ABSTRACT
Introduction: The ongoing pandemic caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has posed important challenges for clinicians and health-care systems worldwide.
Areas covered: The aim of this manuscript is to provide brief guidance for intensive care unit management of mechanically ventilated patients with COVID-19 based on the literature and our direct experience with this population. PubMed, EBSCO, and the Cochrane Library were searched up until 15th of January 2021 for relevant literature.
Expert opinion: Initially, the respiratory management of COVID-19 relied on the general therapeutic principles for acute respiratory distress syndrome; however, recent findings have suggested that the pathophysiology of hypoxemia in patients with COVID-19 presents specific features and changes over time. Several therapies, including antiviral and anti-inflammatory agents, have been proposed recently. The optimal intensive care unit management of patients with COVID-19 remains unclear; therefore, ongoing and future clinical trials are warranted to clarify the optimal strategies to adopt in this cohort of patients.
KEYWORDS: Severe acute respiratory syndrome coronavirus-2, mechanical ventilation, anticoagulation, antibiotic therapy, corticosteroids
1. Introduction
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has led to a worldwide pandemic, overwhelming health-care systems and causing over a million of deaths to date [1,2]. Although patients often present with mild symptoms, such as dry cough, fever, and fatigue, around 20% are hospitalized and 5–10% require intubation and invasive mechanical ventilation for respiratory failure [1–4]. Severe pneumonia induced by SARS-CoV-2 is generally managed in the same way as traditional acute respiratory distress syndrome (ARDS) [5], although it might present specific pathophysiological features [6,7]. The aim of this paper is to provide a brief update on ten things we need to know about intensive care unit (ICU) management of mechanically ventilated patients with COVID-19 (Figure 1).
Figure 1.
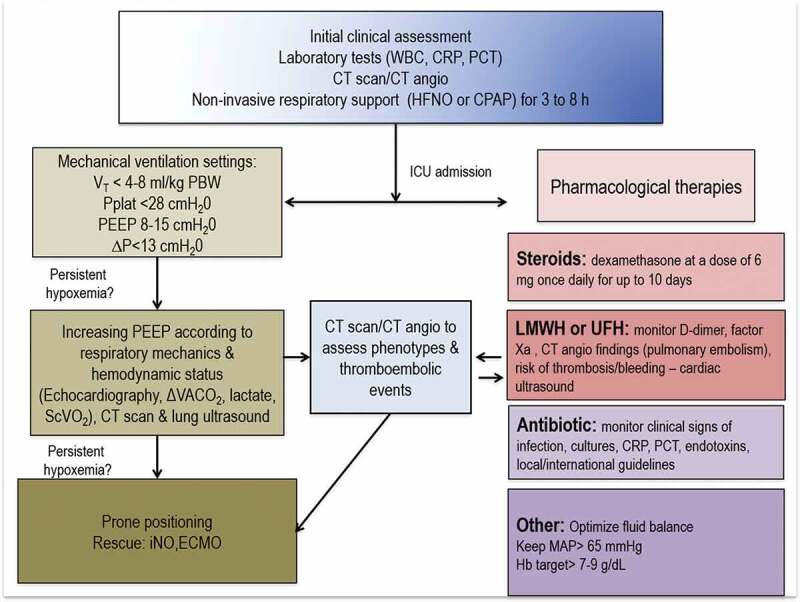
Clinical approach to patients with COVID-19 in the intensive care unit in Genoa, Italy. CPAP, continuous positive airway pressure; CRP, C-reactive protein; CT, computed tomography; ΔVACO2; delta veno-arterial carbon dioxide; P, driving pressure; ECMO, extracorporeal membrane oxygenation membrane; HFNO, high-flow nasal oxygen; iNO, inhaled nitric oxide; LMWH, low-molecular-weight heparin; VT, tidal volume; PBW, predicted body weight; PCT, procalcitonin; PEEP, positive end-expiratory pressure; MAP, mean arterial pressure; Pplat, plateau pressure; ScVO2, ventral venous oxygen saturation; UFH, unfractionated heparin; WBC, white blood cells
1.1. Pathophysiology of COVID-19 ARDS
ARDS may be due to direct (pulmonary) or indirect (extrapulmonary) insult to the alveolar capillary barrier [8]. Pulmonary compared with extrapulmonary ARDS is characterized by increased alveolar epithelial cell damage (both types I and II epithelial cells), alveolar neutrophils, but especially macrophages/monocytes, lymphocyte infiltration, fibrinous exudates, collagen fiber deposition, and alveolar edema. COVID-19 pneumonia shows similar findings to direct ARDS, with pulmonary vascular abnormalities [9,10], alveolar neutrophil infiltration, and fibrosis [3].
Activation of macrophages by COVID-19 leads to the release of pro-inflammatory cytokines, metalloproteinases, and other proteolytic enzymes that can cause thrombi formation, systemic inflammation, intravascular coagulopathy with high risk of thrombotic complications, venous thromboembolism, especially in the lung, thrombosis, and vasculitis [9], and the changes described by Elinoff et al. [11].
Although some authors have suggested that COVID-19 pneumonia is characterized by different phenotypes according to the chest computed tomography (CT) scan and respiratory mechanics characteristics (high vs low compliance) [12], the current literature does not fully support this classification, and the pathophysiology of COVID-19 ARDS is not fully understood [7].
Estimated lung weight of normal lungs is 800 g on average, whereas in traditional ARDS, lung weight increases to 1800 g, with an excess tissue mass of 1000 g [13]. In traditional ARDS, hypoxemia is well explained by increased perfusion in dependent atelectatic and caudal lung regions. Interestingly, in COVID-19 pneumonia, the distribution of perfusion is mainly anti-gravitational, diverted toward non-dependent and caudal lung regions. Furthermore, one-third of non-aerated lung regions can show absence of perfusion associated with thrombosis [14]. Higher positive end-expiratory pressure (PEEP) may lead to a variable amount of alveolar recruitment, and in some cases reduce respiratory system static compliance and increase driving pressure. In traditional ARDS, the prone position leads to recruitment of atelectatic lung regions [15] and maintenance of higher perfusion in dorsal lung, with improvement in regional ventilation, leading to better oxygenation and lower PaCO2. However, in COVID-19 pneumonia, densities are not modified in the prone position; thus, the improvement of oxygenation, if any, is explained by perfusion diversion, which may yield increased PaCO2, because regional ventilation does not change [16]. Moreover, in traditional ARDS, gas-exchange deterioration is accompanied by worsening respiratory mechanics; nevertheless, in COVID-19 pneumonia, deterioration in oxygenation is not necessarily associated with changes in respiratory mechanics, which may lead to so-called ‘happy hypoxia’. Based on these findings, we hypothesize that in COVID-19 pneumonia, ‘happy hypoxia’ can be due to V′/Q′ mismatch, mainly related to increased perfusion rather than increased prevalence of poorly aerated lung areas, resulting in normal or increased static compliance and less inspiratory effort and dyspnea. The natural evolution is toward ‘unhappy hypoxia’ mainly due to (1) increased non-aerated tissue and ‘true shunt’ with consolidated tissue in the dependent lung regions, (2) well-perfused areas combined with low V′/Q′, and (3) higher dead space pulmonary areas [16].
Clinical deterioration from happy hypoxia to unhappy hypoxia should be monitored to assess worsening of non-responsive hypoxia to higher inspired oxygen fractions, decrease of PaCO2 with increased respiratory rate, and activation of accessory muscles (suggesting increased inspiratory effort). In COVID-19 pneumonia, both hypoxemia and PaCO2 changes are well explained by changes in aeration-perfusion distribution due to poorly aerated and non-aerated lung regions. This information is important to optimize and individualize therapy from noninvasive to more invasive respiratory support, although it is clinically difficult to demonstrate when the transition from happy hypoxia and unhappy hypoxia occurs.
1.2. Noninvasive respiratory support
Supplementary oxygen is recommended for patients with COVID-19 with saturation of oxygen (SpO2) below 93%, aiming for a target SpO2 of 92–94% [17]. Noninvasive respiratory support in patients with COVID-19 may include the application of high-flow nasal oxygen (HFNO), continuous positive airway pressure (CPAP), and bilevel positive airway pressure (BiPAP). At present, no definitive evidence exists on whether noninvasive respiratory support is beneficial or harmful for patients with COVID-19. A recent report on 103 critically ill patients with COVID-19 and moderate–severe hypoxemic respiratory failure demonstrated that more than half of the patients undergoing noninvasive respiratory support did not require tracheal intubation and had lower mortality than those patients who were subsequently intubated [2]. Zucman et al. [18], in a retrospective single-center study including 62 patients with COVID-19 treated with HFNO, showed that (1) 34% responded well to HFNO, (2) 63% required intubation [19], and (3) ROX index ([SpO2/FiO2]/respiratory rate) ≥5.37 within 4 h of initiation of HFNO was able to predict a lower risk of intubation. However, increasing evidence on patients with COVID-19 shows great variability regarding the failure rate of noninvasive support and the need for intubation and mechanical ventilation (from 76% of cases to 20–30% cases) [17,20], generating the risk of prolonged and unmeasured increased intrathoracic pressures, thus, potentially leading to further lung damage [21]. NIV and the need for intubation should be evaluated according to specific clinical features as well as specific protocols. Guidelines are warranted regarding the escalation to invasive mechanical ventilation support [7].
Because hypoxemia in patients with COVID-19 can be consequent to the presence of hyperperfused (low V′/Q′) and hypoperfused (high V′/Q′, dead space) ground glass regions, HFNO appears to be the optimal way to improve oxygenation by increasing the inspired oxygen fraction without higher airway pressures and promote CO2 washout. HFNC can improve oxygenation and reduce the respiratory rate [22,23]. In an observational study with a small sample size of critically ill patients with COVID-19 who used HFNC and NIV as first-line therapy, the duration of HFNC + NIV, intubation rate, and mortality did not differ between two groups [24].
Also, a recent systematic review suggested that a high-flow nasal cannula may reduce the need for invasive ventilation and escalation of therapy compared with conventional oxygen therapy in patients with COVID-19 with acute hypoxemic respiratory failure [25]. Thus, HFNO appears to be a feasible method at the bedside, efficient, comfortable for the patient and allowing physiotherapy to be performed [26,27]. Similarly, helmet CPAP has frequently been used during the pandemic, especially outside the ICUs; a recent study reported a rate of success greater than 60% and close to 75% in full treatment patients [28], but these results are not consistent throughout the literature [29].
Noninvasive respiratory support should therefore be considered as a first therapeutic tool for COVID-19–related respiratory failure, but we suggest that patients should be promptly intubated if PaO2/FiO2 does not improve, and/or PaCO2 decreases below 30 mmHg, and/or the respiratory rate increases more than 28 breaths/min with evidence of accessory muscle activity within 3–8 h of treatment [7]. Further research and randomized controlled trials (RCTs) are warranted to understand the role and optimal duration of noninvasive ventilation in this group of patients.
1.3. Lung protective ventilation
The aim of mechanical ventilation is to provide oxygenation, minimizing ventilator-induced lung injury, especially in patients with ARDS [30]. In the early phases of the pandemic, mechanically ventilated patients with COVID-19 were treated as for ARDS, and the main principles for ventilator management were the use of low tidal volumes (VT = 4–8 mL/kg of predicted body weight), low plateau pressure (Pplat <28 cmH2O), and a high PEEP (>10 cmH2O) [31], with occasional recruitment maneuvers. However, patients with COVID-19 do not present traditional ARDS and preserved static compliance may coexist with severe hypoxemia. In this context, although a strategy of protective volumes and plateau pressure is recommended even in patients with COVID-19, the use of high PEEP is controversial. In patients with preserved static compliance and chest CT scans presenting few areas of atelectasis to be recruited but areas with increased perfusion, we suggest that moderate PEEP levels (8–11 cmH2O) may be applied with the aim of redistributing pulmonary blood flow from damaged to non-damaged lungs. In contrast, if atelectasis is present and static compliance is low, in our opinion, moderate PEEP levels (12–15 cmH2O) can be useful to improve lung recruitment; nevertheless, it is important to avoid overdistention and hemodynamic instability [7]. Although no definitive evidence exists regarding the ventilatory strategies to be applied in these patients, authors suggest using lung protective ventilation with tidal volumes ≤6 mL/kg of predicted body weight, driving pressure ≤15 cmH2O, and individualized PEEP titration based on clinical presentation, chest CT scan, and respiratory mechanics [32,33]. Recruitment maneuvers may be considered as a rescue therapy in case of hypoxemia despite optimizing ventilation [34]. RCTs are warranted to assess the role of different ventilator strategies in the specific subpopulation of patients.
1.4. Prone positioning
Prone positioning can be helpful in cases of severe ARDS to reduce atelectasis, redistribute pulmonary blood flow, and improve oxygenation [35], with potential beneficial effect on mortality. Furthermore, the prone position per se might lead to more homogeneous distribution of regional pleural pressure and aeration as well as ventilation, minimizing lung injury. Prone ventilation has been used frequently in patients with COVID-19 (11.5%) [17]. In a recent case series of 44 patients, prone positioning increased oxygenation only in patients with partial pressure of oxygen/fraction of inspired oxygen (PaO2/FiO2) <120 mmHg [36], thus suggesting that the response to pronation strongly depends on the specific pathophysiologic features of each patient with COVID-19 and can help to redistribute pulmonary blood flow and/or reduce atelectasis [7]. Even though prone position has been proposed [37], several reports suggest that the beneficial effects are rapidly lost in most patients when repositioned in the supine position [38]. The optimal timing for prone positioning is unclear in patients with COVID-19. Prone position during noninvasive respiratory support seems to delay intubation [39], which may mask the progressive deterioration of gas exchange. In summary, prone positioning can be considered as rescue therapy in selected patients with COVID-19. The decision to use prone positioning should be related to individualized chest CT features and specific characteristics of respiratory mechanics as well as the hemodynamic status of each patient. It has also recently been suggested that there is a role for the use of awake prone positioning in patients with COVID-19 [40,41]. It has been shown to improved oxygen saturation especially if used in the early phases, but without affecting 28-day mortality and should be therefore taken into consideration, especially considering the lack of medical resources in the pandemic [42,43].
The benefit/lack of benefit of prone positioning is relatively uncertain, but a sub-group of patients seem to benefit from it.
1.5. Nitric oxide, extracorporeal CO2 removal, and extracorporeal membrane oxygenation
Inhaled nitric oxide (iNO) has been proposed as rescue therapy in cases of refractory hypoxemia related to COVID-19 pneumonia [44] in view of the presence of V′/Q′ mismatch with alterations in hypoxic vasoconstriction. However, preliminary reports have shown controversial results regarding the efficacy of iNO in cases of COVID-19 pneumonia. Longobardo et al. [45] retrospectively compared the effects of iNO in patients with COVID-19 versus patients with ARDS, not caused by COVID-19, and found that improvement in the PaO2/FiO2 ratio after iNO administration was lower in patients with COVID-19 compared with traditional ARDS ([3%−17% to 26%] versus 47% [6–54%]; P < 0.05). Similarly, Tavazzi et al. [46] showed that only 4 (25%) patients in their cohort were responders to iNO. The low rate of responders among patients with COVID-19 might result from the severe endothelial injury and micro-thrombosis occurring in the pulmonary middle-small arteries with loss of hypoxic vasoconstriction and lung perfusion regulation. A recent report suggested that high dosage of iNO up to 160 ppm might be effective to reduce the need for mechanical ventilation in selected patients [47].
Veno-venous extracorporeal membrane oxygenation (VV-ECMO) has been increasingly applied since the 2009 H1N1 pandemic [48,49] as respiratory support when other treatments have failed in cases of hypoxemic ARDS. The role of VV-ECMO in selected patients in the context of the current COVID-19 pandemic needs to be more specifically established, especially regarding the criteria for patient selection and timing of initiation. So far, VV-ECMO for COVID-19 has been used in approximately 3% of severe cases with restoration of adequate oxygenation [48,49]. According to the Extracorporeal Life Support Organization (ELSO) dashboard, the survival rate of patients undergoing ECMO was 40%, with VV-ECMO being the most applied (91%); venous-arterial ECMO, which provides cardiorespiratory support, was applied in only a small number (4%) of patients [50]. Although the use of VV-ECMO in previous pandemics has provided preliminary guidance for its use in cases of COVID-19, data on its efficacy are lacking. Moreover, resource constraints and availability issues experienced during the COVID-19 pandemic make it fundamental to use it responsibly in strictly selected patients [51]. An alternative to VV-ECMO is the use of minimally invasive CO2 removal techniques, associated with respiratory dialysis at 100–500 ml/min, which might be incorporated in renal support dialysis machines, or extracorporeal CO2 removal (ECCO2R) at 1000–1500 ml/min. These devices might allow control of PaCO2 and pHa, thus markedly reducing the energy and mechanical power delivered by the mechanical ventilator to the lungs (lower tidal volume, driving pressure, respiratory rate, and plateau pressure) likely reducing lung injury [52]. In particular, ECCO2R and ECMO should be considered with the aim to reduce ventilator-lung injury by allowing the application of super protective ventilation [53,54].
In summary, the role of rescue therapies in COVID-19 is unclear and RCTs are warranted to assess their efficacy in this cohort of patients. A list of the ongoing trials is provided in Appendix 1.
1.6. Antibiotic therapy
In patients with COVID-19, bacterial co-infection at emergency department admission and during the hospital stay is not common [55] and has been reported in 3.5% and 15% of cases, respectively [56]. However, severe disease may be associated with high inflammation marker values, thus making the diagnosis and the decision to start prophylactic antibiotic therapy challenging [56]. Patients with COVID-19 have been often treated with broad-spectrum antibiotics [57–59], but their efficacy is unclear and inappropriate antibiotic therapy can increase antimicrobial resistance rates and hospital-acquired pneumonia caused by resistant pathogens [60]. The most common pathogens responsible for co-infection in COVID-19 are Staphylococcus aureus, Haemophilus influenzae, and less frequently gram-negative bacteria such as Acinetobacter baumannii and Klebsiella pneumoniae [61]. To date, no studies have evaluated the effectiveness of specific antibiotic regimens in patients with COVID-19 and the presence of bacterial co-infection. However, recent published guidelines suggest a restrictive use of antibiotics in patients with COVID-19, but enurage physicians to constantly evaluate the risk for bacterial co-infection and start antibiotic therapy after considering laboratory results (i.e. inflammatory markers such as procalcitonin, C-reactive protein-CRP), clinical criteria, chest radiography or CT findings, Modified Clinical Pulmonary Infection score [62], and immunodepression [56]. A recent study suggests that the absence of increased white blood cells and CRP can exclude bacterial co-infection [63].
Cultures including blood, sputum, and bronchoalveolar samples should be obtained as soon as possible, together with a routine HIV test, pneumococcal and Legionella urinary antigen testing before starting empirical antibiotic therapy, and when the decision to start antibiotic therapy is made, the treatment should be based on the clinical severity and according to local and/or national guidelines [64,65]. Antibiotic treatment should last 5 days in patients with COVID-19 and/or until improvement of clinical, radiologic and laboratory findings [66].
Invasive pulmonary aspergillosis (IPA) is not a frequent complication in patients with severe COVID-19, but it is responsible for increased mortality [67]. Although there is insufficient evidence to confirm that there is a disturbing incidence of pulmonary aspergillosis, this issue should be taken into consideration. A list of the ongoing trials is provided in Appendix 1.
1.7. Anticoagulants
COVID-19 infection causes alterations in the coagulative cascade and fibrinolysis, with release of endotoxin, and interleukins, and consequent neutrophil-platelet aggregation and transcriptional changes in platelets followed by an increase in thrombin and fibrin generation [6,68,69]. Pulmonary postmortem findings have shown a high incidence of platelet-fibrin thrombi with inflammatory infiltrates [70].
As a consequence, a variety of clinical features have been described after COVID-19 infection, ranging from thrombo-hemorrhagic complications to disseminated intravascular coagulation or vasculitis. Although observational clinical data suggest that the use of either prophylactic or intermediate doses of low-molecular-weight heparin (LMWH) in high-risk patients may be associated with better prognosis, the optimal thromboprophylaxis strategy in the critically ill COVID-19 patient population is still unclear [71]. The European Heart Journal – Cardiovascular Pharmacotherapy guidelines suggest the use of therapeutic unfractionated heparin (UFH) infusion titrated to an activated partial thromboplastin time (aPTT) target of 60–85 s for all patients admitted to the ICU with COVID-19 symptoms, with the recommendation to de-escalate treatment to enoxaparin 40 mg twice daily for those with confirmed absence of deep vein thrombosis [72]. However, the Scientific and Standardization Committee of the International Society on Thrombosis and Hemostasis recommends intermediate-dose LMWH in high-risk patients and discourages treatment-dose heparin until the results of RCTs become available [73]. Finally, the CHEST guidelines on Prevention, Diagnosis, and Treatment of VTE in Patients With Coronavirus Disease 2019 recommend using standard dose anticoagulant thromboprophylaxis and do not recommend intermediate (LMWH twice daily or increased weight-based dosing) or full treatment dosing [74]. In the absence of strong evidence, patients’ treatment should be individualized on the basis of the risk of bleeding and thrombosis and optimized using laboratory tests and cardiologic evaluation including cardiac echography.
Recently, an RCT aiming to compare the effectiveness of antithrombotic strategies for the prevention of adverse outcomes in COVID-19-positive inpatients has been paused for futility (ACTIV-4 study), thus suggesting that therapeutic heparin should not be adopted routinely.
1.8. Steroids
Pathophysiologic studies have highlighted the role of inflammatory system activation in the development of respiratory and systemic COVID-19 complications; therefore, the role of adjunctive corticosteroids has been proposed to suppress hyperinflammation cascade consequent to COVID-19 [59,75,76]. However, their use has been shown in observational studies to increase infection rates and mortality during severe acute respiratory syndrome (SARS), and Middle East respiratory syndrome (MERS) pandemics [77], and could potentially increase the risk of Strongyloides stercoralis hyperinfection syndrome [78]. Recent systematic reviews [79,80] showed that the case fatality rate among patients who received corticosteroid therapy was significantly higher than for those not treated with corticosteroids (69 of 443, 15.6% versus 56 of 1310, 4.3%). In addition, a systematic review and meta-analysis suggests that glucocorticoids might be a risk factor for mortality in patients with cancer and COVID-19 [81].
In July 2020, The Randomized Evaluation of COVID-19 Therapy (RECOVERY) trial from the UK [82], which randomly assigned patients to receive oral dexamethasone (at a dose of 6 mg once daily) for up to 10 days or to receive usual care only demonstrated that the administration of dexamethasone reduced mortality (age-adjusted rate ratio, 0.83; 95% confidence interval [CI], 0.75–0.93; P < 0.001). This effect was significant only in patients who were mechanically ventilated (29.3% versus 41.4%; rate ratio, 0.64; 95% CI, 0.51–0.81) and in those receiving oxygen (23.3% versus 26.2%; rate ratio, 0.82; 95% CI, 0.72–0.94) but not in patients who did not receive any form of respiratory support (17.8% versus 14.0%; rate ratio, 1.19; 95% CI, 0.91–1.55). After publication of the RECOVERY findings, three steroid trials, focusing on ICU patients, were stopped prematurely after inclusion of 384 [83], 299 [84], and 149 [84] patients, respectively. Similarly, a recent meta-analysis [85], which pooled data from seven RCTs that evaluated the efficacy of corticosteroids (dexamethasone, hydrocortisone, or methylprednisolone) in 1703 critically ill patients with COVID-19 showed an association between systemic corticosteroid administration and lower all-cause mortality (summary odds ratio, 0.66; 95% CI, 0.53–0.82; P < 0.001) [86]. Moreover, the recently published REMAP-CAP trial showed that treatment with a 7-day course of hydrocortisone, compared with no hydrocortisone, yielded 93% and 80% increased probabilities of improvement in organ support-free days within 21 days [87].
In summary, evidence suggests that the use of steroids should be implemented in the care of critically ill patients with COVID-19 receiving invasive mechanical ventilation and requiring supplementary oxygen. A list of the ongoing trials is provided in Appendix 1.
1.9. Antivirals and other drugs
Several antiretroviral agents have been suggested for the treatment of patients with COVID-19. Remdesivir, a nucleotide analog, has been used as a therapeutic drug for Ebola and Marburg virus infections [88], but results from an RCT published in May 2020 showed that remdesivir was not associated with any statistically significant clinical benefits. A recent randomized, open-label, phase 3 trial [89] randomizing patients to receive remdesivir for 5 or 10 days found no significant difference between a 5-day course and a 10-day course (P = 0.14), and currently, two phase 3, double-blind, RCTs of remdesivir versus placebo are ongoing [90]; at present, the routine use of remdesivir has not been recommended for patients with COVID-19 [91], even though the US Food and Drug Administration approved its use for COVID-19.
Similar results have been obtained from studies on other antiretroviral agents. In a recent RCT, the use of lopinavir–ritonavir was not associated with a significant benefit compared with standard care on patients’ clinical status (hazard ratio for clinical improvement, 1.31; 95% CI, 0.95–1.80), and mortality at 28 days (19.2% versus 25.0%; difference, −5.8 percentage points; 95% CI, −17.3 to 5.7) [92], whereas favipiravir has recently been approved for clinical trials for the management of SARS-CoV-2 [93]. Ongoing studies (Clinicaltrials.gov: NCT04293887, NCT02845843) are currently exploring the role of interferon as adjuvant to reduce the replication of SARS-Cov-2 in combination with antiviral therapy.
Chloroquine and hydroxychloroquine have been widely proposed as potential therapies against COVID-19 due to their immunomodulatory effects and possible in vitro antiviral activity [94]. However, these drugs may carry the risk of cardiovascular complications and arrhythmia [95]. A recent observational study, in which 811 of 1446 patients (58.9%) received hydroxychloroquine, suggested that hydroxychloroquine administration was not associated with either a greatly lowered or an increased risk of the composite endpoint of intubation or death [96]. The recently published RCT Outcomes Related to COVID-19 Treated with Hydroxychloroquine Among Inpatients With Symptomatic Disease (ORCHID) trial demonstrated that treatment with hydroxychloroquine, compared with placebo, did not significantly improve clinical status at day 14 (median [interquartile range] score, 6 [4–7] versus 6 [4–7]; adjusted OR, 1.02 [95%CI, 0.73–1.42]), thus not supporting the use of hydroxychloroquine in this group of patients.
Finally, several molecules have been proposed, especially in research settings, and are in development. Their mechanism of action includes inhibition of viral enzymes, including proteases and components of RNA-dependent RNA polymerase, 3 C-like proteinase, as well as antisense oligonucleotides, interferons, and interferon fusion proteins [5,97].
Convalescent plasma [98] and immunoglobulin therapy are considered potential treatments for patients with COVID-19, but whether convalescent plasma is beneficial for people admitted to hospital with COVID-19 is uncertain [99]. The recently published RCT on convalescent plasma [100] cast serious doubts on its effectiveness; moreover, the concentration of antibodies evaluated, and the dosage and timing of administration are not clear from the literature. Studies using tolicizumab, an inhibitor of the interleukin-6 receptor, for COVID-19 showed controversial results [101], and this drug is not currently used routinely in clinical practice.
Therefore, at present, none of these treatments are currently implemented routinely in clinical practice. A list of the ongoing trials is described in Appendix 1.
1.10. Weaning and extubation
Extubation of patients with COVID-19 has the primary aim to minimize the chance of reintubation, and it is a high-risk procedure for both ICU staff and patients. Readiness to be weaned should be evaluated early during mechanical ventilation and assessed by objective criteria. Factors associated with the risk of reintubation include low PaO2/FiO2 ratio, age, Acute Physiology and Chronic Health Evaluation (APACHE) II score, Rapid Shallow Breathing Index (RSBI), and positive fluid balance [102]. Clinical criteria to be met before starting a spontaneous breathing test (SBT) in cases of COVID-19 include adequate cough, satisfactory oxygenation, hemodynamic stability, adequate level of consciousness, and RSBI <100 after 2 min of SBT [7,28]. However, recent evidence suggests that despite the high mortality of patients with COVID-19 who require mechanical ventilation, most patients are weaned in a relatively short period of time [103]. In this context, the important role of early chest physiotherapy has recently been highlighted [104].
Some precautions should be adopted in the process of weaning patients with COVID-19. A humidifier with a virus-filtering function should be used if modified T-piece ventilation is needed. Also, several strategies to reduce the aerosolization of SARS-CoV-2 have been adopted in recent months [105], including portable barrier hood devices, the use of wet gauze, plastic drape covering the patient’s mouth and nose, the use of masks over tube or extubation boxes, which can potentially reduce the risk of exposure [106].
In addition, the planned use of noninvasive ventilation (CPAP, NIV, or high-frequency oscillatory ventilation) after extubation can be recommended in patients with COVID-19 to reduce the chance of extubation failure. Finally, the role and timing of tracheotomy during the COVID-19 pandemic remains to be determined, because evidence is lacking regarding the effects of tracheostomy on a patient’s clinical course. Although it is not possible to establish an optimal timing for performing tracheostomy [107], early tracheostomy may reduce the time of invasive mechanical ventilation, weaning, with a potential increase in availability of ICU beds during the pandemic [108,109]. Finally, chest physiotherapy has a fundamental role in avoiding extubation failure [28], as well as the availability of a high-dependence unit for post intensive care to monitor patients for 3–10 days after extubation before discharging to the regular ward. This minimizes the possible risks of clinical worsening or deterioration after ICU discharge.
2. Conclusions and future directions
SARS-CoV-2 infection is a complex multiorgan disease with peculiar and specific characteristics that require aggressive individualized treatment, especially in mechanically ventilated patients in the ICU. The fatality rate remains high among those critically ill patients [110]. Although recent research has improved our knowledge regarding the pathophysiology of COVID-19 and clarified the therapeutic role of some specific drugs and ventilator strategies, questions remain regarding the optimal management of these patients. In this context, it is important to better understand the pathophysiology of SARS-CoV-2-induced pneumonia and damage to other organs, the best moment to intubate patients with hypoxemia, parameters to be evaluated at the bedside and ability to mitigate patient self-inflicted lung injury and ventilator-induced lung injury, how to set PEEP in both the supine and prone position, whether dexamethasone presents similar beneficial effects to methylprednisolone, and how to avoid lung fibrosis and strengthen the muscles involved in respiration in mechanically ventilated patients. A large number of RCTs are ongoing (Appendix 1) with the aim of clarifying not only mechanical ventilation strategies but also new therapies such as immunomodulator drugs, the use of interferon, hydroxychloroquine, as well adjuvant strategies such as the use of vitamin C, cholecalciferol probiotics, etc.
3. Expert opinion
The current pandemic caused by SARS-CoV-2 infection is posing important challenges for clinicians. Over the last months, research has improved knowledge of the pathophysiology and treatment principles in this group of patients. Although some aspects have been clarified, questions remain regarding the optimal management of these patients. The application of NIV continues to be controversial. NIV is a safe, feasible, and useful ventilatory strategy that may avoid the complications of tracheal intubation and ventilation in selected patients with COVID-19-associated respiratory failure. However, NIV should not be prolonged to avoid the risk of lung damage for uncontrolled airway pressures. In this context, HFNO can be considered a good option to improve oxygenation and minimize the risk of lung injury. Despite the use of noninvasive respiratory support, a large number of patients with COVID-19 may develop severe respiratory failure requiring admission to the ICU and mechanical ventilation.
The principles of ventilator strategies in this group of patients rely on the specific pathophysiological features of COVID-19 infection, together with CT findings. In general, evaluation of respiratory mechanics enables us to assess the role of different ventilator rescue strategies, such as prone positioning or the use of iNO. The role and optimal settings for PEEP are still unclear; PEEP may be useful not only for reducing atelectasis and recruiting non-aerated areas of the lung but also for redistributing pulmonary flow and reducing shunt. We therefore suggest applying a strategy of lung protective ventilation and considering and individualizing the respiratory settings on a case by case basis according to specific clinical, functional, and imaging features.
In addition, patients with COVID-19 show great complexity due to multiple organ involvement and a dynamic evolution over time. Patients with COVID-19 may develop multiorgan failure, with neurologic, cardiologic, and renal involvement.
To date, there are no specific vaccines or medicines for COVID-19. Treatments are under investigation and will be tested through clinical trials. Different drugs against COVID-19 are classified according to their certain or possible mechanisms of action, including inhibitors of viral replication, inhibitors of viral proteases, inhibitors of viral entry to the host cell, immunoenhancement, immunomodulating, immunosuppressive, anti-inflammatory, and pulmonary vaso-effectors.
At present, RCTs suggest avoiding the use of chloroquine and hydroxychloroquine as well as convalescent plasma. The use of remdesivir is still controversial.
Clinical management includes supportive management of the most common complications of severe COVID-19: pneumonia, hypoxemic respiratory failure/ARDS, sepsis and septic shock, coagulopathy, cardiomyopathy, acute kidney injury, liver failure, and complications from prolonged hospitalization, including secondary bacterial and fungal infections, thromboembolism, gastrointestinal bleeding, as well as neurologic complications and critical illness polyneuropathy/myopathy. Recent studies support the use of dexamethasone 6 mg once daily for up to 10 days, which is currently part of our clinical practice. Anticoagulant treatment should be based on strict monitoring of D-dimer, factor Xa, and CT findings to assess the risk of thromboembolism and bleeding. Strict monitoring of signs of infection, including fungal infection, laboratory tests, and cultures should also be performed so that antibiotic therapy can be started promptly; however, prophylactic antibiotic management is not recommended in these patients. We strongly believe that RCTs are warranted to answer many open questions that need to be addressed. Currently, research is focusing mainly on the safety and efficacy of antiretroviral therapies as well as the use of adjuvant medications and therapies.
Supplementary Material
Acknowledgments
The authors would like to express their gratitude to Mrs. Moira Elizabeth Schottler (Rio de Janeiro) and Lorna O’Brien (authorserv.com) for their assistance in editing the manuscript.
Funding Statement
This paper was funded by the Brazilian Council for Scientific and Technological Development (COVID-19-CNPq) 401700/2020-8 and 403485/2020-7, the Rio de Janeiro State Research Foundation (COVID-19- FAPERJ) E-26/210.181/2020, Funding Authority for Studies and Projects (FINEP) 01200008.00, National Council for Scientific and Technological Development, and Brazilian Ministry of Science, Technology, and Information COVID-19 Network (RedeVírus MCTI).
Article highlights
The pathophysiology of respiratory failure in patients with COVID-19 presents peculiar features that require individualized treatment based on clinical laboratory and radiologic findings.
Non-invasive respiratory support may help to reduce the need for intubation. However, prolonged non-invasive respiratory treatment can lead to patient self-inflicted lung injury and worse outcomes.
Lung protective ventilation is warranted in critically ill patients with COVID-19. The role of PEEP needs further investigations and the optimal level of PEEP probably depends on the patient’s phenotype based on a CT scan and lung mechanics.
Prone positioning, inhaled nitric oxide, respiratory dialysis, extracorporeal CO2 removal and extracorporeal membrane oxygenation can be considered as rescue therapies in selected patients with COVID-19, according to specific clinical and radiologic features.
Bacterial co-infection at hospital admission and during the hospital stay can occur in patients with COVID-19. Strict clinical and laboratory monitoring and evaluation are necessary to decide whether and when to start antibiotic therapy.
Thromboembolic complications are common in patients with COVID-19; in the absence of clear evidence regarding the best prophylactic and therapeutic treatment, patient management should be individualized based on the risk of bleeding and thrombosis and optimized case by case.
Dexamethasone has shown beneficial effects on reducing the 28-day mortality rate and should be recommended in mechanically ventilated patients requiring supplementary oxygen.
There is no evidence for routine use of antiviral and other adjuvant therapies in critically ill patients with COVID-19.
Author contributions
CR, DB, LB, PP, and PRMR contributed to the literature review and the drafting of the manuscript. All authors read and approved the final manuscript.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplementary material
Supplemental data for this article can be accessed here.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.COVID-ICU Group for the R Network and the C-I Investigators . Clinical characteristics and day-90 outcomes of 4,244 critically ill adults with COVID-19: a prospective cohort study. Intensive Care Med. 2020:1–14. DOI: 10.1007/s00134-020-06294-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Richards-Belle A, Orzechowska I, Gould DW, et al. COVID-19 in critical care: epidemiology of the first epidemic wave across England, Wales and Northern Ireland. Intensive Care Med. 2020;46(11):2035–2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vena A, Giacobbe DR, Di Biagio A, et al. Clinical characteristics, management and in-hospital mortality of patients with coronavirus disease 2019 in Genoa, Italy. Clin Microbiol Infect. 2020;26(11):1537–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Battaglini D, Robba C, Ball L, et al. Emerging therapies for COVID-19 pneumonia. Expert Opin Investig Drugs. 2020;29(7):633–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robba C, Battaglini D, Pelosi P, et al. Multiple organ dysfunction in SARS-CoV-2: MODS-CoV-2. Expert Rev Respir Med. 14(9): 865–868. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Although initially described as a viral pneumonia, SARS-CoV-2 infection seems to present features of a multisystem disease. Therefore, a new nomenclature was proposed, which includes the concept of multiple organ damage: multiple organ dysfunction in SARS-CoV-2 (MODS-CoV-2).
- 7.Robba C, Battaglini D, Ball L, et al. Distinct phenotypes require distinct respiratory management strategies in severe COVID-19. Respir Physiol Neurobiol. 2020;279:103455. [DOI] [PMC free article] [PubMed] [Google Scholar]; • COVID-19 pneumonia is characterized by different phenotypes, thus requiring distinct mechanical ventilation strategies.
- 8.Pelosi P, D’Onofrio D, Chiumello D, et al. Pulmonary and extrapulmonary acute respiratory distress syndrome are different. Eur Respir J. 2003;22(Supplement 42):48s–56s. [DOI] [PubMed] [Google Scholar]
- 9.Dorward DA, Russell CD, Um IH, et al. Tissue-specific immmunopathology in fatal COVID-19. Am J Respir Crit Care Med. 2021;203(2):192–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel BV, Arachchillage DJ, Ridge CA, et al. Pulmonary angiopathy in severe COVID-19: physiologic, imaging, and hematologic observations. Am J Respir Crit Care Med. 2020;202(5):690–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elinoff JM, Salit RB, Ackerman HC.. The tumor lysis syndrome. N Engl J Med. 2011;365:571–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gattinoni L, Camporota L, Marini JJ. COVID-19 phenotypes: leading or misleading? Eur Respir J. 2020;56(2):2002195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chiumello D, Busana M, Coppola S, et al. Physiological and quantitative CT-scan characterization of COVID-19 and typical ARDS: a matched cohort study. Intensive Care Med. 2020;46(12):2187–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herrmann J, Mori V, Bates JHT, et al. Modeling lung perfusion abnormalities to explain early COVID-19 hypoxemia. Nat Commun. 2020;11(1):4883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goligher EC, Jonkman AH, Dianti J, et al. Clinical strategies for implementing lung and diaphragm-protective ventilation: avoiding insufficient and excessive effort. Intensive Care Med. 2020;46(12):2314–2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dhont S, Derom E, Van Braeckel E, et al. The pathophysiology of ‘happy’ hypoxemia in COVID-19. Respir Res. 21(1): 198. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This study describe the mechanisms of “happy hypoxemia”.
- 17.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zucman N, Mullaert J, Roux D, et al. Prediction of outcome of nasal high flow use during COVID-19-related acute hypoxemic respiratory failure. Intensive Care Med. 2020;46(10):1924–1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sivaloganathan AA, Nasim-Mohi M, Brown MM, et al. Noninvasive ventilation for COVID-19-associated acute hypoxaemic respiratory failure: experience from a single centre. Br J Anaesth. 2020;125(4):e368–e371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Franco C, Facciolongo N, Tonelli R, et al. Feasibility and clinical impact of out-of-ICU noninvasive respiratory support in patients with COVID-19-related pneumonia. Eur Respir J. 2020;56(5):2002130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brochard L, Slutsky A, Pesenti A. Mechanical ventilation to minimize progression of lung injury in acute respiratory failure. Am J Respir Crit Care Med. 2017;195(4):438–442. [DOI] [PubMed] [Google Scholar]
- 22.Xia J, Zhang Y, Ni L, et al. High-flow nasal oxygen in coronavirus disease 2019 patients with acute hypoxemic respiratory failure: a multicenter, retrospective cohort study. Crit Care Med. 2020;48(11):e1079–e1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang K, Zhao W, Li J, et al. The experience of high-flow nasal cannula in hospitalized patients with 2019 novel coronavirus-infected pneumonia in two hospitals of Chongqing, China. Ann Intensive Care. 2020;10(1):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duan J, Chen B, Liu X, et al. Use of high-flow nasal cannula and noninvasive ventilation in patients with COVID-19: a multicenter observational study. Am J Emerg Med. 2020. DOI: 10.1016/j.ajem.2020.07.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Agarwal A, Basmaji J, Muttalib F, et al. High-flow nasal cannula for acute hypoxemic respiratory failure in patients with COVID-19: systematic reviews of effectiveness and its risks of aerosolization, dispersion, and infection transmission. Can J Anaesth. 2020;67(9):1217–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li J, Fink JB, Ehrmann S. High-flow nasal cannula for COVID-19 patients: low risk of bio-aerosol dispersion. Eur Respir J. 2020;55(5):2000892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Battaglini D, Robba C, Caiffa S, et al. Chest physiotherapy: an important adjuvant in critically ill mechanically ventilated patients with COVID-19. Respir Physiol Neurobiol. 2020;282:103529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bellani G, Grasselli G, Cecconi M, et al. Noninvasive ventilatory support of COVID-19 patients outside the intensive care units (WARd-COVID). Ann Am Thorac Soc. 2021. DOI: 10.1513/AnnalsATS.202008-1080OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aliberti S, Radovanovic D, Billi F, et al. Helmet CPAP treatment in patients with COVID-19 pneumonia: a multicentre cohort study. Eur Respir J. 2020;56(4):2001935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fan E, Del Sorbo L, Goligher EC, et al. An official American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine clinical practice guideline: mechanical ventilation in adult patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2017;195(9):1253–1263. [DOI] [PubMed] [Google Scholar]
- 31.Alhazzani W, Møller MH, Arabi YM, et al. Surviving sepsis campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Crit Care Med. 2020;48(6):e440–e469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Menk M, Estenssoro E, Sahetya SK, et al. Current and evolving standards of care for patients with ARDS. Intensive Care Med. 2020;46(12):2157–2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perchiazzi G, Pellegrini M, Chiodaroli E, et al. The use of positive end expiratory pressure in patients affected by COVID-19: time to reconsider the relation between morphology and physiology. Best Pract Res Clin Anaesthesiol. 2020;34(3):561–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alhazzani W, Møller MH, Arabi YM, et al. Surviving sepsis campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Intensive Care Med. 2020;46(5):854–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guérin C, Reignier J, Richard J-C, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368(23):2159–2168. [DOI] [PubMed] [Google Scholar]
- 36.Gleissman H, Forsgren A, Andersson E, et al. Prone positioning in mechanically ventilated patients with severe acute respiratory distress syndrome and coronavirus disease 2019. Acta Anaesthesiol Scand. 2020;65(3):aas.13741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mittermaier M, Pickerodt P, Kurth F, et al. Evaluation of PEEP and prone positioning in early COVID-19 ARDS. EClinicalMedicine. 2020;28:100579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pelosi P, Brazzi L, Gattinoni L. Prone position in acute respiratory distress syndrome. Eur Respir J. 2002;20(4):1017–1028. [DOI] [PubMed] [Google Scholar]
- 39.Ferrando C, Mellado-Artigas R, Gea A, et al. Awake prone positioning does not reduce the risk of intubation in COVID-19 treated with high-flow nasal oxygen therapy: a multicenter, adjusted cohort study. Crit Care. 24(1): 597. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Even though prone positioning has been used in awake COVID-19 patients, the risk of intubation was not reduced.
- 40.Bower G, He H. Protocol for awake prone positioning in COVID-19 patients: to do it earlier, easier, and longer. Crit Care. 2020;24(1):371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Caputo ND, Strayer RJ, Levitan R. Early self-proning in awake, non-intubated patients in the emergency department: a single ED’s experience during the COVID-19 pandemic. Acad Emerg Med. 2020;27(5):375–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carlos F, Ricard MA, Alfredo G, et al. Awake prone positioning does not reduce the risk of intubation in COVID-19 treated with high-flow nasal oxygen therapy: a multicenter, adjusted cohort study. Crit Care. 2020;24(1):597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koeckerling D, Barker J, Mudalige NL, et al. Awake prone positioning in COVID-19. Thorax. 2020;75(10):833–834. [DOI] [PubMed] [Google Scholar]
- 44.Bagate F, Tuffet S, Masi P, et al. Rescue therapy with inhaled nitric oxide and almitrine in COVID-19 patients with severe acute respiratory distress syndrome. Ann Intensive Care. 2020;10(1):151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Longobardo A, Montanari C, Shulman R, et al. Inhaled nitric oxide minimally improves oxygenation in COVID-19 related acute respiratory distress syndrome. Br J Anaesth. 2020;S0007–0912:30843–30846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tavazzi G, Marco P, Mongodi S, et al. Inhaled nitric oxide in patients admitted to intensive care unit with COVID-19 pneumonia. Crit Care. 2020;24(1):508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wiegand SB, Safaee Fakhr B, Carroll RW, et al. Rescue treatment with high-dose gaseous nitric oxide in spontaneously breathing patients with severe coronavirus disease 2019. Crit Care Explor. 2020;2(11):e0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peek GJ, Mugford M, Tiruvoipati R, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374(9698):1351–1363. [DOI] [PubMed] [Google Scholar]
- 49.Goligher EC, Tomlinson G, Hajage D, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome and posterior probability of mortality benefit in a post hoc Bayesian analysis of a randomized clinical trial. JAMA. 2018;320(21):2251. [DOI] [PubMed] [Google Scholar]
- 50.ELSO . COVID-19 registry dashboard [Internet]; 2020. Available from: https://www.elso.org/Registry/FullCOVID19RegistryDashboard.aspx
- 51.Cho HJ, Heinsar S, Jeong IS, et al. ECMO use in COVID-19: lessons from past respiratory virus outbreaks—a narrative review. Crit Care. 2020;24(1):301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Combes A, Fanelli V, Pham T, et al. Feasibility and safety of extracorporeal CO2 removal to enhance protective ventilation in acute respiratory distress syndrome: the SUPERNOVA study. Intensive Care Med. 2019;45(5):592–600. [DOI] [PubMed] [Google Scholar]
- 53.Amato MBP, Meade MO, Slutsky AS, et al. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med. 2015;372(8):747–755. [DOI] [PubMed] [Google Scholar]
- 54.Schultz MJ, Juffermans NP, Matthay MA, et al. From protective ventilation to super-protective ventilation for acute respiratory distress syndrome. Intensive Care Med. 2013;39(5):963–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Langford BJ, So M, Raybardhan S, et al. Bacterial co-infection and secondary infection in patients with COVID-19: a living rapid review and meta-analysis. Clin Microbiol Infect. 2020;26(12):1622–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sieswerda E, De Boer MGJ, Bonten MMJ, et al. Recommendations for antibacterial therapy in adults with COVID-19 – an evidence based guideline. Clin Microbiol Infect. 2020;27(1):61–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ding Q, Lu P, Fan Y, et al. The clinical characteristics of pneumonia patients coinfected with 2019 novel coronavirus and influenza virus in Wuhan, China. J Med Virol. 2020;92(9):1549–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Du Y, Tu L, Zhu P, et al. Clinical features of 85 fatal cases of COVID-19 from Wuhan. A retrospective observational study. Am J Respir Crit Care Med. 2020;201(11):1372–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bell BG, Schellevis F, Stobberingh E, et al. A systematic review and meta-analysis of the effects of antibiotic consumption on antibiotic resistance. BMC Infect Dis. 2014;14(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63:e61–e111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mason CY, Kanitkar T, Richardson CJ, et al. Exclusion of bacterial co-infection in COVID-19 using baseline inflammatory markers and their response to antibiotics. J Antimicrob Chemother. 2021;dkaa563. DOI: 10.1093/jac/dkaa563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Olson G, Davis AM. Diagnosis and treatment of adults with community-acquired pneumonia. JAMA. 2020;323(9):885. [DOI] [PubMed] [Google Scholar]
- 65.Tanzella G, Motos A, Battaglini D, et al. Optimal approaches to preventing severe community-acquired pneumonia. Expert Rev Respir Med. 2019;13(10):1005–1018. [DOI] [PubMed] [Google Scholar]
- 66.Rello J, Belliato M, Dimopoulos M-A, et al. Update in COVID-19 in the intensive care unit from the 2020 HELLENIC Athens international symposium. Anaesth Crit Care Pain Med. 2020;39(6):723–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dellière S, Dudoignon E, Fodil S, et al. Risk factors associated with COVID-19-associated pulmonary aspergillosis in ICU patients: a French multicentric retrospective cohort. Clin Microbiol Infect. 2020. DOI: 10.1016/j.cmi.2020.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Reyes L, Sanchez-Garcia MA, Morrison T, et al. Proteomics identifies a type I IFN, prothrombotic hyperinflammatory circulating COVID-19 neutrophil signature distinct from non-COVID-19 ARDS. medRxiv. 2020. DOI: 10.1101/2020.09.15.20195305. [DOI] [Google Scholar]
- 69.Klok FA, Kruip MJHA, Van Der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Carsana L, Sonzogni A, Ahmed Nasr A, et al. Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: a two-centre descriptive study. Lancet Infect Dis. 2020;20(10):1135–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tang N, Bai H, Chen X, et al. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 18(5): 1094–1099. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This clinical study reported the importance of anticoagulant treatment to reduce mortality rate in patients with severe COVID-19.
- 72.Atallah B, Mallah SI, AlMahmeed W. Anticoagulation in COVID-19. Eur Heart J Cardiovasc Pharmacother. 2020;6(4):260–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Spyropoulos AC, Levy JH, Ageno W, et al. Scientific and standardization committee communication: clinical guidance on the diagnosis, prevention, and treatment of venous thromboembolism in hospitalized patients with COVID‐19. J Thromb Haemost. 2020;18(8):1859–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhai Z, Li C, Chen Y, et al. Prevention and treatment of venous thromboembolism associated with coronavirus disease 2019 infection: a consensus statement before guidelines. Thromb Haemost. 2020;120(6):937–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shang L, Zhao J, Hu Y, et al. On the use of corticosteroids for 2019-nCoV pneumonia. Lancet. 2020;395(10225):683–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Qu TT, Yang Q, Yu MH, et al. A fatal Strongyloides stercoralis hyperinfection syndrome in a patient with chronic kidney disease: a case report and literature review. Medicine (Baltimore). 2016;95(19):e3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395(10223):473–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yang J-W, Yang L, Luo R-G, et al. Corticosteroid administration for viral pneumonia: COVID-19 and beyond. Clin Microbiol Infect. 2020;26(9):1171–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu Y, Lu H, Wang W, et al. Clinical risk factors for mortality in patients with cancer and COVID-19: a systematic review and meta-analysis of recent observational studies. Expert Rev Anticancer Ther. 2020;21(1):107–119. [DOI] [PubMed] [Google Scholar]
- 82.Horby P, Shen Lim W, Emberson JR, et al. Dexamethasone in hospitalized patients with Covid-19 — preliminary report. N Engl J Med. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]; • The RECOVERY trial established that a moderate dose of dexamethasone (6 mg daily for 10 days) reduced mortality in hospitalized patients with COVID-19 and respiratory failure who required therapy with supplemental oxygen or mechanical ventilation.
- 83.Angus DC, Derde L, Al-Beidh F, et al. Effect of hydrocortisone on mortality and organ support in patients with severe COVID-19. JAMA. 2020;324(13):1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tomazini BM, Maia IS, Cavalcanti AB, et al. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19. JAMA. 2020;324(13):1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sterne JAC, Murthy S, Diaz JV, et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19. JAMA. 2020;324(13):1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.De Backer D, Azoulay E, Vincent J-L. Corticosteroids in severe COVID-19: a critical view of the evidence. Crit Care. 2020;24(1):627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Angus DC, Derde L, Al-Beidh F, et al. Effect of hydrocortisone on mortality and organ support in patients with severe COVID-19: the REMAP-CAP COVID-19 corticosteroid domain randomized clinical trial. JAMA. 2020. October 6;324(13):1317–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang Y, Zhang D, Du G, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395(10236):1569–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Goldman JD, Lye DCB, Hui DS, et al. Remdesivir for 5 or 10 days in patients with severe Covid-19. N Engl J Med. 383(19): 1827–1837. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]; • In this open-label, randomized, multicenter, phase 3 trial among patients with severe Covid-19 pneumonia due to infection with SARS-CoV-2, the efficacy of remdesivir did not differ between 5-day and 10-day courses.
- 90.Wang Y, Zhou F, Zhang D, et al. Evaluation of the efficacy and safety of intravenous remdesivir in adult patients with severe COVID-19: study protocol for a phase 3 randomized, double-blind, placebo-controlled, multicentre trial. Trials. 2020;21(1):422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.WHO . COVID-19 treatment guidelines [Internet]. NIH; 2020. Available from: https://www.covid19treatmentguidelines.nih.gov/immune-based-therapy/ [Google Scholar]
- 92.Cao B, Wang Y, Wen D, et al. A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382(19):1787–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Maxmen A. More than 80 clinical trials launch to test coronavirus treatments. Nature. 2020;578(7795):347–348. [DOI] [PubMed] [Google Scholar]
- 94.Yao X, Ye F, Zhang M, et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin Infect Dis. 2020;71(15):732–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.FDA . FDA cautions against use of hydroxychloroquine or chloroquine for COVID-19 outside of the hospital setting or a clinical trial due to risk of heart rhythm problems; 2020. [cited 2020 July1]. Available from: https://www.fda.gov/drugs/drug-safety-and-availability/fda-cautions-against-use-hydroxychloroquine-or-chloroquine-covid-19-outside-hospital-setting-or
- 96.Geleris J, Sun Y, Platt J, et al. Observational study of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. 2020;382(25):2411–2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ortiz-Alcantara J, Bhardwaj K, Palaninathan S, et al. Small molecule inhibitors of the SARS-CoV Nsp15 endoribonuclease. Virus Adapt Treat. 2010;2:125. [Google Scholar]
- 98.Duan K, Liu B, Li C, et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci. 2020;117(17):9490–9496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chai KL, Valk SJ, Piechotta V, et al. Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a living systematic review. Cochrane Database Syst Rev. 2020;10:CD013600. [DOI] [PubMed] [Google Scholar]
- 100.Li L, Zhang W, Hu Y, et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19. JAMA. 324(5): 460. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]; • In patients with severe COVID-19, convalescent plasma therapy, compared with standard treatment alone, did not result in a statistically significant improvement in time to clinical improvement within 28 days.
- 101.Salvarani C, Dolci G, Massari M, et al. Effect of tocilizumab vs standard care on clinical worsening in patients hospitalized with COVID-19 pneumonia. JAMA Intern Med. 2020:; 181(1): 24-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mitchell V, Dravid R, Patel A, et al. Difficult airway society guidelines for the management of tracheal extubation. Anaesthesia. 2012;67(3):318–340. [DOI] [PubMed] [Google Scholar]
- 103.Ovadya D, Bachar K, Peled M, et al. Weaning of severe COVID-19 mechanically ventilated patients: experience within a dedicated unit in Israel. Isr Med Assoc J. 2020;22(12):733–735. [PubMed] [Google Scholar]
- 104.Lazzeri M, Lanza A, Bellini R, et al. Respiratory physiotherapy in patients with COVID-19 infection in acute setting: a position paper of the Italian Association of Respiratory Physiotherapists (ARIR). Monaldi Arch Chest Dis. 2020;90(1). DOI: 10.4081/monaldi.2020.1285 [DOI] [PubMed] [Google Scholar]
- 105.Kangas-Dick AW, Swearingen B, Wan E, et al. Safe extubation during the COVID-19 pandemic. Respir Med. 2020;170:106038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.D’Silva DF, McCulloch TJ, Lim JS, et al. Extubation of patients with COVID-19. Br J Anaesth. 2020;125(1):e192–e195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Botti C, Lusetti F, Peroni S, et al. The role of tracheotomy and timing of weaning and decannulation in patients affected by severe COVID-19. Ear Nose Throat J. 2020;100(2_suppl):116S-119S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Avilés-Jurado FX, Prieto-Alhambra D, González-Sánchez N, et al. Timing, complications, and safety of tracheotomy in critically ill patients with COVID-19. JAMA Otolaryngol Neck Surg. 2020;147(1):1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.McGrath BA, Brenner MJ, Warrillow SJ, et al. Tracheostomy in the COVID-19 era: global and multidisciplinary guidance. Lancet Respir Med. 2020;8(7):717–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Quah P, Li A, Phua J. Mortality rates of patients with COVID-19 in the intensive care unit: a systematic review of the emerging literature. Crit Care. 2020;24(1):285. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


