Abstract
Background
This study examined the safety and immunogenicity of an inactivated SARS-CoV-2 vaccine.
Method
In a phase I randomized, double-blinded, placebo-controlled trial involving 192 healthy adults 18–59 years old, two injections of three doses (50 EU, 100 EU, 150 EU) of an inactivated SARS-CoV-2 vaccine or placebo were administered intramuscularly at a 2- or 4-week interval. The safety and immunogenicity of the vaccine were evaluated.
Results
Vaccination was completed in 191 subjects. Forty-four adverse reactions occurred within 28 days, most commonly mild pain and redness at the injection site or slight fatigue. At days 14 and 28, the seroconversion rates were 87.5% and 79.2% (50 EU), 100% and 95.8% (100 EU), and 95.8% and 87.5% (150 EU), respectively, with geometric mean titers (GMTs) of 18.1 and 10.6, 54.5 and 15.4, and 37.1 and 18.5, respectively, for the schedules with 2-week and 4-week intervals. Seroconversion was associated with synchronous upregulation of antibodies against the S protein, N protein and virion and a cytotoxic T lymphocyte (CTL) response. No cytokines and immune cells related to immunopathology were observed. Transcriptome analysis revealed the genetic diversity of immune responses induced by the vaccine.
Interpretation
In a population aged 18–59 years in this trial, this inactivated SARS-CoV-2 vaccine was safe and immunogenic.
Trial registration: CTR20200943 and NCT04412538.
Keywords: SARS-CoV-2, Inactivated vaccine, Phase I
1. Introduction
Coronavirus disease 2019 (COVID-19) is caused by a novel member of the Coronaviridae family called severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). From the emergence of COVID-19 at the end of 2019 to September 2020, more than 26 million cases and more than 800,000 deaths had been recorded, indicating that COVID-19 poses a substantial threat to public health worldwide [1]. Because of the highly contagious nature of SARS-CoV-2 and the severe clinical outcomes [2], [3], one of the primary strategies to control the pandemic is to develop an effective vaccine, and within a short period, clinical trials of several vaccines have been initiated [4], [5], [6], [7]. To date, the data obtained from phase I/II clinical trials have focused on serological detection to assess the immunogenicity of these vaccines [4], [7], [8]. The data suggest that SARS-CoV-2, an enveloped virus, possesses various encoded antigenic components, including S (spike), N (nucleocapsid), E (envelope) and M (membrane) antigens [9], all of which might theoretically be recognized by the immune system during infection; however, the key antigen is the S protein, which mediates virion entry into cells by interacting with the ACE2 receptor [10]. Our previous work, based on an analysis of the composition of antibodies in convalescent serum from COVID-19 patients, suggested a technical strategy for the preparation of an inactivated SARS-CoV-2 vaccine in which the inactivation process yields an inactivated virion in which the S, N and other viral antigens are exposed through a patented two-step inactivation with formaldehyde and beta-propiolactone [11]. This vaccine was found to effectively elicit immune protection in nonhuman primates under viral challenge and was approved for a phase I clinical trial (permission number: 2020L00020 by the Chinese Food and Drug Administration (CFDA); clinical trial registration number: CTR20200943 and NCT04412538). Here, we further investigated the safety and immunogenicity of this vaccine in immunized individuals in a phase I trial. The results obtained are encouraging, and further study is needed.
2. Materials and methods
2.1. Viruses and cells
All SARS-CoV-2 virus strains used in this work were isolated from hospitalized patients including domestic and foreign patients with confirmed COVID-19 in Yunnan Hospital of Infectious Diseases from January to May 2020. A strain with a D614G mutation in the S protein was isolated from a pediatric patient who had returned from the U.S. to their hometown and was identified as being infected with SARS-CoV-2 through clinical diagnosis and q-RT-PCR in March 2020. The virus was proliferated in Vero cells (WHO) and was titrated with a microtitration assay. Vero cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Corning, NY, USA) containing 5% fetal bovine serum (FCS; HyClone, Logan, USA).
2.2. Inactivated vaccine
The SARS-CoV-2 inactivated vaccine was developed by the Institute of Medical Biology (IMB), Chinese Academy of Medical Sciences (CAMS) in its facility in accordance with GMP requirements. Briefly, the virus strain, named KMS-1 (GenBank No: MT226610.1), was inoculated into Vero cells for production in an environment that met the BSL requirements. The harvested viruses were inactivated by formaldehyde (v:v = 1:4000) for 48 h to lyse the viral membrane, purified via chromatography and concentrated. A second inactivation with beta-propiolactone (v:v = 1:2000) was performed to destroy the viral genomic structure, followed by a second purification and concentration using the same protocol. The vaccine stock was evaluated using various quality indexes, including antigen content, immunogenicity, sterility and residue testing. The viral antigen content was measured via ELISA. The vaccine contained 50, 100 or 150 EU of inactivated viral antigen adsorbed to 0.25 mg of Al(OH)3 adjuvant and suspended in 0.5 ml of buffered saline for each dose. Before this vaccine was released for clinical study by the CFDA under No. 2020L00020, the protective efficacy of the vaccine was tested in the macaque challenge test [11], [12], and various toxicity studies, including an acute toxicity test, a long-term toxicity test and an allergic test, demonstrated its safety in nonhuman primates and rodents. The placebo contained only the same amount of Al(OH)3 in buffer.
2.3. Study design and participants
The trial was designed based upon the principles of randomization, double-blinding and placebo control. The study protocol was reviewed and approved by the Ethics Committee of the West China Second University Hospital, Sichuan University (Approval Number: Y2020008). An independent data safety monitoring board was established to oversee the safety data during the study, specifically during the 7 days after each inoculation (p.i.). The trial was conducted according to the principles of the Declaration of Helsinki and Good Clinical Practice at the West China Second University Hospital, Sichuan University. Healthy volunteers 18–59 years of age were eligible for enrollment after providing informed consent. The inclusion and exclusion criteria are listed in the supplementary appendix. The enrolled participants were randomly assigned at a ratio of 1:1 to receive two inoculations at an interval of 14 days or 28 days, and the subjects in each schedule were assigned at a ratio of 1:1:1:1 to receive one of the three vaccine doses (50 EU, 100 EU and 150 EU) or the placebo. All the enrolled participants were asked to record solicited and unsolicited adverse events, if any, for a period of 28 days. Study staff visited participants on site to track their health status and determine whether they needed medical care. Blood samples were taken from the enrolled participants on the day before immunization (baseline) and days 7, 14 and 28 (0, 14 schedule) or days 7 and 28 (0, 28 schedule) after the booster immunization to evaluate the immunogenicity of the vaccine at different time points, determine the level of 48 cytokines in the serum and analyze mRNA gene expression in peripheral blood mononuclear cells (PBMCs).
2.4. Randomization and masking
The randomization number for each vaccination schedule was generated by SAS software (version 9.4), and stratified block randomization (block size 8) by subgroups, generated by an independent statistician, was used. Within each randomization block, the ratio of vaccine to placebo was 3:1. A randomization number was sequentially assigned to each participant, and then the participant was injected with a vaccine or placebo with the same number. All participants, investigators, and laboratory staff were blinded to the treatment allocation.
2.5. End points of the clinical trial
The primary end point was the total rate of adverse reactions from 0 to 28 days post-immunization. The secondary end points were serological evidence of the immunogenicity of the vaccine.
2.6. Laboratory detection
2.6.1. Neutralizing antibody test
The neutralizing antibody assay was performed via microtitration, and the neutralizing titer in the sera was determined by CPE observation. Briefly, heat-inactivated serum was diluted and incubated with live virus (100 lgCCID50/well) for 2 h at 37 °C followed by the addition of Vero cells (105/mL), and the mixture was incubated at 37 °C in 5% CO2 for 7 days. The CPEs were observed and assessed to determine the neutralizing antibody titer of the serum. The geometric mean titers (GMTs) of neutralizing antibodies were measured. Antibody titers ≥ 4 were considered positive. Seroconversion was defined as seropositivity after immunization in previously seronegative subjects.
2.6.2. ELISA
ELISAs were conducted with antibodies against the S protein, the N protein and virions that were developed by this institute. S/N protein (Sanyou Biopharmaceuticals Co., Ltd., Shanghai, China) and purified viral antigen were used to coat 96-well ELISA plates (Corning, NY, USA) at a concentration of 5 μg/well, and the plates were incubated at 4 °C overnight. The plates were then blocked with 5% BSA and incubated with serum samples, and immune complexes were visualized using an HRP-conjugated antibody (Abcam, MA, USA) and TMB substrate (Solarbio, Beijing, China) as described in a previous report [13]. The absorbance of each well at 450 nm was measured using an ELISA plate reader (Gene Company, Beijing, China). The antibody serum samples that yielded OD values at least 2.1-fold higher than that of the negative control at a test sample dilution of 1:400 were defined as positive. The endpoint titer (ET) was defined as the highest serum dilution that yielded a positive OD value. The GMT was calculated as the geometric mean of the ETs of the positive serum samples in each group. For neutralizing antibodies, seroconversion was defined as seropositivity after immunization in previously seronegative subjects.
2.6.3. ELISpot assay
An ELISpot assay was performed with a Human IFN-γ ELISpot Kit (Mabtech, Cincinnati, OH, USA) according to the manufacturer’s protocol. PBMCs were isolated from blood obtained from 50% of the subjects in the immunization and placebo groups via lymphocyte isolation (Ficoll-Paque PREMIUM; GE Healthcare, Piscataway, NJ, USA) and plated in duplicate wells. Purified virions, recombinant S protein and recombinant N protein (Sanyou Biopharmaceuticals Co., Ltd.) were added to the wells to stimulate the cells. The positive control was phytohemagglutinin (PHA), and the negative controls included a cell-only control and a medium-only control. The plates were incubated at 37 °C for 24 h, the cells were removed, and the spots were developed. The colored spots were counted using an ELISPOT reader (CTL, Shaker Heights, OH, USA).
2.6.4. Immune cell populations
The isolation of immune cell populations was performed according to a standard protocol. PBMCs were isolated via lymphocyte isolation (Ficoll-Paque PREMIUM; GE Healthcare). Anti-CD3, anti-CD20 and anti-CD16 antibodies (BD558639; BD, USA) were added to the PBMCs. The mixtures were incubated at room temperature (RT) for 30 min in the dark. Reagents for red blood cell lysis (BD, USA) and membrane permeabilization (BD, USA) were added in sequence. After two washes with PBS, the cells were resuspended in PBS and subjected to flow cytometry (BD, USA). T cells (CD3+; anti-human CD3, BD555332), T helper cells (CD3+/CD4+; anti-human CD4, BD566319) and CD8 (+) T cells (CD3+/CD8+; anti-human CD8, BD555368) were evaluated. The T cells were further categorized as T helper (Th)1 and Th2 cells using anti-CD4 (BD550631), anti-IL-4 (BD500824) and anti-IFN-γ (BD506507) antibodies (BD, USA). The percentages of immune cells of each type were determined using a flow cytometer (Beckman Coulter Cytoflex; BD, USA). Th1 cells (CD4+/IFN-γ+) and Th2 cells (CD4+/IL-4+) were assessed.
2.6.5. Cytokine assay
The levels of 48 cytokines in the serum of the subjects were measured using the Bio-Plex Pro Human Cytokine 48-Plex (Bio-Rad, Hercules, California, USA) according to the manufacturer’s protocol. Briefly, 4-fold dilutions of serum samples were added to tubes containing detection beads. The antibodies used for detection were then added, and the mixtures were incubated at RT for 0.5 h in the dark. The beads were then washed, resuspended in wash buffer and measured using a flow cytometer (BD, USA). The concentration of each cytokine was determined based on a calibration curve that was constructed independently for each cytokine. The samples were analyzed in duplicate.
2.6.6. Transcriptome assay
Transcriptome assays were performed by Novogene Co., Ltd., China. To exclude individual differences, each group included two samples (A and B) at each time point, and each sample was mixed with PBMCs from five individuals. Total RNA was extracted from PBMCs using an RNeasy Mini Kit (QIAGEN, GmBH, Germany). The RNA was checked for quality and quantified. Double- and single-stranded DNA and ribosomal RNA (rRNA) were removed. Magnetic beads were then used to purify and recover the reaction products, and sequencing libraries were generated according to the manufacturer’s recommendations. In brief, the reaction products were heated, denatured and circularized using a splint oligo sequence. The RNA-seq libraries were sequenced on an Illumina HiSeq X TEN platform (2 × 150-bp paired-end reads).
The read pairs were filtered using software to remove those with low-quality bases (Phred quality < 5) or with more than 10% uncertain bases. The RNA-seq reads were aligned to the human genome (http://ftp.ensembl.org/pub/release-84/fasta/homo_sapiens/dna/) using Bowtie V2.0.6. The raw count was used by DESeq2 to quantify the gene expression level. Cuffdiff was used to identify genes that were differentially expressed in the vaccine and control samples. Significantly differentially expressed genes were identified at P < 0.05. The raw microarray data are available from the National Genomics Data Center of the China National Center for Bioinformation/Beijing Institute of Genomics, Chinese Academy of Sciences (CNCB-NGDC), under accession number HRA000347.
2.6.7. qRT-PCR
RNA was extracted from samples using TRIzol reagent (Invitrogen Tiangen Biotech, China). Real-time RT-PCR assays were performed using the One-Step PrimeScript RT-PCR Kit (Takara, Shuzo, Japan) and a Real-Time PCR System (Bio-Rad, USA). Primers and probes for SARS-CoV-2 were used to measure mRNA levels; for N, these were forward: 5′-GGGGAACTTCTCCTGCTAGAAT-3′, reverse: 5′-CAGACATTTTGCTCTCAAGCTG-3′, and probe: 5′-TTGCTGCTGCTTGACAGATT-3′, and for ORF 1ab, they were forward: 5′-CCCTGTGGGTTTTACACTTAA-3′, reverse: 5′-ACGATTGTGCATCAGCTGA-3′, and probe: 5′-CCGTCTGCGGTATGTGGAAAGGTTATGG-3′. Primers and probes targeting the 3′ untranslated region (UTR) of DENV serotypes 1–3 were used according to previously described methods [14]. An RT-PCR assay with a universal single probe for the diagnosis of dengue virus infections was performed [14].
2.6.8. Statistical analysis
The sample size was determined based on the requirement of the National Medical Products Administration of China, which requires a minimum sample size of 20–30 participants for each vaccine dose in an immunization schedule in phase 1 clinical trials. Means and standard deviations are used to describe normally distributed continuous variables, and frequencies and proportions are used to describe categorical variables. The safety analysis was performed with the data from the participants who had received at least one dose of the vaccine and for whom safety data were available. The numbers and proportions of participants with adverse reactions or events were summarized. The immunogenicity analysis was conducted with the data from the full cohort, including all participants who received injections and had results for the antibody test. The antibodies against SARS-CoV-2 are summarized as geometric mean titers with 95% CIs, and the cellular responses are presented as the proportion of positive responders. The chi-square test or Fisher’s exact test was used to analyze the seroconversion rate of antibodies against SARS-CoV-2, and the rank-sum test was used to compare antibody titers. When the comparison among all four groups of each schedule showed a significant difference, pairwise comparisons were performed. A P value lower than 0.05 (two-sided) was considered to be significant. The statistical analysis was performed by an independent statistician using GraphPad Prism software (San Diego, CA, USA) and STATA software (Version 15.0; STATA Corp., College Station, TX, USA).
3. Results
3.1. Study participants
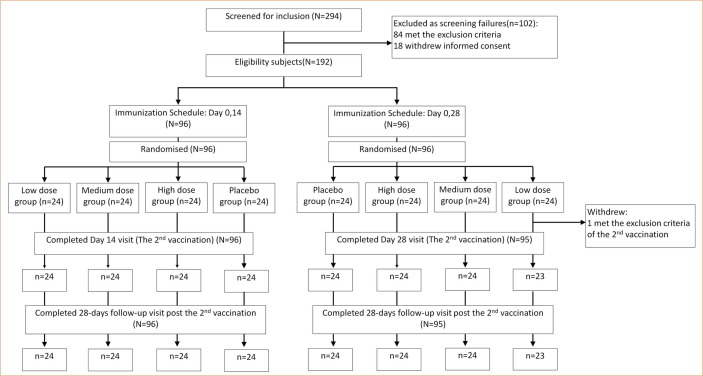
A total of 294 adults aged 18 to 59 years were evaluated for inclusion in this phase I trial. Of these, 102 persons were excluded: 84 were ineligible due to physical unfitness, and 18 withdrew their informed consent. The remaining 192 participants were randomly assigned at a ratio of 1:1 to receive two inoculations with an intervening interval of 14 days or 28 days, and subjects on each schedule were assigned at a ratio of 1:1:1:1 to receive one of the three doses of the vaccine or the placebo (Fig. 1 ).
Fig. 1.
Screening, randomization and inclusion in phase I clinical trial.
From May 2020 through August 2020, all participants who received two inoculations of the vaccine or placebo were monitored for any clinical manifestations and were required to provide blood samples three (0, 14 schedule) or two (0, 28 schedule) times after inoculation. The withdrawal rate was 0.5%: 1 participant in the low-dose group who was assigned to the 0, 28 schedule did not receive the second dose. The 191 participants were divided as follows: 24 in each of the three different dose groups and the control group assigned to the 0, 14 schedule and 23, 24, 24 and 24 in the low-dose, medium-dose, high-dose and control groups assigned to the 0, 28 schedule (Fig. 1). The demographic characteristics of the participants in each group are shown in Table 1 .
Table 1.
Baseline characteristics of the study participants.
| Characteristic |
Immunization Schedule: Day 0,14 (N = 96) |
Immunization Schedule: Day 0,28 (N = 96) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Low dose group (n = 24) | Medium dosegroup (n = 24) | High dose group (n = 24) | Placebo group (n = 24) | Total (n = 96) | Low dose group (n = 24) | Medium dose group (n = 24) | High dose group (n = 24) | Placebo group (n = 24) | Total (n = 96) | ||
| Age | |||||||||||
| 18–29 | 9 (38%) | 7 (29%) | 11 (46%) | 5 (21%) | 32 (33%) | 8 (33%) | 4 (17%) | 7 (29%) | 5 (21%) | 24 (25%) | |
| 30–39 | 4 (17%) | 6 (25%) | 7 (29%) | 10 (42%) | 27 (28%) | 9 (38%) | 7 (29%) | 9 (38%) | 10 (42%) | 35 (36%) | |
| 40–49 | 7 (29%) | 7 (29%) | 3 (13%) | 6 (25%) | 23 (24%) | 7 (29%) | 11 (46%) | 3 (13%) | 7 (29%) | 28 (29%) | |
| 50–59 | 4 (17%) | 4 (17%) | 3 (13%) | 3 (13%) | 14 (15%) | 0 | 2 (8%) | 5 (21%) | 2 (8%) | 9 (9%) | |
| Mean(SD) | 37.0 ± 11.84 | 38.2 ± 10.83 | 33.9 ± 11.00 | 37.6 ± 9.04 | 36.7 ± 10.69 | 34.4 ± 9.21 | 40.1 ± 9.70 | 36.5 ± 11.43 | 37.2 ± 8.67 | 37.0 ± 9.86 | |
| Sex | |||||||||||
| Male | 11 (46%) | 8 (33%) | 10 (42%) | 14 (58%) | 43 (45%) | 9 (38%) | 12 (50%) | 10 (42%) | 12 (50%) | 43 (45%) | |
| Female | 13 (54%) | 16 (67%) | 14 (58%) | 10 (42%) | 53 (55%) | 15 (63%) | 12 (50%) | 14 (58%) | 12 (50%) | 53 (55%) | |
| BMI, kg/m2 | |||||||||||
| Mean(SD) | 23.6 ± 2.80 | 23.0 ± 2.76 | 23.0 ± 2.96 | 23.0 ± 2.70 | 23.1 ± 2.77 | 22.7 ± 2.83 | 22.8 ± 2.73 | 22.6 ± 1.37 | 23.3 ± 2.39 | 22.9 ± 2.38 | |
| Ethnicity | |||||||||||
| Han | 24 (100%) | 22 (92%) | 22 (92%) | 24 (100%) | 92 (96%) | 22 (92%) | 24 (100%) | 24 (100%) | 24 (100%) | 94 (98%) | |
| Man | 0 | 0 | 0 | 0 | 0 | 1 (4%) | 0 | 0 | 0 | 1 (1%) | |
| Hmong | 0 | 1 (4%) | 0 | 0 | 1 (1%) | 0 | 0 | 0 | 0 | 0 | |
| Tujia | 0 | 0 | 1 (4%) | 0 | 1 (1%) | 0 | 0 | 0 | 0 | 0 | |
| Tibetan | 0 | 1 (4%) | 1 (4%) | 0 | 2 (2%) | 1 (4%) | 0 | 0 | 0 | 1 (1%) | |
BMI, body mass index; SD, standard deviation. ‘n’ used as denominator for calculation of percentage values.
3.2. Comprehensive observation of adverse reactions
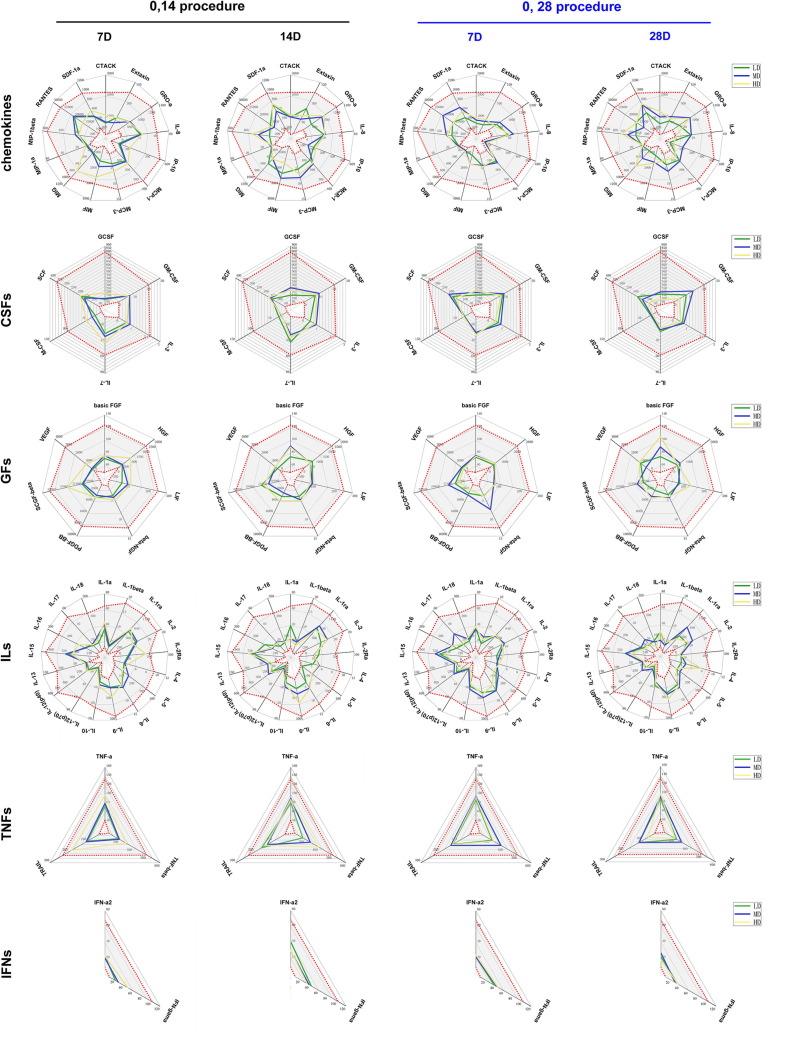
In this trial, we observed not only solicited clinical adverse reactions for 7 days and unsolicited events for 28 days after each inoculation but also variations in cytokine levels in the serum of 50% of the subjects inoculated with the vaccine or placebo. The 18 solicited systemic adverse reactions observed in the 7 days after each inoculation were distributed as follows: 3, 1, 1 and 0 in the low-dose, medium-dose, high-dose and placebo groups assigned to the 0, 14 schedule, and 2, 4, 4, and 3 in low-dose, medium-dose, high-dose and placebo groups assigned to the 0, 28 schedule. Unsolicited reactions were reported by 8.3%, 0.0%, and 8.3% of the participants in the low-, medium- and high-dose groups compared to 0.0% in the placebo group among those assigned to the 0, 14 schedule, and unsolicited reactions were reported by 0.0%, 8.3%, and 12.5% of the participants in the low-, medium- and high-dose groups compared to 4.2% in the placebo group among those assigned to the 0, 28 schedule. The most common adverse reactions were mild pain and redness at the injection site and slight fatigue. No severe (grade 3) adverse reactions or serious reactions were observed within 28 days (Table 2 ). Furthermore, the levels of various cytokines in the sera of immunized subjects in the 3 dose groups following the 2 schedules did not show abnormalities compared to those in the sera from subjects in the placebo groups (Fig. 2 ). There were no significant differences between the vaccine and placebo groups with regard to the counts of various T cell populations in the peripheral blood (Fig. 3 ). These results suggest that immunopathologic events associated with variation of cytokine levels and immune cell populations related to vaccination did not occur.
Table 2.
Adverse reactions in the safety population.
| Adverse reactions | 0,14 procedure |
0,28 procedure |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Low dose group (n = 24) | Middle dose group (n = 24) | High dose group (n = 24) | Placebo group (n = 24) | Low dose group (n = 24) | Middle dose group (n = 24) | High dose group (n = 24) | Placebo group (n = 24) | ||
| number of participants (%) | number of participants (%) | ||||||||
| The first immunization | |||||||||
| All adverse reactions within 0–7 days | 5 (20.8) | 3 (12.5) | 3 (12.5) | 1 (4.2) | 3 (12.5) | 4 (16.7) | 8 (33.3) | 3 (12.5) | |
| Solicited injection site adverse reactions within 0–7 days | |||||||||
| Total | 3 (12.5) | 2 (8.3) | 2 (8.3) | 1 (4.2) | 2 (8.3) | 1 (4.2) | 4 (16.7) | 0 (0.0) | |
| Pain | 1 (4.2) | 2 (8.3) | 2 (8.3) | 1 (4.2) | 2 (8.3) | 1 (4.2) | 4 (16.7) | 0 (0.0) | |
| Itch | 2 (8.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Solicited systemic adverse reactions within 0–7 days | |||||||||
| Total | 3 (12.5) | 1 (4.2) | 1 (4.2) | 0 (0.0) | 1 (4.2) | 3 (12.5) | 4 (16.7) | 2 (8.3) | |
| Fatigue | 2 (8.3) | 1 (4.2) | 0 (0.0) | 0 (0.0) | 1 (4.2) | 3 (12.5) | 3 (12.5) | 1 (4.2) | |
| Diarrhoea | 0 (0.0) | 1 (4.2) | 1 (4.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (4.2) | 0 (0.0) | |
| Fever | 1 (4.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Rash | 1 (4.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (4.2) | |
| Unsolicited adverse reactions within 0–7 days | 2 (8.3) | 0 (0.0) | 1 (4.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (8.3) | 1 (4.2) | |
| Overall adverse reactions within 0–14 days | 8 (33.3) | 3 (12.5) | 4 (16.7) | 2 (8.3) | / | / | / | / | |
| Overall adverse reactions within 0–28 days | / | / | / | / | 3 (12.5) | 4 (16.7) | 8 (33.3) | 3 (12.5) | |
| The booster immunization | |||||||||
| All adverse reactions within 0–7 days | 5 (20.8) | 2 (8.3) | 1 (4.2) | 2 (8.3) | 2 (8.3) | 5 (20.8) | 4 (16.7) | 2 (8.3) | |
| Solicited injection site adverse reactions within 0–7 days | |||||||||
| Total | 5 (20.8) | 2 (8.3) | 0 (0.0) | 2 (8.3) | 1 (4.2) | 3 (12.5) | 3 (12.5) | 0 (0.0) | |
| Pain | 5 (20.8) | 2 (8.3) | 0 (0.0) | 1 (4.2) | 1 (4.2) | 2 (8.3) | 3 (12.5) | 0 (0.0) | |
| Itch | 0 (0.0) | 1 (4.2) | 0 (0.0) | 1 (4.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Redness | 1 (4.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Swelling | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (4.2) | 0 (0.0) | 0 (0.0) | |
| Solicited systemic adverse reactions within 0–7 days | |||||||||
| Total | 1 (4.2) | 1 (4.2) | 0 (0.0) | 0 (0.0) | 1 (4.2) | 2 (8.3) | 1 (4.2) | 1 (4.2) | |
| Fatigue | 1 (4.2) | 1 (4.2) | 0 (0.0) | 0 (0.0) | 1 (4.2) | 1 (4.2) | 1 (4.2) | 1 (4.2) | |
| Diarrhoea | 0 (0.0) | 1 (4.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Fever | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (4.2) | 0 (0.0) | 0 (0.0) | |
| Rash | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Unsolicited adverse reactions within 0–7 days | 0 (0.0) | 0 (0.0) | 1 (4.2) | 0 (0.0) | 0 (0.0) | 2 (8.3) | 2 (8.3) | 1 (4.2) | |
| Overall adverse reactions within 0–28 days | 5 (20.8) | 2 (8.3) | 1 (4.2) | 2 (8.3) | 2 (8.3) | 5 (20.8) | 4 (16.7) | 2 (8.3) | |
No severe (grade 3) adverse reaction and serious adverse events were observed.
Fig. 2.
Variations in the levels of 48 cytokines in sera from immunized individuals. The levels of 48 cytokines in the sera of subjects who received the vaccine or the placebo were monitored. Subjects were assigned to the immunization procedure with an interval of 14 days (black) or with an interval of 28 days (blue). The measured cytokines included chemokines, interleukins (ILs), growth factors (GFs), colony-stimulating factors (CSFs), tumor necrosis factors (TNFs), and interferon (IFNs). The levels of 48 cytokines (pg/ml) in the sera of subjects before receiving the vaccine and placebo are shown as gray intervals between red dots in each figure. Control (Con, 0 EU); low dose (LD, 50 EU); medium dose (MD, 100 EU); and high dose (HD, 150 EU).
Fig. 3.
T cell populations in peripheral blood from individuals immunized with the inactivated vaccine. The percentage of various T cell populations among PBMCs obtained from individuals assigned to the immunization procedure with an interval of 14 days (0, 14 procedure) or with an interval of 28 days (0, 28 procedure) after booster immunization. Control (Con, 0 EU); low dose (LD, 50 EU); medium dose (MD, 100 EU); and high dose (HD, 150 EU).
3.3. Assessment of immunity elicited in the 3 dose groups assigned to the 2 schedules
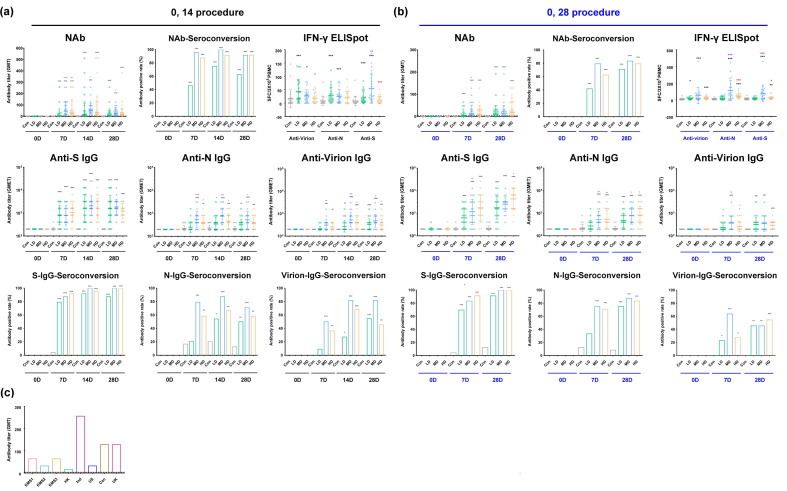
The results obtained for the three dose groups assigned to the two schedules with regard to the neutralizing antibody titers of the immunized subjects in the groups assigned to the 0, 14 schedule showed seroconversion rates of 54.2%, 100% and 87.5% in the low-, medium- and high-dose groups compared to the placebo group at day 7 after the booster, and their geometric mean titers (GMTs) increased by 5.2, 45.1 and 27.4, respectively. Furthermore, at day 14, the seroconversion rates in the three groups reached 87.5%, 100% and 95.8%, with GMTs of 18.1, 54.5 and 37.1, respectively (Fig. 4 a). However, there appeared to be a decreasing trend in the neutralizing antibody titers from day 14 to day 28 (Fig. 4a). In the groups assigned to the 0, 28 schedule, the seroconversion rate of the neutralizing antibodies reached 79.2% (low-dose), 95.8% (medium-dose) and 87.5% (high-dose), with GMTs of 10.6, 15.4 and 18.5, at day 28 after booster immunization. An increasing trend of neutralizing antibodies was observed starting from day 7 after booster immunization, with seroconversion rates of 54.2% (low-dose), 79.2% (medium-dose) and 69.6% (high-dose) and GMTs of 4.2, 11.2 and 8.9 (Fig. 4b). Significantly, ELISA with antibodies against the S protein, N protein and virion showed essentially synchronous increases regardless of the schedule to which the participants were assigned (Fig. 4a, b). The specific positive cytotoxic T lymphocyte (CTL) responses against the S protein, N protein and virion in the ELISpot assay indicated a distinct increase at day 28 after the booster for both schedules (Fig. 4a, b). These results suggest that the vaccine elicits a synchronous dynamic response involving antibodies and CTLs against the viral antigens. The IgG1 subtype was the common subtype against all three antigens (Table S1). Additionally, the immune sera were found to neutralize the pandemic strains in North America (Fig. 4c) with the D614G mutation in the S protein (Supplemental Fig. 1) [15].
Fig. 4.
Immune responses induced in human individuals immunized with inactivated SARS-Cov-2 vaccine in the 0, 14 and 0, 28 schedule groups. (a) Neutralizing antibodies (NAb) and ELISA antibodies (IgGs) against the S protein (Anti-S IgG), N protein (Anti-N IgG) and virion (Anti-Virion IgG) and specific positive CTL responses (IFN-γ ELISpot) against the S, N and virion antigens (displayed on abscissa) induced by the inactivated vaccine in individuals assigned to the immunization procedure with an interval of 14 days after booster immunization. The corresponding seroconversion rate is also shown in the figure. (b) Neutralizing antibodies (NAb) and ELISA antibodies (IgGs) against the S protein (Anti-S IgG), the N protein (Anti-N IgG) and virion (Anti-Virion IgG) and specific positive CTL responses (IFN-γ ELISpot) against the S, N and virion antigens (displayed on abscissa) induced by the inactivated vaccine in individuals assigned to the immunization procedure with an interval of 28 days after booster immunization. The corresponding seroconversion rate is also shown in the figure. (c) Neutralizing antibodies induced by the inactivated vaccine could identify pandemic strains from all over the world, including the strain carrying the D614G mutation from America and strains from Hong Kong, Indonesia, Canada, and the United Kingdom with the same S protein sequence as that of the strain used to produce the vaccine. Control (Con, 0 EU); low dose (LD, 50 EU); medium dose (MD, 100 EU); and high dose (HD, 150 EU). The antibody positive judgment threshold is marked with a dotted line in the figure. *, 0.01 < p < 0.05; **, 0.001 < p < 0.01; ***, p < 0.001. The significant differences compared to the control group (Con) are shown in black, those compared to the low-dose group in purple, and those compared to the middle-dose group in red.
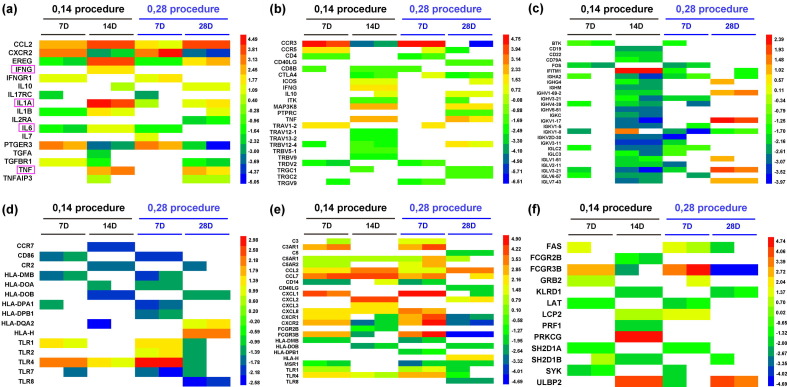
To characterize the immunogenicity of the vaccine, we performed a comparative analysis of the mRNA profile of PBMCs from immunized individuals of the medium-dose group on the two schedules at day 7 and 28 after booster immunization to determine the genetic diversity of the immune response elicited by the vaccine. The results suggest that the vaccine initiates and promotes a series of transcriptional activities in immune cells, which lead to the significant upregulation of many genes related to the immune response. The classification of all the different upregulated genes into various immune functions suggests that immunization with the medium dose of the vaccine using the two schedules results in the specific activation of the innate and adaptive immune responses compared to the placebo control. However, the expression of certain significant cytokine genes, including IL-6, IL-1, IL-2, TNF-α and IFN-γ, which were found to be distinctly increased in the peripheral blood of COVID-19 patients, varied only slightly compared to those in the placebo controls (Fig. 5 a). Furthermore, our data indicate that the genes related to T and B cell activation were upregulated by approximately 40% and 25%, respectively, at day 7 after the booster inoculation and that their expression varied dynamically during the periods from day 7 to day 14 or from day 7 to day 28 for the two schedules (Fig. 5b, c). The genes related to the activation of DCs, mononuclear cells/macrophages and NK cells were upregulated to varying degrees in the same periods (Fig. 5d, e, f).
Fig. 5.
Genetic diversity of genes related to the immune response induced by the inactivated SARS-Cov-2 vaccine. (a) The fold changes in some of the differentially expressed genes involved in cytokine production. Some important genes reported relating to COVID-19 are marked with a pink rectangle. (b) The fold changes in some of the differentially expressed genes involved in T cell activation. (c) The fold changes in some of the differentially expressed genes involved in B cell activation. (d) The fold changes in some of the differentially expressed genes involved in DC cell activation. (e) The fold changes in some of the differentially expressed genes involved in mononuclear cell/macrophage activation. (f) The fold changes in some of the differentially expressed genes involved in NK cell activation. Each row represents one gene, and the samples are depicted in the columns. Red indicates genes that were expressed at higher levels, and blue denotes genes that were expressed at lower levels compared with the control group at the same time point. The color bars represent the log2 fold change.
4. Discussion
Given the urgent need to control the COVID-19 pandemic, vaccine development is being accelerated into the clinical trials phase [4], [7], [8], even though understanding of the immunologic features of the antigens of SARS-CoV-2 remains poor. In this phase I trial, a study was performed to investigate the safety and immunogenicity of this inactivated vaccine in 191 subjects. The data collected show several notable features. First, the clinical safety observations among the 191 subjects suggest that there were no severe adverse reactions related to vaccination, and the most frequently reported events were mild, including redness, itching and swelling at the inoculation site and a few cases of slight fatigue; there were no significant differences between the vaccine and control groups. These data support the clinical safety of this vaccine. However, based on the current concern about the possibility of immunopathology due to SARS-Cov-2 infection [16], we extended our safety observations to the investigation of variations in immune cell populations and cytokine levels in the peripheral blood. The test results suggested that there were no abnormalities in most of the 48 detected cytokines and the proportions of immune cells. Second, not only did serological detection show the presence of neutralizing antibodies, antibodies against the S protein, the N protein and the complete virion antigens were also found in ELISA assays to have been elicited in the vaccinated population, and there were dynamic alterations in the levels of these antibodies based on the dose and the vaccination schedule. However, the medium and high doses in both the 0, 14 and 0, 28 schedule groups led to 100% seroconversion of ELISA anti-S antibody after two inoculations, and interestingly, the medium dose group assigned to the 0, 14 schedule reached 100% seroconversion of the neutralizing antibody with the highest GMT value. However, the high-dose group exhibited lower seroconversion and GMT values. According to our understanding, this result might be due to the medium dose providing suitable signal stimulation to the immune system or the limited sample size. Therefore, further investigations in phase II and III trials are necessary. Additionally, the neutralizing antibody can neutralize different pandemic strains with diverse mutations. However, the GMT of neutralizing antibodies measured in this trial is obviously lower than the GMTs observed in the trials of mRNA vaccines developed by Moderna and Pfizer [17], [18]; this difference suggests that characteristic immune responses are elicited by mRNA vaccines and vaccines against the inactivated virus and that these vaccines should be evaluated based upon their clinical protective efficacy [19]. At the time of the antibody response, a CTL response with IFN-gamma specificity against the S, N and virion antigens was detected in immunized individuals in comparison with individuals receiving the placebo; this suggests that any one of these three antigens enables the specific activation of T cells even if the immune response does not show dose-dependent effects. These immunological indexes indicate that a systemic immune response was elicited by our vaccine in the human population. To examine the genetic diversity of the specific immunity elicited by the vaccine, we examined the mRNA gene profile of the PBMCs from vaccinated individuals and found that most of the expressed mRNA genes were related to various signaling pathways of the innate and adaptive immune systems, and the immune functions were upregulated in comparison with the placebo group. Here, activation of the multiple signaling pathways involved in the immune response resulted in variations in hundreds of genes related to activation of innate immunity at day 7 after booster immunization regardless of the immunization schedule; however, the cytokines that were found to have elevated levels in COVID-19 patients had only mild variations and were at levels similar to those in the placebo control group, which corresponded with those detected in the serum. The activation of genes related to T cells, B cells, DCs and mononuclear cells/macrophages with varying dynamics is evidence of the immune resonse elicited by the vaccine. All the data obtained in this trial support the safety and immunogenicity of this inactivated vaccine and are encouraging with regard to further studies of its efficacy in the future.
A limitation of this study is the lack of analysis of the protective efficacy of the vaccine and the lack of a comparative transcriptional analysis of PBMCs from vaccinated individuals and COVID-19 patients; the latter comparison was not possible because few blood samples have been obtained from COVID-19 patients in mainland China at this time.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
We appreciate the contributions of all investigators at West China Second Hospital of Sichuan University who worked on the trial. This work was supported by the National Key R&D Program of China (2020YFC0849700), the Program of the Chinese Academy of Medicine Science (2020HY320001) and the Major Science and Technology Special Projects of Yunnan Province (202003AC100009).
Author contributions
Conceived of and designed the experiments: QL, CL, YC, and LL. Performed the experiments: JP, QY, ZY, YZ, XL, QY, ZZ, RL, HC, TM, HZ, SF, ZX, LW, ZH, YL, SF, JW, LZ, JL, HZheng, PC, GJ, LG, MX, HY, YG, XX, LC, LY, ZC, and DD. Analyzed the data: QL, CL, YC, LL, JP, YZ and ZZ. Contributed reagents/materials/analysis tools: JR, JZ, CH, HZhao, YL, KM, DLiu, SL, XW and SY. Wrote the paper: QL, YC, YZ and ZZ. The manuscript was drafted by QL.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2021.04.006.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Johns_Hopkins_University. COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU) 2020. (Accessed April 28, 2020, at https://www.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6.)
- 2.Tezer H., Bedir Demirdag T. Novel coronavirus disease (COVID-19) in children. Turk J Med Sci. 2020;50:592–603. doi: 10.3906/sag-2004-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palacios Cruz M., Santos E., Velazquez Cervantes M.A., Leon Juarez M. COVID-19, a worldwide public health emergency. Rev Clin Esp. 2020;221(1):55–61. doi: 10.1016/j.rceng.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xia S., Duan K., Zhang Y., Zhao D., Zhang H., Xie Z., et al. Effect of an inactivated vaccine against SARS-CoV-2 on safety and immunogenicity outcomes: interim analysis of 2 randomized clinical trials. JAMA. 2020;324(10):951–960. doi: 10.1001/jama.2020.15543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Folegatti P.M., Ewer K.J., Aley P.K., Angus B., Becker S., Belij-Rammerstorfer S., et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396:467–478. doi: 10.1016/S0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang F., Kream R.M., Stefano G.B. An evidence based perspective on mRNA-SARS-CoV-2 vaccine development. Med Sci Monit. 2020;26 doi: 10.12659/MSM.924700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu F.C., Guan X.H., Li Y.H., Huang J.Y., Jiang T., Hou L.H., et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2020;396:479–488. doi: 10.1016/S0140-6736(20)31605-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu F.C., Li Y.H., Guan X.H., Hou L.H., Wang W.J., Li J.X., et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. Lancet. 2020;395:1845–1854. doi: 10.1016/S0140-6736(20)31208-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rehman S.U., Shafique L., Ihsan A., Liu Q. Evolutionary trajectory for the emergence of novel coronavirus SARS-CoV-2. Pathogens. 2020;9(3):240. doi: 10.3390/pathogens9030240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lan J., Ge J., Yu J., Shan S., Zhou H., Fan S., et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581:215–220. doi: 10.1038/s41586-020-2180-5. [DOI] [PubMed] [Google Scholar]
- 11.Chen H, Xie Z, Long R, Fan S, Li H, He Z, et al. A valid protective immune response elicited in rhesus macaques by an inactivated vaccine is capable of defending against SARS-CoV-2 infection. bioRxiv. 2020:2020.08.04.235747
- 12.Zheng H., Li H., Guo L., Liang Y., Li J., Wang X., et al. Virulence and pathogenesis of SARS-CoV-2 infection in rhesus macaques: A nonhuman primate model of COVID-19 progression. PLoS Pathog. 2020;16 doi: 10.1371/journal.ppat.1008949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang X., Jiang Q., Xu X., Wang Y., Liu L., Lian Y., et al. Immune mechanisms induced by an HSV-1 mutant strain: Discrepancy analysis of the immune system gene profile in comparison with a wild-type strain. Vaccine. 2018;36:2394–2402. doi: 10.1016/j.vaccine.2018.03.056. [DOI] [PubMed] [Google Scholar]
- 14.Alm E., Lesko B., Lindegren G., Ahlm C., Soderholm S., Falk K.I., et al. Universal single-probe RT-PCR assay for diagnosis of dengue virus infections. PLoS Negl Trop Dis. 2014;8 doi: 10.1371/journal.pntd.0003416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ogawa J, Zhu W, Tonnu N, Singer O, Hunter T, Ryan AL, et al. The D614G mutation in the SARS-CoV2 Spike protein increases infectivity in an ACE2 receptor dependent manner. bioRxiv 2020;21(7):214932.
- 16.Chen F.F., Zhong M., Liu Y., Zhang Y., Zhang K., Su D.Z., et al. The characteristics and outcomes of 681 severe cases with COVID-19 in China. J Crit Care. 2020;60:32–37. doi: 10.1016/j.jcrc.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jackson L.A., Anderson E.J., Rouphael N.G., Roberts P.C., Makhene M., Coler R.N., et al. An mRNA vaccine against SARS-CoV-2 - preliminary report. N Engl J Med. 2020;383:1920–1931. doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mulligan M.J., Lyke K.E., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature. 2020;586:589–593. doi: 10.1038/s41586-020-2639-4. [DOI] [PubMed] [Google Scholar]
- 19.N.L. Quick guide: COVID-19 vaccines in use and how they work 2021.February 2. (Accessed March 4, 2021, at https://www.foxnews.com/health/covid-19-vaccines-in-use-how-they-work-guide?keepThis=true&caption=FOX%20News.)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.