ABSTRACT
Objectives
Currently published papers and clinical guidelines regarding the effects of tocilizumab in severe and critical COVID-19 are contradictory. The aim of this meta-analysis was to combine the results of clinical studies of different designs to investigate the efficacy and safety of tocilizumab in severely-to-critically ill COVID-19 patients.
Methods
A systematic search was performed in PubMed, Embase, CENTRAL, ClinicalTrials.gov, Scopus, and preprint servers up to 26 December 2020. Since a substantial heterogeneity was expected, a random-effects model was applied to calculate the pooled effect size (ES) and 95% confidence interval (CI) for each study outcome.
Results
Forty-five comparative studies involving 13,189 patients and 28 single-arm studies involving 1,770 patients were analyzed. The risk of mortality (RR of 0.76 [95%CI 0.65 to 0.89], P < 0.01) and intubation (RR of 0.48 [95%CI 0.24 to 0.97], P = 0.04) were lower in tocilizumab patients compared with controls. We did not find any significant difference in secondary infections, length of hospital stay, hospital discharge before day 14, and ICU admission between groups.
Conclusion
Tocilizumab can improve clinical outcomes and reduce mortality rates in severe to critical COVID-19 patients. Large-scale randomized controlled trials are still required to improve the statistical power of meta-analysis.
KEYWORDS: ARDS, covid-19, intubation, meta-analysis, mortality, tocilizumab
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) is a newly emerging pathogen. According to a World Health Organization (WHO) report, since its first identification in late 2019, SARS-CoV-2 has caused over 107.8 million confirmed infections and over 2.3 million deaths globally as of 13 February 2021. The WHO declared the coronavirus disease 2019 (COVID-19) a pandemic on 11 March 2020 [1].
The clinical manifestation of COVID-19 ranges from asymptomatic to severe pneumonia and acute respiratory distress syndrome (ARDS) [2]. Several studies have reported a massive release of inflammatory mediators, such as interleukin‐6 (IL‐6), in response to the SARS-COV-2 infection. This may lead to a cytokine storm in severely-to-critically ill COVID-19 patients [3]. The significant role of IL-6 in the COVID-19 inflammatory pathogenesis has been established in the early studies on severely-to-critically ill patients [4]. Hence, tocilizumab, an IL-6 inhibitor with an approved clinical indication in cytokine release syndrome (CRS) [5], has been evaluated in severe to critical COVID-19 patients by various clinical research teams around the world. The China National Health Commission Guidelines were the first to include tocilizumab in the treatment plan of COVID-19 patients [6]. The status of the National Institutes of Health (NIH) was neither for nor against tocilizumab until July 2020; however, NIH decided to recommend against the use of this medication on its later updates [7]. Most recently, the UK’s National Institute for Health Research (NIHR) supported the use of tocilizumab for critically ill COVID-19 patients based on the results of the REMAP-CAP trial (they reported a 24% relative reduction in the risk of mortality; unpublished data) [8].15,44
Data on the clinical safety and efficacy of tocilizumab in COVID-19 patients is rapidly growing. Hence, we performed an updated meta-analysis to combine the results of clinical studies of different designs to further investigate the potential benefits and harms of tocilizumab treatment in severely-to-critically ill COVID-19 patients.
2. Methods
2.1. Protocol and registration
This systematic review and meta-analysis study was conducted and reported following the Preferred Reporting Items for Systematic reviews and Meta-Analyses checklists (PRISMA). The study protocol was prospectively registered in the PROSPERO database (CRD42020203461) and can be accessed on https://www.crd.york.ac.uk/prospero/.
2.2. Eligibility criteria
For this systematic review and meta-analysis, studies were selected based on the following population (P), intervention (I), comparison (C), and outcomes (O) (PICO) criteria: P, hospitalized patients with a confirmed diagnosis of COVID-19; I, intravenous tocilizumab; C, any comparator provided as standard-of-care (SOC) or placebo, and O, mortality rate. We included comparative studies, including randomized controlled trials (RCTs), case–control studies, and cohort studies. Moreover, we analyzed single-arm observational studies in separate analyses. Other published literature, including editorials, letters to the editor, commentaries, case series, case reports, specific populations, and reviews (of any type) were excluded.
COVID-19 patients with an oxygen saturation of 93% or less while breathing room air, a respiratory rate of 30 breaths/min or more, a ratio of arterial oxygen partial pressure to fractional inspired oxygen (PaO2/FIO2) of below 300 mmHg or lung infiltrates of more than 50% were considered as severe. COVID-19 patients with shock, organ failure, or ARDS requiring mechanical ventilation, and any patient requiring admission to the intensive care unit (ICU) were considered as critical [9,10].
2.3. Information sources
Potential studies were identified through a systematic search of online databases, including PubMed, Embase, CENTRAL, ClinicalTrials.gov, Scopus, and preprint servers, including medRxiv, bioRxiv, and SSRN up to 26 December 2020.
2.4. Search
Generally, following search keywords were used: ‘tocilizumab’, ‘actemra’, ‘IL-6 blocker’, ‘IL-6 blockade therapy’, ‘anti-interleukin-6 therapy’, ‘IL-6 inhibitor’, ‘COVID19’, ‘COVID-19’, ‘SARS-CoV-2’, ‘severe acute respiratory syndrome Coronavirus type 2’, ‘Coronavirus disease 2019’, ‘2019-nCoV’, ‘novel coronavirus’, ‘emerging coronavirus’, and ‘Wuhan coronavirus’. Search strategies used in these databases are available in Supplementary file 1.
2.5. Data collection process
Four reviewers (SR, BF, ZM, and HM) independently selected the eligible studies and collected the following data when available: study design, patient demographics, disease characteristics, and the outcomes of interest (mortality, ICU admission, intubation, length of hospital stay, hospital discharge before day 14, clinical improvement, and secondary infections). The reviewers extracted data from the texts, tables, and graphs of the included studies. Any disagreements were resolved by the two senior reviewers (SR and BF).
2.6. Risk of bias in individual studies
Four reviewers (SR, BF, ZM, and HM) independently assessed all the included studies for the risk of bias (RoB). Disagreements regarding RoB were resolved by discussion and consensus. The Cochrane risk-of-bias tool for RCTs (RoB 2) was used to assess the RoB in the RCTs; the Newcastle Ottawa Scale (NOS) tool was used to evaluate the RoB in the comparative observational studies; and an adjusted NOS tool was used to assess the RoB in the single-arm observational studies.
2.7. Summary measures
In this meta-analysis, we calculated the pooled proportions, standardized mean differences (SMDs), and relative risks (RRs) for the study outcomes based on the design of the included studies.
2.8. Synthesis of results
Heterogeneity across the included studies was evaluated using the inconsistency index I2. We used the DerSimonian and Laird random-effects model because of the significant heterogeneity among studies [11–13]. The combined effect size (ES) and its 95% confidence interval (CI) for each outcome of interest were calculated using numbers of events in both tocilizumab cases (tocilizumab plus SOC) and controls (SOC). Subgroup meta-analysis of study outcomes was also performed based on the study design (RCT, cohort, and case–control studies). We also evaluated the single-arm observational studies in addition to the comparative studies to use all the available evidence. To achieve a better understanding of the results, a systematically matched SOC group was created for the single-arm studies using the SOC group of the included comparative studies. Patients in the single-arm studies and the systematically matched SOC group were statistically similar in terms of age, sex, and disease severity. Proportions and means were compared between the single-arm studies and the matched group using a two-proportion z-test and a Student’s t-test, respectively.
2.9. Risk of bias across studies
The potential risk of publication bias was assessed by visually inspecting the funnel plots for each of the study outcomes. In this approach, we plotted the logarithm of the effect sizes against their standard errors.
2.10. Additional analyses
Meta-regression analyses were performed to evaluate the effects of sex, age, study design, and baseline disease stage. All the statistical analyses were conducted using STATA 14. Differences were considered significant if P < 0.050.
3. Results
3.1. Study selection
Figure 1 illustrates the results of our search strategy (PRISMA flow diagram). A total of 3364 articles was identified through a systematic search of online databases. After removing duplications, 1224 articles remained. Based on the eligibility criteria, 73 articles were finally selected for this systematic review and meta-analysis.
Figure 1.
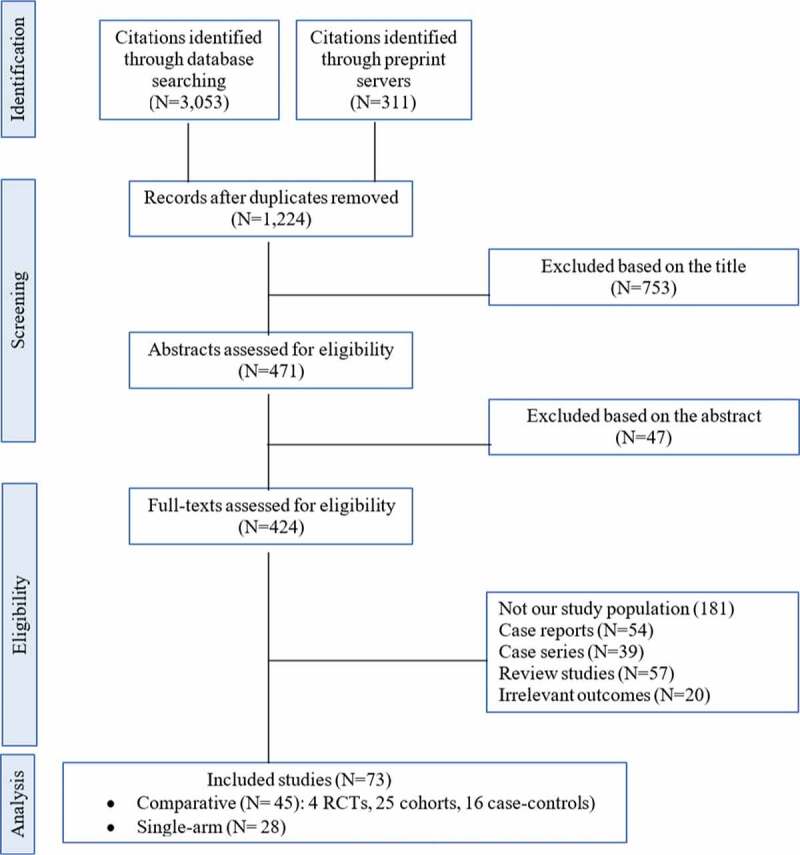
PRISMA Flow diagram of selecting studies for meta-analysis. RCT, randomized controlled trial
3.2. Study characteristics
Of 1224 citations, 45 comparative studies, including four RCTs [14–17], 25 cohort studies [18–42], and 16 case–control studies [43–58], were included. A total of 13,189 patients were involved in these studies, of which 3,999 received tocilizumab plus SOC and 9,190 received SOC alone. All these studies assessed the effect of tocilizumab administration in patients with severe and/or critical COVID-19. Patel et al. [40] reported the clinical outcomes of the two groups of severe and critical COVID-19 patients separately. Therefore, we have considered them as two separate studies. Moreover, 28 single-arm observational studies [59–86] were included in this analysis. Knorr et al. [82] classified the patients into two groups of severe and critical COVID-19, and we have considered these two groups as two separate studies.
The mean age of patients in the studies was 63.14 ± 5.2 years, and more than half (64%) were male. The mean time of follow-up was 27.7 ± 13.4 days. Table 1 illustrates the baseline characteristics of patients in the included comparative studies. Increased serum levels of IL-6 and/or C-reactive protein (CRP), that indicate the presence of CRS, were among the main eligibility criteria for all the included studies.
Table 1.
Baseline characteristics of included comparative studies
| No. | First author | Country | Study Design | Disease stage | Age, mean (SD) |
Group size, N |
Sex, male, % |
|||
|---|---|---|---|---|---|---|---|---|---|---|
| TOZ+SOC | SOC | TOZ+SOC | SOC | TOZ+SOC | SOC | |||||
| 1 | Salvarani | Italy | RCT | Severe | 61 (16.7) | 60 (11.3) | 60 | 63 | 66.7 | 58.7 |
| 2 | Stone | USA | RCT | Severe | 61 (17.4) | 56 (17.4) | 161 | 82 | 59.6 | 54.9 |
| 3 | Hermine | France | RCT | Severe | 65.2 (13) | 64.2 (11.5) | 63 | 67 | 69.8 | 65.7 |
| 4 | Rosas | USA | RCT | Severe-Critical | 60.9 (14.6) | 60.6(13.7) | 294 | 144 | 69.7 | 70.1 |
| 5 | Campochiaro | Italy | Cohort | Severe | 64 (17) | 60 (15.8) | 32 | 33 | 90.6 | 81.8 |
| 6 | Somers | USA | Cohort | Critical | 55 (14.9) | 60 (14.5) | 78 | 76 | 67.9 | 64.5 |
| 7 | Wadud | USA | Cohort | Critical | 71.5 (13) | 84.5 (15.6) | 44 | 50 | 84.1 | 70.0 |
| 8 | Guaraldi | Italy | Cohort | Severe | 64 (13.4) | 69 (15.6) | 179 | 365 | 70.9 | 63.6 |
| 9 | Ip | USA | Cohort | Critical | 62 (12.7) | 69 (14.1) | 134 | 413 | 73.9 | 62.2 |
| 10 | Kimmig | USA | Cohort | Critical | 64 (14.2) | 62 (15.9) | 54 | 57 | 63.0 | 49.1 |
| 11 | Maeda | USA | Cohort | Severe | 66 (NA) | 66 (NA) | 23 | 201 | NA | NA |
| 12 | Moreno-García | Spain | Cohort | Severe-Critical | 61 (12) | 61 (16) | 77 | 94 | 68.8 | 62.8 |
| 13 | Martínez-Sanz | Spain | Cohort | Severe-Critical | 65 (15.6) | 68 (17) | 260 | 969 | 73.5 | 59.2 |
| 14 | Mikulska | Italy | Cohort | Severe | 64.5 (12.4) | 73.5 (14.4) | 130 | 66 | 71.5 | 62.1 |
| 15 | Biran | USA | Cohort | Severe | 62 (13.4) | 65 (13.3) | 210 | 420 | 73.8 | 66.9 |
| 16 | Fisher | USA | Cohort | Critical | 56.2 (14.7) | 60.6 (13.4) | 45 | 70 | 64.4 | 72.9 |
| 17 | Gupta | USA | Cohort | Critical | 62 (14.8) | 62 (14) | 443 | 3491 | 61.2 | 62.9 |
| 18 | Rodríguez-Bano | Spain | Cohort | Severe | 66 (12) | 69 (12.6) | 88 | 344 | 45.5 | 68.9 |
| 19 | Roomi | USA | Cohort | Severe | 65 (NA) | 58 (NA) | 32 | 144 | 187.5 | 16.0 |
| 20 | Rossi | France | Cohort | Severe | 64 (13) | 70 (16.5) | 106 | 140 | 66.0 | 57.9 |
| 21 | Ruiz-Antoran | Spain | Cohort | Severe | 66.6 (10.7) | 67.3 (14.8) | 268 | 238 | 68.7 | 58.8 |
| 22 | Tsai | USA | Cohort | Severe-Critical | 61 (13.5) | 63 (17.2) | 84 | 190 | 75.0 | 55.3 |
| 23 | Zheng | china | Cohort | Severe-Critical | 68 (12.5) | 66 (12.2) | 92 | 89 | 62.0 | 52.8 |
| 24 | Hill | USA | Cohort | Severe | NA | NA | 43 | 45 | 69.8 | 68.9 |
| 25 | Kewan | USA | Cohort | Severe | 62 (4.47) | 68.6 (5.18) | 28 | 23 | 71.4 | 47.8 |
| 26 | Gould | USA | Cohort | Critical | 59.8 (11.7) | 58.8 (12.7) | 52 | 41 | 86.5 | 68.3 |
| 27 | Masia | Spain | Cohort | Severe | 65.4 (15.2) | 65.7 (17.3) | 76 | 62 | 71.1 | 50.0 |
| 28 | Patel | USA | Cohort | Severe | 70.8 (18.7) | 70.0 (18.7) | 21 | 21 | 42.9 | 47.6 |
| Critical | 60.1 (10.5) | 60.3 (11.2) | 21 | 20 | 52.4 | 55.0 | ||||
| 29 | Perrone | Italy | Cohort | Severe-Critical | 63.3 (NA) | 70.3 (NA) | 41 | 38 | 70.7 | 71.1 |
| 30 | Canziani | Italy | Case-control | Severe-Critical | 63 (12) | 64 (8) | 64 | 64 | 73.4 | 73.4 |
| 31 | Capra | Italy | Case-control | Severe | 63.0 (4.0) | 69.3 (6.4) | 62 | 23 | 72.6 | 82.6 |
| 32 | Colaneri | Italy | Case-control | Severe | 62.3 (9.8) | 63.7 (6.7) | 21 | 91 | 90.5 | 69.2 |
| 33 | Carvalho | Brazil | Case-control | Severe | 54.6 (16.3) | 60.2 (15.6) | 29 | 24 | 62.1 | 75.0 |
| 34 | Gokhale | India | Case-control | Severe | 50.9 (9.8) | 56 (12.8) | 70 | 91 | 67.1 | 58.2 |
| 35 | Rojas-Marte | USA | Case-control | Severe-Critical | 58.8 (13.6) | 62 (14) | 96 | 97 | 77.1 | 64.9 |
| 36 | Roumier | France | Case-control | Severe-Critical | 58.8 (12.4) | 71.2 (15.4) | 30 | 29 | 80.0 | 79.3 |
| 37 | Klopfensteina | France | Case-control | Severe | 76.8 (11) | 70.7 (15) | 20 | 25 | NA | NA |
| 38 | Rossotti | Italy | Case-control | Severe-Critical | 60.4 (15.1) | 60.4 (13.4) | 74 | 148 | 82.4 | 81.1 |
| 39 | Ramaswamy | USA | Case-control | Severe | 63.2 (15.6) | 63.8 (15.9) | 21 | 65 | 61.9 | 55.4 |
| 40 | Ramiro | Netherlands | Case-control | Severe-Critical | 67 (12) | 67 (11) | 86 | 86 | 79.1 | 79.1 |
| 41 | Klopfenstein | France | Case-control | Critical | 75.6 (11.3) | 74.3 (11) | 30 | 176 | 70.0 | 59.1 |
| 42 | Menzella | Italy | Case-control | Severe | 63.3 (10.6) | 70.3 (11.3) | 41 | 38 | 70.7 | 71.1 |
| 43 | Nasa | UAE | Case-control | Severe-Critical | 51 (NA) | 52 (NA) | 22 | 63 | 100.0 | 95.2 |
| 44 | Okoh | USA | Case-control | Severe | 53.2 (19.1) | 61.4 (19.2) | 20 | 40 | 50.0 | 60.0 |
| 45 | Pettit | USA | Case-control | Critical | 66 (13.7) | 65 (16.3) | 74 | 74 | 58.1 | 44.6 |
NA, non-available; RCT, randomized controlled trial; SD, standard deviation; SOC, standard-of-care; TOZ, Tocilizumab; UAE, United Arab Emirates; USA, United States of America
Mortality was reported in all the included comparative studies. Secondary infections, hospital discharge before day 14, intubation, length of hospital stay, ICU admission, and clinical improvement were reported in 22, 15, 13, 10, 8, and 6 studies, respectively (Supplementary file 2).
The included single-arm observational studies involved 1,770 tocilizumab-treated patients (69.9% male) with a mean age of 61.3 ± 5.7 years and a mean follow-up time of 22.37 ± 12.64 days (Supplementary file 3). Mortality was reported in all these studies. Clinical improvement, hospital discharge before day 14, intubation, length of hospital stay, and secondary infections were reported in 12, 10, 9, 9, and 5 studies, respectively (Supplementary file 4).
3.3. Risk of bias within studies
Based on the RoB 2 tool, except for the RCT-TCZ-COVID-19 study [14] with a moderate risk of bias, all the included RCTs had a low risk (Supplementary file 5). Based on the NOS risk of bias tool, 22 of the 41 comparative observational studies were at moderate risk of bias, and the other 19 studies were at low risk of bias (Supplementary file 6). All the 28 single-arm studies had a fair methodological quality based on the adjusted NOS tool. Many of the comparative and single-arm trials did not report on patient withdrawals, and this was the main cause of bias among the included studies (Supplementary file 7).
3.4. Results of individual studies and synthesis of results
3.4.1. Mortality
Pooling all the 45 comparative reports (four RCTs, 25 cohorts, and 16 case-controls) yielded a RR of mortality of 0.76 (95% CI 0.65 to 0.89, P < 0.01, I2 = 75.7%), corresponding to a number needed to treat (NNT) of 10 (95% CI 9 to 11) (Figure 2).
Figure 2.
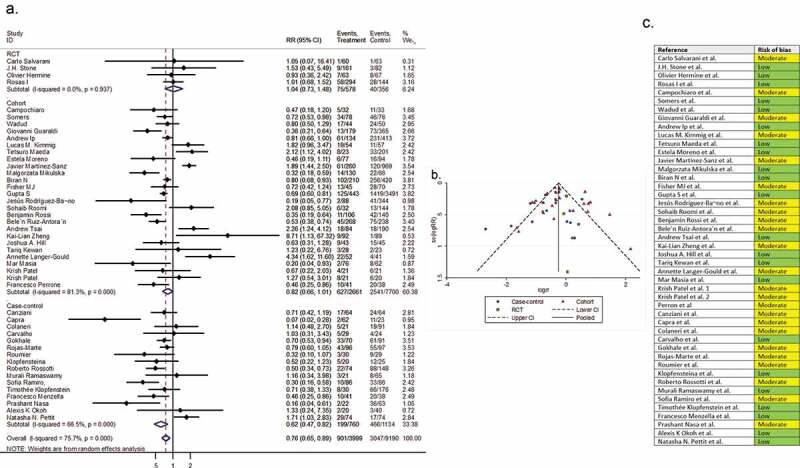
(A) Forest plot of pooled RR of mortality; (B) Funnel plot with pseudo 95% confidence limits; (C) Risk of bias across studies. CI, confidence interval; RCT, randomized controlled trial; RR, relative risk
Pooling all the 29 single-arm observational reports yielded a mortality rate of 0.20 (95% CI 0.15 to 0.26) in tocilizumab-treated patients. The systematically matched SOC group’s mortality rate was 0.27 (95% CI 0.22 to 0.33) (Supplementary file 8, parts A & B). There was no significant difference between tocilizumab-treated patients and the systematically matched SOC group in mortality rates (P = 0.49).
3.4.2. Clinical improvement
Six comparative studies (one RCT, four cohorts, and one case-control) with a total of 487 patients reported clinical improvement as a secondary outcome variable. The pooled RR of clinical improvement was 1.19 (95% CI 1.00 to 1.42; P = 0.05, I2 = 81.2%) (Figure 3).
Figure 3.
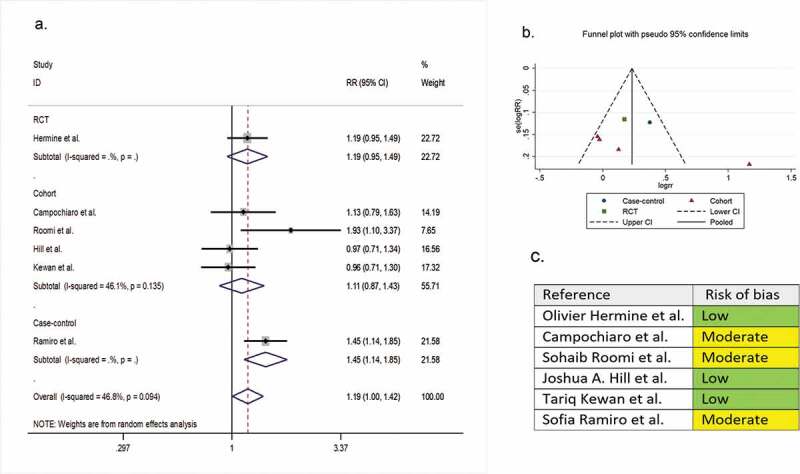
(A) Forest plot of pooled RR of clinical improvement; (B) Funnel plot with pseudo 95% confidence limits; (C) Risk of bias across studies. CI, confidence interval; RCT, randomized controlled trial; RR, relative risk
Twelve single-arm studies with a total of 633 patients reported the clinical improvement as a secondary outcome variable. The pooled proportion of clinical improvement in the tocilizumab-treated patients and the systematically matched SOC group were 0.58 (95% CI 0.44 to 0.71) and 0.55 (95% CI 0.35 to 0.75), respectively (P = 0.90) (Supplementary file 8, parts C & D).
3.4.3. Intubation
Ten comparative studies (two RCTs, one cohort, and seven case-controls) with a total of 1,612 patients reported the need for intubation as a secondary outcome variable. The pooled RR of intubation was 0.48 (95% CI 0.24 to 0.97; P = 0.04; I2 = 74.5%) (Figure 4).
Figure 4.
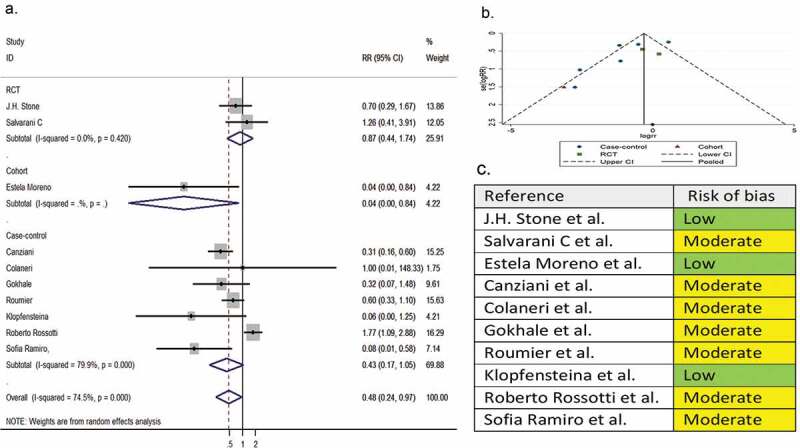
(A) Forest plot of pooled RR of intubation; (B) Funnel plot with pseudo 95% confidence limits; (C) Risk of bias across studies. CI, confidence interval; RCT, randomized controlled trial; RR, relative risk
Nine single-arm observational studies with a total of 641 patients reported intubation as a secondary outcome variable. The pooled proportion of intubation in the tocilizumab-treated patients and the systematically matched SOC group were 0.15 (95%CI 0.09 to 0.20) and 0.17 (95% CI 0.10 to 0.26), respectively (P = 0.90) (Supplementary file 8, parts E & F).
3.4.4. Length of hospital stay
Ten comparative studies (one RCT, three cohorts, and six case-controls) involving 1,583 patients compared the length of hospital stay in the tocilizumab group with the SOC group. The pooled SMD was 0.10 (95% CI −0.38 to 0.58; P = 0.58; I2 = 94.4%) (Figure 5).
Figure 5.
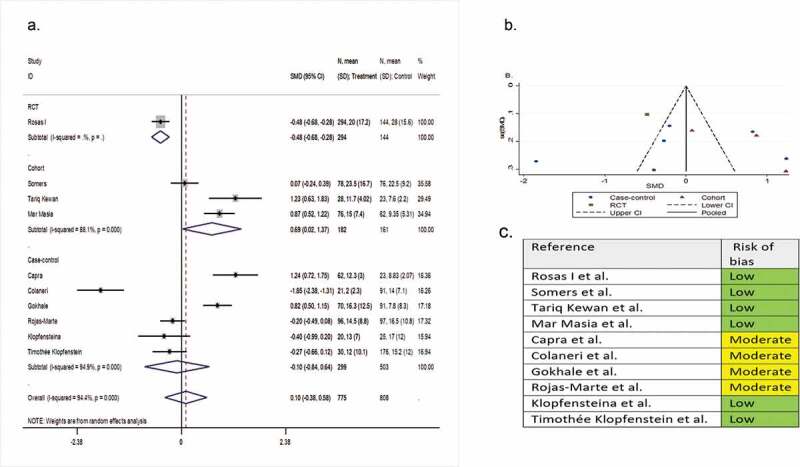
(A) Forest plot of SMD of hospital length of stay; (B) Funnel plot with pseudo 95% confidence limits; (C) Risk of bias across studies. CI, confidence interval; RCT, randomized controlled trial; SMD, standardized mean difference
Seven single-arm observational studies involving 506 patients reported length of hospital stay as a secondary outcome variable. The mean ± SD length of stay was 10.82 ± 5.18 days and 16.56 ± 11.13 days for the tocilizumab-treated and the systematically matched SOC patients, respectively (P = 0.23).
3.4.5. Hospital discharge before day 14
Fifteen comparative studies (two RCTs, nine cohorts, and four case-controls) involving 2,383 patients reported hospital discharge before day 14 as a secondary outcome variable. The pooled RR of hospital discharge before day 14 was 1.09 (95% CI 0.88 to 1.35; P = 0.44; I2 = 79.6%) (Figure 6).
Figure 6.
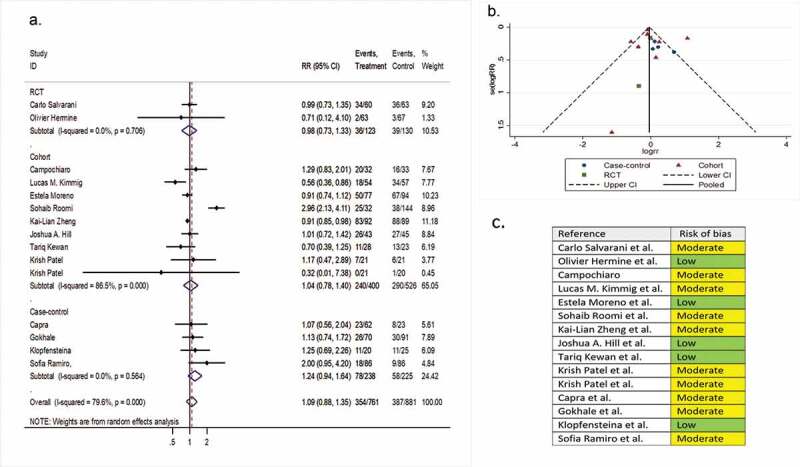
(A) Forest plot of RR of discharging before day 14; (B) Funnel plot with pseudo 95% confidence limits; (C) Risk of bias across studies. CI, confidence interval; RCT, randomized controlled trial; RR, relative risk
Ten single-arm studies involving 624 patients investigated hospital discharge before day 14 as a secondary outcome variable. The pooled proportion of patients discharged before day 14 in the tocilizumab and the systematically matched SOC groups were 0.43 (95% CI 0.28 to 0.60) and 0.42 (95% CI 0.22 to 0.60), respectively (P = 0.96) (Supplementary file 8, part G & H).
3.4.6. ICU admission
Eight comparative studies (two RCTs, three cohorts, and three case-controls) involving 2,233 patients reported ICU admission as a secondary outcome variable. The pooled RR of ICU admission was 0.98 (95% CI 0.36 to 2.66; P = 0.99; I2 = 89.4%) (Figure 7).
Figure 7.
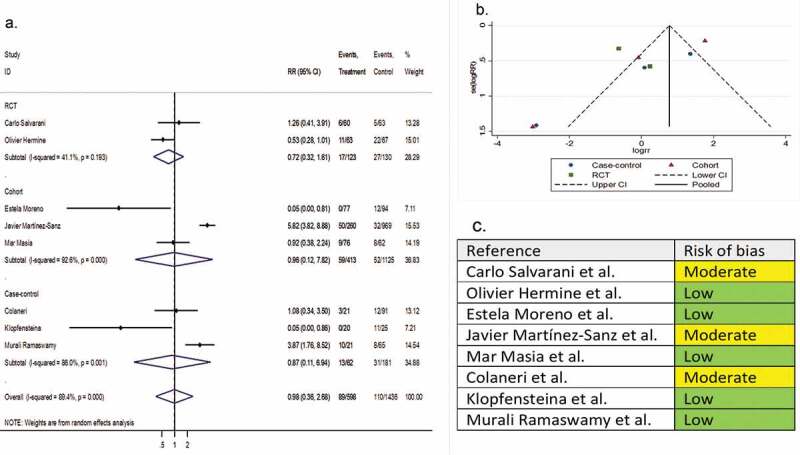
(A) Forest plot of RR of ICU admission; (B) Funnel plot with pseudo 95% confidence limits; (C) Risk of bias across studies. CI, confidence interval; RCT, randomized controlled trial; RR, relative risk
Four single-arm studies involving 248 patients reported the ICU admission rates as a secondary outcome variable. The pooled proportion for the tocilizumab-treated patients and the systematically matched SOC group were 0.21 (95% CI 0.15 to 0.28) and 0.15 (95% CI 0.07 to 0.25), respectively (P = 0.79) (Supplementary file 8, part I & J).
3.4.7. Secondary infections
Twenty-three comparative observational studies (4 RCTs, 12 cohorts, and 7 case-controls) involving 8,660 patients reported infection as a secondary outcome variable. The pooled RR of infection was 1.24 (95% CI 0.98 to 1.56; P = 0.07; I2 = 66.5%) (Figure 8).
Figure 8.
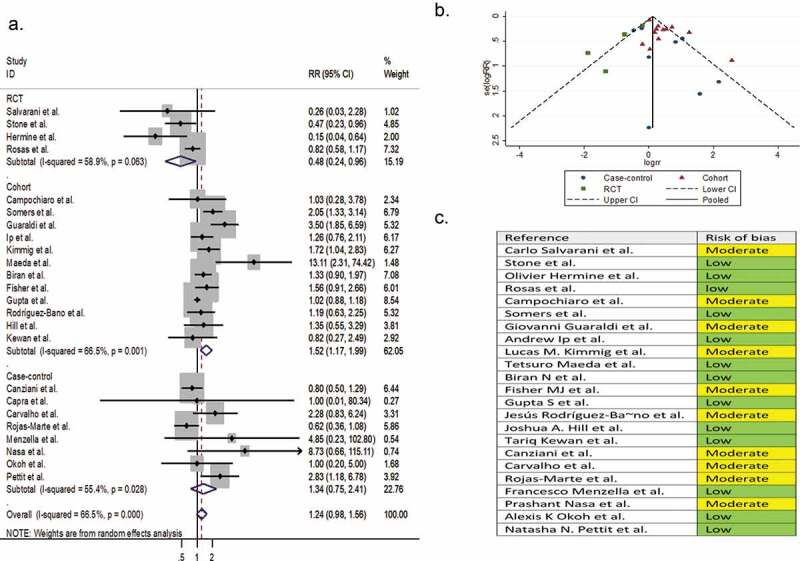
(A) Forest plot of pooled RR of infection; (B) Funnel plot with pseudo 95% confidence limits; (C) Risk of bias across studies. CI, confidence interval; RCT, randomized controlled trial; RR, relative risk
Five single-arm studies involving 404 patients reported infection as a secondary outcome variable. The pooled proportion of infection in the tocilizumab-treated patients and the systematically matched SOC group were 0.16 (95% CI 0.05 to 0.31) and 0.13 (95% CI 0.07 to 0.19), respectively (P = 0.86) (Supplementary file 8, parts K & L).
3.5. Risk of bias across studies
In the assessment of mortality, the symmetry of the funnel plot suggested no publication bias (part B of Figure 2). However, in the assessment of clinical improvement, intubation, secondary infection, length of hospital stay, hospital discharge before day 14, and ICU admission, the asymmetry of the funnel plots suggested possible publication bias (part B of Figure 3–8). In addition, the risk of bias for each outcome of interest in each included study is shown in part C of Figure 2–8.
3.6. Additional analysis
In meta-regression, we did not find any association between the RR of mortality between tocilizumab and control patients and the independent variables of sex, age, study design, and stage of the disease (P > 0.05).
4. Discussion
4.1. Summary of evidence
Since the beginning of the COVID-19 pandemic, several anti-inflammatory agents have been evaluated to dampen the cytokine storm following SARS-CoV-2 infection. Tocilizumab was among the most noticed immunomodulatory drugs. In this systematic review and meta-analysis study, we investigated the potential harms and benefits of the tocilizumab treatment in COVID-19 patients based on the most updated and comprehensive data available in the literature.
Our meta-analysis demonstrated a lower risk of mortality with tocilizumab treatment (RR [95%CI] of 0.76 [0.65, 0.89]). The NNT value of 10 in our analysis suggests that one death is prevented with every 10 severely-to-critically ill patients treated with tocilizumab. Similar results were obtained by previous meta-analysis studies on the effect of tocilizumab treatment on the mortality risk of COVID-19 patients. Khan et al. [87], Rubio-Rivas et al. [88], and Kotak et al. [89] reported RRs [95%CIs] of 0.83 [0.72, 0.96], 0.73 [0.57, 0.93], and 0.56 [0.34, 0.92], respectively, and Zhao et al. [90] and Sarfraz et al. [91] reported odds ratios [95%CIs] of 0.44 [0.36, 0.55] and 0.42 [0.26, 0.69], respectively, with tocilizumab treatment compared with SOC. Despite the promising results in these meta-analysis studies, none of the four published RCTs found a significant beneficial effect on mortality rates for tocilizumab in COVID-19 patients. The subgroup meta-analysis of RCTs (Figure 2) for the risk of death yielded an RR [95%CI] of 1.04 [0.73, 1.48] in our study. Although RCTs and meta-analyses of RCTs are at the top of the hierarchy of evidence for treatment effectiveness, wide 95%CIs reported in the four included RCTs in our meta-analysis show the great levels of uncertainty in their results. Accordingly, these trials cannot rule out either the benefits or harms of tocilizumab treatment on mortality rates in severe to critical COVID-19. Hence, we conducted an updated meta-analysis by combining the results of comparative studies of different types (RCTs, cohort studies, and case–control studies) to provide further conclusions on the impact of tocilizumab treatment on COVID-19 outcomes. Moreover, the comparison of single-arm studies with the systematically matched group showed a 7% reduction in the risk of death with tocilizumab treatment; however, this reduction was not statistically significant.
Regarding secondary efficacy outcomes, our meta-analysis demonstrated that tocilizumab may have some beneficial impacts on the oxygen-support status of severely-to-critically ill COVID-19 patients as it decreased the need for invasive mechanical ventilation with a RR [95%CI] of 0.48 [0.24, 0. 97]. Tleyjeh et al. [92] in the meta-analysis of four RCTs reported a pooled RR [95%CI] of 0.71 [0.52, 0.96] for the effect of tocilizumab on mechanical ventilation. Similarly, Kotak et al. [89] demonstrated a lower risk of the need for intubation with a RR [95%CI] of 0.34 [0.12, 0.99]; and Aziz et al. [93] reported lower rates of mechanical ventilation with a risk difference [95%CI] of −0.11 [−0.19, −0.02] in tocilizumab patients. In a recent retrospective study, Salvati et al. [94] found improved alveolar-arterial oxygen gradient and pulmonary vascular radiologic score in severe COVID-19 patients 1 week after tocilizumab treatment. These data show that tocilizumab may have the potential to improve the lung perfusion in severe COVID-19 patients. Our results indicate no statistically significant differences in the ICU admission rates and length of hospital stay among treatment and control groups. However, Rosas et al. [17] in the COVACTA trial reported a lower time to hospital discharge in the tocilizumab arm (median [95%CI] of 20 [16,26] days), compared to the control group (median [95%CI] of 28 [20, not evaluable] days) with a hazard ratio [95%CI] of 1.35 [1.02 to 1.79]. Single-arm studies indicated potential benefits of tocilizumab treatment in all secondary efficacy outcome measures; however, these differences were not statistically significant.
Regarding safety, almost all published meta-analysis studies reported no significant differences in the rate of clinically important infections between the tocilizumab and SOC groups [89,92,95,96]. Similarly, in our study, no significant association between tocilizumab administration and secondary infections was found (RR [95%CI] of 1.24 [0.98, 1.56]). Even lower rates of infection were reported in tocilizumab cases in the four published RCTs (pooled RR [95%CI] of 0.48 [0.24, 0.96]). In the CORIMUNO-19 trial [16], bacterial and fungal sepsis were more common in the control group (11/67 and 2/67, respectively) compared with the tocilizumab group (2/63 and 0/63, respectively). Similarly, in the RCT-TCZ-COVID-19 study [14], the rate of secondary infections was lower in the tocilizumab group (1.7%, 1/60) compared with the SOC group (6.3%, 4/63). However, the results of cohort and case–control studies regarding the impact of tocilizumab treatment on the rate of secondary infections were conflicting. Recently, Frigault et al. [97] analyzed the risk of infection in 391 patients with hematologic malignancies in the two groups of tocilizumab (n = 166) and control (n = 225) for the management of CRS following chimeric antigen receptor T (CAR T) cell therapy. After 100 days of follow-up, similar rates of clinically significant infections were reported in the tocilizumab (31.3%) and control (29.8%) groups (P = 0.85). Collectively, although mechanistically possible, available data suggest that the short-term use of tocilizumab in COVID-19 patients cannot be associated with a significant increase in the risk of clinically important infections.
4.2. Limitations
Our meta-analysis study carries several limitations. We were not able to conduct our meta-analysis based only on the published RCTs. The four RCTs lacked adequate power to detect any significant impact for tocilizumab on mortality rates and their pooled RR had a very wide 95%CI. Hence, we included observational clinical studies in addition to the RCTs to increase the power of the analysis [98]. The timing of tocilizumab administration with respect to the severity of the disease can significantly influence the effectiveness of the drug. The timing of treatment was not reported in many of the selected studies and, accordingly, could not be evaluated in our meta-analysis. Similarly, concomitant treatments, notably the corticosteroids, were not reported properly in many of the observational trials. Moreover, secondary outcome measures were not available for all the included studies. Accordingly, we performed the meta-analysis of the secondary outcome variables with the data on a lower number of patients compared with the primary outcome variable. Despite these limitations, our study further supports the administration of tocilizumab to decrease mortality rates in severely-to-critically ill COVID-19 patients. Our meta-analysis was strengthened by the large number of studies involving RCTs, cohort studies, case-controlled studies, and uncontrolled studies. So far, our study is the most updated and the most comprehensive meta-analysis on the effects of tocilizumab in severe and critical COVID-19.
5. Conclusions
Tocilizumab can potentially improve clinical outcomes and reduce mortality rates in patients with severe to critical COVID-19. Our meta-analysis involved a large number of studies of different designs. Published RCTs do not have adequate statistical power to detect possible impacts of tocilizumab on mortality rates and hence meta-analysis performed solely based on the RCTs cannot be considered as conclusive. Large-scale RCTs are still required to make more robust conclusions.
Funding Statement
This study was supported by the AryoGen Pharmed Co. under Grant number 99/33022.
Declaration of interest
Nassim Anjidani is the head of the medical department of Orchid Pharmed company, which is in collaboration with AryoGen Pharmed company with respect to conducting clinical trials. Ali Taheri is an employee of Orchid Pharmed company. The remaining authors have no other relevant affiliations or financial involvement with any organization with a financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
A peer reviewer has received honoraria from Roche-Chugai and Sanofi for research and advisory Board. Peer reviewers on this manuscript have no other relevant financial or other relationships to disclose.
Author contributions
MP and FD contributed to conceptualization and design of study. SR and BF contributed to systematic searching, screaning, article selection, data extraction, data analysis, coordination, and drafing of manuscript. ZK and HM contributed to the data extraction and drafting of the manuscript. NA and AT contribute to the data analysis and drafting of the manuscript. MP, FD and RM contributed to the interpretation of results and supervision of execution. All authors critically revised the manuscript and have contributed to the final approval of the version to be submitted.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.(WHO) WHO . Weekly Epidemiological Update: coronavirus disease 2019 (COVID–19).
- 2.Lai -C-C, Liu YH, Wang C-Y, et al. Asymptomatic carrier state, acute respiratory disease, and pneumonia due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): facts and myths. J Microbiol Immunol Infect. 2020;53(3):404–412. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yuan J, Zou R, Zeng L, et al. The correlation between viral clearance and biochemical outcomes of 94 COVID-19 infected discharged patients. Inflammation Res. 2020;69(6):599–606. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coomes EA, Haghbayan H.. Interleukin-6 in Covid-19: a systematic review and meta-analysis. Rev Med Virol. 2020;30(6):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Le RQ, Li L, Yuan W, et al. FDA approval summary: tocilizumab for treatment of chimeric antigen receptor T cell‐induced severe or life‐threatening cytokine release syndrome. Oncologist. 2018;23(8):943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chinese Clinical Guidance for COVID-19 Pneumonia Diagnosis and Treatment. 7th edition. Beijing: China National Health Commission; 2020. [Google Scholar]
- 7.National Institutes of Health (NIH): Coronavirus Disease 2019. (COVID-19) Treatment Guidelines. [PubMed]
- 8.Research N Arthritis drugs effective in improving survival in sickest COVID-19 patients: national Institute for Health Research.
- 9.Organization WH . Clinical management of COVID–19.
- 10.Mehta C, Kataria S, Mehta Y. COVID Special Issue, Part I: management of Coronavirus 2019. J Cardiac Crit Care TSS. 2020;4(1):40. [Google Scholar]
- 11.DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clin Trials. 1986;7(3):177–188. [DOI] [PubMed] [Google Scholar]
- 12.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. [DOI] [PubMed] [Google Scholar]
- 13.Higgins JP, Thomas J, Chandler J, et al. Cochrane handbook for systematic reviews of interventions. Hoboken: John Wiley & Sons; 2019. [Google Scholar]
- 14.Salvarani C, Dolci G, Massari M, et al. Effect of tocilizumab vs standard care on clinical worsening in patients hospitalized with COVID-19 pneumonia: a randomized clinical trial. JAMA Intern Med. 2021;181(1):24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stone JH, Frigault MJ, Serling-Boyd NJ, et al., Efficacy of tocilizumab in patients hospitalized with Covid-19. N Engl J Med. 383(24): 2333–2344. 2020. . [DOI] [PMC free article] [PubMed] [Google Scholar]; • A high quality and well-designed randomized controlled trial with 28 days of follow-up conducted in USA.
- 16.Hermine O, Mariette X, Tharaux P-L, et al. Effect of Tocilizumab vs Usual Care in Adults Hospitalized With COVID-19 and Moderate or Severe Pneumonia: a Randomized Clinical Trial. JAMA Internal Medicine. 2021;181(1):32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosas I, Bräu N, Waters M, et al. Tocilizumab in hospitalized patients with COVID-19 pneumonia. medRxiv. 2020. [Google Scholar]; • A high quality, multi-center, Bayesian randomized controlled trial with 28 days of follow-up conducted in France.
- 18.Campochiaro C, Della-Torre E, Cavalli G, et al. Efficacy and safety of tocilizumab in severe COVID-19 patients: a single-centre retrospective cohort study. Eur J Internal Med. 2020;76:43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Somers EC, Eschenauer GA, Troost JP, et al. Tocilizumab for treatment of mechanically ventilated patients with COVID-19. Clin Infect Dis. 2020. DOI: 10.1093/cid/ciaa954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wadud N, Ahmed N, Shergil M, et al. Improved survival outcome in SARs-CoV-2 (COVID-19) acute respiratory distresssyndrome patients with Tocilizumab administration. medRxiv. 2020. [Google Scholar]
- 21.Guaraldi G, Meschiari M, Cozzi-Lepri A, et al. Tocilizumab in patients with severe COVID-19: a retrospective cohort study. Lancet Rheumatol. 2020;2(8):e474–e484. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ip A, Berry DA, Hansen E, et al. Hydroxychloroquine and tocilizumab therapy in COVID-19 patients—An observational study. PloS One. 2020;15(8):e0237693. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kimmig LM, Wu D, Gold M, et al. IL-6 Inhibition in Critically Ill COVID-19 Patients Is Associated With Increased Secondary Infections. Frontiers in medicine. 2020;7:583897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maeda T, Obata R, Rizk DO D, et al. The association of interleukin-6 value, interleukin inhibitors, and outcomes of patients with COVID-19 in New York City. J Med Virol. 2021;93(1):463–471. . [DOI] [PubMed] [Google Scholar]
- 25.Moreno-García E, Rico V, Albiach L, et al. Tocilizumab is associated with reduced risk of ICU admission and mortality in patients with SARS-CoV-2 infection. medRxiv. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martínez-Sanz J, Muriel A, Ron R, et al. Effects of tocilizumab on mortality in hospitalized patients with COVID-19: a multicentre cohort study. Clin Microbiol Infect. 2021;27(2):238-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mikulska M, Nicolini L, Signori A. Tocilizumab and steroid treatment in patients with severe COVID-19 pneumonia. medRxiv. Preprint Posted. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Biran N, Ip A, Ahn J, et al. Tocilizumab among patients with COVID-19 in the intensive care unit: a multicentre observational study. Lancet Rheumatol. 2020;2(10):e603–e612. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fisher MJ, Raymundo LAM, Monteforte M, et al. Tocilizumab in the treatment of critical COVID-19 pneumonia: a retrospective cohort study of mechanically ventilated patients. Int J Infectious Dis. 2021;103:536–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gupta S, Wang W, Hayek SS, et al. Association between early treatment with tocilizumab and mortality among critically ill patients with COVID-19. JAMA Intern Med. 2021;181(1):41–51. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rodríguez-Baño J, Pachón J, Carratalà J, et al. Treatment with tocilizumab or corticosteroids for COVID-19 patients with hyperinflammatory state: a multicentre cohort study (SAM-COVID-19). Clin Microbiol Infect. 202. 1;27(2), 244-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rossi B, Nguyen LS, Zimmermann P, et al. Effect of tocilizumab in hospitalized patients with severe COVID-19 pneumonia: a case–control cohort study. Pharmaceuticals. 2020;13(10):317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ruiz-Antoran B, Sancho-Lopez A, Torres F. et al. Combination of tocilizumab and steroids to improve mortality in patients with severe COVID-19 infection: a Spanish, multicenter, cohort study. Infectious diseases and therapy. 2021;10(1): 347-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsai A, Diawara O, Nahass RG, et al. Impact of tocilizumab administration on mortality in severe COVID-19. Sci Rep. 2020;10(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zheng K-L, Xu Y, Guo Y-F, et al. Efficacy and safety of tocilizumab in COVID-19 patients. Aging (Albany NY). 2020;12(19):18878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hill JA, Menon MP, Dhanireddy S, et al. Tocilizumab in hospitalized patients with COVID-19: clinical outcomes, inflammatory marker kinetics, and safety.. J Med Virol. 2021;93(4):2270–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Langer-Gould A, Smith JB, Gonzales EG, et al. Early identification of COVID-19 cytokine storm and treatment with anakinra or tocilizumab. Int J Infectious Dis. 2020;99:291–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kewan T, Covut F, Al–Jaghbeer MJ, et al. Tocilizumab for treatment of patients with severe COVID–19: a retrospective cohort study. EClinicalMedicine 2020;24:100418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Masiá M, Fernández-González M, Padilla S, et al. Impact of interleukin-6 blockade with tocilizumab on SARS-CoV-2 viral kinetics and antibody responses in patients with COVID-19: a prospective cohort study. EBioMedicine 2020;60:102999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patel K, Gooley TA, Bailey N, et al. Use of the IL-6R antagonist tocilizumab in hospitalized COVID-19 patients. Journal of internal medicine. 202. 1;289(3):430–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perrone F, Piccirillo MC, Ascierto PA, et al. Tocilizumab for patients with COVID-19 pneumonia. The single-arm TOCIVID-19 prospective trial. J Transl Med. 2020;18(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roomi S, Ullah W, Ahmed F, et al. Efficacy of hydroxychloroquine and tocilizumab in patients with COVID-19: single-center retrospective chart review. J Med Internet Res. 2020;22(9):e21758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Canziani LM, Trovati S, Brunetta E, et al. Interleukin-6 receptor blocking with intravenous tocilizumab in COVID-19 severe acute respiratory distress syndrome: a retrospective case–control survival analysis of 128 patients. J Autoimmun. 2020;114:102511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Capra R, De Rossi N, Mattioli F, et al. Impact of low dose tocilizumab on mortality rate in patients with COVID-19 related pneumonia. Eur J Internal Med. 2020;76:31–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Colaneri M, Bogliolo L, Valsecchi P, et al. Tocilizumab for treatment of severe COVID-19 patients: preliminary results from SMAtteo COvid19 REgistry (SMACORE). Microorganisms. 2020;8(5):695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carvalho V, Turon R, Goncalves B, et al. Effects of tocilizumab in critically ill patients with COVID-19: a quasi-experimental study. medRxiv 2020. [Google Scholar]
- 47.Gokhale Y, Mehta R, Karnik N, et al. Tocilizumab improves survival in patients with persistent hypoxia in severe COVID-19 pneumonia.. EClinicalMedicine 2020;24:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rojas-Marte G, Khalid M, Mukhtar O, et al. Outcomes in patients with severe COVID-19 disease treated with tocilizumab: a case–controlled study. QJM. 2020;113(8):546–550. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roumier M, Paule R, Matthieu G, et al. Interleukin-6 blockade for severe COVID-19. medrxiv 2020. [Google Scholar]
- 50.Klopfenstein T, Zayet S, Lohse A, et al. Impact of tocilizumab on mortality and/or invasive mechanical ventilation requirement in a cohort of 206 COVID-19 patients. Int J Infectious Dis. 2020;99:491–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rossotti R, Travi G, Ughi N, et al. Safety and efficacy of anti-il6-receptor tocilizumab use in severe and critical patients affected by coronavirus disease 2019: a comparative analysis. J Infect. 2020;81(4):e11–e17. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ramaswamy M, Mannam P, Comer R, et al. Off-label real world experience using tocilizumab for patients hospitalized with COVID-19 disease in a regional community health system: a case–control study. medrxiv. 2020. [Google Scholar]
- 53.Ramiro S, Mostard RL, Magro-Checa C, et al. A Pseudo-Randomised Comparison of a Strategy with Intense Immunosuppression vs. Supportive Care Only in Patients with COVID-19-Associated Cytokine Release Syndrome Results of the CHIC-Study. SSRN Electronic Journal. 2020. DOI: 10.2139/ssrn.3627257. [DOI] [Google Scholar]
- 54.Klopfenstein T, Zayet S, Lohse A, et al. Tocilizumab therapy reduced intensive care unit admissions and/or mortality in COVID-19 patients. Médecine et Maladies Infectieuses. 2020;50(5):397–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Menzella F, Fontana M, Salvarani C, et al. Efficacy of tocilizumab in patients with COVID-19 ARDS undergoing noninvasive ventilation. Crit Care. 2020;24(1):1–9. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nasa P, Singh A, Upadhyay S, et al. Tocilizumab Use in COVID-19 Cytokine-release Syndrome: retrospective Study of Two Centers. Indian J Crit Care Med. 2020;24(9):771. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Okoh AK, Bishburg E, Grinberg S, et al. Tocilizumab use in COVID-19-associated pneumonia. J Med Virol. 2021;93(2):1023–1028. . [DOI] [PubMed] [Google Scholar]
- 58.Pettit NN, Nguyen CT, Mutlu GM, et al. Late onset infectious complications and safety of tocilizumab in the management of COVID‐19. J Med Virol. 202. 1;93(3):1459-1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hashimoto S, Kitajima H, Arai T, et al. A retrospective study evaluating efficacy and safety of compassionate use of tocilizumab in 13 patients with severe-to-critically ill COVID-19: analysis of well-responding cases and rapidly-worsening cases after tocilizumab administration. medRxiv. 2020. [Google Scholar]
- 60.Conrozier T, Lohse A, Balblanc J-C. et al. Biomarker variation in patients successfully treated with tocilizumab for severe coronavirus disease 2019 (COVID-19): results of a multidisciplinary collaboration. Clinical and experimental rheumatology. 2020;38(4):742–747. [PubMed] [Google Scholar]
- 61.Rimland CA, Morgan CE, Bell GJ, et al. Clinical characteristics and early outcomes in patients with COVID-19 treated with tocilizumab at a United States academic center. medRxiv. 2020. [Google Scholar]
- 62.Morrison AR, Johnson JM, Griebe KM, et al. Clinical characteristics and predictors of survival in adults with coronavirus disease 2019 receiving tocilizumab. Journal of autoimmunity. 2020;114:102512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sirimaturos M, Gotur DB, Patel SJ, et al. Clinical Outcomes Following Tocilizumab Administration in Mechanically Ventilated Coronavirus Disease 2019 Patients.. Critical Care Explorations. 2020;2(10):10. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jordan SC, Zakowski P, Tran HP, et al. Compassionate use of tocilizumab for treatment of SARS-CoV-2 pneumonia. Clinical Infectious Diseases. 2020;71(12):3168–3173. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gorgolas M, Cabello A, Perez LP, et al. Compassionate Use of Tocilizumab in Severe SARS-CoV2 Pneumonia. When late administration is too late. medRxiv. 2020. [Google Scholar]
- 66.Strohbehn GW, Heiss BL, Rouhani SJ, et al. COVIDOSE: a Phase II Clinical Trial of Low‐Dose Tocilizumab in the Treatment of Noncritical COVID‐19 Pneumonia. Clinical pharmacology and therapeutics. 2020;109(3), 688–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sanchez-Montalva A, Selares-Nadal J, Espinosa-Pereiro J, et al. Early outcomes of tocilizumab in adults hospitalized with severe COVID19. An initial report from the Vall dHebron COVID19 prospective cohort study. medRxiv. 2020. [Google Scholar]
- 68.Corominas H, Castellví I, Pomar V, et al. Effectiveness and safety of intravenous tocilizumab to treat COVID-19-associated hyperinflammatory syndrome: covizumab-6 observational cohort.. Clinical Immunology (Orlando, Fla.). 2021;223:108631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vu CA, DeRonde KJ, Vega AD, et al. Effects of Tocilizumab in COVID-19 patients: a cohort study. BMC infectious diseases. 2020;20(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Quartuccio L, Sonaglia A, Pecori D, et al. Higher levels of IL-6 early after tocilizumab distinguish survivors from nonsurvivors in COVID-19 pneumonia: a possible indication for deeper targeting of IL-6.. Journal of Medical Virology. 2020;92(11):2852–2856. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Morena V, Milazzo L, Oreni L, et al. Off-label use of tocilizumab for the treatment of SARS-CoV-2 pneumonia in Milan, Italy.. European Journal of Internal Medicine. 2020;76(36–42):36–42. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sciascia S, Aprà F, Baffa A, et al. Pilot prospective open, single-arm multicentre study on off-label use of tocilizumab in patients with severe COVID-19.. Clinical and Experimental Rheumatology. 2020;38(3):529–532. [PubMed] [Google Scholar]
- 73.Patel A, Shah K, Dharsandiya M, et al. Safety and efficacy of tocilizumab in the treatment of severe acute respiratory syndrome coronavirus-2 pneumonia: a retrospective cohort study. Indian Journal of medical microbiology. 2020;38(1):116–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.De Cáceres C, Martínez R, Bachiller P, et al. The effect of tocilizumab on cytokine release syndrome in COVID-19 patients.. Pharmacological Reports: PR. 2020;72(6):1529–1537. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Capo A, Patel H, Rajaratnam N, et al. The use of tocilizumab at a community hospital during the COVID-19 pandemic in the epicenter of northern New Jersey. Chest. 2020;158(4):A2430. [Google Scholar]
- 76.Dastan F, Nadji SA, Saffaei A, et al. Tocilizumab administration in a refractory case of COVID-19.. International Journal of Antimicrobial Agents. 2020;56(2):106043. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Petrak RM, Skorodin NC, Van Hise NW, et al. Tocilizumab as a therapeutic agent for critically ill patients infected with sARS‐CoV‐2. Clinical and Translational Science. 2020. DOI: 10.1111/cts.12894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Perrone F, Piccirillo MC, Ascierto PA, et al. Tocilizumab for patients with COVID-19 pneumonia. The TOCIVID-19 phase 2 trial. medRxiv. 2020. [Google Scholar]
- 79.Tomasiewicz K, Piekarska A, Stempkowska-Rejek J, et al. Tocilizumab for patients with severe COVID-19: a retrospective, multi-center study. Expert Review of Anti-infective Therapy. 2021;19(1):93–100. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fernández‐Ruiz M, López‐Medrano F, Pérez‐Jacoiste Asín MA, et al. Tocilizumab for the treatment of adult patients with severe COVID‐19 pneumonia: a single‐center cohort study. Journal of Medical Virology. 2021;93(2):831–842. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Alattar R, Ibrahim TB, Shaar SH, et al. Tocilizumab for the treatment of severe coronavirus disease 2019. Journal of medical virology. 2020;92(10):2042-2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Knorr JP, Colomy V, Mauriello CM, et al. Tocilizumab in patients with severe COVID‐19: a single‐center observational analysis. Journal of Medical Virology. 2020;92(11):2813–2820. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jiménez-Brítez G, Ruiz P, Soler X. Tocilizumab plus glucocorticoids in severe and critically COVID-19 patients. A single center experience. Medicina Clínica. 2020;155(9):410-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Price CC, Altice FL, Shyr Y, et al. Tocilizumab treatment for cytokine release syndrome in hospitalized patients with coronavirus disease 2019: survival and clinical outcomes. Chest. 2020;158(4):1397–1408. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Luo P, Liu Y, Qiu L, et al. Tocilizumab treatment in COVID‐19: a single center experience. Journal of Medical Virology. 2020;92(7):814–818. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nasir N, Mahmood SF, Habib K, et al. Treatment of ARDS and hyperinflammation in COVID-19 with IL-6 antagonist Tocilizumab: a tertiary care experience from Pakistan. medRxiv. 2020. [Google Scholar]
- 87.Khan F, Stewart I, Fabbri L, et al. A systematic review and meta-analysis of Anakinra, Sarilumab, Siltuximab and Tocilizumab for Covid-19. medRxiv. 2020. [DOI] [PubMed] [Google Scholar]
- 88.Rubio-Rivas M, Mora-Lujan JM, Montero A, et al. Beneficial and harmful outcomes of tocilizumab in severe COVID-19: a systematic review and meta-analysis. medRxiv 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kotak S, Khatri M, Malik M, et al. Use of Tocilizumab in COVID-19: a systematic review and meta-analysis of current evidence. Cureus 2020;12(10):10. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhao M, Lu J, Tang Y, et al. Tocilizumab for treating COVID-19: a systemic review and meta-analysis of retrospective studies. Eur J Clin Pharmacol. 202. 1;77(3):311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sarfraz A, Sarfraz Z, Sarfraz M, et al. Tocilizumab and COVID-19: a Meta-Analysis of 2120 Patients with Severe Disease and Implications for Clinical Trial Methodologies. Turkish journal of medical sciences.;2020. 10.3906/sag-2010-131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tleyjeh IM, Kashour Z, Damlaj M, et al. Efficacy and safety of tocilizumab in COVID-19 patients: a living systematic review and meta-analysis. Clinical Microbiology and Infection. 2020;27(2):215–227. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Aziz M, Haghbin H, Abu Sitta E, et al. Efficacy of tocilizumab in COVID‐19: a systematic review and meta‐analysis. Journal of Medical Virology. 2020;93(3):1620–1630. . [DOI] [PubMed] [Google Scholar]
- 94.Salvati L, Occhipinti M, Gori L, et al. Pulmonary vascular improvement in severe COVID-19 patients treated with tocilizumab. Immunol Lett. 2020;228:122–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li Z, Che Q, Li M, et al. Efficacy and Safety of Tocilizumab in Patients with COVID-19: a Systematic Review and Meta-Analysis. Research Square. 2020. [Google Scholar]
- 96.Malgie J, Schoones JW, Pijls BGJCID. Decreased mortality in coronavirus disease 2019 patients treated with tocilizumab: a rapid systematic review and meta-analysis of observational studies. Clinical Infectious Diseases. 2020. 10.1093/cid/ciaa1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Frigault MJ, Nikiforow S, Mansour MK, et al. Tocilizumab not associated with increased infection risk after CAR T-cell therapy: implications for COVID-19? Blood J Am Soc Hematol. 2020;136(1):137–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shrier I, Boivin J-F, Steele RJ, et al., Should meta-analyses of interventions include observational studies in addition to randomized controlled trials? A critical examination of underlying principles. Am J Epidemiol. 166(10): 1203–1209. 2007. . [DOI] [PubMed] [Google Scholar]; •• According to this paper, along with the randomized controlled trials, observational studies should be included in the meta-analysis studies in order to make use of all available evidence in the area.


