Abstract
In March 2020, COVID-19 infection caused by SARS-CoV-2 has been declared to be a global pandemic, where its complications, severity and mortality are reported to be due to the released inflammatory cytokines or the so-called cytokine storm. This is quite similar to that observed in the autoimmune and chronic inflammatory rheumatic disease, rheumatoid arthritis (RA). It was hypothesized that RA patients are at a higher risk of acquiring COVID-19; however, recent studies reported that they are not when compared to the rest of the population. In this review, we aim to highlight the mutual pathological features, cytokine profiles and risk factors between COVID-19 and RA. Also, many researchers are currently working to explore therapeutic agents that could aid in the eradication of COVID-19 infection. Due to the similarity between the inflammation status in COVID-19 and RA, many anti-rheumatic drugs such as hydroxychloroquine, tocilizumab, baricitinib and anakinra were proposed to be therapeutic modalities for COVID-19 infection.
Keywords: Rheumatoid arthritis, COVID-19, anti-rheumatic, cytokines, DMARDs
1. COVID-19 infection
Coronaviruses (CoVs) compromise a family of large positive single-stranded RNA viruses that may cause respiratory diseases in humans1. CoVs nomenclature is based on their morphology as spherical virions with a core-shell and large bulbous surface projections, meaning in Latin “virus with a crown”2. SARS-CoV-2 belongs to the subgroup of beta‐coronaviruses and is very closely related to the severe acute respiratory distress syndrome virus (SARS-CoV), that was reported in 20023–5. Last December, COVID-19 infection caused by SARS-CoV-2 virus was first reported in the Huanan seafood market in Wuhan, China6. A few months later, the World Health Organization (WHO) has declared it to be a global pandemic. The mode of transmission of SARS-CoV-2 is through infectious droplets produced by coughing or sneezing that could be present in the air7, and could spread across distances and remain viable on surfaces8.
SARS-CoV-2 uses multiple host receptors for cell entry in the lungs such as angiotensin-converting enzyme 2 (ACE2) and the cellular serine protease TMPRSS29–13. ACE2 is expressed in various organs including lung and alveolar epithelial cells which makes it more susceptible to COVID-19 viral infection14. Upon infection with SARS-CoV-2, there is a downregulation of ACE2 receptor expression along with increased production of angiotensin II by the related enzyme ACE and enhanced activity of the renin-angiotensin system thus inducing pulmonary vascular permeability and lung damage15,16.
The clinical symptoms could vary from fatigue, fever, headache, dyspnea, nasal congestion, nausea, diarrhea and cough3. The most severe complication of COVID-19 is pneumonia that could consequently lead to acute respiratory distress syndrome (ARDS) accompanied by neutrophilia, lymphopenia, and thrombocytopenia17–20. Being a viral infection that is typically associated with a strong immune response and inflammation, markers such as C‐reactive protein (CRP), erythrocyte sedimentation rate (ESR) and proinflammatory cytokines are elevated17. Extreme high concentrations of cytokines could lead to the so-called “cytokine storm” as observed in severe cases of COVID-19 patients and were correlated with disease severity, viral replication and lung injury12,21. The cytokines profile observed in COVID-19 was found to show some similarities to that of secondary hemophagocytic lymphohistiocytosis (sHLH), the hyperinflammatory syndrome associated with an excessive cytokine and multi-organ failure22,23. These inflammatory cytokines and chemokines include IL-1β, IL-2, IL-6, IL-7, IL-8, IL-10, granulocyte colony-stimulating factor (G-CSF), tumor necrosis factor-alpha (TNFα), CCL2, CCL3, and CXCL1012,21. Furthermore, an elevation of ferritin level and presence of proinflammatory state was reported in sHLH, or the so-called macrophage activation syndrome (MAS), which is quite similar to that observed in COVID-1924.
COVID-19 complications could be due to the CRS and the extravagant immune response against the SARS-CoV-2 virus21,25,26, suggesting that management of this storm is quite critical. One would speculate that rheumatoid arthritis (RA) patients may be at higher risk of COVID-19 infection, due to the inflammation status and the use of immunomodulatory drugs. However, recent data has shown that the prevalence of COVID-19 in RA patients is the same as in the general population. In this review, we aim to highlight the mutual features between COVID-19 and the chronic inflammatory rheumatic disease, rheumatoid arthritis (RA), as well as the possible use of anti-rheumatic drugs as a therapeutic modality for COVID-19 infection.
2. Rheumatoid arthritis
Rheumatoid arthritis is a chronic and systemic autoimmune disease affecting multiple joints that could lead to progressive disability, systemic complications, socioeconomic costs and burden. RA is accompanied by systemic manifestations such as cardiovascular diseases that might lead to high mortality and morbidity. It is characterized by joint inflammation associated with hyperplasic synovium, cytokine and chemokine production, detection of autoantibodies like rheumatoid factor (RF) and anticitrullinated peptide antibody (ACPA). Moreover, these are accompanied by osteoclastogenesis, angiogenesis and systemic manifestations affecting the cardiovascular, pulmonary, neurovascular and skeletal symptoms27. Furthermore, symmetric polyarticular arthritis and persistent inflammation in the synovium might lead to pannus formation and thus joint destruction28. Early diagnosis and immediate effective therapy are crucial for the prevention of joint deterioration, functional disability and unfavorable, even fatal disease outcomes. An approach which is known as T2T (treat to target) can be implemented when the target is the disease activity remission or low disease activity.
Therapy of RA aims at improving the patient’s quality of life and joint function as well as reducing the inflammatory state, pain and disease progression28,29. There are several pharmacological agents known as disease-modifying antirheumatic drugs (DMARDs) including methotrexate, sulfasalazine, and hydroxychloroquine that slow down the progression of RA30. Several cytokines and chemokines play a role in the pathogenesis of RA through regulation of inflammation, autoimmunity and joint destruction, including IL-1, IL-6, IL-12, IL-17, IL-18, IL-23, and TNFα as well as the chemokines CCL2, CCL3, CCL4, CXCL8 and CXCL1031,32. Hence, the biologicals agents mainly act by dampening the host inflammatory response. The biological agents are engineered drugs that target specific inflammatory cells, cytokines and receptors that mediate RA inflammation and tissue damage. Thus, they reduce the symptoms associated with RA and disease progression33. These agents include IL-1 receptor antagonist (anakinra), rituximab (anti-CD20, B cell depleting agent) and TNF antagonists (infliximab, etanercept, golimumab, adalimumab), IL-6 receptor blockers (tocilizumab) and the JAK inhibitors (Upadacitinib, baricitinib and tofacitinib)34,35. Such biologics have shown to be highly effective in reducing RA symptoms, leading to amelioration of physical function and quality of life36,37.
3. Common factors between COVID-19 and rheumatoid arthritis
Despite the multiple differences between COVID-19 and RA in terms of etiology, epidemiology, clinical features, organ involvement, and prognosis, they seem to have some similarities in the pathogenesis and risk factors associated with the disease. For example, some external microorganisms could cause acute and chronic arthritis, either by the direct presence in the joints or the aberrant autoimmune reaction induced by the host. On the other hand, the absence of the commensal bacteria present in the microbiome was found to lead to disease amelioration due to reduction in the pro-inflammatory Th17 response38–42. On the other hand, patients suffering from inflammatory arthritis are at high risk of infections as it could lead to disease flares43–45. Additionally, RA patients often have co-morbidities such as diabetes mellitus, cardiovascular disease and pulmonary diseases which further increases the risk of viral infections43,46,47. This increased risk of infection is associated with certain risk factors similar to those reported in COVID-1943,48,49.
One of the most consistent associated comorbidities in RA is hypertension50. Recent data indicated that hypertension was significantly associated with the increased risk of adverse outcomes in COVID-19 patients and that hypertension is an independent risk factor for predicting the severity and mortality of COVID-19 patients51. Additionally, the prevalence of diabetes mellitus is controversial among RA, but definitely, RA patients are more predisposed to developing both insulin resistance and type 2 diabetes mellitus52–54. The second most common comorbidity associated with a worse outcome in COVID-19 patients is diabetes55–57, probably due to higher ACE2 expression in diabetic patients58.
Regarding risk factors, old age seems to increase the likelihood of RA development. This could be due to the dramatic changes in the lymphocyte populations and phenotypes that could possibly cause an increase in reactivity to self-tissue antigens28. Similarly, age over 65 years might increase the risk of acquiring COVID-19 infection59. Another reported risk factor is smoking where smokers were reported to experience seropositive, erosive RA disease with extraarticular manifestations60. This could be attributed to the effect of the posttranslational modifications such as citrullination of the mucosal proteins including collagen, fibrinogen, enolase and fibrinogen61–63. Likewise, smoking increases the risk and severity of COVID-19, which could be attributed to lung injury and reduced lung capacity64.
There are many discrepancies between RA and COVID-19 in terms of etiology, disease progression, risk factors and demographic factors. For instance, it seems that the gender susceptibility between RA and COVID-19 is quite the opposite. Women are more prone to RA possibly due to the X chromosomes or their female hormones as suggested by some studies where estrogen and prolactin stimulate autoantibody production28,65,66. In contrast, males are more at risk for worse outcomes and high mortality with COVID-19, which could be due to higher expression levels of ACE267.
Another common feature among RA and COVID-19 infection is vasculitis. Systemic rheumatoid vasculitis is among the most serious complications of RA. It is characterized by inflammation of mid-size arteries and capillaries, which could lead to deep cutaneous ulcers, gangrene, and neuropathy, which is associated with poor outcomes and mortality68. Recently, some case reports highlighted the presence and association of vasculitis in COVID-19 patients69,70. Also, the development of RA is accompanied by a disturbance of the coagulation system and elevation in the blood coagulation state and fibrinolysis, which is probably due to excessive stimulation of inflammatory pathways71,72. Likewise, coagulopathies were observed in COVID-19 infection as reported by the prominent elevation of D-dimer and fibrin/fibrinogen-degradation products. This was proposed to be a result of the hypoxia and the inflammatory response to SARS-CoV-2 causing thrombo-inflammation and thrombosis73.
Most importantly, the cytokine imbalance in COVID-19 infection is quite similar to that observed in inflammatory rheumatic diseases. This includes the pro-inflammatory and pyrogenic IL-1, IL-6 and TNF-α cytokines, similar to what has been previously observed in other coronavirus infections SARS and MERS (middle east respiratory syndrome), as well as the inflammatory chemokines CCL2 and CXCL1021,25,32,74. It has been observed that a reduction in CD4+ and CD8+ T cells is found in severe COVID-19 patients along with higher serum levels of TNF-α, IL-1 and IL-620. It is worth mentioning that IL-6 is one of the crucial inflammatory mediators in COVID-19 as its levels are correlated with SARS-CoV-2 viral load75. Besides, COVID-19 and RA share another feature, i.e. genetic host characteristics such as IL-6 gene polymorphisms, that can contribute to SARS-CoV-2 susceptibility and RA76,77. Such gene markers could be used as predictors of response to anti-IL-6 treatment during the COVID-19 pandemic78.
4. Is there an increased risk of COVID-19 in patients with rheumatoid arthritis?
The shared immune and genetic mechanisms as well as clinical aspects between inflammatory diseases and COVID-19, propose that SARS-CoV-2 could be a trigger for the development of excessive inflammatory disorders, especially in susceptible individuals79. Therefore, arthritis patients should be informed to maintain their treatments during the pandemic, adhere to self-protection principles for COVID-19, and consult their rheumatologists in case of any doubt of infection, preferably via telemedicine if available80. There are various risk factors associated with COVID-19, such as pulmonary and cardiovascular diseases as well as diabetes mellitus81,82. However, it remains unclear if chronic rheumatic diseases such as RA increase the risk of COVID-19 compared to the general population83. Such a risk has been proposed to be due to the immune dysregulation and the use of chemical and biological anti-rheumatic agents that generally increase the risk of infections due to the state of being immunocompromised along with associated comorbidities25,84–86. In particular, RA patients suffered from a malfunction in the thymus, increased turnover of peripheral T cells and dysfunction of circulating T cells, which make these patients more prone to infections87–89. Additionally, nonsteroidal anti-inflammatory drugs (NSAIDs) such as ibuprofen, which might be used as an adjunct treatment to reduce arthritic pain90, have been demonstrated to induce ACE2 overexpression and hence might increase the SARS-CoV-2 infection susceptibility. These worsen the clinical course or even mask some symptoms that aid in the diagnosis of COVID-1991. Other studies claimed that NSAIDs are not associated with the odds of hospitalization, unlike glucocorticoid exposure which seems to increase the hospitalization chances in patients with rheumatic diseases92.
However, several recent studies report that patients diagnosed with rheumatic diseases, especially patients with chronic inflammatory arthritis taking chemical and bDMARDS, are not at a higher risk of acquiring COVID-19 infection compared to the rest of the population5,93–97. This goes in line with previous reports during SARS and MERS, where patients with autoimmune diseases did not show any increase in mortality rates93,98. On the other hand, a case report has proposed that patients with rheumatic immune diseases are more likely to progress into severe/critical COVID-19, as these two diseases overlap in pathogenesis and therapeutic agents used99. Moreover, a study by Akiyama et al. showed that the prevalence of COVID-19 in patients with autoimmune diseases was higher than in other populations. Additionally, the use of glucocorticoids increased the risk of severe outcomes, whereas anti-TNF therapy reduced the risk of severe COVID-19100. Initially, patients with rheumatic disease and COVID-19 infection were reported to be more likely to require mechanical ventilation but had the same hospitalization rate101. However, an update of this study indicated that patients with rheumatic disease are not at a higher risk of hospitalization, intensive care unit admission, mechanical ventilation or death102.
5. Remitters: anti-rheumatic and anti-COVID-19 agents
Currently, many researchers across the globe are investigating potential therapeutic agents that can be utilized for the treatment of COVID-19. The standard care comprises supplemental oxygen, ventilation, and antibiotic agents103. Until now, several attempts include the use of plasma containing antibodies from recovered COVID-19 patients to severe and deteriorating patients infected with COVID-19104. Moreover, anti-viral agents that have previously worked against other RNA viruses such as remdesivir and lopinavir/ritonavir showed potential therapeutic efficacy against SARS-CoV-2 virus in vitro105–108. However, other studies have suggested that lopinavir/ritonavir did not reduce mortality, hospitalization duration, or clinical progression in hospitalized COVID-19 patients (Chinese Clinical Trial Register number, ChiCTR2000029308)103,106. Furthermore, the RECOVERY trial showed that lopinavir/ritonavir did not cause any reductions in 28-day mortality, duration of hospitalization, or risk of progressing to invasive mechanical ventilation or death109. Therefore, WHO decided to discontinue the use of lopinavir/ritonavir in hospitalized COVID-19 patients; however, this does not include non-hospitalized COVID-19 patients110.
As mentioned earlier, there is a similarity in the cytokine imbalance of COVID-19 and inflammatory rheumatic disorders, thus some of the anti-rheumatic drugs had been re-directed in the treatment of this pandemic. Furthermore, many of the anti-rheumatic drugs especially the biological agents, are currently under investigation to be used in the treatment protocols for COVID-19111,112. It is well known for decades that steroids such as prednisolone can be used in RA as it reduces pain and flares of synovitis as well as modify the progression of disease via affecting joint tenderness113,114. Corticosteroids may be recently introduced for COVID-19 therapy, as the corticosteroid dexamethasone was suggested to have anti-inflammatory and immunosuppressive roles by limiting the activity of inflammatory cytokines, T and B cells. Furthermore, dexamethasone may reduce the mortality of severe, intubated COVID-19 patients115,116. The RECOVERY trial further supported the use of dexamethasone which resulted in decline in mortality rate among the COVID-19 patients receiving either invasive mechanical ventilation or oxygen alone117.
During the initial phase of the COVID-19 pandemic, hydroxychloroquine and chloroquine have been suggested to inhibit SARS-CoV-2 replication and activity, especially in the cases of COVID-19 pneumonia107,108,118. This is attributed to the previously reported antiviral mechanism of action of chloroquine against various viruses including H5N1 avian influenza, Zika, Ebola, and SARS-CoV119–122. Chloroquine acts by increasing the endosomal pH required for the fusion of virus and host cell as well as interfering with the glycosylation of the cellular receptor ACE2, thus negatively influencing the virus-receptor binding118,122,123. Hydroxychloroquine is a more potent derivative than chloroquine and was suggested for the management of SARS-CoV-2 infection124. This could be due to its multiple mechanisms of action: anti-viral, immunomodulatory, anti-inflammatory and anti-thrombotic that are needed to resolve the clinical symptoms in COVID-19 infection124,125. However, if the use of hydroxychloroquine is uncontrolled, side effects such as retinopathy and potential cardiac damage could be observed126,127. Initial in vitro studies revealed hydroxychloroquine to reduce SARS-CoV-2 viral load128,129. Moreover, hydroxychloroquine could block antigen presentation to CD4+ T cells, and cause a reduction in the production of type I IFNs, TNF-α, IL-6, GM-CSF, and IL-1β130,131. Recently, WHO has announced that hydroxychloroquine will not be included in the international Solidarity clinical trial of COVID-19 therapy for hospitalized patients110. This was due to the evidence proven by multiple studies where there were no observed differences in the clinical status or mortality for mild-moderate hospitalized COVID-19 patients receiving hydroxychloroquine with or without macrolide therapy compared to standard care (ClinicalTrials.gov number, NCT04322123)132–134. However, it is worth mentioning that hydroxychloroquine could have a beneficial effect in the therapy of non-hospitalized COVID-19 patients as well as being under investigations to be used in a prophylactic approach for COVID-19135,136. Nevertheless, hydroxychloroquine did not show a preventive effect against SARS-CoV-2 infection in patients with rheumatological conditions92,137,138.
Furthermore, due to the presence of inflammatory cytokines in the plasma and bronchoalveolar lavage fluid of COVID-19 patients, targeting such markers using bio-therapies such as IL-6 receptor blocker tocilizumab was proposed139. Tocilizumab is a recombinant human IL-6 monoclonal antibody that blocks its signaling pathway and the inflammatory response140. Tocilizumab was one of the most effective therapeutics to relieve the symptoms of RA patients and their quality of life141,142. Being an IL-6- receptor blocker, tocilizumab inhibits the inflammatory storm observed in COVID-19 thus halting alveolar-capillary blood-gas exchange dysfunction, pulmonary fibrosis and organ failure143–145. Based on this, tocilizumab can be a suitable and effective drug for COVID-19 patients, especially those with ARDS, via resolving fever and oxygen saturation and restoring CRP levels and lymphocytes count146. Tocilizumab showed controversial data on COVID-19 patients. For instance, a retrospective study by Guaraldi et al. and another multi-center observational study showed that the risk of invasive mechanical ventilation, ICU admission or death in patients with severe COVID-19 pneumonia was reduced upon tocilizumab therapy147,148. Similarly, tocilizumab reduced the need of mechanical ventilation or death, but did not improve the survival of COVID-19 patients with pneumonia who were not receiving mechanical ventilation149. On the other hand, another study highlighted that tocilizumab did not seem to be reducing the mortality of hospitalized patients with COVID-19 pneumonia (ClinicalTrials.gov number, NCT04356937)150–152. This was further highlighted in other studies where tocilizumab did not show to be effective in prevention of intubation or death in moderately ill hospitalized COVID-19 patients150–153.
It is worth mentioning that excessive activity of tocilizumab can cause damage such as autoimmunity154. Multiple health centers have used and recommended tocilizumab for COVID-19 therapy but further studies and trials are still needed to confirm the positive therapeutic effect in COVID-19 patients154. Single-nucleotide polymorphisms (SNPs) in various genes have been recognized to predict response to tocilizumab therapy in RA and correlated with low DAS28 score155–157. Additionally, the IL-6R gene SNPs were found to be associated with response to tocilizumab therapy in RA158,159. It is interesting to investigate these genetic markers that can be useful in understanding SARS-CoV-2 mechanisms and hence allow targeted therapy in COVID-19 infection78.
Another inhibitor of the IL-6 pathway is sarilumab, an inhibitor of soluble and membrane IL-6Rα receptor that could be used to reduce the severity of the pulmonary complications of COVID-1919. Recent reports claim that sarilumab is only effective in critical but not moderate/severe COVID-19 patients, while others have reported that sarilumab is a potential therapeutic approach resulting in a clinical benefit with good safety in severe COVID-19 patients160. This has been emphasized by others where clinical improvement and mortality in severe COVID-19 patients were unaffected upon sarilumab therapy and standard of care161. Nevertheless, sarilumab was associated with faster recovery in a subset of patients showing minor lung consolidation. Also, the use of IL-1 blockers was proposed as a possible therapeutic strategy for COVID-19162.
Previously, the SARS viral spike protein was reported to induce TNF-α-converting enzyme-dependent shedding of the ACE2 ectodomain, thus affecting viral entry163,164. This suggests that a similar effect could be suggested in SARS-CoV-2, hence linking its mechanism of entry to TNF-α production. Therefore, the use of TNF inhibitors such as adalimumab may be effective in resolving COVID-19 infection165–168. Additionally, other biologic drugs such as the JAK inhibitor baricitinib that is used for treating RA patients, is suggested for controlling SARS-CoV-2 viral replication and showed positive therapeutic efficacies139,169,170. This could be mediated via the inhibition of the key regulators of endocytosis, AP2-associated protein kinase 1 (AAK1), and the binding cyclin G-associated kinase (GAK), thus blocking viral entry into the lungs171,172. Furthermore, JAK inhibitors could dampen the signaling pathways associated with the cytokine storm, the main player of severe symptoms of COVID-19169. Baricitinib in combination to remdesivir was superior to the use of remdesivir alone in improving the clinical status, among patients receiving oxygen or mechanical ventilation, with minimal side effects173.
Colchicine, which is used for the treatment of some rheumatic conditions, was proposed for COVID-19 therapy. A possible mechanism of action could be via non-selective inhibition of NLRP3 inflammasome, a major inflammatory marker in the COVID-19 infection174.
Rheumatologists play a crucial role in this COVID-19 pandemic as they are the most acquainted with these therapeutic agents such as chloroquine, hydroxychloroquine, JAK inhibitors and anti-IL-1 and IL-6 agents, that are typically prescribed in rheumatic diseases175. Rheumatologists should share their knowledge and experience to fight the COVID-19 pandemic. Also, they should maintain the management and counseling of patients through telemedicine, in order to avoid the abrupt withdrawal of DMARDs. This would aid in avoiding relapses, flares and development of associated morbidities of such rheumatic diseases, as recommended by EULAR and ACR organizations80,96,176,177. A major concern about the common remission agents of COVID-19 and RA is the possibility of a shortage of such drugs that are quite critical for the management of patients with rheumatic diseases178.
6. Conclusions
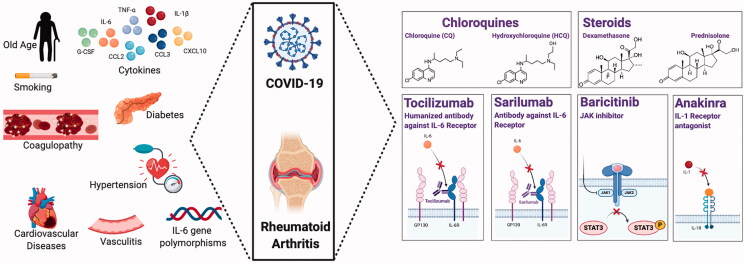
It seems that COVID-19 infection revisits inflammatory pathways such as those found in rheumatoid arthritis. Additionally, COVID-19 and RA share mutual features and risk factors that promote the use of synthetic and biological anti-rheumatic agents, for COVID-19 therapy (Figure 1). Current and future studies are working on the efficacy of these agents in COVID-19 infection.
Figure 1.
Mutual features between COVID-19 infection and rheumatoid arthritis. The left panel shows the cytokines, risk factors and co-morbidities associated with both diseases, while the right panel displays the remedies that may have potential therapeutic effects in COVID-19 and RA
Transparency
Declaration of funding
The authors have no funding to declare.
Declaration of financial/other relationships
The authors declare that the work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
All authors were involved in the conception and design, as well as the drafting and revision of the paper.
References
- 1.Rota PA, Oberste MS, Monroe SS, et al. Characterization of a Novel Coronavirus associated with severe acute respiratory syndrome. Science. 2003;300(5624):1394–1399. [DOI] [PubMed] [Google Scholar]
- 2.Guo Y-R, Cao Q-D, Hong Z-S, et al. The origin, transmission and clinical therapies on Coronavirus disease 2019 (COVID-19) outbreak – an update on the status. Mil Med Res. 2020;7(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Velavan TP, Meyer CG.. The COVID-19 epidemic. Trop Med Int Health. 2020;25(3):278–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou P, Yang X-L, Wang X-G, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quartuccio L, Valent F, Pasut E, et al. Prevalence of COVID-19 among patients with chronic inflammatory rheumatic diseases treated with biologic agents or small molecules: a population-based study in the first two months of COVID-19 outbreak in Italy. Joint Bone Spine. 2020;87(5):439–443. doi:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang C, Horby PW, Hayden FG, et al. A novel coronavirus outbreak of global health concern. The Lancet. 2020;395(10223):470–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rothe C, Schunk M, Sothmann P, et al. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N Engl J Med. 2020;382(10):970–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kampf G, Todt D, Pfaender S, et al. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J Hosp Infect. 2020;104(3):246–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prabakaran P, Xiao X, Dimitrov DS.. A model of the ACE2 structure and function as a SARS-CoV receptor. Biochem Biophys Res Commun. 2004;314(1):235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li W, Moore MJ, Vasilieva N, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426(6965):450–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yan R, Zhang Y, Li Y, et al. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367(6485):1444–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel Coronavirus in Wuhan, China. The Lancet. 2020;395(10223):497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao Y, Zhao Z, Wang Y, et al. Single-cell RNA expression profiling of ACE2, the receptor of SARS-CoV-2. bioRxiv. 2020. 2020.01.26.919985. [DOI] [PMC free article] [PubMed]
- 15.Zou Z, Yan Y, Shu Y, et al. Angiotensin-converting enzyme 2 protects from lethal avian influenza A H5N1 infections. Nat Commun. 2014;5(1):3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Favalli EG, Ingegnoli F, De Lucia O, et al. COVID-19 infection and rheumatoid arthritis: Faraway, so close!. Autoimmun Rev. 2020;19(5):102523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guan W-j, Ni Z-y, Hu Y, et al. Clinical characteristics of 2019 novel coronavirus infection in China. medRxiv. 2020. 2020.02.06.20020974.
- 18.Chen F, Liu ZS, Zhang FR, et al. [First case of severe childhood novel coronavirus pneumonia in China]. Zhonghua Er Ke Za Zhi. 2020;58(0):E005. [DOI] [PubMed] [Google Scholar]
- 19.Marotto D, Sarzi-Puttini P.. What is the role of rheumatologists in the era of COVID-19? Autoimmun Rev. 2020;19(6):102539–102539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with Coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020;71(15):762–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mehta P, McAuley DF, Brown M, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. The Lancet. 2020;395(10229):1033–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yildiz H, Van Den Neste E, P. Defour J, et al. Adult haemophagocytic lymphohistiocytosis: a review. Int J Med. 2020;21(14):11. [DOI] [PubMed] [Google Scholar]
- 23.Ramos-Casals M, Brito-Zerón P, López-Guillermo A, et al. Adult haemophagocytic syndrome. The Lancet. 2014;383(9927):1503–1516. [DOI] [PubMed] [Google Scholar]
- 24.Ruscitti P, Cipriani P, Di Benedetto P, et al. Increased level of H-ferritin and its imbalance with L-ferritin, in bone marrow and liver of patients with adult onset Still’s disease, developing macrophage activation syndrome, correlate with the severity of the disease. Autoimmun Rev. 2015;14(5):429–437. [DOI] [PubMed] [Google Scholar]
- 25.Sepriano A, Kerschbaumer A, Smolen JS, et al. Safety of synthetic and biological DMARDs: a systematic literature review informing the 2019 update of the EULAR recommendations for the management of rheumatoid arthritis. Ann Rheum Dis. 2020;79(6):760–770. [DOI] [PubMed] [Google Scholar]
- 26.Wan S, Yi Q, Fan S, et al. Characteristics of lymphocyte subsets and cytokines in peripheral blood of 123 hospitalized patients with 2019 novel coronavirus pneumonia (NCP). medRxiv. 2020. 2020.02.10.20021832.
- 27.McInnes IB, Schett G.. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365(23):2205–2219. [DOI] [PubMed] [Google Scholar]
- 28.Alam J, Jantan I, Bukhari SNA.. Rheumatoid arthritis: recent advances on its etiology, role of cytokines and pharmacotherapy. Biomed Pharmacother. 2017;92:615–633. [DOI] [PubMed] [Google Scholar]
- 29.Smolen JS, Landewé RBM, Bijlsma JWJ, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis. 2020;79(6):685–699. [DOI] [PubMed] [Google Scholar]
- 30.Jones G, Halbert J, Crotty M, et al. The effect of treatment on radiological progression in rheumatoid arthritis: a systematic review of randomized placebo-controlled trials. Rheumatology. 2003;42(1):6–13. [DOI] [PubMed] [Google Scholar]
- 31.McInnes IB, Schett G.. Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunol. 2007;7(6):429–442. [DOI] [PubMed] [Google Scholar]
- 32.Elemam NM, Hannawi S, Maghazachi AA.. Role of chemokines and chemokine receptors in rheumatoid arthritis. Immunotargets Ther. 2020;9:43–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Curtis JR, Singh JA.. Use of biologics in rheumatoid arthritis: current and emerging paradigms of care. Clin Ther. 2011;33(6):679–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jones G, Sebba A, Gu J, et al. Comparison of tocilizumab monotherapy versus methotrexate monotherapy in patients with moderate to severe rheumatoid arthritis: the AMBITION study. Ann Rheum Dis. 2010;69(01):88–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee EB, Fleischmann R, Hall S, et al. Tofacitinib versus methotrexate in rheumatoid arthritis. N Engl J Med. 2014;370(25):2377–2386. [DOI] [PubMed] [Google Scholar]
- 36.Smolen JS, Aletaha D, Koeller M, et al. New therapies for treatment of rheumatoid arthritis. Lancet. 2007;370(9602):1861–1874. [DOI] [PubMed] [Google Scholar]
- 37.Strand V, Singh JA.. Improved health-related quality of life with effective disease-modifying antirheumatic drugs: evidence from randomized controlled trials. The American journal of managed care. Suppl 9. 2007;13:S237–S251. [PubMed] [Google Scholar]
- 38.Mathew AJ, Ravindran V.. Infections and arthritis. Best Pract Res Clin Rheumatol. 2014;28(6):935–959. [DOI] [PubMed] [Google Scholar]
- 39.Bogdanos DP, Smyk DS, Invernizzi P, et al. Infectome: a platform to trace infectious triggers of autoimmunity. Autoimmun Rev. 2013;12(7):726–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu HJ, Ivanov II, Darce J, et al. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity. 2010;32(6):815–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Belkaid Y, Hand TW.. Role of the microbiota in immunity and inflammation. Cell. 2014;157(1):121–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li S, Yu Y, Yue Y, et al. Microbial infection and rheumatoid arthritis. J Clin Cell Immunol. 2013;4(6):174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Listing J, Gerhold K, Zink A.. The risk of infections associated with rheumatoid arthritis, with its comorbidity and treatment. Rheumatology. 2013;52(1):53–61. [DOI] [PubMed] [Google Scholar]
- 44.Galloway JB, Hyrich KL, Mercer LK, et al. Anti-TNF therapy is associated with an increased risk of serious infections in patients with rheumatoid arthritis especially in the first 6 months of treatment: updated results from the British Society for Rheumatology Biologics Register with special emphasis on risks in the elderly. Rheumatology. 2011;50(1):124–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Widdifield J, Bernatsky S, Paterson JM, et al. Serious infections in a population-based cohort of 86,039 seniors with rheumatoid arthritis. Arthritis Care Res. 2013;65(3):353–361. [DOI] [PubMed] [Google Scholar]
- 46.Dougados M, Soubrier M, Antunez A, et al. Prevalence of comorbidities in rheumatoid arthritis and evaluation of their monitoring: results of an international, cross-sectional study (COMORA). Ann Rheum Dis. 2014;73(1):62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ranganath VK, Maranian P, Elashoff DA, et al. Comorbidities are associated with poorer outcomes in community patients with rheumatoid arthritis. Rheumatology. 2013;52(10):1809–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Apicella M, Campopiano MC, Mantuano M, et al. COVID-19 in people with diabetes: understanding the reasons for worse outcomes. Lancet Diabetes Endocrinol. 2020;8(9):782–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bansal M. Cardiovascular disease and COVID-19. Diabetes Metab Syndr. 2020;14(3):247–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kłodziński Ł, Wisłowska M.. Comorbidities in rheumatic arthritis. Reumatologia. 2018;56(4):228–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liang X, Shi L, Wang Y.. The association of hypertension with the severity and mortality of COVID-19 patients: evidence based on adjusted effect estimates. J Infect. 2020;81(3):e44–e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pradhan AD, Manson JE, Rifai N, et al. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286(3):327–334. [DOI] [PubMed] [Google Scholar]
- 53.Xu H, Barnes GT, Yang Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112(12):1821–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Han C, Robinson DW, Jr., Hackett MV, et al. Cardiovascular disease and risk factors in patients with rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. J Rheumatol. 2006;33(11):2167–2172. [PubMed] [Google Scholar]
- 55.Mazucanti CH, Egan JM.. SARS-CoV-2 disease severity and diabetes: why the connection and what is to be done? Immun Ageing. 2020;17:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang J-J, Dong X, Cao Y-y, et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020;75(7):1730–1741. [DOI] [PubMed] [Google Scholar]
- 57.Yang J, Zheng Y, Gou X, et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wysocki J, Ye M, Soler MJ, et al. ACE and ACE2 activity in diabetic mice. Diabetes. 2006;55(7):2132–2139. [DOI] [PubMed] [Google Scholar]
- 59.Zheng Z, Peng F, Xu B, et al. Risk factors of critical & mortal COVID-19 cases: a systematic literature review and meta-analysis. J Infect. 2020;S0163-4453(20):30234–30236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Karlson EW, Lee IM, Cook NR, et al. A retrospective cohort study of cigarette smoking and risk of rheumatoid arthritis in female health professionals. Arthritis Rheum. 1999;42(5):910–917. [DOI] [PubMed] [Google Scholar]
- 61.van der Woude D, Rantapää-Dahlqvist S, Ioan-Facsinay A, et al. Epitope spreading of the anti-citrullinated protein antibody response occurs before disease onset and is associated with the disease course of early arthritis. Ann Rheum Dis. 2010;69(8):1554–1561. [DOI] [PubMed] [Google Scholar]
- 62.Mahdi H, Fisher BA, Källberg H, et al. Specific interaction between genotype, smoking and autoimmunity to citrullinated alpha-enolase in the etiology of rheumatoid arthritis. Nat Genet. 2009;41(12):1319–1324. [DOI] [PubMed] [Google Scholar]
- 63.Anzilotti C, Merlini G, Pratesi F, et al. Antibodies to viral citrullinated peptide in rheumatoid arthritis. J Rheumatol. 2006;33(4):647–651. [PubMed] [Google Scholar]
- 64.Patanavanich R, Glantz SA.. Smoking is associated with COVID-19 progression: a meta-analysis. Nicotine Tob Res. 2020;22(9):1653–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Peeva E, Venkatesh J, Michael D, et al. Prolactin as a modulator of B cell function: implications for SLE. Biomed Pharmacother. 2004;58(5):310–319. [DOI] [PubMed] [Google Scholar]
- 66.Oliver JE, Silman AJ.. Why are women predisposed to autoimmune rheumatic diseases? Arthritis Res Ther. 2009;11(5):252–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jin J-M, Bai P, He W, et al. Gender differences in patients with COVID-19: focus on severity and mortality. [10.3389/fpubh.2020.00152].Front Public Health. 2020;8:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Makol A, Crowson CS, Wetter DA, et al. Vasculitis associated with rheumatoid arthritis: a case-control study. Rheumatology. 2014;53(5):890–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Castelnovo L, Capelli F, Tamburello A, et al. Symmetric cutaneous vasculitis in COVID-19 pneumonia. J Eur Acad Dermatol Venereol. 2020;34(8):16589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.de Perosanz-Lobo D, Fernandez-Nieto D, Burgos-Blasco P, et al. Urticarial vasculitis in COVID-19 infection: a vasculopathy-related symptom? J Eur Acad Dermatol Venereol. 2020;34(10):e566–e568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang P, Liu J, Tan B.. Hypercoagulation in patients with rheumatoid arthritis correlates with activation of Act1/NF-kb signaling pathway. J Rheum Dis Treat. 2015;1(4):024. [Google Scholar]
- 72.Ichikawa Y, Hoshina Y, Horiki T, et al. Molecular markers of coagulation and fibrinolysis as indicators for the disease activity of rheumatoid arthritis. Jpa J Rheumatol. 1997;7(3):173–181. [Google Scholar]
- 73.Connors JM, Levy JH.. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135(23):2033–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sarzi-Puttini P, Giorgi V, Sirotti S, et al. COVID-19, cytokines and immunosuppression: what can we learn from severe acute respiratory syndrome? Clin Exp Rheumatol. 2020;38(2):337–342. [PubMed] [Google Scholar]
- 75.Chen X, Zhao B, Qu Y, et al. Detectable serum SARS-CoV-2 viral load (RNAaemia) is closely associated with drastically elevated interleukin 6 (IL-6) level in critically ill COVID-19 patients. medRxiv. 2020. 2020.02.29.20029520.
- 76.Li F, Xu J, Zheng J, et al. Association between interleukin-6 gene polymorphisms and rheumatoid arthritis in Chinese Han population: a case-control study and a meta-analysis. Sci Rep. 2014;4:5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kirtipal N, Bharadwaj S.. Interleukin 6 polymorphisms as an indicator of COVID-19 severity in humans. J Biomol Struct Dyn. 2020:1–3. [DOI] [PubMed] [Google Scholar]
- 78.Perricone C, Conigliaro P, Ciccacci C, et al. The differential response to anti IL-6 treatment in COVID-19: the genetic counterpart. Clin Exp Rheumatol. 2020;38(3):580–32452345. [PubMed] [Google Scholar]
- 79.Caso F, Costa L, Ruscitti P, et al. Could Sars-coronavirus-2 trigger autoimmune and/or autoinflammatory mechanisms in genetically predisposed subjects? Autoimmun Rev. 2020;19(5):102524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Smith AC, Thomas E, Snoswell CL, et al. Telehealth for global emergencies: implications for coronavirus disease 2019 (COVID-19). J Telemed Telecare. 2020;26(5):309–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bae S, Kim SR, Kim M-N, et al. Impact of cardiovascular disease and risk factors on fatal outcomes in patients with COVID-19 according to age: a systematic review and meta-analysis. Heart. 2021;107(5):373–380. [DOI] [PubMed] [Google Scholar]
- 82.Lim S, Bae JH, Kwon H-S, et al. COVID-19 and diabetes mellitus: from pathophysiology to clinical management. Nat Rev Endocrinol. 2021;17(1):11–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hyrich KL, Machado PM.. Rheumatic disease and COVID-19: epidemiology and outcomes. Nat Rev Rheumatol. 2021;17(2):71–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Favalli EG, Agape E, Caporali R.. Incidence and clinical course of COVID-19 in patients with connective tissue diseases: a descriptive observational analysis. J Rheumatol. 2020;47(8):1296–1296. [DOI] [PubMed] [Google Scholar]
- 85.Hsu CY, Ko CH, Wang JL, et al. Comparing the burdens of opportunistic infections among patients with systemic rheumatic diseases: a nationally representative cohort study. Arthritis Res Ther. 2019;21(1):211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mehta B, Pedro S, Ozen G, et al. Serious infection risk in rheumatoid arthritis compared with non-inflammatory rheumatic and musculoskeletal diseases: a US national cohort study. RMD Open. 2019;5(1):e000935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Blumentals WA, Arreglado A, Napalkov P, et al. Rheumatoid arthritis and the incidence of influenza and influenza-related complications: a retrospective cohort study. BMC Musculoskelet Disord. 2012;13:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Koetz K, Bryl E, Spickschen K, et al. T cell homeostasis in patients with rheumatoid arthritis. Proc Natl Acad Sci USA. 2000;97(16):9203–9208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Atzeni F, Masala IF, di Franco M, et al. Infections in rheumatoid arthritis. Curr Opin Rheumatol. 2017;29(4):323–330. [DOI] [PubMed] [Google Scholar]
- 90.Crofford LJ. Use of NSAIDs in treating patients with arthritis. Arthritis Res Ther. 2013;15(Suppl 3): 4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fang L, Karakiulakis G, Roth M.. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8(4):e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gianfrancesco M, Hyrich KL, Al-Adely S, et al. Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis. 2020;79(7):859–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Monti S, Balduzzi S, Delvino P, et al. Clinical course of COVID-19 in a series of patients with chronic arthritis treated with immunosuppressive targeted therapies. Ann Rheum Dis. 2020;79(5):667–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sanchez-Piedra C, Diaz-Torne C, Manero J, et al. Clinical features and outcomes of COVID-19 in patients with rheumatic diseases treated with biological and synthetic targeted therapies. Ann Rheum Dis. 2020;79(7):988–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Favalli EG, Monti S, Ingegnoli F, et al. Incidence of COVID-19 in patients with rheumatic diseases treated with targeted immunosuppressive drugs: what can we learn from observational data? Arthritis Rheumatol. 2020;72(10):1600–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zen M, Fuzzi E, Astorri D, et al. SARS-CoV-2 infection in patients with autoimmune rheumatic diseases in northeast Italy: a cross-sectional study on 916 patients. J Autoimmun. 2020;112:102502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Stradner MH, Dejaco C, Zwerina J, et al. Rheumatic musculoskeletal diseases and COVID-19 a review of the first 6 months of the pandemic. Front Med. 2020;7:562142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.D’Antiga L. Coronaviruses and immunosuppressed patients: the facts during the third epidemic. Liver Transpl. 2020;26(6):832–834. [DOI] [PubMed] [Google Scholar]
- 99.Cheng C, Li C, Zhao T, et al. COVID-19 with rheumatic diseases: a report of 5 cases. Clin Rheumatol. 2020;39(7):2025–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Akiyama S, Hamdeh S, Micic D, et al. Prevalence and clinical outcomes of COVID-19 in patients with autoimmune diseases: a systematic review and meta-analysis. Ann Rheum Dis. 2021;80(3):384–391. [DOI] [PubMed] [Google Scholar]
- 101.D’Silva KM, Serling-Boyd N, Wallwork R, et al. Clinical characteristics and outcomes of patients with coronavirus disease 2019 (COVID-19) and rheumatic disease: a comparative cohort study from a US ‘hot spot’. Ann Rheum Dis. 2020;79(9):1156–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Serling-Boyd N, D’Silva KM, Hsu TYT, et al. Coronavirus disease 2019 outcomes among patients with rheumatic diseases 6 months into the pandemic. Ann Rheum Dis. 2020;annrheumdis-2020-219279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cao B, Wang Y, Wen D, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382(19):1787–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cao W, Liu X, Bai T, et al. High-dose intravenous immunoglobulin as a therapeutic option for deteriorating patients with coronavirus disease 2019. Open Forum Infect Dis. 2020;7(3):102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chu CM, Cheng VCC, Hung IFN, et al. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59(3):252–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Stower H. Lopinavir-ritonavir in severe COVID-19. Nat Med. 2020;26(4):465–465. [DOI] [PubMed] [Google Scholar]
- 107.World Health Organization . Clinical management of severe acute respiratory infection when novel coronavirus (2019-nCoV) infection is suspected: interim guidance. 2020. p. 21–21.
- 108.Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel Coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Horby PW, Mafham M, Bell JL, et al. ritonavir in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. The Lancet. 2020;396(10259):1345–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.World Health Organization. WHO discontinues hydroxychloroquine and lopinavir/ritonavir treatment arms for COVID-19 . 2020; [cited 2021 Mar 22]. Available from: https://www.who.int/news-room/detail/04-07-2020-who-discontinues-hydroxychloroquine-and-lopinavir-ritonavir-treatment-arms-for-covid-19.
- 111.Benucci M, Damiani A, Infantino M, et al. Old and new antirheumatic drugs for the treatment of COVID-19. Joint Bone Spine. 2020;87(3):195–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Quartuccio L, Semerano L, Benucci M, et al. Urgent avenues in the treatment of COVID-19: Targeting downstream inflammation to prevent catastrophic syndrome. Joint Bone Spine. 2020;87(3):191–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gøtzsche PC, Johansen HK.. Meta-analysis of short-term low dose prednisolone versus placebo and non-steroidal anti-inflammatory drugs in rheumatoid arthritis. BMJ. 1998;316(7134):811–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dennison EM, Cooper C.. Corticosteroids in rheumatoid arthritis. BMJ. 1998;316(7134):789–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Theoharides TC, Conti P.. Dexamethasone for COVID-19? Not so fast. J Biolog Regul Homeos Ag. 2020;34(3):1–5. [DOI] [PubMed] [Google Scholar]
- 116.Selvaraj V, Dapaah-Afriyie K, Finn A, et al. Short-term dexamethasone in SARS-CoV-2 patients. Rhode Island Med J. 2020;103(6):39–43. [PubMed] [Google Scholar]
- 117.The RECOVERY Collaborative Group . Dexamethasone in hospitalized patients with Covid-19 — Preliminary Report. New Eng J Med. 2020;384:693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Devaux CA, Rolain J-M, Colson P, et al. New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19? Int J Antimicrob Agents. 2020;55(5):105938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yan Y, Zou Z, Sun Y, et al. Anti-malaria drug chloroquine is highly effective in treating avian influenza A H5N1 virus infection in an animal model. Cell Res. 2013;2013/02/0123(2):300–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dowall SD, Bosworth A, Watson R, et al. Chloroquine inhibited Ebola virus replication in vitro but failed to protect against infection and disease in the in vivo guinea pig g model. J Gen Virol. 2015;96(12):3484–3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Li C, Zhu X, Ji X, et al. Chloroquine, a FDA-approved drug, prevents Zika virus infection and its associated congenital microcephaly in mice. EBioMedicine. 2017;24:189–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Vincent MJ, Bergeron E, Benjannet S, et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2(1):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Savarino A, Di Trani L, Donatelli I, et al. New insights into the antiviral effects of chloroquine. Lancet Infect Dis. 2006;6(2):67–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yao X, Ye F, Zhang M, et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2). Clin Infect Dis. 2020;71(15):732–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Liu J, Cao R, Xu M, et al. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020;6(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kim AHJ, Sparks JA, Liew JW, et al. A rush to judgment? Rapid reporting and dissemination of results and its consequences regarding the use of hydroxychloroquine for COVID-19. Ann Intern Med. 2020;172(12):819–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Guastalegname M, Vallone A.. Could chloroquine/hydroxychloroquine be harmful in Coronavirus Disease 2019 (COVID-19) treatment? Clin Infect Dis. 2020;71(15):888–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gautret P, Lagier J-C, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020;56(1):105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 129.Georgiev T. Coronavirus disease 2019 (COVID-19) and anti-rheumatic drugs. Rheumatol Int. 2020;40(5):825–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.van den B, Be Dijkmans BA, de Rooij HH, et al. Chloroquine and hydroxychloroquine equally affect tumor necrosis factor-alpha, interleukin 6, and interferon-gamma production by peripheral blood mononuclear cells. J Rheumatol. 1997;24(1):55–60. [PubMed] [Google Scholar]
- 131.Han J, Zhou Q, Li X, et al. Novel function of hydroxychloroquine: down regulation of T follicular helper cells in collagen-induced arthritis. Biomed Pharmacother. 2018;97:838–843. [DOI] [PubMed] [Google Scholar]
- 132.Rosenberg ES, Dufort EM, Udo T, et al. Association of treatment with hydroxychloroquine or azithromycin with in-hospital mortality in patients with COVID-19 in New York State. JAMA. 2020;323(24):2493–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Li X, Wang Y, Agostinis P, et al. Is hydroxychloroquine beneficial for COVID-19 patients? Cell Death Dis. 2020;11(7):512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cavalcanti AB, Zampieri FG, Rosa RG, et al. Hydroxychloroquine with or without azithromycin in mild-to-moderate Covid-19. N Engl J Med. 2020;383(21):2041–2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.The Pharmaceutical Journal. Down , but not out: hydroxychloroquine could still have a role against COVID-19. 2020.
- 136.Monti M, Vertogen B, Masini C, et al. Hydroxychloroquine as prophylaxis for COVID-19: a review. Front Pharmacol. 2020;11:2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gentry CA, Humphrey MB, Thind SK, et al. Long-term hydroxychloroquine use in patients with rheumatic conditions and development of SARS-CoV-2 infection: a retrospective cohort study. Lancet Rheumatol. 2020;2(11):e689–e697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Konig MF, Kim AHJ, Scheetz MH, et al. Baseline use of hydroxychloroquine in systemic lupus erythematosus does not preclude SARS-CoV-2 infection and severe COVID-19. Ann Rheum Dis. 2020;79(10):1386–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Luo P, Liu Y, Qiu L, et al. Tocilizumab treatment in COVID-19: a single center experience. J Med Virol. 2020;92(7):814–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Choy EH, De Benedetti F, Takeuchi T, et al. Translating IL-6 biology into effective treatments. Nat Rev Rheumatol. 2020;16(6):335–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kremer JM, Blanco R, Brzosko M, et al. Tocilizumab inhibits structural joint damage in rheumatoid arthritis patients with inadequate responses to methotrexate: results from the double-blind treatment phase of a randomized placebo-controlled trial of tocilizumab safety and prevention of structural joint damage at one year. Arthritis Rheum. 2011;63(3):609–621. [DOI] [PubMed] [Google Scholar]
- 142.Emery P, Keystone E, Tony HP, et al. IL-6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-tumour necrosis factor biologicals: results from a 24-week multicentre randomised placebo-controlled trial. Ann Rheum Dis. 2008;67(11):1516–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sheppard M, Laskou F, Stapleton PP, et al. Tocilizumab (Actemra). Hum Vaccin Immunother. 2017;13(9):1972–1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hu B, Zeng L-P, Yang X-L, et al. Discovery of a rich gene pool of bat SARS-related coronaviruses provides new insights into the origin of SARS coronavirus. PLoS Pathog. 2017;13(11):e1006698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Michot JM, Albiges L, Chaput N, et al. Tocilizumab, an anti-IL-6 receptor antibody, to treat COVID-19-related respiratory failure: a case report. Ann Oncol. 2020;31(7):961–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Rizk JG, Kalantar-Zadeh K, Mehra MR, et al. Pharmaco-immunomodulatory therapy in COVID-19. Drugs. 2020;80(13):1267–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Guaraldi G, Meschiari M, Cozzi-Lepri A, et al. Tocilizumab in patients with severe COVID-19: a retrospective cohort study. Lancet Rheumatol. 2020;2(8):e474–e484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Biran N, Ip A, Ahn J, et al. Tocilizumab among patients with COVID-19 in the intensive care unit: a multicentre observational study. Lancet Rheumatol. 2020;2(10):e603–e612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Salama C, Han J, Yau L, et al. Tocilizumab in patients hospitalized with Covid-19 pneumonia. N Engl J Med. 2021;384(1):20–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Stone JH, Frigault MJ, Serling-Boyd NJ, et al. Efficacy of tocilizumab in patients hospitalized with Covid-19. N Engl J Med. 2020;383(24):2333–2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Salvarani C, Dolci G, Massari M, et al. Effect of tocilizumab vs standard care on clinical worsening in patients hospitalized with COVID-19 pneumonia: a randomized clinical trial. JAMA Intern Med. 2021;181(1):24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Rosas I, Bräu N, Waters M, et al. Tocilizumab in hospitalized patients with COVID-19 Pneumonia. medRxiv. 2020. 2020.08.27.20183442.
- 153.Hermine O, Mariette X, Tharaux P-L, et al. Effect of tocilizumab vs usual care in adults hospitalized with COVID-19 and moderate or severe pneumonia: a randomized clinical trial. JAMA Intern Med. 2021;181(1):32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Samaee H, Mohsenzadegan M, Ala S, et al. Tocilizumab for treatment patients with COVID-19: recommended medication for novel disease. Int Immunopharmacol. 2020;89(Pt A):107018–107018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wang J, Bansal AT, Martin M, et al. Genome-wide association analysis implicates the involvement of eight loci with response to tocilizumab for the treatment of rheumatoid arthritis. Pharmacogenomics J. 2013;13(3):235–241. [DOI] [PubMed] [Google Scholar]
- 156.Maldonado-Montoro M, Cañadas-Garre M, González-Utrilla A, et al. Genetic and clinical biomarkers of tocilizumab response in patients with rheumatoid arthritis. Pharmacol Res. 2016;111:264–271. [DOI] [PubMed] [Google Scholar]
- 157.Jiménez Morales A, Maldonado-Montoro M, Martínez de la Plata JE, et al. FCGR2A/FCGR3A Gene polymorphisms and clinical variables as predictors of response to tocilizumab and rituximab in patients with rheumatoid arthritis. J Clin Pharmacol. 2019;59(4):517–531. [DOI] [PubMed] [Google Scholar]
- 158.Luxembourger C, Ruyssen-Witrand A, Ladhari C, et al. A single nucleotide polymorphism of IL6-receptor is associated with response to tocilizumab in rheumatoid arthritis patients. Pharmacogenomics J. 2019;19(4):368–374. [DOI] [PubMed] [Google Scholar]
- 159.Maldonado-Montoro M, Cañadas-Garre M, González-Utrilla A, et al. Influence of IL6R gene polymorphisms in the effectiveness to treatment with tocilizumab in rheumatoid arthritis. Pharmacogenomics J. 2018;18(1):167–172. [DOI] [PubMed] [Google Scholar]
- 160.Gremese E, Cingolani A, Bosello SL, et al. Sarilumab use in severe SARS-CoV-2 pneumonia. EClinicalMedicine. 2020;27:100553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Della-Torre E, Campochiaro C, Cavalli G, et al. Interleukin-6 blockade with sarilumab in severe COVID-19 pneumonia with systemic hyperinflammation: an open-label cohort study. Ann Rheum Dis. 2020;79(10):1277–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Cavalli G, De Luca G, Campochiaro C, et al. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. Lancet Rheumatol. 2020;2(6):e325–e331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Haga S, Yamamoto N, Nakai-Murakami C, et al. Modulation of TNF-alpha-converting enzyme by the spike protein of SARS-CoV and ACE2 induces TNF-alpha production and facilitates viral entry. Proc Natl Acad Sci USA. 2008;105(22):7809–7814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wang W, Ye L, Ye L, et al. Up-regulation of IL-6 and TNF-alpha induced by SARS-Coronavirus spike protein in murine macrophages via NF-kappaB pathway. Virus Res. 2007;128(1–2):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Mahase E. Covid-19: what treatments are being investigated? BMJ. 2020;368:m1252. [DOI] [PubMed] [Google Scholar]
- 166.Robinson PC, Liew DFL, Liew JW, et al. The potential for repurposing anti-TNF as a therapy for the treatment of COVID-19. Med. 2020;1(1):90–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Robinson PC, Richards D, Tanner HL, et al. Accumulating evidence suggests anti-TNF therapy needs to be given trial priority in COVID-19 treatment. Lancet Rheumatol. 2020;2(11):e653–e655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Feldmann M, Maini RN, Woody JN, et al. Trials of anti-tumour necrosis factor therapy for COVID-19 are urgently needed. Lancet. 2020;395(10234):1407–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Richardson P, Griffin I, Tucker C, et al. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet. 2020;395(10223):e30–e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Stebbing J, Krishnan V, Bono S, et al. Mechanism of baricitinib supports artificial intelligence-predicted testing in COVID-19 patients. EMBO Mol Med. 2020;12(8):2020697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sorrell Fiona J, Szklarz M, Abdul Azeez Kamal R, et al. Family-wide structural analysis of human numb-associated protein kinases. Structure. 2016;24(3):401–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kalil AC, Patterson TF, Mehta AK, et al. Baricitinib plus Remdesivir for hospitalized adults with Covid-19. N Engl J Med. 2021;384(9):795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Deftereos SG, Siasos G, Giannopoulos G, et al. The Greek study in the effects of colchicine in COvid-19 complications prevention (GRECCO-19 study): rationale and study design. Hellenic J Cardiol. 2020;61(1):42–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ferro F, Elefante E, Baldini C, et al. COVID-19: the new challenge for rheumatologists. Clin Exp Rheumatol. 2020;38(2):175–180. [PubMed] [Google Scholar]
- 176.Décary S, Barton JL, Proulx L, et al. How to effectively support patients with rheumatic conditions now and beyond COVID-19. ACR Open Rheuma. 2020;2(9):505–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Zhang Y, Wang J, Zhao L, et al. Online management of rheumatoid arthritis during COVID-19 pandemic. Ann Rheum Dis. 2021;80(1):e4–e4. [DOI] [PubMed] [Google Scholar]
- 178.McInnes IB. COVID-19 and rheumatology: first steps towards a different future? Ann Rheum Dis. 2020;79(5):551–552. [DOI] [PubMed] [Google Scholar]