ABSTRACT
Objective: Efficacy and safety of Itolizumab, an immunomodulatory mAb, in treating moderate-to-severe acute respiratory distress syndrome (ARDS) due to cytokine release in COVID-19 patients was evaluated in a multi-centric, open-label, two-arm, controlled, randomized, phase-2 study.
Methods: Patients were randomized (2:1) to Arm-A (best supportive care [BSC]+Itolizumab) and Arm-B (BSC). Primary outcome of interest was reduction in mortality 30-days after enrollment.
Results: Thirty-six patients were screened, five treated as first-dose-sentinels and rest randomized, while four patients were screen-failures. Two patients in Arm-A discontinued prior to receiving one complete infusion and were replaced. At end of 1-month, there were three deaths in Arm-B, and none in Arm-A (p = 0.0296; 95% CI = −0.3 [−0.61, −0.08]). At end of study, more patients in Arm-A had improved SpO2 without increasing FiO2 (p = 0.0296), improved PaO2 (p = 0.0296), and reduction in IL-6 (43 vs 212 pg/ml; p = 0.0296) and tumor necrotic factor-α (9 vs 39 pg/ml; p = 0.0253) levels. Transient lymphopenia (Arm-A: 11 patients) and infusion reactions (7 patients) were commonly reported treatment-related safety events.
Conclusion: Itolizumab is a promising, safe and effective immunomodulatory therapy for treatment of ARDS due to cytokine release in COVID-19 patients, with survival and recovery-benefit.
KEYWORDS: Itolizumab, COVID-19, coronavirus, acute respiratory distress syndrome, cytokine release syndrome, immunotherapy, anti-CD6, immune hyperactivation
1. Introduction
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2; COVID-19) poses a serious global concern for public health, with nearly 125 million people infected worldwide and more than 2.7 million dead [1,2]. Among other things, the SARS-CoV-2 infection sets off an inflammatory cascade resulting in an increased release of pro-inflammatory cytokines and chemokines, especially IL-1, IL-6, IL-12, IFN-γ, and TNF-α during the third phase of SARS-CoV-2 disease progression [3,4]. These proinflammatory molecules potentiate a Th1 (T helper-1) response, causing the recruitment of monocytes and T-lymphocytes resulting in peripheral lymphopenia and higher neutrophil:lymphocyte ratio typically observed in patients suffering from COVID-19 [5–7]. If untreated, this cytokine release syndrome may lead to vascular hypo-responsiveness, increased endothelial permeability, hypercoagulation, multi-organ dysfunction and eventually death [8,9].
Targeting T-cells and their involvement in excessive cytokine release during the management of SARS-CoV-2 disease has been one of the therapeutic strategies adopted to improve survival rates and reduce mortality [10–12]. CD6 is a costimulatory receptor differentially expressed on T-cells, subsets of innate lymphoid and natural killer cells, but not on T-regulatory cells (Tregs) [13]. The binding of CD6 to the activated leukocyte cell adhesion molecule (ALCAM), expressed in both the antigen presenting cells and endothelial/epithelial tissue, including the blood-brain barrier, skin, gut, lung and kidney, can modulate T-cell activity and trafficking [14–16]. CD6-ALCAM pathway is now being unraveled as central to immune-hyperinflammation with clinical efficacy and safety established in psoriasis and rheumatoid arthritis, trials ongoing for its involvement in Systemic Lupus Erythematosus and Lupus Nephritis among other autoimmune conditions (NCT04128579; https://clinicaltrials.gov/ct2/show/NCT04128579).
Itolizumab is a humanized IgG1 kappa anti-CD6 monoclonal antibody that binds to domain 1 of human CD6. It selectively targets the CD6-ALCAM pathway resulting in decreased levels of IFN-γ, IL-6, and TNF-α through Th-1 pathway and IL-17, IL-6, TNFα through Th-17 pathway [14,15]. Itolizumab thereby leads to a reduction in the T-cell infiltration at the sites of inflammation, without inducing T-cell or B-cell depletion [14].
Itolizumab has been approved in India for the treatment of moderate-to-severe chronic plaque psoriasis since 2013 [17–20]. It has shown promising results in managing psoriatic arthritis [21,22] and rheumatoid arthritis [23,24] in addition to a favorable safety profile in Phase 2 and Phase 3 trials. Based on the periodic reviews of safety data, the overall safety profile evaluation for Itolizumab has remained consistent over the years. The most commonly reported adverse evets (AEs), occurring in ≥5% chronic plaque psoriasis patients, in Phase 3, have been infusion reactions, pyrexia, upper respiratory tract infections, and pruritus with the majority of the events being mild or moderate in severity [25].
Based on its mechanism of action (Figure 1) we hypothesized that Itolizumab would control the pro-inflammatory cytokine release in COVID-19 patients by immunomodulation of (Teff) effector function and trafficking to the inflammation site while sparing Tregs and preserving the anti-viral response, thereby reducing morbidity and mortality. The current study was undertaken to estimate the efficacy and safety of Itolizumab in the management of cytokine release due to moderate to severe acute respiratory distress in COVID-19 patients.
Figure 1.
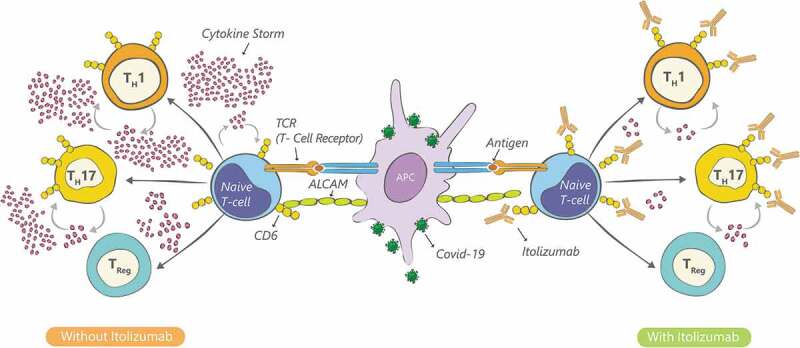
Itolizumab mechanism of action in COVID-19 infection
2. Methods
2.1. Study design
This was an open-label, two-arm, randomized, controlled, multi-centric, phase-2 study conducted in four designated COVID-19 hospitals in India. Initial dosing was done for the first five patients in a staggered manner wherein after a patient was dosed, safety was monitored for 24–48 hours prior to dosing the next patient. Once all five patients were dosed in this staggered manner, subsequent patients were enrolled in a way that the study had patients randomized in a 2:1 ratio. Randomization was centrally done using computer-generated sequences (SAS version 9.4). Patients who were randomized, but did not receive the full infusion, were considered unevaluable and the same randomization code was allocated to the next patient enrolled by the study site. The CONSORT flow diagram for the study is summarized in Figure 2. The study was initiated on 2 May 2020 and all patients were followed up for 30 days. The study closed-out on 7 July 2020 after the follow-up period of the final patient was completed, and all patients in the trial had either been discharged from clinical care or had died of COVID-19 complications.
Figure 2.
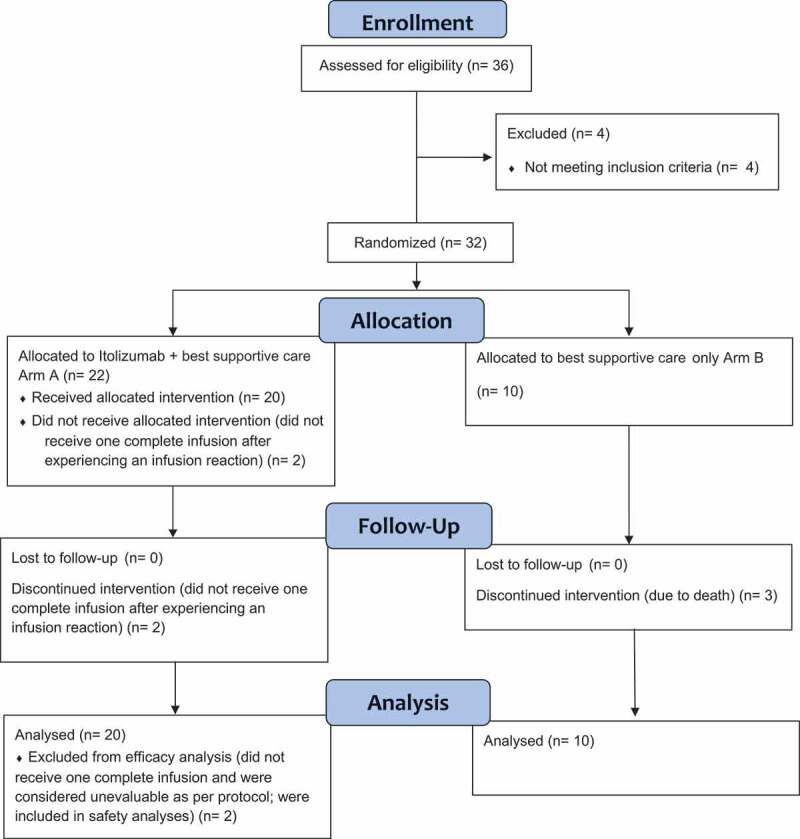
CONSORT 2010 flow diagram
2.2. Study subjects
Adult male or female patients above 18 years, who tested positive for virologic diagnosis of SARS-CoV-2 infection (RT-PCR), and who were hospitalized due to clinical worsening with oxygen saturation ≤94% at rest in ambient air, were eligible for randomization if they had either moderate to severe ARDS and/or high levels of proinflammatory markers. Patients were defined to have moderate to severe ARDS if they had PaO2/Fio2 ratio of <200 or more than 25% deterioration from the immediate previous value. Alternatively, the proinflammatory markers included were baseline serum ferritin level ≥400 ng/mL or IL-6 levels greater than 4 times of upper limits of normal value.
Major exclusion criteria included – known severe allergic reactions to monoclonal antibodies, an active tuberculosis (TB) infection/inadequately treated tuberculosis/latent tuberculosis, those on oral anti-rejection or any immune-suppressive drug in the last 6 months and those who had participated in any drug clinical trial using anti-IL-6 therapy. Patients with a known history of Hepatitis B, Hepatitis C or HIV, absolute neutrophil count (ANC) <1000/mm3, platelet count <50,000/mm3 and absolute lymphocyte count (ALC) <500/mm3 were also excluded.
2.3. Study settings
The study was carried out at four COVID-19 specific hospitals in India. Two of these sites were in New Delhi and two were in Mumbai. All four sites were tertiary, teaching hospitals, with considerable experience of undertaking clinical trials.
2.4. Treatments
Oxygen, antibiotics, hydroxychloroquine, antivirals, steroids, low-molecular-weight heparin, and vitamin supplements were used as a part of the best supportive care in both treatment arms. Supplementary Table 1 shows the best supportive care received by both the arms.
Table 1.
Participant disposition, demographic and baseline characteristics
|
Variable |
Arm-A(N = 20) |
Arm-B(N = 10) |
|---|---|---|
| Participant disposition | ||
| FAS Population,* n (%) | 20 (100) | 10 (100) |
| Safety Population,** n (%) | 22 (110) | 10 (100) |
| Completed the study, n (%) | ||
| Completed 30 days follow up in hospital | 4 (20) | 1 (10) |
| Early discharged** | 16 (80) | 6 (60) |
| Discontinued, n (%) | - | 3 (30) |
| Reasons for Discontinuation, n (%) | ||
| Death |
- |
3 (30) |
|
Demographic and baseline characteristics | ||
| Age (years) | ||
| N | 20 | 10 |
| Mean (SD) | 49.55 (12.49) | 48.30 (14.62) |
| Sex, n (%) | ||
| Female | 1 (5) | 3 (30) |
| Male | 19 (95) | 7 (70) |
| Race, n (%) | ||
| Asian | 20 (100) | 10 (100) |
| Ethnicity, n (%) | ||
| South Asian | 19 (95) | 10 (100) |
| Southeast Asian | 1 (5) | - |
| D-Dimer (mcg/ml (FEU)) Mean (SD) |
3.50 (4.87) | 5.15 (7.85) |
| D-Dimer (mcg/ml (FEU)) Median (Range) |
1.86 (0.28–20.0) | 1.59 (0.28–20.0) |
| Ferritin (ng/ml) Mean (SD) |
943.34 (756.06) | 577.95 (336.73) |
| Ferritin (ng/ml) Median (Range) |
669.79 (100–2550.7) | 496.93 (90.70–1290.2) |
| LDH (U/L) Mean (SD) |
533.3 (206.85) | 645.3 (292.79) |
| LDH (U/L) Median (Range) |
512 (254–1125) | 555 (375–1150) |
| C-Reactive Protein (mg/L) Mean (SD) |
73.74 (71.84) | 103.88 (87.89) |
| C-Reactive Protein (mg/L) Median (Range) |
58.15 (5.47–254.3) | 76.9 (19.90–275.4) |
| Duration of COVID-19 related symptoms at enrollment (in Days) Mean (SD) |
8.55 (6.21) | 5.60 (2.59) |
| Duration of COVID-19 related symptoms at enrollment (in Days) Median (Range) |
7.5 (1–26) | 5.5 (3–11) |
| Absolute Lymphocyte Count Mean (SD) |
969.85 (407.70) | 1357.3 (492.30) |
*FAS: Full analysis set defined as all patients randomized and those who received at least one full dose of Itolizumab (Arm A); Safety population was defined as all patients randomized (in Arm B) and those who received partial or full dose of Itolizumab (in Arm A)
** 2 subjects could not complete even one dosing and were replaced as per protocol
At randomization, patients requiring oxygen therapy were either on non-rebreather mask (NRBM), Venturi Mask, noninvasive ventilation (Bilevel positive airway pressure [BiPAP], continuous positive airway pressure [CPAP]) or a face mask (FM). Supplementary Table 2 gives the patient oxygen delivery mode at randomization.
Table 2.
Patients with stable/improved SpO2 without increasing FiO2 and patients with stable/improved PaO2 without increasing FiO2.
|
Patients with Stable/Improved SpO2 without Increasing FiO2a | |||
|---|---|---|---|
| Visit | Arm-A(N = 20) | Arm-B(N = 10) | P-value |
| Day 7, n (%) | 17 (85) | 5 (50) | 0.0778 |
| Day 14b, n (%) | 19 (95) | 7 (70) | 0.0952 |
| Day 21, n (%) | 20 (100) | 7 (70) | 0.0296 |
| Day 30, n (%) | 20 (100) | 7 (70) | 0.0296 |
| Patients with Stable/Improved PaO2 Without Increasing FiO2c | |||
| Visit | Arm-A(N = 20) | Arm-B(N = 10) | P-value |
| Day 7, n (%) | 18 (90) | 6 (60) | 0.1413 |
| Day 14d, n (%) | 19 (95) | 7 (70) | 0.0952 |
| Day 21, n (%) | 20 (100) | 7 (70) | 0.0296 |
| Day 30, n (%) | 20 (100) | 7 (70) | 0.0296 |
aStable SpO2 was defined as absence of increase in FiO2 to maintain Spo2 ≥ 92% and improvement of SpO2 was defined as decrease in FiO2 to maintain SpO2 > 92%.
bPatients improved/weaned off O2, the observation was carried forward; three patients in Arm-B died on Day 4, 5 and 12; p-value between arm is estimated using Fisher’s exact test (p-value <0.05 is considered significant)
cStable PaO2 was defined as up to 10% change in PaO2/FiO2 ratio from baseline while an improvement of PaO2 was defined as >10% improvement in PaO2/FiO2 ratio from baseline (including patients weaned off oxygen).
dPatients improved/weaned-off oxygen, the observation was carried forward; three patients in Arm-B died on Days 4, 5 and 12; p-value between arm is estimated using Fisher’s exact test (p-value <0.05 is considered significant)
Since Itolizumab was re-purposed for the management of COVID-19 complications, its clinically relevant immunomodulatory dose was calculated based on previous clinical experience and in vitro data. Itolizumab dose ranges from 0.4 to 1.6 mg/kg which is equivalent to 10–40 μg/ml dose in vitro [14]. Receptor occupancy studies indicate that CD6 receptors are fully saturated at this range of Itolizumab. In addition, it has been shown that the maximum inhibitory potential of Itolizumab also lies within this range [17]. Doses of 0.8 mg/kg and 1.6 mg/kg have been previously used in rheumatoid arthritis and chronic plaque psoriasis patients [19,20,23–26].
Itolizumab was diluted in 250 ml of normal (0.9%) saline and was allowed to reach room temperature prior to infusion. Itolizumab infusion in Arm-A started after pre-medication with Hydrocortisone 100 mg i.v (or equivalent short acting glucocorticoid) and Pheniramine 30 mg i.v. about 30 ± 10 minutes prior to infusion. Patients were initiated on 1.6 mg/kg i.v infusion of Itolizumab and continued with a 0.8 mg/kg dose weekly regimen, if required, based on previous clinical experience, as stated above. If the patient recovered, the subsequent doses were modified, deferred, or stopped as per the investigator’s discretion. As Itolizumab was being used in COVID-19 indication for the first time, post the first infusion, the second Itolizumab dose was repeated after 7 days, as per the investigator’s discretion, and so were the subsequent doses. Investigators' decision was based on the levels of inflammatory markers, clinical status, oxygen requirement and safety concerns (if any). The maximum number of doses that could be administered was 4. Supplementary Table 3 mentions the number of doses provided to each patient.
Table 3.
Mean change from baseline values for inflammatory markers
| Ferritin (ng/mL) | |||||
|---|---|---|---|---|---|
| Day 7 | Day 14 | Day 21 | Day 30 | ||
| Arm-A | −117.8 | −713.9 | −780.9 | −479.3 | |
| Na | 18 | 15 | 11 | 3 | |
| Arm-B | −87.05 | −209.6 | 4238 | −234.4 | |
| Nb | 7 | 5 | 3 | 2 | |
| D-dimer (µg/mL FEU) | |||||
| Day 7 | Day 14 | Day 21 | Day 30 | ||
| Arm-A | −1.43 | −0.45 | −4.35 | −2.63 | |
| Na | 18 | 12 | 11 | 3 | |
| Arm-B | 2.3 | −0.68 | 8.54 | −0.35 | |
| Nb | 7 | 4 | 2 | 2 | |
| LDH (U/L) | |||||
| Day 7 | Day 14 | Day 21 | Day 30 | ||
| Arm-A | −134 | −195.8 | −308.1 | −212.7 | |
| Na | 18 | 15 | 11 | 3 | |
| Arm-B | −44.29 | −195.2 | 155.3 | −97 | |
| Nb | 7 | 5 | 3 | 2 | |
| CRP (mg/L) | |||||
| Day 7 | Day 14 | Day 21 | Day 30 | ||
| Arm-A | −61.69 | −81.65 | −90.99 | −103.2 | |
| Na | 18 | 16 | 11 | 3 | |
| Arm-B | −103.6 | −107.2 | −127.5 | −127.6 | |
| Nb | 8 | 5 | 3 | 2 | |
aNumber of patients in Arm-A at given time points.
bNumber of patients in Arm-B at given time points.Values calculated based on number of patients at each time point.
The Itolizumab infusion was administered over a period of not less than 120 minutes, using an infusion set with an in-line, sterile, non-pyrogenic, low protein-binding filter (pore size of 1.2 μm or less). Approximately 50 mL of the infusion solution was administered during the first hour, followed by the remaining solution (approximately 200 mL) in the next hour. Infusion period could be extended up to 8 hours for medical reasons, particularly if the patient experienced infusion-related reactions, which needed medical attention prior to re-initiation of infusion. Itolizumab was not infused concomitantly in the same i.v line with any other agents.
2.5. Ethics
This study was carried out in accordance with the ethical principles described in the Declaration of Helsinki (64th WMA General Assembly, Fortaleza, Brazil, October 2013), the International Council for Harmonization Good Clinical Practice (ICH GCP) E6 (R2), and New Drugs and Clinical Trials Rule 2019 issued by the Government of India. The study received approvals from the IECs/IRBs of all the participating sites. All the IECs/IRBs had active CDSCO registration at the time of approving this study. Subjects provided written informed consent prior to initiation of the study procedures. Study data were periodically reviewed by a data and safety monitoring board (DSMB).
2.6. Study objectives and endpoints
The current study was undertaken to evaluate the efficacy and safety of Itolizumab in the management of patients with cytokine release syndrome (CRS) in moderate to severe acute respiratory distress syndrome (ARDS) due to COVID-19. Secondary Objective was to assess possible correlations/associations between cytokine markers and clinical efficacy/safety.
The study’s primary outcome measures included:
1. Reduction in mortality 1 month after randomization
2. Reduction in the proportion of patients with deteriorating lung functions, as measured by:
a. Stable SpO2 without increasing FiO2
b. Stable PaO2 without increasing FiO2
3. Reduction in proportion of patients who needed noninvasive ventilation, invasive mechanical ventilation/endotracheal intubation, and high flow nasal oxygen.
4. eduction in inflammatory markers: Ferritin, D-dimer, LDH, CRP.
Key secondary outcome measures included measurement of:
Biomarkers such as IL-6, TNF-α, IL-17A
Absolute lymphocyte count
PaO2/FiO2 ratio calculated from arterial blood gas analyses
Safety: Number of participants with treatment-related side effects as assessed by Common Terminology Criteria for Adverse Event (CTCAE) version 5.0
2.7. Biomarker assessments
Blood samples were collected for analysis of cytokines/chemokines.
2.8. Statistical analysis
For this Phase 2 study, carried out amidst pandemic conditions, we considered enrolling 30 patients. Continuous variables were summarized using descriptive statistics such as mean, standard deviation, 95% confidence interval (CI), or median with range, as appropriate. Categorical variables were summarized using proportions (counts and percentages). Comparisons between proportions were done using Fisher’s exact test since the sample size was small. While the chi-squared test relies on an approximation, Fisher’s exact test is an exact test, especially, when more than 20% of cells have expected frequencies <5. We have used Fisher’s exact test because applying the approximation method is inadequate & Fisher’s exact test assesses the null hypothesis of independence by applying hypergeometric distribution of the numbers in the cells of the table. For continuous variables, change from baseline or trends in change over time were tabulated, as appropriate. All statistical tests were performed at 5% level of significance (two-sided test) and p-value <0.05 was considered statistically significant. All statistical analysis was performed using SAS® (version 9.4) software. In this study, the 95% CI were estimated using the NCSS software. 95% CI of the difference between proportions was estimated using Chen’s Quasi-Exact Method. This method produces intervals that are close to unconditional exact intervals. Chen’s method inverts a hypothesis test based on Farrington and Manning’s method.
2.9. Trial registration details
The trial protocol was registered with the Clinical Trials Registry of India (CTRI). The CTRI registration number is CTRI/2020/05/024959. The trial was prospectively registered with the CTRI, which is the government mandated registry for trials in India.
3. Results
3.1. Participant disposition and baseline characteristics
A total of 36 patients were screened, of which 4 were considered screen failures; 1 patient was COVID-19 negative and ALC count of 3 patients was <500 cells/cu.mm. A total of 32 patients were randomized: 22 patients in Arm-A and 10 patients in Arm-B (Table 1). Two patients from Arm-A discontinued treatment prior to completion of first dosing due to infusion-related reactions and were replaced as defined above. The events of infusion-related reactions (IRRs) were resolved on the same day and the patients continued to receive best supportive care. A total of 27 patients (Arm-A: 20 and Arm-B: 7) completed the study; 3 patients in Arm-B discontinued due to death. The deaths happened on days 4, 5 and 12 despite the best supportive care as per the Institute’s protocol. All 20 patients in Arm-A had at least one complete infusion of Itolizumab; of these, 7 patients had two infusions; 3 patients had three infusions and 4 patients had four infusions.
The mean age of patients in Arm-A was 49.5 years and in Arm-B was 48.3 years (Table 1). All patients were of Asian ethnicity with most of the patients being male (Arm-A: 95% and Arm-B: 70%).
The mean duration of COVID-19 related symptoms at enrollment was 8.6 days and 5.6 days for Arms A and B, respectively, and the difference was not statistically significant. The most frequently reported COVID-19 related symptoms in both treatment arms were fever and dyspnea, followed by cough and tachypnea. Hypertension was the most common active co-morbid condition (20% in each arm).
3.2. Primary outcome measures
3.2.1. Mortality at 1 month
Itolizumab treatment had a noticeable improvement onpatients' survival through reduction in 1-month mortality rate. A statistically significant difference (p = 0.029; 95% CI = −0.3 [−0.61, −0.08]) in the 1-month mortality rate was observed between the 2 treatment arms. Three deaths were reported in Arm-B on Days 4, 5 and 12 (1 due to acute respiratory distress and 2 due to respiratory failure). There were no deaths in Arm-A.
3.2.2. Lung function determined by SpO2 and PaO2
3.2.2.1. Stable/improved SpO2
A higher proportion of patients in Arm-A had stable/improved SpO2 without increasing FiO2 in all post-baseline assessment visits in comparison to Arm-B (Table 2). A significant difference was observed between the two arms from Day 21 onwards; 100% of the participants in Arm-A showed favorable outcomes compared to only 70% in Arm-B (p = 0.029).
3.2.2.2. Stable/improved PaO2
A higher proportion of patients in Arm-A had stable PaO2 without increasing FiO2 in all the post-baseline assessment visits in comparison to Arm-B (Table 2). Significant difference was observed Day 21 onwards; 100% in Arm-A compared to 70% in Arm-B (p = 0.029)
3.2.3. Noninvasive ventilation, invasive mechanical ventilation/endotracheal intubation, and high flow nasal oxygen
In Arm-A, there were 5 patients on NIV (BiPAP or CPAP) at baseline who improved and came off NIV by Day 14. In Arm-B, there were 4 patients on NIV at baseline of which one patient improved and came off NIV by Day 14. Condition of the three remaining patients in Arm-B, which included one patient who continued to be on NIV and 2 patients who progressed to IMV before Day 7, further worsened and all of them died by Day 12. All patients in Arm-A were progressively weaned off oxygen by Day 30, with 5, 14, 18 and 20 patients getting weaned by days 7, 14, 21 and 30, respectively. In Arm-B, 2, 4, 6 and 7 patients were progressively weaned off oxygen on Days 7, 14, 21 and 30, respectively. Supplementary Table 4 mentions the number of patients going from noninvasive ventilation to no requirement of oxygen.
Table 4.
Mean PaO2/FiO2 ratio over time
| - | Baseline | Day 7 | Day 14 | Day 21 | Day 30/EOS |
|---|---|---|---|---|---|
| Arm-A (n) | 20 | 16 | 14 | 8 | 3 |
| Mean (SD) | 126.57(38.31) | 203.50(95.51) | 283.43(104.26) | 350.25(70.36) | 397.67(15.63) |
| Arm-B (n) | 10 | 6 | 5 | 3 | 0 |
| Mean (SD) | 114.05(30.93) | 184.53(95.51) | 338.40(42.57) | 398.33(24.01) | |
| p-value | 0.34606 | 0.68263 | 0.12243 | 0.12566 | NA |
3.2.4. Inflammatory markers (related to primary outcomes)
3.2.4.1. Ferritin
Baseline ferritin was high in Arm-A compared to Arm-B (943.34 ng/mL vs 577.95 ng/mL). In Arm-A, the mean ferritin reduced to 303.50 (SD 210.93) ng/dL and 189.22 (SD 129.96) ng/dL on Day 14 and 30, respectively. In Arm-B, ferritin was 367.68 (SD 130.22) ng/dL and 285.25 (SD 157.76) ng/dL on Days 14 and 30, respectively. A greater reduction from baseline in serum ferritin levels was seen in Arm-A (−479.3 (620.95) ng/dL) in comparison to Arm-B (−234.4 (405.67) ng/dL) at day 30 (p-value = 0.078).
3.2.4.2. D-dimer
Baseline D-dimer was higher in Arm-B (5.15 (SD 7.85) µg/mL) compared to Arm-A (3.50 (SD 4.87) µg/mL). In Arm-A, mean D-dimer reduced to 2.83 (SD 5.46) µg/mL and 0.41 (SD 0.18) µg/mL on Days 14 and 30, respectively. In Arm-B, mean D-dimer was 0.86 (SD 0.71) µg/mL and 1.15 (SD 0.37) µg/mL on Days 14 and 30, respectively. Eight patients in Arm-A and five patients in Arm-B received low-molecular-weight heparin. P-values for D-dimer between two groups were non-significant (0.483).
3.2.4.3. LDH
Baseline lactate dehydrogenase (LDH) was comparable in both arms; 533.30 (SD 206.85) U/L in Arm-A and 645.30 (SD 292.79) U/L in Arm-B. In Arm-A, mean LDH reduced to 381.47 (SD 181.45) U/L and 208.67 (SD 40.72) U/L on Days 14 and 30, respectively. In Arm-B, mean LDH was 330.20 (SD 91.63) U/L and 456.50 (SD 173.24) U/L on Days 14 and 30, respectively. P-value for LDH between two groups was non-significant (0.298).
3.2.4.4. CRP
Baseline C-reactive protein (CRP) was numerically higher in Arm-B. It was 73.74 (SD 71.84) mg/L in Arm-A vs 103.88 (SD 87.89) mg/L in Arm-B. In Arm-A, mean CRP reduced to 6.45 (SD 4.14) mg/L and 13.69 (SD 20.45) mg/L on Days 14 and 30, respectively. In Arm-B, mean CRP was 14.36 (9.29) mg/L and 3.05 (2.62) mg/L on Days 14 and 30, respectively. P-value for CRP between the two groups was non-significant (p-value = 0.362).
The mean change from baseline values, over time, is captured in Table 3. The number of patients at each time point varied due to patients reaching the end of follow-up either due to discharge from clinical care or death.
3.3. Secondary outcome measures
3.3.1. Biomarkers
3.3.1.1. IL-6
Mean baseline value of IL-6 was comparable in both arms; 159.09 pg/mL in Arm-A and 162.16 pg/mL in Arm-B. A significant decline (p = 0.027) in mean IL-6 levels post first infusion was seen in Arm-A (42.98 pg/mL) compared to Arm-B (211.52 pg/mL) (Figure 3). P-value for IL-6 between the two groups was significant (0.027).
Figure 3.

Mean IL-6 values
3.3.1.2. TNF-α
Mean baseline value of TNF-α was higher in Arm-A (43.64 pg/mL) than in Arm-B (11.26 pg/mL). A significant decline (p = 0.025) in mean TNF-α levels post first infusion was seen in Arm-A (8.87 pg/mL) compared to Arm-B (39.19 pg/mL) (Figure 4). P-value for TNF-α between the two groups was significant (0.025).
Figure 4.
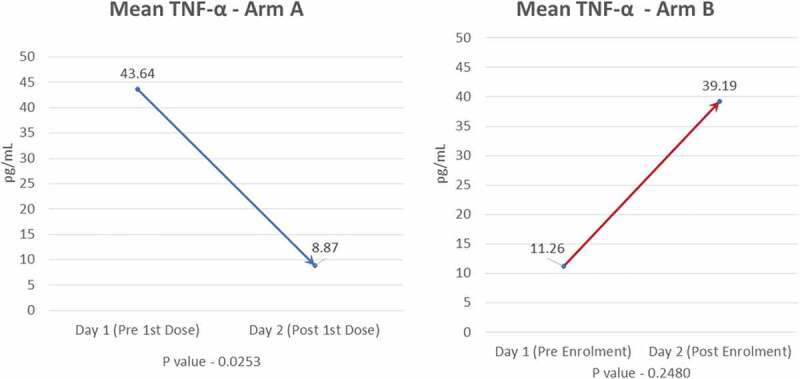
Mean TNF-α
3.3.1.3. IL-17A
Mean baseline value of IL-17A was comparable in both arms; 10.36 pg/mL in Arm-A and 9.83 pg/mL in Arm-B. A notable decline in mean IL-17A levels post first infusion was seen in Arm-A (6.75 pg/mL) unlike in Arm-B, where there was an increase (14.75 pg/mL). P-value for IL-17A between the two groups was non-significant (0.418).
3.3.2. Absolute lymphocyte count
Baseline ALC was numerically lower in Arm-A (969.85 cells per mm3) than in Arm-B (1357.3 cells per mm3). A gradual increase over time in mean ALC was seen in Arm-A in comparison to Arm-B (Figure 5). Eleven patients in Arm-A and two patients in Arm-B had a grade 3 event of post-infusion lymphopenia, which was transient and recovered spontaneously by day 7.
Figure 5.
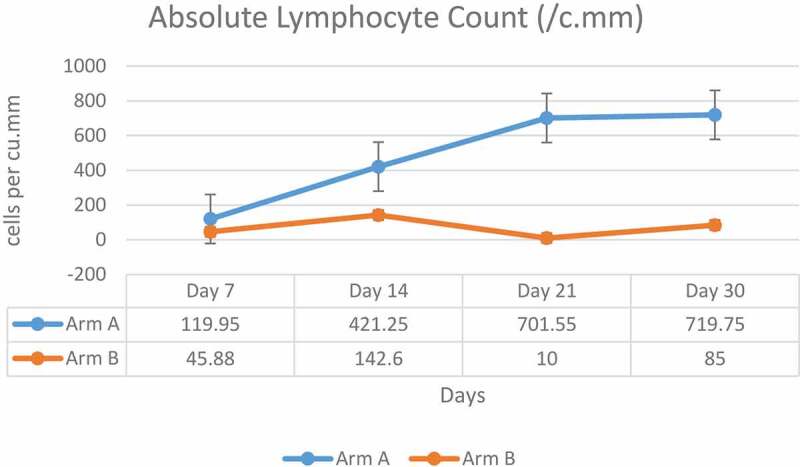
Mean absolute lymphocyte count
3.3.3. PaO2/FiO2 ratio
At baseline, mean PaO2/FiO2 ratio was numerically higher in Arm-A (126.57; SD 38.31) vs Arm-B (114.05; SD 30.93). PaO2/FiO2 ratio gradually increased over time in Arm-A in comparison to Arm-B. The change from baseline observed at various time points is mentioned in Table 4. Given that there were censoring events (discharged from care or death) over time, the number of patients in each arm varied at each time point.
3.3.4. Safety
During the treatment period, a total of 22 patients experienced at least one treatment emergent adverse event (TEAE); 18 (81.8%) patients in Arm-A and 4 (40%) patients in Arm-B. Transient lymphocyte count decrease was the most commonly reported TEAE in both arms in addition to lower respiratory tract infection, ARDS and respiratory failure in Arm-B (Table 5). Lymphocyte count decrease was the most frequently reported study drug-related TEAE reported in 50% of patients in Arm-A (n = 11). These events were reported between Day 2 to Day 4 and returned to normal by Day 7. The hyperglycemic events reported in four patients in Arm-A were considered as not related to study drug. There was an event of sinus tachycardia reported in one patient in Arm-A during the study. The patient recovered and the event was considered as not related to the study drug. Other TEAEs are listed in Table 5.
Table 5.
Treatment emergent adverse events by treatment group (Safety population)
|
System Organ Class (n, %) Preferred Term (n, %) |
Arm-A(N = 22)a | Arm-B(N = 10) |
|---|---|---|
| Cardiac disorders | 2 (9.1) | - |
| Pericardial effusionb | 1 (4.5) | - |
| Sinus tachycardia | 1 (4.5) | - |
| Endocrine disorders | 1 (4.5) | - |
| Hypothyroidismb | 1 (4.5) | - |
| Gastrointestinal disorders | 1 (4.5) | - |
| Constipation | 1 (4.5) | - |
| General disorders and administration site conditions | 5 (22.7) | - |
| Chills | 5 (22.7)c | - |
| Immune system disorders | 1 (4.5) | - |
| Anaphylactic reaction | 1 (4.5)c | - |
| Infections and infestations | 1 (4.5) | 3 (30) |
| Fungal infection | - | 1 (10) |
| Lower respiratory tract infection | - | 2 (20) |
| Urinary tract infection | 1 (4.5) | - |
| Injury, poisoning and procedural complications | 1 (4.5) | - |
| Infusion related reaction | 1 (4.5)c | - |
| Investigations | 12 (54.5) | 2 (20) |
| Alanine aminotransferase increased | 1 (4.5) | - |
| Fibrin D dimer increased | 1 (4.5) | - |
| Low density lipoprotein increased | 1 (4.5) | - |
| Lymphocyte count decreased | 11 (50)c | 2 (20) |
| Non-high-density lipoprotein cholesterol increased | 1 (4.5) | - |
| Platelet count decreased | 1 (4.5)c | - |
| Metabolism and nutrition disorders | 6 (27.3) | 1 (10) |
| Hyperglycemia | 4 (18.2) | 1 (10) |
| Hypertriglyceridemia | 2 (9.1) | 1 (10) |
| Respiratory, thoracic, and mediastinal disorders | - | 3 (30) |
| Acute respiratory distress syndrome | - | 2 (20) |
| Respiratory failure | - | 2 (20) |
a2 subjects could not complete even one dosing and were replaced as per protocol. They are part of safety population set till their discontinuation
bPericardial effusion was considered due to underlying hypothyroidism (grade 2). The patient was treated with levothyroxine and recovered. The event was considered not related to the study drug.
cRelated to the study drug.
Five patients (two patients in Arm-A out of the patients who received complete infusion and three patients in Arm-B) reported serious adverse events (SAEs) during the study. The SAEs reported in Arm-A were anaphylactic reaction and pericardial effusion. Anaphylactic reaction resolved on the same day with medical intervention and was considered as related to the study drug infusion. Pericardial effusion was considered due to underlying hypothyroidism (grade 2). The patient was treated with levothyroxine and recovered. The event was considered not related to the study drug.
Three deaths were reported in Arm-B. The first death was due to lower respiratory tract infection with ARDS; the second was due to type 1 respiratory failure with ARDS, with lower respiratory tract infection; and the third was due to respiratory failure. No fatal TEAEs were reported in Arm-A.
As defined in the protocol, patients who did not complete one full dose were considered unevaluable and were replaced. Two patients randomized to Arm-A, experienced an infusion reaction shortly after initiation of drug and did not complete the first dose and withdrew from the study. The event of infusion reaction was resolved on the same day in both patients. Subsequently, one patient recovered from COVID-19 in approximately 2 weeks and was discharged from the hospital. The second patient developed further complications of COVID-19 related ARDS and died 9 days after discontinuation, and the event was deemed not related to the study drug.
All infusion reactions (5 events) were considered related to study medication and resolved within a few hours with symptomatic management. These infusion reactions occurred when the drug was given over 2 hours. However, these reduced if the infusion was given over 5–6 hours.
No notable differences were seen between the arms in vital parameters and clinical laboratory (hematology and biochemistry) evaluations except lymphocyte count decrease, which was seen in 11 (50%) patients in Arm-A and 2 (20%) patients in Arm-B. The events in Arm-A, although severe in nature, were transient, without any clinical consequences, and considered to be related to the study drug. The events can be attributed to the mechanism of action of the drug and are expected in keeping with the known safety profile of the drug.
4. Discussion
Progression of COVID-19 complications is phasic and is associated with systemic hyperinflammation and elevation of inflammatory markers in the second phase [27]. As a consequence of this exaggerated immune response, there is a pro-inflammatory release of cytokines, which is associated with high morbidity and mortality in COVID-19 patients [26]. In patients in intensive care, who are critically ill (requiring ventilation) and seriously ill (requiring oxygen support), there is an increased systemic concentration of IL-6, TNF-a and other cytokines [27]. This inflammatory pathophysiology of COVID-19 has encouraged research into the use of several immunomodulatory treatments, including Itolizumab, in moderate to severe cases of COVID-19 [11,12,26]. Furthermore, the mechanism of action of Itolizumab acting upstream on the Th1 and Th17 pathways, may provide additional and broader immunomodulatory benefits over other similar agents [26,28,29].
Building on the experience of using Itolizumab, its documented safety profile, and its mechanism of action, we undertook this study to explore its potential to prevent cytokine release syndrome and reduce mortality in moderate-to-severe ARDS in COVID-19 patients. The current effort provides encouraging results, particularly with respect to mortality noted at the end of 30 days follow-up, and there is a need to replicate these findings either through additional, larger clinical trials or post-marketing surveillance studies.
With the global caseload of COVID-19 soon to be edging past 125 million and the death tally of more than 2.7 million, it is imperative to explore therapeutic alternatives which not only prevent progression to severe disease but also reduce mortality and morbidity, as the vaccination of global population will take a long time [1,30]. We identified a mortality benefit in the current study which we interpret cautiously considering the small sample size and usual limitations of undertaking an open-label study, conducted within the restrictions imposed by an ongoing pandemic [31]. We further acknowledge the limitation that the open-label design is also known to yield slightly larger estimates of effect size, but these can be overcome by blinded, larger clinical trials [32]. However, what is encouraging in the current data is that in addition to the mortality benefit, we also identified favorable outcomes related to improved lung function, biomarker profile and clinical resolution, especially with respect to respiratory/ventilatory support requirements. These are encouraging enough to warrant more investigations in order to ensure generalizability and external validity. The recent emergency use authorization accorded to Itolizumab for use in COVID-19 patients in India and Cuba provides a window of opportunity to conduct larger, global trials. A phase 4 study to evaluate the safety and efficacy of Itolizumab for the management of excessive release of cytokines in moderate to severe ARDS patients due to COVID-19 is currently ongoing [CTRI/2020/09/027941; http://ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=46023&EncHid=&userName=027941].
Systemic vasculitis and cytokine-mediated coagulation disorders have been recognized as significant factors for multi-organ failure in patients with severe COVID-19 complications [33]. In patients with COVID-19, levels of organ dysfunction markers such as D-dimer and LDH and surrogate markers of systemic inflammation or endothelial/cellular damage, such as ferritin and CRP, need to be closely monitored as these are considered as markers for potential progression to critical illness [34–36]. Elevated LDH levels indicate acute inflammation and have been associated with a 6-fold increase in the odds of progressing to severe COVID-19 and a 16-fold increase in odds of dying from COVID-19 [37]. D-dimer levels indicate coagulopathy and higher values have been shown to be associated with poorer clinical outcomes [38,39]. Serum ferritin levels indicate RBC damage and have been identified to be independently associated with the development of severe COVID-19 [40]. Plasma CRP levels have also been associated with CT confirmed moderate to severe pneumonia in COVID-19 patients [41]. In the current study, encouraging trends were noted for all these biomarkers in Arm-A patients, who received Itolizumab in addition to best supportive care. A consistent reduction in D-dimer and LDH levels was seen in Arm-A unlike in Arm-B where no pattern was observed. The mean change in ferritin from baseline was higher in Arm-A at all timepoints. Furthermore, decreasing levels of these markers was accompanied with clinical improvement in patients receiving Itolizumab.
TNF-α showed a four-fold decrease in Arm-A after administration of Itolizumab (p = 0.025), whereas a three-fold increase in TNF-α was seen in Arm-B. A four-fold decrease was seen in IL-6 levels in Arm-A after the administration of Itolizumab (p = 0.027) while a 30% (1.3-fold) increase was observed in Arm-B. Our findings are in agreement with recent trials held in Cuba, where reduction in IL-6 levels was seen in COVID-19 patients treated with Itolizumab [42–44]. Saavedra et al. have reported that one dose of Itolizumab reduced the baseline serum levels of IL-6 in 24 critically and severely ill COVID-19 patients as well as stabilized the baseline low levels in moderately ill elderly COVID-19 patients [42]. The Cuban expanded access trial in elderly patients with co-morbidities reported that timely use of Itolizumab in combination with other antivirals reduced COVID-19 disease worsening and mortality [44].
A decrease in lymphocyte count has been observed in COVID-19 patients [45]. Absolute lymphocyte count is considered as an important prognostic marker in COVID-19 infection [46]. In Arm-A, a transient reduction in ALC was seen by Day 7, which was considered related to the study drug. However, the levels increased back from Day 7 to Day 30 and were comparable to Arm-B, and no resulting adverse clinical outcomes such as infections were observed.
A total of five serious TEAEs were reported in the study of which three deaths were reported in Arm-B. Of the two serious TEAEs reported in Arm-A, one (pericardial effusion) was related to underlying comorbidity (hypothyroidism) and was unrelated to the drug. The other was anaphylaxis due to infusion reaction, which is a known adverse effect of Itolizumab. The reported anaphylactic reaction was due to the shorter duration of infusion (2 hours). Other than the serious case of infusion reaction, non-serious treatment-related infusion reactions were reported which abated with the extension of the infusion period to 5–6 hours and with premedication protocol. The TEAEs, such as infusion reactions and related events, reported in the study were those expected for a monoclonal antibody (in treatment of psoriasis with the study drug, 10–15% of patients experienced infusion-related reaction) [19]. The other treatment related AE was lymphocyte count decrease which was transient, and the patients recovered. In general, immunomodulatory drugs are expected to increase the risk of infection by acting on the immune system. However, in this study only one case of unrelated infection was reported in Arm-A. These results are in line with the earlier findings with Itolizumab [14,18,20,47,48]. Itolizumab has now been administered to more than 2000 COVID-19 patients with moderate to severe ARDS and has been found to be safe and well tolerated.
Several ‘repurposed’, ‘emergency use’ or ‘off-label’ drugs have been considered as treatment alternatives for managing early phases of the disease, wherein interfering with viral replication provides clinical benefits. As the trial was conducted between 2 May 2020 and 7 July 2020 Hydroxychloroquine had been shown to have limited clinical effectiveness [49–55], and Remdesivir [56,57] Favipiravir [58,59] and convalescent plasma [60,61] were still under investigation. Remdesivir had not yet received approval for COVID-19 treatment and the results of RECOVERY trial (dexamethasone usage) were not yet available [56,57,62]. In this study, antiviral drugs like lopinavir/ritonavir/oseltamivir to reduce viral load, steroids such as methyl prednisolone 40 mg BID/dexamethasone 8 mg IV to reduce systemic inflammation and LMW heparin at the dose of 40 mg SC BID to prevent thrombosis and antibiotics such as azithromycin and ivermectin to reduce infections, were used as best supportive care.
A recent report by Gore et al. (2021) states that a single dose of Itolizumab accelerated recovery in 25 adult patients with COVID-19 by controlling immune hyperactivation and clinical improvement was demonstrated by reduction in inflammatory markers, getting weaned-off from oxygen, reduced length of hospital stay and improvement of ordinal score [63]. Another recent evidence by Thacker et al. (2021) shows treatment of moderate-to-severe COVID-19 disease with Itolizumab reduced inflammatory markers and improved oxygen saturation levels in 27 ARDS patients [64]. Furthermore, as per Thacker et al. (2021), Itolizumab accelerated recovery time in hospitalized patients, with no serious adverse events and no mortality [64].
5. Conclusion
The current investigation highlights the potential of Itolizumab as a promising, safe and effective immunomodulatory therapy for the treatment of CRS leading to ARDS in COVID-19 patients, with survival and recovery benefit. The encouraging results from this study and RWE call for continued investigation of Itolizumab through larger controlled trials in controlling the hyperinflammatory immune response leading to ARDS in COVID-19.
Acknowledgments
Medical writing assistance was provided by Shivani Mittra, PhD and Ubhayabharathi Gurunath, M.Sc (Biocon Biologicals Ltd.).
Clinical support was provided by Arpitkumar Prajapati, M.D., Radhika A, M.D., and Sarika S Deodhar, M.D. (Biocon Biologicals Ltd.).
Trial operation support was provided by Anirudh Sahoo, M.Pharm (Biocon Biologicals Ltd).
Sandeep N. Athalye, as the guarantor of this work, takes full responsibility for the work, including the study design, access to data, and the decision to submit and publish the manuscript.
Funding Statement
The study was funded by Biocon Biologics India Limited and the funders did not have any role in patient recruitment and management.
Author contributions
Sandeep N. Athalye, Subramanian Loganathan, Shashank R. Joshi were involved in study conceptualization and design. Suresh Kumar, Rosemarie de Souza, Milind Nadkar, Randeep Guleria and Anjan Trikha were study investigators. Ashwani Marwah was involved in data curation and data validation. Sivakumar Vaidyanathan was involved in project administration. All authors analyzed and interpreted the study data and results. All authors participated in the preparation and review of the manuscript. All authors read and approved the final version of the manuscript.
Declaration of interest
Suresh Kumar, Rosemarie de Souza, Milind Nadkar, Randeep Guleria and Anjan Trikha report no competing interests. Ashwani Marwah, Subramanian Loganathan and Sandeep N. Athalye are employees of Biocon Biologics Ltd. and hold stocks in Biocon. Shashank R. Joshi has received Speaker/Advisory/Research Grants from Abbott, Astra, Biocon, Boehringer Ingelheim, Eli Lilly, Franco Indian, Glenmark, Lupin, Marico, MSD, Novartis, Novo Nordisk, Roche, Sanofi, Serdia and Zydus. Sivakumar Vaidyanathan is an employee of Biocon Biologics Ltd. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial relationships or otherwise to disclose.
Reference
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Johns Hopkins CSSE . Coronavirus (COVID-19) global cases: center for systems science and engineering, Johns Hopkins University. 2020. Available: https://coronavirus.jhu.edu/map.html [cited 2021Mar 24] [Google Scholar]
- 2.Chatterjee P, Nagi N, Agarwal A, et al. The 2019 novel coronavirus disease (COVID-19) pandemic: a review of the current evidence. Indian J Med Res. 2020. DOI: 10.4103/ijmr.IJMR_519_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Costela-Ruiz VJ, Illescas-Montes R, Puerta-Puerta JM, et al. SARS-CoV-2 infection: the role of cytokines in COVID-19 disease. Cytokine Growth Factor Rev. 2020. cited 2021 February26;54:62–75. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• COVID-19 and cytokine storm.
- 4.Moore JB, June CH.. Cytokine release syndrome in severe COVID-19. Sci. 2020. May 1;368(6490):473–474. [DOI] [PubMed] [Google Scholar]
- 5.Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–422. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Immunopathogenesis of acute respiratory distress syndrome associated with COVID-19.
- 6.Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020;71(15):762–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fu L, Wang B, Yuan T, et al. Clinical characteristics of coronavirus disease 2019 (COVID-19) in China: a systematic review and meta-analysis. J Infect. 2020;80(6):656–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siddiqi HK, Mehra MR. COVID-19 illness in native and immunosuppressed states: a clinical–therapeutic staging proposal. J Heart Lung Transplant. 2020;39(5):405–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Webb BJ, Peltan ID, Jensen P, et al. Clinical criteria for COVID-19-associated hyperinflammatory syndrome: a cohort study. Lancet Rheumatol. 2020. December 1;2(12):e754–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhong J, Tang J, Ye C, et al. The immunology of COVID-19: is immune modulation an option for treatment? Lancet Rheumatol. 2020. May 20;2(7):e428-e436. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Immune modulation as a therapeutic option in COVID-19.
- 11.Horby PW, Pessoa-Amorim G, Peto L, et al. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): preliminary results of a randomised, controlled, open-label, platform trial. Medrxiv. 2021. January 1. DOI: 10.1101/2021.02.11.21249258. [DOI] [Google Scholar]
- 12.REMAP-CAP Investigators . Interleukin-6 receptor antagonists in critically ill patients with Covid-19–Preliminary report. N Engl J Med. 2021. February 25. doi: 10.1056/NEJMoa2100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia Santana CA, Tung JW, Gulnik S. Human treg cells are characterized by low/negative CD6 expression. Cytometry A. 2014;85(10):901–908. [DOI] [PubMed] [Google Scholar]
- 14.Bughani U, Saha A, Kuriakose A, et al. T cell activation and differentiation is modulated by a CD6 domain 1 antibody Itolizumab. PLoS ONE. 2017;12(7):e0180088. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Immunomodulatory role of Itolizumab, an anti-CD6 humanized IgG1 monoclonal antibody.
- 15.Li Y, Singer NG, Whitbred J, et al. CD6 as a potential target for treating multiple sclerosis. Proc Nat Acad Sci. 2017. March 7;114(10):2687–2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cayrol R, Wosik K, Berard JL, et al. Activated leukocyte cell adhesion molecule promotes leukocyte trafficking into the central nervous system. Nat Immunol. 2008. February;9(2):137–145. [DOI] [PubMed] [Google Scholar]
- 17.Nair P, Melarkode R, Rajkumar D, et al. CD6 synergistic co-stimulation promoting proinflammatory response is modulated without interfering with the activated leucocyte cell adhesion molecule interaction. Clin Exp Immunol. 2010;162(1):116–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Menon R, David BG. Itolizumab - a humanized anti-CD6 monoclonal antibody with a better side effects profile for the treatment of psoriasis. Clin Cosmet Investig Dermatol. 2015;8:215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krupashankar DS, Dogra S, Kura M, et al. Efficacy and safety of itolizumab, a novel anti-CD6 monoclonal antibody, in patients with moderate to severe chronic plaque psoriasis: results of a double-blind, randomized, placebo-controlled, phase-III study. J Am Acad Dermatol. 2014;71(3):484–492. [DOI] [PubMed] [Google Scholar]; •• Phase 3 efficacy and safety results of Itolizumab in moderate to severe plaque psoriasis.
- 20.Dogra S, D S K, Budamakuntla L, et al. Long-term efficacy and safety of itolizumab in patients with moderate-to-severe chronic plaque psoriasis: A double-blind, randomized-withdrawal, placebo-controlled study. J Am Acad Dermatol. 2015; 73: 331–3.e1. doi: 10.1016/j.jaad.2015.03.040 [DOI] [PubMed] [Google Scholar]
- 21.Dogra S, D S, Rajagopalan M. Anti-CD6 mAbs for the treatment of psoriasis. Expert Opin Biol Ther. 2020;1–7. DOI: 10.1080/14712598.2020.1776254 [DOI] [PubMed] [Google Scholar]
- 22.Srivastava A. Itolizumab in psoriasis. Indian J Dermatol. 2017. Jul-Aug;62(4):418–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chopra A, Chandrashekara S, Iyer R, et al. Itolizumab in combination with methotrexate modulates active rheumatoid arthritis: safety and efficacy from a phase 2, randomized, open-label, parallel-group, dose-ranging study. Clin Rheumatol. 2016;35(4):1059–1064. [DOI] [PubMed] [Google Scholar]
- 24.Rodríguez PC, Prada DM, Moreno E, et. al. The anti-CD6 antibody itolizumab provides clinical benefit without lymphopenia in rheumatoid arthritis patients: results from a 6-month, open-label Phase I clinical trial. Clin Exp Immunol. 2018; 191: 229–39. doi: 10.1111/cei.13061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.https://www.biocon.com/docs/domestic-market-pi/ccd/ALZUMAB% 20PI%2025% 20mg.pdf [cited 26 Feb 2021
- 26.Loganathan S, Athalye SN, Joshi SR. Itolizumab, an anti-CD6 monoclonal antibody, as a potential treatment for COVID-19 complications. Expert Opin Biol Ther. 2020;1–7. DOI: 10.1080/14712598.2020.1798399 [DOI] [PubMed] [Google Scholar]; • Mini-review on role of Itolizumab in COVID-19 complications.
- 27.Mehta P, McAuley DF, Brown M, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tay MZ, Poh CM, Rénia L, et al. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20(6):363–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Budamakuntla L, Madaiah M, Sarvajnamurthy S, et al. Itolizumab provides sustained remission in plaque psoriasis: a 5-year follow-up experience. Clin Exp Dermatol. 2015;40(2):152–155. [DOI] [PubMed] [Google Scholar]
- 30.Agarwal A, Nagi N, Chatterjee P, et al. Guidance for building a dedicated health facility to contain the spread of the 2019 novel coronavirus outbreak. Indian J Med Res. 2020. Epub ahead of print. DOI: 10.4103/ijmr.IJMR_518_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schulz KF, Grimes DA. Blinding in randomised trials: hiding who got what. Lancet. 2002;359(9307):696–700. [DOI] [PubMed] [Google Scholar]
- 32.Schulz KF, Chalmers I, Hayes RJ, et al. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA. 1995;273(5):408–412. [DOI] [PubMed] [Google Scholar]
- 33.Fadel R, Morrison A, Vahia A, et al. Early short course corticosteroids in hospitalized patients with COVID-19. medRxiv. 2020;5(4):20074609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Henry BM, De Oliveira MHS, Benoit S, et al. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med. 2020;58(7):1021–1028. [DOI] [PubMed] [Google Scholar]
- 35.Zhang C, Wu Z, Li J-W, et al. Cytokine release syndrome in severe COVID-19: interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int J Antimicrob Agents. 2020;55(5):105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tang N, Li D, Wang X, et al. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Henry BM, Aggarwal G, Wong J, et al. Lactate dehydrogenase levels predict coronavirus disease 2019 (COVID-19) severity and mortality: a pooled analysis. Am J Emerg Med. 2020;38(9):1722–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vidali S, Morosetti D, Cossu E, et al. D-dimer as an indicator of prognosis in SARS-CoV-2 infection: a systematic review. ERJ Open Res. 2020;6(2):00260–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tang N, Bai H, Chen X, et al. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020. DOI: 10.1111/jth.14817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin Z, Long F, Yang Y, et al. Serum ferritin as an independent risk factor for severity in COVID-19 patients. J Infect. 2020;81(4):647–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen W, Zheng KI, Liu S, et al. Plasma CRP level is positively associated with the severity of COVID-19. Ann Clin Microbiol Antimicrob. 2020;19(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saavedra D, Añé-Kourí AL, Sánchez N, et al. An anti-CD6 monoclonal antibody (itolizumab) reduces circulating IL-6 in severe COVID-19 elderly patients. Immunity Ageing. 2020. December;17(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Effect of Itolizumab in reducing circulating IL-6 in severe COVID-19 elderly patients.
- 43.Filgueira LM, Cervantes JB, Lovelle OA, et al. An anti-CD6 antibody for the treatment of COVID-19 patients with cytokine-release syndrome: report of three cases. Immunotherapy. 2021. March;13(4):289–295. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Treatment of Cytokine release syndrome in COVID-19 patients with Itolizumab.
- 44.Díaz Y, Ramos-Suzarte M, Martín Y, et al. Use of a humanized anti-CD6 monoclonal antibody (itolizumab) in elderly patients with moderate COVID-19. Gerontology. 2020;66(6):553–561. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Treatment of COVID-19 with Itolizumab in elderly patients: a Cuban study.
- 45.Tavakolpour S, Rakhshandehroo T, Wei EX, et al. Lymphopenia during the COVID-19 infection: what it shows and what can be learned. Immunol Lett. 2020;225:31–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wagner J, DuPont A, Larson S, et al. Absolute lymphocyte count is a prognostic marker in Covid-19: a retrospective cohort review. Int J Lab Hematol. n/a. DOI: 10.1111/ijlh.13288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Braun M, Müller B, Ter Meer D, et al. The CD6 scavenger receptor is differentially expressed on a CD56 natural killer cell subpopulation and contributes to natural killer-derived cytokine and chemokine secretion. J Innate Immun. 2011;3(4):420–434. [DOI] [PubMed] [Google Scholar]
- 48.Parthasaradhi A. Safety and efficacy of Itolizumab in the treatment of psoriasis: a case series of 20 patients. J Clin Diagn Res. 2016;10(11):WD01–WD03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.COVID-19 drugs trial rolled out across UK homes and communities. University of Oxford. [cited 2021 February26]. Available: https://www.ox.ac.uk/news/2020-05-12-covid-19-drugs-trial-rolled-out-across-uk-homes-and-communities [Google Scholar]
- 50.Chatterjee P, Anand T, Singh K, et al. Healthcare workers & SARS-CoV-2 infection in India: a case-control investigation in the time of COVID-19. Indian J Med Res. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.NIH begins clinical trial of hydroxychloroquine and azithromycin to treat COVID-19. In: National Institutes of Health (NIH) [Internet]. 14 May 2020. [cited 2021 February26]. Available: https://www.nih.gov/news-events/news-releases/nih-begins-clinical-trial-hydroxychloroquine-azithromycin-treat-covid-19 [Google Scholar]
- 52.Wright JK, Tan DHS, Walmsley SL, et al. Protecting frontline health care workers from COVID-19 with hydroxychloroquine pre-exposure prophylaxis: a structured summary of a study protocol for a randomised placebo-controlled multisite trial in Toronto, Canada. Trials. 2020;21(1):647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mahase E. Hydroxychloroquine for covid-19: the end of the line? BMJ. 2020;369:m2378. [DOI] [PubMed] [Google Scholar]
- 54.Skipper CP, Pastick KA, Engen NW, et al. Hydroxychloroquine in nonhospitalized adults with early COVID-19: a randomized trial. Ann Intern Med. 2020;173(8):623–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Boulware DR, Pullen MF, Bangdiwala AS, et al. A randomized trial of hydroxychloroquine as postexposure prophylaxis for Covid-19. N Engl J Med. 2020;383(6):517–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Goldman JD, Lye DCB, Hui DS, et al. Remdesivir for 5 or 10 days in patients with severe Covid-19. N Engl J Med. 2020;383(19):1827–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19 - preliminary report. N Engl J Med. 2020;383(19):1813–1826. [DOI] [PubMed] [Google Scholar]
- 58.Glenmark Pharmaceuticals . Glenmark to commence new phase 3 clinical trial on combination of two anti-viral drugs favipiravir and umifenovir in hospitalized patients of moderate COVID-19 in India. In: PR Newswire [Internet]. [cited 2021 February26]. Available: https://www.prnewswire.com/in/news-releases/glenmark-to-commence-new-phase-3-clinical-trial-on-combination-of-two-anti-viral-drugs-favipiravir-and-umifenovir-in-hospitalized-patients-of-moderate-covid-19-in-india-836904730.html
- 59.Chen C, Zhang Y, Huang J, et al. Favipiravir versus Arbidol for COVID-19: a randomized clinical trial. medRxiv. 2020;3(17):20037432. [Google Scholar]
- 60.Gharbharan A, Jordans CCE, GeurtsvanKessel C, et al. Convalescent plasma for COVID-19. A randomized clinical trial. medRxiv. 2020;7(1):20139857. [Google Scholar]
- 61.Joyner MJ, Klassen SA, Senefeld J, et al. Evidence favouring the efficacy of convalescent plasma for COVID-19 therapy. medRxiv. 2020;7(29):20162917. [Google Scholar]
- 62.RECOVERY Collaborative Group . Dexamethasone in hospitalized patients with Covid-19—preliminary report. N Engl J Med. 2020. July 17. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gore V, Kshirsagar DP, Bhat SM, et al. Itolizumab treatment for cytokine release syndrome in moderate to severe acute respiratory distress syndrome due to COVID-19: clinical outcomes, a retrospective study. J Assoc Physicians India. 2021;69:43–48. [PubMed] [Google Scholar]; •• Itolizumab treatment of cytokine release syndrome in COVID-19 patients with moderate to severe acute respiratory distress syndrome.
- 64.Thacker HP, Halnor D, Dhekane A, et al. An early experience of Itolizumab with best supportive care in treatment of moderate to severe COVID-19 patients: a retrospective study. Indian J Resp Care in Press. [Google Scholar]; •• Treatment of moderate to severe COVID-19 with Itolizumab.


