Abstract
Beta 2 adrenergic receptor (β2 AR) activation in the central and peripheral nervous system has been implicated in nociceptive processing in acute and chronic pain settings with anti-inflammatory and anti-allodynic effects of β2-AR mimetics reported in several pain states. In the current study, we examined the therapeutic efficacy of the β2-AR agonist clenbuterol in a rat model of persistent postsurgical hypersensitivity induced by disruption of descending noradrenergic signaling in rats with plantar incision. We used growth curve modeling of ipsilateral mechanical paw withdrawal thresholds following incision to examine effects of treatment on postoperative trajectories. Depletion of spinal noradrenergic neurons delayed recovery of hypersensitivity following incision evident as a flattened slope compared to non-depleted rats (-1.8 g/day with 95% CI -2.4 to -1.085, p < 0.0001). Chronic administration of clenbuterol reduced mechanical hypersensitivity evident as a greater initial intercept in noradrenergic depleted (6.2 g with 95% CI 1.6 to 10.8, p = 0.013) and non-depleted rats (5.4 g with 95% CI 1.2 to 9.6, p = 0.018) with plantar incision compared to vehicle treated rats. Despite a persistent reduction in mechanical hypersensitivity, clenbuterol did not alter the slope of recovery when modeled over several days (p = 0.053) or five weeks in depleted rats (p = 0.64). Systemic clenbuterol suppressed the enhanced microglial activation in depleted rats and reduced the density of macrophage at the site of incision. Direct spinal infusion of clenbuterol failed to reduce mechanical hypersensitivity in depleted rats with incision suggesting that beneficial effects of β2-AR stimulation in this model are largely peripherally mediated. Lastly, we examined β2-AR distribution in the spinal cord and skin using in-situ hybridization and IHC. These data add to our understanding of the role of β2-ARs in the nervous system on hypersensitivity after surgical incision and extend previously observed anti-inflammatory actions of β2-AR agonists to models of surgical injury.
Keywords: Postoperative pain, acute to chronic pain transition, glial plasticity, growth curve modeling, surgery
Introduction
Chronic postsurgical pain (CPSP) remains a significant public health concern occurring in 10–50% of patients undergoing surgery. The primary risk factors associated with the development of CPSP remain preexisting pain and severe acute postoperative pain following the surgical procedure.1 Recently, clinical and preclinical studies have suggested that impaired endogenous analgesia2–4 and an amplified neuroimmune or inflammatory responses in the spinal cord5 and periphery6–8 are key mechanisms driving the transition from an acute to a chronic postsurgical pain state.9 Therefore, new pharmacological approaches that augment endogenous analgesia or modulate the innate immune response to surgical tissue injury might be beneficial for promoting postsurgical pain resolution or preventing pain chronicity.
Beta 2-adrenoreceptor agonists are bronchodilators routinely administered to provide symptom relief for several respiratory conditions. These agents also have anti-inflammatory effects on activated macrophage and microglia mediated largely but not exclusively via canonical Gs protein coupled signaling mechanisms involving activation of cyclic AMP and protein kinase A10 . Previous studies indicate that β2-AR activation in lipopolysaccharide (LPS)-stimulated macrophage suppresses the production of pro-inflammatory mediators (TNFα, IL-6 and IL1β)11–14 and potentiates the rapid release of the anti-inflammatory cytokine IL-1013,15,16 essentially shifting macrophage polarization from pro-inflammatory M1 to an anti-inflammatory M2 phenotype.16–18 Similar results have been observed in primary spinal microglia, whereby norepinephrine suppresses p38 mitogen activated protein kinase (MAPK) signaling and expression of TNF-α induced by ATP in a β-AR dependent manner.19 Pharmacological agents that stimulate β2-ARs possess anti-inflammatory and anti-nociceptive effects in several pain conditions in vivo. The administration of β2-AR agonists terbutaline and salbutamol decrease edema and cartilage degeneration when administered after induction of arthritis in rodents.20–23 Likewise, systemic administration of salbutamol in rats reduces hind paw swelling and mechanical hyperalgesia following intra-plantar carrageenan injection.13,24 Anti-allodynic effects of β2-AR mimetics have also been reported in rodent models of nerve injury. Chronic administration of β2-AR agonists or antidepressants that stimulate β2-ARs suppress mechanical hypersensitivity in nerve injured mice in part by inhibiting peripheral release of TNF-α from DRG satellite cells.25–27 Spinal effects of β2-AR agonists have been reported as direct acute spinal administration of terbutaline in mice with spinal nerve ligation reverses mechanical allodynia via inhibitory effects on microglial and astrocyte signaling.28 Conversely, pro-nociceptive effects of β2-AR stimulation have been reported following acute dermal administration of epinephrine29,30 and in mouse models of complex pain syndromes31–33 Collectively, these studies suggest that endogenous and exogenous β2-AR agonists can act on multiple cell types to produce anti-nociceptive or pro-nociceptive effects and the analgesic efficacy of β2-AR mimetics may be pain state dependent.34
Notably, the therapeutic potential of β2-AR agonists have not been tested in incisional pain models. The plantar incision model has a unique pathophysiology and is mechanistically distinct from inflammatory and neuropathic pain (for review see Pogatzki-Zahn et al.35) In our previous study, we observed that disruption of noradrenergic input to the spinal cord significantly delayed recovery of mechanical hypersensitivity for several weeks following plantar incision in rats.36 This delay was associated with enhanced spinal glial activation.36 The goal of the current study was to examine the ability of the β2-AR agonist clenbuterol to promote resolution of mechanical hypersensitivity following incisional surgery including in rats with impaired spinal noradrenergic tone. We hypothesized that administration of the β2-AR agonist clenbuterol would reduce mechanical hypersensitivity by reducing the response of peripheral macrophage to tissue injury and potentially by inhibiting spinal microglial activation. Additionally, we used IHC and fluorescent in situ hybridization (FISH) to better define the cellular distribution of β2-AR in the lumbar spinal cord and skin of the rat to gain insight into the potential site of action of clenbuterol.
Methods
Animals. A total of 104 male Sprague–Dawley rats (Harlan Industries, Indianapolis, IN), weighing 180–250 g, were used for experiments. All studies conformed to the Wake Forest University Guidelines on the ethical use of animals, and studies were performed under Animal Care and Use Committee (Winston-Salem, North Carolina) approval. Animals were housed under a 12-hour light–dark cycle, with food and water ad libitum.
Drugs. For ablative studies, anti-DβH-saporin and control immunoglobulin G (IgG)-saporin were obtained from Advanced Targeting Systems, San Diego, CA and injected intrathecally at a dose of 5 μg in 10 μl of sterile saline via percutaneous lumbar puncture at the L5-L6 interspace two weeks prior to incision surgery. Successful puncture of the dura was confirmed by the presence of a tail flick. Previous studies demonstrate that spinal anti-DβH-saporin at this dose reduces nearly all spinally projecting noradrenergic neurons including those originating from the locus coeruleus (A6), A5 and A7 cell groups. Supraspinally, there is a reduction of DβH immunoreactive fibers in the cerebral cortex and thalamic nuclei, however noradrenergic fibers in the paraventricular hypothalamic nuclei are largely preserved.37,38 Previous studies have demonstrated that dopaminergic and serotonergic innervation are not altered by spinal DβH-saporin.38 Clenbuterol hydrochloride (Cat no C5423, Sigma-Aldrich, St. Louis, Missouri, USA) was prepared in sterile saline solution at a concentration of 5 mg/ml and delivered systemically twice a day at 8:00 am and 8:00 pm beginning 6 days prior to and 8 days post plantar incision at dose of 0.5 mg/kg (i.p). The dose chosen for systemic delivery are based on previous studies that demonstrated anti-inflammatory effects in rodent models of injury.39–42 For studies involving chronic spinal drug delivery, rats were implanted with intrathecal catheters and drug was delivered via mini-osmotic pumps as previously described.36 Briefly, a small incision was made at the back of the neck and a puncture was made in the atlanto-occipital membrane of the cisterna magnum. A polyethylene catheter (external diameter: 0.23 mm, internal volume: 6 μL; ReCathCo LLC, Allison Park, PA, USA) was inserted for 7.5 cm caudal so that the tip reached the lumbar enlargement of the spinal cord, and then it was secured on the fascia of paravertebral muscle and the tip of the catheter was internalized and the incision skin incision was enclosed with sutures. Seven days after intrathecal catheterization (three days prior to incision) a small incision was made at the base of the neck and mini-osmotic pumps (Model 2002, ALZET Osmotic Pumps, Cupertino, CA, USA) were attached to the catheter and implanted for continual drug infusion for 14 days at a rate of 0.5 μ//hour. Pumps were preloaded with clenbuterol at concentrations to deliver 100 ng/hour (2.4 μg/day), 10 ng/hour (240 ng/day), and 1 ng/hour (24 ng/day). These doses are similar to those previously shown to enhance fear or emotional memory consolidation following infusion into the basolateral amygdala of Sprague Dawley rats.43,44
Plantar incision surgery. Plantar incision was performed as previously described45 In brief, rats were anesthetized with 2–3% of isoflurane. The plantar aspect of the left hind paw was prepared in a sterile manner with a 10% povidone-iodine solution. A 1-cm midline incision was made using a No. 11 surgical blade starting 0.5 cm from the proximal edge of the heel. The plantaris muscle was elevated with curved forceps and incised longitudinally. The model includes surgical incision of the skin, muscle, and fascia of the rat hind paw. The wound was closed with two 5-0 nylon mattress sutures and covered with triple antibiotic ointment. Sham surgery for plantar incision consisted of all perioperative procedures including inhalational isoflurane without incision of the skin.
Behavioral analysis. For all behavioral analysis, individuals who conducted assays were blinded to the group allocation. Because the incision was on the plantar aspects of the paw, the examiner could not be blinded to surgery. Paw withdrawal thresholds to mechanical stimuli were assessed using von Frey filaments (Stoelting Co., Wood Dale, IL, USA) using the up-down statistical method.37 In brief, filaments were applied to the hindpaw medial to the incision to the bending point for 6 second and a brisk paw withdrawal was considered a positive response.
Tissue preparation and immunohistochemistry. Rats were anesthetized with sodium pentobarbital (i.p.; 100 mg/kg), the thorax was opened, and 0.1 M phosphate-buffered saline (PBS, pH 7.4) followed by fixative (4% paraformaldehyde in 0.1 M PBS, pH 7.4) was perfused through the left ventricle with a peristaltic pump (20 ml/min). The lumbar spinal cord and plantar skin were removed, immersed in fixative for 1 h at 4 °C, then immersed in 30% sucrose at 4 °C for cryoprotection until ready to be sectioned. We determined in preliminary studies that post-fixation greater than 4 hours significantly blocked antigenicity for β2-AR resulting in nearly complete loss of staining. Transverse spinal cord sections were cut at 40 µms using a cryostat (Leica CM3050S, Leica Biosystems GmbH, Wetzlar, Germany) and mounted to non-plus slides to be processed as free-floating sections. Skin sections were cut at 20 µms and mounted to Superfrost™ Plus slides (Fisher Scientific, Pittsburgh, PA, USA). Free-floating and slide-mounted sections were blocked with 3% normal donkey serum in 0.3% Triton X-100 for 1 h at room temperature and incubated overnight at 4 °C with primary antibodies. We used a previously characterized antibody against β2 adrenergic receptors (AAR-016, β2-AR, 1:1000, rabbit anti-mouse, Alomone Labs; Jerusalem, Israel). This antibody is highly specific antibody directed against an extracellular epitope of the mouse β2-AR corresponding to amino acid residues 15–30 (Peptide (C) NGSRAPDHDVTQERDE) with sequence homology of 15/16 amino acid residues with human and 14/16 residues with rat sequence. We also validated specificity in rat spinal cord and DRG tissue based on loss of staining following preabsorption of the primary antibody with the corresponding peptide. Cell type specific antibodies included ionizing calcium binding adapter molecule 1 (IBA1; 1:1000, goat anti-rat; Abcam) and CD11b (clone OX-42, 1:500, mouse anti-rat, Bio-Rad, Hercules, CA, USA) for microglia; CD68 (clone ED1, 1:2000, mouse anti-rat, Bio-Rad, Hercules, CA, USA) for activated M1 macrophage, NeuN (mouse anti-rat NeuN) to label neurons and DβH (1:500, mouse anti-rat; Millipore, Billerica, MA, USA) for noradrenergic neurons and terminals. After incubation with primary antibodies, sections were washed three times for 10–15 min each in PBS. Subsequently, sections were incubated for 2–3 h at room temperature with Cy3- and biotin-conjugated secondary antibody (1:500; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA). After washing three times, streptavidin-Cy2 was used for 1 h at room temperature and followed with another three 10-min washes. Finally, the free-floating sections were mounted on Superfrost™ Plus slides, and then run them through 70%, 95%, 100% ETOH, and xylene for 2 min each, and cover slipped with DPX.
In situ hybridization. For ISH, we used the QuantiGene ViewRNA tissue assay (Affymetrix Panomics) according to the manufacturer's instructions, with a probe set designed by Affymetrix for hybridization to the rat β2-AR coding region. Briefly, freshly dissected lumbar spinal cord was sectioned at 16 μm, collected on Superfrost Plus slides, and stored at −80°C until use. Sections were postfixed for 1 hour in 4% formaldehyde then treated with Protease QF for 20 min and then incubated with RNA probes for 3 h at 40 °C. After hybridization, washing, preamplifier hybridization, amplifier hybridization, and hybridization with an alkaline phosphatase-labeled probe, the signal was developed via reaction with fast red. Sections were counterstained with 4’, 6-diamidino-2-phenylindole (DAPI, 1:10,000, Invitrogen, Carlsbad, CA, USA) to label all nuclei
Image analysis. Images of the spinal cord were captured on a Nikon Eclipse Ni fluorescent microscope fitted with a NIkon DS Qi1MCdigital camera using a 10× objective or an Olympus Fluoview 1200 laser scanning confocal system. Quantification for IBA1 immunostaining was performed in 5 randomly selected sections from each animal. Image analysis software (Nikon Element Basic Research version 4.3) was used to quantify immunofluorescence. As in our previous study36 the upper and lower threshold optical densities were adjusted to match positive immunoreactivity for each antibody. The image thresholds were determined and applied uniformly to all sections. A fixed area (250 × 250 μm2) was positioned in the dorsal medial and central third of the spinal cord dorsal horn (corresponding to the topography of the central projections of sensory neurons that innervate the incised region of the hindpaw) 38 and the number of pixels within the threshold value was quantified. Data are expressed as number of pixels in the area. To limit variability in immunohistochemical measurements, all groups of rats were processed on the same day and the same threshold value was applied to all images for a given antibody. We also quantified the number of p-p38-iR cellular profiles in the dorsal (laminae I-III) spinal cord. Counts were restricted to p-p38-IR cellular profiles that colocalized with the microglial marker CD11b. The individuals quantifying the images were blind to the treatment group. For analysis of macrophage in the skin of rats with incision, the density of total IBA1+ monocyte/macrophage and CD68+ activated M1 macrophage was quantified in two regions of the epidermis (200 µm × 200 µm area) and dermis (400 µm × 400 µm area) adjacent to the site of incision. The area of labeling within a defined intensity range or threshold was measured in the respective regions and reported as mean ± SEM.
Experimental design and group size. Behavioral studies consisted of an initial cohort of 24 rats randomly assigned to 4 treatment groups (clenbuterol treated DβH-saporin incision, clenbuterol treated IgG-saporin, vehicle treated DβH-saporin and vehicle treated IgG saporin, n = 6 per group) in order to examine the effects of chronic perioperative clenbuterol on trajectory of postsurgical recovery of mechanical hypersensitivity through eight days following plantar incision. Spinal cord and skin tissue were collected from this same cohort of rats to examine the effects of treatment on spinal glial activation and infiltration of macrophage at the incision site. A second cohort of NE depleted incision rats was randomly assigned to treatment (clenbuterol treated DβH-saporin, n = 16 and vehicle treated DβH-saporin, n = 8) to examine long term effects of systemic clenbuterol on postsurgical recovery of hypersensitivity over 5 weeks postoperatively. A third cohort of NE depleted incision rats was randomly assigned to one of four treatment groups (n = 5 per group) for spinal infusion of clenbuterol or vehicle. For studies examining the effects of clenbuterol treatment on spinal microglial and peripheral macrophage infiltration, spinal cord and skin tissue was collected and analyzed from the initial behavioral cohort of rats (n = 6 per group). In previous studies, we determined that microglial activation induced by plantar incision was most prominent in the L4 region. Therefore, the six rats in each group were further divided into two sets with a sample size of three for each set of markers (DβH/IBA1 and p-p38/CD11b). For qualitative examination of β2-AR-IR and β2 mRNA in rat spinal cord , tissue was collected from a total of 4 naïve rats for IHC and in situ hybridization. For qualitative examination of β2-AR-IR in the skin, hind paw skin tissue was obtained from naïve (n = 4) and rats two days following plantar incision (n = 4), a time point shown to coincide with maximal monocyte/macrophage infiltration following plantar incision in previous rodent studies.46 For immunohistochemical and behavioral analysis in treated rats, we did not define a priori a minimum biologically important difference and determine group size based on a formal power analysis or simulations. Rather, we used group sizes typical for these kind of experiments.3,47
Data analysis. The primary outcome measure was reduction in mechanical hypersensitivity over time as determined from modeled trajectories of postsurgical mechanical paw withdrawal thresholds in the ipsilateral paw of rats with plantar incision. Longitudinal measures of mechanical withdrawal threshold were modeled using growth curve analysis as previously described.47 Linear and quadratic models were employed to model individual change over time and the linear model was chosen based on Bayesian Information Criterion (BIC) fit statistic. This analysis gives rise to intercept (initial pain at time 0) and slope (linear rate of change in pain measure) estimates. The intercept and slope terms can vary across individual rats (random effects) and as a function of treatment group or condition (fixed effects) allowing us to examine the ability of interventions to impair or improve several aspects of postoperative pain trajectory. Bonferroni correction was made for the within group models considering p < 0.025 to be statistically significant. Group averaged trajectories are presented with 95% confidence limits and were analyzed using SAS® v9.4 software (SAS Institute Inc., Cary, NC, USA). Mechanical withdrawal thresholds were also analyzed using a two-way repeated measures analysis of variance (RM-ANOVA) where group (DβH-saporin or IgG-saporin) and treatment (clenbuterol or vehicle) were considered as independent variables in the model. Bonferroni post hoc correction was also made here to adjust for multiple comparisons. Immunohistochemical data was analyzed using one or two-way ANOVA followed by the Student Newman Keuls (SNK) post hoc test for multiple comparisons among different groups. Immunohistochemical data are presented as mean ± SEM and analyzed using Sigma Plot (Version 12.0; Systat Inc., San Jose, CA). All reported P values are two-tailed and a P value of less than 0.05 is accepted for statistical significance in the immunohistochemical analysis.
Results
Effects of perioperative systemic and spinal β2-AR agonist clenbuterol on resolution of mechanical hypersensitivity in rats following plantar incision
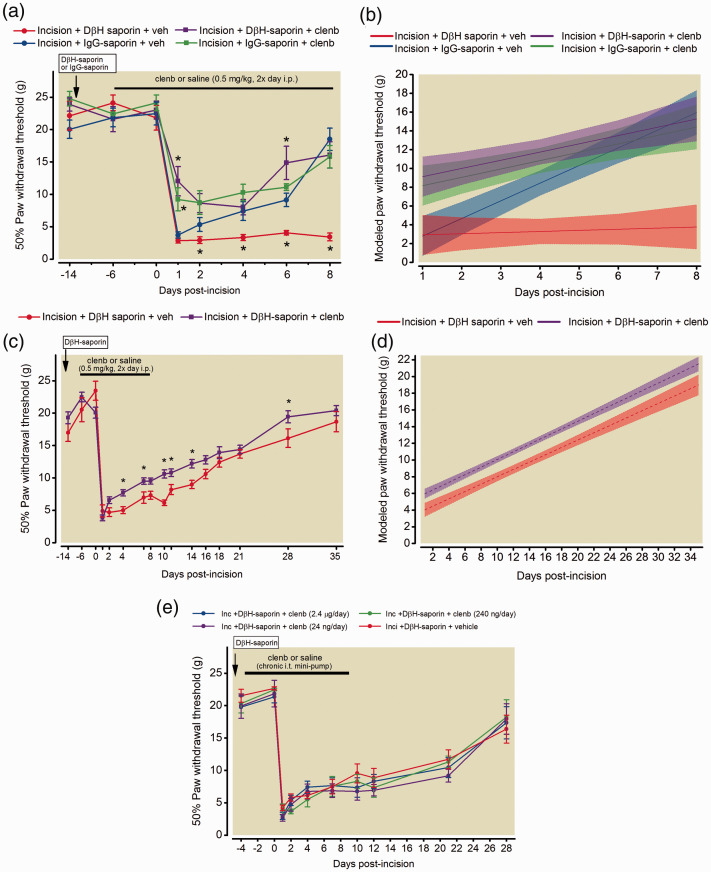
Systemic administration of clenbuterol for six days prior to surgery did not significantly alter baseline thresholds in any of the groups (22.47 ± 1.8 Incision/IgG-sap/vehicle; 21.81 ± 1.9 Incision/DβH-sap/Vehicle 24.12 ± 1.2 Incision/IgG-sap/clenbuterol, 21.81 ± 1.9 Incision/DβH-sap/Vehicle; p = 0.98, Figure 1(a)). Continued systemic administration of clenbuterol for 8 days post-incision significantly reduced postsurgical hypersensitivity in both DβH-saporin treated and control IgG-saporin treated rats one day following incision (Figure 1(a)). DβH-saporin incision rats administered with vehicle had greater mechanical hypersensitivity compared to IgG-saporin incision vehicle treated rats on days 2,4,6, and 8 following surgery (P < 0.001). This increased acute mechanical hypersensitivity from day 2 to 8 post-incision was not observed in DβH-saporin incision rats administered with clenbuterol. We used mixed effects growth curve modeling of longitudinal paw withdrawal thresholds to examine effects of clenbuterol on postoperative pain trajectories. Postoperative trajectories of DβH-saporin incision rats treated with vehicle displayed lower intercept (greater initial hypersensitivity) and nearly flat slope indicating a slower rate of recovery during the first eight days after incision compared to control IgG-saporin incision rats administered vehicle (Figure 1(b), Table 1) similar to our previous study.36 Trajectories of DβH-saporin and IgG-saporin incision rats treated with clenbuterol displayed greater intercepts (less initial hypersensitivity) and flat slopes compared to control IgG-saporin incision rats administered vehicle (Figure 1(b), Table 1) indicating an acute attenuation of mechanical hypersensitivity over several days.
Figure 1.
Effects of clenbuterol on resolution of mechanical hypersensitivity in a rat model of persistent postsurgical pain. Fourteen days prior to plantar incision surgery, rats received spinal DβH-saporin (5 µg/10 µl, i.t.) to deplete spinal noradrenergic fibers or IgG-saporin (5 µg/10 µl, i.t.) as a control. Male Sprague Dawley rats were administered clenbuterol (0.5 mg/kg, 2×/day, i.p.) or saline beginning six days prior to surgery and mechanical paw withdrawal thresholds were assessed longitudinally in the ipsilateral paw for eight days postoperatively (a). Data are expressed as mean ± SEM (n = 6 per group, Two way RM-ANOVA with Bonferroni comparisons *P < 0.05 within time point versus IgG-saporin incision + vehicle. Longitudinal behavioral data beginning at day 1 following incisions was also analyzed using mixed effects growth curve modeling to examine differences in acute postoperative pain trajectories (b). Data expressed as group averaged mean trajectories with 95% confidence intervals. Modeled acute postoperative pain trajectories indicated significant differences in initial mechanical hypersensitivity [(Group) p = 0.003] and slope [(Group x Time) p < 0.0001] comparing DβH-saporin vehicle treated rats to IgG-saporin vehicle treated rats. Clenbuterol treated DβH saporin rats had significantly greater intercept [(Group) p = 0.01] but similar slope or rate of recovery [(Group x Time) p = 0.053] compared to DβH-saporin incision vehicle treated rats. In a separate cohort of DβH-saporin incision rats, long term effects of clenbuterol treatment were assessed until 35 days postoperatively (c). Data are expressed as mean ± SEM (Two-way RM-ANOVA with Bonferroni comparisons *P < 0.05 within time point versus DβH-saporin incision + vehicle; n = 8 vehicle threated group; n = 16 clenbuterol treated group). Modeled postoperative pain trajectories indicated significant differences in degree of mechanical hypersensitivity [(Group) p = 0.0014] but similar slopes or rate of recovery over 35 days postoperatively [(Group x Time) p = 0.63] compared to vehicle treated rats (d). Spinal administration of varying doses of clenbuterol for 14 days by mini-osmotic pump beginning four days prior to surgery in DβH-saporin incision rats (f). Data are expressed as mean ± SEM. Two-way RM ANOVA indicate effect of time: p < 0.001 but not dose/group: p = 0.600 or interaction: p = 0.995.
Table 1.
Short term study: Growth curve modeling of ipsilateral mechanical withdrawal thresholds in rats with plantar incision over 8 days postoperatively.
| Predictor | Parameter | Estimate | (Lower bound, upper bound) | p |
|---|---|---|---|---|
| Entire population | Intercept | 2.803 | (0.013, 5.592) | 0.049 |
| Entire population | Slope | 1.880 | (1.404, 2.356) | <0.001 |
| Group | Intercept | |||
| DβH-saporin + clenb | 6.320 | (2.375, 10.264) | 0.003 | |
| IgG-saporin + clenb | 5.370 | (1.425, 9.314) | 0.010 | |
| DβH-saporin + vehicle | 0.124 | (–3.820, 4.069) | 0.948 | |
| IgG-saporin + vehicle | REF | |||
| Group x Time | Slope | |||
| DβH-saporin + clenb | –1.00 | (–1.675, –.0.330) | 0.004 | |
| IgG-saporin + clenb | –0.988 | (–1.660, –0.315) | 0.005 | |
| DβH-saporin + vehicle | –1.758 | (–2.431, –1.085) | <0.0001 | |
| IgG-saporin + vehicle | REF |
Comparison of growth curve parameters for NE depleted (DβH-saporin) and intact (IgG-saporin) rats following plantar incision and treatment with clenbuterol (0.5 mg/kg, i.p. 2× day) or saline vehicle from 6 days prior to incision through 8 days postoperatively. Growth curve analysis of paw withdrawal thresholds (Day 1–8) best fit a linear model giving rise to an intercept (hypersensitivity at time 0), slope (linear rate of change in hypersensitivity). clen = clenbuterol, REF = reference value.
In a separate cohort of rats, we assessed mechanical hypersensitivity for several weeks after discontinuing drug to examine long term effects of clenbuterol on recovery in NE depleted DβH-saporin incision rats (Figure 1(c)). Similar to the shorter time course study, we observed reduced mechanical hypersensitivity for several days after incision (Figure 1(c), Two-way RM ANOVA, group x time: F (15,330) = 2.28, p = 0.004). DβH-saporin incision rats administered vehicle had greater mechanical hypersensitivity compared to DβH-saporin incision clenbuterol treated rats on days 4,7,10, 11, 14 and 28 following surgery (P < 0.05). Similar to the above results, postoperative trajectories of clenbuterol treated DβH-saporin incision rats had a significantly greater intercept or reduced initial mechanical hypersensitivity but the slope or rate of recovery over 5 weeks was not significantly different compared to vehicle treated DβH-saporin incision rats (Figure 1(d), Table 2). To determine if spinal delivery of clenbuterol produced similar reductions in mechanical hypersensitivity in DβH-saporin incision rats, we chronically infused varying doses of clenbuterol or saline directly into the spinal cord of rats following DβH-saporin treatment and four days prior to plantar incision. Prior to incision, spinal clenbuterol did not alter baseline mechanical withdrawal thresholds (22.66 ± 0.2 g Incision/DβH-sap/vehicle; 21.35 ± 0.9 g Incision/DβH-sap/clenbuterol – 2.4 µg/day; 24.47 ± 0.2 g Incision/DβH-sap/clenbuterol-240 ng/day; 21.86 ± 2.0 g Incision/DβH-sap/clenbuterol-24 ng/day; p = 0.97, Figure 1(e)). Unlike systemic administration, continued spinal infusion for ten days postoperatively did not significantly reduce mechanical hypersensitivity in rats with incision at any dose tested (Figure 1(e)).
Table 2.
Long term study: Growth curve modeling of ipsilateral mechanical withdrawal thresholds in rats with plantar incision over 5 weeks postoperatively.
| Predictor | Parameter | Estimate | (Lower bound, upper bound) | p |
|---|---|---|---|---|
| Entire population | Intercept | 4.031 | (3.140, 4.921) | <0.0001 |
| Entire population | Slope | 0.440 | (0.383, 0.498) | <0.0001 |
| Group | Intercept | |||
| DβH-saporin + clenb | 1.926 | (0.835, 3.016) | 0.001 | |
| DβH-saporin + vehicle | REF | |||
| Group x Time | Slope | |||
| DβH-saporin + clenb | 0.017 | (-0.054, 0.088) | 0.635 | |
| DβH-saporin + vehicle | REF |
Comparison of growth curve parameters for spinally NE depleted (DβH-saporin) and intact rats following plantar incision and treatment with clenbuterol (0.5 mg/kg, i.p. 2× day) or saline vehicle from 6 days prior to incision through 8 days postoperatively. Growth curve analysis of paw withdrawal thresholds (Day 1–35) best fit a linear model giving rise to an intercept (hypersensitivity at time 0), slope (linear rate of change in hypersensitivity). clen = clenbuterol, REF = reference value.
Impact of perioperative systemic β2 adrenergic receptor agonist clenbuterol on spinal microglial activation in rats with incision
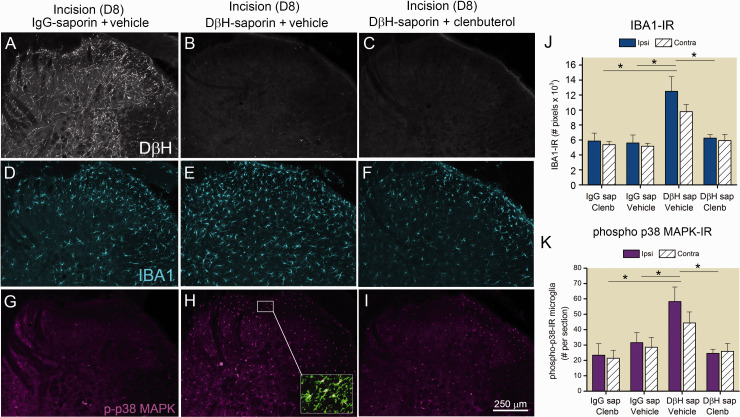
In the same rats used for short term behavioral studies (Figure 1(a) and (b)), we examined the spinal cord of rats eight days following incision to determine if clenbuterol treatment impacted microglial activation. We confirmed spinal depletion of noradrenergic terminals in DβH-saporin treated rats based on nearly complete loss of labeling for DβH (Figure 2(b) and (c)) compared to control IgG-saporin treated rats (Figure 2(a)). We observed a near two-fold greater density of IBA1-IR (Figure 2(d) to (f) and (j)) and p38-IR microglia (Figure 2(g) to (i) and (k)) in the medial aspects of the dorsal spinal cord of DβH-saporin incision vehicle treated rats compared to IgG-saporin incision vehicle treated rats similar to our previous results.44 Clenbuterol treatment prevented the increased IBA1-IR (Figure 2(f) and (j)) and p38-IR microglia (Figure 2(i) and (k)) in DβH-saporin treated incision rats.
Figure 2.
Effects of β2 adrenergic receptor (AR) agonist on enhanced spinal microglia activation in a rat model of persistent postoperative pain. Sections of spinal cord were collected from rats 8 days following plantar incision and following treatment with DβH-saporin to deplete spinal noradrenergic terminals or control IgG-saporin. Rats were chronically administered clenbuterol (0.5 mg/kg, 2×/day, i.p.) or saline vehicle 6 days prior to and for 8 days after plantar incision. Depletion of spinal noradrenergic fibers was verified immunohistochemically with an antibody against dopamine β hydroxylase (DβH, (a)–(c)). Representative confocal images of IBA1-IR (blue, (d)–(f)) and phospho-p38 MAPK-IR (purple, (g)–(i)) in the ipsilateral spinal cord of incision rats. Localization of p38 MAPK in microglia was confirmed by colocalization with an antibody against the cell surface antigen CD11b (green, inset in (h)). Quantification of IBA1-IR in ipsilateral and contralateral spinal cord of rats with incision (j). Data represent mean ± SEM, n = 3 rats per group. Two way ANOVA indicated effect of group: p < 0.001 but not side p = 0.184 or interaction: p = 0.59 with SNK pairwise comparisons *p < 0.001 versus Incision+ DβH-saporin+ vehicle. Quantification of phospho-p38 MAPK microglial in the ipsilateral and contralateral spinal cord of rats with incision (k). Data represent mean ± SEM, n = 3 rats per group. Two way ANOVA indicate effect of group: p = 0.002 but not side p = 0.398 or interaction: p = 0.67 with SNK pairwise comparisons * p < 0.005 versus Incision+ DβH-saporin+ vehicle.
Impact of perioperative systemic β2 -AR agonist clenbuterol on activation of skin resident monocytes/macrophage
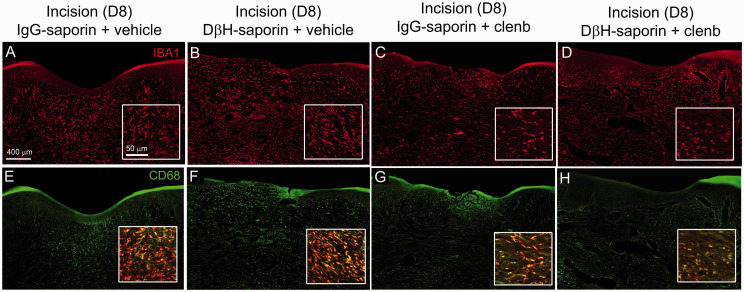
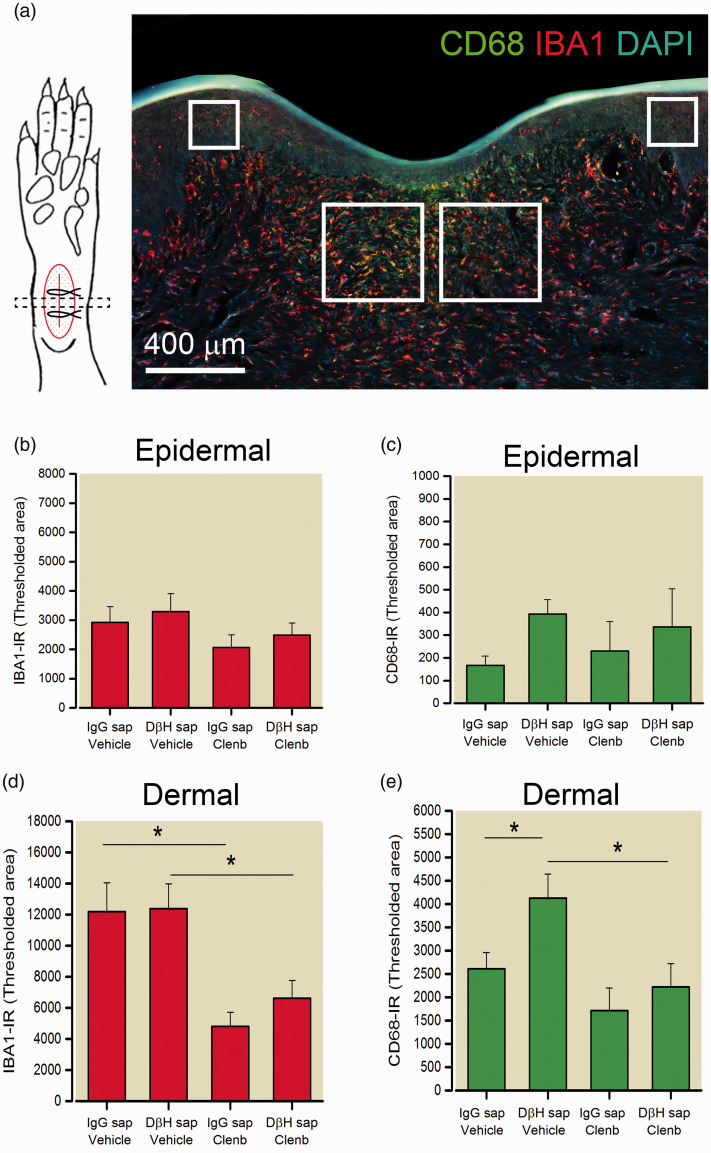
As previous studies suggest that β2-AR activation has immunomodulatory and anti-inflammatory effects on macrophage, we examined the distribution (Figure 3) and quantified the immunodensity (Figure 4) of IBA1 positive monocyte/macrophage and activated CD68 positive macrophage in the epidermal and dermal skin layers eight days following incision in the plantar aspects of the ipsilateral paw of rats from the short-term behavioral studies above. Both DβH-saporin and IgG saporin treated rats administered saline had a high density of IBA1-IR monocytes/macrophage in the dermal region adjacent to the incision site (Figure 3). We observed significant reduction in the levels of IBA1-IR in the dermis of clenbuterol treated rats (Figure 3(a) to (d); Figure 4(d)). Similarly, the density of CD68-IR macrophage was reduced in the dermis of clenbuterol treated rats (Figure 3(e) to (h); Figure 4(e)). CD68-IR was slightly greater in vehicle treated DβH-saporin rats versus vehicle treated IgG-saporin treated rats. The levels of IBA-IR and CD68-IR observed in the epidermal layers of the skin were not significantly different between groups (Figure 4(b) and (c)).
Figure 3.
Confocal images of IBA1-IR monocytes/macrophage and activated CD68-IR macrophage at the incision site in treated rats. Transverse sections of skin were collected from rats 8 days following plantar incision. Sections were labeled with an antibody against IBA1 (red, (a)–(d)) to label all monocytes/macrophage and an antibody against CD68 (green, (e)–(h)) to label M1 or activated macrophage in DβH-saporin and IgG saporin incision rats treated chronically with clenbuterol (0.5 mg/kg 2× day) or vehicle from 6 days prior to 8 days after surgery. Higher magnification insets were obtained from the dermal layer adjacent to the incision site to more clearly show density and morphology of IBA1-IR macrophage ((a)–(d)) and colocalization of CD68 with IBA1-IR in macrophage ((e)–(h)). Note reduced density of IBA1-IR cellular profiles in the dermal skin layer of clenbuterol treated DβH-saporin and IgG saporin incision rats.CD68-IR was also reduced in the dermal skin layer of clenbuterol treated DβH-saporin and IgG saporin incision rats compared to vehicle controls.
Figure 4.
Quantification of macrophage density and activation at the incision site in treated rats. (a) Illustration of approach for sampling and analyzing transverse skin sections including representative image of IBA1-IR (red), CD68-IR (green) and DAPI positive cellular nuclei (blue) in a transverse section of skin eight days following plantar incision. Immunodensity of IBA1-IR ((b) and (d)) and CD68-IR ((c) and (e)) was quantified in two regions of the epidermis (200 µm2 × 200 µm2 area) and dermis (400 µm2 × 400 µm2 area) indicated by boxes adjacent to the site of incision. The area of labeling within a defined intensity range or threshold was measured in the respective regions and reported as mean ± SEM. n = 6 rats per group. For IBA1 dermal: Two-way ANOVA with SNK pairwise comparisons indicate main effect of treatment: p < 0.001 but not group p = 0.489 **p < 0.002, * p < 0.05 versus vehicle. For CD68 dermal: Two-way ANOVA with Bonferroni contrasts indicates main effect of treatment: p = 0.007 and group p = 0.043. * p < 0.05 versus vehicle. # p < 0.05 versus DβH-saporin.
Distribution of β2-AR in the spinal cord and skin of naïve and incision rats
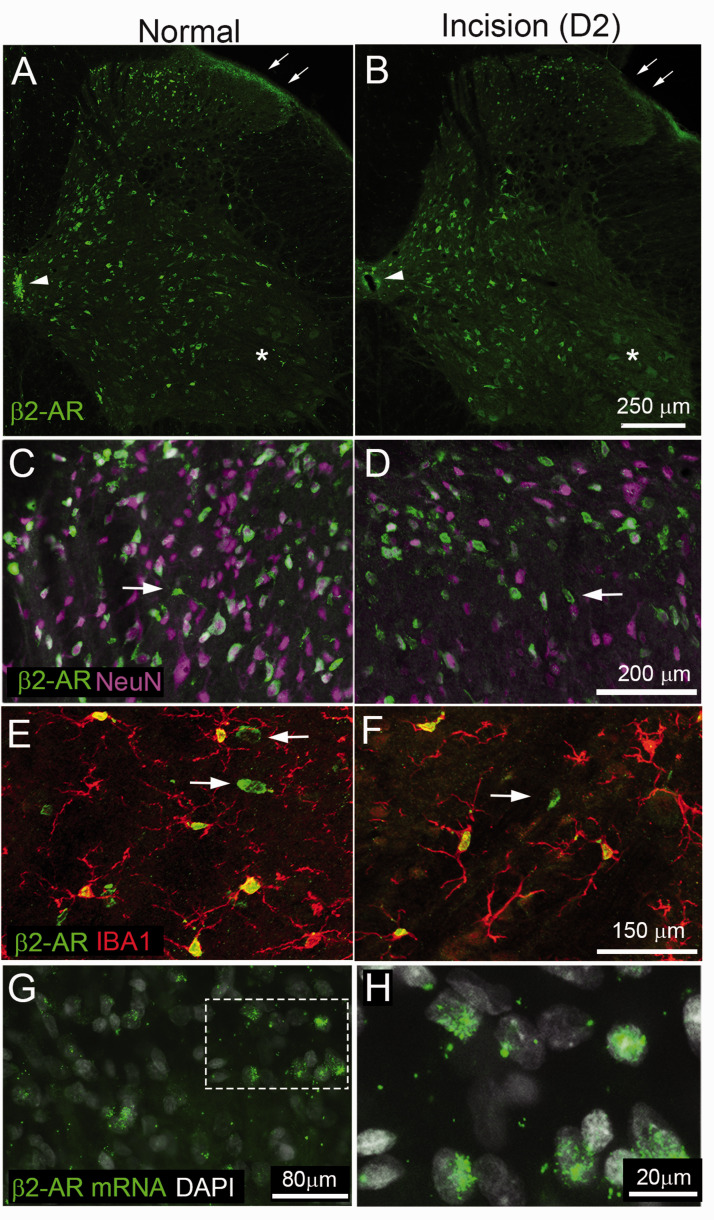
We used IHC and in situ hybridization to confirm the cellular localization of β2-ARs in the spinal cord of rats under naïve conditions and two days following plantar incision. Within the naïve rat spinal cord, we observed β2-AR-IR in a subpopulation of neuronal soma in the dorsal and ventral horn as well as fibers within mediolateral aspects of the superficial dorsal horn (Figure 5(a) and (b)). β2-AR-IR was also present in ependymal cells in the vicinity of the central canal (Figure 5(a) and (b), arrowhead). β2-AR-IR was not present in motor neurons within the spinal cord (Figure 5(a) and (b), asterisk). In higher magnification confocal images, we observed colocalization of β2-AR-IR in neurons (NeuN-IR) and in a few non-neuronal cells (Figure 5(c) and (d)). Non-neuronal labeling in the spinal cord colocalized primarily with the microglial marker IBA1 but not GFAP (data not shown) (Figure 5(e) and (f)). β2-AR mRNA was associated with a subset of nuclei within the dorsal spinal cord of naïve rats (Figure 5(g) and (h)) in a similar pattern as immunoreactivity.
Figure 5.
Beta 2-adrenergic receptor immunoreactivity in the spinal cord of rats under naïve conditions and two days following plantar incision. Transverse section of L4 spinal cord of rat reacted with antibody against β2 adrenergic receptor ((a), β2-AR, green). There is a high density of immunoreactivity in cellular profiles throughout dorsal and ventral horn. There is also dense immunoreactivity in axon terminals within the lateral portion of the superficial laminae (arrow) and ependymal cells in the vicinity of the central canal (Arrowhead). Note lack of staining for β2-AR in motor neurons within the ventral horn (asterisk). Higher magnification confocal images show β2-AR-IR ((c), green) is present in a subpopulation of neurons ((d), NeuN, purple) in the dorsal spinal cord. Most β2-AR-IR cellular profiles colocalized with NeuN with the exception of a few non-neuronal profiles with morphology typical of microglia (arrows, (c)–(f)). β2-AR-IR non-neuronal cellular profiles in the spinal cord colocalized with the microglial marker IBA1 (red, (e) and (f)). Arrows in F indicate IBA1 negative neuronal cellular profiles. Representative images of β2 mRNA and DAPI in the dorsal spinal cord (g) with high power image showing colocalization with a subset of nuclei (h).
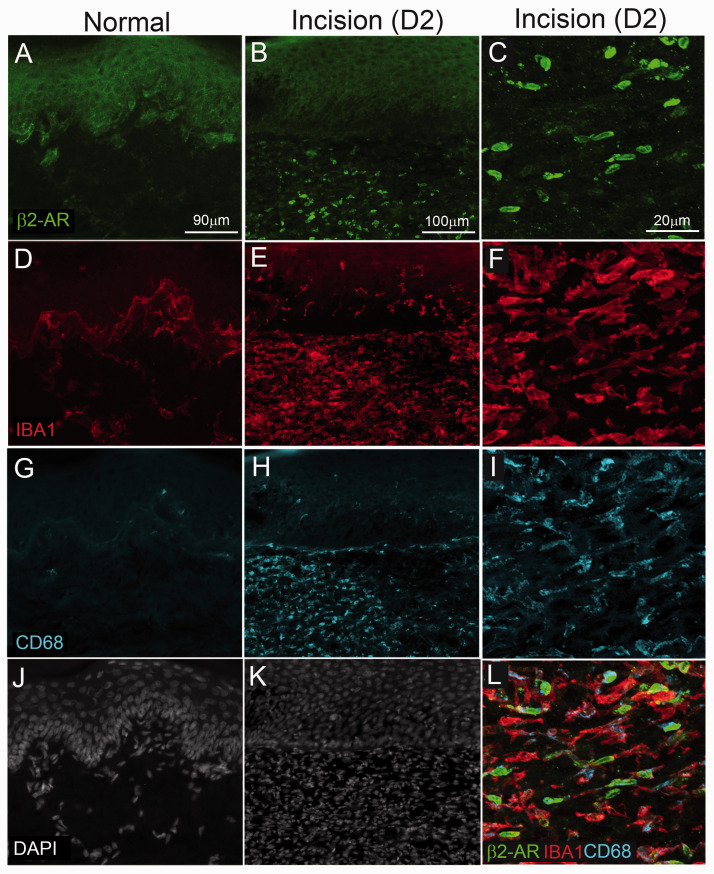
We also used IHC to confirm the cellular localization of β2-AR IR in the skin of rats under naïve conditions and two days following plantar incision. In normal rats, β2-AR-IR was prominent in keratinocytes within the epidermis but not present on resident IBA1-IR monocytes/macrophages or the few detectable CD68-IR activated macrophage located at the epidermal/dermal interface (Figure 6(a), (d), (g), and (j)). Following plantar incision, β2-AR-IR was present on keratinocytes and a subpopulation of IBA1-IR monocyte/macrophage in the vicinity of the incision site (Figure 6(b), (e), (h), and (k)). The majority of β2-AR cells in the skin co-expressed IBA1-IR and CD68-IR (Figure 6(c), (f), (i), and (l)).
Figure 6.
β2-adrenergic receptor immunoreactivity (β2AR-IR) in hindpaw of rats under naïve conditions and following plantar incision. Skin sections were obtained from the hind paw of naïve rats and incision rats two days following surgery. Sixteen-μm-thick sections were stained with antibodies against β2AR-IR (green, (a)–(c)), IBA1 (red, (d)–(f)) to label all monocytes/and macrophage, CD68 (blue, (g)–(i)) for activated M1 macrophage) and DAPI ((j) and (k)) to label all nuclei. β2-AR IR was present in keratinocytes of both naïve and incision rats. Two days following plantar incision there were increased β2-AR IR cellular profiles in predominantly the dermal layers of the skin. Higher magnification confocal images ((c), (f), (i), and (l)) indicate colocalization of β2-AR in IBA1+ cells and a subset of which express CD68-IR. Note in naïve skin IBA1-IR was primarily present at the epidermal/dermal interface and had reduced dermal cellularity (DAPI+ cells) compared to skin adjacent to the wound in incision rats.
Discussion
In the current study, our key finding was that perioperative administration of clenbuterol attenuated post-incisional mechanical hypersensitivity and altered postoperative trajectories particularly in rats with impaired spinal noradrenergic tone. The ability of clenbuterol to attenuate mechanical hypersensitivity was most pronounced acutely within days of plantar incision but persisted throughout the recovery period. Systemic clenbuterol reduced microglial activation in the spinal cord and macrophage infiltration at the site of incision. In contrast, direct spinal infusion of varying doses of clenbuterol over two weeks failed to reduce mechanical hypersensitivity in rats with incision suggesting that effects of systemic β2 stimulation on mechanical hypersensitivity were peripherally mediated. We also conducted secondary analysis characterizing the cell type specific distribution of β2-ARs within the spinal cord and skin of rats. In the rat spinal cord, β2-AR IR was present on ependymal cells, microglia and a subpopulation of neurons in normal and incision rats. Within the skin, β2-AR IR was present in keratinocytes and increased on infiltrating macrophage at the site of incision following plantar incision.
Distribution and cell type expression of β2-AR activation in spinal cord of normal and incision rats
β2-ARs have been described throughout the peripheral and central nervous system.48–50 In peripheral tissue, β2-ARs have been localized in keratinocytes,32,51 myeloid cells including macrophage and dendritic cells,52,53 satellite glial cells25and sensory neurons.30,54–56 In the spinal cord, we observed β2-AR-IR on subpopulations of neurons, microglia but not astrocytes in the normal and incision rats. Previous studies have reported β2-AR mRNA in primary spinal microglia19 and in cultured astrocytes.57 β2-AR-IR has previously been reported in vivo on astrocyte process within the visual cortex of rats based on IHC with custom antibodies.58 It is not clear why we failed to observe clear localization in astrocytes. Prior studies used a primary antibody that targeted a C-terminal portion of β2-AR selectively expressed in astrocytes, whereas the current study used an antibody that targets an N-terminal sequence.58 Cell type selective differences in amino acid sequences within regions of β2-AR might explain differences between ours and this previous study. We observed β2-AR-IR on a subpopulation of neurons in the dorsal horn of the spinal cord. Previous studies have reported β2-AR mRNA and IR in neurons and ependymal cells within the spinal cord of rats50,59 consistent with our results. Generally, we did not observe qualitative differences in spinal cord β2-AR-IR between naïve or rats two days following plantar incision a time point when mechanical hypersensitivity was at a maximum. Within the skin, we observed immunoreactivity for β2-AR in keratinocytes of naïve and incision rats. Notably we observed an increaseof β2-AR-IR on immune cells around the site of incision two days postoperatively. These cells predominantly colocalized with IBA1 and CD68 suggesting that they were myeloid lineage monocytes and macrophage and a likely site of action of β2-AR agonists. An important future direction of research will be to examine a more complete time course of alterations in β2-ARs following incision on discrete subsets of spinal neurons and glial cells and their function in nociceptive processing.
Perioperative administration of clenbuterol has immunomodulatory effects in the skin and spinal cord of rats with incision
β2-AR stimulation contributes to a variety of immune related responses following surgical incision including wound healing,60,61neovascularization62 and potentially immune cell mediated sensitization of sensory neurons as several studies in rodents provide evidence that monocytes/macrophage at the site of injury contribute to mechanical hypersensitivity following plantar incision.46,63 In the current study, we observed a nearly two-fold reduction in the density of macrophage near the site of incision following clenbuterol treatment. The mechanism responsible for this effect was not examined, however β2 AR stimulation of activated macrophage in vitro reduces the synthesis and release of CCL2,14 a well-known chemotactic molecule involved In trafficking of immune cells to sites of injury. β2-AR stimulation also has well known ability to reduce the release of proinflammatory mediators (TNFα, IL-6 and IL1β) 11,12,14,64 and increase the release of anti-inflammatory mediators (IL-10) in activated macrophage.13,15 Several of these pro-inflammatory mediators have an established role in driving peripheral sensitization by direct actions on sensory neurons65 and IL-10 derived from monocytes/macrophage has been shown to reduce mechanical hypersensitivity under inflammatory conditions.66 Recent studies in mice demonstrate that genetic knockdown of NOD-like receptor 3 (NLRP3) inflammasome signaling reduces infiltration of innate immune cells, wound IL-1β levels and mechanical hypersensitivity in male but not female mice.63 As β2-AR agonists potently inhibit NLRP3 inflammasomes and IL1β release in activated macrophage,64 it is plausible that the reduced mechanical hypersensitivity in clenbuterol treated rats could be due to reducing infiltration and anti-inflammatory effects on macrophage at the site of incision.
Similar to our previous results, we observed an enhanced microglial response to plantar incision in rodents with disrupted noradrenergic inhibition36 evident as increased cellular levels of IBA1 and number of microglia that express p-p38 MAPK. Microglial activation and p38 signaling have been shown to enhance excitatory neurotransmission and contribute to central sensitization in models of post-incisional pain.67–69 β2-AR agonists suppress microglial activation in vitro and in vivo. Spinal terbutaline reduced mechanical allodynia in mice with partial sciatic nerve ligation by inhibiting phosphorylation of microglial p38 MAPK and astrocyte Jun N-terminal kinase (JNK) signaling.28 The reductions in mechanical hypersensitivity we observed with systemic administration of clenbuterol could be in part due to central effects on spinal microglia since clenbuterol readily crosses the blood brain barrier.70 However, based on the lack of effect of chronic spinal β2 AR stimulation on mechanical hypersensitivity over a wide dose range spinal β2 AR stimulation may not be sufficient to attenuate mechanical hypersensitivity in the current model. It is possible that reductions in microglial activation observed with systemic clenbuterol may be indirect due to reduced activation of sensory neurons and spinal release of neurotransmitters that drive microglial activation. More studies are needed to determine the precise cellular mechanisms involved in the anti-allodynic effects of clenbuterol in the current study. Studies utilizing cell type specific knockdown of β2-ARs71 or peripherally restricted β2 agonists may shed light on the site of action and cellular effects of β2 agonists in various postsurgical pain states.
Impact of clenbuterol on postoperative trajectory and speed of recovery in rats with plantar incision
Clinically, acute pain postoperative trajectories are increasingly being examined as a means to identify patients at risk of developing persistent pain and poor functional recovery. A few studies indicate that high intensity non-resolving pain scores over days and weeks may be predictive of greater pain and disability months later72,73 and therapeutic interventions that alter acute pain trajectories and speed recovery may improve long term outcomes.74–76 For this reason, we examined post-incisional trajectories of mechanical hypersensitivity as our primary outcome in the current study. Similar to our previous results, we observed that depletion of spinal NE using a targeted intrathecal anti-DβH-saporin toxin delayed resolution of mechanical hypersensitivity following incision for several weeks evident as a lower intercept and a slower resolving flat slope of recovery compared to non-depleted incision rats administered control IgG-saporin.36 When we modeled paw withdrawal thresholds over eight days or several weeks postoperatively, we observed a clear attenuation of mechanical hypersensitivity at early time points in clenbuterol compared to vehicle treated rats; however, the slope of recovery between clenbuterol and vehicle treated rats was similar. There are several potential explanations for the failure of clenbuterol to speed recovery in rats with impaired noradrenergic tone. First, β2-AR stimulation may be insufficient to prevent the enhanced spinal sensitization that occurs with the loss of descending noradrenergic inhibition. It is well established that presynaptic and postsynaptic α2-ARs are required for inhibitory effects of norepinephrine in the spinal cord.77 In our previous study, we demonstrated a critical role for spinal α2-AR in both the initial hypersensitivity and resolution of incisional pain as chronic administration of the α2-AR antagonist atipamezole dose dependently increased both the magnitude and duration of mechanical hypersensitivity.36 Several recent studies demonstrate an impairment of descending spinal noradrenergic inhibition in rats with chronic spinal nerve ligation (seven weeks after surgery) and therapies including antidepressants like duloxetine that increase spinal noradrenaline levels can restore the impaired descending noradrenergic system and reverse mechanical hypersensitivity. This restoration requires α2-ARs.78 Secondly, while β2-AR stimulation showed clear spinal and peripheral immunomodulatory effects eight days after incision this treatment regimen may be insufficient to produce lasting effects during later stages of recovery. In our previous study, we observed an enhanced microglial activation in the spinal cord of NE depleted rats as late as three weeks following incision.36 A longer treatment regimen may be required in NE depleted rats to effectively suppress the prolonged spinal inflammatory response in this model. Alternatively, chronic administration of clenbuterol may cause desensitization of β2ARs on immune cells limiting there anti-inflammatory and anti-allodynic effects during later stages of recovery. Additionally, the acute anti-inflammatory effect of clenbuterol may be maximal during the early immune response to surgery when peripheral macrophage activation is most pronounced, but these immune related mechanisms may be less relevant to the speed of recovery during the more chronic phase. In recent studies, behavioral resolution of mechanical hypersensitivity parallels closely key alterations in peripheral sensory neuron physiology.79
A key aspect of future studies will be to determine the influence of clenbuterol or other β2-AR agonists on the physiological response of sensory neurons particularly in NE depleted rats at various stages of recovery.
Limitations and conclusions
There are some limitations to the current study. First, we focus solely on mechanical hypersensitivity as an outcome. In our previous study, we observed minimal effects of disrupting spinal noradrenergic input on spontaneous guarding, a measure of ongoing pain at rest following incision. This suggests clinically β2-AR agonists may be more effective at reducing hypersensitivity around surgical wounds rather than ongoing pain. Additionally, the current anatomical and behavioral studies are limited to male Sprague Dawley rats. As mentioned above, preclinical studies have described sex dependent differences in mechanisms of mechanical hypersensitivity associated with plantar incision in mice. Future studies are needed to determine if β2-AR agonists have equivalent analgesic efficacy in females and in other species. Nonetheless, the current results expand our understanding of the role of β2-ARs in mechanical hypersensitivity associated with plantar incision and point to the potential importance of targeting β2-ARs to modulate the innate immune response and reduce mechanical hypersensitivity associated with surgical injury.
Footnotes
Authors' Note: Carlos Eduardo Morado-Urbina is now affiliated with Department of Physiology and Pharmacology and Center for Molecular Medicine, Karolinska Institutet, Stockholm, Sweden.
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: In the past 36 months JCE has consulted to Adynxx (San Francisco, CA, USA) and TEVA Pharmaceutical Industries (North Wales, PA, USA) regarding preclinical and clinical analgesic development of pharmaceuticals not related to the current publication. The remaining authors declare no conflicts of interest.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work is supported in part by grant R01GM099863 to CMP and P01GM113852 to JCE from the National Institute of Health, Bethesda, MD.
ORCID iD: Christopher M Peters https://orcid.org/0000-0001-5268-9506
References
- 1.Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet 2006; 367: 1618–1625. [DOI] [PubMed] [Google Scholar]
- 2.De Felice M, Sanoja R, Wang R, Vera-Portocarrero L, Oyarzo J, King T, Ossipov MH, Vanderah TW, Lai J, Dussor GO, Fields HL, Price TJ, Porreca F. Engagement of descending inhibition from the rostral ventromedial medulla protects against chronic neuropathic pain. Pain 2011; 152: 2701–2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peters CM, Hayashida K, Suto T, Houle TT, Aschenbrenner CA, Martin TJ, Eisenach JC. Individual differences in acute pain-induced endogenous analgesia predict time to resolution of postoperative pain in the rat. Anesthesiology 2015; 122: 895–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yarnitsky D, Crispel Y, Eisenberg E, Granovsky Y, Ben-Nun A, Sprecher E, Best LA, Granot M. Prediction of chronic post-operative pain: pre-operative DNIC testing identifies patients at risk. Pain 2008; 138: 22–28. [DOI] [PubMed] [Google Scholar]
- 5.Haight ES, Forman TE, Cordonnier SA, James ML, Tawfik VL. Microglial modulation as a target for chronic pain: from the bench to the bedside and back. Anesth Analg 2019; 128: 737–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gaudilliere B, Fragiadakis GK, Bruggner RV, Nicolau M, Finck R, Tingle M, Silva J, Ganio EA, Yeh CG, Maloney WJ, Huddleston JI, Goodman SB, Davis MM, Bendall SC, Fantl WJ, Angst MS, Nolan GP. Clinical recovery from surgery correlates with single-cell immune signatures. Sci Transl Med 2014; 6: 255ra131–255ra131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ji RR, Xu ZZ, Gao YJ. Emerging targets in neuroinflammation-driven chronic pain. Nat Rev Drug Discov 2014; 13: 533–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fragiadakis GK, Gaudilliere B, Ganio EA, Aghaeepour N, Tingle M, Nolan GP, Angst MS. Patient-specific immune states before surgery are strong correlates of surgical recovery. Anesthesiology 2015; 123: 1241–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richebe P, Capdevila X, Rivat C. Persistent postsurgical pain: pathophysiology and preventative pharmacologic considerations. Anesthesiology 2018; 129: 590–607. [DOI] [PubMed] [Google Scholar]
- 10.Theron AJ, Steel HC, Tintinger GR, Feldman C, Anderson R. Can the anti-inflammatory activities of beta2-agonists be harnessed in the clinical setting? Drug Des Devel Ther 2013; 7: 1387–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Izeboud CA, Mocking JA, Monshouwer M, van Miert AS, Witkamp RF. Participation of beta-adrenergic receptors on macrophages in modulation of LPS-induced cytokine release. J Recept Signal Transduct Res 1999; 19: 191–202. [DOI] [PubMed] [Google Scholar]
- 12.Izeboud CA, Monshouwer M, van Miert AS, Witkamp RF. The beta-adrenoceptor agonist clenbuterol is a potent inhibitor of the LPS-induced production of TNF-alpha and IL-6 in vitro and in vivo. Inflamm Res 1999; 48: 497–502. [DOI] [PubMed] [Google Scholar]
- 13.Keranen T, Hommo T, Hamalainen M, Moilanen E, Korhonen R. Anti-inflammatory effects of beta2-receptor agonists salbutamol and terbutaline are mediated by MKP-1. PLoS One 2016; 11: e0148144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keranen T, Hommo T, Moilanen E, Korhonen R. beta2-receptor agonists salbutamol and terbutaline attenuated cytokine production by suppressing ERK pathway through cAMP in macrophages. Cytokine 2017; 94: 1–7. [DOI] [PubMed] [Google Scholar]
- 15.Ağaç D, Estrada LD, Maples R, Hooper LV, Farrar JD. The beta2-adrenergic receptor controls inflammation by driving rapid IL-10 secretion. Brain Behav Immun 2018; 74: 176–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lamkin DM, Srivastava S, Bradshaw KP, Betz JE, Muy KB, Wiese AM, Yee SK, Waggoner RM, Arevalo JMG, Yoon AJ, Faull KF, Sloan EK, Cole SW. C/EBPbeta regulates the M2 transcriptome in beta-adrenergic-stimulated macrophages. Brain Behav Immun 2019; 80: 839–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bacou E, Haurogne K, Allard M, Mignot G, Bach JM, Herve J, Lieubeau B. beta2-adrenoreceptor stimulation dampens the LPS-induced M1 polarization in pig macrophages. Dev Comp Immunol 2017; 76: 169–176 [DOI] [PubMed] [Google Scholar]
- 18.Sloan EK, Priceman SJ, Cox BF, Yu S, Pimentel MA, Tangkanangnukul V, Arevalo JM, Morizono K, Karanikolas BD, Wu L, Sood AK, Cole SW. The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res 2010; 70: 7042–7052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morioka N, Tanabe H, Inoue A, Dohi T, Nakata Y. Noradrenaline reduces the ATP-stimulated phosphorylation of p38 MAP kinase via beta-adrenergic receptors-cAMP-protein kinase A-dependent mechanism in cultured rat spinal microglia. Neurochem Int 2009; 55: 226–234. [DOI] [PubMed] [Google Scholar]
- 20.Lorton D, Bellinger DL, Schaller JA, Shewmaker E, Osredkar T, Lubahn C. Altered sympathetic-to-immune cell signaling via beta(2)-adrenergic receptors in adjuvant arthritis. Clin Dev Immunol 2013; 2013: 764395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lubahn CL, Lorton D, Schaller JA, Sweeney SJ, Bellinger DL. Targeting alpha- and beta-Adrenergic receptors differentially shifts Th1, Th2, and inflammatory cytokine profiles in immune organs to attenuate adjuvant arthritis. Front Immunol 2014; 5: 346–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malfait AM, Malik AS, Marinova-Mutafchieva L, Butler DM, Maini RN, Feldmann M. The beta2-adrenergic agonist salbutamol is a potent suppressor of established collagen-induced arthritis: mechanisms of action. J Immunol 1999; 162: 6278–6283. [PubMed] [Google Scholar]
- 23.Wu H, Chen J, Song S, Yuan P, Liu L, Zhang Y, Zhou A, Chang Y, Zhang L, Wei W. beta2-adrenoceptor signaling reduction in dendritic cells is involved in the inflammatory response in adjuvant-induced arthritic rats. Sci Rep 2016; 6: 24548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uzkeser H, Cadirci E, Halici Z, Odabasoglu F, Polat B, Yuksel TN, Ozaltin S, Atalay F. Anti-inflammatory and antinociceptive effects of salbutamol on acute and chronic models of inflammation in rats: involvement of an antioxidant mechanism. Mediators Inflamm 2012; 2012: 438912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bohren Y, Tessier LH, Megat S, Petitjean H, Hugel S, Daniel D, Kremer M, Fournel S, Hein L, Schlichter R, Freund-Mercier MJ, Yalcin I, Barrot M. Antidepressants suppress neuropathic pain by a peripheral beta2-adrenoceptor mediated anti-TNFalpha mechanism. Neurobiol Dis 2013; 60: 39–50. [DOI] [PubMed] [Google Scholar]
- 26.Kremer M, Yalcin I, Goumon Y, Wurtz X, Nexon L, Daniel D, Megat S, Ceredig RA, Ernst C, Turecki G, Chavant V, Theroux JF, Lacaud A, Joganah LE, Lelievre V, Massotte D, Lutz PE, Gilsbach R, Salvat E, Barrot M. A dual noradrenergic mechanism for the relief of neuropathic allodynia by the antidepressant drugs duloxetine and amitriptyline. J Neurosci 2018; 38: 9934–9954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yalcin I, Tessier LH, Petit-Demouliere N, Waltisperger E, Hein L, Freund-Mercier MJ, Barrot M. Chronic treatment with agonists of beta(2)-adrenergic receptors in neuropathic pain. Exp Neurol 2010; 221: 115–121. [DOI] [PubMed] [Google Scholar]
- 28.Zhang FF, Morioka N, Abe H, Fujii S, Miyauchi K, Nakamura Y, Hisaoka-Nakashima K, Nakata Y. Stimulation of spinal dorsal horn beta2-adrenergic receptor ameliorates neuropathic mechanical hypersensitivity through a reduction of phosphorylation of microglial p38 MAP kinase and astrocytic c-jun N-terminal kinase. Neurochem Int 2016; 101: 144–155. [DOI] [PubMed] [Google Scholar]
- 29.Chen X, Levine JD. Epinephrine-induced excitation and sensitization of rat C-fiber nociceptors. J Pain 2005; 6: 439–446. [DOI] [PubMed] [Google Scholar]
- 30.Khasar SG, McCarter G, Levine JD. Epinephrine produces a beta-adrenergic receptor-mediated mechanical hyperalgesia and in vitro sensitization of rat nociceptors. J Neurophysiol 1999; 81: 1104–1112. [DOI] [PubMed] [Google Scholar]
- 31.Ciszek BP, O'Buckley SC, Nackley AG. Persistent Catechol-O-methyltransferase-dependent pain is initiated by peripheral beta-adrenergic receptors. Anesthesiology 2016; 124: 1122–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li W, Shi X, Wang L, Guo T, Wei T, Cheng K, Rice KC, Kingery WS, Clark JD. Epidermal adrenergic signaling contributes to inflammation and pain sensitization in a rat model of complex regional pain syndrome. Pain 2013; 154: 1224–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nackley AG, Tan KS, Fecho K, Flood P, Diatchenko L, Maixner W. Catechol-O-methyltransferase inhibition increases pain sensitivity through activation of both beta2- and beta3-adrenergic receptors. Pain 2007; 128: 199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Segall SK, Maixner W, Belfer I, Wiltshire T, Seltzer Z, Diatchenko L. Janus molecule I: dichotomous effects of COMT in neuropathic vs nociceptive pain modalities. CNS Neurol Disord Drug Targets 2012; 11: 222–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pogatzki-Zahn EM, Segelcke D, Schug SA. Postoperative pain-from mechanisms to treatment. Pain Rep 2017; 2: e588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arora V, Morado-Urbina CE, Aschenbrenner CA, Hayashida K, Wang F, Martin TJ, Eisenach JC, Peters CM. Disruption of spinal noradrenergic activation delays recovery of acute Incision-Induced hypersensitivity and increases spinal glial activation in the rat. J Pain 2016; 17: 190–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 1994; 53: 55–63. [DOI] [PubMed] [Google Scholar]
- 38.Swett JE, Woolf CJ. The somatotopic organization of primary afferent terminals in the superficial laminae of the dorsal horn of the rat spinal cord. J Comp Neurol 1985; 231: 66–77. [DOI] [PubMed] [Google Scholar]
- 39.Gleeson LC, Ryan KJ, Griffin EW, Connor TJ, Harkin A. The beta2-adrenoceptor agonist clenbuterol elicits neuroprotective, anti-inflammatory and neurotrophic actions in the kainic acid model of excitotoxicity. Brain Behav Immun 2010; 24: 1354–1361. [DOI] [PubMed] [Google Scholar]
- 40.Griffin EW, Yssel JD, O'Neill E, Ryan KJ, Boyle N, Harper P, Harkin A, Connor T. The beta2-adrenoceptor agonist clenbuterol reduces the neuroinflammatory response, neutrophil infiltration and apoptosis following intra-striatal IL-1beta administration to rats. Immunopharmacol Immunotoxicol 2018; 40: 99–106. [DOI] [PubMed] [Google Scholar]
- 41.Izeboud CA, Hoebe KH, Grootendorst AF, Nijmeijer SM, van Miert AS, Witkamp RR, Rodenburg RJ. Endotoxin-induced liver damage in rats is minimized by beta 2-adrenoceptor stimulation. Inflamm Res 2004; 53: 93–99. [DOI] [PubMed] [Google Scholar]
- 42.Ryan KJ, Griffin E, Yssel JD, Ryan KM, McNamee EN, Harkin A, Connor TJ. Stimulation of Central beta2-adrenoceptors suppresses NFkappaB activity in rat brain: a role for IkappaB. Neurochem Int 2013; 63: 368–378. [DOI] [PubMed] [Google Scholar]
- 43.McReynolds JR, Anderson KM, Donowho KM, McIntyre CK. Noradrenergic actions in the basolateral complex of the amygdala modulate arc expression in hippocampal synapses and consolidation of aversive and non-aversive memory. Neurobiol Learn Mem 2014; 115: 49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roozendaal B, Schelling G, McGaugh JL. Corticotropin-releasing factor in the basolateral amygdala enhances memory consolidation via an interaction with the beta-adrenoceptor-cAMP pathway: dependence on glucocorticoid receptor activation. J Neurosci 2008; 28: 6642–6651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brennan TJ, Vandermeulen EP, Gebhart GF. Characterization of a rat model of incisional pain. Pain 1996; 64: 493–502. [DOI] [PubMed] [Google Scholar]
- 46.Ghasemlou N, Chiu IM, Julien JP, Woolf CJ. CD11b+Ly6G- myeloid cells mediate mechanical inflammatory pain hypersensitivity. Proc Natl Acad Sci U S A 2015; 112: E6808–E6817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aschenbrenner CA, Houle TT, Gutierrez S, Eisenach JC. Modeling individual recovery after peripheral nerve injury in rats and the effects of parturition. Anesthesiology 2014; 121: 1056–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nicholas AP, Pieribone VA, Elde R, Hokfelt T. Initial observations on the localization of mRNA for alpha and beta adrenergic receptors in brain and peripheral tissues of rat using in situ hybridization. Mol Cell Neurosci 1991; 2: 344–350. [DOI] [PubMed] [Google Scholar]
- 49.Nicholas AP, Pieribone VA, Hokfelt T. Cellular localization of messenger RNA for beta-1 and beta-2 adrenergic receptors in rat brain: an in situ hybridization study. Neuroscience 1993; 56: 1023–1039. [DOI] [PubMed] [Google Scholar]
- 50.Nicholson R, Dixon AK, Spanswick D, Lee K. Noradrenergic receptor mRNA expression in adult rat superficial dorsal horn and dorsal root ganglion neurons. Neurosci Lett 2005; 380: 316–321. [DOI] [PubMed] [Google Scholar]
- 51.Sivamani RK, Lam ST, Isseroff RR. Beta adrenergic receptors in keratinocytes. Dermatol Clin 2007; 25: 643–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grisanti LA, de Lucia C, Thomas TP, Stark A, Strony JT, Myers VD, Beretta R, Yu D, Sardu C, Marfella R, Gao E, Houser SR, Koch WJ, Hamad EA, Tilley DG. Prior beta-blocker treatment decreases leukocyte responsiveness to injury. JCI Insight 2019; 4: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lorton D, Lubahn C, Bellinger DL. Potential use of drugs that target neural-immune pathways in the treatment of rheumatoid arthritis and other autoimmune diseases. Curr Drug Targets Inflamm Allergy 2003; 2: 1–30. [DOI] [PubMed] [Google Scholar]
- 54.Hoffman BU, Baba Y, Griffith TN, Mosharov EV, Woo SH, Roybal DD, Karsenty G, Patapoutian A, Sulzer D, Lumpkin EA. Merkel cells activate sensory neural pathways through adrenergic synapses. Neuron 2018; 100: 1401–1413.e1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ochoa-Cortes F, Guerrero-Alba R, Valdez-Morales EE, Spreadbury I, Barajas-Lopez C, Castro M, Bertrand J, Cenac N, Vergnolle N, Vanner SJ. Chronic stress mediators act synergistically on colonic nociceptive mouse dorsal root ganglia neurons to increase excitability. Neurogastroenterol Motil 2014; 26: 334–345. [DOI] [PubMed] [Google Scholar]
- 56.Wang S, Zhu HY, Jin Y, Zhou Y, Hu S, Liu T, Jiang X, Xu GY. Adrenergic signaling mediates mechanical hyperalgesia through activation of P2X3 receptors in primary sensory neurons of rats with chronic pancreatitis. Am J Physiol Gastrointest Liver Physiol 2015; 308: G710–G719. [DOI] [PubMed] [Google Scholar]
- 57.Sugimoto T, Morioka N, Sato K, Hisaoka K, Nakata Y. Noradrenergic regulation of period1 expression in spinal astrocytes is involved in protein kinase A, c-Jun N-terminal kinase and extracellular signal-regulated kinase activation mediated by alpha1- and beta2-adrenoceptors. Neuroscience 2011; 185: 1–13. [DOI] [PubMed] [Google Scholar]
- 58.Aoki C, Pickel VM. C-terminal tail of beta-adrenergic receptors: immunocytochemical localization within astrocytes and their relation to catecholaminergic neurons in N. tractus solitarii and area postrema. Brain Res 1992; 571: 35–49. [DOI] [PubMed] [Google Scholar]
- 59.Mizukami T. Immunocytochemical localization of beta2-adrenergic receptors in the rat spinal cord and their spatial relationships to tyrosine hydroxylase-immunoreactive terminals. Kurume Med J 2004; 51: 175–183. [DOI] [PubMed] [Google Scholar]
- 60.Le Provost GS, Pullar CE. beta2-adrenoceptor activation modulates skin wound healing processes to reduce scarring. J Invest Dermatol 2015; 135: 279–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Romana-Souza B, Santos JS, Monte-Alto-Costa A. Beta-1 and beta-2, but not alpha-1 and alpha-2, adrenoceptor blockade delays rat cutaneous wound healing. Wound Repair Regen 2009; 17: 230–239. [DOI] [PubMed] [Google Scholar]
- 62.Pullar CE, Le Provost GS, O'Leary AP, Evans SE, Baier BS, Isseroff RR. beta2AR antagonists and beta2AR gene deletion both promote skin wound repair processes. J Invest Dermatol 2012; 132: 2076–2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cowie AM, Menzel AD, O'Hara C, Lawlor MW, Stucky CL. NOD-like receptor protein 3 inflammasome drives postoperative mechanical pain in a sex-dependent manner. Pain 2019; 160: 1794–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Song N, Fang Y, Sun X, Jiang Q, Song C, Chen M, Ding J, Lu M, Hu G. Salmeterol, agonist of beta2-aderenergic receptor, prevents systemic inflammation via inhibiting NLRP3 inflammasome. Biochem Pharmacol 2018; 150: 245–255. [DOI] [PubMed] [Google Scholar]
- 65.Goncalves Dos Santos G, Delay L, Yaksh TL, Corr M. Neuraxial cytokines in pain states. Front Immunol 2019; 10: 3061–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Willemen HL, Eijkelkamp N, Garza Carbajal A, Wang H, Mack M, Zijlstra J, Heijnen CJ, Kavelaars A. Monocytes/macrophages control resolution of transient inflammatory pain. J Pain 2014; 15: 496–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huang L, Gao YJ, Wang J, Strichartz G. Shifts in cell-type expression accompany a diminishing role of spinal p38-mapkinase activation over time during prolonged postoperative pain. Anesthesiology 2011; 115: 1281–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Peters CM, Eisenach JC. Contribution of the chemokine (C-C motif) ligand 2 (CCL2) to mechanical hypersensitivity after surgical incision in rats. Anesthesiology 2010; 112: 1250–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wen YR, Suter MR, Ji RR, Yeh GC, Wu YS, Wang KC, Kohno T, Sun WZ, Wang CC. Activation of p38 mitogen-activated protein kinase in spinal microglia contributes to incision-induced mechanical allodynia. Anesthesiology 2009; 110: 155–165. [DOI] [PubMed] [Google Scholar]
- 70.Botterblom MH, Feenstra MG, Erdtsieck-Ernste EB. Determination of propranolol, labetalol and clenbuterol in rat brain by high-performance liquid chromatography. J Chromatogr 1993; 613: 121–126. [DOI] [PubMed] [Google Scholar]
- 71.Stowell RD, Sipe GO, Dawes RP, Batchelor HN, Lordy KA, Whitelaw BS, Stoessel MB, Bidlack JM, Brown E, Sur M, Majewska AK. Noradrenergic signaling in the wakeful state inhibits microglial surveillance and synaptic plasticity in the mouse visual cortex. Nat Neurosci 2019; 22: 1782–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ocay DD, Li MMJ, Ingelmo P, Ouellet JA, Page MG, Ferland CE. Predicting acute postoperative pain trajectories and long-term outcomes of adolescents after spinal fusion surgery. Pain Res Manag 2020; 2020: 9874739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Thomazeau J, Rouquette A, Martinez V, Rabuel C, Prince N, Laplanche JL, Nizard R, Bergmann JF, Perrot S, Lloret-Linares C. Predictive factors of chronic Post-Surgical pain at 6 months following knee replacement: influence of postoperative pain trajectory and genetics. Pain Physician 2016; 19: E729–E741. [PubMed] [Google Scholar]
- 74.Albayrak Y, Saglam MB, Yildirim K, Karatay S, Polat B, Uslu T, Suleyman H, Akcay F. Effects of epinephrine and cortisol on the analgesic activity of metyrosine in rats. Arch Pharm Res 2011; 34: 1519–1525. [DOI] [PubMed] [Google Scholar]
- 75.Althaus A, Arranz Becker O, Moser KH, Lux EA, Weber F, Neugebauer E, Simanski C. Postoperative pain trajectories and pain chronification-an empirical typology of pain patients. Pain Med 2018; 19: 2536–2545. [DOI] [PubMed] [Google Scholar]
- 76.Althaus A, Arranz B. O a, Neugebauer E. Distinguishing between pain intensity and pain resolution: using acute post-surgical pain trajectories to predict chronic post-surgical pain. Eur J Pain 2014; 18: 513–521. [DOI] [PubMed] [Google Scholar]
- 77.Hayashida KI, Obata H. Strategies to treat chronic pain and strengthen impaired descending noradrenergic inhibitory system. IJMS 2019; 20: 822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ito S, Suto T, Saito S, Obata H. Repeated administration of duloxetine suppresses neuropathic pain by accumulating effects of noradrenaline in the spinal cord. Anesth Analg 2018; 126: 298–307. [DOI] [PubMed] [Google Scholar]
- 79.Boada MD, Martin TJ, Parker R, Houle TT, Eisenach JC, Ririe DG. Recovery from nerve injury induced behavioral hypersensitivity in rats parallels resolution of abnormal primary sensory afferent signaling. Pain 2020; 161: 949–959. [DOI] [PMC free article] [PubMed] [Google Scholar]