Abstract
Among COVID-19 hospitalized patients, high incidence of alterations in inflammatory and coagulation biomarkers correlates with a poor prognosis. Comorbidities such as chronic degenerative diseases are frequently associated with complications in COVID-19 patients. The aim of this study was to evaluate inflammatory and procoagulant biomarkers in COVID-19 patients from a public hospital in Mexico. Blood was sampled within the first 48 h after admission in 119 confirmed COVID-19 patients that were classified in 3 groups according to oxygen demand, evolution and the severity of the disease as follows: 1) Non severe: nasal cannula or oxygen mask; 2) Severe: high flow nasal cannula and 3) Death: mechanical ventilation eventually leading to fatal outcome. Blood samples from 20 healthy donors were included as a Control Group. Analysis of inflammatory and coagulation biomarkers including D-dimer, interleukin 6, interleukin 8, PAI-1, P-selectin and VWF was performed in plasma. Routine laboratory and clinical biomarkers were also included and compared among groups. Concentrations of D-dimer (14.5 ± 13.8 µg/ml) and PAI-1 (1223 ± 889.6 ng/ml) were significantly elevated in severe COVID-19 patients (P < 0.0001). A significant difference was found in interleukin-6, PAI-1 and P-selectin in non-severe and healthy donors when compared to Severe COVID-19 and deceased patients (P < 0.001). VWF levels were also significantly different between severe patients (153.5 ± 24.3 UI/dl) and non-severe ones (133.9 ± 20.2 UI/dl) (P < 0.0001). WBC and glucose levels were also significantly elevated in patients with Severe COVID-19. Plasma concentrations of all prothrombotic biomarkers were significantly higher in patients with a fatal outcome.
Keywords: COVID-19, immunothrombosis, D dimer, interleukin 6, interleukin 8, PAI-1, P-selectin, von Willebrand factor
Introduction
Coronavirus disease 2019 (COVID-19), caused by SARS-CoV-2 and declared pandemic by the World Health Organization in March 2020, has affected tens of millions of people.1 Complications of this disease have been correlated with an exacerbated state of inflammation, characterized by the elevation of certain cytokines such as interleukin 1 (IL-1), interleukin 6 (IL-6), interleukin 8 (IL-8), tumor necrosis factor α (TNF-α) and gamma interferon (INF-γ), in a process called “cytokine storm”.2–4 Also, an elevation in coagulation-related biomarkers levels has been reported, highlighting increased fibrinogen concentrations, D-dimer, von Willebrand factor (vWF) and prolongation in prothrombin time,5,6,7 as well as an increase in plasma concentrations of some coagulation regulators such as type 1 plasminogen activator inhibitor 1 (PAI-1) and tissue-activating plasminogen (tPA),8 in hospitalized patients with COVID-19. This close relationship between cytokines and procoagulant mediators results in a hypercoagulable state that has been associated with an enhanced risk for thrombotic events and mortality.9 The International Society of Thrombosis and Hemostasis has issued recommendations for the management of the coagulopathy present in COVID-19 based on some of these prothrombotic markers.10
It has been described that patients with chronic degenerative diseases, such as metabolic syndrome, obesity and/or diabetes mellitus (DM), when affected with COVID-19, trend to be associated with a poorer prognosis.11,12 These diseases share a chronic inflammatory state that, coupled with the severe inflammation of COVID-19, may promote an immunothrombotic status,12–14 nevertheless, the mechanism of the viral infection disrupting the coagulation system has not been described.
Mexico has shown one of the highest rates of mortality in the world, reaching 1.9 million COVID-19 cases and 165 000 deaths in February 2021. Mexico also shows one of the highest rates of obesity and diabetes, which could partially explain these high numbers. Information on the immunothrombotic status in Mexican patients is very scarce.
This article aims to describe the behavior of inflammatory and procoagulant biomarkers in hospitalised COVID-19 patients and its association with the severity of the disease in a public community hospital in Mexico.
Patients and Methods
Patients
A total of 150 patients who were admitted with a clinical diagnosis of COVID-19 in the months of July to September 2020 at the General Hospital “Dr. Miguel Silva” in Morelia, Mexico were included. From the initial cohort, 119 patients had a positive diagnosis of SARS-CoV-2 infection, which was confirmed by reverse transcription polymerase chain reaction (RT-PCR) by the State Public Health Laboratory. Samples from 20 healthy donors were included as a Control Group, samples of this group were obtained from August to December 2019 as control group for other studies and stored in our biobank, before the COVID-19 pandemic so no SARS-CoV2 infection is possible in them. All patients were older than 18 years. The study was approved by the Ethics and Research Committee of the General Hospital “Dr. Miguel Silva” with the registration number 530/01/20. Blood samples from all patients were obtained upon admission to the emergency department and at the most within the first 48 hours after their admission to the hospital; only those samples from confirmed SARS-CoV-2 infection were included in the study and were classified according to their evolution into 3 groups: Non-Severe COVID-19, Severe COVID-19 and Death Group.
Samples
Whole human blood was obtained from all patients by clean venipuncture after signing the informed consent form. Blood for plasma samples was collected in vacutainer tubes containing 3.2% sodium citrate (Becton Dickinson, Franklin Lakes, NJ, USA) and processed within 2 h after collection. Blood samples for Complete blood cell count, ABO group, glucose and other routine chemical tests were also obtained. Serum and plasma were obtained by centrifugation at 3500 rpm for 10 minutes at room temperature and preserved at -70°C until its use for biomarker assessment.
Assessment of Immunothrombotic Biomarkers
The following immunothrombotic biomarkers were analized: Platelet count, D dimer, Interleukin 6 (IL-6), P-selectin (P. Sel), type 1 plasminogen activator inhibitor (PAI-1). Plasma assessment of biomarkers was performed by flow cytometry using the LEGENDplex Kit™ Human Thrombosis Panel Standard from BioLegend® following the instructions suggested by the supplier. The samples were read on a CytoFLEX equipment, BECKMAN COULTER®. Briefly, plasma was incubated with beads that are differentiated by size and internal fluorescence intensities. Each bead set is conjugated with a specific antibody on its surface and acts as the capture bead for that particular analyte, so each analyte will bind to its specific capture bead. After washing, a biotinylated detection antibody cocktail is added, and each detection antibody in the cocktail will bind to its specific analyte bound on the capture beads, forming a capture bead-analyte-detection antibody sandwich. Streptavidin-phycoerythrin (SA-PE) is subsequently added and after 30 minutes of incubation, samples were taken to the CytoFLEX equipment for analysis.
D-Dimer
D-Dimer was evaluated by 2 independent methods: an automated coagulometric method using STA Compact Max2, Stago® using STA-Liatest D-Di PLUS as reagent and by flow cytometry with antibody beads alongside the rest of the immunothrombotic biomarkers.
Von Willebrand Factor
Von Willebrand Factor was assessed in plasma samples using the BIOMEDICA DIAGNOSTICS IMUBIND® vWF ELISA kit. This is a “sandwich” ELISA that uses a goat polyclonal antibody as capture antibody. Samples are incubated in the precoated micro-test wells and a polyclonal antibody conjugated with horseradish peroxidase (HRP) is used to detect the bound vWF antigen. The addittion of perborate/3,3´,5.5´-tetramethylbenzidine (TMB) substrate and its subsequent reaction with the HRP, creates a blue stained solution; finally, the assay is stopped by a pH change. All assays were performed following specifications and recommendations issued by the supplier. ELISA plates were read using a Thermo Scientific Multiskan FC reader® at a wavelength of 450 nm.
Statistical Analysis
Sociodemographic and categorical variables are expressed in frequencies and percentages. Continuous variables are expressed using mean, standard deviation, median, minimum and maximum values. The results obtained were expressed in standard means and deviations. Shapiro Wilk test was used for normality test. A variance analysis was used to assess differences between groups and multiple comparisons were evaluated using the Kruskal-Wallis test. A p value <0.05 was considered to be statistically significant. GraphPad 6.0 software was used for the analysis (San Diego, CA).
Ethics
The study was approved by an appropriate Institutional Review Board: the Ethics and Research Committee of the General Hospital “Dr. Miguel Silva” with the registration number 530/01/20. Written informed consent was obtained from all patient(s) or from a legally authorized representative for their anonymized information to be published in this article.
Results
One hundred and fifty hospitalized patients with clinical suspicion of COVID-19 were enrolled in the study, all of them admitted in the General Hospital “Dr Miguel Silva” in the city of Morelia Michoacan, Mexico. Main criterion for admission to hospital was hypoxemia. Diagnosis of SARS-CoV2 infection was confirmed in 119 patients by means of a positive real-time reverse transcriptase (RT-PCR) test. Confirmed positive SARS-CoV2 patients were classified into 3 groups according to the severity of the disease, a total of 37 (31.0%) patients had mild and moderate disease, these patients required oxygen support with nasal cannula or oxygen mask and were classified into the Non-Severe COVID-19 group, 64 (53.7%) patients who had severe disease, required support with high flow nasal cannula and were part of the Severe COVID-19 group, and 18 (15.1%) patients who had critically severe disease requiring assisted mechanical ventilation, were admitted in the Intensive Care COVID Unit (ICU) and finally had a fatal outcome, were part of the Death Group. The Control Group was composed by 20 volunteers who were healthy at the moment of the study, samples of this group were obtained as a control group for other studies and stored in our bank before the COVID-19 pandemic so no SARS-CoV2 infection is possible in them. The clinical and demographic characteristics of the study population are shown in Table 1.
Table 1.
Clinical and Demographic Characteristics of the Study Population.
| Variable | Healthy donors (n = 20) |
Non severe covid (n = 37) | Severe covid-19 (n = 64) | Death by covid-19 (n = 18) | P value |
|---|---|---|---|---|---|
| Age (years, mean ± SD) | 41.40 ± 14.9 | 44.52 ± 12.7 | 55.53 ± 12.3 | 63.77 ± 13.8 | 0.063 |
| Male n (%) | 8 (40%) | 26 (62.16%) | 39 (60.9%) | 9 (50) | 0.604 |
| Diabetes mellitus n (%) | N/A | 15 (40.54%) | 21 (32.8%) | 10 (55.6%) | 0.085 |
| Hypertension n (%) | N/A | 10 (27.02%) | 14 (21.9%) | 9 (5%) | 0.495 |
| Obesity n (%) | N/A | 4 (10.81%) | 9 (14.1%) | 4 (22.2%) | 0.851 |
| COPD n (%) | N/A | 0 (0.0%) | 4 (6.3%) | 1 (5.5%) | 0.092 |
| WBC (x109/L, mean ± SD) | 6.14 ± 1.1 | 8.12 ± 3.1 | 12.27 ± 5.9 | 14.99 ± 6.2 | 0.0001 |
| Hb (g/dL mean ± SD) | 14.52 ± 2.1 | 14.25 ± 1.6 | 13.79 ± 2.5 | 13.25 ± 2.0 | 0.312 |
| Lymphocyte (x109/L mean ± SD) | 5.67 ± 0.9 | 18.03 ± 8.2 | 15.48 ± 12.6 | 7.05 ± 4.96 | 0.003 |
| Platelets ( x109/L mean ± SD) | 245.3 ± 66.8 | 287.88 ± 104.8 | 294.07 ± 117.5 | 262.11 ± 117.0 | 0.590 |
| MPV (fL mean ± SD) | 8.30 ± 1.3 | 10.45 ± 1.0 | 10.65 ± 0.99 | 10.60 ± 1.1 | 0.637 |
| D-dimer (µg/ml mean ± SD) | N/A | 0.8221 ± 0.6 | 2.84 ± 3.9 | 2.61 ± 3.7 | 0.013 |
| Fibrinogen (mg/dL mean ± SD) | N/A | 630.34 ± 143.8 | 625.84 ± 215.0 | 780.54 ± 356.3 | 0.039 |
| Glucose (mg/dL mean ± SD) | 88.75 ± 6.7 | 113.75 ± 64.5 | 151.64 ± 100.2 | 219.82 ± 200.3 | 0.008 |
| Tryglicerides (mg/dL mean ± SD) | 114.5 ± 48.6 | 171.15 ± 55.4 | 202.43 ± 97.9 | 261.42 ± 197.6 | 0.028 |
| Cholesterol (mg/dL mean ± SD) | 173.6 ± 37.7 | 150.26 ± 40.9 | 147.26 ± 41.9 | 137.29 ± 32.9 | 0.558 |
* ANOVA test was used to evaluate the comparison between groups.
* COPD: Chronic Obstructive Pulmonary Disease.
* D-dimer results by coagulometric method.
The mean ages of the patients were not significantly different among the patients groups (non severe COVID-19, severe COVID-19, and Death Group) (p = 0.063), however the group of critical patients with fatal outcome had a mean age of 63.7 ± 13.8 and an age range of 31 to 85 years, which upper limit is higher than the rest of the groups. The presence of hypertension, diabetes mellitus, obesity and COPD were found as the most frequent comorbidities associated with COVID and they were compared according to the evolution of the disease without finding significant differences among the 3 groups. The main differences that were observed were found in the complete blood cell count; particularly the white blood cell count (WBC) was significantly higher in the Severe COVID-19 group (12.2 ± 5.9 x 109/L) and the Death Group (14.9 ± 6.1 x 109/L) when compared to the Non-Severe COVID-19 group (8.1 ± 3.0 x 109/L, p = 0.0001). If we analyze the lymphocyte count (%), it was significantly reduced in the Death Group (7.0 ± 4.9 x 109/L) compared to patients in the Severe COVID-19 group (15.4 ± 12.5 x 109/L) and patients of the Non-Severe COVID-19 group (18.0 ± 8.1 x 109/L). Glucose and triglycerides were significantly higher in the Severe COVID-19 group and in the Death Group vs non severe COVID-19 group. Demographic data and results from routine laboratory tests are shown in Table 1.
One of the most common complications in COVID-19 patients is the excessive inflammatory response that leads to a cytokine storm that triggers tissue damage, platelet activation, and thrombosis in critically ill patients. For this reason, circulating cytokine concentrations of several immune response: IL-6 and IL-8 and prothrombotic biomarkers: D-dimer, vWF, PAI-1 and P-selectin were evaluated in plasma samples from patients infected with COVID-19 and a group of healthy donors.
D-Dimer
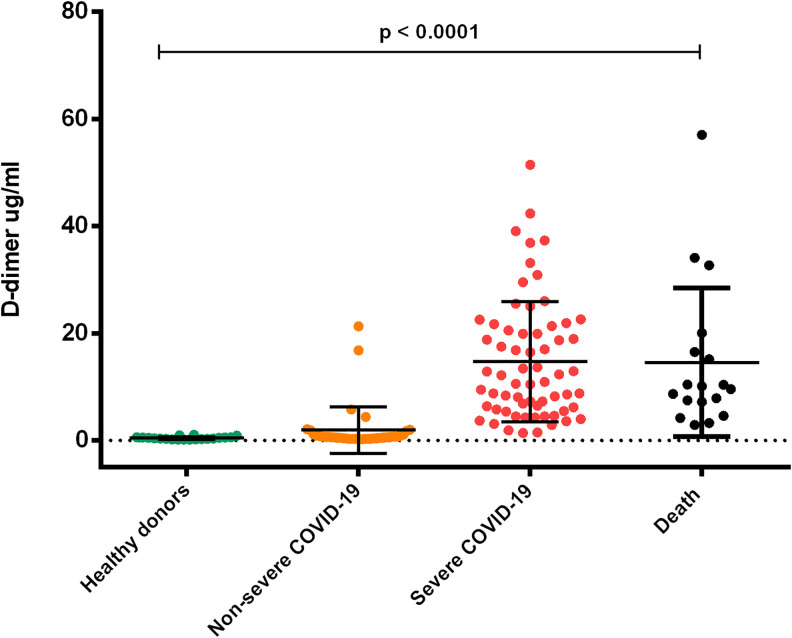
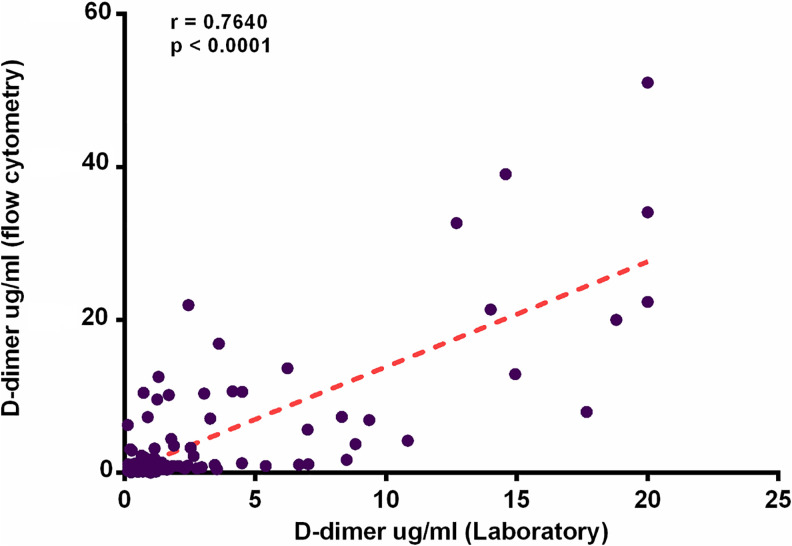
D-dimer has been linked to a notable increase in mortality in COVID-19 patients. We evaluated the plasma concentrations of D-dimer by 2 independent methods: the traditional coagulometric method and a flow cytometry method based in antibody coated beads. Evaluation of D-dimer by the coagulometric method was performed in the hospital laboratory to patients with a positive diagnosis for COVID-19. Results were (mean ± SD) 0.8221 ± 0.6 µg/ml in the NonSevere COVID-19 group; 2.84 ± 3.8 µg/ml in Severe COVID-19 group and 2.61 ± 3.66 µg/ml in the Death group, the difference among groups was significant (p = 0.013) (Table1). We also evaluated the D-dimer concentrations by flow cytometry alongside other prothrombotic biomarkers in the 3 study groups of patients and in the group of healthy donors. Very high D-dimer concentrations were observed in the Severe COVID-19 (14.7 ± 11.2 µg/ml) and Death group (14.5 ± 13.8 µg/ml) when compared with the Non-Severe COVID-19 group (1.9 ± 4.3 µg/ml) and healthy donors (0.4 ± 0.2 µg/ml) with a significant difference p = 0.0001. (Figure 1). We performed a correlation test between both methodologies obtaining an r value of 0.7 (p ≤ 0.0001). (Figure 2)
Figure 1.
Comparison of plasma concentrations of D-dimer in patients with COVID-19 according to the severity of the disease. An increase in plasma D-dimer concentrations is observed in patients infected with COVID-19. The severe COVID-19 and death groups showed concentrations above 20 ug/ml, while the group with non-severe COVID-19 showed a mean non greater than 2 ug/ml (ANOVA p < 0.0001). 131 × 110 mm (300 × 300 DPI).
Figure 2.
Correlation between plasmatic concentration of D-dimer measured by flow cytometry vs coagulometric method. D-Dimer concentrations measured by flow cytometry correlated positively with D-Dimer concentrations evaluated by a coagulometric method (r = 0.7649, p < 0.0001). 152 × 103mm (220 × 220 DPI).
Interleukins
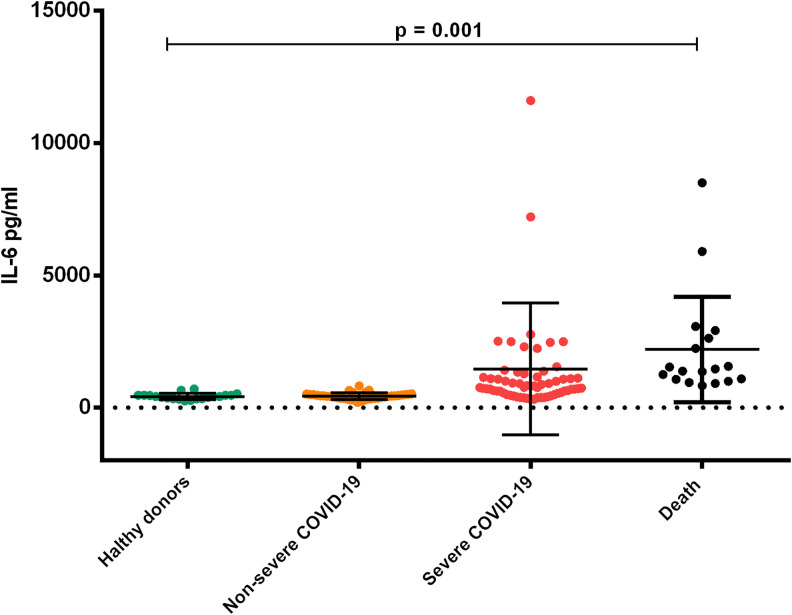
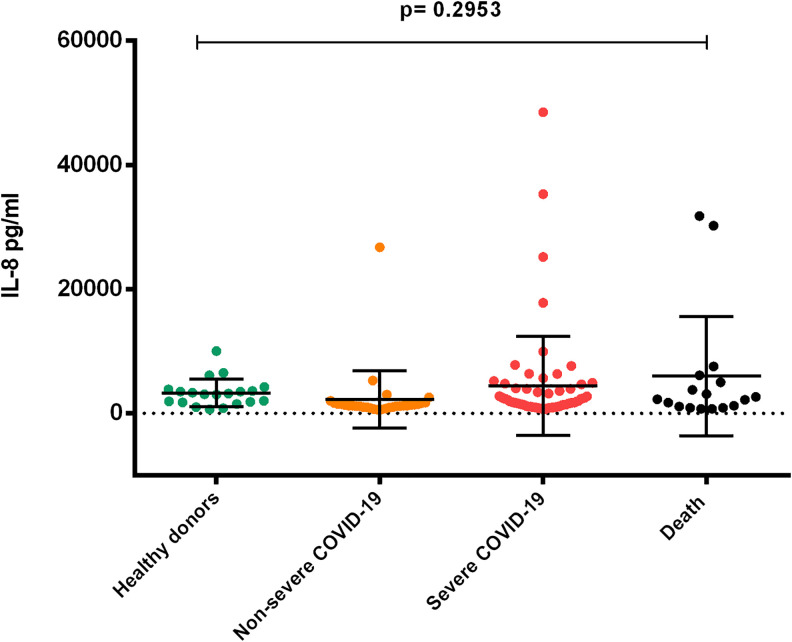
Our results showed similar IL-6 concentrations in the Non-Severe COVID-19 group (430.3 ± 122.9 pg/ml) and Control group (419.5 ± 112.8 pg/ml), however the concentrations of IL-6 in Severe COVID-19 (1463 ± 2501 pg/ml) and the Death Group (2200 ± 1994 pg/ml), were significantly higher (p = 0.001). (Figure 3). IL-8 levels were higher in the Severe group (4420 ± 7977 pg/mL) and the Death group (6000 ± 9618 pg/mL) when compared to Non-Severe COVID-19 (2270 ± 4626 pg/mL) and control group (3285 ± 2233 pg/mL), although differences were not significant (p = 0.2953). (Figure 4)
Figure 3.
Comparison of plasma concentrations of IL-6 in patients with COVID-19 according to the degree of severity. The group of healthy donors and the non-severe covid-19 group showed similar IL-6 concentrations. Plasmatic levels of IL-6 increased in the severe covid-19 group and the death group (ANOVA p = 0.001). 144 × 117 mm (300 × 300 DPI).
Figure 4.
Comparison of plasma concentrations of IL-8 in patients with COVID-19 according to the severity of the disease. Non-severe COVID-19 and healthy subjects in the control group. Concentrations of IL-8 showed lower levels when compared to complicated COVID-19 patients. No statistical difference was found among groups. (ANOVA p = 0.2953). 134 × 112 mm (300 × 300 DPI).
Prothrombotic Biomarkers von Willebrand Factor
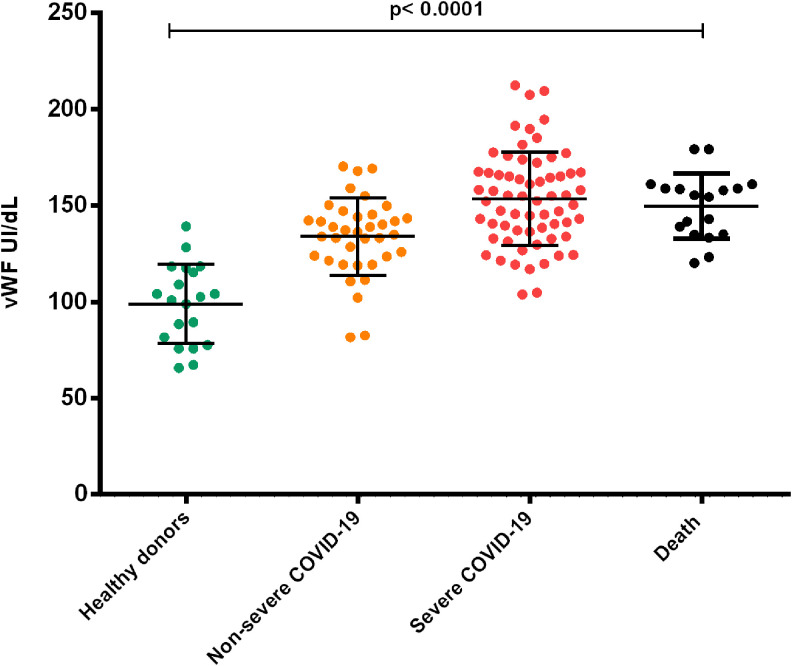
VWF plasma concentration was significantly higher in patients with Severe COVID-19 group (153.5 ± 24.3 UI/dL) and Death group (149.8 ± 17 UI/dL) when compared to patients with non-severe COVID-19 (133.9 ± 20.18 UI/dL) and with healthy donors (98.9 ± 20.7 UI/dL) p ≤ 0.0001, with concentrations increasing according to the severity of the disease (Figure 5).
Figure 5.
Comparison of plasma concentrations of vWF in patients with COVID-19 according to the severity of the disease. vWF plasma concentrations from all 3 groups of patients showed a significant difference when compared with the group of healthy donors. The figure shows how as the severity of the disease increases, vWF levels also significantly increase. The groups with severe COVID-19 and the death group showed the highest concentrations (ANOVA p < 0.0001). 130 × 114mm (300 × 300 DPI).
PAI-1
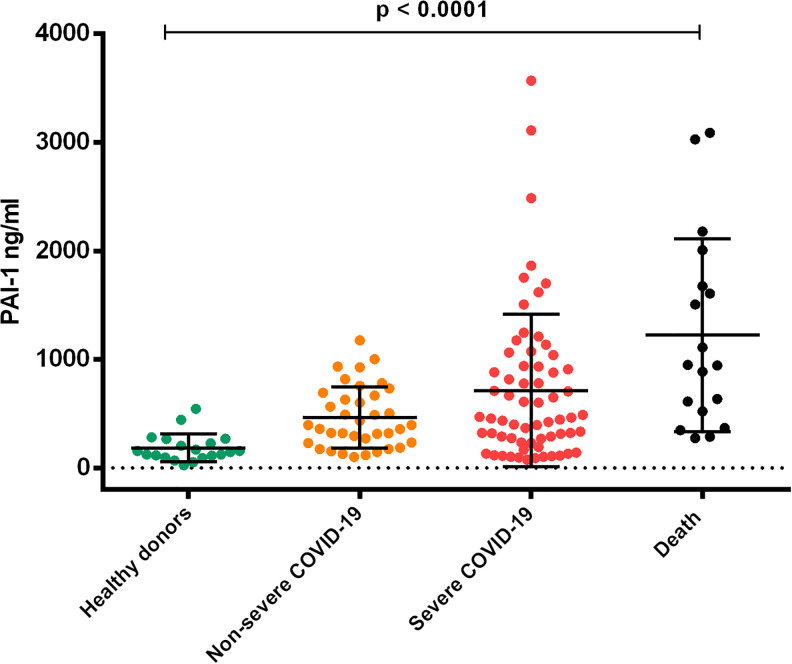
Significantly increased plasma concentrations of PAI -1 were detected in patients in the Severe COVID-19 group (713.3 ± 702.5 ng / ml) and the Death group (1223.5 ± 889.6 ng / ml) when compared to patients in the Non-Severe COVID-19 group (465.2 ± 282.1 ng / ml) and healthy donors (183.7 ± 129.1 ng / ml) p ≤ 0.0001. (Figure 6).
Figure 6.
Plasmatic concentrations of PAI-1 in patients with covid-19. Plasma concentrations of PAI-1 increased significantly in patients infected with Covid-19 as the degree of severity of the groups progressed. The results were compared with a group of healthy donors (ANOVA p < 0.0001). 128 × 111 mm (300 × 300 DPI).
P-Selectin
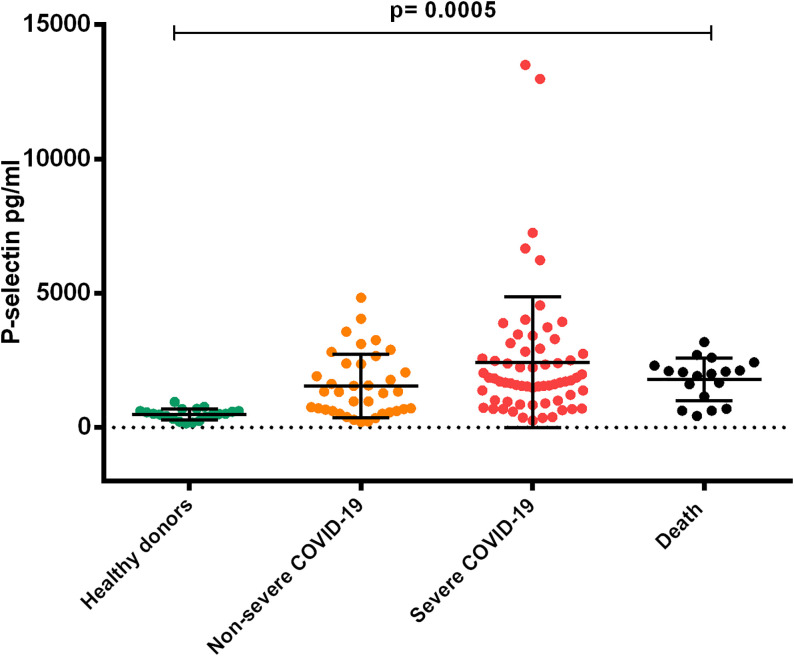
We observed higher P-Selectin concentrations in the Severe and Death COVID-19 groups (2430 ± 2434 pg/mL) (1795 ± 794.4 pg/mL) compared with patients in the Non-Severe COVID-19 group (1553 ± 1180 pg/mL) and healthy donors (496.2 ± 204 pg/mL) (p < 0.0005). It can be observed that severity of the disease increases significantly P-selectin levels (Figure 7).
Figure 7.
Comparison of plasma concentrations of P-selectin in patients with COVID-19 according to the degree of severity. Plasma concentrations of P-selectin showed significant difference in COVID-19 patients when compared to healthy donors. It is observed that severity of the disease increases significantly P-selectin levels. (ANOVA p < 0.0005) 128 × 109mm (300 × 300 DPI).
Discussion
The ongoing COVID-19 pandemic has rushed medical doctors and researchers in order to find effective disease severity predictors that can help palliate the disease and to clarify its pathophysiological mechanisms. The aim of this research was to identify inflammatory and prothrombotic biomarkers associated with the severity of the disease that could be helpful for clinicians to establish risk stratification and clinical management of patients. In this study, we provide evidence that inflammatory biomarkers such as IL-6 and IL-8 and even more, prothrombotic biomarkers including D-Dimer, vWF, PAI-1, and P-Selectin are associated with the severity of COVID-19 and could predict a higher risk of death irrespective of age, sex or chronical comorbidities. Additional studies are needed to determine whether any of the observed changes in inflammatory and/or prothrombotic biomarkers are, in fact, predictive of death.
The proinflammatory cytokine IL-6 is critical in the progression of acute inflammatory diseases such as sepsis and ARDS. Hyperinflammation in ARDS is characterized by an excessive increase in cytokines, the so called “cytokine storm”. Consistent with previous reports, our study shows concentrations of IL-6 were increased in Severe COVID-19 patients and deceased patients but not in non-severe patients. It has been proposed that IL-6 signaling plays a central role in the production of cytokines and PAI-1 during COVID-19. IL-8 is also produced in vascular endothelial cells, nevertheless we did not found a significant difference in IL-8 among our study groups. These results are consistent with those found by McElvany et al15 who also found unlike IL-6, the IL-8 response to infection was not more pronounced in severe COVID-19. In the first months of the COVID-19 pandemic, these data provided some insight regarding potential therapeutic options using IL-6 and other specific cytokine inhibitors such as tocilizumab, anakinra or infliximab, nevertheless clinical trials such as the COVACTA study16 have recently proved these inhibitors do not offer broad spectrum anti-inflammatory protection and have proved not to be useful in the treatment of COVID-19 patients. The situation with anti-inflammatory medication is still evolving and further research and clinical trials are needed until a definitive therapy and vaccines are developed.
It has already been described that several biomarkers are associated with severity and poor outcome, among them is elevated CRP, ferritin, fibrinogen and D-Dimer.17 Among these, Fibrinogen and D-Dimer are associated with a hypercoagulable state and thrombotic risk in COVID-19.6 D-dimer is a measure of the coagulation and fibrinolytic system and is often used to assess the severity of the host response in infectious diseases, playing an important role in the risk stratification of patients with sepsis to improve clinical management. Several studies showed that the higher the D-dimer levels, the greater the risk of sepsis and septic shock for patients.18 Increased concentrations of D dimer have been associated with adverse outcomes in COVID-19.19 In the present study, we evaluated concentrations of D-Dimer by 2 independent methods: the coagulometric method and flow cytometry method using antibody coated beads. Our results concur with those previously described, finding significantly higher D-dimer levels in patients from the Severe-COVID and Death groups when compared with Non-Severe patients.
A hallmark of Acute Respiratory Distress Syndrome (ARDS) is the increased alveolar-capillary permeability triggered by exudation of fluid rich in cell and plasma proteins, including albumin, fibrinogen, proinflammatory cytokines and coagulation factors.20 This leads to recruiting of inflammatory cells including neutrophils, macrophages, monocytes and platelets. During sepsis, there is an upregulation of tissue factor resulting in a downregulation of anti-thrombin leading to increase in plasma thrombin. At the same time, endothelial damage increases the release of anti-fibrinolytic mediators such as PAI-1 and further inhibition of fibrinolysis, leading to deposition of fibrin in the intra-alveolar space. Collectively, all these changes induce a hypercoagulable state that is also found in COVID-19.21 On the other hand, the vascular endothelium is emerging as a leading player in COVID-19. SARS-CoV-2 enters the human body via the angiotensin converting enzyme 2 (ACE2) receptor, which is widely expressed in several organs including the lung (alveolar epithelial cells), heart, kidney and intestine, but also in vascular endothelial cells, COVID-19 patients exhibit severe endotheliopathy in the lungs, including the presence of microthrombi.22 COVID-19 endothelitis could explain the systemic impaired microcirculatory function in different vascular beds, their clinical sequelae and the increased severity of the disease found in patients with pre-existing endothelial dysfunction, which is associated with male sex, hypertension, diabetes, obesity and previous cardiovascular disease, all of them associated with poor prognosis in COVID-19.23 In this study, we studied inflammatory and prothrombotic biomarkers that are widely recognized biomarkers of endothelial dysfunction and showed increasing concentrations associated with the severity of the disease.
Prothrombotic Biomarkers
The entry of the virus to the endothelial cell could contribute to inflammation and damage causing release of prothrombotic mediators such as vWF which is stored in the Weibel-Palade storage bodies and exposing underlying collagen to which vWF binds.24 High circulating vWF levels have been reported in patients with severe COVID-19 infection and suggest vWF might be playing a significant role in the progression and prognosis of COVID-19. In this study we found significantly higher concentrations of vWF both in the Severe COVID group that included patients that required support with high flow nasal cannula and the Death groups. Our results concur with those found by Rauch et al, who found that high plasma concentrations of vWF are independent predictors of increased oxygen requirements and poor outcomes in COVID-1925 and with those from Ladikou et al, who found median vWF levels were significantly higher in patients that died compared to the ones that remained alive24 Under high shear stress conditions, vWF plays a key role in mediating platelet adhesion and aggregation, but in the presence of high concentrations of vWF these interactions with platelets can occur even in the absence of high shear stress conditions. The high levels of vWF in COVID-19 patient enhance its spontaneous interaction with circulating platelets and damaged endothelium leading to a prothrombotic state. Accumulation of neutrophils in the lung vasculature also contributes to the damage; neutrophils release their nuclear content including chromatin, DNA and histones forming web like traps called NETs: neutrophil extracellular traps in a process known as NETosis. NETs play an important role in the fight against invading pathogens including bacteria and viruses. However, uncontrolled NETs can contribute to the pathogenesis of diseases exacerbating coagulation and inflammation. vWF and Fibrin interactions with NETs are key mechanisms in the intricate relation between the coagulation system and the innate immune system called immunothrombosis with vWF directly participating alongside NETs in providing a scaffold for procoagulant effectors that leads to the direct activation of the contact pathway of coagulation, further enhancing the hypercoagulation state.26
Endothelial damage also promotes release of PAI-1, additionally, infiltration of platelets may result in local release. Elevated levels of PAI-1 are associated with platelet activation and depression of urokinase Plasminogen Activator activity in bronchoalveolar fluid.27 Importantly, a hypofibrinolytic state and increased PAI-1 was observed in the SARS-CoV epidemic in 2002 in a study performed by Gralinski et al28 they found fibrin persistence was mediated by overexpression of PAI-1 which overcomes local uPA and tissue-type plasminogen activator (tPA). These studies point at a relevant role of PAI-1 in the etiology of COVID-19 and suggest it is a key protein contributing to abnormal deposition of fibrin in the alveolar space.29 Additionally, plasma concentrations of PAI-1 have been reported as a potential biomarker for predicting disease progression in ARDS and a positive predictor of mortality.30,31 Our results agree with these previous studies showing significantly elevated plasma concentrations of PAI-1 even in moderate/mild COVID-19 patients, with concentrations increasing according to the severity of the disease and patients of the Death Group showing the higher concentrations.
Increased levels of P selectin have been previously found in COVID-19 patients, P selectin contributes to a prothrombotic state by supporting platelet-leucocyte heteroaggregate formation and thrombi stabilization through the interaction of P-selectin on the surface of platelets and leukocyte surface P-selectin ligand (PSGL-1). Plasma P selectin may originate also from activated platelets and not only from endothelial cells. Platelet activation by SARS-CoV 2 has been proposed as being associated with thrombosis or death in patients hospitalized with COVID-19 and may contribute to adverse events.32
Prophylactic treatment of COVID-19 patients using indirect anticoagulants such as heparin, particularly low molecular weight heparin (LMWH) is important to limit the hypercoagulable state in this disease. Recent recommendations for the use of LMWH in hospitalized patients with COVID-19 have been released.33–35 Heparin treatment also reduces levels of inflammatory biomarkers and therefore may be beneficial in reducing the inflammatory state in COVID-19.
Conclusions
There is increasing evidence of the importance of immunothrombosis in COVID-19, not only for the development of thrombotic complications but for its contribution to the pathophysiology of the disease. Several prothrombotic biomarkers have been identified as poor outcome predictors in COVID-19. Further studies are needed to evaluate the prognostic value of them, specially vWF as a measure to stratify not only thrombotic risk but also severity and adverse outcomes in this pandemic disease.
Footnotes
Authorship Contributions: SELC and NGL contributed equally to this work. MEVS designed the research, MEVS., SLC, CAM, NGL., conceived and designed the experiments; SLC, CAM and LDCH obtained ethical approval, recruited patients and clinical data, NGL, GDV, performed flow cytometry experiments and data analysis; MEVS and SLC performed project management. ACM, GDV, KBA, NGL, APM, obtained samples, performed laboratory studies and built the data base, NGL performed statistical analysis, NGL, SLC, MEVS performed data analysis, NGL, ACM, SLC and MEVS wrote the original manuscript and MEVS and CAM reviewed and edited the manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by a grant from Consejo Nacional de Ciencia y Tecnologia, Convocatoria 2020-1 “Apoyo para proyectos de investigación científica, desarrollo tecnológico e innovación en salud ante la contingencia por COVID-19” project number 312999, MEVS was responsable of this project. NGL., ACM, KBA and GDV are recipients of CONACYT-Mexico fellowships for post grade students. The sponsors of this study are public or non-profit organizations that support science in general and had no role in gathering, analysing, interpreting the data or in the writing of the manuscript.
ORCID iD: Martha Eva Viveros-Sandoval  https://orcid.org/0000-0002-2801-643X
https://orcid.org/0000-0002-2801-643X
References
- 1. Rabi FA, Al Zoubi MS, Al-Nasser AD, Kasasbeh GA, Salameh DM. Sars-cov-2 and coronavirus disease 2019: what we know so far. Pathogens. 2020;9(3):231. doi:10.3390/pathogens9030231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mahmudpour M, Roozbeh J, Keshavarz M, Farrokhi S, Nabipour I. COVID-19 cytokine storm: the anger of inflammation. Cytokine. 2020;133:155151. doi:10.1016/j.cyto.2020.155151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ye Q, Wang B, Mao J. The pathogenesis and treatment of the “Cytokine Storm” in COVID-19. J Infect. 2020;80(6):607–613. doi:10.1016/j.jinf.2020.03.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Herold T, Jurinovic V, Arnreich C, et al. Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19. J Allergy Clin Immunol. 2020;146(1):128–136. doi:10.1016/j.jaci.2020.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Helms J, Tacquard C, Severac F, et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46(6):1089–1098. doi:10.1007/s00134-020-06062-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Han H, Yang L, Liu R, et al. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin Chem Lab Med. 2020;58(7):1116–1120. doi:10.1515/cclm-2020-0188 [DOI] [PubMed] [Google Scholar]
- 7. Fogarty H, Townsend L, Ni Cheallaigh C, et al. COVID19 coagulopathy in Caucasian patients. Br J Haematol. 2020;189(6):1044–1049. doi:10.1111/bjh.16749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kang S, Tanaka T, Inoue H, et al. IL-6 trans-signaling induces plasminogen activator inhibitor-1 from vascular endothelial cells in cytokine release syndrome. Proc Natl Acad Sci U S A. 2020;117(36):22351–22356. doi:10.1073/pnas.2010229117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844–847. doi:10.1111/jth.14768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Barrett CD, Moore HB, Yaffe MB, Moore EE. ISTH interim guidance on recognition and management of coagulopathy in COVID-19: a comment. J Thromb Haemost. 2020;18(8):2060–2063. doi:10.1111/jth.14860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yang J, Hu J, Zhu C. Obesity aggravates COVID-19: a systematic review and meta-analysis. J Med Virol. 2020;10.1002/jmv.26237. doi:10.1002/jmv.26237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pal R, Bhadada SK. COVID-19 and diabetes mellitus: an unholy interaction of two pandemics. Diabetes Metab Syndr. 2020;14(4):513–517. doi:10.1016/j.dsx.2020.04.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Singh AK, Gupta R, Ghosh A, Misra A. Diabetes in COVID-19: prevalence, pathophysiology, prognosis and practical considerations. Diabetes Metab Syndr. 2020;14(4):303–310. doi:10.1016/j.dsx.2020.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kumar A, Arora A, Sharma P, et al. Is diabetes mellitus associated with mortality and severity of COVID-19? a meta-analysis. Diabetes Metab Syndr. 2020;14(4):535–545. doi:10.1016/j.dsx.2020.04.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McElvaney OJ, McEvoy NL, McElvaney OF, et al. Characterization of the inflammatory response to severe COVID-19 Illness. Am J Respir Crit Care Med. 2020;202(6):812–821. doi:10.1164/rccm.202005-1583OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Furlow B. COVACTA trial raises questions about tocilizumab’s benefit in COVID-19. Lancet Rheumatol. 2020;2(10):e592. doi:10.1016/s2665-9913(20)30313 -1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yin S, Huang M, Li D, Tang N. Difference of coagulation features between severe pneumonia induced by SARS-CoV2 and non-SARS-CoV2. J Thromb Thrombolysis. 2020;1–4. doi:10.1007/s11239-020-02105-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rodelo JR, De La Rosa G, Valencia ML, et al. D-dimer is a significant prognostic factor in patients with suspected infection and sepsis. Am J Emerg Med. 2012;30(9):1991–1999. doi:10.1016/j.ajem.2012.04.033 [DOI] [PubMed] [Google Scholar]
- 19. Iba T, Levy JH, Levi M, Connors JM, Thachil J. Coagulopathy of coronavirus disease 2019. Crit Care Med. 2020;48(9):1358–1364. doi:10.1097/CCM.0000000000004458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Meduri GU, Annane D, Chrousos GP, Marik PE, Sinclair SE. Activation and regulation of systemic inflammation in ARDS: rationale for prolonged glucocorticoid therapy. Chest. 2009;136(6):1631–1643. doi:10.1378/chest.08-2408 [DOI] [PubMed] [Google Scholar]
- 21. Buja LM, Wolf D, Zhao B, et al. The emerging spectrum of cardiopulmonary pathology of the coronavirus disease 2019 (COVID-19): report of 3 autopsies from Houston, Texas, and review of autopsy findings from other United States cities. Cardiovasc Pathol. 2020;48:107233. doi:10.1016/j.carpath.2020.107233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383(2):120–128. doi:10.1056/nejmoa2015432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. doi:10.1016/S0140-6736(20)30937-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ladikou EE, Sivaloganathan H, Milne KM, et al. Von Willebrand factor (VWF): marker of endothelial damage and thrombotic risk in COVID-19? Clin Med J R Coll Physicians London. 2020;20(5):e178–e182. doi:10.7861/CLINMED.2020-0346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rauch A, Labreuche J, Lassalle F, et al. Coagulation biomarkers are independent predictors of increased oxygen requirements in COVID-19. J Thromb Haemost. 2020;18(11):2942–2953. doi:10.1111/jth.15067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jayarangaiah A, Kariyanna PT, Chen X, Jayarangaiah A, Kumar A. COVID-19-associated coagulopathy: an exacerbated immunothrombosis response. Clin Appl Thromb. 2020;26:1076029620943293. doi:10.1177/1076029620943293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bertozzi P, Astedt B, Zenzius L, et al. Depressed bronchoalveolar urokinase activity in patients with adult respiratory distress syndrome. N Engl J Med. 1990;322(13):890–897. doi:10.1056/nejm199003293221304 [DOI] [PubMed] [Google Scholar]
- 28. Gralinski LE, Bankhead A, Jeng S, et al. Mechanisms of severe acute respiratory syndrome coronavirus-induced acute lung injury. MBio. 2013;4(4):e00271–e00313. doi:10.1128/mBio.00271-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Whyte CS, Morrow GB, Mitchell JL, Chowdary P, Mutch NJ. Fibrinolytic abnormalities in acute respiratory distress syndrome (ARDS) and versatility of thrombolytic drugs to treat COVID-19. J Thromb Haemost. 2020;18(7):1548–1555. doi:10.1111/jth.14872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sapru A, Curley MAQ, Brady S, Matthay MA, Flori H. Elevated PAI-1 is associated with poor clinical outcomes in pediatric patients with acute lung injury. Intensive Care Med. 2010;36(1):157–163. doi:10.1007/s00134-009-1690-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Prabhakaran P, Ware LB, White KE, Cross MT, Matthay MA, Olman MA. Elevated levels of plasminogen activator inhibitor-1 in pulmonary edema fluid are associated with mortality in acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2003;285(1):L20–L28. doi:10.1152/ajplung.00312.2002 [DOI] [PubMed] [Google Scholar]
- 32. Barrett TJ, Lee AH, Xia Y, et al. Platelet and vascular biomarkers associate with thrombosis and death in coronavirus disease. Circ Res. 2020;127(7):945–947. doi:10.1161/CIRCRESAHA.120.317803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Thachil J, Tang N, Gando S, et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020;18(5):1023–1026. doi:10.1111/jth.14810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(5):1094–1099. doi:10.1111/jth.14817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kollias A, Kyriakoulis KG, Dimakakos E, Poulakou G, Stergiou GS, Syrigos K. Thromboembolic risk and anticoagulant therapy in COVID-19 patients: emerging evidence and call for action. Br J Haematol. 2020;189(5):846–847. doi:10.1111/bjh.16727 [DOI] [PMC free article] [PubMed] [Google Scholar]