Abstract
Background:
The limb symmetry index may overestimate the recovery of quadriceps muscle strength after anterior cruciate ligament reconstruction. Comparison of individuals who have had anterior cruciate ligament reconstruction with age-, sex-, and activity-matched individuals might be more appropriate to guide rehabilitation interventions.
Purpose:
To compare the quadriceps strength between the injured limb of people with anterior cruciate ligament reconstruction and the limb of an age-, sex-, and activity-matched control group.
Study Design:
Systematic review; Level of evidence, 3.
Methods:
MEDLINE, CINAHL, EMBASE, SCOPUS, and SPORTDiscus were searched between inception and April 2019. Studies were included if they reported the peak quadriceps strength for persons with anterior cruciate ligament reconstruction and age-, sex-, and activity-matched control groups measured using isometric or isokinetic dynamometry. Risk of bias was assessed, and meta-analyses and metaregression (for effect of time since surgery) were performed.
Results:
A total of 2759 studies were identified and 21 were included for analyses. Quadriceps strength was lower in the limbs with anterior cruciate ligament reconstruction compared with the limb from matched controls within 6 months of anterior cruciate ligament reconstruction (standardized mean difference [SMD], –1.42; 95% CI, –1.62 to –1.23), 6 to 18 months after anterior cruciate ligament reconstruction (SMD, –0.92; 95% CI, –1.18 to –0.66), and >18 to 48 months after anterior cruciate ligament reconstruction (SMD, –0.38; 95% CI, –0.79 to 0.03). Results of the metaregression were significant, with the difference between anterior cruciate ligament reconstruction and matched controls decreasing with time since surgery (P < .001).
Conclusion:
In people with anterior cruciate ligament reconstruction, the injured limb had lower quadriceps strength compared with the limb of age-, sex-, and activity-matched controls up to 4 years after surgery. Clinicians should consider comparison with matched cohorts for return to sports decision making.
Keywords: return to sports, rehabilitation, limb symmetry index, ACL
Given the ubiquity of quadriceps muscle weakness after anterior cruciate ligament (ACL) injury and surgery, the recovery of quadriceps strength is a key goal of the rehabilitation protocols for individuals who undergo reconstruction (ACLR).15,31 The recovery of quadriceps strength before returning to sports after ACLR is often assessed by comparing the strength of the injured limb with the strength of the uninjured limb, a measure known as the quadriceps limb symmetry index (LSI).12,13 Achieving a predetermined quadriceps LSI, usually 90%, is used to determine readiness to return to sports.12,52 Although current protocols recommend using LSI as a criterion for return to sports, quadriceps limb symmetry measures may overestimate the recovery of quadriceps function in the injured limb.4,8,56 Hence, it is important to establish more objective measures of quadriceps strength recovery in this population.
A previous systematic review reported persistent asymmetry in quadriceps strength between the injured and contralateral limbs 12 months after ACLR.31 It is likely that there are bilateral deficits in quadriceps function among individuals with ACLR.14,35,47,56 Athletes who return to sports with inadequate quadriceps strength or other neuromuscular deficits in quadriceps function may be placing themselves at increased risk for ACL reinjury or posttraumatic osteoarthritis.13,56 Therefore, rather than comparing quadriceps strength of the limb with ACLR with that of the uninjured contralateral extremity, comparisons with the uninjured limb of individuals of similar age, sex, and activity levels may be needed to help accurately assess strength recovery.56 A recent systematic review compared isometric quadriceps strength in both ACLR and contralateral limbs with that in the limb of control participants.32 The authors reported isometric quadriceps strength was lower in both ACLR and contralateral limbs than in the limb of the control participants. However, in this review, studies were not selected to ensure that the control group was matched for age, sex, and activity levels to the ACLR participants. Furthermore, the authors in the prior review only included studies that assessed isometric quadriceps strength when isokinetic measures are recommended while assessing return to sports in individuals with ACLR.13
The primary objectives of our review were (1) to compare the quadriceps strength between the injured limb in individuals with ACLR and the limb of age-, sex-, and activity-matched controls using meta-analyses of published data and (2) to assess the effect of time since surgery on the difference between the ACLR and control groups using metaregression analyses of published data. We hypothesized that quadriceps strength would be lower in the injured limb of individuals after ACLR than in the limb of matched controls and that this difference between groups would decrease as time since surgery increased. A secondary objective was to compare quadriceps strength between the uninjured contralateral limb and the limb of the age-, sex-, and activity-matched control group. We also explored the effects of graft type and strength measurement technique (isometric or isokinetic) where possible.
Methods
The study protocol was developed according to guidelines provided by the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement. The protocol was prospectively registered on the PROSPERO international register for systematic reviews (https://www.crd.york.ac.uk/PROSPERO) (CRD42018083765).
Search Strategy
We followed guidelines provided by the Cochrane Collaboration and developed a search strategy of the following databases: MEDLINE, EMBASE, SPORTDiscus, CINAHL, and SCOPUS.16 Search terms were entered into each database as follows: (anterior cruciate OR anterior cruciate ligament OR ACL) AND (reconstruct* OR surg*) AND (quadricep* OR extens*) AND (strength OR power OR torque). Additional terms were added to EMBASE, CINAHL, and SCOPUS search strings in order to exclude MEDLINE results. Three researchers (C.B., L.M., D.K.) screened titles and abstracts from the retrieved results. All identified studies that appeared suitable for inclusion were independently reviewed by at least 2 researchers (C.B., D.K.) for confirmation. The complete search strategy is shown in Appendix Table A1.
Study Selection
We included studies based on the following criteria: case-control, cross-sectional, cohort, or randomized clinical trial (RCT) studies that included participants with a primary ACLR who were on average within 4 years of surgery and a healthy uninjured control group. Only studies that included peak isometric and/or isokinetic quadriceps strength outcomes measured using an instrumented dynamometer were included. Only studies that reported a priori matching of experimental and control groups for age, sex, and activity or had statistically insignificant differences in these categories were included. The search included studies published from inception until April 2019. Only studies published in the English language were included. For RCTs, only baseline data were included, and these were only included when an uninjured matched control group was also available.
We excluded studies based on the following criteria: studies using handheld dynamometry or manual muscle testing; studies that included individuals who underwent revision ACLR, bilateral ACLR, or other bony procedures at the same time as the primary ACLR (eg, osteotomy); studies that did not report peak torque data; and studies that did not attempt to quantify the patient’s level of activity through established scales or other methods. We excluded case reports, systematic reviews and meta-analyses, conference abstracts, and gray literature. We excluded studies that included participants who were skeletally immature, which we operationally defined as age <13 years. We set no limits on publication date, study size, graft type, sex, or recruitment method. We excluded studies that used a handheld dynamometer (HHD) for assessing quadriceps strength because strength assessment using an isokinetic dynamometer is considered the gold standard; there can be considerable variability in how HHD is used (eg, with or without belt stabilization), the correlation between HHD and isokinetic measures has been reported to be poor to moderate, and the measurements collected using a HHD have been reported to be lower than those collected using an isokinetic dynamometer.5,33,38
Risk of Bias Assessment
We used a modified Downs and Black checklist to assess study quality and risk of bias.9 As applied in recent publications, 16 questions were included in the modified checklist (Appendix Table A2).21,41 Item 27 was used to assess the power of the study. Similar to previous research, the maximum score for item 27 was modified to be 1 (a power analysis was conducted) instead of 5.21,23,41 The total possible score was 17; studies with scores of 11 or greater (≥65%) were considered to have a low risk of bias (LR), and studies with scores <11 (<65%) were considered to have a high risk of bias (HR).3 Two investigators independently assessed each included study using this checklist (C.B., L.M.). Any disagreements between the 2 investigators were resolved in a consensus meeting that involved a third investigator (D.K.).
Data Management and Statistical Analysis
Relevant data were independently extracted by 2 reviewers (C.B., D.K.) to ensure accuracy. Data were extracted for the primary outcomes (mean and SD of peak quadriceps torque for ACLR and control groups, sample size) and participant characteristics (age, sex, weight, height, body mass index, Tegner activity scale score, graft type, time since surgery). Other information recorded included study characteristics (design, setting, population description, inclusion criteria, exclusion criteria, method of recruitment) and strength measurement characteristics (isokinetic/isometric, measurement angle/speed, use of gravity correction, use of warm-up trials, number of repetitions, and duration of rest period between repetitions).
Data were pooled for analyses using RevMan V5.3. Primary analyses compared the peak quadriceps strength between the ACLR and matched control groups stratified by time since surgery: 0 to 6 months, 6 to 18 months, and >18 months. A meta-analysis of previous studies has reported that the mean time to return to sports can range from 6 to 13 months after ACLR.27 Hence, these time periods reflect the early rehabilitation phase, return-to-sports phase, and longer-term recovery phase. Standardized mean differences (SMDs) and 95% CIs were calculated for peak quadriceps strength because units of measurement difference across studies. Pooled analyses were only conducted when at least 4 studies were available for pooling. Random-effects models were used for the meta-analyses. Heterogeneity across the studies was assessed using the I2 statistic. The pooled SMD was interpreted using Cohen criteria, where SMD ≥0.8 indicated a large effect, SMD between 0.5 and 0.8 indicated a medium effect, and SMD between 0.2 and 0.5 indicated a small effect. Forest plots were developed for the pooled analyses, and funnel plots and the Egger test were used to assess for bias. To explore the effects of time since surgery on the differences between the ACLR and control groups, a metaregression was performed.
Secondary analyses included comparison of the contralateral uninjured limb in people with ACLR with the limb of matched controls. Exploratory sensitivity analyses were performed by repeating the primary analyses while only including studies with patients who received an autogenous patellar tendon or hamstring tendon graft and to assess differences in quadriceps strength between the ACLR and matched control groups stratified by method of strength assessment (isometric or isokinetic). Studies that did not describe the graft type or included participants who received allografts were excluded from this sensitivity analysis.
Level of Evidence
Level of evidence was determined as done in previous studies.21,41,54 Specifically, the following definitions were used for pooled analyses: (1) Strong evidence was defined as pooled results derived from ≥3 studies, including a minimum of 2 LR studies, which are statistically homogeneous (P > .05) and may be associated with a statistically significant or nonsignificant result. (2) Moderate evidence was defined as statistically significant pooled results derived from multiple studies, including at least 1 LR study, which are statistically heterogeneous (P < .05), or from multiple HR studies that are statistically homogeneous (P > .05). (3) Limited evidence was defined as results from multiple HR studies that are statistically heterogeneous (P < .05) or from 1 LR study. (4) Very limited evidence was defined as results from 1 HR study. (5) Conflicting evidence was defined as pooled results that are insignificant and derived from multiple studies, regardless of quality, which are statistically heterogeneous (P < .01 or I2 > 60%).
Results
Search Results
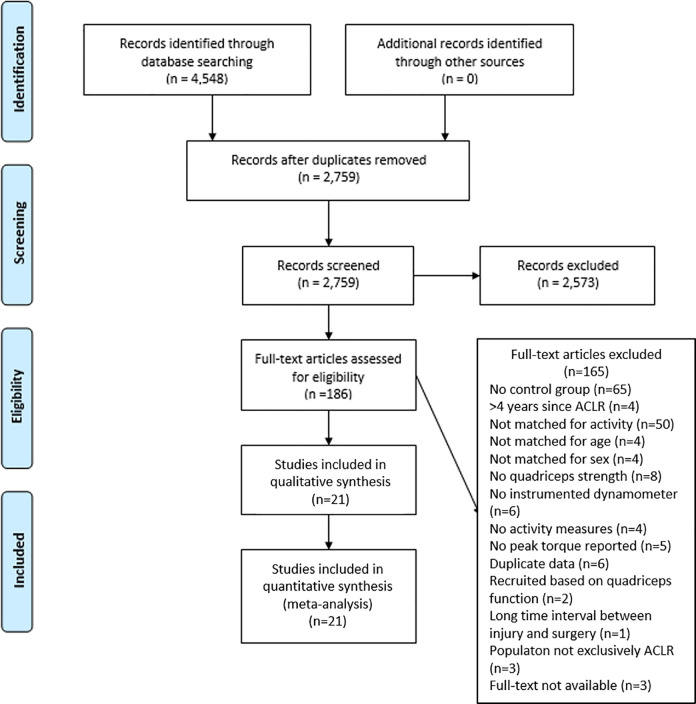
The search strategy yielded 4548 titles (Figure 1). After the removal of duplicates, 2759 titles and abstracts were screened. The full texts of 186 papers were retrieved, and 21 studies met the study criteria and were included for the meta-analyses.∥
Figure 1.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) diagram. ACLR, anterior cruciate ligament reconstruction.
Risk of Bias
The results from the study quality assessment using a modified Downs and Black checklist are shown in Appendix Table A3. Total scores ranged from 11 to 16 out of a maximum possible score of 17. All 21 included studies were rated as having an LR.
Included Studies
The characteristics of the 21 included studies are summarized in Tables 1 and 2. Outcomes were reported for 730 participants with ACLR and 590 age-, sport-, and sex-matched uninjured controls. For the primary analysis of the 21 studies, we used data from isometric quadriceps strength testing from 10 studies and data from isokinetic testing from 11 studies. The studies reported strength in newton meters, newton meters per kilogram, newtons, or newtons per kilogram. Eight studies included patients who received autogenous patellar tendon grafts, 3 studies included patients who received autogenous hamstring tendon grafts, 6 studies included patients with a combination of graft types (with 3 using autogenous hamstring or patellar tendon grafts and 3 using autogenous hamstring tendon grafts, autogenous patellar tendon grafts, and allografts), and 4 studies did not report graft type (Table 1). The follow-up length ranged from 3 to 48 months.
Table 1.
Study Design, Graft Type, Time Since Surgery, Strength Measurement, and Limb Information for Included Studies (in Reverse Chronological Order)a
| Lead Author, Year | Design | Graft Type | Time Since Surgery, mo | Strength Measurement Type | Difference in Strength, % |
|---|---|---|---|---|---|
| Almeida, 20181 | Case-control | Hamstring tendon | 6 | Isokinetic at 60 (used) and 240 deg/s | 18 (ACLR < C) |
| Boo, 20186 | Cross-sectional | Not reported | 7.3 | Isokinetic at 60 deg/s | 24 (ACLR < C) |
| Garrison, 201810 | Cross-sectional | Not reported | 3 | Isokinetic at 60 deg/s | 45 (ACLR < C) |
| Johnson, 201819 | Cross-sectional | Patellar tendon | 7.5 ± 1.4 | Isometric at 90° (used); isokinetic at 60 deg/s | 25 (ACLR < C) |
| O’Malley, 201840 | Cross-sectional | Patellar tendon | 6.6 ± 1 | Isokinetic at 60 deg/s | 23 (ACLR < C) |
| Pamukoff, 201842 | Cross-sectional | Combination of graft types (patellar tendon, hamstring tendon, or allograft) | 48.0 ± 25.0 | Isometric at 45° (used); isokinetic at 60, 180, and 240 deg/s | 10 (ACLR < C) |
| Pelegrinelli, 201845 | Case-control | Hamstring tendon | 5 (4.5-6)b | Isokinetic at 60, 120, and 300 deg/s | 18 (ACLR < C) |
| Mirkov, 201735 | Cross-sectional | Patellar tendon | 4 ± 0.3 | Isometric at 45° | 23 (ACLR < C) |
| Kuenze, 201725 | Clinical trial | Combination of graft types (patellar tendon or hamstring tendon) | 27.9 ± 16 | Isometric at 90° | 20 (ACLR < C) |
| Goetschius, 201611 | Cross-sectional | Not reported | 44.1 ± 29.9 | Isometric at 90° | 13 (ACLR < C) |
| Zwolski, 201659 | Cross-sectional | Not reported | 9.2 ± 2.2 | Isometric at 60° | 17 (ACLR < C) |
| Lepley, 201530 | Case-control | Combination of graft types (patellar tendon or hamstring tendon) | Presurgery and 6.5 post-ACLR (used) | Isometric at 90° | 28 (ACLR < C) |
| Kuenze, 201526 | Cross-sectional | Combination of graft types (patellar tendon or hamstring tendon) | 31.5 ± 23.5 | Isometric at 90° | 10 (ACLR < C) |
| Chung, 20157 | Cohort | Hamstring tendon | 3 (used), 6, 12, and 24 (used) | Isokinetic at 60 (used) and 180 deg/s | 39 (pre-RTS: ACLR < C); 17 (post-RTS: ACLR < C) |
| Hsieh, 201517 | Cross-sectional | Combination of graft types (patellar tendon, hamstring tendon, or allograft) | 2.8 | Isokinetic at 60 deg/s | 36 (ACLR < C) |
| Lepley, 201429 | Cross-sectional | Combination of graft types (patellar tendon, hamstring tendon, or allograft) | 48.2 ± 35.5 | Isometric at 90° | 15 (ACLR < C) |
| Mohammadi, 201336
|
Randomized controlled trial | Patellar tendon and hamstring tendon (separate cohorts) | Patellar tendon, 8.1 ± 1.4; hamstring tendon, 8.2 ± 1.8 |
Isokinetic at 60 (used) and 180 deg/s | 15 (ACLR < C) |
| Xergia, 201358 | Cross-sectional | Patellar tendon | 7 ± 0.9 | Isokinetic at 120 (used), 180, and 300 deg/s | 24 (ACLR < C) |
| Thomas, 201350 | Cross-sectional | Patellar tendon | Presurgery and 7 post-ACLR (used) | Isokinetic at 60 deg/s | 17 (ACLR < C) |
| Rudroff, 200348 | Case-control | Patellar tendon | 24 | Isometric at 90° | 12 (ACLR > C) |
| Mattacola, 200234 | Cross-sectional | Patellar tendon | 18 ± 10 | Isokinetic at 120 (used) and 240 deg/s | 15 (ACLR < C) |
aACLR, anterior cruciate ligament reconstruction; C, control; RTS, return to sport. Used indicates that data from that particular test were included in the meta-analyses.
bReported as median.
Table 2.
Age, Sex, and Activity Information for Included Studies (in Reverse Chronological Order)a
| Lead Author, Year | n | Age (y) | Sex (% Female) | Activity Level | ||||
|---|---|---|---|---|---|---|---|---|
| ACLR | Control | ACLR | Control | ACLR | Control | ACLR | Control | |
| Almeida, 20181 | 20 | 20 | 21 (18-28) | 20.5 (18-34) | 0 | 0 | Professional soccer players | Professional soccer players |
| Boo, 20186 | 17 | 17 | 14.7 ± 1 | 14.5 ± 1.1 | 100 | 100 | Level 1 sports | Level 1 sports |
| Garrison, 201810 | 24 | 24 | 15.5 ± 1 | 15.5 ± 1.2 | Not available | Not available | Level 1 or 2 sports | Level 1 or 2 sports |
| Johnson, 201819 | 67 | 10 | 21.34 ± 5 | 23.5 ± 3.44 | 36 | 40 | Tegner, 6.38 ± 1.89 | Tegner, 6.1 ± 2.42 |
| O’Malley, 201840 | 118 | 44 | 23.6 ± 5 | 24.1 ± 3.6 | 0 | 0 | Multidirectional athletes | Multidirectional athletes |
| Pamukoff, 201842 | 38 | 38 | 21.9 ± 2 | 21.9 ± 1.3 | 76 | 76 | Tegner, 7 ± 1.7 | Tegner, 6.8 ± 1.1 |
| Pelegrinelli, 201845 | 7 | 7 | 23 (19-25) | 21.8 (19-24) | 0 | 0 | Professional soccer players and active individuals who play amateur soccer | Professional soccer players and active individuals who play amateur soccer |
| Mirkov, 201735 | 19 | 16 | 23 ± 3 | 23 ± 1 | 0 | 0 | Similar International Physical Activity Questionnaire scores | Similar International Physical Activity Questionnaire scores |
| Kuenze, 201725 | 10 | 10 | 21 ± 2.8 | 22.2 ± 3.2 | 90 | 90 | Tegner, 7.9 ± 1.3 | Tegner, 7.1 ± 1.6 |
| Goetschius, 201611 | 53 | 50 | 23.4 ± 4.9 | 23.3 ± 4.4 | 49 | 44 | Tegner, 6.8 ± 1.8 | Tegner, 6.8 ± 1.8 |
| Zwolski, 201659 | 15 | 15 | 18.2 ± 2.6 | 17.9 ± 2.2 |
100 | 100 | Level 1 or 2 sports | Level 1 or 2 sports |
| Lepley, 201530 | 20 | 20 | 20.9 ± 4.4 | 21.7 ± 3.7 | 45 | 45 | Tegner, 6.2 ± 0.9 | Tegner, 6.4 ± 0.9 |
| Kuenze, 201526 | 22 | 24 | 22.5 ± 5 | 21.7 ± 3.6 | 45 | 50 |
Tegner, 6.4 ± 1.2 | Tegner, 6.1 ± 1.7 |
| Chung, 20157 | 75 | 75 | 27.9 ± 8.6 | 27.6 ± 7 | 15 | 15 | Tegner, 6.3 ± 0.9 | Tegner, 6.4 ± 0.8 |
| Hsieh, 201517 | 28 | 28 | 19.6 ± 4.5 | 20 ± 4.3 | 50 | 50 | Level 1 and 2 sports | Level 1 and 2 sports |
| Lepley, 201429 | 29 | 29 | 21.5 ± 3.7 | 21.2 ± 2.7 | 69 | 69 | Tegner, 5.9 ± 2 | Tegner, 6 ± 1.4 |
| Mohammadi, 201336
|
Patellar tendon, 21; hamstring tendon, 21 | 21 | Patellar tendon, 24.9 ± 2; hamstring tendon, 25.2 ± 2.4 | 25.4 ± 2.9 |
0 | 0 | Tegner, 9 | Tegner, 9 |
| Xergia 201358 | 22 | 22 | 28.8 ± 11.2 | 24.8 ± 9.1 |
0 | 0 | Tegner, 7.5 | Tegner, 8 |
| Thomas, 201350 | 15 | 15 | 20.27 ± 5.4 | 24.73 ± 3.4 | 47 | 53 | Matched on Tegner (±1) | Matched on Tegner (±1) |
| Rudroff, 200348 | Patellar tendon,15; hamstring tendon, 15 | 10 |
Patellar tendon, 32.6 ± 4; hamstring tendon, 29.1 ± 6.7 |
31.1 ± 4.7 | 0 | 0 | Soccer players | Soccer players |
| Mattacola, 200234 | 20 | 20 | 25.8 ± 8.1 | 24.5 ± 6.9 | 45 | 45 | Matched using sections B and C of the Sports Participation Survey | Matched using sections B and C of the Sports Participation Survey |
aACLR, anterior cruciate ligament reconstruction.
In the case of multiple quadriceps torque measurements in a study (eg, isometric and isokinetic, multiple isokinetic speeds), we used the most common measurement across all of the included studies to minimize heterogeneity for the primary analysis (Table 1). If separate data were available for >1 graft type (2 studies), data from the patellar tendon graft cohort were included for the primary analysis, as this was most common across the included studies.36,48 The study by Chung et al7 was longitudinal in nature and reported data from multiple time points. Hence, data from this study were included in 2 strata, that is, 0 to 6 months after ACLR and >18 months after ACLR. However, for the metaregression, only the later time point was included.
Quadriceps Strength in the Injured Limb for ACLR Compared With Matched Controls
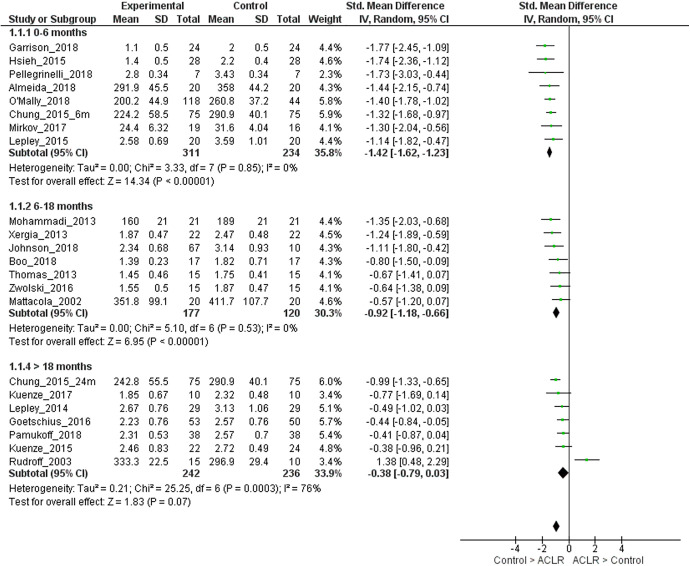
The mean time since surgery was 5 months (0- to 6-month strata), 9.2 months (6- to 18-month strata), and 35.4 months (>18-month strata). For 0 to 6 months after ACLR, strong evidence with a large effect size (8 LR studies, homogeneous) showed that limbs with ACLR had lower quadriceps strength compared with matched control limbs (SMD, –1.42; 95% CI, –1.62 to –1.23; I2 = 0%) (Figure 2).1,7,10,17,30,35,40,45 For 6 to 18 months after ACLR, strong evidence with a large effect size (7 LR studies, homogeneous) showed that ACLR limbs had lower quadriceps strength compared with matched control limbs (SMD, –0.92; 95% CI, –1.18 to –0.66; I2 = 0%) (Figure 2).6,19,34,36,50,58,59 For >18 months after ACLR, moderate evidence (7 LR studies, heterogeneous) with a small effect size showed that ACLR limbs had lower quadriceps strength when compared with matched control limbs (SMD, –0.38; 95% CI, –0.79 to 0.03; I2 = 76%) (Figure 2).7,11,25,26,29,42,48 It was seen that 1 study was causing the heterogeneity.48 On removing this study, strong evidence (6 LR studies, homogeneous) with a moderate effect size showed that ACLR limbs had lower quadriceps strength when compared with matched control limbs (SMD, –0.60; 95% CI, –0.83 to –0.37; I2 = 29%).
Figure 2.
Forest plot for comparison of quadriceps strength in the injured limb of participants with anterior cruciate ligament reconstruction (ACLR) and the control group, stratified by time since surgery. IV, inverse variance; Std, standardized.
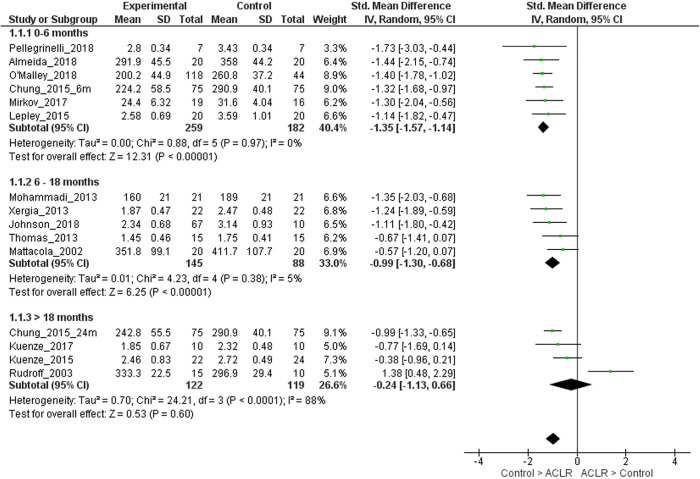
We performed sensitivity analyses including only studies with patients who received an autogenous patellar tendon or hamstring tendon graft (ie, excluding studies that did not report graft type or included patients with other graft types in addition to autogenous patellar tendon or hamstring tendon grafts). For 0 to 6 months after ACLR, strong evidence with a large effect size (6 LR studies, homogeneous) showed that limbs with ACLR had lower quadriceps strength compared with matched control limbs (SMD, –1.35; 95% CI, –1.57 to –1.14; I2 = 0%) (Appendix Figure A1).1,7,30,35,40,45 For 6 to 18 months after ACLR, strong evidence (5 LR studies, homogeneous) with a large effect size showed that ACLR limbs had lower quadriceps strength compared with matched control limbs (SMD, –0.99; 95% CI, –1.30 to –0.68; I2 = 5%) (Appendix Figure A1).19,34,36,50,58 For >18 months after ACLR, moderate evidence (4 LR studies, heterogeneous) showed that quadriceps strength was similar in ACLR and matched control limbs (SMD, –0.24; 95% CI, –1.13 to 0.66; I2 = 88%).7,25,26,48 It was seen that 1 study was causing the heterogeneity.48 On removing this study, only 3 studies remained, so pooled analyses were not performed.
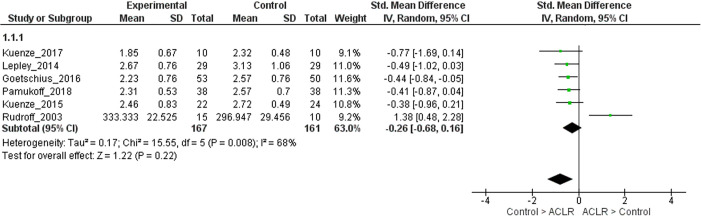
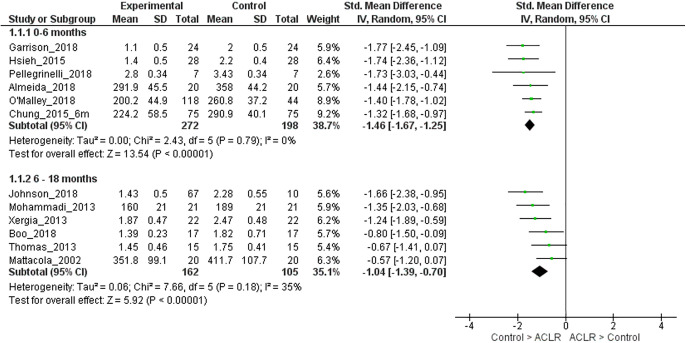
We performed sensitivity analyses to assess differences in quadriceps strength between ACLR and matched control limbs stratified by method of strength assessment (isometric or isokinetic). When the analyses were restricted to studies that reported isometric quadriceps strength, pooled analyses were not performed for the 0- to 6-month strata (only 2 studies were available)30,35 and 6- to 18-month strata (only 2 studies were available).19,59 For >18 months after ACLR, moderate evidence (6 LR studies, heterogeneous) showed that ACLR limbs had similar quadriceps strength when compared with matched control limbs (SMD, –0.26; 95% CI, –0.68 to 0.16; I2 = 68%) (Appendix Figure A2).11,25,26,29,42 It was seen that 1 study was causing the heterogeneity.48 On removing this study, strong evidence (5 LR studies, homogeneous) with a small effect size showed that ACLR limbs had lower quadriceps strength when compared with matched control limbs (SMD, –0.46; 95% CI, –0.68 to –0.23; I2 = 0%). When the analyses were restricted to studies that reported isokinetic quadriceps strength, for 0 to 6 months after ACLR, strong evidence with a large effect size (6 LR studies, homogeneous) showed that limbs with ACLR had lower isokinetic quadriceps strength compared with matched control limbs (SMD, –1.46; 95% CI, –1.67 to –1.25; I2 = 0%) (Appendix Figure A3).1,7,10,17,40,45 For the 6- to 18-month strata, strong evidence with a large effect size (6 LR studies, homogeneous) showed that ACLR limbs had lower isokinetic quadriceps strength compared with matched control limbs (SMD, –1.04; 95% CI, –1.39 to –0.70; I2 = 35%) (Appendix Figure A3).6,19,34,36,50,58 For >18 months after ACLR, pooled analysis was not performed because only 3 studies were available.7,11,42
Effect of Time Since Surgery on Difference in Quadriceps Strength Between ACLR Limbs and Matched Control Limbs
The results from the metaregression (Figure 3) showed that there was a significant association of time since surgery on the effect size (P < .001), with studies that included participants with a shorter time since ACLR showing a greater difference in quadriceps strength between the ACLR limb and matched control limb in favor of the control limb. The funnel plot for the pooled analyses can be seen in Figure 4. The Egger test was nonsignificant (P = .998), suggesting that publication bias was unlikely to have influenced these results.
Figure 3.
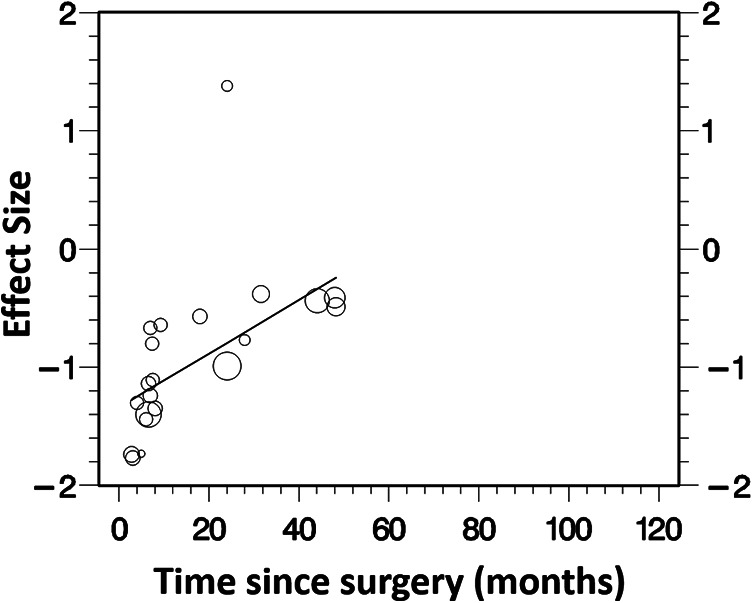
Results of metaregression for association of time since surgery with effect size.
Figure 4.
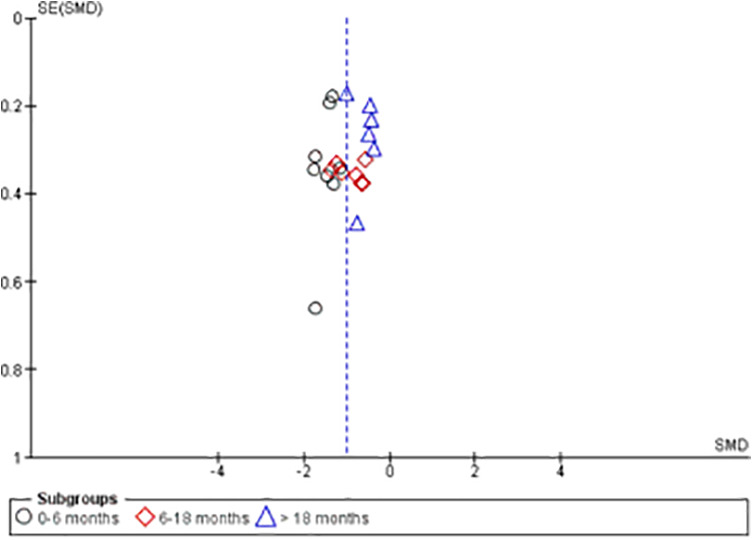
Funnel plot for included studies. SMD, standardized mean difference.
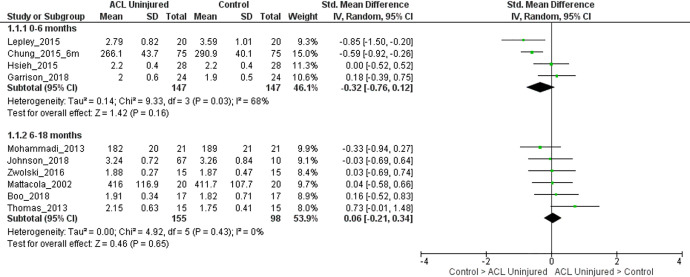
Quadriceps Strength in the Uninjured Limb of Persons With ACLR Compared With Matched Control Limbs
For 0 to 6 months after ACLR, moderate evidence (4 LR studies, heterogeneous) showed that the quadriceps strength in the uninjured limbs of persons with ACLR was similar to that in matched control limbs (SMD, –0.32; 95% CI, –0.76 to 0.12; I2 = 68%) (Figure 5).7,10,17,30 For 6 to 18 months after ACLR, strong evidence (6 LR studies, homogeneous) showed that the quadriceps strength in the uninjured limbs of persons with ACLR was similar to that in matched control limbs (SMD, 0.06; 95% CI, –0.21 to 0.34; I2 = 0%) (Figure 5).6,19,34,36,50,59 For >18 months after ACLR, pooled analyses were not performed because only 3 studies were available.7,42,48
Figure 5.
Forest plot for comparison of quadriceps strength in the uninjured limb of participants with anterior cruciate ligament (ACL) reconstruction and the control group, stratified by time since surgery. IV, inverse variance; Std, standardized.
Discussion
Our objective was to compare quadriceps strength between the injured limb of individuals with ACLR and the limb of age-, sex-, and activity-matched uninjured controls. The results showed that, regardless of time since surgery (up to 4 years after ACLR), individuals with ACLR had lower quadriceps strength in their injured limb when compared with matched healthy controls. These results suggest that the quadriceps strength deficits are evident for many years in people with ACLR despite surgery and rehabilitation when compared with an appropriate control group. Because of the critical role of knee musculature stability of the joint, especially after a ligamentous injury, deficits in muscle strength may place the patient at an increased risk for reinjury and posttraumatic knee osteoarthritis.
In our analyses, quadriceps strength deficits were evident when comparing the ACLR group with the age-, sex-, and activity-matched control group within 6 months of surgery, 6 to 18 months after surgery, and >18 months after surgery. In a recent meta-analysis of 28 studies,32 the authors compared isometric quadriceps strength in individuals with ACLR and controls and reported a pooled effect size of –0.78 (95% CI, –0.99 to –0.58) in favor of the control group. However, in our analyses, the effect sizes were larger than those reported previously when we pooled data for people within 6 months of ACLR (SMD, –1.42) and 6 to 18 months of ACLR (SMD, –0.92), and smaller than those previously reported when we pooled data for people >18 months after ACLR (SMD, –0.38). The previous review included studies with time since surgery as short as 1 month, those that did not stratify the analyses by time since surgery, and those that only included studies reporting isometric quadriceps torque. These differences in the methodologies preclude direct comparisons. However, the results show overall that people with ACLR have weaker quadriceps in their injured limb when compared with controls. The persistence of strength deficits over many years after ACLR could be related to peripheral changes in the quadriceps muscle, including chronic atrophy and changes in the architecture and composition of the muscle,24,39 activation deficits,15 and so forth. Our results showing the persistence of quadriceps strength deficits in people with ACLR when compared with age-, sex-, and activity-matched controls are based on moderate to strong evidence with an LR.
Our primary analyses do not appear to have been affected by publication bias, as can be seen in the funnel plot and the Egger test. All studies were determined to be at LR, leading to moderate to strong evidence for most of the analyses reported. Heterogeneity for the primary analyses was low, except for the 6- to 18-month strata. However, this was because of a single outlier study,48 and exclusion of this study resulted in homogeneous findings. Hence, the reported results from the primary analyses can be considered robust. While we observed significant differences in quadriceps strength between the injured limb after ACLR and matched control limbs, the effect size decreased as the time since surgery increased. This was confirmed in the metaregression, which showed a significant effect of time since surgery on the difference in quadriceps strength between groups. In a narrative review by Petersen et al,46 the authors concluded that muscular deficits are greater within the first 6 months after surgery and they can persist up to 2 years or longer. These findings are aligned with our meta-analyses. While it was not possible to determine the reasons for the gradual reduction of effect with time, it could be assumed that the return to sports and increase in activity could provide further stimulus for quadriceps strengthening. However, the fact that these stimuli appear to be insufficient to promote full recovery of quadriceps strength is concerning. While delaying return to sports is not often feasible, an increased awareness and reassessment of the postoperative rehabilitation after the athlete returns to play may be important.
Quadriceps strength recovery, measured as LSI, is considered a key criterion while making return-to-sports decisions.12,13 In a systematic review, achieving an LSI >90 was the most common criterion used to make return-to-sports decisions.55 However, studies have suggested that comparisons with the uninjured limb may not be appropriate given the presence of bilateral neuromuscular deficits after ACLR. Multiple experimental studies47,51,53 and a systematic review17 have reported bilateral deficits in quadriceps activation among people with ACLR. Mirkov et al35 reported a lower quadriceps rate of force development in the uninjured limb of individuals with ACLR than in control limbs. Hence, using LSI as a criterion for making return-to-sports decisions might be a flawed strategy and could have significant negative clinical consequences. Wellsandt et al56 reported that achieving LSI >90% for quadriceps strength, among other measures, does not guarantee that functional levels equivalent to preinjury levels are met. The authors suggested using preinjury data as the most appropriate comparison, and because such data are usually not available, comparison with age-, sex-, and activity-matched normative data should be considered. The results from our study provide further guidance for clinicians by demonstrating the persistence of quadriceps strength deficits in the injured limb of people with ACLR when compared with an appropriate control group. These results suggest that current return-to-sports criteria that rely on LSI may need to be reconsidered and comparison with a matched control group might be more appropriate. The persistence of the strength deficits beyond 6 months and after return to sports also suggests that athletes may benefit from continued rehabilitation after returning to their everyday activities at full capacity. However, further work is needed to better understand the mechanisms underlying persistent weakness so that the rehabilitation intervention can be appropriately targeted. In addition to clinical implications, these analyses may offer guidance for the design of future studies examining quadriceps strength after ACLR. Future studies should consider incorporating a matched control group when investigating quadriceps recovery after ACLR. While the recruitment methods varied across the studies that we included in our analyses, one approach was to recruit uninjured teammates of participants who underwent ACLR.36 This could be an effective way to ensure matching in future studies. These data also did not allow for a determination of what may be an acceptable magnitude of deficit in quadriceps strength in the injured limbs of people with ACLR when compared with the limbs of matched controls to allow for return to sports. This could be investigated in future studies.
As a secondary objective, we compared the quadriceps strength between the uninjured limb of people with ACLR and the limb of matched controls. These differences in quadriceps strength between the uninjured limb in people with ACLR and the matched control limb at 0 to 6 months and from 6 to 18 months were small. Fewer studies were available to be pooled than were available for the primary analyses because not all studies reported peak quadriceps strength data for the uninjured leg. It should be noted that we did not specifically select studies to address this question. Hence, our effect estimates may have been somewhat biased. However, our results are in agreement with a recent review that also did not observe a significant difference between the uninjured contralateral limb and the control limb (SMD, –0.24; 95% CI, –0.68 to 0.19).32 Importantly, the authors did observe a lower central activation ratio in the contralateral uninjured limb (SMD, –0.73; 95% CI, –1.39 to –0.07) compared with the control limb. While this might suggest the presence of bilateral neuromuscular deficits in individuals with ACLR, further research is needed to compare participants with ACLR with age-, sex-, and activity-matched controls to investigate the magnitude and type of deficits in the contralateral limb. This is important given the higher rate of reinjury in the contralateral limb than the ACLR limb,57 especially in women.43,44 Future studies should consider reporting data from both legs in individuals with ACLR while comparing them with an appropriate control group to further evaluate this question.
In the exploratory analyses assessing differences in quadriceps strength between groups when measured using isometric dynamometry and when measured using isokinetic dynamometry, there was an insufficient number of studies available to allow for pooled analyses across all strata. However, when studies could be pooled and the outlier study could be excluded, the results were aligned with the primary analysis, with lower quadriceps strength seen for ACLR limbs compared with control limbs irrespective of the measurement technique. While we did not have sufficient data to explore the effects of each graft type given that some studies included patients with different graft types and others did not report graft type, we did perform sensitivity analyses including only studies with patients with patellar tendon or hamstring tendon grafts. The results from the >18-month strata do not appear to be robust given the high heterogeneity and small number of studies. Otherwise, the results are similar to the primary analyses, suggesting that our findings are generalizable. Furthermore, the literature appears equivocal on the effects of graft type on the recovery of quadriceps strength after ACLR.2,18,22,28 However, further work may still be warranted to specifically assess the effects of quadriceps tendon grafts, allografts, and contralateral tendon harvest on the recovery of quadriceps strength.
Important limitations need to be considered while interpreting the results from these analyses. Although we observed statistical significance, the clinical significance is unclear because many patients do return to sports after ACLR. Future research may need to be developed to demonstrate clinical meaningfulness. We did not have data or know the preinjury quadriceps strength in patients who tore their ACL. Thus, they may have had a weaker quadriceps than did their matched controls. This may, in fact, have put them at risk for ACL tears. Although the heterogeneity was low for the primary analyses, important differences were seen across studies, particularly for methodologies used to measure quadriceps strength. These differences included the type of strength assessment (isometric, isokinetic), angles and velocities, positioning, the use of practice trials, the duration and reporting of rest periods, and so forth. Our exploratory analyses stratifying the studies into those using isometric or isokinetic measurements suggested that the differences due to type of strength assessment were unlikely to affect the primary results. However, it was not possible to fully explore the effects of all differences in quadriceps strength testing protocols across the studies. It was not possible to make inferences about various factors related to the magnitude of initial injury, the type of surgical procedures, rehabilitation protocols, and so forth, as these varied across the included studies. Another factor that might be important but could not be assessed because of the lack of reporting was the possible effects of biological sex on the reported differences. While a few studies only included men or women, most included a mixed group, precluding any analyses by sex. Given the strong effects of sex on ACL injury risk,37 outcomes after ACLR,49 and risk of posttraumatic osteoarthritis,20 it would be important to further evaluate the effect of sex on the recovery of quadriceps strength as compared with matched controls.
Conclusion
We observed that quadriceps strength was lower in the injured limb of people with ACLR than in the limbs of age-, sex-, and activity-matched controls. These deficits persisted for many years after ACLR. The results have implications for current rehabilitation protocols and return-to-sports criteria.
Acknowledgment
The authors would like to thank the researchers whose publications were included in our analyses.
Appendix
Table A1.
Complete Search Strategy
| Database | Search String | Comments |
|---|---|---|
| PUBMED | (anterior cruciate OR anterior cruciate ligament OR ACL) AND (reconstruct* OR surg*) AND (quadricep* OR extens*) AND (strength OR power OR torque) | Limited to English language |
| SPORTDiscus | (anterior cruciate OR anterior cruciate ligament OR ACL) AND (reconstruct* OR surg*) AND (quadricep* OR extens*) AND (strength OR power OR torque) | Limited to English language |
| EMBASE | “anterior cruciate ligament” AND (“reconstruct*” OR “surgery”) AND (“quadricep*” OR “exten*”) AND (“strength” OR “power” OR “torque”) AND [english]/lim AND [embase]/lim | Limited to English language and excluded MEDLINE results |
| CINAHL | (anterior cruciate OR anterior cruciate ligament OR ACL) AND (reconstruct* OR surg*) AND (quadricep* OR extens*) AND (strength OR power OR torque) | Limited to English language and excluded MEDLINE results |
| SCOPUS | “anterior cruciate ligament” OR “ACL” AND reconstruction AND quadricep* AND strength OR power OR torque AND NOT INDEX (medline) AND (LIMIT-TO (LANGUAGE, “English”)) | Limited to English language and excluded MEDLINE results |
Table A2.
Modified Downs and Black Checklista
| Category | Original Item No. | Question | Scoring |
|---|---|---|---|
| Reporting | 1. | Is the hypothesis/aim/objective of the study clearly described? | 1 = hypothesis/aim/objective described 0 = no description |
| 2. | Are the main outcomes to be measured clearly described in the introduction or Methods section? | 1 = outcome measures described in the introduction or Methods | |
| 0 = described in Results | |||
| 3. | Are the characteristics of the patients included in the study clearly described? | 1 = inclusion/exclusion criteria provided for both groups | |
| 0 = criteria not provided | |||
| 5. | Are the distributions of principal confounders for each group to be compared clearly described? | 2 = age, sex, activity level/sports, BMI described for both groups | |
| 1 = age, BMI, activity level described for both groups | |||
| 0 = missing confounders | |||
| 6. | Are the main findings of the study clearly described? | 1 = findings clearly described | |
| 0 = no description | |||
| 7. | Does the study provide estimates of the random variability in the data for the main outcomes? | 1 = measures of variability (standard deviation, standard error, or confidence intervals) provided | |
| 0 = no information provided | |||
| 10. | Have actual probability values been reported (eg, .035 rather than <.05) for the main outcomes except where probability value is <.001? | 1 = if actual P values reported | |
| 0 = if actual P values not reported | |||
| External validity | 11. | Were the participants asked to participate in the study representative of the entire population from which they were recruited? | 1 = if participants were from the community |
| 0 = no description or unable to determine | |||
| 12. | Were those participants who were prepared to participate representative of the entire population from which they were recruited? | 1 = if participants enrolled represented source community | |
| 0 = no description or unable to determine | |||
| Internal validity | 15. | Was an attempt made to blind those measuring main outcomes of the intervention? | 1 = if assessors blinded |
| 0 = if blinding not described | |||
| 16. | If any of the results of the study were based on “data dredging,” was this made clear? | 1 = planned analyses described clearly | |
| 0 = data dredging present | |||
| 18. | Were the statistical tests used to assess the main outcomes appropriate? | 1 = appropriate statistical tests used | |
| 0 = inappropriate statistical tests used or no information provided | |||
| 20. | Were the main outcome measures used accurate (valid and reliable)? | 1 = if reference provided for reliability or validity of the outcome measures used | |
| 0 = no description | |||
| 21. | Were the participants of the 2 groups recruited from the same population? | 1 = if participants recruited from the same population (eg, soccer players) | |
| 0 = if no information provided or not recruited from same population | |||
| 25. | Was there adequate adjustment for the confounding in the analysis from which the findings were drawn? | 1 = if groups matched on age, sex, activity level | |
| 0 = not matched | |||
| Power | 27. | Were appropriate power calculations reported? | 1 = power calculation provided |
| 0 = no information |
aBMI, body mass index.
Source: Checklist modified from Downs and Black.9
Table A3.
Study Quality Assessed Using Modified Downs and Black Checklista
| Original Item No. | Score | Risk of Bias | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reporting | External Validity | Internal Validity | Power | ||||||||||||||||
| Lead Author, Year | 1 | 2 | 3 | 5 | 6 | 7 | 10 | 11 | 12 | 15 | 16 | 18 | 20 | 21 | 25 | 27 | Total | % | |
| Almeida, 20181 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 13 | 76 | LR |
| Boo, 20186 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 12 | 71 | LR |
| Garrison, 201810 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 11 | 65 | LR |
| Johnson, 201819 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 12 | 71 | LR |
| O’Malley, 201840 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 12 | 71 | LR |
| Pamukoff, 201842 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 13 | 76 | LR |
| Pelegrinelli, 201845 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 11 | 65 | LR |
| Mirkov, 201735 | 1 | 1 | 1 | 2 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 12 | 71 | LR |
| Kuenze, 201725 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 12 | 71 | LR |
| Goetschius, 201611 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 14 | 82 | LR |
| Zwolski, 201659 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 13 | 76 | LR |
| Lepley, 201530 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 13 | 76 | LR |
| Kuenze, 201526 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 13 | 76 | LR |
| Chung, 20157 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 12 | 71 | LR |
| Hsieh, 201517 | 1 | 1 | 1 | 2 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 16 | 94 | LR |
| Lepley, 201429 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 15 | 88 | LR |
| Mohammadi, 201336 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 13 | 76 | LR |
| Xergia, 201358 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 12 | 71 | LR |
| Thomas, 201350 | 1 | 1 | 0 | 2 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 11 | 65 | LR |
| Rudroff, 200348 | 1 | 1 | 1 | 2 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 12 | 71 | LR |
| Mattacola, 200234 | 1 | 1 | 1 | 2 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 11 | 65 | LR |
aLR, low risk of bias.
Figure A1.
Forest plot for comparison of quadriceps strength in the injured limb of participants with anterior cruciate ligament reconstruction (ACLR) who received patellar tendon or hamstring tendon grafts and the control group, stratified by time since surgery. IV, inverse variance; Std, standardized.
Figure A2.
Forest plot for comparison of isometric quadriceps strength in the injured limb of participants with anterior cruciate ligament reconstruction (ACLR) and the control group, stratified by time since surgery. IV, inverse variance; Std, standardized.
Figure A3.
Forest plot for comparison of isokinetic quadriceps strength in the injured limb of participants with anterior cruciate ligament reconstruction (ACLR) and the control group, stratified by time since surgery. IV, inverse variance; Std, standardized.
Footnotes
Final revision submitted September 24, 2020; accepted November 18, 2020
The authors declared that they have no conflicts of interest in the authorship and publication of this contribution. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
References
- 1. Almeida AM, Santos Silva PR, Pedrinelli A, Hernandez AJ. Aerobic fitness in professional soccer players after anterior cruciate ligament reconstruction. PLoS One. 2018;13(3):e0194432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aune AK, Holm I, Risberg MA, Jensen HK, Steen H.Four-strand hamstring tendon autograft compared with patellar tendon-bone autograft for anterior cruciate ligament reconstruction: a randomized study with two-year follow-up. Am J Sports Med. 2001;29(6):722–728. [DOI] [PubMed] [Google Scholar]
- 3. Barton CJ, Lack S, Malliaras P, Morrissey D. Gluteal muscle activity and patellofemoral pain syndrome: a systematic review. Br J Sports Med. 2013;47(4):207–214. [DOI] [PubMed] [Google Scholar]
- 4. Benjaminse A, Holden S, Myer GD. ACL rupture is a single leg injury but a double leg problem: too much focus on “symmetry” alone and that’s not enough! Br J Sports Med. 2018;52(16):1029–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bohannon RW, Bubela DJ, Wang YC, Magasi SR, Gershon RC. Adequacy of belt-stabilized testing of knee extension strength. J Strength Cond Res. 2011;25(7):1963–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Boo ME, Garrison JC, Hannon JP, et al. Energy absorption contribution and strength in female athletes at return to sport after anterior cruciate ligament reconstruction: comparison with healthy controls. Orthop J Sports Med. 2018;6(3):2325967118759522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chung KS, Ha JK, Yeom CH, et al. Are muscle strength and function of the uninjured lower limb weakened after anterior cruciate ligament injury? Two-year follow-up after reconstruction. Am J Sports Med. 2015;43(12):3013–3021. [DOI] [PubMed] [Google Scholar]
- 8. Dingenen B, Gokeler A. Optimization of the return-to-sport paradigm after anterior cruciate ligament reconstruction: a critical step back to move forward. Sports Med (Auckland, NZ). 2017;47(8):1487–1500. [DOI] [PubMed] [Google Scholar]
- 9. Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Commun Health. 1998;52(6):377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Garrison JC, Hannon J, Goto S, et al. Participants at three months post-operative anterior cruciate ligament reconstruction (ACL-R) demonstrate differences in lower extremity energy absorption contribution and quadriceps strength compared to healthy controls. Knee. 2018;25(5):782–789. [DOI] [PubMed] [Google Scholar]
- 11. Goetschius J, Hart JM. Knee-extension torque variability and subjective knee function in patients with a history of anterior cruciate ligament reconstruction. J Athl Train. 2016;51(1):22–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gokeler A, Welling W, Zaffagnini S, Seil R, Padua D. Development of a test battery to enhance safe return to sports after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2017;25(1):192–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Grindem H, Snyder-Mackler L, Moksnes H, Engebretsen L, Risberg MA. Simple decision rules can reduce reinjury risk by 84% after ACL reconstruction: the Delaware-Oslo ACL cohort study. Br J Sports Med. 2016;50(13):804–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Grooms DR, Page SJ, Onate JA. Brain activation for knee movement measured days before second anterior cruciate ligament injury: neuroimaging in musculoskeletal medicine. J Athl Train. 2015;50(10):1005–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hart JM, Pietrosimone B, Hertel J, Ingersoll CD. Quadriceps activation following knee injuries: a systematic review. J Athl Train. 2010;45(1):87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. (editors). Cochrane Handbook for Systematic Reviews of Interventions. 2nd Edition. Chichester (UK): John Wiley & Sons, 2019. [Google Scholar]
- 17. Hsieh CJ, Indelicato PA, Moser MW, Vandenborne K, Chmielewski TL. Speed, not magnitude, of knee extensor torque production is associated with self-reported knee function early after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2015;23(11):3214–3220. [DOI] [PubMed] [Google Scholar]
- 18. Huber R, Viecelli C, Bizzini M, et al. Knee extensor and flexor strength before and after anterior cruciate ligament reconstruction in a large sample of patients: influence of graft type. Phys Sportsmed. 2019;47(1):85–90. [DOI] [PubMed] [Google Scholar]
- 19. Johnson AK, Palmieri-Smith RM, Lepley LK. Contribution of neuromuscular factors to quadriceps asymmetry after anterior cruciate ligament reconstruction. J Athl Train. 2018;53(4):347–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jones MH, Spindler KP. Risk factors for radiographic joint space narrowing and patient reported outcomes of post-traumatic osteoarthritis after ACL reconstruction: data from the MOON cohort. J Orthop Res. 2017;35(7):1366–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kaur M, Ribeiro DC, Theis JC, Webster KE, Sole G. Movement patterns of the knee during gait following ACL reconstruction: a systematic review and meta-analysis. Sports Med (Auckland, NZ). 2016;46(12):1869–1895. [DOI] [PubMed] [Google Scholar]
- 22. Keays SL, Bullock-Saxton JE, Keays AC, Newcombe PA, Bullock MI. A 6-year follow-up of the effect of graft site on strength, stability, range of motion, function, and joint degeneration after anterior cruciate ligament reconstruction: patellar tendon versus semitendinosus and gracilis tendon graft. Am J Sports Med. 2007;35(5):729–739. [DOI] [PubMed] [Google Scholar]
- 23. Korakakis V, Whiteley R, Tzavara A, Malliaropoulos N. The effectiveness of extracorporeal shockwave therapy in common lower limb conditions: a systematic review including quantification of patient-rated pain reduction. Br J Sports Med. 2018;52(6):387–407. [DOI] [PubMed] [Google Scholar]
- 24. Krishnan C, Williams GN. Factors explaining chronic knee extensor strength deficits after ACL reconstruction. J Orthop Res. 2011;29(5):633–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kuenze C, Eltoukhy M, Kelly A, Kim CY. Impact of quadriceps strengthening on response to fatiguing exercise following ACL reconstruction. J Sci Med Sport. 2017;20(1):6–11. [DOI] [PubMed] [Google Scholar]
- 26. Kuenze C, Hertel J, Saliba S, et al. Clinical thresholds for quadriceps assessment after anterior cruciate ligament reconstruction. J Sport Rehabil. 2015;24(1):36–46. [DOI] [PubMed] [Google Scholar]
- 27. Lai CCH, Ardern CL, Feller JA, Webster KE. Eighty-three per cent of elite athletes return to preinjury sport after anterior cruciate ligament reconstruction: a systematic review with meta-analysis of return to sport rates, graft rupture rates and performance outcomes. Br J Sports Med. 2018;52(2):128–138. [DOI] [PubMed] [Google Scholar]
- 28. Lephart SM, Kocher MS, Harner CD, Fu FH. Quadriceps strength and functional capacity after anterior cruciate ligament reconstruction: patellar tendon autograft versus allograft. Am J Sports Med. 1993;21(5):738–743. [DOI] [PubMed] [Google Scholar]
- 29. Lepley AS, Ericksen HM, Sohn DH, Pietrosimone BG. Contributions of neural excitability and voluntary activation to quadriceps muscle strength following anterior cruciate ligament reconstruction. Knee. 2014;21(3):736–742. [DOI] [PubMed] [Google Scholar]
- 30. Lepley AS, Gribble PA, Thomas AC, et al. Quadriceps neural alterations in anterior cruciate ligament reconstructed patients: a 6-month longitudinal investigation. Scand J Med Sci Sports. 2015;25(6):828–839. [DOI] [PubMed] [Google Scholar]
- 31. Lepley LK. Deficits in quadriceps strength and patient-oriented outcomes at return to activity after ACL reconstruction: a review of the current literature. Sports Health. 2015;7(3):231–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lisee C, Lepley AS, Birchmeier T, O’Hagan K, Kuenze C. Quadriceps strength and volitional activation after anterior cruciate ligament reconstruction: a systematic review and meta-analysis. Sports Health. 2019;11(2):163–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Martin HJ, Yule V, Syddall HE, et al. Is hand-held dynamometry useful for the measurement of quadriceps strength in older people? A comparison with the gold standard Bodex dynamometry. Gerontology. 2006;52(3):154–159. [DOI] [PubMed] [Google Scholar]
- 34. Mattacola CG, Perrin DH, Gansneder BM, et al. Strength, functional outcome, and postural stability after anterior cruciate ligament reconstruction. J Athl Train. 2002;37(3):262–268. [PMC free article] [PubMed] [Google Scholar]
- 35. Mirkov DM, Knezevic OM, Maffiuletti NA, et al. Contralateral limb deficit after ACL-reconstruction: an analysis of early and late phase of rate of force development. J Sports Sci. 2017;35(5):435–440. [DOI] [PubMed] [Google Scholar]
- 36. Mohammadi F, Salavati M, Akhbari B, et al. Comparison of functional outcome measures after ACL reconstruction in competitive soccer players: a randomized trial. J Bone Joint Surg Am. 2013;95(14):1271–1277. [DOI] [PubMed] [Google Scholar]
- 37. Montalvo AM, Schneider DK, Yut L, et al. “What’s my risk of sustaining an ACL injury while playing sports?” A systematic review with meta-analysis. Br J Sports Med. 2019;53(16):1003–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Muff G, Dufour S, Meyer A, et al. Comparative assessment of knee extensor and flexor muscle strength measured using a hand-held vs. isokinetic dynamometer. J Phys Ther Sci. 2016;28(9):2445–2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Noehren B, Andersen A, Hardy P, et al. Cellular and morphological alterations in the vastus lateralis muscle as the result of ACL injury and reconstruction. J Bone Joint Surg Am. 2016;98(18):1541–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. O’Malley E, Richter C, King E, et al. Countermovement jump and isokinetic dynamometry as measures of rehabilitation status after anterior cruciate ligament reconstruction. J Athl Train. 2018;53(7):687–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pairot-de-Fontenay B, Willy RW, Elias ARC, et al. Running biomechanics in individuals with anterior cruciate ligament reconstruction: a systematic review. Sports Med (Auckland, NZ). 2019;49(9):1411–1424. [DOI] [PubMed] [Google Scholar]
- 42. Pamukoff DN, Montgomery MM, Choe KH, et al. Bilateral alterations in running mechanics and quadriceps function following unilateral anterior cruciate ligament reconstruction. J Orthop Sports Phys Ther. 2018;48(12):960–967. [DOI] [PubMed] [Google Scholar]
- 43. Paterno MV, Rauh MJ, Schmitt LC, Ford KR, Hewett TE. Incidence of contralateral and ipsilateral anterior cruciate ligament (ACL) injury after primary ACL reconstruction and return to sport. Clin J Sport Med. 2012;22(2):116–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Paterno MV, Rauh MJ, Schmitt LC, Ford KR, Hewett TE. Incidence of second ACL injuries 2 years after primary ACL reconstruction and return to sport. Am J Sports Med. 2014;42(7):1567–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pelegrinelli ARM, Guenka LC, Dias JM, et al. Isokinetic muscle performance after anterior cruciate ligament reconstruction: a case-control study. Int J Sports Phys Ther. 2018;13(5):882–889. [PMC free article] [PubMed] [Google Scholar]
- 46. Petersen W, Taheri P, Forkel P, Zantop T. Return to play following ACL reconstruction: a systematic review about strength deficits. Arch Orthop Trauma Surg. 2014;134(10):1417–1428. [DOI] [PubMed] [Google Scholar]
- 47. Pietrosimone BG, Lepley AS, Ericksen HM, et al. Neural excitability alterations after anterior cruciate ligament reconstruction. J Athl Train. 2015;50(6):665–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rudroff T. Functional capability is enhanced with semitendinosus than patellar tendon ACL repair. Med Sci Sports Exerc. 2003;35(9):1486–1492. [DOI] [PubMed] [Google Scholar]
- 49. Tan SH, Lau BP, Khin LW, Lingaraj K. The importance of patient sex in the outcomes of anterior cruciate ligament reconstructions: a systematic review and meta-analysis. Am J Sports Med. 2016;44(1):242–254. [DOI] [PubMed] [Google Scholar]
- 50. Thomas AC, Villwock M, Wojtys EM, Palmieri-Smith RM. Lower extremity muscle strength after anterior cruciate ligament injury and reconstruction. J Athl Train. 2013;48(5):610–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Thomas AC, Wojtys EM, Brandon C, Palmieri-Smith RM. Muscle atrophy contributes to quadriceps weakness after anterior cruciate ligament reconstruction. J Sci Med Sport. 2016;19(1):7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Thomeé R, Kaplan Y, Kvist J, et al. Muscle strength and hop performance criteria prior to return to sports after ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2011;19(11):1798–1805. [DOI] [PubMed] [Google Scholar]
- 53. Urbach D, Nebelung W, Weiler HT, Awiszus F. Bilateral deficit of voluntary quadriceps muscle activation after unilateral ACL tear. Med Sci Sports Exerc. 1999;31(12):1691–1696. [DOI] [PubMed] [Google Scholar]
- 54. van Tulder M, Furlan A, Bombardier C, Bouter L. Updated method guidelines for systematic reviews in the Cochrane Collaboration back review group. Spine. 2003;28(12):1290–1299. [DOI] [PubMed] [Google Scholar]
- 55. Webster KE, Hewett TE. What is the evidence for and validity of return-to-sport testing after anterior cruciate ligament reconstruction surgery? A systematic review and meta-analysis. Sports Med (Auckland, NZ). 2019;49(6):917–929. [DOI] [PubMed] [Google Scholar]
- 56. Wellsandt E, Failla MJ, Snyder-Mackler L. Limb symmetry indexes can overestimate knee function after anterior cruciate ligament injury. J Orthop Sports Phys Ther. 2017;47(5):334–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wiggins AJ, Grandhi RK, Schneider DK, et al. Risk of secondary injury in younger athletes after anterior cruciate ligament reconstruction: a systematic review and meta-analysis. Am J Sports Med. 2016;44(7):1861–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Xergia SA, Pappas E, Zampeli F, Georgiou S, Georgoulis AD. Asymmetries in functional hop tests, lower extremity kinematics, and isokinetic strength persist 6 to 9 months following anterior cruciate ligament reconstruction. J Orthop Sports Phys Ther. 2013;43(3):154–162. [DOI] [PubMed] [Google Scholar]
- 59. Zwolski C, Schmitt LC, Thomas S, Hewett TE, Paterno MV. The utility of limb symmetry indices in return-to-sport assessment in patients with bilateral anterior cruciate ligament reconstruction. Am J Sports Med. 2016;44(8):2030–2038. [DOI] [PubMed] [Google Scholar]