Abstract
Coenzyme Q10 (CoQ10) is a strongly hydrophobic lipid that functions in the electron transport chain and as an antioxidant. CoQ10 was conferred with aqueous solubility by incorporation into nanoparticles containing phosphatidylcholine (PtdCho) and apolipoprotein (apo) A-I. These particles, termed CoQ10 nanodisks (ND), contain 1.0 mg CoQ10/5 mg PtdCho/2 mg apoA-I (97% CoQ10 solubilization efficiency). UV/Vis absorbance spectroscopy of CoQ10 ND revealed a characteristic absorbance peak centered at 275 nm. Incorporation of CoQ10 into ND resulted in quenching of apoA-I tryptophan fluorescence emission. Gel filtration chromatography of CoQ10 ND gave rise to a single major absorbance peak and HPLC of material extracted from this peak confirmed the presence of CoQ10. Incubation of cultured cells with CoQ10 ND, but not empty ND, resulted in a significant increase in the CoQ10 content of mitochondria as well as enhanced oxidative phosphorylation, as observed by a ~24% increase in maximal oxygen consumption rate. Collectively, a facile method to solubilize significant quantities of CoQ10 in lipid nanoparticles has been developed. The availability of CoQ10 ND provides a novel means to investigate biochemical aspects of CoQ10 uptake by cells and/or administer it to subjects deficient in this key lipid as a result of inborn errors of metabolism, statin therapy, or otherwise.
Keywords: Coenzyme Q, HepG2, Mitochondria, Nanodisc, Reconstituted HDL
Introduction
In humans, coenzyme Q10 (CoQ10), or ubiquinone, is a key component of the mitochondrial electron transport chain (ETC). CoQ10 accepts electrons from Complex I or Complex II and transfers them to Complex III. In doing so, CoQ10 undergoes an oxidation/reduction cycle wherein it is capable of transitioning between three distinct states; fully oxidized ubiquinone, a semiquinone, and the reduced state, ubiquinol. The ability of CoQ10 to act both as a two electron carrier (from ubiquinone to ubiquinol) and a one electron carrier (from semiquinone to either ubiquinone or ubiquinol) is central to its role in the ETC as well as its function as a free radical scavenging antioxidant. Structurally, CoQ10 is composed of a modestly polar benzoquinone ring linked to a polyisoprene tail that, in humans, is 10 isoprene units (50 carbons) in length.
The benzoquinone moiety of CoQ10 is synthesized from tyrosine while its polyisoprene tail arises from acetyl CoA via the mevalonate pathway. These components are synthesized separately, and ultimately condense to form the product, CoQ10. The presence of a 50-carbon isoprene chain confers considerable hydrophobicity to CoQ10. As such, CoQ10 is completely insoluble in aqueous solutions. Given its hydrophobic nature, bioavailability of CoQ10 is poor (Zaki, 2016). Indeed, most CoQ10 is generated via de novo biosynthesis rather than from dietary sources. This poses a problem for individuals who take “statin” drugs to control their plasma cholesterol levels. Although statin-mediated inhibition of 3-hydroxy, 3-methylglutaryl CoA (HMG CoA) reductase is an effective way to inhibit cholesterol biosynthesis, the same pathway is essential for CoQ10 biosynthesis. Thus, statin therapy can lead to a decline in CoQ10 levels. Given the critical role it plays in energy metabolism and as an antioxidant, CoQ10 dietary supplements are often recommended for individuals prescribed statin therapy (Taylor, 2018). Unfortunately, poor bioavailability of oral CoQ10 limits the potential benefit of this strategy. To circumvent this problem, several approaches have been devised to improve CoQ10 bioavailability (Villalba et al., 2010). Products designed to increase oral bioavailability of CoQ10 exist as structural analogs of CoQ10 that are less hydrophobic yet manifest similar oxidation/reduction properties.
In the present study, we report solubilization of CoQ10 in stable, nascent high-density lipoprotein (HDL)-like nanoparticles composed of egg yolk phosphatidylcholine (PtdCho) and recombinant human apoA-I. The product particles, termed CoQ10 nanodisks (ND), are disk-shaped bilayers whose periphery is circumscribed by apoA-I, an amphipathic “scaffold” protein that interacts with otherwise exposed phospholipid fatty acyl chains at the edge of the bilayer. It is postulated that CoQ10 intercalates between phospholipids that comprise the bilayer component of ND in a manner that is similar to its association with membranes in vivo. Findings with CoQ10 reported herein extend the repertoire of hydrophobic bioactive agents to be successfully incorporated into ND (Ryan, 2010). For example, the polyene antibiotic, amphotericin B (Oda et al., 2006), the polyphenol phytonutrient, curcumin (Ghosh et al., 2011), the bioactive sphingolipid, sphingadiene (Zhao et al., 2018), contrast agents for medical diagnostics (Kornmueller et al., 2019), and the cholesterol synthesis inhibitor, simvastatin (Duivenvoorden et al., 2014), have been formulated into ND and characterized in terms of their biological activity. In general, hydrophobic bioactive agent incorporation into ND results in retention, if not enhancement, of biological activity. Moreover, bioactive agent release from ND delivery vehicles leads to cellular uptake and subsequent intracellular trafficking (Crosby et al., 2015; Ghosh and Ryan, 2014). Studies reported herein reveal that CoQ10 can be effectively solubilized in ND. As such, these particles provide a potentially useful way to administer CoQ10 and, thereby, improve its bioavailability.
Materials and Methods
CoQ10 ND Formulation
Five milligrams egg yolk PtdCho (Sigma), dissolved in chloroform–methanol (3:1 vol/vol) was dried under a stream of N2 gas, forming a thin film of lipid on the vessel wall. Residual organic solvent was removed under vacuum. The prepared lipid was then dispersed in 0.5 mL phosphate buffered saline (PBS; 20 mM sodium phosphate, 150 mM sodium chloride, pH 7.4) and, unless otherwise indicated, 1.0 mg CoQ10 was then added to the sample (from a 10 mg/mL stock solution in dimethylformamide, DMF). Following this, 2 mg recombinant human apoA-I (Ryan et al., 2003) was added from a 4.0 mg/mL stock solution in PBS and the sample (1.2 mL final volume) subjected to bath sonication between 43 and 47 °C. During sonication, the turbid mixture clarified, indicating apolipoprotein-phospholipid complexes (i.e. ND) had formed. Empty ND were prepared in the same manner, except that CoQ10 was omitted. Following formulation, ND samples were centrifuged at 13,000g for 5 min and the supernatant dialyzed against PBS to remove residual DMF solvent. CoQ10 was purchased from Sigma and CoQ9 was from Cayman Chemical. For solubilization efficiency determinations, samples containing different combinations of individual CoQ10 ND formulation components were subjected to bath sonication and centrifugation, as described above. CoQ9 internal standard was added to the supernatant fractions prior to extraction with 1-propanol. The extracted material was filtered through a 0.22 μm Polyvinylidene fluoride (PVDF) membrane prior to HPLC analysis. Protein concentrations were determined using the BCA Protein Assay (ThermoFisher) with bovine serum albumin as standard.
CoQ10 Detection and Quantitation by HPLC
CoQ10 ND extracts in 1-propanol were chromatographed on a Shimadzu Prominence HPLC fitted with a Kinetex® 5 μm EVO C18 100 Å, 150 × 4.6 mm reversed phase column (Phenomenex) and a SecurityGuard™ ULTRA for EVO C18 guard column. Samples were separated using an isocratic mobile phase composed of methanol and 2-propanol (2:1 vol/vol) eluted at 0.7 mL/min at a column temperature of 30 °C. An SPD-M20A photodiode array detector was used to monitor absorbance at 275 nm.
UV/Vis and Fluorescence Spectroscopy
Absorbance spectra were obtained for CoQ10 alone (75 μg in 95% EtOH) as well as CoQ10 ND in PBS. The CoQ10 ND samples were formulated with 0 mg CoQ10, 0.25 mg CoQ10, and 0.5 mg CoQ10 (in PBS), respectively. Spectra were recorded from 250 to 350 nm on a Spectramax M5 instrument using a quartz cuvette. In the fluorescence mode, samples were excited at 280 nm and emission monitored from 300 to 430 nm.
Fast protein liquid chromatography (FPLC) Gel Filtration Chromatography
A sample of CoQ10 ND, formulated as above, was centrifuged at 14,000g for 2 min immediately prior to gel permeation chromatography on a Superose 6 Increase 10/300 GL column using a GE AKTA Pure FPLC instrument. Two hundred and fifty μL ND (corresponding to 0.4 mg apoA-I or 0.2 mg CoQ10), were applied to the column. The column was eluted with PBS at a flow rate of 0.5 mL/min. Absorbance was monitored at 280 nm with collection of 1.0 mL fractions.
Cellular Uptake Studies
HepG2 cells were purchased from Biosciences Divisional Services at UC Berkeley and cultured in Earl’s minimum essential medium (MEM, Caisson Laboratories, Smithfield, UT) supplemented with 10% FBS (Peak Serum), 50 U/mL penicillin, 50 μg/mL streptomycin, 1:100 dilution GlutaMax, 1:100 dilution MEM nonessential amino acids (ThermoFisher Scientific, Waltham, MA), and 1:100 dilution sodium pyruvate (Life Technologies, Carlsbad, CA). Cells were incubated in a humidified incubator with 5% CO2 at 37 °C. For CoQ10 uptake studies, the cells were plated at 1 × 106 cells per 10 mL MEM in three 100 × 15 mm petri dishes and allowed to adhere to the plate overnight at 37 °C. Cells were incubated with (1) 8 μM CoQ10 ND (as CoQ10), (2) an equivalent volume of empty ND, and (3) no additions for 72 h at 37 °C.
Mitochondrial Isolation
Following incubation with CoQ10 ND or empty ND, HepG2 cells were washed twice with 10 mL PBS and centrifuged at 700g for 5 min. The cells were suspended in 3 mM HEPES, pH 7.4, 210 mM mannitol, 70 mM sucrose, 0.2 mM EGTA, 100x ProteaseArrest (G-Biosciences), and 4 mg/mL fatty acid-free albumin. The cell suspension was transferred to a 2 mL Dounce homogenizer on ice and homogenized with 50 strokes. The homogenate was transferred to a conical tube, 2 mL HEPES buffer (diluted 1:1 with deionized H2O) was added and the sample inverted 3–4 times. The mixture was centrifuged at 700 x g for 10 min at 4 °C and the supernatant transferred to 1.5 mL microfuge tubes. To pellet mitochondria, the sample was centrifuged at 3000 x g for 15 min at 4 °C. The resulting pellet was resuspended in 1 mL PBS and a 50 μL aliquot removed to assay protein content.
CoQ10 Extraction from Mitochondria
Mitochondria were washed with 1 mL deionized H2O, centrifuged at 3000 x g for 5 min at 4 °C, and the H2O decanted from the pellet. This step was repeated twice. The pellet was then dried using a CentriVap Benchtop Vacuum Concentrator. Three hundred μg CoQ9 in 1-propanol was added to the dried mitochondrial preparation and the sample disrupted in a hand held homogenizer. The sample was then transferred to 1.5 mL microfuge tubes and centrifuged at 3000g for 5 min. The 1-propanol supernatants were filtered through a 0.22 μm PVDF membrane prior to HPLC as described above.
C2C12 Cell Culture and Incubation with ND
Mouse C2C12 myoblast cells were obtained from UC Berkeley Biosciences Divisional Services and stored in liquid nitrogen. The myoblasts were counted and plated at a final cell density 25,000 cells/well in a 24-well XF24 cell culture microplate plate (Agilent Technologies, Santa Clara, CA) containing Dulbecco’s minimal essential medium (DMEM) supplemented with 10% fetal bovine serum, 50 U/mL penicillin, and 50 μg/mL streptomycin. After 2-days in culture at 37 °C with 5% CO2, DMEM was replaced with DMEM containing 2.5 mM GlutaMAX, 1% HEPES, and 2% horse serum to promote cell differentiation into multinucleated myotubes. The cells were subsequently cultured for an additional 3–5 days to promote myotube differentiation with media changes every 2 days. On day 6, triplicate wells of myotubes were incubated with either CoQ10 ND (20 μM CoQ10) or an equivalent amount of empty ND for 72 h.
Mitochondrial Oxygen Consumption Rate Analysis
In order to assess whether exogenous CoQ10 NDs have an effect on mitochondrial function, we analyzed oxygen consumption rates (OCR) in C2C12 mouse myotubes grown in an XF24 cell culture microplate and treated with empty ND or CoQ10 ND. Analysis was performed using a XF24 Seahorse Extracellular Flux Analyzer (Agilent Technologies). Wells marked B4 and C3, of the XF24 cell culture microplate were assigned as blanks (lacking cells) in order to determine background OCR. A calibration plate was also prepared by adding 1 mL calibrant fluid (Seahorse Bioscience, North Billerica, MA, USA) into wells of the plate 1 day prior to conducting the mitochondrial stress assay. On the day of the assay, the cells were washed three times with XF Media (Seahorse Bioscience), glucose (4.5 g/L), 1 mM sodium pyruvate, and 1 mM glutamine. OCRs were analyzed at baseline for at least four cycles, after which 1 μm oligomycin, 1 μm FCCP, and 1 μm rotenone/antimycin A was sequentially injected into each well to assess ATP-linked OCR, maximal OCR, and mitochondrially-derived OCR, respectively (Lujan et al., 2016; Sacoman et al., 2017; Zhang et al., 2019). In the present study, at the end of each assay, cells in the XF24 cell culture microplate were fixed in 4% paraformaldehyde for 30 min and washed sequentially with PBS and 1.25 μg/mL 4′ 6-diamidino-2-phenylindole (DAPI) to stain nuclei. The DAPI-stained nuclei were then visualized and imaged using an EVOS-FL Cell Imaging System, equipped with EVOS Light cubes specific for UV (Ex/Em of 357/447), at a magnification of 20x (0.45 numerical aperture), as previously described (Dagda et al., 2014). Up to 10 different regions of DAPI-stained cells per well were analyzed blindly to the observer by counting the number of nuclei per field by using NIH Image J version 1.44 (Bethesda, MD, USA) The raw OCR values for each parameter were analyzed and normalized to the average number of nuclei per field.
Statistical Methods
Statistical analyses were performed by two-way ANOVA to compare treatment samples against control samples. Statistical tests were performed using GraphPad Prism version 8.02 for Windows (GraphPad Software, San Diego, CA, USA).
Results
Reconstituted HDL (rHDL) harboring one or more extraneous lipophilic components have been termed ND (Ryan, 2010). In the present case, such particles provide a unique model system to investigate the nature of CoQ10 interactions with bilayer membranes (Fig. 1). The complexes are disk-shaped phospholipid bilayers whose otherwise solvent exposed perimeter is stabilized by interaction with an apolipoprotein scaffold protein (e.g. apoA-I). In general, these particles range in diameter from 12–30 nm and are the thickness of a single bilayer.
Fig. 1.
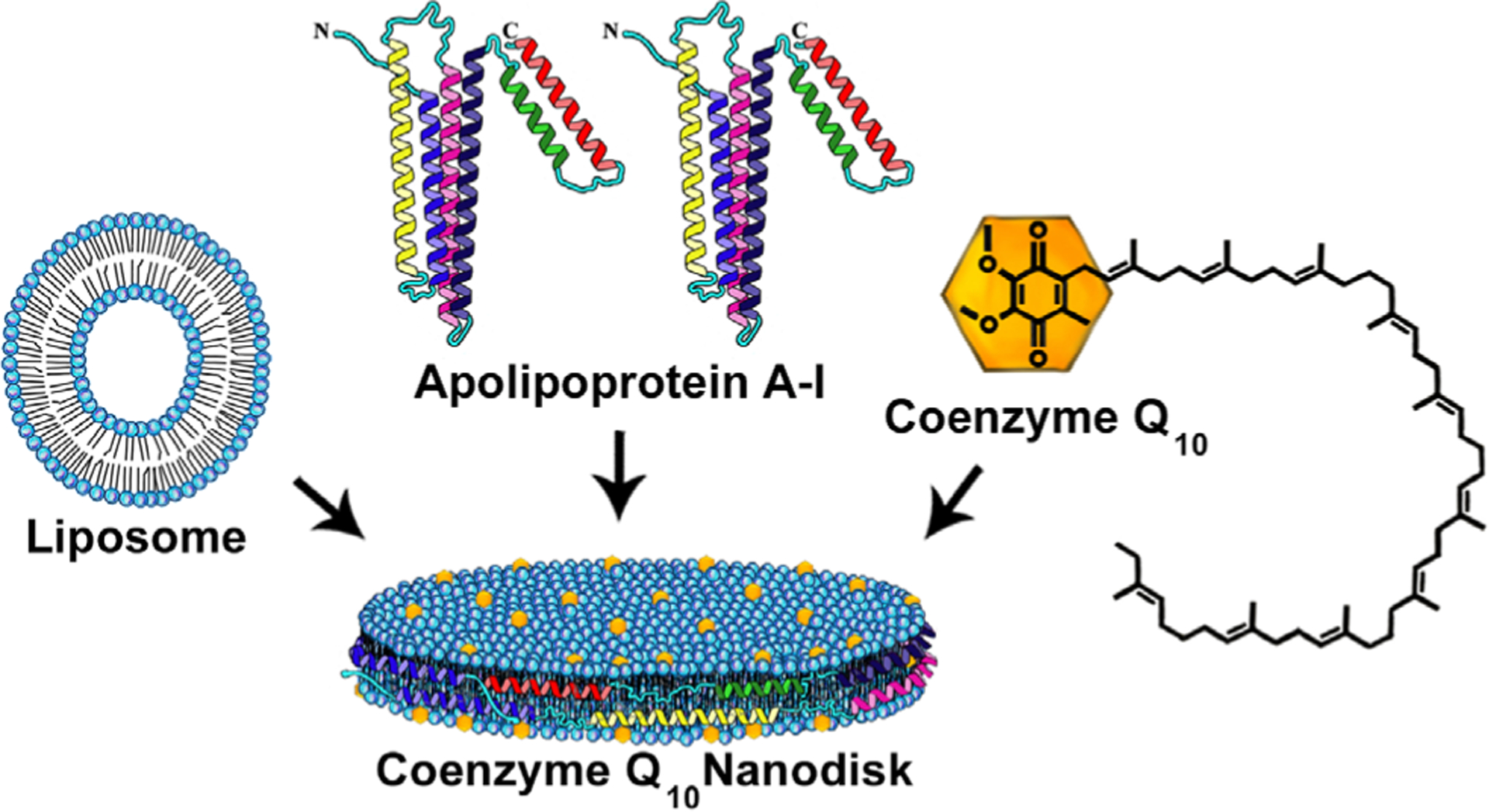
Model structure of CoQ10 ND. In this depiction, PtdCho is organized as a disk-shaped bilayer whose perimeter is circumscribed, and stabilized, by apoA-I, which contacts PtdCho fatty acyl chains at the edge of the disk. CoQ10, depicted as yellow dots, intercalates between phospholipid molecules in the ND structure
Solubilization Efficiency Studies
To determine the extent to which CoQ10 can be solubilized in ND comprised apoA-I and egg PtdCho, various amounts of CoQ10 were introduced to separate formulation reactions. At the concentrations used, DMF was found to have no effect on the formulation reaction. During formulation, the starting turbid incubation mix was transformed into a clarified product solution. Compared to empty ND (composed of apoA-I and PtdCho only), CoQ10 ND were slightly yellow in color and this yellowish hue was retained following dialysis of the sample. Compared to empty ND, CoQ10 ND formulation reaction mixtures were slower to clarify during bath sonication although, at CoQ10 concentrations up to 2.0 mg/5 mg PC (w/w), complete sample clarification was achieved. To determine the effect of various reaction components on CoQ10 solubilization efficiency (Table 1), reaction tubes containing different combinations of reaction components were sonicated under conditions that induce ND formation. Following sonication, each sample was centrifuged at 13,000 x g for 5 min to pellet insoluble material. Supernatant fractions were collected and, following addition of internal standard (CoQ9), the samples were extracted and analyzed by reversed phase HPLC to determine the amount of CoQ10 solubilized. In incubations containing CoQ10, PtdCho and apoA-I, ~97% of the original CoQ10 were recovered in the supernatant fraction. By contrast, in incubations containing CoQ10 and egg PtdCho only, CoQ10 and apoA-I only or CoQ10 alone in buffer, virtually no CoQ10 was recovered in the supernatant fraction.
Table 1.
Effect of egg PtdCho and apoA-I on CoQ10 solubilization efficiency
| Componentsa | Solubilization efficiencyb (%) |
|---|---|
| CoQ10 + egg PtdCho+ apoA-I | 97 |
| CoQ10 + egg PtdCho | <0.1 |
| CoQ10 + apoA-I | <0.1 |
| CoQ10 | <0.1 |
The indicated components were added to PBS and processed as described in Materials and Methods for CoQ10 ND formulation. Individual samples contained 1 mg CoQ10 alone or 1 mg CoQ10 plus 5 mg PC and/or 2 mg apoA-I. Following bath sonication, the samples were stored at 4 °C for 16 h. The samples were then centrifuged at 13,000 x g for 5 min and the supernatant collected. Following this, CoQ9 internal standard was added, the samples were extracted with 1-propanol, and analyzed by reversed phase HPLC as described.
Solubilization efficiency (%) = the amount of CoQ10 recovered in the supernatant fraction/CoQ10 added to the incubation × 100.
UV/Vis Absorbance Spectroscopy
UV/Vis absorbance spectra of CoQ10 were collected in ethanol and compared to spectra of empty ND and CoQ10 ND in PBS (Fig. 2). In ethanol, CoQ10 gives rise to a spectrum with a single major peak centered around 275 nm. Subsequently, spectra of fresh ND were collected in PBS. The ND samples contained variable amounts of CoQ10, including 0, 0.25, and 0.5 mg CoQ10 per 5 mg phospholipid. Whereas empty ND (0 mg CoQ10) give rise to a relatively small absorbance peak centered around 280 nm (attributable to apoA-I Trp residue absorbance), ND formulated with increasing amounts of CoQ10 (0.25 mg CoQ10 and 0.5 mg) yielded spectra with a corresponding increase in absorbance maxima.
Fig. 2.

UV/Vis absorbance spectrum of CoQ10 ND. ND were formulated with 0, 0.25 and 0.5 mg CoQ10, respectively. UV/Vis absorbance scans were then obtained on equivalent aliquots of each ND formulation (from 250 to 500 nm) on a Spectramax spectrophotometer. Curve (a) 75 μg CoQ10 in ethanol solvent; curve (b) CoQ10 ND (formulated with 0.5 mg CoQ10) in PBS; curve (c) CoQ10 ND (formulated with 0.25 mg CoQ10) in PBS; curve (d) empty ND (formulated with 0 mg CoQ10) in PBS. AU, absorbance units; PBS, phosphate buffered saline
Effect of CoQ10 Incorporation into ND on apoA-I Trp Fluorescence Emission
Whereas CoQ10 has negligible intrinsic fluorescence, the apoA-I component of ND emits a strong fluorescence signal centered around 340 nm (excitation 280 nm). When emission spectra of empty ND were recorded from 300 to 450 nm (Fig. 3), a characteristic emission spectrum was observed. As the CoQ10 content of ND was increased, however, a corresponding decrease in apoA-I fluorescence emission quantum yield was observed. Thus, it may be concluded that CoQ10 quenches apoA-I Trp fluorescence emission. Moreover, the data indicate the benzoquinone ring of CoQ10, when present in ND, resides in close proximity to apoA-I. Thus, these results provide additional evidence that CoQ10 has been incorporated into ND as an integral component of the particle structure.
Fig. 3.

Effect of CoQ10 incorporation on ND-associated apoA-I tryptophan fluorescence emission. Empty ND (curve a), CoQ10 ND harboring 0.25 mg CoQ10 (curve b), and CoQ10 ND harboring 0.5 mg CoQ10 (curve c), were excited at 280 nm and emission scanned from 300 to 425 nm. RFU, relative fluorescence units
FPLC Gel Filtration Chromatography of CoQ10 ND
CoQ10 ND and empty egg PtdCho ND, formulated as above, were analyzed by FPLC gel filtration chromatography (Fig. 4). The data reveal that both ND samples elute from the column in a single major peak, indicating a single particle population. Based on comparison of this elution profile with that of cardiolipin ND (Fox et al., 2019), which are 18–31 nm in diameter (Ikon et al., 2015), the data indicate that CoQ10 ND have particle diameters in this range. Absorbance was monitored at 280 nm, with two components (apoA-I and CoQ10) contributing to this signal in CoQ10 ND and only one component contributing (apoA-I) in the empty ND. As a result, empty egg PC ND gave rise to a comparatively smaller 280 nm absorbance peak, as well as an elution volume consistent with a smaller particle size. To verify the presence of CoQ10 in elution fractions collected following chromatography of CoQ10 ND, fractions corresponding to the main absorbance peak were extracted and analyzed by reversed phase HPLC. The data obtained indicated these fractions were positive for CoQ10 (data not shown).
Fig. 4.

FPLC size exclusion chromatography profile of CoQ10 ND. ND were formulated with 1 mg CoQ10 or 0 mg CoQ10 as described. Aliquot of each ND sample (corresponding to 0.2 mg CoQ10 and an equivalent amount of empty ND) in PBS, was applied to a Superose 6 increase 10/300 GL column and elution monitored at 280 nm with collection of 1 mL fractions. PBS, phosphate buffered saline
ND-Mediated Delivery of CoQ10 to HepG2 Cell Mitochondria
To evaluate the ability of ND to serve as a CoQ10 delivery vehicle, HepG2 cells were incubated in the presence and absence of CoQ10 ND for 72 h. Following incubation, the cells were washed to remove unincorporated CoQ10. Mitochondria were isolated by differential centrifugation, CoQ9 internal standard was added, and the sample was dried and extracted with 1-propanol. Reversed phase HPLC analysis of the extracts (Fig. 5) revealed that, compared to control cells incubated with empty ND, incubation with CoQ10 ND led to an increase in the peak corresponding to CoQ10. To verify this result, incubations were conducted on three separate occasions, mitochondria isolated and the content of mitochondrial protein and CoQ10 determined (Fig. 6). The data show that incubation of HepG2 cells with CoQ10 ND leads to a significant increase in the amount of CoQ10 recovered per mg mitochondrial protein.
Fig. 5.

HPLC analysis of CoQ10 extracted from mitochondria. HepG2 cells were incubated with empty ND (dashed line) or CoQ10 ND (solid line) for 72 h. Following incubation, mitochondria were isolated and CoQ9 internal standard added. The mitochondrial preparations were extracted with 1-propanol and aliquots applied to a C18 revered phased column and eluted with methanol and 2-propanol (2:1 vol/vol). IS, internal standard
Fig. 6.

Effect of incubation with CoQ10 ND on mitochondrial CoQ10 content. HepG2 cells were incubated with medium only, empty ND and CoQ10 ND for 72 h as described in materials and methods. Following incubation, the cells were disrupted and mitochondria isolated by differential centrifugation. A protein assay was performed on the isolated mitochondria samples prior to addition of CoQ9 internal standard and extraction with 1-propanol. The extracts were subjected to HPLC to determine the CoQ10 content. Values reported are the mean ± standard error (n = 3) * p < 0.05 vs control and empty PtdCho ND
Biological Effect of ND-Mediated CoQ10 Enrichment of C2C12 Cells
Given that CoQ10 accumulates in mitochondria (Fig. 6), we sought to determine the extent to which exogenous CoQ10 may elicit a measurable improvement in mitochondrial function. To this end, C2C12 myotubes were incubated with empty egg PtdCho ND or CoQ10 ND for 72 h and, following this, mitochondrial function was assessed by measuring OCR values on a XF24 Seahorse Analyzer. The results obtained (Fig. 7) revealed a 23.9 ± 0.9% (n = 3) increase in FCCP-induced maximal OCR in CoQ10 ND-treated cells compared to cells exposed to empty ND. At the same time, baseline OCR values were unaffected. Thus, the present bioenergetics data suggest that incubation of C2C12 myotubes with CoQ10 ND leads to a measurable increase in cellular respiration.
Fig. 7.

Effect of CoQ10 ND on oxygen consumption rates (OCR) of C2C12 myotubes. Differentiated C2C12 myotubes were incubated with DMEM supplemented with either 20 μM CoQ10 ND (open bars) or an equivalent amount of empty ND (filled bars) for 72 h. Following incubation, cells were subjected to a mitochondrial stress test assay as described in the materials and methods. Upon completion of the mitochondrial stress test, OCR were determined for each well and normalized to nucleus count (as visualized following DAPI stain). The graph depicts (1) basal respiration rates and (2) maximal respiration rates for C2C12 cells incubated with control empty ND (filled bars) or with CoQ10 ND (20 μM CoQ10). Values reported are the mean ± standard error (n = 3) * p < 0.05 vs empty PtdCho ND. DMEM, Dulbecco’s minimal essential medium
Discussion
rHDL are fully biocompatible, nanoscale sized particles that arise from the complementary properties of PtdCho and apoA-I (Simonsen, 2016). The most abundant phospholipid in mammalian cells, PtdCho has a natural tendency to form a bilayer in aqueous media. By organizing into a bilayer state, PtdCho fatty acyl chains are effectively protected from contact with the aqueous milieu. This well-known property of PtdCho normally leads to formation of fully sealed spheroidal vesicles or liposomes. When apoA-I is introduced, however, a disc-shaped bilayer is able to form because the apolipoprotein’s amphipathic α-helices promote its function as a scaffold that circumscribes the edge of the bilayer (Lund-Katz and Phillips, 2010). In the absence of a scaffold protein, a planar bilayer would be unstable because phospholipid fatty acyl chains at the edge of the disc would be exposed to the aqueous environment. In rHDL, however, this situation is overcome by interaction of apolipoprotein α-helices with fatty acyl chains at the edge of the discoidal bilayer. This interaction is via the hydrophobic face of apoA-I α-helices, while their opposing, polar faces contact the surrounding aqueous solution. The finding that these two components (i.e. PtdCho and apoA-I) spontaneously assemble into rHDL upon bath sonication, indicates the product particles are inherently stable. When such binary complexes of phospholipid and apolipoprotein are generated in the presence of a third component (usually a hydrophobic bioactive agent), ternary complexes can form wherein the third component stably associates with the discoidal bilayer. In order to distinguish between natural rHDL and complexes engineered to possess additional bioactive components, the term nanodisk (ND) is used in lieu of rHDL. As described in the present report, CoQ10 represents a hydrophobic bioactive molecule capable of stable incorporation into ND. Both the observed incorporation efficiency, as well as the amount of CoQ10 that can be incorporated per ND, are substantial (Table 1).
It is worth noting that, classically, there are two well accepted procedures to generate rHDL. The cholate dialysis method (Jonas, 1986) has seen widespread use and is routinely employed in experiments designed to incorporate transmembrane proteins into the miniature bilayer of rHDL (Tsujita et al., 2018). This method, however, has not been successfully used to generate rHDL that harbor extraneous hydrophobic bioactive lipids (i.e. ND). The most likely explanation for this is that hydrophobic bioactive lipids escape during the dialysis step required to remove cholate. Instead, the direct solubilization method (Ryan, 2008) is usually employed. Until the present report, this method was largely restricted to phospholipids that possess a uniform fatty acyl chain composition, often, myristic acid. In the case of the commonly used dimyristoylphosphatidylcholine, ND formation proceeds optimally at ~23 °C, corresponding to the gel to liquid crystalline phase transition temperature of this lipid. In the present study, we observed that rHDL, or CoQ10 ND, can be prepared using egg PtdCho (mixed fatty acid chains comprised predominantly of palmitic and oleic acids) by conducting the bath sonication at elevated temperature (43–46 °C). This finding extends the utility of the direct solubilization method by expanding the repertoire of phospholipid molecular species that can be employed in formulation reactions.
ND provide a potentially useful model system to investigate the nature of CoQ10 association with natural membranes. For example, CoQ10 exists in relatively high concentrations in the inner mitochondrial membrane (IMM). The IMM is unique in that it is comprised of ~75% protein and ~25% lipid, making it one the most protein-rich membranes in nature. Another important feature of the IMM is that it is a highly curved membrane, containing phospholipids that impose negative membrane curvature pressure on bilayers (Ikon and Ryan, 2017). It is currently unknown precisely how CoQ10 is accommodated in membranes, whether it is unusually long, 50-carbon isoprene tail is fully extended, folds back on itself or both. Likewise, the extent to which the benzoquinone “head group” is exposed to the aqueous environment is not known, nor whether the benzoquinone head group transitions from one side of the bilayer to the other as a normal part of its function. These processes may be affected by the redox state of CoQ10 and are likely important parameters that relate to the function of CoQ10 in the ETC. Regardless of the orientation by which CoQ10 embeds in the ND bilayer, CoQ10 is released into cells and enhances mitochondrial respiration in live cells exposed to ND CoQ10 (Fig. 7), presumably by successfully incorporating into the IMM.
CoQ10 ND should provide a useful vehicle for studies of the mechanism of CoQ10 uptake by cells. Anderson et al. (2015) showed that, in brown adipose tissue, CoQ10 uptake is mediated by the scavenger receptor, CD36. Interestingly, receptors in this class, such as the scavenger receptor class B1 (Cifarelli and Abumrad, 2018) are known to interact with plasma lipoproteins, often promoting nonendocytic uptake of hydrophobic biomolecules. Insofar as ND are structurally similar to nascent HDL particles, these complexes provide a native-like ligand for studies of its receptor interactions. If so, new insight into the mechanism whereby exogenous CoQ10 traffics from plasma to the IMM may be possible. In the present study, when CoQ10 ND were incubated with cultured HepG2 cells, evidence was obtained that, not only is CQ10 taken up, it is capable of trafficking to mitochondria. An interesting and important question is whether administration of CoQ10 ND to cells deficient in CoQ10 will lead to improved mitochondrial function. In humans, inborn errors of metabolism exist that result in CoQ10 deficiency. In these rare disorders, exogenous CoQ10 uptake is of critical importance (Acosta et al., 2016). It is conceivable that intravenous administration of CoQ10 ND may overcome the poor oral bioavailability of CoQ10 and provide a means for enhanced delivery to tissues and organs that rely on CoQ10 for efficient aerobic respiration. Indeed, evidence to this effect was obtained in the present study. Upon incubation of cultured C2C12 myotubues with CoQ10 ND, an ~24% increase in maximal OCR was observed, as compared to control cells incubated with empty PtdCho ND. These data suggest that delivery of CoQ10 to cells upon incubation with CoQ10 ND elicits a beneficial effect on oxidative phosphorylation, presumably the result of increased electron flow from complex I/II to III, which leads to an increase in OCR. However, it has yet to be determined whether the observed enhancement in maximal OCR induced by CoQ10 ND leads to an increase in the rate of ATP production.
In summary, data presented in this study indicate that ND can function as a water soluble delivery vehicle for CoQ10. In cell culture studies, incubation of HepG2 cells with CoQ10 ND led to an increase in mitochondrial CoQ10 content as well as an increase in cellular respiration capacity, as compared to incubations with control empty ND. Given that CoQ10 synthesis decreases with aging (Bentinger et al., 2010; Turunen et al., 2004), potential advantages of CoQ10 ND for in vivo delivery applications include the inherent biocompatibility of the components, their structural similarity to natural nascent HDL particles as well as their nanoscale size. Whereas oral ingestion of CoQ10 ND is unlikely to enhance bioavailability due to anticipated disruption of the particles in the gut, intravenous, intramuscular, or transdermal delivery provide potentially attractive options.
Acknowledgements
This work was support by a grant from the National Institutes of Health (R37 HL64159). The authors thank Colin Fox for helpful suggestions, assistance with technical aspects and troubleshooting, Irina Romenskaia for assistance with cell culture, and Sharon Young and Leanne Perez for guidance in mitochondrial isolations.
Abbreviations
- CoQ10
coenzyme Q10
- DMF
dimethylformamide
- HMG CoA
3-hydroxy, 3-methylglutaryl CoA
- IMM
inner mitochondrial membrane
- ND
nanodisks
- PtdCho
phosphatidylcholine
- rHDL
reconstituted HDL
Footnotes
Conflict of Interest The authors declare that they have no conflict of interest.
References
- Acosta MJ, Vazquez Fonseca L, Desbats MA, Cerqua C, Zordan R, Trevisson E, & Salviati L (2016) Coenzyme Q biosynthesis in health and disease. Biochimica et Biophysica Acta, 1857:1079–1085. [DOI] [PubMed] [Google Scholar]
- Anderson CM, Kazantzis M, Wang J, Venkatraman S, Goncalves RL, Quinlan CL, … Stahl A (2015) Dependence of brown adipose tissue function on CD36-mediated coenzyme Q uptake. Cell Reports, 10:505–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentinger M, Tekle M, & Dallner G (2010) Coenzyme Q—biosynthesis and functions. Biochemical and Biophysical Research Communications, 396:74–79. [DOI] [PubMed] [Google Scholar]
- Cifarelli V, & Abumrad NA (2018) Intestinal CD36 and other key proteins of lipid utilization: Role in absorption and gut homeostasis. Comprehensive Physiology, 8:493–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosby NM, Ghosh M, Su B, Beckstead JA, Kamei A, Simonsen JB, … Ryan RO (2015) Anti-CD20 single chain variable antibody fragment-apolipoprotein A-I chimera containing nanodisks promote targeted bioactive agent delivery to CD20-positive lymphomas. Biochemistry and Cell Biology, 93: 343–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagda RK, Pien I, Wang R, Zhu J, Wang KZ, Callio J, … Chu CT (2014) Beyond the mitochondrion: Cytosolic PINK1 remodels dendrites through protein kinase A. Journal of Neurochemistry, 128:864–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duivenvoorden R, Tang J, Cormode DP, Mieszawska AJ, Izquierdo-Garcia D, Ozcan C, … Mulder WJM (2014) A statin-loaded reconstituted high-density lipoprotein nanoparticle inhibits atherosclerotic plaque inflammation. Nature Communications, 5:3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox CA, Ellison P, Ikon N, & Ryan RO (2019) Calcium-induced transformation of cardiolipin nanodisks. Biochemistry et Biophysica Acta, 1861:1030–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh M, & Ryan RO (2014) ApoE enhances nanodisk-mediated curcumin delivery to glioblastoma multiforme cells. Nanomedicine (London), 9:763–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh M, Singh AT, Xu W, Sulchek T, Gordon LI, & Ryan RO (2011) Curcumin nanodisks: Formulation and characterization. Nanomedicine, 7:162–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikon N, & Ryan RO (2017) Cardiolipin and mitochondrial cristae organization. Biochimica et Biophysica Acta, 1859:1156–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikon N, Su B, Hsu FF, Forte TM, & Ryan RO (2015) Exogenous cardiolipin localizes to mitochondria and prevents TAZ knockdown-induced apoptosis in myeloid progenitor cells. Biochemical and Biophysical Research Communications, 464: 580–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas A (1986) Reconstitution of high-density lipoproteins. Methods in Enzymology, 128:553–582. [DOI] [PubMed] [Google Scholar]
- Kornmueller K, Vidakovic I, & Prassl R (2019) Artificial high density lipoprotein nanoparticles in cardiovascular research. Molecules, 24:E2829. 10.3390/molecules24152829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lujan B, Kushmerick C, Banerjee TD, Dagda RK, & Renden R (2016) Glycolysis selectively shapes the presynaptic action potential waveform. Journal of Neurophysiology, 116: 2523–2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund-Katz S, & Phillips MC (2010) High density lipoprotein structure-function and role in reverse cholesterol transport. Subcellular Biochemistry, 51:183–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda MN, Hargreaves PL, Beckstead JA, Redmond KA, van Antwerpen R, & Ryan RO (2006) Reconstituted high density lipoprotein enriched with the polyene antibiotic amphotericin B. Journal of Lipid Research, 47:260–267. [DOI] [PubMed] [Google Scholar]
- Ryan RO (2008) Nanodisks: Hydrophobic drug delivery vehicles. Expert Opinion on Drug Delivery, 5:343–351. [DOI] [PubMed] [Google Scholar]
- Ryan RO (2010) Nanobiotechnology applications of reconstituted high density lipoprotein. Journal of Nanobiotechnology, 8:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan RO, Forte TM, & Oda MN (2003) Optimized bacterial expression of human apolipoprotein A-I. Protein Expression and Purification, 27:98–103. [DOI] [PubMed] [Google Scholar]
- Sacoman JL, Dagda RY, Burnham-Marusich AR, Dagda RK, & Berninsone PM (2017) Mitochondrial O-GlcNAc transferase (mOGT) regulates mitochondrial structure, function, and survival in HeLa cells. Journal of Biological Chemistry, 292:4499–4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen JB (2016) Evaluation of reconstituted high-density lipoprotein (rHDL) as a drug delivery platform—A detailed survey of rHDL particles ranging from biophysical properties to clinical implications. Nanomedicine, 12:2161–2179. [DOI] [PubMed] [Google Scholar]
- Taylor BA (2018) Does coenzyme Q10 supplementation mitigate statin-associated muscle symptoms? Pharmacological and methodological considerations. American Journal of Cardiovascular Drugs, 18:75–82. [DOI] [PubMed] [Google Scholar]
- Tsujita M, Wolska A, Gutmann DAP, & Remaley AT (2018) Reconstituted discoidal high-density lipoproteins: Bioinspired nanodiscs with many unexpected applications. Current Atherosclerosis Reports, 20:59. [DOI] [PubMed] [Google Scholar]
- Turunen M, Olsson J, & Dallner G (2004) Metabolism and function of coenzyme Q. Biochimica et Biophysica Acta, 1660: 171–199. [DOI] [PubMed] [Google Scholar]
- Villalba JM, Parrado C, Santos-Gonzalez M, & Alcain FJ (2010) Therapeutic use of coenzyme Q10 and coenzyme Q10-related compounds and formulations. Expert Opinion on Investigational Drugs, 19:535–554. [DOI] [PubMed] [Google Scholar]
- Zaki NM (2016) Strategies for oral delivery and mitochondrial targeting of CoQ10. Drug Delivery, 23:1868–1881. [DOI] [PubMed] [Google Scholar]
- Zhang B, Li F, Chen Z, Shrivastava IH, Gasanoff ES, & Dagda RK (2019) Naja mossambica mossambica cobra cardiotoxin targets mitochondria to disrupt mitochondrial membrane structure and function. Toxins (Basel), 11:E152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao P, Aguilar AE, Lee JY, Paul LA, Suh JH, Puri L, … Saba JD (2018) Sphingadienes show therapeutic efficacy in neuroblastoma in vitro and in vivo by targeting the AKT signaling pathway. Investigational New Drugs, 36:743–754. [DOI] [PMC free article] [PubMed] [Google Scholar]


