Abstract
Background:
Male breast carcinoma (male BC) is an uncommon neoplasia without individualized strategies for diagnosis and therapeutics. Low overall survival (OS) rates have been reported, mostly associated with patients’ advanced stage and older age. Intratumoral heterogeneity versus homogeneity of malignant epithelial cells seems to be an important factor to consider for the development of combination therapies with curative intention.
Objective:
In this preliminary study, we aim to provide valuable insight into the distinct clinicopathologic features of male BC.
Material and methods:
In a series of 40 male BC patients, we evaluated by immunohistochemistry androgen receptor; activating transcription factor 3 (ATF3); p16; cyclin D1; fatty acid synthase (FASN); fatty acid transport protein 1 (FATP1); β1, β3, β4, and β6 integrins; collagen I and collagen IV; and their interactions. Kaplan-Meier survival curves and log-rank tests were assessed for statistical analysis.
Results:
Homogeneous epithelial staining of p16, ATF3, β6 integrin, FASN, and FATP1 was found to be significantly intercorrelated, and associated with high Ki67. These markers also stained tumor stromal fibroblasts. The prognostic analysis showed statistically significant associations of FASN with disease-free survival (DFS) and OS, as well as of ATF3 with OS and collagen IV with DFS.
Conclusions:
This study highlights, as a novel finding, the relevance of FASN, ATF3, and collagen IV immunophenotypes, which may have innovative application in the clinical management of male BC.
Keywords: Male breast carcinoma, gynecomastia, immunohistochemistry, molecular markers, prognosis
Introduction
Male breast carcinoma (male BC) is a complex group of malignant epithelial proliferations with specific stromal microenvironment. Although rare, the incidence has been rising by 20% to 25% in the past few decades,1 in part because of the aging population and the obesity trend.2-4 In addition, lower overall survival (OS) rates in male BC as compared with women BC have been reported, mostly associated with patients’ advanced stage and older age.2 Despite recent improvements, current clinical and pathologic parameters are still insufficient for a personalized and accurate treatment efficacy, and male BC requires comprehensive studies to identify additional markers able to assure an optimal clinical care.
Intratumoral heterogeneity of neoplastic tissues is a main challenge in cancer biology, by including diverse and dynamic interacting epithelial and stromal subpopulations, namely the fibroblasts. The intratumoral heterogeneity versus homogeneity of malignant epithelial cells seems to be critical in the management and prognosis of BC, and is an important factor to consider in the efforts to develop combination therapies with curative purposes.5
In this series of previously evaluated estrogen receptor alpha (ERα) and progesterone receptor (PR) male BC,6 we sought to complete the hormonal characterization by performing androgen receptor (AR) analysis, and make a comprehensive study of the eventual interactions between cell cycle regulation proteins, cell-surface proteins, fatty acid metabolism, and components of extracellular matrix. We chose these molecules, which have been little studied in male BC because of their functional interplay and potential relationship with cell proliferation.
Immunohistochemistry (IHC) has shown increasing relevance in breast pathology for solving diagnostic difficulties, being used as a surrogate tool for mutational evaluation in determining response to therapy and prognosis.7 The IHC analysis of ERα, PR, human epidermal growth factor receptor-2/Erb-B2 Receptor Tyrosine Kinase 2 (HER2/ERBB2), and Ki67 is now the standard care to evaluate BC. The tumoral phenotypical IHC expression can also contribute to understand molecular characteristics of malignant epithelial cells and stroma, and to assess intratumoral heterogeneity versus homogeneity.5,7 Diverse biomarkers appraised by IHC technique have been implicated in distinct carcinogenesis steps, and considered potential therapy targets.8
In this study, we analyzed the IHC pattern of the following molecular markers: AR, a nuclear transcription factor member of the steroid hormone nuclear receptor family; activating transcription factor 3 (ATF3), a member of the activator protein 1 family of transcription factors; cell cycle regulation proteins, p16 and cyclin D1; cell-surface proteins, β1, β3, β4, and β6 integrins; fatty acid synthase (FASN), the enzyme for endogenous synthesis of fatty acids; fatty acid transport protein 1 (FATP1), the first described element of the 6 members of FATP family; and the structural components of the extracellular matrix collagen I and collagen IV. Our intention was to evaluate IHC positivity as the result of the widely common dichotomic expression “all or not all.” Therefore, we assessed different cores from different morphologic tumoral areas to reveal the presence of homogeneous versus heterogeneous positivity.
We aim to identify IHC epithelial and stromal patterns that could contribute to better characterize the biology of male BC and, consequently, may represent promising tools for improving the clinical management in this subtype of BC.
Material and Methods
Study cohort
This study comprises 40 invasive male BC selected from a series of 198 male BC patients diagnosed and treated at the Portuguese Institute of Oncology (IPO) Lisbon Center. The selection was rigorous and the patients included were retrieved from the larger series using the following criteria: (a) no neoadjuvant therapy management to avoid predictive bias on survival analyses and (b) diagnosis within the last 10 years to homogenize the fixation conditions. All cases were reviewed to ensure standardized characterization.
The Institutional Ethical Committee of IPO Lisbon Center approved the study (UIC/821). The clinical data were obtained by review of the clinical records. All male BC were previously characterized by histologic type and grading, and staged according to the Tumor. Node, Metastasis/American Joint Committee (TNM/AJCC) on Cancer classification system (8th edition).9,10 Previous IHC analysis of ERα, PR, ERBB2, and Ki-67 allowed the identification of clinically defined, treatment-oriented subtypes (surrogate subtypes). Germinal mutational BRCA (gBRCA) status had been formerly evaluated on 26 cases of male BC, as described in our previous study.6
Tissue microarrays
Representative formalin-fixed, paraffin-embedded tissue cores were inserted in 4 tissue microarray paraffin blocks. Three or four 1.5-mm-diameter cores for each case were included to account for the heterogeneity of the lesions and used to perform an additional immunohistochemical study.
Antibody reagents and conditions
Immunohistochemistry used a peroxidase-indirect-polymer technique performed on a Ventana Benchmark ULTRA instrument (Ventana Medical Systems, Inc; Roche Diagnostics, Basel, Switzerland). Paraffin sections (3 µm) were stained with hematoxylin and eosin staining (Hematoxylin, Cat. Number CS700, Dako; and Eosin, Cat. Number CS701, Dako). We performed IHC with AR; ATF3; p16INK4a; cyclin D1; β1, β3, β4, and β6 integrins; FASN; FATP1; collagen I; and collagen IV in an automatic staining platform (Ventana Medical Systems), using OptiView DAB IHC Detection Kit (Ventana Medical Systems) with diaminobenzidine as the chromogen to detect antigen expression (Table 1).
Table 1.
Antibody reagents and conditions.
| Antibody | Manufacturer | Clone | Dilution | Pretreatment |
|---|---|---|---|---|
| ERα | Ventana Medical Systems, Inc; Roche Diagnostics | SP1 | Prediluted 28 min | ULTRA CC1-64 min |
| PR | Ventana Medical Systems, Inc; Roche Diagnostics | IE2 | Prediluted 36 min | ULTRA CC1-64 min |
| ERBB2 | Ventana Medical Systems, Inc; Roche Diagnostics | 4B5 | Prediluted 60 min | ULTRA CC1-76 min |
| Ki67 | Ventana Medical Systems, Inc; Roche Diagnostics | 30-9 | Prediluted 20 min | ULTRA CC1-40 min |
| AR | Ventana Medical Systems, Inc; Roche Diagnostics | SP107 | Prediluted 32 min | ULTRA CC1-64 min |
| ATF3 | Santa Cruz Biotechnology | C19 | 1:150—28 min | CC1-56 min |
| p16 | CINtec Histology | E6H4 | Prediluted 32 min | ULTRA CC1-64 min |
| Cyclin D1 | Thermo Scientific | SP4 | 1:30—20 min | CC1-20 min |
| β1 integrin | Cell Signaling | D2E5 | 1:100—28 min | ULTRA CC1-92 min |
| β3 integrin | Cell Signaling | D7X3P | 1:100—28 min | ULTRA CC1-56 min |
| β4 integrin | Atlas antibodies | ITGB4 | 1:200—28 min | ULTRA CC1-56 min |
| β6 integrin | Atlas antibodies | ITGB6 | 1:350—28 min | ULTRA CC1-48 min |
| FASN | Sigma | Not indicated | 1:800—28 min | CC1-56 min |
| FATP1 | R&D system | 308420 | 1:200—16 min | CC1-24 min |
| Collagen I | Abcam | EPR7785 | 1:300—20 min | ULTRA CC1-16 min |
| Collagen IV | DAKO | CIV22 | 1:10—20 min | ULTRA CC1-16 min |
Abbreviations: AR, androgen receptor; ATF3, activating transcription factor 3; ER, estrogen receptor; ERBB2, Erb-B2 Receptor Tyrosine Kinase 2; FASN, fatty acid synthase; FATP1, fatty acid transport protein 1; PR, progesterone receptor.
Tissue sections were counterstained with Mayer hematoxylin before mounting. All antibody dilutions were made in Antibody Diluent Reagent Solution (Cat. Number 003218, Life Technologies). Image acquisition was performed in Digital Microimaging Device Leica DMD108 (version 1.15 Build 704, Leica Microsystems).
Scoring criteria and patterns
The staining pattern was recorded in the malignant epithelial cells, stromal fibroblasts, and interstitial stroma. Some of the antibodies also marked vessels and adipose cells, but these structures were not evaluated. The malignant epithelial cell immunoexpression was scored in 3 subgroups: 1 (“homogeneous phenotype”)—positive staining in ⩾95% of epithelial cells with strong or moderate intensity; 2 (“heterogeneous phenotype”)—positive staining in ⩾1% and <95% of epithelial cells with weak or moderate, or focally strong intensity; 3 (negative)—no staining or staining in <1% of cells with weak intensity.
The fibroblast immunoexpression was also scored in 3 subgroups: 1—positive staining in >10% of fibroblasts with strong or moderate intensity; 2—positive staining in ⩾1% and ⩽10% of fibroblasts with strong and moderate intensity; 3—negative in <1% of stained fibroblasts.
Interstitial stroma was classified into 2 subgroups: 1—positive diffuse staining with strong, moderate, or weak intensity and 2—negative staining.
The antibody expression patterns were the following: ARs, ATF3, and cyclin D1 were present as a nuclear staining in epithelial cells, and in fibroblasts as well. p16, when present in epithelial cells, showed a nuclear and a cytoplasmatic staining. β1, β3, and β4 were expressed in the cell membranes of epithelial malignant cells or around epithelial malignant cell clusters. Some stromal fibroblast expression was observed with β1, β3, and β4 integrins. β6 integrin was expressed in epithelial cytoplasmatic staining with cell membrane reinforcement or as a granular cytoplasmatic staining. Fatty acid synthase showed an epithelial cytoplasmatic staining with cell membrane reinforcement and fibroblast expression as well. Fatty acid transport protein 1 was expressed as nuclear staining in epithelial cells and fibroblasts, and occasionally, as cytoplasmatic staining in the epithelial cells. Collagen I and collagen IV could have a stromal diffuse staining and had no epithelial cell expression.
Data and statistical analysis
For male BC, we performed a descriptive analysis, and subsequently, used nonparametric, semiparametric and parametric statistical techniques, employing the software R Core Team 2018.11 The Fisher exact test was used to evaluate the association between variables because it is suitable for the small sample size of the series. Survival curves were based on the Kaplan-Meier nonparametric estimator, and differences among the category curves were evaluated by the log-rank test. Tests with P < .05 were considered significant. Disease-free survival (DFS) corresponded to the remission time up to recurrence, and OS to the interval since pathologic diagnosis until the occurrence of death due to male BC. Patients without disease relapse during the study period and those who died from other causes or were lost for follow-up were considered censored observations. A Cox simple regression model was fitted for each clinicopathologic and IHC variable to evaluate their prognostic influence on both DFS and OS. Following the determination of significant variables, a Cox regression model was performed with all variables simultaneously, as a multiple regression analysis. Because the evaluation of fibroblasts was difficult in some cases, we did not consider the immunophenotypical subgroups of fibroblasts for statistical analysis.
Results
Descriptive clinicopathologic analysis
Male BC clinicopathologic characteristics are summarized in Table 2. The mean age at diagnosis was 66.7 years (range, 37-84 years). Obesity was recorded in clinical files in 9 patients (22.5%). Three patients (7.5%) have metachronous bilateral carcinomas, and prostate carcinoma was the most frequent nonbreast primary neoplasia found (3 cases). One of the patients with prostate carcinoma had also bilateral BC. gBRCA2 mutations were presented in 26.9% (7 of 26 tested patients). The remaining patients refused to perform the genomic test. No gBRCA1 mutations were found. At diagnosis, no patient had distant metastasis. All patients underwent mastectomy. Adjuvant radiotherapy was used in 26 patients (65%), adjuvant hormonotherapy in 36 (90%), adjuvant chemotherapy in 22 (55%), and ERBB2-target agents in 2 patients (5%). Most male BC (60%) is luminal B-like (HER2 negative). No triple-negative carcinomas were diagnosed. Nine patients (22.5%) with male BC also had gynecomastia.
Table 2.
Clinicopathologic characteristics of male BC patients (n = 40).
| Characteristics | Number (%) |
|---|---|
| Age (years) | |
| <70 | 21 (52.5) |
| ⩾70 | 19 (47.5) |
| Family history | |
| No | 29 (72.5) |
| Yes | 11 (27.5) |
| gBRCA2 mutations | |
| Not evaluated | 14 (35) |
| Indeterminate | 19 (47.5) |
| Positive | 7 (17.5) |
| Bilaterality | |
| No | 37 (92.5) |
| Yes | 3 (7.5) |
| Nonbreast primary neoplasms | |
| No | 32 (80) |
| Yes | 8 (20) |
| Tumor size (pT) | |
| pT1 | 11 (27.5) |
| pT2 | 15 (37.5) |
| pT3 | 2 (5) |
| pT4 | 12 (30) |
| Axillary nodal status (pN) | |
| pN0 | 17 (42.5) |
| pN1 | 23 (57.5) |
| Anatomic stage | |
| I | 8 (20) |
| II | 18 (45) |
| III | 14 (35) |
| Histologic type | |
| Invasive no special type | 34 (85) |
| Other invasive subtypes | 6 (15) |
| Histologic grade (G) | |
| G1 | 1 (2.5) |
| G2 | 26 (65) |
| G3 | 13 (32.5) |
| Estrogen receptor α | |
| Positive | 40 (100) |
| Progesterone receptors | |
| Positive | 36 (90) |
| Negative | 4 (10) |
| ERBB2 (IHC + ISH) | |
| Negative | 37 (92.5) |
| Positive | 3 (7.5) |
| Ki67 | |
| Low | 13 (32.5) |
| High | 27 (67.5) |
| Clinically defined subtypes | |
| Luminal A-like | 13 (32.5) |
| Luminal B-like (HER2-negative) | 24 (60.0) |
| Luminal B-like (HER2-positive) | 3 (7.5) |
Abbreviations: BC, breast carcinoma; ERBB2, Erb-B2 Receptor Tyrosine Kinase 2; IHC, immunohistochemistry; ISH, in situ hybridization.
Immunohistochemical staining
The IHC markers’ staining results in male BC are summarized in Table 3. AR, ATF3, p16, and cyclin D1 stainings are shown in Figure 1; β1, β3, β4, and β6 integrins are shown in Figure 2; and FASN, FATP1, collagen IV, and collagen I are depicted in Figure 3.
Table 3.
Immunohistochemical marker staining in male BC.
| Biomarker | Number of cases, percentage, and staining pattern | |||||
|---|---|---|---|---|---|---|
| Malignant epithelial cells | Stromal fibroblasts/Interstitial stromaa | |||||
| AR | 14 | 35% | Homogeneous | 35 | 87.5% | Subgroup 1 |
| 21 | 52.5% | Heterogeneous | ||||
| 5 | 12.5% | Negative | ||||
| ATF3 | 8 | 20.5% | Homogeneous | 21 | 53.8% | Subgroup 1 |
| 13 | 33.3% | Heterogeneous | ||||
| 18 | 46.2% | Negative | ||||
| p16 | 7 | 17.5% | Homogeneous | 40 | 100% | Subgroup 1 |
| 28 | 70.0% | Heterogeneous | ||||
| 5 | 12.5% | Negative | ||||
| Cyclin D1 | 30 | 75.0% | Homogeneous | 39 | 97.5% | Subgroup 1 |
| 9 | 22.5% | Heterogeneous | ||||
| 1 | 2.5% | Negative | ||||
| β1 integrin | 1 | 2.5% | Heterogeneous | 7 | 17.5% | Subgroup 1 |
| 32 | 80.0% | Negative | ||||
| β3 integrin | 2 | 5% | Heterogeneous | 9 | 22.5% | Subgroup 1 |
| 38 | 95% | Negative | ||||
| β4 integrin | 3 | 7.7% | Heterogeneous | 13 | 33.3% | Subgroup 1 |
| 23 | 59.0% | Negative | ||||
| β6 integrin | 12 | 31.6% | Homogeneousb | 24 | 63.2% | Subgroup 1 |
| 12 | 31.6% | Granularc | ||||
| 14 | 36.8% | Negative | ||||
| FASN | 16 | 40% | Homogeneous | 39 | 97.5% | Subgroup 2 |
| 23 | 57.5% | Heterogeneous | ||||
| 1 | 2.5% | Negative | ||||
| FATP1 | 9 | 22.5% | Homogeneous | 23 | 57.5% | Subgroup 1 |
| 14 | 35.0% | Heterogeneous | ||||
| 17 | 42.5% | Negative | ||||
| Collagen I | 39 | 100% | Negative | 34 | 61.5% | Positive intense |
| 15 | 38.5% | Positive weak | ||||
| Collagen IV | 37 | 100% | Negative | 11 | 29.7% | Positive intense |
| 26 | 70.3% | Negative | ||||
Abbreviations: AR, androgen receptor; ATF3, activating transcription factor 3; BC, breast carcinoma; FASN, fatty acid synthase; FATP1, fatty acid transport protein 1.
On collagens I and IV, the staining refers to the interstitial stroma.
Cell membrane.
Cytoplasm.
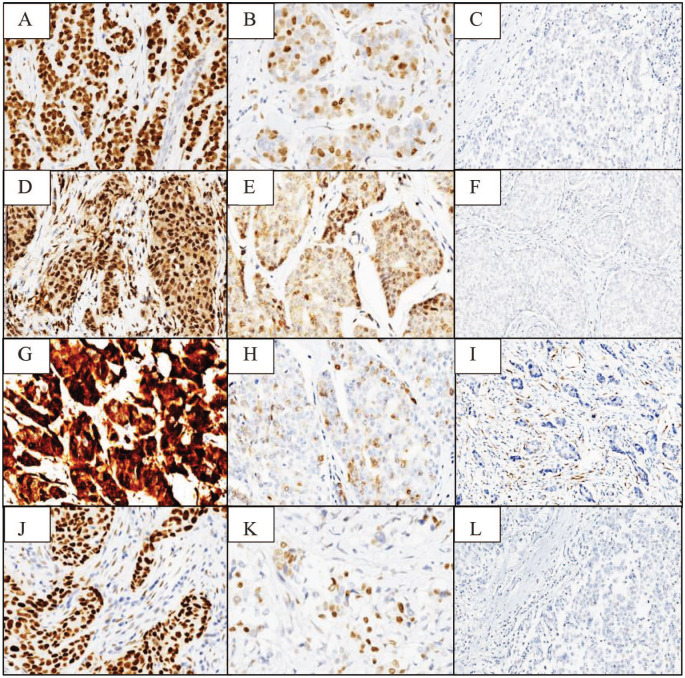
Figure 1.
Androgen receptors (A—positive homogeneous epithelial staining 400×, B—positive heterogeneous 400×, C—negative 100×); ATF3 (D—positive homogeneous epithelial staining 400×, E—positive heterogeneous 400×, F—negative 400×); p16 (G—positive homogeneous epithelial staining 400×, H—positive heterogeneous 400×, I—negative 100×); and cyclin D1 (J—positive homogeneous epithelial staining 400×, K—positive heterogeneous 400×, L—negative 100×).
ATF3 indicates activating transcription factor 3.
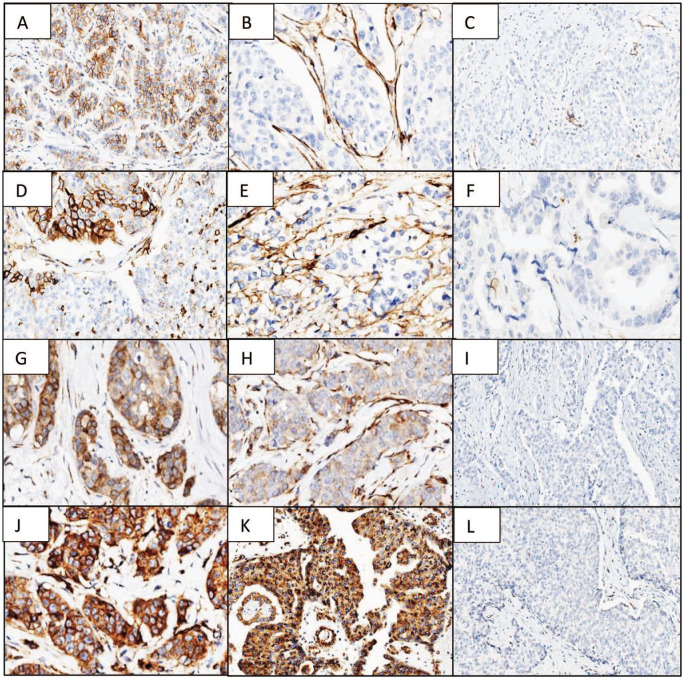
Figure 2.
β1 integrin (A—positive heterogeneous epithelial staining 400×, B—positive stromal staining 400×, C—negative 100×); β3 integrin (D—positive heterogeneous epithelial staining 400×, E—positive stromal staining 400×, F—negative 400×); β4 integrin (G—positive heterogeneous epithelial staining 400×, H—positive stromal staining 400×, I—negative 100×); and β6 integrin (J—positive homogeneous epithelial staining 400×, K—positive heterogeneous epithelial granular staining 100×, L—negative 100×).
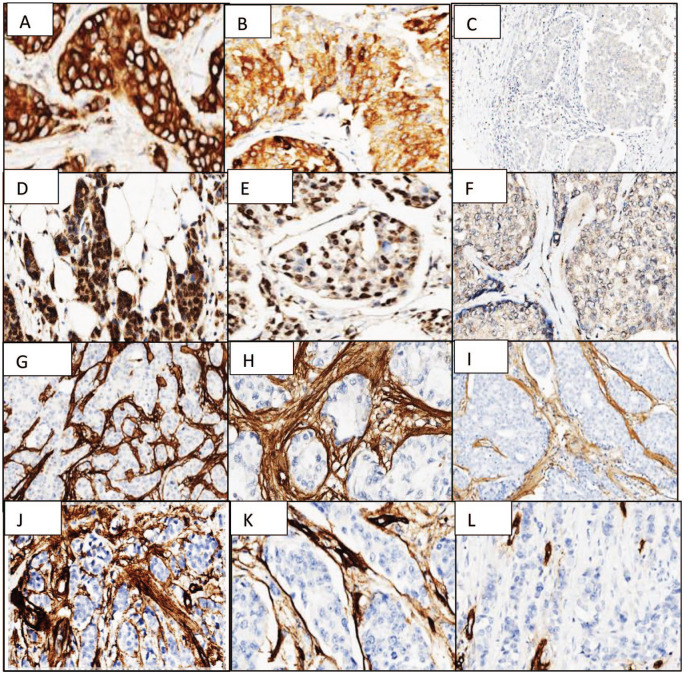
Figure 3.
FASN (A—positive homogeneous epithelial staining 400×, B—positive heterogeneous epithelial staining 400×, C—negative 100×); FATP1 (D—positive homogeneous epithelial staining 400×, E—positive heterogeneous epithelial staining 400×, F—negative 400×); collagen I (G—stromal diffuse and intense staining 100×, H—diffuse and intense stromal staining 400×, I—weak/moderate diffuse stromal staining 100×; and collagen IV (J—diffuse and intense stromal staining 100×; K—diffuse and intense stromal staining 400×, L—negative 100×).
FASN indicates fatty acid synthase; FATP1, fatty acid transport protein 1.
Androgen receptor had a malignant epithelial nuclear positivity in 87.5% of the cases, with homogeneous phenotype in 35% (Figure 1A) and heterogeneous phenotype in 52.5% of the cases (Figure 1B). Stromal fibroblasts were included in subgroup 1 (>10% staining) in malignant epithelial positive cases and were negative in malignant epithelial negative cases (Figure 1C).
Activating transcription factor 3 was negative in malignant epithelial cells in almost half of the male BC cases (46.2%). Positive nuclear malignant epithelial homogeneous phenotype was observed in 20.5% of the cases (Figure 1D) and heterogeneous phenotype in 33.3% (Figure 1E). Fibroblast-positive cases were included in subgroup 1 in epithelial positive cases and were negative in malignant epithelial negative cases (Figure 1F).
p16 was positive in a similar percentage as cyclin D1 (87.5%), but with a reverse pattern, homogeneous phenotype in 17.5% (Figure 1G) and heterogeneous phenotype in 70% of the cases (Figure 1H). Stromal fibroblasts were positive in all cases, even in the malignant epithelial cells negative cases (Figure 1I).
Cyclin D1 was positive in almost all cases, with a malignant epithelial homogeneous phenotype in 75% (Figure 1J) and malignant heterogeneous phenotype in 22.5% (Figure 1K). Fibroblast-positive cases were included in subgroup 1 in positive malignant epithelial cells cases and were negative in malignant epithelial negative cases (Figure 1L).
β1 (Figure 2A to C), β3 (Figure 2D to F), and β4 (Figure 2G to I) integrin chains had an identical staining pattern in most of the cases. Malignant epithelial cells were negative in the majority of the cases (80%, 95%, and 59%, respectively) and stromal fibroblasts, when positive, were located around malignant epithelial cell clusters in 17.5%, 22.5%, and 33.3% of the cases, respectively. β6 integrin showed 2 staining patterns, 1 cytoplasmatic with cell membrane reinforcement (Figure 2J) and the other having a granular cytoplasmatic staining (Figure 2K). Both types were present in one third of the cases (31.6%). Positive stromal fibroblasts (subgroup 1) were present in all positive malignant epithelial cells cases and fibroblasts did not stain in negative cases (Figure 2L).
Fatty acid synthase stained the epithelial cell cytoplasm (with a cell membrane reinforcement) in almost all cases, with a homogeneous phenotype in 40% of the cases (Figure 3A) and heterogeneous phenotype in 57.5% (Figure 3B). Rare scattered fibroblasts were positive (subgroup 2) in all cases with positive epithelial expression and did not stain in negative cases (Figure 3C).
Regarding FATP1 expression, we found a nuclear homogeneous phenotype staining the epithelial cells in 22.5% of the cases (Figure 3D) and heterogeneous phenotype in 35% (Figure 3E). Cytoplasmatic staining was predominantly absent, but occasionally present (Figure 3F). Numerous fibroblasts (subgroup 1) were positive in all male BC cases with positive epithelial expression and did not stain in negative cases (Figure 3G).
Collagen I had a stromal diffuse staining, intense in 61.5% (Figure 3H) or weak/moderate in 38.5% (Figure 3I) of the cases. Collagen IV was present with a diffuse intense stromal staining in 29.7% of the cases (Figure 3J and K) and negative in the remaining (Figure 3L). Only 5 cases shared an intense stroma staining with both collagen types. No collagen I and collagen IV was present in the epithelial cells.
Significant associations between malignant epithelial phenotypes
In this series of male BC previously characterized6 by ERα positivity (100% of the cases), PR positivity (90%), and high Ki67 (67.5%) (Table 2), AR is positive in 87.5% of the cases. We obtained few significant associations between IHC patterns and the clinicopathologic variables, but many significant associations between the IHC evaluated biomarkers. All the statistically significant associations found are shown in Table 4, and the most relevant highlighted.
Table 4.
Significant associations between biomarkers and clinicopathologic features in male BC.
| Biomarkers | P value (Fisher exact test) |
|---|---|
| ATF3 | |
| pN | .025 |
| Stage | .027 |
| Ki67 | <.001 |
| Surrogate subtypes | .006 |
| β6 integrin | .039 |
| Collagen I | .042 |
| FASN | .011 |
| FATP1 | <.001 |
| BRCA2 | .032 |
| p16 | |
| Ki67 | .004 |
| β6 integrin | <.001 |
| FASN | .002 |
| FATP1 | <.001 |
| β6 integrin | |
| Ki67 | .020 |
| ATF3 | .039 |
| p16 | <.001 |
| FATP1 | <.001 |
| FASN | |
| Ki67 | .015 |
| AR | .011 |
| ATF3 | .002 |
| p16 | .001 |
| FATP1 | .001 |
| FATP1 | |
| Stage | .026 |
| Ki67 | .005 |
| Surrogate subtypes | .010 |
| p16 | <.001 |
| ATF3 | <.001 |
| β6 integrin | <.001 |
| FASN | .001 |
Abbreviations: AR, androgen receptor; ATF3, activating transcription factor 3; BC, breast carcinoma; FASN, fatty acid synthase; FATP1, fatty acid transport protein 1.
Values of P ⩽ .001 are in bold.
Considering the positive homogeneous epithelial phenotype, the following associations reached high statistical significance (P ⩽ .001): (a) ATF3 and FATP1, (b) p16, β6 integrin, FATP1, and FASN, and (c) FATP1, β6 integrin, and FASN. The positive homogeneous epithelial phenotype of all these biomarkers was also associated with high Ki67.
Activating transcription factor 3 and FATP1 were the only biomarkers significantly associated with anatomical stage (stage III) (P = .027 and .026, respectively) and surrogate subtypes (luminal B-like HER2-negative) (P = .006 and .010, respectively). Activating transcription factor 3 was also associated with pN1 status (P = .025) and the presence of gBRCA2 mutations (P = .032). Of note, we found statistically significant associations of gBRCA2 mutations with patients aged <70 years, positive family history, and luminal B-like (HER2-negative) status.
Survival analysis
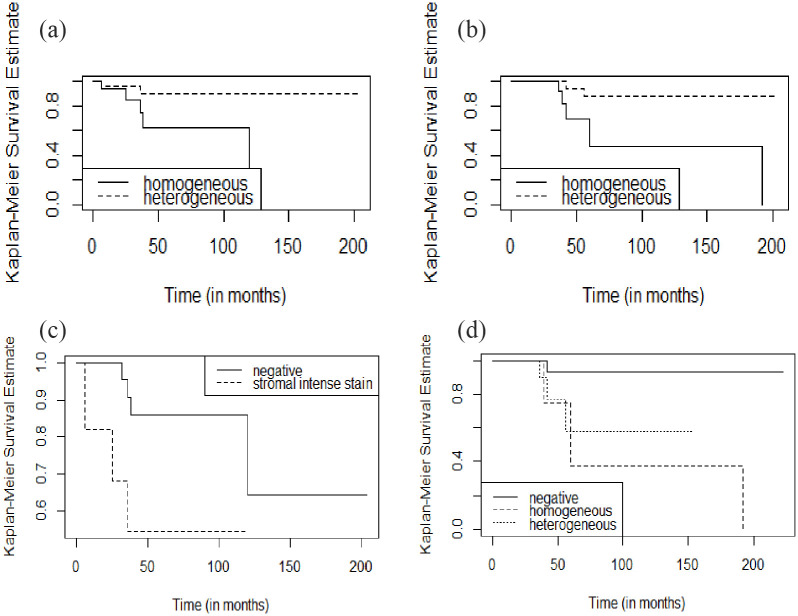
Seven of the 40 patients with male BC (17.5%) had disease recurrence and died of disease. Mean and median remission time were 56.9 and 41 months (range, 6-204), and mean and median survival time were 67.7 and 50 months (range, 7-223), respectively. Beyond the expected and confirmed significant prognostic value of “classic” parameters (pT, anatomic stage, grade), Kaplan-Meier estimates (log-rank test) indicate that male BC patients with FASN homogeneous phenotype had shorter DFS (Figure 4A; P = .04) and OS (Figure 4B; P = .03). Moreover, patients with collagen IV strong stromal immunoexpression staining had a shorter DFS (Figure 4C; P = .05). A worse OS was observed in patients with tumors with an ATF3 homogeneous phenotype (Figure 4D; P = .02). The univariate (simple Cox model) analysis (Table 5) was consistent with and confirmed the Kaplan-Meier/log-rank tests. In multivariate analysis (Table 6), collagen IV was the only of these markers significantly related with DFS (P = .032).
Figure 4.
Kaplan-Meier survival curves of male BC for FASN, collagen IV, and ATF3: (A)—patients with strong homogeneous FASN membrane cytoplasmatic staining have a significantly shorter DFS (P = .04); (B)—patients with strong homogeneous FASN membrane cytoplasmatic staining have a significantly shorter OS (P = .03); (C)—patients with strong stromal staining for collagen IV have a significantly worse DFS (P = .05); (D)—patients with homogeneous staining for ATF3 have a significantly worse OS (P = .02).
ATF3 indicates activating transcription factor 3; BC, breast carcinoma; DFS, disease-free survival; FASN, fatty acid synthase; OS, overall survival.
Table 5.
Univariate Cox regression analysis in relation to DFS and OS.
| Variables | DFS | OS | ||||
|---|---|---|---|---|---|---|
| RR | 95% CI | P value | RR | 95% CI | P value | |
| Androgen receptors | ||||||
| Negative | 1 | – | – | 1 | – | – |
| Homogeneous | 0.61 | 0.12-3.04 | .548 | 1.82 | 0.22-15.3 | .580 |
| ATF3 | ||||||
| Negative | 1 | – | – | 1 | – | – |
| Homogeneous | 5.53 | 0.91-33.64 | .063 | 13.13 | 1.32-130.3 | .027 |
| Heterogeneous | 3.27 | 0.54-19.85 | .197 | 9.16 | 0.92-90.89 | .058 |
| β6 integrin | ||||||
| Negative | 1 | – | – | 1 | – | – |
| Homogeneous | 1.20 | 0.19-7.56 | .848 | 0.70 | 0.07-7.06 | .763 |
| Heterogeneous | 1.13 | 0.23-5.63 | .879 | 1.13 | 0.23-5.72 | .874 |
| FASNa | ||||||
| Homogeneous | 1 | – | – | 1 | – | – |
| Heterogeneous | 0.19 | 0.04-0.99 | .049 | 0.15 | 0.03-0.84 | .030 |
| Collagen I | ||||||
| Negative | 1 | – | – | 1 | – | – |
| Homogeneous | 0.73 | 0.18-2.98 | .666 | 1.57 | 0.35-7.14 | .555 |
| Collagen IV | ||||||
| Negative | 1 | – | – | 1 | – | – |
| Homogeneous | 3.82 | 0.94-15.51 | .061 | 3.54 | 0.7-7.79 | .125 |
| Bilaterality | ||||||
| No | 1 | – | – | 1 | – | – |
| Yes | 6.17 | 1.42-26.79 | .015 | 0.64 | 0.06-7.27 | .720 |
| Family history | ||||||
| No | 1 | – | – | 1 | – | – |
| Yes | 3.81 | 0.94-15.41 | .061 | 2.21 | 0.46-10.8 | .324 |
| Grade (G) | ||||||
| G1-2 | 1 | – | – | 1 | – | – |
| G3 | 5.92 | 1.39-25.23 | .016 | 8.22 | 1.57-42.92 | .012 |
| Tumor size (pT) | ||||||
| pT1-2 | 1 | – | – | 1 | – | – |
| pT3-4 | 16.44 | 2.01-134.5 | .009 | 12.74 | 1.5-108.4 | .020 |
| Stage | ||||||
| I-II | 1 | – | – | 1 | – | – |
| III | 7.65 | 1.53-38.33 | .013 | 14.16 | 1.7-120.2 | .015 |
Abbreviations: ATF3, activating transcription factor 3; CI, confidence interval; DFS, disease-free survival; FASN, fatty acid synthase; OS, overall survival; RR, relative risk.
To avoid predictive bias, we deleted the only FASN-negative case from the statistical analysis.
Table 6.
Multiple Cox regression analysis in relation to DFS and OS.
| Variables | DFS | OS | ||||
|---|---|---|---|---|---|---|
| RR | 95% CI | P value | RR | 95% CI | P value | |
| Collagen IV | ||||||
| Negative | 1 | – | – | |||
| Homogeneous | 8.29 | 1.19-57.53 | .032 | |||
| Tumor size (pT) | ||||||
| pT1-2 | 1 | – | – | 1 | – | – |
| pT3-4 | 57.81 | 3.16-1058 | .006 | 22.86 | 1.0-4.24 | .051 |
| Grade (G) | ||||||
| G1-2 | 1 | – | – | 1 | – | – |
| G3 | 77.01 | 1.78-294 | .016 | 16.99 | 1.77-163 | .014 |
Abbreviations: CI, confidence interval; DFS, disease-free survival; OS, overall survival; RR, relative risk.
We found high RR and CI values in the statistical analysis because the variables’ subcategories had few observations.
Discussion
In this preliminary exploratory study, our objective was to identify IHC molecular biomarkers that could contribute to better characterize the male BC biology, being potentially eligible for further larger and more complex studies to improve the clinical management of this entity.
In male BC, biological specifiers for an effective personalized care and cure are undetermined.6 In addition, the incidence of this rare disease is rising, and persistent poor outcomes have been reported.1,6,12 Understanding and simplifying the complexity of epithelial-stromal interaction and the relevance of phenotypical epithelial homogeneity or heterogeneity burden in tumor progression may be a clue for a new clinical management.13-16
Gynecomastia, a nonneoplastic, often reversible, growth of the mammary tissue, due to proliferation of ductal and mesenchymal components, is the most common benign disease in male breast. Like male BC, gynecomastia is a multifactorial condition, and both entities share risk factors related to high estrogen levels and old age. Although gynecomastia is not considered a premalignant lesion, in this cohort of 40 cases, we found that male BC was associated with gynecomastia in 22.5% of the cases. In the literature, this association has been stated as from nearly nonexistent to being present in 20% to 40% of the cases.9 Complex etiopathogenesis and the sharing of risk factors by both conditions are cumbersome barriers to clarify this association.
Androgen receptor expression was described as having an antiproliferative role in normal breast tissue.17 In female BC, its expression has been linked to a favorable prognosis.18 Some studies emphasize the role of AR in the regulation of tumorigenesis in female BC via epithelial-mesenchymal signaling, but there are very few data regarding the stromal-epithelial interactions in this condition.17 In male BC, AR immunoexpression in the malignant epithelial cells ranges from 34% to 95% and shows conflicting data in relation to its prognostic value.19 In this series, malignant epithelial cells and fibroblasts express AR in most male BC, and the epithelial phenotype was not associated with any prognostic factor. Although the value of AR expression as response predictor to therapy with AR antagonists was not established in male BC patients, some authors19 proposed that stromal-epithelial interactions might have important effects on their action.
Activating transcription factor 3 is considered a mediator of cellular stress response. In female BC, several functions were attributed to ATF3, including a role in epithelial cell proliferation20 and in promotion of tumor progression as a “breast stroma related gene.”21,22 No previous studies evaluated ATF3 expression in male BC. In the present series, ATF3-positive homogeneous phenotype, together with numerous positive fibroblasts, was found in 20.5% of the tumors, and significantly associated with BRCA2 germline mutations, pN1, anatomic stage III, luminal B-like (HER2-negative) subtype, and high Ki67 expression. Accordingly, patients with tumor ATF3 homogeneous score also had a shorter OS. Wang et al22 reported the existence of an interaction of ATF3 with AR, and the eventual use of this link to develop a stromal-target therapy in male BC. In our series, however, no correlation between ATF3 and AR immunophenotype was found (P = .30) to support their results.
p16 and cyclin D1 have important roles in cell cycle regulation. In female BC, overexpression of cyclin D1 in epithelial cells has been reported to occur in 35% to 81% of carcinomas and to be correlated with ERα/PR expression and luminal subtypes, in contrast to p16, which is usually related to high proliferation activity.23-25 In addition, Pestell et al26 demonstrated that stromal cyclin D1 drives tumor microenvironment signaling and promotes BC growth. In male BC, Kanthan et al27 reported similar positive rates for both markers. In this study, cyclin D1 positivity was associated with better outcome, and the p16 expression did not show prognostic significance.27 In our series, cyclin D1 is positive in epithelial and stromal fibroblasts in almost all cases, with a homogeneous phenotype in three quarters of the cases in the epithelial compartment, favoring an important protagonism in male BC. p16 homogeneous phenotype, found in less than a quarter of the cases, is associated with high Ki67 expression and with β6 integrin, FASN, and FATP1 homogeneous phenotype staining. Of note, p16 is the only marker with positive stromal fibroblasts in all cases, even in negative malignant epithelial cells cases.
Integrins are glycoproteins composed by 18α and 8β chains that pair and incorporate 24 different heterodimers. They mediate epithelial cell-to-cell and epithelial cell-extracellular matrix adhesion and organization of the intracellular cytoskeleton. β1, β3, and β4 integrins maintain tissue architecture and contribute to the function of normal breast tissue.28,29 In female BC, they are linked to tumor progression, immune responses, and drug resistance and may be important when considering therapeutic options oriented to tumoral stroma blockage.28-32 In male BC, β1, β3, and β4 integrins have a similar malignant epithelial cell membrane staining, consistent with cell-to-cell adhesion, in very few cases. All these integrins have an identical peculiar reinforcement in the stroma around aggregates of malignant epithelial cells in some negative epithelial cases, in an identical percentage, although in different cases. In our series, β6 integrin staining patterns are different from the other integrins because 2 different patterns of positivity in malignant epithelial cells are present: cell membrane homogeneous phenotype and granular cytoplasmatic staining. These results may be related with β6 integrin participation in different heterodimers. β6 integrin expression was reported to be associated with unfavorable prognosis in different cancer types.33 Although in this series, probably due to the small number of cases, β6 integrin is not directly associated with prognosis, its homogeneous phenotype is associated with high Ki67, as well as with ATF3, p16, and FATP1 homogeneous phenotype, which are related to worse prognosis.
Fatty acid synthase is highly expressed in many conditions, including female BC carcinomas. Fatty acid synthase is a multifunctional protein, involved in the synthesis of long-chain saturated fatty acids. It was reported to be expressed in some benign and preinvasive female breast in basal/suprabasal cells.34 This marker has also been significantly correlated with grade, stage, and worse OS in cancers such as ovarian cancer.35 In triple-negative female BC, FASN was significantly associated with positive lymph nodes, but not with OS or DFS.36 In proliferating neoplastic cells, fatty acids can be synthesized de novo to provide lipids for membrane formation and energy production.37 In female BC, Menendez and Lupu38 described a complex molecular interaction, occurring at multiple levels, between endogenous fatty acid metabolism and ERα signaling, and the capacity of FASN to regulate ERα may represent an effective therapeutic strategy involving FASN inhibitors. Interestingly, in our series of ERα-positive male BC cases, FASN epithelial homogeneous phenotype is significantly associated with shorter DFS and worse OS, in contrast to the reported in triple-negative female BC. Moreover, FASN is also associated with Ki67, AR, ATF3, p16, and FATP1 expression. Fatty acid synthase is rarely positive in fibroblasts in male BC, suggesting that its particular relevance is limited to the epithelial cell compartment.
Fatty acid transport protein 1, encoded by the SLC27a1 gene, was reported to be expressed in cells and tissues with high-level fatty acid import for metabolism or storage, like the adipose tissue.39 The present series is the only study evaluating FATP1 immunophenotype pattern in BC epithelial cells and fibroblasts. Our group has unraveled, in vitro, a role for FATP1 in the metabolic cross-talk between female BC cells MDA-MB-231 and cancer-associated fibroblasts.40 In this study, according to in vitro results, FATP1 stains numerous stromal fibroblasts in male BC, which are positive in the malignant epithelial cells as well, favoring the FATP1 modulation between epithelial and stromal components in these conditions. In normal tissues, lipid droplets are storage organelles for lipids and proteins. These lipids and proteins can traffic between lipid droplets and endoplasmic reticulum, and FATP1 may have a role in facilitating lipid droplet transport at this interface.41-44 The origin and significance of nuclear lipid droplets is uncertain, but the inner nuclear membrane can metabolize lipids and regulate transcription in response to lipid availability.45,46 Recent understanding of the mechanisms of interaction between chromatin and lipids suggests that small lipid molecules can regulate main nuclear functions. Lipids that bind to nucleosomes and affect chromatin are likely to be valuable as tools to modify phenotypes at a molecular level.47,48 Remarkably, in this study, FATP1 has an unexpected nuclear staining, although a concomitant diffuse cytoplasmatic staining with variable intensity was observed in some cases. Fatty acid transport protein 1 homogeneous phenotype is significantly associated with high Ki67, FASN, p16, integrin β6, and ATF3 malignant epithelial homogeneous phenotype, and high-stage and luminal B-like (HER2-negative) carcinomas. The association with high Ki67 supports the fact that, in highly proliferative lesions, cells have increased metabolic demands undertaken by fatty acid metabolism pathway. This finding should be confirmed by other studies, as clinical inhibitors for different steps in fatty acids pathways already exist,49 and their use might be applied in male BC patients.
Collagens are the major structural component of the stroma and may modulate the genesis and progression of carcinomas. There are 28 collagens organized into subgroups, including the fibrillar-forming collagens and the network-forming collagens. Collagen I is a fibrillar-forming collagen and collagen IV forms an interlaced network at basement membrane, found at the basal surface of epithelial and endothelial cells, and essential for tissue polarity and molecular filtration function.50-53 Compared with normal tissue, the amount of collagen I was reported to be augmented in female BC and its dysregulation may affect the behavior of malignant cells.53 In the present series, collagen I strong intensity is significantly associated with ATF3 epithelial homogeneous phenotype, although collagen I is not associated with prognosis. However, the intense and diffuse homogeneous collagen IV stromal immunophenotype is significantly associated with a shorter DFS. This finding may be associated with intense stromal remodulation and supports previous studies that showed the potential value of inhibiting collagen IV synthesis or deposition to control female BC progression.54
As final considerations, we would like to underline that most patients included in this study had clinicopathologic features classically associated with “good prognosis,” such as luminal-like subtypes and anatomic stage I/II. In consequence, we found a low percentage of disease recurrence and death (17.5%). However, these conditions are commonly found in the current clinical management of BC, emphasizing the importance of the results obtained. The molecular markers p16, ATF3, β6 integrin, FASN, and FATP1 are significantly intercorrelated, if homogeneous epithelial staining is present. They are all also significantly related to high cell proliferation, as assessed by Ki67. With the exception of FASN, all these biomarkers stained >10% of fibroblasts in malignant epithelial positive cases, and p16 also stained fibroblasts in malignant epithelial negative cases. Of note, the study reveals the significant associations of homogeneous malignant epithelial staining of FASN with DFS and OS, as well as of ATF3 with OS and collagen IV with DFS.
Conclusions
This study is preliminary, in part limited by its small sampling size, and therefore does not allow definitive assumptions. However, it highlights the potential value of intratumoral epithelial cell homogeneity of biomarkers with distinct biological functions, their interactions, and significant association with high Ki67 cell proliferation. As novel finding, the prognostic relevance of the homogeneous phenotype of FASN and ATF3, as well as the diffuse and intense collagen IV stromal staining, identifies male BC patients with worse outcome. These biomarkers deserve further investigation and may have innovative application in the clinical management of male BC.
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Giovani L. Silva was supported by the Fundação para a Ciência e Tecnologia (FCT) under Project UIDB/00006/2020.
Declaration of Conflicting Interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: SA and AEP participated in the project design and data analysis and wrote the article. GLS performed the statistical analyses. FS and JS performed and evaluated the IHC analyses. AF participated in the coordination of the project and review of the article. All authors read and approved the final article.
ORCID iD: Giovani L Silva  https://orcid.org/0000-0002-7434-2383
https://orcid.org/0000-0002-7434-2383
References
- 1. Gao Y, Heller SL, Moy L. Male breast cancer in the age of genetic testing: an opportunity for early detection, tailored therapy, and surveillance. Radiographics. 2018;38:1289-1311. doi: 10.1148/rg.2018180013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Abdelwahab Yousef AJ. Male breast cancer; Epidemiology and risk factors. Semin Oncol. 2017;44:267-272. doi: 10.1053/j.seminoncol.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 3. Lees T, Cullinane A, Condon A, Shabaan AM, Humphries MP, Speirs V. Characterising the adipose-inflammatory microenvironment in male breast cancer. Endocr Relat Cancer. 2018;25:773-781. doi: 10.1530/ERC-17-0407. [DOI] [PubMed] [Google Scholar]
- 4. Shaaban AM. Pathology of the male breast. Diagn Histopathol. 2019;25:138-142. [Google Scholar]
- 5. Aleskandarany MA, Vandenberghe ME, Marchiò C, Ellis IO, Sapino A, Rakha EA. Tumour heterogeneity of breast cancer: from morphology to personalised medicine. Pathobiology. 2018;85:23-34. doi: 10.1159/000477851. [DOI] [PubMed] [Google Scholar]
- 6. André S, Pereira T, Silva F, et al. Male breast cancer: specific biological characteristics and survival in a Portuguese cohort. Mol Clin Oncol. 2019;10:644-654. doi: 10.3892/mco.2019.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zaha DC. Significance of immunohistochemistry in breast cancer. World J Clin Oncol. 2014;5:382-392. doi: 10.5306/wjco.v5.i3.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Köbel M, Piskorz AM, Lee S, et al. Optimized p53 immunohistochemistry is an accurate predictor of TP53 mutation in ovarian carcinoma. J Pathol Clin Res. 2016;2:247-258. doi: 10.1002/cjp2.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lakhani SR, Ellis IO, Schnitt SJ, Tan PH, van de Vijver MJ, eds. World Health Organization Classification of Tumors of the Breast. WHO Classification of Tumours. Lyon, France: IARC; 2012. [Google Scholar]
- 10. Amin MB, Edge SB, Greene FL, et al. AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer; 2017. doi: 10.1007/978-3-319-40618-3. [DOI] [Google Scholar]
- 11. Schäler J, Thaller G, Hinrichs D. A Language and Environment for Statistical Computing. Vol. 9. Vienna, Austria. R Foundation for Statistical Computing; 2018. [Google Scholar]
- 12. Liu N, Johnson KJ, Ma CX. Male breast cancer: an updated surveillance, epidemiology, and end results data analysis. Clin Breast Cancer. 2018;18:e997-e1002. doi: 10.1016/j.clbc.2018.06.013. [DOI] [PubMed] [Google Scholar]
- 13. Werb Z, Lu P. The role of stroma in tumor development. Cancer J. 2015;21:250-253. doi: 10.1097/PPO.0000000000000127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. LeBleu VS, Kalluri R. A peek into cancer-associated fibroblasts: origins, functions and translational impact. Dis Model Mech. 2018;11:dmm029447. doi: 10.1242/dmm.029447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang Y, Xu H, Zhu B, Qiu Z, Lin Z. Systematic identification of the key candidate genes in breast cancer stroma. Cell Mol Biol Lett. 2018;23:44. doi: 10.1186/s11658-018-0110-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Reiter JG, Baretti M, Gerold JM, et al. An analysis of genetic heterogeneity in untreated cancers. Nat Rev Cancer. 2019;19:639-650. doi: 10.1038/s41568-019-0185-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nieto CM, Rider LC, Cramer SD. Influence of stromal-epithelial interactions on androgen action. Endocr Relat Cancer. 2014;21:T147-T160. doi: 10.1530/ERC-14-0138. [DOI] [PubMed] [Google Scholar]
- 18. Giovannelli P, Di Donato M, Galasso G, Di Zazzo E, Bilancio A, Migliaccio A. The androgen receptor in breast cancer. Front Endocrinol (Lausanne). 2018;9:492. doi: 10.3389/fendo.2018.00492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Di Lauro L, Barba M, Pizzuti L, et al. Androgen receptor and antiandrogen therapy in male breast cancer. Cancer Lett. 2015;368:20-25. doi: 10.1016/j.canlet.2015.07.040. [DOI] [PubMed] [Google Scholar]
- 20. Song Q, Chen Q, Wang Q, et al. ATF-3/miR-590/GOLPH3 signaling pathway regulates proliferation of breast cancer. BMC Cancer. 2018;18:255. doi: 10.1186/s12885-018-4031-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Buganim Y, Madar S, Rais Y, et al. Transcriptional activity of ATF3 in the stromal compartment of tumors promotes cancer progression. Carcinogenesis. 2011;32:1749-1757. doi: 10.1093/carcin/bgr203. [DOI] [PubMed] [Google Scholar]
- 22. Wang H, Jiang M, Cui H, et al. The stress response mediator ATF3 represses androgen signaling by binding the androgen receptor. Mol Cell Biol. 2012;32:3190-3202. doi: 10.1128/MCB.00159-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lebok P, Roming M, Kluth M, et al. P16 overexpression and 9p21 deletion are linked to unfavorable tumor phenotype in breast cancer. Oncotarget. 2016;7:81322-81331. doi: 10.18632/oncotarget.13227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Di Sante G, Di Rocco A, Pupo C, Casimiro MC, Pestell RG. Hormone-induced DNA damage response and repair mediated by cyclin D1 in breast and prostate cancer. Oncotarget. 2017;8:81803-81812. doi: 10.18632/oncotarget.19413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ortiz AB, Garcia D, Vicente Y, Palka M, Bellas C, Martin P. Prognostic significance of cyclin D1 protein expression and gene amplification in invasive breast carcinoma. PLoS ONE. 2017;12:e0188068. doi: 10.1371/journal.pone.0188068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pestell TG, Jiao X, Kumar M, et al. Stromal cyclin D1 promotes heterotypic immune signaling and breast cancer growth. Oncotarget. 2017;8:81754-81775. doi: 10.18632/oncotarget.19953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kanthan R, Fried I, Rueckl T, Senger JL, Kanthan SC. Expression of cell cycle proteins in male breast carcinoma. World J Surg Oncol. 2010;8:10. doi: 10.1186/1477-7819-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nisticò P, Di Modugno F, Spada S, Bissell MJ. β1 and β4 integrins: from breast development to clinical practice. Breast Cancer Res. 2014;16:459. doi: 10.1186/s13058-014-0459-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pan B, Guo J, Liao Q, Zhao Y. β1 and β3 integrins in breast, prostate and pancreatic cancer: a novel implication. Oncol Lett. 2018;15:5412-5416. doi: 10.3892/ol.2018.8076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Eiro N, Gonzalez LO, Fraile M, Cid S, Schneider J, Vizoso FJ. Breast cancer tumor stroma: cellular components, phenotypic heterogeneity, intercellular communication, prognostic implications and therapeutic opportunities. Cancers (Basel). 2019;11:664. doi: 10.3390/cancers11050664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Harjunpää H, Llort Asens M, Guenther C, Fagerholm SC. Cell adhesion molecules and their roles and regulation in the immune and tumor microenvironment. Front Immunol. 2019;10:1078. doi: 10.3389/fimmu.2019.01078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sökeland G, Schumacher U. The functional role of integrins during intra- and extravasation within the metastatic cascade. Mol Cancer. 2019;18:12. doi: 10.1186/s12943-018-0937-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Niu J, Li Z. The roles of integrin αvβ6 in cancer. Cancer Lett. 2017;403:128-137. doi: 10.1016/j.canlet.2017.06.012. [DOI] [PubMed] [Google Scholar]
- 34. Jensen KC, Schaeffer DF, Cheang M, et al. Characterization of a novel anti-fatty acid synthase (FASN) antiserum in breast tissue. Mod Pathol. 2008;21:1413-1420. doi: 10.1038/modpathol.2008.163. [DOI] [PubMed] [Google Scholar]
- 35. Cai Y, Wang J, Zhang L, et al. Expressions of fatty acid synthase and HER2 are correlated with poor prognosis of ovarian cancer. Med Oncol. 2015;32:391. doi: 10.1007/s12032-014-0391-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Giró-Perafita A, Sarrats A, Pérez-Bueno F, et al. Fatty acid synthase expression and its association with clinico-histopathological features in triple-negative breast cancer. Oncotarget. 2017;8:74391-74405. doi: 10.18632/oncotarget.20152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Baenke F, Peck B, Miess H, Schulze A. Hooked on fat: the role of lipid synthesis in cancer metabolism and tumour development. Dis Model Mech. 2013;6:1353-1363. doi: 10.1242/dmm.011338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Menendez JA, Lupu R. Fatty acid synthase regulates estrogen receptor-α signaling in breast cancer cells. Expert Opin Ther Targets. 2017;21:1001-1016. doi: 10.1038/oncsis.2017.4. [DOI] [PubMed] [Google Scholar]
- 39. Wu Q, Ortegon AM, Tsang B, Doege H, Feingold KR, Stahl A. FATP1 is an insulin-sensitive fatty acid transporter involved in diet-induced obesity. Mol Cell Biol. 2006;26:3455-3467. doi: 10.1128/MCB.26.9.3455-3467.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lopes-Coelho F, André S, Félix A, Serpa J. Breast cancer metabolic cross-talk: fibroblasts are hubs and breast cancer cells are gatherers of lipids. Mol Cell Endocrinol. 2018;462:93-106. doi: 10.1016/j.mce.2017.01.031. [DOI] [PubMed] [Google Scholar]
- 41. Xu N, Zhang SO, Cole RA, et al. The FATP1-DGAT2 complex facilitates lipid droplet expansion at the ER-lipid droplet interface. J Cell Biol. 2012;198:895-911. doi: 10.1083/jcb.201201139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Barbosa AD, Siniossoglou S. Function of lipid droplet-organelle interactions in lipid homeostasis. Biochim Biophys Acta Mol Cell Res. 2017;1864:1459-1468. doi: 10.1016/j.bbamcr.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 43. Wu H, Carvalho P, Voeltz GK. Here, there, and everywhere: the importance of ER membrane contact sites. Science. 2018;361:eaan5835. doi: 10.1126/science.aan5835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Henne WM, Goodman JM, Hariri H. Spatial compartmentalization of lipid droplet biogenesis. Biochim Biophys Acta Mol Cell Biol Lipids. 2020;1865:158499. doi: 10.1016/j.bbalip.2019.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Merta H, Bahmanyar S. The inner nuclear membrane takes on lipid metabolism. Dev Cell. 2018;47:397-399. doi: 10.1016/j.devcel.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 46. Sołtysik K, Ohsaki Y, Tatematsu T, Cheng J, Fujimoto T. Nuclear lipid droplets derive from a lipoprotein precursor and regulate phosphatidylcholine synthesis. Nat Commun. 2019;10:473. doi: 10.1038/s41467-019-09294-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Esteves A, Knoll-Gellida A, Canclini L, Silvarrey MC, André M, Babin PJ. Fatty acid binding proteins have the potential to channel dietary fatty acids into enterocyte nuclei. J Lipid Res. 2016;57:219-232. doi: 10.1194/jlr.M062232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fernandes V, Teles K, Ribeiro C, Treptow W, Santos G. Fat nucleosome: role of lipids on chromatin. Prog Lipid Res. 2018;70:29-34. doi: 10.1016/j.plipres.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 49. Currie E, Schulze A, Zechner R, Walther TC, Farese RV, Jr. Cellular fatty acid metabolism and cancer. Cell Metab. 2013;18:153-161. doi: 10.1016/j.cmet.2013.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fang M, Yuan J, Peng C, Li Y. Collagen as a double-edged sword in tumor progression. Tumour Biol. 2014;35:2871-2882. doi: 10.1007/s13277-013-1511-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kim SH, Lee HY, Jung SP, et al. Role of secreted type I collagen derived from stromal cells in two breast cancer cell lines. Oncol Lett. 2014;8:507-512. doi: 10.3892/ol.2014.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Natal RA, Paiva GR, Pelegati VB, et al. Exploring collagen parameters in pure special types of invasive breast cancer. Sci Rep. 2019;9:7715. doi: 10.1038/s41598-019-44156-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Nissen NI, Karsdal M, Willumsen N. Collagens and cancer associated fibroblasts in the reactive stroma and its relation to cancer biology. J Exp Clin Cancer Res. 2019;38:115. doi: 10.1186/s13046-019-1110-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Revert F, Revert-Ros F, Blasco R, et al. Selective targeting of collagen IV in the cancer cell microenvironment reduces tumor burden. Oncotarget. 2018;9:11020-11045. doi: 10.18632/oncotarget.24280. [DOI] [PMC free article] [PubMed] [Google Scholar]