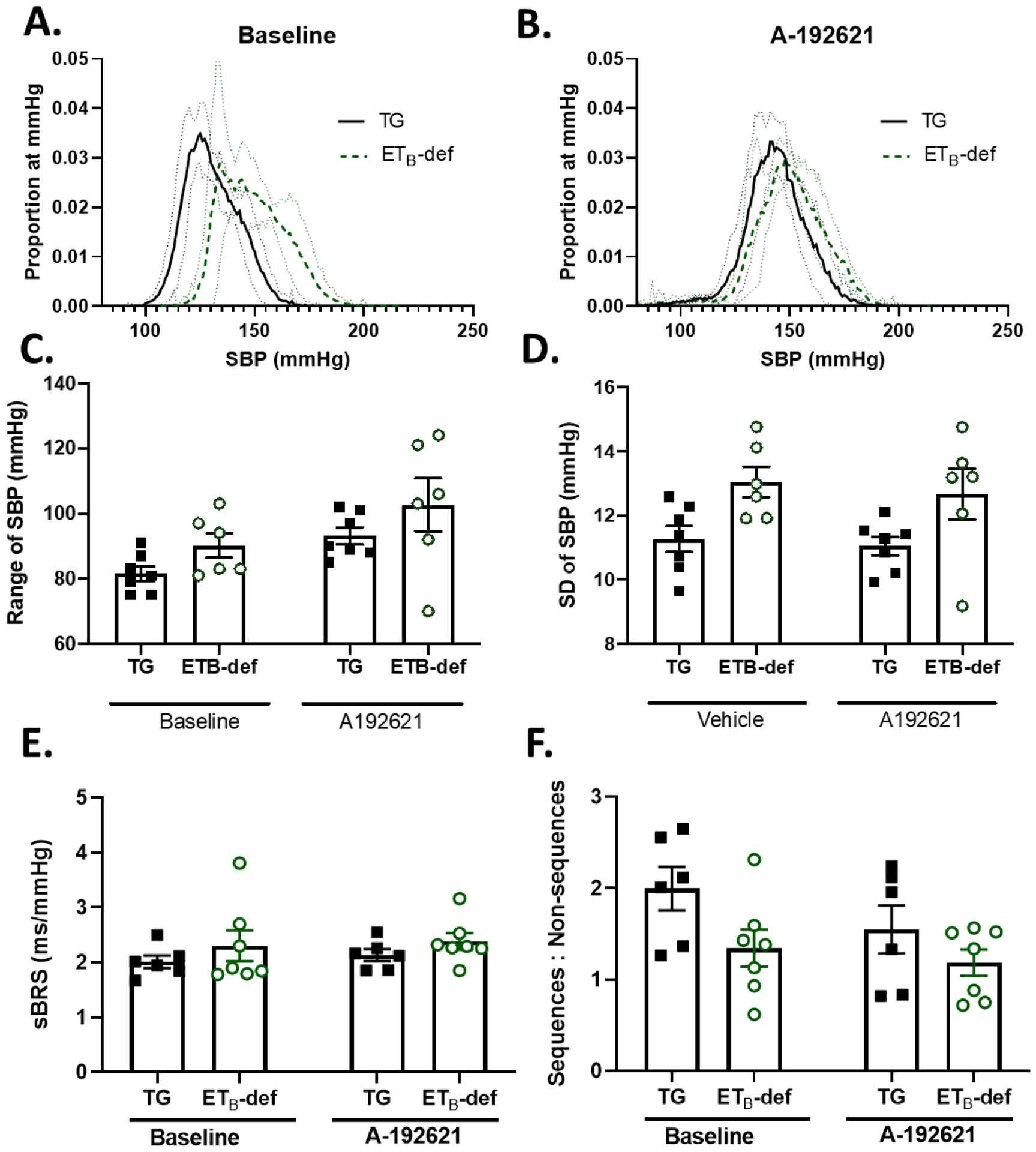
A. Histograms of proportion of instances at a given SBP (number of instances of 10-second bins at each mmHg / total number of bins) during baseline and following 3–5 days of selective endothelin B antagonism with A-192621. Solid line represents mean ± 95% confidence interval. B. 24-hour average SBP at baseline and following A-192621. C. Range of SBP before and after A-192621. 2-way repeated measures ANOVA PA-192621 = 0.0130; Pgenotype = 0.0917; Pinteraction = 0.9112, with Sidak’s test for multiple comparisons. D. Standard deviation (SD) of SBP before and after A-192621. 2-way repeated measures ANOVA PA-192621 = 0.3368; Pgenotype = 0.0224; Pinteraction = 0.8057, with Sidak’s test for multiple comparisons. E. spontaneous baroreflex (sBRS) gain before and after 3–5 days of A-192621. 2-way repeated measures ANOVA PA-192621 = 0.3768; Pgenotype = 0.2905; Pinteraction = 0.8513, with Sidak’s test for multiple comparisons. F: ratio of true baroreflex sequences to non-baroreflex sequences during baseline and after A-192621. 2-way repeated measures ANOVA PA-192621 = 0.0969; Pgenotype = 0.0661; Pinteraction = 0.4078, with Sidak’s test for multiple comparisons.
4. Discussion:
The major findings of the current study are: 1) ETB-def rats display increased blood pressure lability and impaired heart rate baroreflex control, which are augmented during high salt diet, 2) pharmacological blockade of the ETB receptor decreased lability and increased the ratio of true:non-baroreflex sequences even in the face of elevated blood pressure in Sprague-Dawley rats, 3) However, pharmacological blockade of ETB receptors had no significant effect on blood pressure lability or baroreflex control in ETB-def or TG rats. These findings suggest that ETB receptors influence baroreflex control, particurly during high salt diet..
The role of the endothelin system and specifically the role of ETB receptors in baroreflex control has not been well defined. Furthermore, there remains a large amount of uncertainty regarding the interplay between endothelin-1 and the ETA and ETB receptors in modulating various autonomic functions. Some of the challenge in elucidating the role of endothelin receptors in autonomic function likely lies in the potency of vascular responses to activation of either receptor, as this can obscure or complicate attribution of hemodynamic responses to autonomic activity (Davenport et al., 2016). Results from the current study suggest that activation of ETB receptors increases efferent sympathetic nerve activity. Prior work has demonstrated an increase in superoxide production in sympathetic ganglia following activation of ETB receptors (Dai et al., 2004; Lau et al., 2006), and activation of ETB receptors augments the vasoconstriction of vessels to application of norepinephrine (Kita et al., 1998) and/or angiotensin II (Gossl et al., 2004). We have also previously shown direct effects of activating neuronal ETB receptors to increase blood pressure using the ETB-def rats (Becker et al., 2017b). In ETB-def rats with ganglionic blockade, activation of ETB receptors with the highly selective agonist, sarafotoxin 6c, caused an alpha1-adrenergic dependent increase blood pressure. Interestingly, higher doses of sarafotoxin 6c resulted in a tachycardia that was blocked by beta-adrengeric receptor blockade. These findings indicated that activation of ETB receptors on sympathetic neurons caused adrenergic-dependent increases in blood pressure and heart rate. In this current study we demonstrated that the ETB-def model, which only has functional endothelin B receptors on efferent sympathetic nerves, displays baroreflex dysfunction following high salt diet.
We observed an increased blood pressure lability in our ETB-def rats indicated by elevated 24-hour SD and range of SBP that was further augmented by placing the rats on a high salt diet. Our observed difference in SD of SBP between ETB-def and TG control rats during normal salt diet was many orders of magnitude smaller than that seen following complete sinoarotic denervation in studies such as those done by Osborn and Hornfeldt (approximately 20% vs. 200%, respectively) (1998). This highlights that ETB-def rats still have intact and partially functioning baroreflexes, which is further evidenced by the similar spontaneous baroreflex sensitivity between ETB-def and TG controls (Becker et al., 2017a). These differences become exaggerated following high salt diet and approach approximately 60% difference between ETB-def and TG controls suggesting a strong interaction between salt diet and ETB receptors mediating baroreflex dysfunction. High salt diet has been shown to increase renal levels of endothelin-1 (Becker et al., 2017a; Pollock et al., 2001; Speed et al., 2018) and ETB-def rats have elevated circulated levels of endothelin-1 compared to TG controls under both normal and high salt diets. In the DOCA-salt model, endothelin-1 levels are similar to controls in celiac ganglia; however, there is a significant increase in ETB receptor expression (Dai et al., 2004). It is unknown what effect salt diet has on other sympathetic ganglia in terms of endothelin-1 or receptor expression, but it is likely a similar increase in expression may occur in cardiac ganglia. Future work is needed elucidating this potential interaction between ETB receptor expression in various autonomic ganglia and salt diet.
We also observed an increase in the lability of SBP following high salt diet in the TG controls. This increase in lability in a salt-resistant animal has also recently been shown by Simmons et al (Simmonds et al., 2014) with an isolated increase in blood pressure lability during the animals’ active period following high salt diet. Consistent with these findings, we observed an interaction between high salt diet and time of day in both TG and ETB-def rats. These findings along with the increased blood pressure amplitude in ETB-def rats (Becker et al., 2017a; Speed et al., 2018) and sinoaortic denervated rats (Osborn et al., 1998) pose a potential interaction between high salt diet and diurnal baroreflex and blood pressure control. The available rodent model data seem to indicate that salt-sensitive blood pressure is associated with increased diurnal blood pressure rhythms, which is in contrast with human data demonstrating a reduction in blood pressure rhythms or “dipping” in salt-sensitive individuals (Higashi et al., 1997; Kimura et al., 2010). The reasons for this difference between humans and rodent models are unknown but worthy of further investigation because dipping status in humans is a strong prognostic indicator of cardiovascular morbidity and mortality (Douma et al., 2018). Furthermore, high salt diet appeared to cause a higher diurnal difference in frequency distribution across blood pressure in both genotypes (Figure 1B). This divergence created the presence of a hump in the 24-hour distribution curve, although there were no statistically significant differences between genotypes. Future work will be instrumental in elucidating these interactions between the endothelin system and diurnal control of blood pressure.
Although to a lesser magnitude than ETB-def counterparts, the TG rats also had a remarkable inhibition of the heart rate response to modified Oxford baroreflex testing in anesthetized preparations following high salt diet. This could potentially be a feature of the transgene expression in both the ETB-def and TG animals. As the ETB receptor expression is no longer under its native control but is driven by the dopamine beta-hydroxylase promotor, it is feasible there is increased expression of ETB receptors in sympathetic ganglia and central sites important in baroreflex control compared to non-transgenic animals. There is some preliminary evidence that this occurs in the ETB-def and TG line, specifically in the stellate ganglia (Wehrwein et al., 2007), which would explain the differential responses to high salt diet between cardiac and renal sympathetic beds to baroreflex testing (Figure 5). This further emphasizes that the role of ETB receptors on efferent sympathetic nerves themselves is responsible for the observed baroreflex dysfunction and not primarily a sensory defect in baroreceptor afferents. However, the current study is limited in that we are unable to conclusively determine whether the defect in baroreflex function is due to baroreceptor afferents, cardiac efferents, or central neuronal pathways. It is also possible there are localized vascular defects in the ETB-def rats in the baroreceptors that blunt effective signal transduction. One of the major roles of ETB receptors in the vascular endothelium is the production of nitric oxide and other vasodilators. As nitric oxide signaling is an important component of baroreceptor function, it is possible that ETB-def animals lack this signaling pathway. In order to test for the possible contribution of ETB receptors on vascular tone or baroreflex afferents vs. efferents, we subjected Sprague-Dawley rats to global ETB receptor blockade. As has been previously demonstrated, global ETB receptor blockade raised blood pressure; however, even in the face of increased blood pressure we observed a reduction in lability as evidenced by a lowering of the SD of SBP (Figure 6). ETB receptor blockade also failed to significantly alter spontaneous baroreflex sensitivity but did increase the ratio of true to non-baroreflex sequences (Figure 8). Because these effects are in direct contrast to those observed in the ETB-def rat, this would indicate that the primary baroreflex defect in ETB-def rats is the presence of ETB receptors on sympathetic efferents and not the lack of ETB receptors on afferent nerves. However, blocking ETB receptors in ETB-def and TG rats had little observable effect on either blood pressure lability or ratio of true to non-baroreflex sequences (Figure 9), although there was a trend toward a reduction of the true to non-baroreflex sequences in TG controls. This would indicate that an absence of ETB receptors on sensory pathways is responsible for the genotype difference as antagonism had virtually no effect on ETB-def rats. These observed effects were limited to normal salt diet and may be further augmented during high salt diet. Future work will be instrumental in dissecting how high salt diet affects receptor expression and function and how interactions between ETB receptors on sensory vs efferent pathways contribute to baroreflex dysfunction.
Why is the gain of the spontaneous baroreflex not altered even though the modified Oxford technique demonstrated a large effect of ETB receptors on baroreflex function? There is evidence that blocking muscarinic receptors with atropine has a strong effect on reducing spontaneous baroreflex gain, but blocking adrenergic receptors with propranolol does not have a significant effect (Stauss et al., 2006). This would imply that the sequence technique is more apt for observing alterations in parasympathetic/vagal modulation of the baroreflex and may be relatively insensitive to sympathetic influences. As our data indicate altered sympathetic tone to the heart due to ETB receptor activity, we may not be able to observe this in the gain of the spontaneous baroreflex analysis due to these limitations of the system. We therefore extended our evaluation of the spontaneous baroreflex sensitivity by evaluating non-baroreflex sequences, i.e. instances where blood pressure and HR both increase or decrease together rather than reciprocally. There has been some debate regarding the interpretation of non-baroreflex events with the general theory being that they represent a type of feedforward mechanism driven primarily by sympathetic activity to both the vasculature and SA node vs. the feedback mechanism of a classical baroreflex. Investigations that seek to specify the balance between feedforward and feedback mechanisms in an intact animal (closed loop system) are notoriously difficult to fully interpret (Kamiya et al., 2011; Kawada et al., 2016). One potential hypothesis is that under rest and normal conditions, feedforward mechanisms driven by sympathetic activity prevail and the feedback mechanisms are reactionary, responding to alterations in hemodynamic status (Kamiya et al., 2011; Tan et al., 2011). These feedforward sequences may then represent central outflow of sympathetic tone, which is supported by observations of fewer non-baroreflex sequences following sympathetic or ganglionic blockade but no changes in non-baroreflex sequence number following muscarinic blockade (Legramante et al., 1999; Legramante et al., 2009). This interpretive framework fits well in our observations of increased non-baroreflex sequences in ETB-def rats during high salt diet and decreased non-baroreflex sequences following ETB receptor blockade further demonstrating that ETB receptors on sympathetic nerves contributes to increased sympathetic drive.
Our study has a number of limitations that are worthy of discussion. As already briefly mentioned, the nature of the genetic model used (ETB-def and TG) rat is both a strength and weakness of the study. The expression of ETB receptors via a transgene utilizing DβH promotor may result in expression of ETB receptors where they normally would not be expressed under native conditions. Although this selective expression on adrenergic tissue allows us isolate the effects of ETB receptor activity, it may not be representative of normal physiology. Furthermore, our results indicate a connection between ETB receptors and baroreflex dysfunction but do not specifically identify the location of ETB receptors responsible such as sensory or efferent autonomic pathways. Future studies are needed to clarify the specific interactions involved.
Another limitation is issues regarding diurnal variability in our experiments. Telemetry analysis allows us to monitor animals throughout the 24 hrs of the day, but our acute baroreflex measurements were conducted during the animals inactive period. As some of our observed effects, such as the robust increase in SBP phenomenon following high salt diet, occur primarily during the animals active period, this may result in an underestimation of parameters collected during this time. Additionally, the effect of anesthesia on our baroreflex studies cannot be excluded either. Future work utilizing techniques such as reverse lighting in animal housing facilities or indwelling catheters to conduct multiple baroreflex experiments on conscious animals during different periods of the day will be useful in establishing a larger framework of circadian control of baroreflex function.
In conclusion, we have shown that the ETB receptors are important mediators of baroreflex dysfunction that is augmented by high salt diet and which likely contributes, in part, to the salt-sensitive hypertension of the model. Because baroreflex dysfunction is commonly associated with salt-sensitive hypertension in humans and animal models, further work investigating the ability to modulate baroreflex through the endothelin system in various other models of salt-sensitive hypertension will yield greater understanding of the interplay of this important physiological feedback system.
Acknowledgements:
The authors wish to express their appreciation to Jackson Colson, John Miller Allan, and McKenzie King for their help with animal colony management and genotyping. JGJ current affiliation is: 1) Department of Medicine, Division of Nephrology, Hypertension, and Renal Transplantation, University of Florida, Gainesville, FL and 2) North Florida/South Georgia Veterans Health System, Gainesville, FL.
Grants:
This study was supported by National Heart, Lung, and Blood Institute Grant P01 HL069999 and P01 HL136267 (to D. M. Pollock). B.K.B. was supported by an AHA postdoctoral training fellowship (19POST34380109). J.G.J. was supported by the UAB Training Program in Cardiovascular Pathophysiology (T32 HL007918) and an AHA predoctoral training fellowship (15PRE25560074).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
No conflicts of interest, financial or otherwise, are declared by the author(s). C.Y. contributed to this work as a part of the University of Alabama at Birmingham KURE program, Kidney Undergraduate Research Experience.
References:
- Babcock MC, Brian MS, Watso JC, Edwards DG, Stocker SD, Wenner MM, Farquhar WB 2018. Alterations in dietary sodium intake affect cardiovagal baroreflex sensitivity. Am J Physiol Regul Integr Comp Physiol 315, R688–R695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backs J, Bresch E, Lutz M, Kristen AV, Haass M 2005. Endothelin-1 inhibits the neuronal norepinephrine transporter in hearts of male rats. Cardiovasc Res 67, 283–290. [DOI] [PubMed] [Google Scholar]
- Becker BK, Feagans AC, Chen D, Kasztan M, Jin C, Speed JS, Pollock JS, Pollock DM 2017a. Renal denervation attenuates hypertension but not salt sensitivity in ETB receptor-deficient rats. Am J Physiol Regul Integr Comp Physiol 313, R425–R437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker BK, Speed JS, Powell M, Pollock DM 2017b. Activation of neuronal endothelin B receptors mediates pressor response through alpha-1 adrenergic receptors. Physiol Rep 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker BK, Tian C, Zucker IH, Wang HJ 2016. Influence of brain-derived neurotrophic factor-tyrosine receptor kinase B signalling in the nucleus tractus solitarius on baroreflex sensitivity in rats with chronic heart failure. J Physiol 594, 5711–5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertinieri G, di Rienzo M, Cavallazzi A, Ferrari AU, Pedotti A, Mancia G 1985. A new approach to analysis of the arterial baroreflex. J Hypertens Suppl 3, S79–81. [PubMed] [Google Scholar]
- Bugenhagen SM, Cowley AW Jr., Beard DA 2010. Identifying physiological origins of baroreflex dysfunction in salt-sensitive hypertension in the Dahl SS rat. Physiol Genomics 42, 23–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coruzzi P, Parati G, Brambilla L, Brambilla V, Gualerzi M, Novarini A, Castiglioni P, Di Rienzo M 2005. Effects of salt sensitivity on neural cardiovascular regulation in essential hypertension. Hypertension 46, 1321–1326. [DOI] [PubMed] [Google Scholar]
- Dai X, Galligan JJ, Watts SW, Fink GD, Kreulen DL 2004. Increased O2*- production and upregulation of ETB receptors by sympathetic neurons in DOCA-salt hypertensive rats. Hypertension 43, 1048–1054. [DOI] [PubMed] [Google Scholar]
- Davenport AP, Hyndman KA, Dhaun N, Southan C, Kohan DE, Pollock JS, Pollock DM, Webb DJ, Maguire JJ 2016. Endothelin. Pharmacol Rev 68, 357–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douma LG, Gumz ML 2018. Circadian clock-mediated regulation of blood pressure. Free Radic Biol Med 119, 108–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gariepy CE, Ohuchi T, Williams SC, Richardson JA, Yanagisawa M 2000. Salt-sensitive hypertension in endothelin-B receptor-deficient rats. J Clin Invest 105, 925–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gariepy CE, Williams SC, Richardson JA, Hammer RE, Yanagisawa M 1998. Transgenic expression of the endothelin-B receptor prevents congenital intestinal aganglionosis in a rat model of Hirschsprung disease. J Clin Invest 102, 1092–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossl M, Mitchell A, Lerman A, Opazo Saez A, Schafers RF, Erbel R, Philipp T, Wenzel RR 2004. Endothelin-B-receptor-selective antagonist inhibits endothelin-1 induced potentiation on the vasoconstriction to noradrenaline and angiotensin II. J Hypertens 22, 1909–1916. [DOI] [PubMed] [Google Scholar]
- Han SY, Bouwer GT, Seymour AJ, Korpal AK, Schwenke DO, Brown CH 2015. Induction of hypertension blunts baroreflex inhibition of vasopressin neurons in the rat. Eur J Neurosci 42, 2690–2698. [DOI] [PubMed] [Google Scholar]
- Higashi Y, Oshima T, Ozono R, Nakano Y, Matsuura H, Kambe M, Kajiyama G 1997. Nocturnal decline in blood pressure is attenuated by NaCl loading in salt-sensitive patients with essential hypertension: noninvasive 24-hour ambulatory blood pressure monitoring. Hypertension 30, 163–167. [DOI] [PubMed] [Google Scholar]
- Hildebrandt DA, Irwin ED, Cates AW, Lohmeier TE 2014. Regulation of renin secretion and arterial pressure during prolonged baroreflex activation: influence of salt intake. Hypertension 64, 604–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt DA, Irwin ED, Lohmeier TE 2016. Prolonged Baroreflex Activation Abolishes Salt-Induced Hypertension After Reductions in Kidney Mass. Hypertension 68, 1400–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang BS, Leenen FH 1994. Dietary Na and baroreflex modulation of blood pressure and RSNA in normotensive vs. spontaneously hypertensive rats. Am J Physiol 266, H496–502. [DOI] [PubMed] [Google Scholar]
- Iliescu R, Tudorancea I, Irwin ED, Lohmeier TE 2013. Chronic baroreflex activation restores spontaneous baroreflex control and variability of heart rate in obesity-induced hypertension. Am J Physiol Heart Circ Physiol 305, H1080–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya A, Kawada T, Shimizu S, Sugimachi M 2011. Closed-loop spontaneous baroreflex transfer function is inappropriate for system identification of neural arc but partly accurate for peripheral arc: predictability analysis. J Physiol 589, 1769–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawada T, Sugimachi M 2016. Open-loop static and dynamic characteristics of the arterial baroreflex system in rabbits and rats. J Physiol Sci 66, 15–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura G, Dohi Y, Fukuda M 2010. Salt sensitivity and circadian rhythm of blood pressure: the keys to connect CKD with cardiovascular events. Hypertens Res 33, 515–520. [DOI] [PubMed] [Google Scholar]
- Kita S, Taguchi Y, Matsumura Y 1998. Endothelin-1 Enhances Pressor Responses to Norepinephrine: Involvement of Endothelin-B Receptor. Journal of Cardiovascular Pharmacology 31, S119–S121. [DOI] [PubMed] [Google Scholar]
- Kohan DE, Inscho EW, Wesson D, Pollock DM 2011. Physiology of endothelin and the kidney. Compr Physiol 1, 883–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau YE, Galligan JJ, Kreulen DL, Fink GD 2006. Activation of ETB receptors increases superoxide levels in sympathetic ganglia in vivo. Am J Physiol Regul Integr Comp Physiol 290, R90–95. [DOI] [PubMed] [Google Scholar]
- Legramante JM, Raimondi G, Massaro M, Cassarino S, Peruzzi G, Iellamo F 1999. Investigating feed-forward neural regulation of circulation from analysis of spontaneous arterial pressure and heart rate fluctuations. Circulation 99, 1760–1766. [DOI] [PubMed] [Google Scholar]
- Legramante JM, Sacco S, Raimondi G, Di Lecce VN, Pallante M, Di Nardo P, Galante A 2009. Investigating feedforward neural regulation of circulation from analysis of spontaneous arterial pressure and heart rate fluctuations in conscious rats. Am J Physiol Heart Circ Physiol 296, H202–210. [DOI] [PubMed] [Google Scholar]
- Lehmann LH, Stanmore DA, Backs J 2014. The role of endothelin-1 in the sympathetic nervous system in the heart. Life Sci 118, 165–172. [DOI] [PubMed] [Google Scholar]
- Lipphardt M, Koziolek MJ, Lehnig LY, Schafer AK, Muller GA, Luders S, Wallbach M 2019. Effect of baroreflex activation therapy on renal sodium excretion in patients with resistant hypertension. Clin Res Cardiol 108, 1287–1296. [DOI] [PubMed] [Google Scholar]
- Matsumura Y, Kuro T, Kobayashi Y, Konishi F, Takaoka M, Wessale JL, Opgenorth TJ, Gariepy CE, Yanagisawa M 2000. Exaggerated vascular and renal pathology in endothelin-B receptor-deficient rats with deoxycorticosterone acetate-salt hypertension. Circulation 102, 2765–2773. [DOI] [PubMed] [Google Scholar]
- Ohkita M, Wang Y, Nguyen ND, Tsai YH, Williams SC, Wiseman RC, Killen PD, Li S, Yanagisawa M, Gariepy CE 2005. Extrarenal ETB plays a significant role in controlling cardiovascular responses to high dietary sodium in rats. Hypertension 45, 940–946. [DOI] [PubMed] [Google Scholar]
- Osborn JW, Hornfeldt BJ 1998. Arterial baroreceptor denervation impairs long-term regulation of arterial pressure during dietary salt loading. Am J Physiol 275, H1558–1566. [DOI] [PubMed] [Google Scholar]
- Pollock DM, Pollock JS 2001. Evidence for endothelin involvement in the response to high salt. Am J Physiol Renal Physiol 281, F144–150. [DOI] [PubMed] [Google Scholar]
- Sheng CS, Li FK, Cheng YB, Wei FF, Huang JF, Guo QH, Zhang DY, Wang Y, An DW, Huang QF, Li Y, Wang JG 2020. Blood pressure and heart rate variability and baroreflex sensitivity in white-coat, masked, and sustained hypertension. Hypertens Res. [DOI] [PubMed] [Google Scholar]
- Simmonds SS, Lay J, Stocker SD 2014. Dietary salt intake exaggerates sympathetic reflexes and increases blood pressure variability in normotensive rats. Hypertension 64, 583–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speed JS, Hyndman KA, Roth K, Heimlich JB, Kasztan M, Fox BM, Johnston JG, Becker BK, Jin C, Gamble KL, Young ME, Pollock JS, Pollock DM 2018. High dietary sodium causes dyssynchrony of the renal molecular clock in rats. Am J Physiol Renal Physiol 314, F89–F98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauss HM, Moffitt JA, Chapleau MW, Abboud FM, Johnson AK 2006. Baroreceptor reflex sensitivity estimated by the sequence technique is reliable in rats. Am J Physiol Heart Circ Physiol 291, H482–483. [DOI] [PubMed] [Google Scholar]
- Tan CO, Taylor JA 2011. Feedback and feedforward sympathetic haemodynamic control: chicken or egg? J Physiol 589, 1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Geldern TW, Tasker AS, Sorensen BK, Winn M, Szczepankiewicz BG, Dixon DB, Chiou WJ, Wang L, Wessale JL, Adler A, Marsh KC, Nguyen B, Opgenorth TJ 1999. Pyrrolidine-3-carboxylic acids as endothelin antagonists. 4. Side chain conformational restriction leads to ET(B) selectivity. J Med Chem 42, 3668–3678. [DOI] [PubMed] [Google Scholar]
- Wehrwein EA, Parker LM, Esfahanian M, Gariepy CE, Watts SW, Kreulen DL 2007. ETB receptor deficient rats have an elevation of ETB receptor and norepinephrine transporter protein in stellate ganglia. The FASEB Journal 21, A1264–A1264. [Google Scholar]
- Wessale JL, Adler AL, Novosad EI, Calzadilla SV, Dayton BD, Marsh KC, Winn M, Jae HS, von Geldern TW, Opgenorth TJ, Wu-Wong JR. Pharmacology of endothelin receptor antagonists ABT-627, ABT-546, A-182086 and A-192621: ex vivo and in vivo studies. Clin Sci (Lond). 2002. August;103 Suppl 48:112S–117S. [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Matsumoto N, Kanazawa M, Fujita M, Takaoka M, Gariepy CE, Yanagisawa M, Matsumura Y 2005. Different contributions of endothelin-A and endothelin-B receptors in postischemic cardiac dysfunction and norepinephrine overflow in rat hearts. Circulation 111, 302–309. [DOI] [PubMed] [Google Scholar]
- Zucker IH, Patel KP, Schultz HD 2012. Neurohumoral stimulation. Heart Fail Clin 8, 87–99. [DOI] [PMC free article] [PubMed] [Google Scholar]


