Abstract
Maternal obesity is associated with the development of a variety of neuropsychiatric disorders; however, the mechanisms behind this association are not fully understood. Comparison between maternal immune activation and maternal obesity reveals similarities in associated impairments and maternal cytokine profile. Here, we present a summary of recent evidence describing how inflammatory processes contribute towards the development of neuropsychiatric disorders in the offspring of obese mothers. This includes discussion on how maternal cytokine levels, fatty acids and placental inflammation may interact with foetal neurodevelopment through changes to microglial behaviour and epigenetic modification. We also propose an exosome-mediated mechanism for the disruption of brain development under maternal obesity and discuss potential intervention strategies.
Keywords: Maternal obesity, neuropsychiatric disorders, neurodevelopment, inflammation, fatty acids, microglia, placenta
Introduction
During the extremely dynamic and plastic foetal period, neural cells proliferate, migrate and generate the blueprint for functional neuronal circuits. Importantly, recent advances in neurosciences and psychiatry point to an early origin of neuropsychiatric disorders. This early life period appears to be highly sensitive to both genetic and environmental stress. Early life stressors are thought to impair these processes to ‘prime’ the brain for later psychiatric disorders, extending the Developmental Origin of Health and Disease (DOHaD) concept to brain disorders. Accordingly, large-scale population studies demonstrate that maternal obesity increases the risk for neuropsychiatric disorders in offspring (Rivera et al., 2015). These increased risks apply to neurodevelopmental disorders (ASD: autism spectrum disorders, DD: developmental delay, ID: intellectual disability and ADHD: attention deficit hyperactivity disorder) (Heikura et al., 2008; Krakowiak et al., 2012; Rodriguez et al., 2008) but also to schizophrenia and depression (Edlow, 2017). These epidemiological findings are supported by studies in animal models of maternal obesity, in which behavioural deficits in offspring recapitulate the main features of these disorders. Given the current obesity pandemic – 650 million adults obese in 2016 and 340 million children between 5 and 19 overweight or obese (World Health Organization (WHO)) – and continuously rising trends, understanding the mechanisms underlying the transgenerational effect of maternal obesity on the developing brain is a major challenge.
Neuroinflammation, including in early life, recently gained much interest in neuropsychiatry, providing new pathophysiological mechanisms and hope for new treatments (Brown and Derkits, 2010; Brown and Meyer, 2018; Gumusoglu and Stevens, 2019). Because obesity is associated with chronic low-grade inflammation, exploring a neuroinflammatory hypothesis for the increased risks of mental diseases in the offspring of maternal obesity is appealing. In this review, we will present evidence for inflammation during pregnancy while obese and compare findings in animal models of maternal obesity and maternal immune activation (MIA). We will then discuss how this might deregulate the foetal immune system and ‘prime’ the developing brain with a particular focus on microglia, the resident brain macrophages. The role of microbiota, a possible link between inflammation and some neurobehavioral outcomes, will not be discussed here as this highly debated topic would deserve a full article. Interested readers are referred to recent comprehensive reviews (Codagnone et al., 2019; Vuong and Hsiao, 2017).
Behavioural outcomes in animal models of maternal obesity and maternal infection
Infection during pregnancy is associated with increased risk for neurodevelopmental and neuropsychiatric disorders including ASD, schizophrenia, childhood depression and bipolar disorders (Brown and Meyer, 2018; Estes and McAllister, 2016). While associations between maternal obesity and mental disorders overlap with those of infection (e.g. ASD or schizophrenia) (Edlow, 2017), respective risks likely differ, although no studies directly compared the two maternal conditions. These epidemiological findings are supported by a number of preclinical studies using animal models of maternal high-fat diet (MHFD) and MIA. We will focus here on recent findings in rodent models, although behavioural alterations have been identified in several other animal models including non-human primates (Thompson et al., 2017, 2018). A common challenge to MHFD and MIA models resides in differences in methodologies and designs including the use of bacterial (LPS, lipopolysaccharide) or viral (Poly: IC, Polyinosinic: polycytidylic acid) mimics for MIA, high-fat or high-fat/high-sugar diets, concentrations and timing, as well as behavioural testing setups. In addition, extrapolating performance of rodents in behavioural testing to psychiatric phenotypes, in particular mood and social behaviours, is limited as it does not faithfully replicate the complexity of human disorders. Disregarding these limitations both in terms of modelling the initial insult (i.e. maternal obesity and maternal infection) and behavioural outcomes, preclinical studies have been instrumental in increasing our understanding of the pathophysiological mechanisms underlying associations between maternal inflammation and neuropsychiatric disorders in the offspring.
Despite some conflicting results, a common framework of behavioural alterations exists in each model. MIA is commonly used to model environment-induced impairments observed in schizophrenia and ASD. One of the most consistent outcomes in offspring of MIA is social deficits. Both Poly: IC and LPS administration during pregnancy lead to decreased communication and social interactions in mouse and rat models (Gumusoglu and Stevens, 2019). Similar findings were observed in mouse MHFD offspring (Buffington et al., 2016; Kang et al., 2014). Rodent MIA models also exhibit core behavioural phenotypes related to schizophrenia, including sensorimotor gating deficits, attention impairments, increased anxiety and cognitive impairments (Brown and Meyer, 2018; Gumusoglu and Stevens, 2019). Within this set of behavioural deficits only anxiety and cognition has been extensively studied in MHFD models, potentially because of the lower association between schizophrenia and maternal obesity in epidemiological studies (Jones et al., 1998). Several studies demonstrated increased anxiety-like behaviours in adults and juvenile MHFD offspring (Abuaish et al., 2018; Manti et al., 2018; Yan et al., 2017). The few conflicting results showing no effect or decreased anxiety might come from reduced exposure period and/or milder enrichment in dietary fat (40% vs 60% of kcal) (Balsevich et al., 2016; Hiramatsu et al., 2017). Cognitive defects are also commonly observed in MHFD offspring with impaired spatial learning and reduced learning rates (McKee et al., 2017; Park and Kim, 2017; Sanguinetti et al., 2019). In addition, these animals show increased motor activity and heightened hedonic behaviour (Balsevich et al., 2016; Fernandes et al., 2012; Hiramatsu et al., 2017; Peleg-Raibstein et al., 2016). Together, these studies suggest that MIA and MHFD impair common traits in offspring such as social, anxiety and cognition-related deficits, yet they might retain some specificities, for example, regarding complex behavioural impairments observed in schizophrenia. While consistent with epidemiological studies showing that both maternal obesity and maternal infection associate with higher risk for several neuropsychiatric disorders, standardised systematic studies are required to clearly establish similarities and differences between the two models.
Immunomodulation in pregnancy and obesity
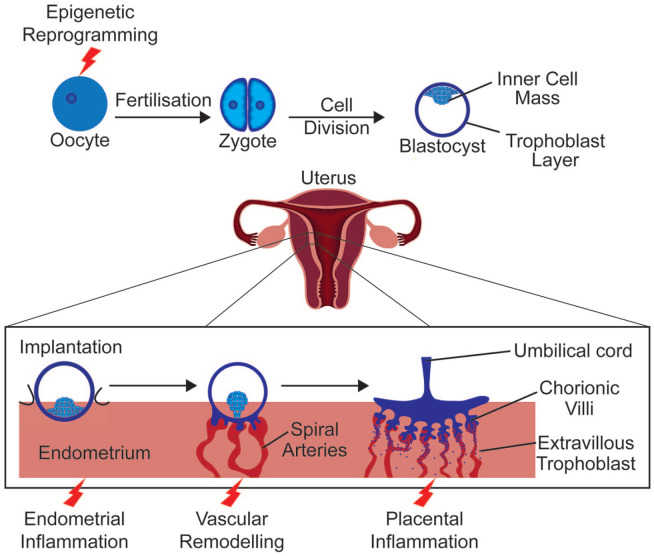
During pregnancy, the immune system must ensure protection from external pathogens as well as prevent rejection of the semi-allogenic foetus. Research in the field has provided conflicting results in part owing to the use of animal models with species-specific differences in immunology and placental anatomy (Ander et al., 2019). Even comparison between human studies has limitations due to variation in study design, such as the period studied, cytokines measured or biological variation between ethnic groups (Gillespie et al., 2016; Graham et al., 2017). Nevertheless, consensus has moved from a predominantly immune-suppressant model to a more complex explanation. During implantation, when the blastocyst adheres to the uterine epithelium and begins to invade the endometrium, an inflammatory reaction, associated with an upregulation of interleukin-6 (IL-6) and tumour necrosis factor (TNF) among other cytokines (Griffith et al., 2017), allows the repair of the uterine epithelium following blastocyst invasion (Figure 1) (Yockey and Iwasaki, 2018). Post implantation, a preponderance of anti-inflammatory cytokines promotes tolerance of foetal antigens (Graham et al., 2017). Then, as parturition begins, an upregulation of pro-inflammatory signalling including IL-6 and TNF assists with the progression of labour (Rinaldi et al., 2017).
Figure 1.
Early stages of pregnancy.
After formation of the zygote upon fusion of the oocyte with a sperm cell, the zygote undergoes several rounds of cell division to form a blastocyst – a trophoblast cell layer surrounding an inner cell mass and cavity. Inner cell mass adjacent trophoblast cells interact with the uterine endometrium. Implantation follows with the breakdown of endometrial cells and endometrial invasion. Trophoblast cells continue to divide forming cytotrophoblast and syncytiotrophoblast cells of the foetal placenta. Red lightning symbols indicate key events which may be affected by maternal obesity or maternal immune activation.
IL-6 and TNF-α are classical immune mediators involved in acute inflammation and are upregulated after infection in both humans and animal models (Glass et al., 2019; Holub et al., 2013). Other cytokines elevated after infection include C-reactive protein (CRP), predominantly after microbial infection in humans, although its role seems to be limited in mouse models (Holub et al., 2013; Torzewski et al., 2014). Evidence has also accumulated for the dysregulation of the immune system during obesity. Termed ‘meta-inflammation’ this inflammatory response is brought about by metabolic signals engaging inflammatory pathways in adipose tissues leading to increased immune cell infiltration. This results in increased pro-inflammatory cytokine production and further immune cell infiltration (Gregor and Hotamisligil, 2011; Weisberg et al., 2003). This meta-inflammation is chronic and low-grade and thus differs from the acute injury–induced or infection-induced inflammation (Gregor and Hotamisligil, 2011). Yet, these differing inflammatory conditions involve the very same cytokines. The study of obese rodent models first demonstrated elevated levels of TNF-α (Hotamisligil et al., 1993). Further studies in both animal models and humans identified other inflammatory mediators dysregulated by obesity, including IL-6, transforming growth factor β (TGF-β) and CRP (Khaodhiar et al., 2004; Samad et al., 1997).
During pregnancy, the impact of pathogen-induced immune activation on cytokine levels is largely the same as in non-gravid conditions, with increases in IL-6, TNF-α and CRP observed in both human and animal work (Fricke et al., 2018; Singh et al., 2018). The inflammatory state during maternal obesity is more controversial than during maternal infection. Animal literature is contradictory, with some papers finding increases in pro-inflammatory cytokines (Kim et al., 2014), while others find no change despite elevated foetal pro-inflammatory cytokines (Crew et al., 2016; Desai et al., 2013). Contrasting results could reflect inter-species variation or differences in diets as both high-fat and high-sugar/high-fat diets are used to induce obesity. As described for healthy pregnancy, human studies are complicated by study design limitations and biological variation. Three heavily measured cytokines, IL-6, TNF-α and CRP, report conflicting associations with body mass index (BMI) (Pendeloski et al., 2017; Sureshchandra et al., 2019). IL-6 is consistently higher in obese pregnant women than lean counterparts and concentration varies with gestation (Christian and Porter, 2014; Friis et al., 2013; Sen et al., 2014). An association between elevated CRP and maternal BMI is supported by most studies (Christian and Porter, 2014; Sen et al., 2014), yet a large longitudinal study did not corroborate this (Friis et al., 2013). Interestingly, levels of CRP closely correlated with gestational weight gain, suggesting a dose–response relationship, unlike IL-6 or TNF-α (Hrolfsdottir et al., 2016). The association of TNF-α with maternal obesity is least supported with many studies finding no correlation (Sen et al., 2014; Zembala-Szczerba et al., 2017). These findings highlight the complexity of the inflammatory state when obesity and pregnancy interact and point towards some level of immune dysregulation during maternal obesity.
Effects of obesity on placental inflammation
Obesity is a risk factor for pregnancy complications such as preeclampsia and adverse foetal outcomes like preterm birth (Papachatzi et al., 2013; Sohlberg et al., 2012; Spradley et al., 2015). The materno-foetal interface of the placenta plays a crucial role in pregnancy success by setting the intrauterine environment for the foetus. A recent genome-wide association study highlighted that genetic risk for schizophrenia is higher in patients with history of early life complications (Ursini et al., 2018). The genes conveying this early life complications–associated genetic risk converge on hypoxic stress, metabolism and immune function pathways in the placenta suggesting they might be key in ‘malprogramming’ the brain for later dysfunction.
Interestingly, placentae from obese mothers or after episodes of maternal infection show higher signs of hypoxic stress and impaired vasculature (Gohir et al., 2019; Kalk et al., 2017; Moeller et al., 2019). In MHFD mouse models, the number of foetal endothelial cells in the placenta is reduced (Kretschmer et al., 2020); placental blood vessels are dysfunctional and lack pericytes (Gohir et al., 2019). Accordingly, transcriptomic studies comparing placental tissue from lean and obese women identified modules of differentially expressed genes related to immune function and blood vessel formation, suggesting defective vascularisation and immunity are critical impairments in obesity-induced placental defects (Cox et al., 2019).
Immunomodulation is essential for the development of the placenta, which relies on complex interactions between the foetal tissue and the uterine mucosa. Recently, complex ligand–receptor pairs controlling interactions between maternal immune cells and invading foetal trophoblast cells were identified (Vento-Tormo et al., 2018). Tissue-resident (decidual) natural killer cells (dNK) interact with trophoblasts and are thought to promote their migration and recruitment through chemokine signalling. In return, invading trophoblasts express immunomodulatory molecules that bind dNK’s killer immunoglobulin-like receptors (KIR), preventing inflammatory reactions. Acute and low-grade inflammation associated with infection and obesity, respectively, likely perturbs this highly orchestrated process. Indeed, MIA leads to an acute increase in activated decidual immune cells, including dNK (Hsiao and Patterson, 2011) and maternal obesity increased the number of activated dNK in the placenta both in human and mouse studies (Baltayeva et al., 2020; Castellana et al., 2018). Intriguingly, only subtle differences in transcriptional programmes were detected between obese and lean dNKs, possibly due to a lack of cellular resolution. It would be interesting to revisit this issue in light of the most recent single-cell advances in the field.
dNK are not the only immune cells affected by maternal obesity in the placenta. A large study on placentae of 400 pregnant women indicates that obesity associates with chronic villitis (Leon-Garcia et al., 2016), an inflammation of the chorionic villi that provide essential exchanges between maternal and foetal blood. Both CD3+ T cells and CD68+ macrophages infiltrated the villi, likely secondary to the initial inflammation. Interestingly, chronic villitis is a risk factor for cerebral palsy and neonatal encephalopathy (Chen and Roberts, 2018; Redline, 2005), highlighting the link between placental inflammation and insults to the developing brain. The scenario leading to placental inflammation is unclear, but a tentative hypothesis is that initial defects in communication between dNK and trophoblasts perturb proper development of the materno-foetal interface, including blood vessels, which would trigger hypoxia and placental inflammatory events. While the placenta itself produces cytokines that might enter foetal circulation and impair brain development, it is not clear how maternal obesity affects this process.
Maternal obesity and the role of cytokines in neural development
In addition to their roles in pregnancy, cytokines are involved in brain development. IL-6 and members of TGF and TNF families regulate many developmental processes from initial central nervous system (CNS) formation to synaptogenesis (Deverman and Patterson, 2009). Studies examining associations between cytokine genetic polymorphisms and neuropsychiatric disorders suggest a link between higher levels and increased risk for these disorders. The TNF-α variant -238 G>A, for example, which may increase circulating TNF-α levels, was associated with schizophrenia in a Tunisian population (Inoubli et al., 2018) but not in Indian Bengalee patients (Debnath et al., 2013). Studies on genetic variation in IL-6 have provided similarly conflicting results. However, a recent study identified a single nucleotide polymorphism (rs2228145) influencing IL-6 receptor concentrations, as being significantly elevated in French early-onset bipolar disorder patients and trending to increase in Indian Tamil patients. This suggests a trans-ethnic association between bipolar disorder and this polymorphism (Sundaresh et al., 2019). Given the importance of these signalling molecules in development, one could hypothesise that their dysregulation through maternal obesity may contribute to increased neurodevelopmental disorder susceptibility in offspring.
IL-6 belongs to the neuropoietic cytokine family which regulates self-renewal of neuroepithelial cells (Deverman and Patterson, 2009). IL-6 also regulates axon development by reducing neurite outgrowth of cultured hypothalamic tissue. Interestingly, circulating IL-6 is increased in the newborn offspring of obese mice and reduced innervation of the hypothalamic paraventricular nucleus in these animals suggests dysregulated cytokine levels in offspring of obese mothers may impair formation of hypothalamic circuits (Sanders et al., 2014). TNF-α is another cytokine shown to be involved in neurite outgrowth. Soluble TNF-α reduced neurite outgrowth during a specific period of late embryonic and early postnatal development (Nolan et al., 2014). Reductions in axon growth were also observed in neuronal populations within the sympathetic nervous system upon TNF exposure (Erice et al., 2019). These findings raise the possibility that increased TNF-α expression resulting from maternal obesity could affect neurite growth, at least within the sympathetic nervous system.
TGF-β signalling is involved in much of early CNS development (Meyers and Kessler, 2017). Dopaminergic neurons, for example, require TGF-β signalling for differentiation, maintenance, axon growth and regulation of synaptic electrophysiology (Farkas et al., 2003; Luo et al., 2016). TGF-β was also recently shown to regulate dendritic spine morphology in midbrain neurons (Yoon et al., 2020). Interestingly, a common TGFB1 polymorphism, correlated with increased TGF-β production, was associated with increased schizophrenia susceptibility (Frydecka et al., 2013). This is corroborated by elevated TGF-β expression observed in circulating monocytes of treatment-free schizophrenia patients (Amoli et al., 2019). Decreases in hippocampal TGF-β expression were found in male offspring of MIA rats with repetitive behaviours and reduced measures of social interaction associated with ASD (Fortunato et al., 2017). Decreased TGF-β levels have also been identified in children with ASD (El Gohary et al., 2015). This suggests a fine balance of TGF-β levels is required for typical neurodevelopment, a balance that can be affected by obesity. Importantly, the brain’s resident immune cells, microglia, require TGF-β signalling for development and function (Butovsky et al., 2014; Lively et al., 2018). Modulation of microglia represents another mechanism by which inflammatory cytokines could impact brain development. Most research into the effect of cytokines on neurons has been conducted in adult models. While the impact of viral infection on foetal neurodevelopment through interferons is better understood, more research is required on how other cytokines affected by maternal obesity directly influence neurodevelopment.
The impact of maternal obesity on microglia
Microglia are heavily involved in brain development, as covered recently by many excellent reviews (Hammond et al., 2018; Li and Barres, 2018; Thion et al., 2018a). Microglia have also been implicated in the pathogenesis of ASD, schizophrenia and major depressive disorder among other conditions (Tay et al., 2017). Their presence from the beginnings of brain formation and ability to sense the environment place them at a key position to mediate the effects of maternal obesity. As resident immune cells of the CNS, microglia must react to signs of pathogen invasion. Pathogen-associated molecular patterns like LPS trigger acute systemic and CNS inflammation. While vital for the removal of pathogens, immune activation can interfere with the developmental responsibilities of microglia, as covered in Box 1.
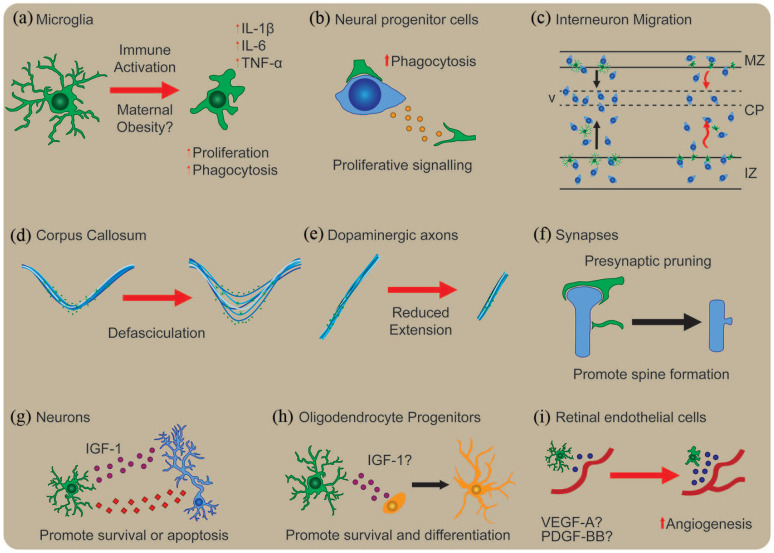
Box 1.
Microglia’s developmental roles and how they are affected by maternal immune activation.
Maternal obesity primes microglia’s response to activation and is hypothesised to interfere with microglia’s developmental functions. Some of the key functions of microglia’s role during brain development and the impact of immune activation are illustrated above. Upon interaction with pathogen-associated molecular patterns, microglia become activated and increase expression of interleukin (IL)-1β, IL-6 and tumour necrosis factor-α (TNF-α). Postnatal cerebellar microglia show increased proliferation and phagocytosis and become ameboid in morphology upon LPS stimulation (a). Microglia’s developmental function starts early in the brain where they regulate neural progenitor cell population size likely through increased phagocytosis (b). Prenatal microglia are thought to regulate cortical interneuron migration. Microglia invade the developing cortical plate at the same time as Lhx6 interneurons which accumulate in layer V. Maternal immune activation induces premature migration of Lhx6-expressing interneurons into the cortical plate and reduced interneuron accumulation at layer V (c). Microglia associate closely with a subset of axonal tracts and play a critical role in their development. In the corpus callosum, both MIA and microglial depletion result in axon tract defasciculation (d). By contrast, MIA reduced the extension of dopaminergic axons into the subpallium, an effect opposite to microglial depletion (e). During postnatal development, microglia remove pre-synaptic structures through trogocytosis and induce spine head formation upon contact (f). They also support survival of postnatal layer V neurons through insulin-like growth factor 1 (IGF-1) signalling and induce apotosis in postnatal cerebellar neurons (g). Similar trophic support for oligodendrocyte precursor survival and differentiation has been proposed within the postnatal corpus callosum (h). Finally, immune activation increased microglial vascular endothelial growth factor A (VEGF-A) and platelet-derived growth factor BB (PDGF-BB) expression and induced angiogenesis in retinal endothelial cells (i). CP: cortical plate; MZ: marginal zone; IZ: intermediate zone; V: layer V.
Maternal obesity can prime microglia towards a pro-inflammatory phenotype postnatally, as demonstrated in rats exposed to maternal obesity with increased levels of microglial activation marker CD11b within the hippocampus at birth (Bilbo and Tsang, 2010). Subsequent LPS stimulus induced exaggerated hippocampal microglia activation and increased IL-1β production. These defects were accompanied by increased anxious behaviour among male offspring. Maldonado-Ruiz et al. (2019a) demonstrated that maternal obesity-exposed adult hypothalamic microglia become increasingly activated following grehlin-mediated neuron activation. Less is known about the impact of maternal obesity on foetal microglial populations. One key study found that while maternal obesity did not induce a pro-inflammatory state, foetal microglia were ‘primed’ to produce more TNF-α upon LPS stimulation than controls (Edlow et al., 2019). Interestingly, placental macrophage populations strongly correlated with foetal microglia in LPS-stimulated TNF-α production. Prenatal priming also occurs in MIA (Mattei et al., 2017). This may increase susceptibility to neuropsychiatric disorder through interactions with insults later in life. Interactions between MIA and later life psychological trauma do appear to synergistically increase risk for schizophrenia in males (Debost et al., 2017).
Several mechanisms may contribute to the impact of maternal obesity on microglial biology. For example, the adipose-derived hormone leptin regulates microglial activation state in the rat hypothalamus and induces inflammatory cytokine production in vitro (Gao et al., 2014). Leptin receptor deficiency in myeloid cells, generated by Cre-Lox recombination, impairs microglial phagocytosis and decreased mouse hypothalamic neuron number (Gao et al., 2018). Interestingly, leptin circulating levels are increased in the offspring of obese dams (Chen et al., 2008). This suggests that leptin signalling might regulate microglia phenotype in the developing brain of obese offspring.
Another example is the role of microglia metabolism. Milanova et al. (2019) show that microglia, but not circulating myeloid cells, in obese rats increase lipid metabolism–associated gene expression and decrease expression of glycolytic genes. Metabolic reprogramming plays a key role in regulating macrophage phenotype and plasticity. The initial view of pro-inflammatory glucose metabolism versus anti-inflammatory fatty acid oxidation has moved to a more complex picture of metabolic flexibility driving macrophage states (Van den Bossche et al., 2017). Recent studies started to examine this phenomenon specifically in microglia. Pro-inflammatory microglia have increased glucose metabolism that preferentially fuels the tricarboxylic acid (TCA) cycle, which is also fuelled by increased glutamine oxidation. Indeed, glutamine is used as an alternate carbon source upon acute hypoglycaemia to maintain microglia surveillance function (Bernier et al., 2020). Both mitochondrial fatty acid oxidation and fatty acid synthesis are higher in anti-inflammatory polarised microglia and decreased in inflammatory states. On the other hand, lipid droplets are frequently found upon inflammation in immune cells and in microglia after LPS stimulation or in aging brain (Marschallinger et al., 2020). These lipid droplet–containing microglia have impaired phagocytic function and a pro-inflammatory secretome. Metabolic disruptions during maternal obesity might therefore impact microglial metabolic plasticity in the offspring that would in turn impair neurodevelopment.
The immunomodulatory roles of fatty acids
In addition to increased levels of inflammatory cytokines, obese women show an altered profile of circulating metabolites, including fatty acids (Tu et al., 2019). Among these, saturated fatty acids (SFAs) and polyunsaturated fatty acids (PUFAs) and their derivatives have the best characterised immunomodulatory properties.
SFA gavage increases microglial expression of activation markers, including TNF-α, without increasing peripheral inflammatory cytokine levels in adult mice (Valdearcos et al., 2014). Moreover, microglial depletion reduced hypothalamic inflammation and neuronal stress marker Hsp72 demonstrating microglia are responsible for SFA-mediated hypothalamic neuronal stress. As microglia are brain-resident macrophages, the mechanisms of SFA-induced activation are likely similar to those in other macrophage populations. SFAs were thought to cause macrophage activation as agonists of Toll-like receptor 4. However, Lancaster et al. (2018) provide compelling evidence that this effect may be mediated by changes in macrophage metabolism. Recent evidence suggests fatty acid metabolism is involved in regulating activation through modulation of plasma membrane properties, rather than directly inducing polarisation or binding Toll-like receptors (Divakaruni et al., 2018; Köberlin et al., 2015). As SFAs are elevated in obese versus lean pregnancies (Vidakovic et al., 2015), these bioactive lipids may contribute to developmental inflammation. SFAs have been shown to induce pro-inflammatory cytokine release in placental trophoblast cells which, as mentioned previously, may contribute towards neurodevelopmental disorder (Yang et al., 2015). Yet, evidence for the transport of SFAs across the placenta is limited, with human studies finding no correlation between circulating maternal and foetal SFA levels (Liu et al., 2017; Meher et al., 2016). Further studies should determine whether and how SFA can cross the placental barrier and how they affect inflammatory processes in the foetus.
The major source of PUFAs in mammals comes from the diet as precursors found in vegetable oils or directly from meat (n-6) and fish (n-3). In a schematic view, n-6 PUFAs are converted into eicosanoids and prostaglandins with pro-inflammatory properties. By contrast, n-3 (also termed omega-3) PUFAs have anti-inflammatory effects through conversion into specialised pro-resolving mediators such as resolvins (Rey et al., 2019). Importantly, maternal dietary manipulations of PUFA content induce modifications in the lipid profile of the developing brain. An imbalanced diet with high levels of n-6 and low levels of n-3 PUFAs results in corresponding changes in the foetal brain, without affecting global levels of saturated or monounsaturated fatty acids (Sakayori et al., 2016). Maternal dietary manipulations alter PUFA content of microglia in the offspring brain with high n-6/n-3 PUFA ratio leading to a corresponding increase in n-6 PUFAs in microglia membrane. Conversely, n-3 PUFA supplemented diet with low n-6/n-3 PUFA ratio resulted in higher n-3 PUFA membrane content. Intriguingly, n-3 PUFA supplemented diet had a stronger effect on microglia lipid composition, including phospholipid classes (Rey et al., 2018). These changes might be linked to impaired microglia function, as maternal dietary n-3 PUFA deficiency impaired microglial process motility and regulated expression of inflammation-related genes (Madore et al., 2014). In addition, n-3 PUFA deficiency exacerbated inflammation and behavioural defects induced by MIA (Labrousse et al., 2018). While these studies clearly show the adverse effect of maternal dietary PUFA deficiency on prenatal brain development and behavioural outcome in offspring, our understanding of the contribution of PUFAs to foetal development and cognitive outcome in maternal obesity is much more limited.
MHFD models rely on diets usually enriched in SFAs with a high n-6/n-3 PUFA ratio, reflecting the Western diet. Indeed, obese pregnant women consistently have an elevated n-6/n-3 PUFA ratio (Benaim et al., 2018; Vidakovic et al., 2015). Interestingly, a study in Hawaiian pregnant women, whose fish consumption is likely higher than in the typical Western diet, showed that obese women had higher n-3 PUFA levels than obese women from Ohio, albeit lower than their lean counterparts (Alvarado et al., 2018). Placenta from Hawaiian obese women did not show signs of lipid accumulation and had similar levels of TNF-α, suggesting that higher intake of n-3 PUFAs might have a protective effect in the placenta. Interestingly, dietary PUFAs also seem to have a protective effect against changes in the epigenetic landscape of the placenta. Female rats fed a high-fat diet enriched in SFAs had increased methylation but reduced histone acetylation in the placenta, compared to those fed a diet enriched in long-chain or very-long-chain PUFAs (Ramaiyan and Talahalli, 2018). Human studies of maternal n-3 PUFA supplementation yielded controversial results regarding beneficial effects on offspring cognitive outcome (Colombo et al., 2019; Ramakrishnan et al., 2016). One reason for this might be that beneficial effects are more likely to be observed in adverse conditions such as maternal obesity. Maternal dietary n-3 PUFA deficiency and maternal obesity lead to similar defects in prenatal brain development and behavioural impairments in animal models. Hence, they might share common pathways worth investigating further to provide new leads for intervention.
Maternal obesity alters the epigenetic landscape in offspring
Cellular metabolism is tightly linked to epigenetic modifications. Lipid pathways provide acetyl-coA and β-hydroxybutyrate which are involved in acetylation/deacetylation reactions and the mitochondrial TCA cycle also provides molecular substrate for DNA methylation. Thus, altered metabolism in the context of obesity might affect epigenetic processes throughout foetal development.
Shortly after fertilisation, a global demethylation accompanied by oxidation of 5-methylcytosine (producing 5hmC marks) contributes to resetting the paternal genome. By contrast, the maternal pronucleus is protected from active demethylation early on by the DNA-binding protein Stella and demethylation is achieved by dilution in subsequent divisions (Lee et al., 2014; Smith and Meissner, 2013). Han et al. (2018) show in an elegant study that this epigenetic remodelling is altered under MHFD. The epigenetic asymmetry usually observed between maternal and paternal genomes is lost in MHFD zygotes, with increased hypomethylation across the genome in particular at transposon elements. They further show that Stella levels are decreased in MHFD oocytes and restoring Stella levels prevents the early loss of epigenetic asymmetry contributing to the maintenance of the early embryonic methylome (Han et al., 2018). Thus, defects in the maternal epigenetic machinery due to diet-induced obesity have long-term consequences on the epigenetic programme required for proper embryonic development.
Strikingly, a global increase in methylation with concurrent decrease in hydroxymethylation of DNA across the genome is observed in placenta from obese women (Mitsuya et al., 2017; Ramaiyan and Talahalli, 2018), potentially regulating immune response, placental and foetal development gene programmes. A recent study showed that epigenetic modifications also occur in the immune cell lineage during development. Umbilical cord blood monocytes from babies of mothers with pregravid obesity had decreased methylation in promoter regions of genes involved in inflammatory response. These changes in the epigenetic profile of umbilical monocytes did not affect their transcriptional programme at resting state but blunted their response to LPS stimulation and the associated transcriptional changes (Sureshchandra et al., 2019).
How these global alterations specifically affect inflammation in the developing brain is unclear. Dynamic changes in the epigenome are a major feature of proper microglia development. The landscape of accessible chromatin regions is strikingly different between embryonic and adult microglia (Matcovitch-Natan et al., 2016). Genetic deletion prenatally of two histone deacetylase genes Hdac1 and Hdac2 impaired microglia development with long-lasting effects later in life, contrasting with little effect when performed postnatally (Datta et al., 2018). Moreover, the unique signature of microglia heavily relies on environment-dependent epigenetic regulation, which critically is lost when they are placed in culture (Gosselin et al., 2017). Importantly, the genes downregulated in cultured microglia largely overlap with those upregulated during in vivo development, indicating that microglial developmental programmes require interaction with the environment. Indeed, the epigenetic landscape of embryonic microglia is sensitive to environmental changes such as absent maternal microbiota (Thion et al., 2018b). Given the widespread alterations in DNA methylation upon exposure to maternal obesity and the tight link between DNA methylation and histone modifications, it is likely that microglia’s epigenetic landscape is impaired in offspring of obese mothers. Surprisingly, while MIA induces changes in embryonic microglia’s transcriptome, modifications in their epigenetic programme have not been reported. These MIA-induced transcriptomic changes seem to be transient as adult microglia show only mild differences in gene expression (Matcovitch-Natan et al., 2016; Mattei et al., 2017).
A role for extracellular vesicles in mediating the impact of maternal obesity on the developing brain?
The question of how dysregulated signals from obese mothers reach the developing brain is a complex unanswered issue. Circulating cytokines and nutrients might enter foetal circulation after tight control at the level of the placenta, but this process is likely compound specific and our understanding is still limited. Another layer of complexity comes from extracellular vesicles (EVs). EVs describe a broad range of cell-released membranous structures. EV nomenclature is ambiguous with the term exosome used to describe EV origin or size – usually 30–100 nm for exosomes. To address this, EV nomenclature guidelines have recently been produced by experts in the field (Théry et al., 2018). Here, the term exosome is used only where authors have experimentally established endosomal origin of the EV. EVs are involved in intercellular signalling during many processes, including embryonic and neurodevelopment (Gong et al., 2016; Greening et al., 2016). Signalling occurs through transport of RNAs, DNA, proteins or bioactive lipids from donor to recipient cells or through ligand–receptor interactions at the EV and cell surface (Iraci et al., 2016). Exosomes are a specific type of EV of endosomal origin that have received much attention for their roles in cell–cell signalling. Importantly, exosomes can cross both the placental (Sheller-Miller et al., 2019) and blood–brain barriers when injected systemically (Alvarez-Erviti et al., 2011).
During brain development, EVs are released by neurons, glia, oligodendrocytes and astrocytes. These EVs are involved in several processes including trophic support and inflammation (Sharma et al., 2013). Human macrophage EVs, for example, increase pro-inflammatory cytokine release in placental trophoblast explants (Holder et al., 2016). EVs may therefore contribute towards placental inflammation during maternal infection leading to neurodevelopmental disorder as mentioned earlier. The transfer of Zika virus between murine cortical neurons has been shown to occur through EVs and some studies indicate trans-placental transport of Zika virus containing EVs (Zhang et al., 2016; Zhou et al., 2019). In addition to its direct effect on neural progenitor survival, Zika infection of cranial neural crest cells increased cytokine production in vitro leading to altered neurogenesis and apoptosis of neural progenitors (Bayless et al., 2016).
Developmentally EVs transport morphogens such as Wnt and Sonic Hedgehog (Shh), which are instrumental in vertebrate brain development influencing cell proliferation, patterning and differentiation (Memi et al., 2018). Shh-containing exosomes are released by chick embryo notochord cells and human cell lines, initiating differentiation of mouse embryonic stem cells into motor neurons (Vyas et al., 2014). Interestingly, exosomal Shh released by adipocytes induces pro-inflammatory polarisation of macrophages and maternal obesity increases human circulating pro-inflammatory exosome number (Elfeky et al., 2017; Song et al., 2018). Expression of Shh is also increased in the subcutaneous fat of obese mice (Yao et al., 2019). Combined, these data present the possibility that maternal obesity–induced changes in Shh exosomes impact foetal neurodevelopment by directly modifying neural Shh signalling or by inducing a pro-inflammatory state in brain macrophages.
Adipose tissue may be an important source of exosomal signalling, including via micro-RNAs (miRNAs) (Thomou et al., 2017). Exosomes from obese individuals contained 55 differentially expressed miRNAs, relative to lean counterparts, some of which target TGF-β signalling (Ferrante et al., 2015). Obesity-induced changes to exosome profile have a functional impact on macrophages. Increased expression of miRNA-34a in mouse adipose tissue exosomes enhanced pro-inflammatory polarisation of adipose macrophages (Pan et al., 2019), another potential mechanism by which brain macrophage behaviour could be altered. Furthermore, adipocytes may have an exosome-mediated effect on macrophage differentiation during obesity (Flaherty et al., 2019). Microglia differentiation in particular is susceptible to TGF-β signalling modification which may be impacted by exosomes in obesity (Ferrante et al., 2015; Utz et al., 2020).
Findings summarised here suggest exosome signalling during obesity causes changes to macrophage behaviour and inflammatory status. In maternal obesity, these changes could be mirrored in the foetal brain resulting in neurodevelopmental abnormalities (Figure 2). Indeed, modulation of maternal exosome–derived miRNAs through metabolic dysfunction is already known to induce developmental defects in other systems (Shi et al., 2017). It has also been proposed that hyperlipidaemia might cause release of mitochondrial DNA–containing EVs (Maldonado-Ruiz et al., 2019b). Serum of children with ASD is known to contain EVs with elevated mitochondrial DNA able to induce microglial pro-inflammatory cytokine expression (Tsilioni and Theoharides, 2018). Further investigations are needed to better understand the role of exosomes in transmitting signals of maternal obesity to the developing brain.
Figure 2.
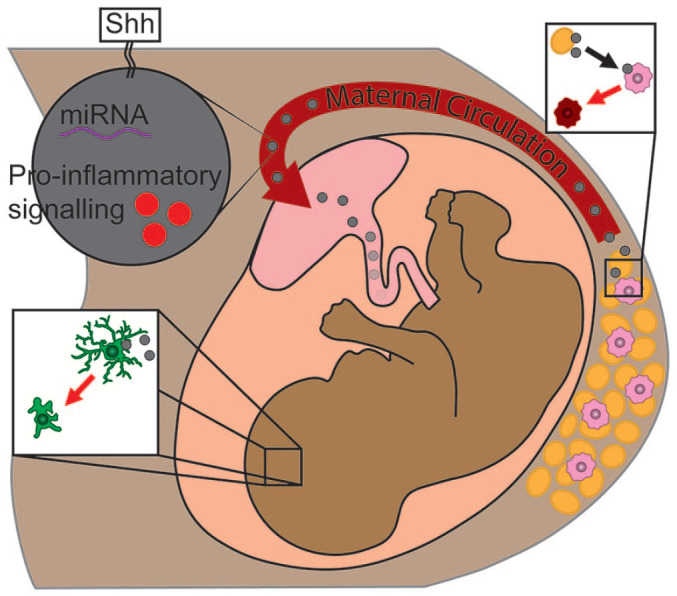
Proposed mechanism for exosome-mediated microglia activation in maternal obesity.
Adipocytes release pro-inflammatory exosomes (in grey) able to signal local macrophages (in pink) to become pro-inflammatory (in red). These exosomes enter maternal circulation and are able to cross the placenta into foetal circulation. Microglia (in green) are suspected to become pro-inflammatory upon interaction with maternal adipocyte–derived exosomes, interfering with microglia’s developmental roles.
Future directions and intervention strategies
A number of inflammation-related systems are dysregulated under obesity, systems with a major role in successful offspring development. This is evident from observed similarities in neuropsychiatric outcomes between MIA and maternal obesity. Several hurdles lie in the path to fully understanding the mechanisms involved in the inflammatory impact of maternal obesity on neurodevelopment. Clearing up contradictory findings in cytokine circulation under maternal obesity would be an excellent start. Longitudinal studies measuring cytokine levels in obese women at regular intervals throughout pregnancy and afterwards, as carried out by Graham et al. (2017) for healthy pregnancy, could begin to clarify understanding provided ethnic differences are accounted for. Differences in diet between subjects may also need to be controlled for given the modulatory effects of n-3/n-6 PUFAs on inflammation (Kanerva et al., 2014; Shivappa et al., 2018). In addition, measuring cytokine levels in placental tissue and cord blood under these conditions could shed light on the relationship between maternal, placental and foetal cytokine profiles during maternal obesity. Knowledge in this field may allow better targeting of any anti-inflammatory therapies to particular stages of pregnancy or women with particular complications.
Further research is also required within the field of microglial biology to understand the role of leptin signalling and metabolic dysfunction in the developmental activity of microglia. Further investigation into dietary modifications with a focus on microglia could provide intervention opportunities. Low carbohydrate ketogenic diet metabolites, for example, reduce microglial inflammation but are likely not appropriate during pregnancy (Huang et al., 2018). Studies examining changes in microglial metabolism under maternal obesity could also help uncover epigenetic changes in microglia, given the close relationship between the two. In addition, investigating the crosstalk between microglia and other cells that contribute to brain inflammation including astrocytes will shed light on cellular interactions critical for sustained effect on brain functions.
Initial studies into the role of exosome signalling in microglia behaviour are also required. The potential for exosomes in the treatment of many disorders has begun to be explored. Preclinical investigations suggest these vesicles may be useful in the treatment of ASD (Perets et al., 2018). Although great care is needed when applying preventive treatments to pregnant women, the fact that exosomes that are naturally involved in pregnancy may reduce the risk of complications if applied to maternal obesity. The targeting of inflamed regions, including during neuroinflammation, by certain exosomes makes anti-inflammatory exosome treatment an interesting avenue of investigation (Perets et al., 2019).
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: J.D was supported by a Hodge Foundation PhD studentship. E.M. is a recipient of the Hodge Foundation Fellowship and received support from a Springboard Award from the Academy of Medical Sciences (SBF005\1100).
ORCID iD: Erik Mire  https://orcid.org/0000-0002-6793-0566
https://orcid.org/0000-0002-6793-0566
References
- Abuaish S, Spinieli RL, McGowan PO. (2018) Perinatal high fat diet induces early activation of endocrine stress responsivity and anxiety-like behavior in neonates. Psychoneuroendocrinology 98: 11–21. [DOI] [PubMed] [Google Scholar]
- Alvarado FL, Calabuig-Navarro V, Haghiac M, et al. (2018) Maternal obesity is not associated with placental lipid accumulation in women with high omega-3 fatty acid levels. Placenta 69: 96–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Erviti L, Seow Y, Yin H, et al. (2011) Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nature Biotechnology 29(4): 341–345. [DOI] [PubMed] [Google Scholar]
- Amoli MM, Khatami F, Arzaghi SM, et al. (2019) Over-expression of TGF-β1 gene in medication free Schizophrenia. Psychoneuroendocrinology 99: 265–270. [DOI] [PubMed] [Google Scholar]
- Ander SE, Diamond MS, Coyne CB. (2019) Immune responses at the maternal-fetal interface. Science Immunology 4(31): eaat6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsevich G, Baumann V, Uribe A, et al. (2016) Prenatal exposure to maternal obesity alters anxiety and stress coping behaviors in aged mice. Neuroendocrinology 103(3–4): 354–368. [DOI] [PubMed] [Google Scholar]
- Baltayeva J, Konwar C, Castellana B, et al. (2020) Obesogenic diet exposure alters uterine natural killer cell biology and impairs vasculature remodeling in micedagger. Biology of Reproduction 102(1): 63–75. [DOI] [PubMed] [Google Scholar]
- Bayless NL, Greenberg RS, Swigut T, et al. (2016) Zika virus infection induces cranial neural crest cells to produce cytokines at levels detrimental for neurogenesis. Cell Host & Microbe 20(4): 423–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benaim C, Freitas-Vilela AA, Pinto TJP, et al. (2018) Early pregnancy body mass index modifies the association of pre-pregnancy dietary patterns with serum polyunsaturated fatty acid concentrations throughout pregnancy in Brazilian women. Maternal & Child Nutrition 14(1): e12480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier LP, York EM, Kamyabi A, et al. (2020) Microglial metabolic flexibility supports immune surveillance of the brain parenchyma. Nature Communications 11(1): 1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbo SD, Tsang V. (2010) Enduring consequences of maternal obesity for brain inflammation and behavior of offspring. The FASEB Journal 24(6): 2104–2115. [DOI] [PubMed] [Google Scholar]
- Brown AS, Derkits EJ. (2010) Prenatal infection and schizophrenia: A review of epidemiologic and translational studies. American Journal of Psychiatry 167(3): 261–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS, Meyer U. (2018) Maternal immune activation and neuropsychiatric illness: A translational research perspective. American Journal of Psychiatry 175(11): 1073–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffington SA, Di Prisco GV, Auchtung TA, et al. (2016) Microbial reconstitution reverses maternal diet-induced social and synaptic deficits in offspring. Cell 165(7): 1762–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovsky O, Jedrychowski MP, Moore CS, et al. (2014) Identification of a unique TGF-β-dependent molecular and functional signature in microglia. Nature Neuroscience 17(1): 131–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellana B, Perdu S, Kim Y, et al. (2018) Maternal obesity alters uterine NK activity through a functional KIR2DL1/S1 imbalance. Immunology and Cell Biology 96(8): 805–819. [DOI] [PubMed] [Google Scholar]
- Chen A, Roberts DJ. (2018) Placental pathologic lesions with a significant recurrence risk: What not to miss! APMIS 126(7): 589–601. [DOI] [PubMed] [Google Scholar]
- Chen H, Simar D, Lambert K, et al. (2008) Maternal and postnatal overnutrition differentially impact appetite regulators and fuel metabolism. Endocrinology 149(11): 5348–5356. [DOI] [PubMed] [Google Scholar]
- Christian LM, Porter K. (2014) Longitudinal changes in serum proinflammatory markers across pregnancy and postpartum: Effects of maternal body mass index. Cytokine 70(2): 134–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codagnone MG, Spichak S, O’Mahony SM, et al. (2019) Programming bugs: Microbiota and the developmental origins of brain health and disease. Biological Psychiatry 85(2): 150–163. [DOI] [PubMed] [Google Scholar]
- Colombo J, Shaddy DJ, Gustafson K, et al. (2019) The Kansas University DHA outcomes study (KUDOS) clinical trial: Long-term behavioral follow-up of the effects of prenatal DHA supplementation. American Journal of Clinical Nutrition 109(5): 1380–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox B, Tsamou M, Vrijens K, et al. (2019) A co-expression analysis of the placental transcriptome in association with maternal pre-pregnancy BMI and newborn birth weight. Frontiers in Genetics 10: 354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crew RC, Waddell BJ, Mark PJ. (2016) Maternal obesity induced by a ‘cafeteria’ diet in the rat does not increase inflammation in maternal, placental or fetal tissues in late gestation. Placenta 39: 33–40. [DOI] [PubMed] [Google Scholar]
- Datta M, Staszewski O, Raschi E, et al. (2018) Histone deacetylases 1 and 2 regulate microglia function during development, homeostasis, and neurodegeneration in a context-dependent manner. Immunity 48(3): 514.e–529.e. [DOI] [PubMed] [Google Scholar]
- Debnath M, Mitra B, Bera NK, et al. (2013) Lack of association of IL-6 (-174 G>C) and TNF-α (-238 G>A) variants with paranoid schizophrenia in Indian Bengalee population. Cytokine 61(2): 455–458. [DOI] [PubMed] [Google Scholar]
- Debost JP, Larsen JT, Munk-Olsen T, et al. (2017) Joint effects of exposure to prenatal infection and peripubertal psychological trauma in schizophrenia. Schizophrenia Bulletin 43(1): 171–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai N, Roman A, Rochelson B, et al. (2013) Maternal metformin treatment decreases fetal inflammation in a rat model of obesity and metabolic syndrome. American Journal of Obstetrics and Gynecology 209(2): e131–e139. [DOI] [PubMed] [Google Scholar]
- Deverman BE, Patterson PH. (2009) Cytokines and CNS development. Neuron 64(1): 61–78. [DOI] [PubMed] [Google Scholar]
- Divakaruni AS, Hsieh WY, Minarrieta L, et al. (2018) Etomoxir inhibits macrophage polarization by disrupting CoA homeostasis. Cell Metabolism 28(3): 490–503e497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlow AG. (2017) Maternal obesity and neurodevelopmental and psychiatric disorders in offspring. Prenatal Diagnosis 37(1): 95–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlow AG, Glass RM, Smith CJ, et al. (2019) Placental macrophages: A window into fetal microglial function in maternal obesity. International Journal of Developmental Neuroscience 77: 60–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Gohary TM, El Aziz NA, Darweesh M, et al. (2015) Plasma level of transforming growth factor β 1 in children with autism spectrum disorder. Egyptian Journal of Ear, Nose, Throat and Allied Sciences 16(1): 69–73. [Google Scholar]
- Elfeky O, Longo S, Lai A, et al. (2017) Influence of maternal BMI on the exosomal profile during gestation and their role on maternal systemic inflammation. Placenta 50: 60–69. [DOI] [PubMed] [Google Scholar]
- Erice C, Calhan OY, Kisiswa L, et al. (2019) Regional differences in the contributions of TNF reverse and forward signaling to the establishment of sympathetic innervation. Developmental Neurobiology 79(4): 317–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes ML, McAllister AK. (2016) Maternal immune activation: Implications for neuropsychiatric disorders. Science 353(6301): 772–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas LM, Dünker N, Roussa E, et al. (2003) Transforming growth factor-beta(s) are essential for the development of midbrain dopaminergic neurons in vitro and in vivo. Journal of Neuroscience 23(12): 5178–5186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes C, Grayton H, Poston L, et al. (2012) Prenatal exposure to maternal obesity leads to hyperactivity in offspring. Molecular Psychiatry 17(12): 1159–1160. [DOI] [PubMed] [Google Scholar]
- Ferrante SC, Nadler EP, Pillai DK, et al. (2015) Adipocyte-derived exosomal miRNAs: A novel mechanism for obesity-related disease. Pediatric Research 77(3): 447–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherty SE, 3rd Grijalva A, Xu X, et al. (2019) A lipase-independent pathway of lipid release and immune modulation by adipocytes. Science 363(6430): 989–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortunato JJ, da Rosa N, Martins Laurentino AO, et al. (2017) Effects of ω-3 fatty acids on stereotypical behavior and social interactions in Wistar rats prenatally exposed to lipopolysaccarides. Nutrition 35: 119–127. [DOI] [PubMed] [Google Scholar]
- Fricke EM, Elgin TG, Gong H, et al. (2018) Lipopolysaccharide-induced maternal inflammation induces direct placental injury without alteration in placental blood flow and induces a secondary fetal intestinal injury that persists into adulthood. American Journal of Reproductive Immunology 79(5): e12816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friis CM, Paasche Roland MC, Godang K, et al. (2013) Adiposity-related inflammation: Effects of pregnancy. Obesity 21(1): E124–E130. [DOI] [PubMed] [Google Scholar]
- Frydecka D, Misiak B, Beszlej JA, et al. (2013) Genetic variants in transforming growth factor-β gene (TGFB1) affect susceptibility to schizophrenia. Molecular Biology Reports 40(10): 5607–5614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Ottaway N, Schriever SC, et al. (2014) Hormones and diet, but not body weight, control hypothalamic microglial activity. Glia 62(1): 17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Vidal-Itriago A, Milanova I, et al. (2018) Deficiency of leptin receptor in myeloid cells disrupts hypothalamic metabolic circuits and causes body weight increase. Molecular Metabolism 7(C): 155–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie SL, Porter K, Christian LM. (2016) Adaptation of the inflammatory immune response across pregnancy and postpartum in Black and White women. Journal of Reproductive Immunology 114: 27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass R, Norton S, Fox N, et al. (2019) Maternal immune activation with staphylococcal enterotoxin A produces unique behavioral changes in C57BL/6 mouse offspring. Brain, Behavior, and Immunity 75: 12–25. [DOI] [PubMed] [Google Scholar]
- Gohir W, Kennedy KM, Wallace JG, et al. (2019) High-fat diet intake modulates maternal intestinal adaptations to pregnancy and results in placental hypoxia, as well as altered fetal gut barrier proteins and immune markers. Journal of Physiology 597(12): 3029–3051. [DOI] [PubMed] [Google Scholar]
- Gong J, Körner R, Gaitanos L, et al. (2016) Exosomes mediate cell contact-independent ephrin-Eph signaling during axon guidance. Journal of Cell Biology 214(1): 35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosselin D, Skola D, Coufal NG, et al. (2017) An environment-dependent transcriptional network specifies human microglia identity. Science 356(6344): eaal3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham C, Chooniedass R, Stefura WP, et al. (2017) In vivo immune signatures of healthy human pregnancy: Inherently inflammatory or anti-inflammatory? PLoS ONE 12(6): e0177813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greening DW, Nguyen HP, Elgass K, et al. (2016) Human endometrial exosomes contain hormone-specific cargo modulating trophoblast adhesive capacity: Insights into endometrial-embryo interactions. Biology of Reproduction 94(2): 38. [DOI] [PubMed] [Google Scholar]
- Gregor MF, Hotamisligil GS. (2011) Inflammatory mechanisms in obesity. Annual Review of Immunology 29: 415–445. [DOI] [PubMed] [Google Scholar]
- Griffith OW, Chavan AR, Protopapas S, et al. (2017) Embryo implantation evolved from an ancestral inflammatory attachment reaction. Proceedings of the National Academy of Sciences of the United States of America 114(32): e6566–e6575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumusoglu SB, Stevens HE. (2019) Maternal inflammation and neurodevelopmental programming: A review of preclinical outcomes and implications for translational psychiatry. Biological Psychiatry 85(2): 107–121. [DOI] [PubMed] [Google Scholar]
- Hammond TR, Robinton D, Stevens B. (2018) Microglia and the brain: Complementary partners in development and disease. Annual Review of Cell and Developmental Biology 34: 523–544. [DOI] [PubMed] [Google Scholar]
- Han L, Ren C, Li L, et al. (2018) Embryonic defects induced by maternal obesity in mice derive from Stella insufficiency in oocytes. Nature Genetics 50(3): 432–442. [DOI] [PubMed] [Google Scholar]
- Heikura U, Taanila A, Hartikainen AL, et al. (2008) Variations in prenatal sociodemographic factors associated with intellectual disability: A study of the 20-year interval between two birth cohorts in northern Finland. American Journal of Epidemiology 167(2): 169–177. [DOI] [PubMed] [Google Scholar]
- Hiramatsu L, Kay JC, Thompson Z, et al. (2017) Maternal exposure to Western diet affects adult body composition and voluntary wheel running in a genotype-specific manner in mice. Physiology & Behavior 179: 235–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holder B, Jones T, Sancho Shimizu V, et al. (2016) Macrophage exosomes induce placental inflammatory cytokines: A novel mode of maternal-placental messaging. Traffic 17(2): 168–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holub M, Lawrence DA, Andersen N, et al. (2013) Cytokines and chemokines as biomarkers of community-acquired bacterial infection. Mediators of Inflammation 2013: 190145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS, Shargill NS, Spiegelman BM. (1993) Adipose expression of tumor necrosis factor-alpha: Direct role in obesity-linked insulin resistance. Science 259(5091): 87–91. [DOI] [PubMed] [Google Scholar]
- Hrolfsdottir L, Schalkwijk CG, Birgisdottir BE, et al. (2016) Maternal diet, gestational weight gain, and inflammatory markers during pregnancy. Obesity 24(10): 2133–2139. [DOI] [PubMed] [Google Scholar]
- Hsiao EY, Patterson PH. (2011) Activation of the maternal immune system induces endocrine changes in the placenta via IL-6. Brain, Behavior, and Immunity 25(4): 604–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Wang P, Xu X, et al. (2018) The ketone body metabolite β-hydroxybutyrate induces an antidepression-associated ramification of microglia via HDACs inhibition-triggered Akt-small RhoGTPase activation. Glia 66(2): 256–278. [DOI] [PubMed] [Google Scholar]
- Inoubli O, Jemli A, Ben Fredj S, et al. (2018) Haplotypes of TNFα/β genes associated with sex-specific paranoid schizophrenic risk in Tunisian population. Disease Markers 2018: 3502564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iraci N, Leonardi T, Gessler F, et al. (2016) Focus on extracellular vesicles: Physiological role and signalling properties of extracellular membrane vesicles. International Journal of Molecular Sciences 17(2): 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones PB, Rantakallio P, Hartikainen AL, et al. (1998) Schizophrenia as a long-term outcome of pregnancy, delivery, and perinatal complications: A 28-year follow-up of the 1966 north Finland general population birth cohort. American Journal of Psychiatry 155(3): 355–364. [DOI] [PubMed] [Google Scholar]
- Kalk E, Schubert P, Bettinger JA, et al. (2017) Placental pathology in HIV infection at term: A comparison with HIV-uninfected women. Tropical Medicine & International Health 22(5): 604–613. [DOI] [PubMed] [Google Scholar]
- Kang SS, Kurti A, Fair DA, et al. (2014) Dietary intervention rescues maternal obesity induced behavior deficits and neuroinflammation in offspring. Journal of Neuroinflammation 11: 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaodhiar L, Ling PR, Blackburn GL, et al. (2004) Serum levels of interleukin-6 and C-reactive protein correlate with body mass index across the broad range of obesity. Journal of Parenteral and Enteral Nutrition 28(6): 410–415. [DOI] [PubMed] [Google Scholar]
- Kim DW, Young SL, Grattan DR, et al. (2014) Obesity during pregnancy disrupts placental morphology, cell proliferation, and inflammation in a sex-specific manner across gestation in the mouse. Biology of Reproduction 90(6): 130. [DOI] [PubMed] [Google Scholar]
- Köberlin MS, Snijder B, Heinz LX, et al. (2015) A conserved circular network of coregulated lipids modulates innate immune responses. Cell 162(1): 170–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakowiak P, Walker CK, Bremer AA, et al. (2012) Maternal metabolic conditions and risk for autism and other neurodevelopmental disorders. Pediatrics 129(5): e1121–e1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretschmer T, Schulze-Edinghausen M, Turnwald EM, et al. (2020) Effect of maternal obesity in mice on IL-6 levels and placental endothelial cell homeostasis. Nutrients 12(2): 296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrousse VF, Leyrolle Q, Amadieu C, et al. (2018) Dietary omega-3 deficiency exacerbates inflammation and reveals spatial memory deficits in mice exposed to lipopolysaccharide during gestation. Brain, Behavior, and Immunity 73: 427–440. [DOI] [PubMed] [Google Scholar]
- Lancaster GI, Langley KG, Berglund NA, et al. (2018) Evidence that TLR4 is not a receptor for saturated fatty acids but mediates lipid-induced inflammation by reprogramming macrophage metabolism. Cell Metabolism 27(5): 1096–1110e1095. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Hore TA, Reik W. (2014) Reprogramming the methylome: Erasing memory and creating diversity. Cell Stem Cell 14(6): 710–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon-Garcia SM, Roeder HA, Nelson KK, et al. (2016) Maternal obesity and sex-specific differences in placental pathology. Placenta 38: 33–40. [DOI] [PubMed] [Google Scholar]
- Li Q, Barres BA. (2018) Microglia and macrophages in brain homeostasis and disease. Nature Reviews Immunology 18(4): 225–242. [DOI] [PubMed] [Google Scholar]
- Liu K, Ye K, Han Y, et al. (2017) Maternal and cord blood fatty acid patterns with excessive gestational weight gain and neonatal macrosomia. Asia Pacific Journal of Clinical Nutrition 26(2): 291–297. [DOI] [PubMed] [Google Scholar]
- Lively S, Lam D, Wong R, et al. (2018) Comparing effects of transforming growth factor β1 on microglia from rat and mouse: Transcriptional profiles and potassium channels. Frontiers in Cellular Neuroscience 12: 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo SX, Timbang L, Kim JI, et al. (2016) TGF-β signaling in dopaminergic neurons regulates dendritic growth, excitatory-inhibitory synaptic balance, and reversal learning. Cell Reports 17(12): 3233–3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SE, Grissom NM, Herdt CT, et al. (2017) Methyl donor supplementation alters cognitive performance and motivation in female offspring from high-fat diet-fed dams. The FASEB Journal 31(6): 2352–2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madore C, Nadjar A, Delpech JC, et al. (2014) Nutritional n-3 PUFAs deficiency during perinatal periods alters brain innate immune system and neuronal plasticity-associated genes. Brain, Behavior, and Immunity 41: 22–31. [DOI] [PubMed] [Google Scholar]
- Maldonado-Ruiz R, Cárdenas-Tueme M, Montalvo-Martínez L, et al. (2019. a) Priming of hypothalamic ghrelin signaling and microglia activation exacerbate feeding in rats’ offspring following maternal overnutrition. Nutrients 11(6): 1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado-Ruiz R, Garza-Ocañas L, Camacho A. (2019. b) Inflammatory domains modulate autism spectrum disorder susceptibility during maternal nutritional programming. Neurochemistry International 126: 109–117. [DOI] [PubMed] [Google Scholar]
- Manti M, Fornes R, Qi X, et al. (2018) Maternal androgen excess and obesity induce sexually dimorphic anxiety-like behavior in the offspring. The FASEB Journal 32(8): 4158–4171. [DOI] [PubMed] [Google Scholar]
- Marschallinger J, Iram T, Zardeneta M, et al. (2020) Lipid-droplet-accumulating microglia represent a dysfunctional and proinflammatory state in the aging brain. Nature Neuroscience 23(2): 194–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matcovitch-Natan O, Winter DR, Giladi A, et al. (2016) Microglia development follows a stepwise program to regulate brain homeostasis. Science 353(6301): aad8670. [DOI] [PubMed] [Google Scholar]
- Mattei D, Ivanov A, Ferrai C, et al. (2017) Maternal immune activation results in complex microglial transcriptome signature in the adult offspring that is reversed by minocycline treatment. Translational Psychiatry 7(5): e1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meher A, Randhir K, Mehendale S, et al. (2016) Maternal fatty acids and their association with birth outcome: A prospective study. PLoS ONE 11(1): e0147359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memi F, Zecevic N, Radonjić N. (2018) Multiple roles of Sonic Hedgehog in the developing human cortex are suggested by its widespread distribution. Brain Structure and Function 223(5): 2361–2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers EA, Kessler JA. (2017) TGF-β family signaling in neural and neuronal differentiation, development, and function. Cold Spring Harbor Perspectives in Biology 9(8): a022244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milanova IV, Kalsbeek MJT, Wang XL, et al. (2019) Diet-induced obesity disturbs microglial immunometabolism in a time-of-day manner. Frontiers in Endocrinology 10: 424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuya K, Parker AN, Liu L, et al. (2017) Alterations in the placental methylome with maternal obesity and evidence for metabolic regulation. PLoS ONE 12(10): e0186115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller SL, Nyengaard JR, Larsen LG, et al. (2019) Malaria in early pregnancy and the development of the placental vasculature. Journal of Infectious Diseases 220(9): 1425–1434. [DOI] [PubMed] [Google Scholar]
- Nolan AM, Collins LM, Wyatt SL, et al. (2014) The neurite growth inhibitory effects of soluble TNFα on developing sympathetic neurons are dependent on developmental age. Differentiation 88(4–5): 124–130. [DOI] [PubMed] [Google Scholar]
- Pan Y, Hui X, Hoo RLC, et al. (2019) Adipocyte-secreted exosomal microRNA-34a inhibits M2 macrophage polarization to promote obesity-induced adipose inflammation. Journal of Clinical Investigation 129(2): 834–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papachatzi E, Dimitriou G, Dimitropoulos K, et al. (2013) Pre-pregnancy obesity: Maternal, neonatal and childhood outcomes. Journal of Neonatal-Perinatal Medicine 6(3): 203–216. [DOI] [PubMed] [Google Scholar]
- Park HS, Kim TW. (2017) Paternal physical exercise improves spatial learning ability by enhancing hippocampal neuroplasticity in male pups born from obese maternal rats. Journal of Exercise Rehabilitation 13(3): 266–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peleg-Raibstein D, Sarker G, Litwan K, et al. (2016) Enhanced sensitivity to drugs of abuse and palatable foods following maternal overnutrition. Translational Psychiatrya 6(10): e911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendeloski KPT, Ono E, Torloni MR, et al. (2017) Maternal obesity and inflammatory mediators: A controversial association. American Journal of Reproductive Immunology 77(5): 12674. [DOI] [PubMed] [Google Scholar]
- Perets N, Betzer O, Shapira R, et al. (2019) Golden exosomes selectively target brain pathologies in neurodegenerative and neurodevelopmental disorders. Nano Letters 19(6): 3422–3431. [DOI] [PubMed] [Google Scholar]
- Perets N, Hertz S, London M, et al. (2018) Intranasal administration of exosomes derived from mesenchymal stem cells ameliorates autistic-like behaviors of BTBR mice. Molecular Autism 9: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaiyan B, Talahalli RR. (2018) Dietary unsaturated fatty acids modulate maternal dyslipidemia-induced DNA methylation and histone acetylation in placenta and fetal liver in rats. Lipids 53(6): 581–588. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan U, Gonzalez-Casanova I, Schnaas L, et al. (2016) Prenatal supplementation with DHA improves attention at 5 y of age: A randomized controlled trial. American Journal of Clinical Nutrition 104(4): 1075–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redline RW. (2005) Severe fetal placental vascular lesions in term infants with neurologic impairment. American Journal of Obstetrics and Gynecology 192(2): 452–457. [DOI] [PubMed] [Google Scholar]
- Rey C, Delpech JC, Madore C, et al. (2019) Dietary n-3 long chain PUFA supplementation promotes a pro-resolving oxylipin profile in the brain. Brain, Behavior, and Immunity 76: 17–27. [DOI] [PubMed] [Google Scholar]
- Rey C, Nadjar A, Joffre F, et al. (2018) Maternal n-3 polyunsaturated fatty acid dietary supply modulates microglia lipid content in the offspring. Prostaglandins, Leukotrienes & Essential Fatty Acids 133: 1–7. [DOI] [PubMed] [Google Scholar]
- Rinaldi SF, Makieva S, Saunders PT, et al. (2017) Immune cell and transcriptomic analysis of the human decidua in term and preterm parturition. Molecular Human Reproduction 23(10): 708–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera HM, Christiansen KJ, Sullivan EL. (2015) The role of maternal obesity in the risk of neuropsychiatric disorders. Frontiers in Neuroscience 9: 194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez A, Miettunen J, Henriksen TB, et al. (2008) Maternal adiposity prior to pregnancy is associated with ADHD symptoms in offspring: Evidence from three prospective pregnancy cohorts. International Journal of Obesity 32(3): 550–557. [DOI] [PubMed] [Google Scholar]
- Sakayori N, Kikkawa T, Tokuda H, et al. (2016) Maternal dietary imbalance between omega-6 and omega-3 polyunsaturated fatty acids impairs neocortical development via epoxy metabolites. Stem Cells 34(2): 470–482. [DOI] [PubMed] [Google Scholar]
- Samad F, Yamamoto K, Pandey M, et al. (1997) Elevated expression of transforming growth factor-beta in adipose tissue from obese mice. Molecular Medicine 3(1): 37–48. [PMC free article] [PubMed] [Google Scholar]
- Sanders TR, Kim DW, Glendining KA, et al. (2014) Maternal obesity and IL-6 lead to aberrant developmental gene expression and deregulated neurite growth in the fetal arcuate nucleus. Endocrinology 155(7): 2566–2577. [DOI] [PubMed] [Google Scholar]
- Sanguinetti E, Guzzardi MA, Tripodi M, et al. (2019) Microbiota signatures relating to reduced memory and exploratory behaviour in the offspring of overweight mothers in a murine model. Science Reports 9(1): 12609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen S, Iyer C, Meydani SN. (2014) Obesity during pregnancy alters maternal oxidant balance and micronutrient status. Journal of Perinatology 34(2): 105–111. [DOI] [PubMed] [Google Scholar]
- Sharma P, Schiapparelli L, Cline HT. (2013) Exosomes function in cell-cell communication during brain circuit development. Current Opinion in Neurobiology 23(6): 997–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheller-Miller S, Choi K, Choi C, et al. (2019) Cyclic-recombinase-reporter mouse model to determine exosome communication and function during pregnancy. American Journal of Obstetrics and Gynecology 221(5): 502.e1–502.e12. [DOI] [PubMed] [Google Scholar]
- Shi R, Zhao L, Cai W, et al. (2017) Maternal exosomes in diabetes contribute to the cardiac development deficiency. Biochemical and Biophysical Research Communications 483(1): 602–608. [DOI] [PubMed] [Google Scholar]
- Singh KP, Shakeel S, Naskar N, et al. (2018) Role of IL-1β, IL-6 and TNF-α cytokines and TNF-α promoter variability in Plasmodium vivax infection during pregnancy in endemic population of Jharkhand, India. Molecular Immunology 97: 82–93. [DOI] [PubMed] [Google Scholar]
- Smith ZD, Meissner A. (2013) DNA methylation: Roles in mammalian development. Nature Reviews Genetics 14(3): 204–220. [DOI] [PubMed] [Google Scholar]
- Sohlberg S, Stephansson O, Cnattingius S, et al. (2012) Maternal body mass index, height, and risks of preeclampsia. American Journal of Hypertension 25(1): 120–125. [DOI] [PubMed] [Google Scholar]
- Song M, Han L, Chen FF, et al. (2018) Adipocyte-derived exosomes carrying Sonic Hedgehog mediate M1 macrophage polarization-induced insulin resistance via Ptch and PI3K pathways. Cellular Physiology and Biochemistry 48(4): 1416–1432. [DOI] [PubMed] [Google Scholar]
- Spradley FT, Palei AC, Granger JP. (2015) Increased risk for the development of preeclampsia in obese pregnancies: Weighing in on the mechanisms. American Journal of Physiology – Regulatory, Integrative and Comparative Physiology 309(11): R1326–R1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaresh A, Oliveira J, Chinnadurai RK, et al. (2019) IL6/IL6R genetic diversity and plasma IL6 levels in bipolar disorder: An Indo-French study. Heliyon 5(1): e01124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sureshchandra S, Marshall NE, Messaoudi I. (2019) Impact of pregravid obesity on maternal and fetal immunity: Fertile grounds for reprogramming. Journal of Leukocyte Biology 106(5): 1035–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay TL, Béchade C, D’Andrea I, et al. (2017) Microglia gone rogue: Impacts on psychiatric disorders across the lifespan. Frontiers in Molecular Neuroscience 10: 421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Théry C, Witwer KW, Aikawa E, et al. (2018) Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. Journal of Extracellular Vesicles 7(1): 1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thion MS, Ginhoux F, Garel S. (2018. a) Microglia and early brain development: An intimate journey. Science 362(6411): 185–189. [DOI] [PubMed] [Google Scholar]
- Thion MS, Low D, Silvin A, et al. (2018. b) Microbiome influences prenatal and adult microglia in a sex-specific manner. Cell 172(3): 500.e–516.e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomou T, Mori MA, Dreyfuss JM, et al. (2017) Adipose-derived circulating miRNAs regulate gene expression in other tissues. Nature 542(7642): 450–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JR, Gustafsson HC, DeCapo M, et al. (2018) Maternal diet, metabolic state, and inflammatory response exert unique and long-lasting influences on offspring behavior in non-human primates. Frontiers in Endocrinology 9: 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JR, Valleau JC, Barling AN, et al. (2017) Exposure to a high-fat diet during early development programs behavior and impairs the central serotonergic system in juvenile non-human primates. Frontiers in Endocrinology 8: 164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torzewski M, Waqar AB, Fan J. (2014) Animal models of C-reactive protein. Mediators of Inflammation 2014: 683598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsilioni I, Theoharides TC. (2018) Extracellular vesicles are increased in the serum of children with autism spectrum disorder, contain mitochondrial DNA, and stimulate human microglia to secrete IL-1β. Journal of Neuroinflammation 15(1): 239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursini G, Punzi G, Chen Q, et al. (2018) Convergence of placenta biology and genetic risk for schizophrenia. Nature Medicine 24(6): 792–801. [DOI] [PubMed] [Google Scholar]
- Utz SG, See P, Mildenberger W, et al. (2020) Early fate defines microglia and non-parenchymal brain macrophage development. Cell 181(3): 557.e–573.e. [DOI] [PubMed] [Google Scholar]
- Valdearcos M, Robblee MM, Benjamin DI, et al. (2014) Microglia dictate the impact of saturated fat consumption on hypothalamic inflammation and neuronal function. Cell Reports 9(6): 2124–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Bossche J, O’Neill LA, Menon D. (2017) Macrophage immunometabolism: Where are we (going)? Trends in Immunology 38(6): 395–406. [DOI] [PubMed] [Google Scholar]
- Vento-Tormo R, Efremova M, Botting RA, et al. (2018) Single-cell reconstruction of the early maternal-fetal interface in humans. Nature 563(7731): 347–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidakovic AJ, Jaddoe VW, Gishti O, et al. (2015) Body mass index, gestational weight gain and fatty acid concentrations during pregnancy: The Generation R Study. European Journal of Epidemiology 30(11): 1175–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuong HE, Hsiao EY. (2017) Emerging roles for the gut microbiome in autism spectrum disorder. Biological Psychiatry 81(5): 411–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas N, Walvekar A, Tate D, et al. (2014) Vertebrate Hedgehog is secreted on two types of extracellular vesicles with different signaling properties. Science Reports 4: 7357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisberg SP, McCann D, Desai M, et al. (2003) Obesity is associated with macrophage accumulation in adipose tissue. Journal of Clinical Investigation 112(12): 1796–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z, Jiao F, Yan X, et al. (2017) Maternal chronic folate supplementation ameliorates behavior disorders induced by prenatal high-fat diet through methylation alteration of BDNF and Grin2b in offspring hippocampus. Molecular Nutrition & Food Research 61(12): 00461. [DOI] [PubMed] [Google Scholar]
- Yang X, Haghiac M, Glazebrook P, et al. (2015) Saturated fatty acids enhance TLR4 immune pathways in human trophoblasts. Human Reproduction 30(9): 2152–2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Q, Liu J, Xiao L, et al. (2019) Sonic hedgehog signaling instigates high-fat diet-induced insulin resistance by targeting PPARγ stability. Journal of Biological Chemistry 294(9): 3284–3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yockey LJ, Iwasaki A. (2018) Interferons and proinflammatory cytokines in pregnancy and fetal development. Immunity 49(3): 397–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon S, Parnell E, Penzes P. (2020) TGF-β-induced phosphorylation of Usp9X stabilizes ankyrin-G and regulates dendritic spine development and maintenance. Cell Reports 31(8): 107685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zembala-Szczerba M, Jaworowski A, Huras H, et al. (2017) Low-grade metabolically-induced inflammation mediators interleukin-6, adiponectin, and TNF-α serum levels in obese pregnant patients in the perinatal period. Medical Science Monitor Basic Research 23: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZW, Li ZL, Yuan S. (2016) The role of secretory autophagy in Zika virus transfer through the placental barrier. Frontiers in Cellular and Infection Microbiology 6: 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Woodson M, Sherman MB, et al. (2019) Exosomes mediate Zika virus transmission through SMPD3 neutral Sphingomyelinase in cortical neurons. Emerging Microbes & Infections 8(1): 307–326. [DOI] [PMC free article] [PubMed] [Google Scholar]