Abstract
Background:
People with HIV (PWH) have increased risk for adiposity and sarcopenia, despite effective antiretroviral therapy. Our objective was to compare the effects of prescribed exercise on body composition in older PWH and uninfected controls.
Setting:
Academic medical center
Methods:
Sedentary PWH (n=27) and uninfected controls (n=28) aged 50-75 completed 24 weeks of cardiovascular and resistance exercise. Participants completed 12 weeks of moderate-intensity exercise then were randomized to moderate- or high-intensity exercise for 12 additional weeks. Total lean (LEAN) and fat mass (FAT) and visceral adipose tissue area (VAT) were measured using DXA at baseline and 24 weeks; baseline and intervention differences were compared by HIV serostatus using multivariable regression analyses adjusted for baseline values, age, and exercise adherence.
Results:
At baseline, PWH had significantly lower FAT (P=0.003), but no significant differences in LEAN or VAT compared to controls (P>0.20). Changes over 24 weeks were not significantly different by HIV serostatus, though controls tended to gain more LEAN (0.8 kg [0, 1.6; P=0.04] than PWH (0.6 kg [−0.2, 1.4; P=0.12]) and lose less FAT and VAT (controls (−0.9 [−1.8, 0.0] kg; −10.3 [−19.6, 1.0] cm2; both P = 0.03 vs PWH −2.0 [−2.9, −1.1] kg; −17.7 [−27.1, −8.2] cm2; both P< 0.001). Exercise intensity differences were not apparent for LEAN, FAT, or VAT.
Conclusion:
Exercise reduced total and visceral fat in older PWH and controls. Minimal gains in lean mass suggest that greater emphasis on resistance exercise may be needed to effectively increase muscle in PWH.
Keywords: resistance exercise, endurance exercise, aging, HIV, sarcopenia, adiposity
Introduction
Antiretroviral therapy (ART) has decreased mortality for people with HIV (PWH) and, with newer ART agents, AIDS-associated complications including muscle wasting or lipoatrophy are less frequent compared to the early years of ART. With age, many people with HIV experience age-related changes in body composition, including sarcopenia (loss of muscle mass and function) and increased central adiposity. Studies by our group and others have found that age-related sarcopenia and adiposity are associated with impairments in physical function among older adults with HIV1–3. Furthermore, emerging data suggest that ART regimens that include integrase strand transfer inhibitors (INSTIs)4–6 may be associated with greater weight gain than other ART classes. Thus interventions to simultaneously reduce sarcopenia and obesity are needed to improve the health of adults aging with HIV.
The U.S. Department of Health and Human Services (DHHS)7 recommends regular aerobic and resistance exercise to increase muscle mass and function and reduce body fat in healthy older adults and adults with chronic health conditions; evidence for these adaptations in older PWH has been equivocal8,9. Even with effectively suppressed HIV-1, exposure to earlier generation ART 10,11 that were toxic to mitochondria or newer ART12–14 associated with weight gain, chronic inflammation, comorbidities, and viral reservoirs14–16 could interfere with the potential beneficial effects of exercise in PWH.
We have previously reported that older PWH had similar improvements in physical function as controls without HIV following a 24-week, moderate- or high-intensity exercise training intervention17. Here, in this secondary analysis, we sought to compare the adaptations in body composition in response to exercise in older PWH and controls, and to identify what, if any, adaptations were unique to older PWH. Further, we evaluated whether the changes in body composition were related to circulating testosterone and insulin-like growth factor 1 (IGF-1) because of their anabolic effects on skeletal muscle and changes with aging.
Methods
The Exercise for Healthy Aging Study enrolled PWH and HIV-uninfected controls from April 2014 to May 2017 (NCT02404792). The inclusion and exclusion criteria and study design have been published previously17. Briefly, participants were aged 50 to 75 years, sedentary (< 60 minutes of physical activity per week for preceding 6 months by self-report), had a body mass index (BMI) between 20 and 40 kg/m2, and no contraindications to exercise. PWH were on stable ART with undetectable HIV-1 RNA for >2 years and CD4+ T-cell count > 200 cells/µL. The study procedures were approved by the Colorado Multiple Institution Review Board. Written informed consent was obtained from all participants.
Study Procedures
Prior to training and at week 12, peak cardiorespiratory capacity (VO2 peak) was measured using a graded treadmill test. Maximum muscle strength was measured using the one-repetition maximum (1-RM; maximum weight that can be lifted only one time) at baseline and every 3 weeks. Participants attended supervised exercise sessions three times/week for 24 weeks at the University of Colorado-Anschutz Medical Campus Exercise Research Laboratory. Each session included treadmill walking and four weight-assisted machine (Cybex) exercises (bench press, leg press, lateral pulldown, and a rotating 4th exercise). Exercise time gradually increased to a target of 50 minutes/session. The first 12 weeks targeted moderate intensity (40–50% VO2 peak) treadmill walking and 3 sets of 8 repetitions of moderate intensity resistance exercises (60–70% 1-RM) each session. At week 12, participants were randomized to continue moderate-intensity training or advance to high-intensity training (60–70% of week 12 VO2 peak and > 80% 1-RM). Participants were encouraged to consume a healthy diet but no specific or personalized dietary guidance was given.
Body Composition Measures
Before and at week 24 of exercise training, total body bone-free lean mass (LEAN), fat mass (FAT), and visceral adipose tissue (VAT) area were measured by dual-energy x-ray absorptiometry (DXA) using either the Hologic Discovery W (Apex 4.0.1; n=42) or Horizon W (Apex 5.6.05; n=13) instrument (Hologic, Inc., Bedford MA). Each participant had pre- and post-training measures on the same instrument. In our laboratory, the coefficient of variation (95% CI) for LEAN and FAT are 0.7% (0.5, 0.8%) and 1.7% (1.3, 2.1%), respectively. Appendicular lean mass (ALM) was calculated as the sum of the limb lean mass (kg), then normalized to height in meters-squared (ALM/m2). VAT area included the fat within the abdominal muscle walls of the abdominal cavity, between the superior border of the pelvis and the inferior border of the rib cage. (Hologic Series User Guide, Whole Body Examination, MAN-03644 Revision 006). DXA scan reports were reviewed by one investigator (CMJ) for quality assurance.
At baseline and 24 weeks, BMI was calculated using body mass from DXA divided by baseline height squared (kg/m2), with height measured using a wall-mounted stadiometer. Participants were classified as sarcopenic using the criteria of Baumgartner et al: ALM/m2 < 7.26 for men and < 5.45 for women 18. Lipodystrophy among the PWH was determined at baseline using the criteria of Bonnet et al (ratio of percent fat of the trunk to percent fat of the lower limbs ≥ 1.961 for males; ≥ 1.329 for females)19.
Anabolic hormones
Fasted blood samples were collected at baseline and 24 weeks for measurement of total testosterone (T; males only) and IGF-1. Serum sex hormone binding globulin (SHBG) and albumin were measured and used to calculate free T20. Serum was separated and stored at −80°C for batch analyses. All samples from the same participant were analyzed in the same batch. T was measured using liquid chromatography-tandem mass spectrometry. The intra- and inter-assay co-efficients of variation (CVs) were < 2% and < 8%, respectively. SHBG and IGF-1 were measured by chemiluminescence (SHBG: Beckman Coulter; intra- and inter-assay CVs 3.6% and 5.7%, respectively; IGF-1: Immunodiagnostics Systems; intra- and inter-assay CVs 2.0% and 9.5%, respectively). Albumin was measured by enzymatic assay (Beckman Coulter; intra- and inter-assay CVs 2.3% and 4.9%, respectively).
Comorbidity Assessment
The Veterans Aging Cohort Risk Index (VACS Index) was used to quantify comorbidities at baseline, with higher scores indicating greater comorbidity. The VACS index reliably predicts HIV and non-HIV related mortality21.
Statistical analyses
Study data were managed using the secure, web-based software platform Research Electronic Data Capture (REDCap) hosted at the University of Colorado22,23. The primary body composition outcome was the change from baseline in LEAN. The effect of exercise intensity was considered a secondary outcome given the limited duration of the higher intensity training and the time necessary to measure changes in body composition. Additional outcomes, including differences in free T and IGF-1 levels, were considered complementary. The randomization to exercise intensity arms was balanced by HIV serostatus, gender, and age. Log transformations were performed due to right skew, as appropriate, and results reported as geometric means. Baseline measures were compared via t-tests or Fischer’s Exact Test.
The primary predictor was HIV serostatus. All models were adjusted for the respective baseline value, age, and exercise adherence, with percent exercise adherence calculated as [1- (72 sessions - # sessions attended)/72 sessions] x 100. Exercise intensity models were similarly adjusted for baseline values, age and percent adherence. Separate models tested the effect of adjusting for VACS (in place of age) and baseline free T (males only), and a sensitivity analysis compared responses by use of testosterone supplementation. Additionally, we implemented a model that adjusted for DXA instrument. Two-sided tests are reported assuming a 0.05 significance level. Outcomes were considered complementary and reported without adjustment for multiple comparisons24–26. Results are expressed as the mean and 95% confidence interval unless otherwise specified. Analyses utilized R (v. 3.4.2) and SAS (v. 9.4).
Results
Sixty-nine participants began the study and 56 completed, of whom 55 contributed body composition data before and after exercise training (27 PWH [15 high-intensity, 13 moderate-intensity], 28 HIV-uninfected controls [14 high-intensity, 14 moderate-intensity]). Of the completers, baseline characteristics are provided in Table 1. Compared to controls, more PWH had a higher VACS score (P= 0.02). The prevalence of current smoking, use of testosterone supplementation, and statin use were not significantly different in PWH and controls (P>0.2). Adherence to the exercise intervention (median, [IQR]) was 89% [82, 92%] in PWH and 87% [81, 92%] in controls.
Table 1.
Participant Characteristics (median [IQR] or %)
| People with HIV (n=27) | Uninfected Controls (n=28) | |
|---|---|---|
| Age (years) | 56 [52, 61] | 61 [54, 64] |
| Gender (% male) | 93 | 93 |
| Race (% white) | 70 | 89 |
| Non-Hispanic (%) | 85 | 86 |
| Current Smoker (%) | 15 | 11 |
| Testosterone use (% males) | 20 | 8 |
| Statin use (%) | 48 | 43 |
| VACS-1 > 20 points (%) | 59* | 25* |
| Unintentional weight lossa (n) | 6 | 1 |
| Years since HIV diagnosis | 23 [17, 28] | --- |
| CD4 count (cells/µL) | 546 [416, 772] | --- |
| ART duration (years) | 17 [9, 19] | --- |
| Current use INSTI (%) | 59 | --- |
| Current use TAF (%) | 4 | --- |
| Current or prior use of thymidine analogues (%) | 68 | --- |
Year prior to intervention, self-report.
P<0.05 for between group difference
ART, antiretroviral therapy; BMI, body mass index; INSTI, integrase strand transfer inhibitors; TAF, tenofovir alafenamide fumarate; VACS-1, Veterans Administration Comorbidity Score-1
Baseline Body Composition
At baseline (Table 2), PWH had lower BMI (P=0.018) and FAT (P=0.003) than controls whereas LEAN, ALM/m2, and VAT area were not significantly different between groups (P>0.20). Four PWH and one control met criteria for sarcopenia, and 7 of 27 PWH had lipodystrophy.
Table 2.
Baseline Body Composition by HIV Serostatus [Mean (95% CI) or %]
| People with HIV (n=27) | Uninfected Controls (n=28) | P-value | |
|---|---|---|---|
| Body Mass Index (kg/m2) | 26.9 (25.3, 28.4) |
29.6 (27.9, 31.3) |
0.018 |
| Total Body Lean Mass (kg) | 57.7 (54.2, 61.1) |
60.0 (56.4, 63.6) |
0.34 |
| ALM/m2 | 8.6 (8.1, 9.1) |
8.9 (8.5, 9.3) |
0.26 |
| Fat Mass (kg) | 20.6 (17.7, 23.6) |
27.2 (24.0, 30.4) |
0.003 |
| VAT area (cm2) | 184 (151, 217) |
211 (184, 237) |
0.20 |
| Sarcopeniaa (%) | 15 | 4 | 0.19 |
| Lipodystrophyb (%) | 26 | __ |
According to the criteria of Baumgartner et al (1998).
According to the criteria of Bonnet et al (2005).
ALM/m2, appendicular lean mass relative to height; VAT, visceral adipose tissue
Changes in Body Composition
The main effect of HIV status was not significant for any changes in BMI and body composition after adjustment for baseline values, age, and exercise adherence (Figure 1; P>0.09). The decrease in BMI was significant for PWH (−0.48 [−0.87, −0.08]kg/m2; P=0.02) but not controls (−0.15 [−0.54, 0.24]kg/m2; P=0.43). LEAN increased in controls (0.8 [0, 1.6]kg; P=0.04) and PWH (0.6 [−0.2, 1.4]kg; P=0.12) but reached significance only in controls. Increases in ALM/m2 approached significance (P=0.06), with similar changes in each group (PWH, 0.14 [−0.01, 0.30]kg/m2; controls 0.14 [−0.01, 0.29]kg/m2). After the intervention, two of the four PWH and the one control no longer met criteria for sarcopenia.
Fig 1.
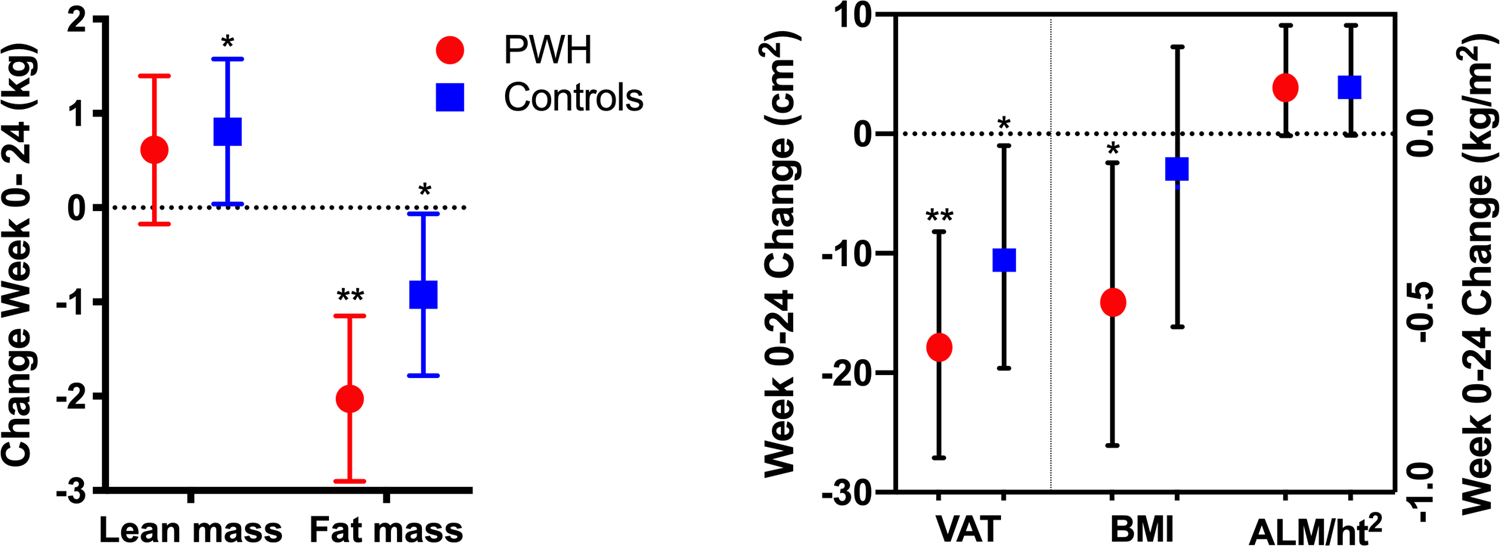
Changes in body composition in response to the intervention, adjusted for baseline values, age, and exercise adherence. * P < 0.05; ** P < 0.001 for changes within groups.
ALM, appendicular lean mass; BMI, body mass index; PWH, people with HIV; VAT, visceral adipose tissue area.
Total and visceral adiposity decreased within both groups in response to the intervention. FAT decreased 2.0 kg (−2.9, −1.1 kg; P < 0.001) in PWH and 0.9 kg (−1.8, −0.06 kg; P=0.04) in controls, but the between group difference was not significant (P=0.09). VAT decreased 18 cm2 (−27, −8.2 cm2; P< 0.001) in PWH and 10 cm2 (−20, −1.0 cm2; P= 0.03) in controls. The between group difference in the changes in VAT was not significant (P=0.28).
Exercise intensity in weeks 13–24 (high vs. moderate) was not related to any changes to body composition (P>0.12). Inclusion of the VACS Index in the any of the models did not meaningfully alter the estimates of the intervention on body composition outcomes (≤10% difference in the estimates). Greater exercise adherence was significantly associated with changes in FAT (−0.1 kg [−0.2, 0], P=0.008), but did not meaningfully change the effect estimates. Adjustment for DXA instrument did not appreciably alter the body composition results.
Anabolic hormones
At baseline, serum total T was significantly greater in male PWH than controls (P=0.006) but free T was not different between groups (P=0.35; Supplemental Table). When participants using testosterone supplementation were excluded from the analyses, total T remained significantly greater in PWH (P=0.002) and free testosterone tended to be greater in PWH than controls (P=0.063). In response to the intervention, the decreases in free T were not significant within (PWH, P=0.27; controls, P= 0.68) or between groups (P=0.62). When testosterone supplementation was excluded from the analysis, free T decreased significantly within PWH (P=0.02) and controls (P< 0.001) but these changes were not different between groups (P=0.32). Baseline free T was not significantly associated with the changes in body composition (P= 0.21 to 0.36) in males. Baseline IGF-1 did not differ between PWH and controls (P=0.472), the changes in IGF-1 from baseline to week 24 were not significant within PWH (P=0.10) or controls (P=0.73), and the changes due to training were not significantly different between PWH and controls (P=0.16).
Discussion
The goals of this study were to 1) compare the adaptations in body composition in response to exercise in older PWH and controls; 2) identify whether any adaptations were unique to older PWH; and 3) evaluate the whether the changes in body composition were related to systemic testosterone and IGF-1. The side-by-side comparisons of PWH and uninfected controls in a supervised resistance and cardiovascular endurance exercise intervention that was consistent with the 2008 U.S. DHHS recommendations7 provided a rigorous examination of body composition adaptations among older adults with and without HIV infection. Overall, we found that HIV infection did not significantly interfere with the reduced adiposity seen with training, but may blunt increases in lean mass.
First, we found similar, highly favorable reductions in total body and visceral fat in both PWH and HIV-uninfected controls that could reduce long-term cardiometabolic disease risk. The reduction in VAT area (using DXA) in PWH was comparable to that reported in a systematic review of supervised exercise trials in middle-aged adults without HIV infection (15.3 ± 40.4 cm2; using computed tomography)27. The reduced BMI in PWH following the exercise intervention was attributed to a decrease in fat mass and maintenance of lean mass.
Second, the reduction in fat mass and visceral fat that we found was consistent with three other recent studies of younger (average ages 46–49 years) PWH who completed combined aerobic and resistance exercise training. Notably, none of these studies included an HIV-uninfected group for comparison. Guariglia et al28 found that fat mass decreased approximately 1.5 kg (9%) and android fat mass (measured by DXA) decreased 0.2 g (10%) in PWH (N=25) during 16 weeks of exercise training. Cutrono et al8 found that waist circumference decreased approximately 0.5 inch in PWH after 12 weeks of combined moderate-intensity exercise. In a study of PWH with lipodystrophy (N=26), Bonato et al 9 found that DXA-measured fat mass decreased approximately 0.1 kg, lean mass increased 1.8 kg (both P<0.05), and waist circumference decreased by 1.0 cm (P=0.05) from baseline to 12 weeks of exercise. The participants in Bonato et al9 were also guided to consume ≤ 2000 kcal/day, including 15–30% of energy as protein and 20–30% as fat during the intervention, which may explain the greater increase in lean mass compared to the PWH in our study (0.6 kg). Although PWH acknowledge the importance of exercise and diet for their overall health, evidence-based interventions are needed to promote and then maintain these behavior changes over the long-term29.
Weight loss is often accompanied by reductions in muscle mass, with the concern for potential long-term functional consequences. Our findings of maintenance or modestly increased lean mass reassured that aerobic and resistance exercise among older PWH can improve cardiometabolic risk with decreased fat mass while also preserving lean mass and improving physical function, as we previously reported17. Appendicular lean mass adjusted for height, an index of sarcopenia, improved in both groups, albeit not significantly, and three of five participants who were sarcopenic at baseline became non-sarcopenic after training. The blunted increase in lean mass in PWH may be related to persistent mitochondrial dysfunction due to previous exposure to thymidine analogues or HIV-infection 30–32. Neither greater comorbidity (VACS Index) nor prevalence of smoking explained the limited increase in lean mass in PWH. The anabolic hormonal milieu, albeit limited to circulating free testosterone in males and IGF-1 in all participants, did not have detectable effects on the changes in body composition. The exercise intervention included only four resistance exercises, three of which involved large muscle groups. Hence, the stimulus for skeletal muscle hypertrophy was also modest. Exercise recommendations for older PWH may need to place greater emphasis on resistance exercise to increase muscle mass, although improvements in physical function indicate improved muscle quality.
Lastly, greater loss of fat mass in PWH and controls was not related to exercise intensity, but was significantly associated with exercise adherence. As we have previously published, however, higher intensity exercise did not deter adherence to training and resulted in greater improvements in some physical function outcomes17. The association of greater exercise adherence with improved outcomes is not surprising and could be a motivating message for PWH to start and maintain an exercise habit. The DHHS guidelines for exercise7,33 can and should be recommended for older PWH, particularly with the growing prevalence of obesity and physical function impairments in this population, however barriers to starting and maintaining a regular exercise habit in older PWH are substantial34. We have described35 several behavior change strategies that health care providers can recommend to encourage and maintain physical activity behaviors among PWH. A program of supervised exercise training that transitions to self-managed exercise may improve the uptake and maintenance of exercise in older PWH36, thereby leading to favorable changes in body composition.
The side-by-side comparisons of PWH and uninfected controls in a supervised exercise intervention provides a rigorous examination of the effects of resistance and cardiovascular endurance exercise on body composition among older adults with and without HIV, however several limitations of the study should be highlighted. The study sample was predominantly male, thus limiting generalization to women aging with HIV. The study was not adequately powered to rule out an interaction of exercise intensity and HIV status on body composition. The operational definition of sarcopenia18 included lean tissue mass but no indicator of muscle quality37,38. Lastly, the effects of dietary changes outside of the exercise intervention cannot be discerned. Participating in supervised exercise may have prompted conscious or unconscious dietary habits that could have contributed to changes in body composition. As the primary focus of this study was on changes in physical function, we did not collect detailed dietary logs throughout the intervention.
In summary, our findings provide evidence that moderate- to high-intensity cardiovascular and resistance exercise reduces abdominal and total body adiposity and maintains lean mass in middle-aged and older PWH. Larger studies with greater racial, ethnic, and gender diversity are needed to confirm the present findings, and to determine if there is a dose-response between exercise intensity and body composition changes in older PWH. The etiology of the suggested attenuated response of lean mass to exercise among PWH is unknown, but may be due to primary muscle pathology, altered myokine/cytokine responses, or mitochondrial dysfunction. A more intensive resistance exercise intervention (e.g., additional exercises, longer duration, or greater frequency) or adjuvant therapies (e.g., high protein diet) may be needed to increase lean mass in older PWH. Lastly, behavior change strategies to promote long-term adherence to exercise are imperative to sustaining improvements in physical function and body composition beyond the duration of supervised interventions.
Supplementary Material
Sources of Funding
KME has received funding to the University of Colorado from Gilead Sciences Research Scholars Program in HIV, from the National Institute on Aging of the National Institutes of Health [K23AG050260], and has consulted for ViiV and Gilead Sciences. TTB has received funding from the National Institute of Allergy and Infectious Diseases [K24 AI120834] and served as a consultant to Gilead Sciences, Merck, ViiV, Theratechnologies, and EMD-Serono. The work was supported by grants from NCATS Colorado CTSA [UL1TR002535] and the National Institute of Diabetes, Digestive, and Kidney Disorders [DK048520].
Footnotes
Parts of the data were presented at the International Conference on Frailty and Sarcopenia Research, Miami Beach, FL, Feb 22, 2019 and the International Workshop on HIV & Aging, New York, NY, Oct 10, 2019.
Conflicts of Interest
CMJ, SM, MPW, TBC, WK, and RSS have no conflicts of interest.
References
- 1.Hawkins KL, Zhang L, Ng DK, et al. Abdominal obesity, sarcopenia, and osteoporosis are associated with frailty in men living with and without HIV. AIDS. 2018;32(10):1257–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Erlandson KM, Allshouse AA, Jankowski CM, MaWhinney S, Kohrt WM, Campbell TB. Functional impairment is associated with low bone and muscle mass among persons aging with HIV infection. J Acquir Immune Defic Syndr. 2013;63(2):209–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Erlandson KM, Wu K, Koletar SL, et al. Association Between Frailty and Components of the Frailty Phenotype With Modifiable Risk Factors and Antiretroviral Therapy. J Infect Dis. 2017;215(6):933–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bourgi K, Rebeiro PF, Turner M, et al. Greater Weight Gain in Treatment Naive Persons Starting Dolutegravir-Based Antiretroviral Therapy. Clin Infect Dis. 2019. [DOI] [PMC free article] [PubMed]
- 5.Bakal DR, Coelho LE, Luz PM, et al. Obesity following ART initiation is common and influenced by both traditional and HIV−/ART-specific risk factors. J Antimicrob Chemother. 2018;73(8):2177–2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Norwood J, Turner M, Bofill C, et al. Brief Report: Weight Gain in Persons With HIV Switched From Efavirenz-Based to Integrase Strand Transfer Inhibitor-Based Regimens. J Acquir Immune Defic Syndr. 2017;76(5):527–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DHHS. 2008 Physical Activity Guidelins for Americans. In. Rockville, MD: U.S. Department of Health and Human Services; 2008. [Google Scholar]
- 8.Cutrono SE, Lewis JE, Perry A, Signorile J, Tiozzo E, Jacobs KA. The Effect of a Community-Based Exercise Program on Inflammation, Metabolic Risk, and Fitness Levels Among Persons Living with HIV/AIDS. AIDS Behav. 2016;20(5):1123–1131. [DOI] [PubMed] [Google Scholar]
- 9.Bonato M, Galli L, Passeri L, et al. A pilot study of brisk walking in sedentary combination antiretroviral treatement (cART)- treated patients: benefit on soluble and cell inflammatory markers. BMC Infect Dis. 2017;17(1):61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carr A, Miller J, Law M, Cooper DA. A syndrome of lipoatrophy, lactic acidaemia and liver dysfunction associated with HIV nucleoside analogue therapy: contribution to protease inhibitor-related lipodystrophy syndrome. AIDS. 2000;14(3):F25–32. [DOI] [PubMed] [Google Scholar]
- 11.Mallal SA, John M, Moore CB, James IR, McKinnon EJ. Contribution of nucleoside analogue reverse transcriptase inhibitors to subcutaneous fat wasting in patients with HIV infection. AIDS. 2000;14(10):1309–1316. [DOI] [PubMed] [Google Scholar]
- 12.McComsey GA, Moser C, Currier J, et al. Body Composition Changes After Initiation of Raltegravir or Protease Inhibitors: ACTG A5260s. Clin Infect Dis. 2016;62(7):853–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McComsey GA, Kitch D, Sax PE, et al. Peripheral and central fat changes in subjects randomized to abacavir-lamivudine or tenofovir-emtricitabine with atazanavir-ritonavir or efavirenz: ACTG Study A5224s. Clin Infect Dis. 2011;53(2):185–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guaraldi G, Stentarelli C, Zona S, et al. The natural history of HIV-associated lipodystrophy in the changing scenario of HIV infection. HIV Med. 2014;15(10):587–594. [DOI] [PubMed] [Google Scholar]
- 15.Agarwal N, Iyer D, Patel SG, et al. HIV-1 Vpr induces adipose dysfunction in vivo through reciprocal effects on PPAR/GR co-regulation. Sci Transl Med. 2013;5(213):213ra164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lagathu C, Cossarizza A, Bereziat V, Nasi M, Capeau J, Pinti M. Basic science and pathogenesis of ageing with HIV: potential mechanisms and biomarkers. AIDS. 2017;31 Suppl 2:S105–S119. [DOI] [PubMed] [Google Scholar]
- 17.Erlandson KM, MaWhinney S, Wilson M, et al. Physical function improvements with moderate or high-intensity exercise among older adults with or without HIV infection. AIDS. 2018;32(16):2317–2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baumgartner RN, Koehler KM, Gallagher D, et al. Epidemiology of sarcopenia among the elderly in New Mexico. American journal of epidemiology. 1998;147(8):755–763. [DOI] [PubMed] [Google Scholar]
- 19.Bonnet E, Delpierre C, Sommet A, et al. Total body composition by DXA of 241 HIV-negative men and 162 HIV-infected men: proposal of reference values for defining lipodystrophy. J Clin Densitom. 2005;8(3):287–292. [DOI] [PubMed] [Google Scholar]
- 20.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84(10):3666–3672. [DOI] [PubMed] [Google Scholar]
- 21.Justice AC, McGinnis KA, Skanderson M, et al. Towards a combined prognostic index for survival in HIV infection: the role of ‘non-HIV’ biomarkers. HIV Med. 2010;11(2):143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform. 2019;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1(1):43–46. [PubMed] [Google Scholar]
- 25.Saville DJ. Multiple Comparison Procedures: The Practical Solution. The American Statistician. 1990;44:174–180. [Google Scholar]
- 26.Motulsky HJ. “When it makes sense to not correct for multiple comparisons”, GraphPad Statistics Guide. In.
- 27.Rao S, Pandey A, Garg S, et al. Effect of Exercise and Pharmacological Interventions on Visceral Adiposity: A Systematic Review and Meta-analysis of Long-term Randomized Controlled Trials. Mayo Clin Proc. 2019;94(2):211–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guariglia DA, Pedro RE, Deminice R, Rosa FT, Peres SB, Franzoi De Moraes SM. Effect of combined training on body composition and metabolic variables in people living with HIV: A randomized clinical trial. Cytokine. 2018;111:505–510. [DOI] [PubMed] [Google Scholar]
- 29.Webel AR, Perazzo JD, Dawson-Rose C, et al. A multinational qualitative investigation of the perspectives and drivers of exercise and dietary behaviors in people living with HIV. Appl Nurs Res. 2017;37:13–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haugaard SB, Andersen O, Pedersen SB, et al. Depleted skeletal muscle mitochondrial DNA, hyperlactatemia, and decreased oxidative capacity in HIV-infected patients on highly active antiretroviral therapy. J Med Virol. 2005;77(1):29–38. [DOI] [PubMed] [Google Scholar]
- 31.Ortmeyer HK, Ryan AS, Hafer-Macko C, Oursler KK. Skeletal muscle cellular metabolism in older HIV-infected men. Physiol Rep. 2016;4(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin H, Stankov MV, Hegermann J, et al. Zidovudine-Mediated Autophagy Inhibition Enhances Mitochondrial Toxicity in Muscle Cells. Antimicrob Agents Chemother. 2019;63(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DHHS. Physical Activity Guidelines for Americans. In: Services USDoHaH, ed. 2 ed. Washington, D.C.: U.S. Departmat of Health and Human Services; 2018. [Google Scholar]
- 34.Neff HA, Kellar-Guenther Y, Jankowski CM, et al. Turning disability into ability: barriers and facilitators to initiating and maintaining exercise among older men living with HIV. AIDS Care. 2019;31(2):260–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Montoya JL, Jankowski CM, O’Brien KK, et al. Evidence-informed practical recommendations for increasing physical activity among persons living with HIV. AIDS. 2019;33(6):931–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Webel AR, Moore SM, Longenecker CT, et al. Randomized Controlled Trial of the SystemCHANGE Intervention on Behaviors Related to Cardiovascular Risk in HIV+ Adults. J Acquir Immune Defic Syndr. 2018;78(1):23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Correa-de-Araujo R, Harris-Love MO, Miljkovic I, Fragala MS, Anthony BW, Manini TM. The Need for Standardized Assessment of Muscle Quality in Skeletal Muscle Function Deficit and Other Aging-Related Muscle Dysfunctions: A Symposium Report. Front Physiol. 2017;8:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


