Abstract
The type II protein arginine methyltransferase 5 (PRMT5) has been engaged in various human cancer development and progression types. Nevertheless, few studies uncover the biological functions of PRMT5 in the epithelial-mesenchymal transition (EMT) of human lung cancer cells, and the associated molecular mechanisms and signaling cascades are entirely unknown. Here, we show that PRMT5 is the ectopic expression in human lung cancer tissues and cell lines. Further study reveals that silencing PRMT5 by lentivirus-mediated shRNA or blocking of PRMT5 by specific inhibitor GSK591 attenuates the expression levels of EMT-related markers in vivo, using the xenograft mouse model. Moreover, our results show that down-regulation of PRMT5 impairs EGFR/Akt signaling cascades in human lung cancer cells, whereas re-expression of PRMT5 recovers those changes, suggesting that PRMT5 regulates EMT probably through EGFR/Akt signaling axis. Altogether, our results demonstrate that PRMT5 serves as a critical oncogenic regulator and promotes EMT in human lung cancer cells. More importantly, our findings also suggest that PRMT5 may be a potential therapeutic candidate for the treatment of human lung cancer.
Keywords: PRMT5, EMT, Aktm, EGFR, GSK3β, lung cancer
Introduction
Human lung cancer, a highly deadly and aggressive tumor, is the most common malignancy of the lung with a dismal 5-year survival rate of less than 15%–17%, especially non-small cell lung cancer (NSCLC). Although human lung cancer is a chronic disease and its development is prolonged in most people over a decade, lung cancer is the primary leading cause of death and the most frequently diagnosed cancer in China. Also, the conventional therapeutic methods, such as radiotherapy, chemotherapy, surgery, and combination therapy, have generally had little effect on this aggressive cancer, despite making many efforts over the many years. Furthermore, most patients with lung cancer cannot be treated with currently available therapeutic methods. Thus, the accurate detection and diagnosis of human lung cancer early and discovering the new therapeutic targets are incredibly urgent.
Protein arginine methyltransferase 5 (PRMT5) belongs to the type II arginine methyltransferase that produces the symmetrical dimethylation of histone or non-histone protein substrates at the arginine residues. PRMT5 is engaged in various cellular processes, including cell metabolism1, proliferation2, anti-apoptosis3, RNA splicing4, chromatin remodeling5, cell cycle progression6, ribosome biogenesis7, and signaling transduction8. The accumulating evidence has shown that PRMT5 is involved in different types of human cancer and development, including liver cancer, breast cancer, bladder cancer, colorectal cancer, lymphoma, neuroblastoma, prostate cancer, and lung cancer. Previous studies have shown that PRMT5 regulates lung cancer cell proliferation and growth via PI3K/Akt signaling pathway2, and down-regulation of PRMT5 promotes lung cancer cell death induced by resveratrol through Akt/GSK3β signaling axis3. Recent studies have shown that PRMT5 was participated in tumorigenesis9 and repressed tumor suppressor FBW7 to enhance c-Myc expression and promotes pancreatic cancer cell proliferation and growth10. Nevertheless, so far, no studies have revealed the biological functions of PRMT5 in the regulation of EMT in human lung cancer, and the related molecular mechanisms are completely unknown.
In the current study, we uncovered the crucial role of PRMT5 in EMT in human lung cancer and revealed the underlying molecular mechanism. Our findings showed that PRMT5 was not only overexpressed in human lung cancer tissues but also highly expressed in lung cancer cell lines. Moreover, down-regulation or inhibition of PRMT5 by shRNA or specific inhibitor GSK591 attenuated the EMT in vivo, using the PRMT5 depletion xenograft mouse model. More importantly, PRMT5 controlled EMT probably via regulation of EGFR/Akt signaling cascades in lung cancer cells. Our results suggest that PRMT5 is a pivotal upstream regulator for EMT in human lung cancer, and targeting PRMT5 may help improve therapeutic efficacy in clinical treatment.
Materials and Methods
Cancer Tissues Collection
Fresh human lung cancer tissues and matched adjacent normal tissues were collected between January 2018 and January 2020 from Department of Pulmonary and Critical Care Medicine, Shanghai East Hospital, Tongji University School of Medicine. The human samples were obtained from the patients with informed consent. The study was approved by the Ethics Review Board of Shanghai East Hospital at Tongji University (Shanghai, China).
Cell Culture and Chemicals
The normal human lung fibroblast cells (IMR90) were purchased from Sigma (cat# 85020204) and cultured in EMEM (EBSS) plus 2 mM Glutamine plus 1% Non Essential Amino Acids (NEAA) plus 10% Foetal Bovine Serum (FBS, Sigma cat# F2442). The human lung cancer cell lines: A549, PC14, H322, and H1299 cells were purchased from American Type Culture Collection (ATCC; Manassas, VA, USA), and the cells were grown in the indicated cell culture media containing 10% FBS. The cells were maintained at 37 °C with 5% CO2. PRMT5 specific inhibitor GSK591 was purchased from Sigma (cat# SML-1751).
Plasmids
In order to down-regulation of PRMT5 expression in cells, we generated the lentivirus containing human PRMT5-shRNA2. For lentivirus packaging, the helper plasmids MD2G and PAX2 were purchased from Addgene. The targeting sequences of human PRMT5 were shown: shRNA1, 5′-GGATAAAGCTGTATGCTGT-3′; shRNA2: 5′-GCCATCTATAAATGTCTGCTA-3′. For the gain-of-function experiments, we generated the Flag-human PRMT5 plasmid. The human PRMT5 cDNA was subcloned into the Flag vector and verified by sequencing.
Gene Expression Analysis
For the analysis of gene expression, the total RNA was extracted from human normal and lung cancer tissues or IMR90, PC14, H322, H1299, and A549 cells using TRIzol reagent (Cat# 15596-018; Invitrogen) according to the manufacturer’s protocol. Next, an equal amount of RNA was used to conduct the reverse transcription with Bio-Red PCR thermal cycler (C1000). Subsequently, the SYBR green fluorescent Dye (cat# 1725272; Bio-Rad) was subjected to the quantitative real-time PCR (qRT-PCR) for gene expression analysis with an ABI7500 PCR machine (Applied BiosystemsTM). The primers were used in this study were shown below: human PRMT5 forward: 5′-CCTGTGGAGGTGAACACAGT-3′ and revise: 5′-AGAGGATGGGAAACCATGAG-3′; β-catenin forward: 5′-AAAGCGGCTGTTAGTCACTGG-3′ and revise: 5′-CGAGTCATTGCATACTGTCCAT-3′; Collagen I forward: 5′-GAGGGCCAAGACGAAGACATC-3′ and revise: 5′-CAGATCACGTCATCGCACAAC-3; Vimentin forward: 5′-AGTCCACTGAGTACCGGAGAC-3′ and revise: 5′-CATTTCACGCATCTGGCGTTC-3′;
ZEB1 forward: 5′-GCACCTGAAGAGGACCAGAG-3′ and revise: 5′- TGCATCTGGTGTTCCATTTT-3′; GAPDH, forward: 5′-CCATGTTCGTCATGGGTGTG-3′ and revise: 5′-CAGGGGTGCTAAGCAGTTGG-3′. GAPGH was used as an internal control. Relative mRNA expression level was calculated by the method of ΔΔ-Ct.
Generation of PRMT5 Stable Knockdown Cell Line
To generate a PRMT5 stable depletion cell line, the lentivirus containing Scramble or PRMT5-shRNAs was co-transfected along with the helper plasmids MD2G and PAX2 into 293 T cells using lipofectamineTM 2000 transfection reagent (Invitrogen, cat# 11668019). After 48 h, the culture media was collected, and the viral titer was pre-determined. In order to generate the PRMT5 stable knockdown cell line, A549 cells were infected with the indicated lentivirus with an equal amount of virus particles. These cells were then selected with puromycin (1 μg/mL, Sigma, cat# p9620) for 48 h, and the non-infected cells were killed. Then, the PRMT5 depletion stable cells were used for the indicated experiments.
Xenograft Mouse Model
The animal protocol was approved by the Institutional Animal Care and Use Committee of Tongji University School of Medicine, Shanghai, China. The PRMT5 stable knockdown A549 cells or control A549 cells containing scramble-shRNA (1 × 106 cells per site) were subcutaneously injected into 6-week-old BALB/c nude mice (Shanghai SLAC Laboratory Animal Co., Ltd) to generate xenografts. To test the effects of PRMT5 inhibitor GSK591 on the changes of EMT markers, the A549 cells (2 × 106 cells per site) were subcutaneously injected into 6-week-old BALB/c nude mice to generate xenografts, which were then treated with vehicle or GSK591 for 2 weeks. There are six male mice for each treatment. Post-treatment, the mice were sacrificed, and the tumors were harvested for further analysis.
Cell Transfection
A549 cells were seeded into the 6-well plates and were transfected with flag-vector or flag-human PRMT5 plasmids when the cells were grown around 70% using the LipofectamineTM 3000 (cat# L3000015, Invitrogen) transfection reagent. After forty-eight-hour post-transfection, the cell were collected for Western blotting analysis.
Western Blotting Analysis
Western blotting was performed as described previously11. Briefly, the total proteins were extracted from normal human tissues and lung cancer tissues or the indicated cell lines using the lysis buffer (20 mmol/L Tris, PH 7.4, two mmol/L EDTA, two mmol/L EGTA, one mmol/L sodium orthovanadate, 1% Triton X-100, 150 mmol/L NaCl, 50 mmol/L sodium fluoride, 0.1% SDS, and 100 mmol/L phenylmethylsulfonyl fluoride) and then were centrifuged for 10 min at 4°C. The proteins were separated in sodium dodecyl sulphate/polyacrylamide gel electrophoresis (SDS/PAGE) and transferred to the PVDF membranes (cat#1620177; Bio-Red). Then, the membranes were washed twice with TBST for 5 min and blocked with 5% non-fat milk for 1 h at room temperature. The membranes were incubated with indicated antibodies: PRMT5 (cat# sc-376937; Santa Cruz Biotechnology), Symmetric Di-Methyl Arginine Motif [sdme-RG] MultiMab™ (cat# 13222; Cell Signaling Technology), phospho-Thr308-Akt (cat# 13038; Cell Signaling Technology), phospho-Ser473-Akt (cat# 4060; Cell Signaling Technology), total Akt (cat# 4691; Cell Signaling Technology), phospho-Ser9-GSK3β (cat# 5558; Cell Signaling Technology), total GSK3β (cat# 12456; Cell Signaling Technology), phospho-Tyr-1068-EGFR (cat# 3777; Cell Signaling Technology) total EGFR (cat# 2085; Cell Signaling Technology), phospho-Ser2448-mTOR (cat# 5536; Cell Signaling Technology), total mTOR (cat# 2983; Cell Signaling Technology), PTEN (cat# 9188; Cell Signaling Technology), β-catenin (cat# 8480; Cell Signaling Technology), Collagen I (cat# ab34710; abcam), ZEB1 (cat# 70512; Cell Signaling Technology), and β-tubulin (cat# 5666; Cell Signaling Technology). After incubation, the membranes were washed with TBST and labeled with goat anti-mouse conjugated to HRP secondary antibody (cat# sc-2005; Santa Cruz Biotechnology) or goat anti-rabbit conjugated to HRP secondary antibody (cat# sc-2004; Santa Cruz Biotechnology). Immunoreactivity was detected by SuperSignal West Pico Chemiluminescent Substrate Western blotting detection reagents (cat# 34580; Thermo Fisher Scientific).
Statistical Analysis
All experiments were carried out in triplicate under identical conditions, and data were represented as means ± SEM. Differences between two groups were analyzed by unpaired two-tailed Student’s t test. The difference with P < 0.05 was considered statistically significant.
Results
PRMT5 is the Ectopic Expression in Human Lung Cancer Tissues and CELL lines
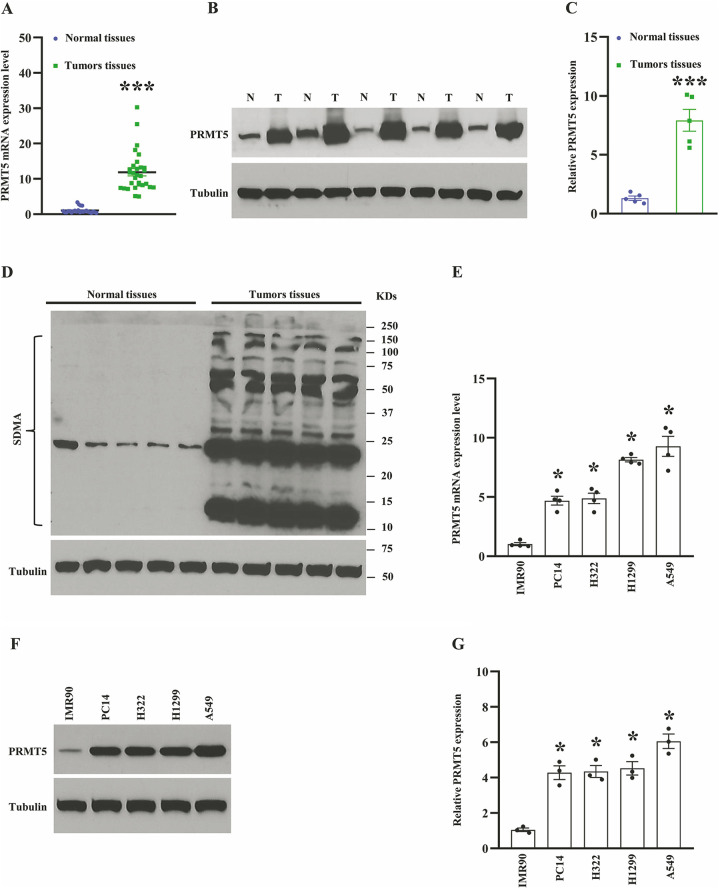
The accumulated evidence has been shown that PRMT5 is an oncogene and engaged in different types of human cancer development, including lung cancer2. To explore the biological roles of PRMT5 in human lung cancer, we assessed the PRMT5 expression level in both mRNA and protein level using human lung cancer tissues and adjacent normal tissues. As seen in Fig. 1A, PRMT5 mRNA expression level was dramatically enhanced in the lung cancer tissues compared to adjacent normal tissues. Subsequently, the PRMT5 protein expression level was measured by Western blotting, and we found that PRMT5 was highly expressed in lung cancer tissues compared with the adjacent normal tissues (Fig. 1B, C). Previous studies showed that methylation of arginine residues by PRMT5 plays a pivotal role in the control of gene transcription and protein modification12,13. Furthermore, PRMT5 produces the most symmetric di-methylarginine (SDMA) in cells, which is closely related to the enzymatic activity of PRMT5 and can be detected by global SDMA. As seen in Fig. 1D, the expression level of SDMA was distinctly increased in the lung cancer tissues compared with the adjacent normal tissues, implying that the enzymatic activity of PRMT5 is higher in the lung cancer tissues. To further confirm our hypothesis that PRMT5 is the ectopic expression in human lung cancer cells, we examined the PRMT5 expression level in both mRNA and protein levels using various human lung cancer cell lines. As seen in Fig. 1E, PRMT5 mRNA expression level was significantly increased in human lung cancer cell lines compared with normal human lung fibroblast cells (IMR90). Next, we assessed the PRMT5 protein expression level in those human lung cancer cell lines. As seen in Fig. 1F, G, the PRMT5 protein expression level was markedly enhanced in various lung cancer cell lines compared with IMR90 cells, implying that PRMT5 plays an essential role in human lung cancer. These results collectively suggest that PRMT5 is overexpressed in human lung cancer tissues and cell lines, and the enzymatic activity of PRMT5 is linked to human lung cancer.
Figure 1.
PRMT5 is the ectopic expression in human lung cancer tissues and cells. (A) The mRNA expression level of PRMT5 in human lung cancer tissues and adjacent normal tissues. (n = 25–30). ***P < 0.001 vs. normal tissues. (B) PRMT5 protein expression level was detected by Western blotting in human lung cancer tissues and adjacent normal tissues. (C) PRMT5 protein expression level was quantified. ***P < 0.001 vs. adjacent normal tissues. (D) The global symmetric dimethylarginine (SDMA) is detected by Western blotting in human lung cancer tissues and adjacent normal tissues. (E) PRMT5 mRNA expression level was measured by qRT-PCR in different 10 human lung cancer cell lines and normal human lung fibroblast cells (IMR90) (n = 4). *P < 0.05 vs. IMR90 cells. (F) PRMT5 protein expression level was detected by Western blotting in human lung cancer cells and IMR90 cells. Representative pictures were shown. (G) PRMT5 protein expression level was quantified. *P < 0.05 vs. IMR90 cells.
Depletion of PRMT5 Attenuated EMT In Vivo
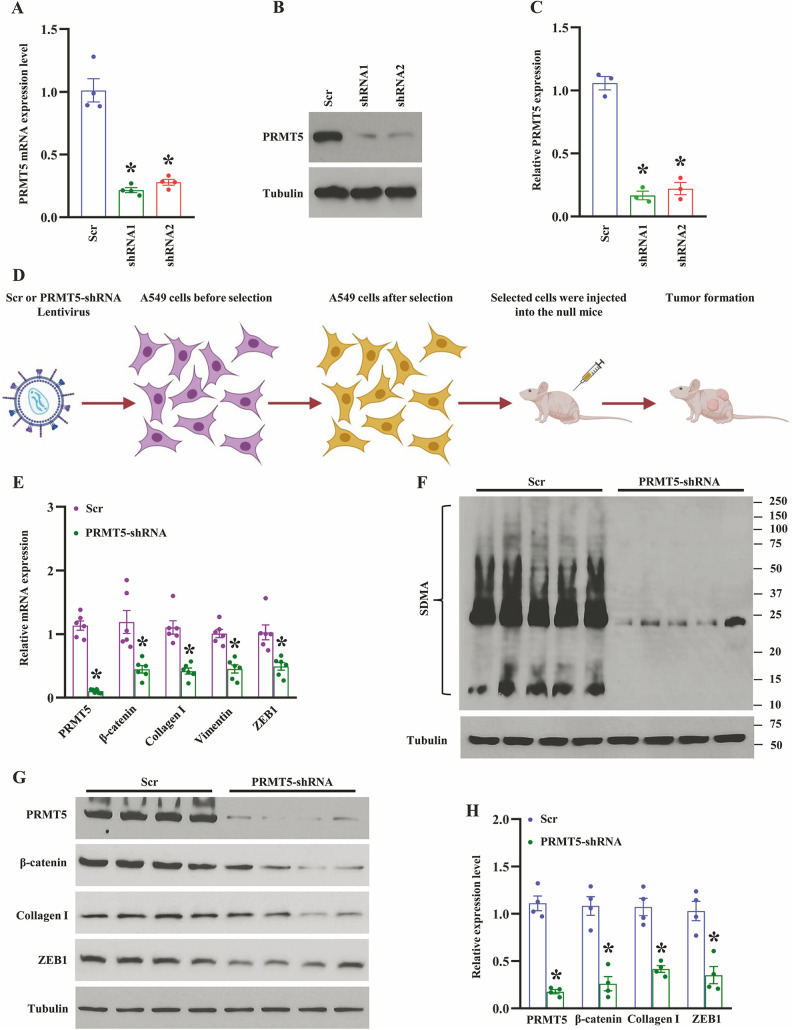
Previous studies have shown that PRMT5 is involved in cancer cell proliferation, growth, and apoptosis13. Nevertheless, how does PRMT5 promote EMT and the related molecular mechanisms are still obscure. To address this question, we first generate the PRMT5 stable knockdown cell line (A549 cells) using lentivirus containing Scramble or PRMT5-shRNAs. As seen in Fig. 2A, the PRMT5 mRNA expression level was dramatically decreased in A549 cells compared with Scramble. To further confirm the PRMT5 depletion in A549 cells, we examined the PRMT5 protein expression level in the stable knockdown cell line, as well. As seen in Figs. 2B and 2C, the PRMT5 protein expression level was significantly reduced in the A549 cells compared with Scramble. In order to explore the precise role of PRMT5 in EMT, we generated the xenograft mouse model using PRMT5 depletion cells (Fig. 2D), and the EMT-related genes and proteins were detected, respectively. As seen in Fig. 2E, the qRT-PCR analysis showed that down-regulation of PRMT5 markedly reduced the EMT genes, such as β-catenin, collagen I, vimentin, and ZEB1. Subsequently, we evaluated the expression level of SDMA, which is related to the enzymatic activity of PRMT5. As shown in Fig. 2F, the SDMA expression level was dramatically impaired in PRMT5-depletion tumors compared with control tumors, indicating that the enzymatic activity of PRMT5 was reduced. We also detected the EMT markers in the PRMT5-depletion tumors. As seen in Fig. 2G, H, PRMT5 protein expression level was dramatically reduced in the tumors and the EMT-related proteins, β-catenin, collagen I, and ZEB1, were also distinctly decreased. Altogether, our findings indicate that PRMT5 regulates EMT markers in human lung cancer.
Figure 2.
Silencing of PRMT5 reduces EMT-related markers in vivo. (A) The mRNA expression level of PRMT5 was detected by qRT-PCR in A549 cells. *P < 0.05 vs. Scr, n = 4. (B) PRMT5 protein expression level was detected by Western blotting in A549 cells. Representative pictures were shown. (C) PRMT5 protein expression level was quantified. *P < 0.05 vs. Scr, n = 3. (D) The graphical summary of PRMT5 knockdown xenograft mouse model. (E) qRT-PCR analysis of indicated genes in Scr or PRMT5 knockdown tumors. *P < 0.05 vs. Scr, n = 6. (F) The global symmetric dimethylarginine (SDMA) was detected by Western blotting in in Scr or PRMT5 knockdown tumors. (G) Western blotting analysis of indicated proteins in Scr or PRMT5 knockdown tumors. Representative pictures were shown. (H) The indicated proteins were quantified. *P < 0.05 vs. Scr, n = 4.
Inhibition of PRMT5 Blocked EMT In Vivo
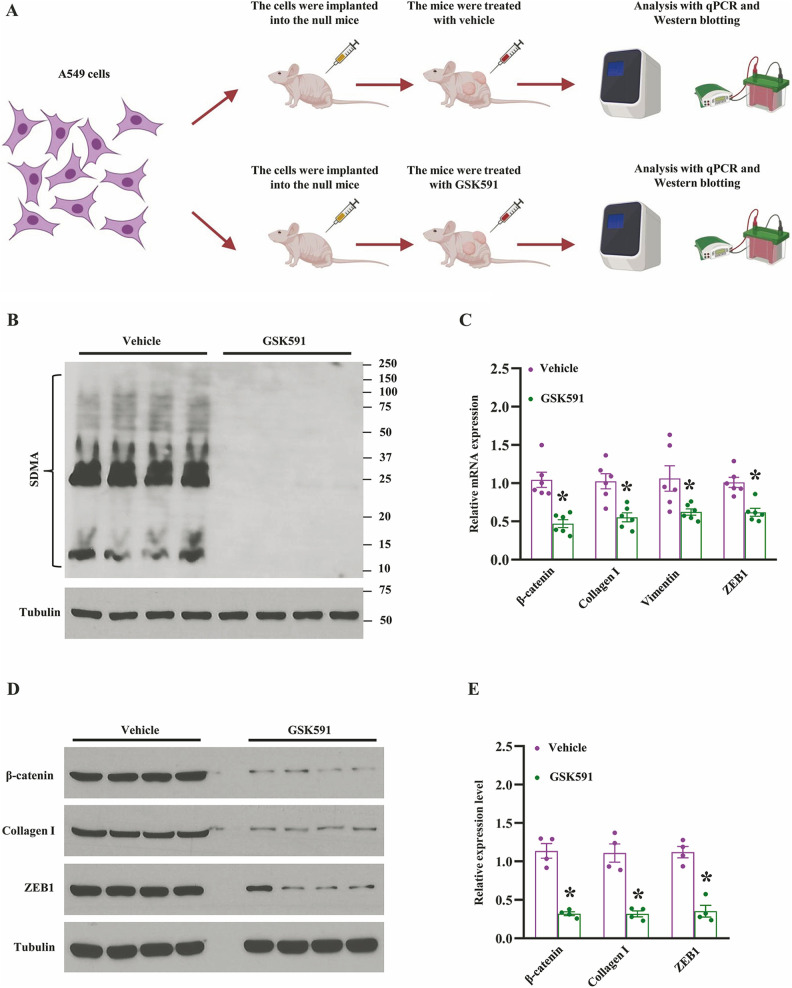
The emerging evidence has shown that PRMT5 controls cancer cell chromatin remodeling, gene expression, and non-histone protein modification by methylation14. Thus, the enzymatic activity of PRMT5 is crucial for the methylation of DNA and histone or non-histone proteins during human cancer development. In order to further explore the enzymatic activity of PRMT5 was required or not for the lung cancer EMT, we generated the xenograft mouse model using normal A549 cells, and the null mice were treated with vehicle or PRMT5 specific inhibitor GSK591. The procedures were shown in Fig. 3A. Firstly, we evaluated the expression level of SDMA, which is related to the enzymatic activity of PRMT5. As shown in Fig. 3B, the SDMA expression level was almost diminished in GSK591-treated tumors compared with vehicle treatment, indicating that the enzymatic activity of PRMT5 was blocked entirely. Next, we assessed the EMT-related gene expression using the same samples, and as shown in Fig. 3C, blocking of PRMT5 enzymatic activity markedly reduced the EMT genes, such as β-catenin, collagen I, vimentin, and ZEB1. To further confirm our findings, those proteins engaged in EMT were detected by Western blotting. As shown in Fig. 3D, E, EMT-related proteins, β-catenin, collagen I, and ZEB1, were distinctly decreased as well, indicating that enzymatic activity of PRMT5 is required to regulate EMT markers in human lung cancer. Collectively, our findings indicate that the enzyme activity of PRMT5 is closely related to EMT in human lung cancer.
Figure 3.
Inhibition of PRMT5 blocks EMT-related markers in vivo. (A) The graphical summary of the xenograft mouse model and the administration of PRMT5 inhibitor GSK591. (B) The global symmetric dimethylarginine (SDMA) was detected by Western blotting in tumors treated with vehicle or GSK591. (C) qRT-PCR analysis of indicated genes in tumors treated with vehicle or GSK591. *P < 0.05 vs. vehicle, n = 6. (D) Western blotting analysis of indicated proteins in tumors treated with vehicle or GSK591. Representative pictures were shown. (E) The indicated proteins were quantified. *P < 0.05 vs. vehicle, n = 4.
PRMT5 Regulates EMT via EGFR/Akt Signaling Pathway
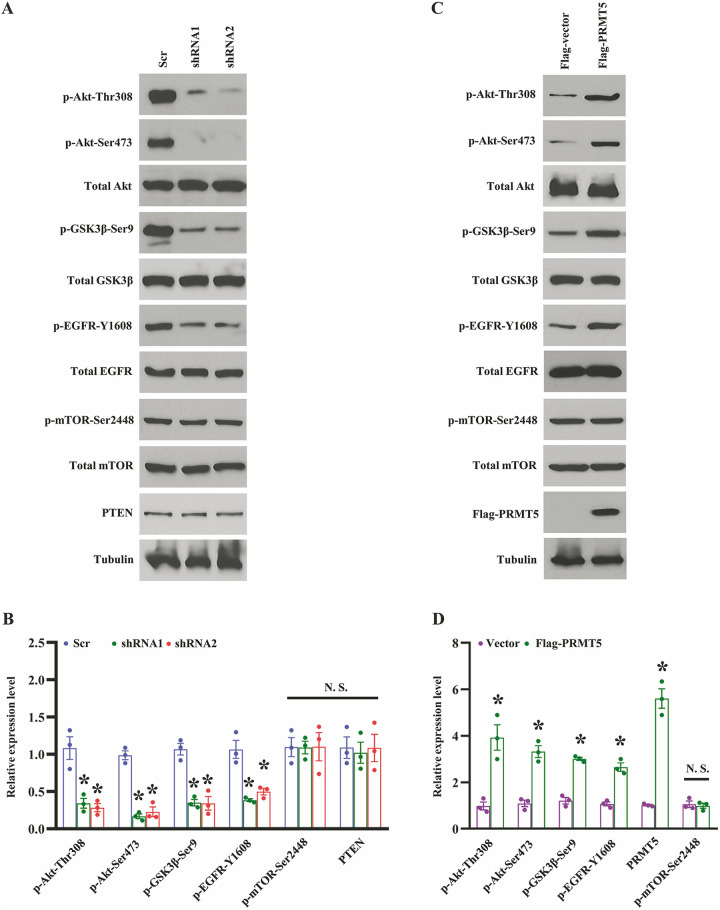
Although our previous studies have shown that PRMT5 regulates Akt activation in human lung cancer cells2, how does PRMT5 regulate EMT and the associated mechanisms are still unclear. Additionally, Akt is a crucial regulator for EMT in many types of human cancers15. Nevertheless, it is not clear whether PRMT5/Akt signaling pathway is engaged in EMT in human lung cancer. To this end, we determined the several essential proteins that regulated EMT in PRMT5-depletion A549 cells. As shown in Fig. 4A, B, the Akt phosphorylation at Thr308 and Ser473 was significantly reduced when PRMT5 was down-regulated. The total Akt expression was unchanged. We also found that the GSK3β phosphorylation at Ser9, a negative regulator for EMT, dramatically decreased, whereas the total GSK3β expression was unchanged. Strikingly, we found that the EGFR phosphorylation at Y1608 was markedly impaired, whereas the total EGFR expression was unchanged. Moreover, the mTOR phosphorylation at Ser2448, total mTOR, and PTEN, the upstream regulators of Akt, were unchanged. These results uncovered that PRMT5 regulates EMT via EGFR/Akt/GSK3β signaling axis in human lung cancer. To further confirm our hypothesis, we re-introduced the exogenous PRMT5 into the PRMT5-depletion A549 cells. As shown in Fig. 4C, D, the re-expression of PRMT5 dramatically increased the Akt phosphorylation at Thr308 and Ser 473 and the GSK3β phosphorylation at Ser9, whereas the total Akt and GSK3β expression were unchanged. We also found that re-expression of PRMT5 distinctly enhanced the EGFR phosphorylation at Y1608, whereas the total EGFR expression was unchanged. Furthermore, the mTOR phosphorylation at Ser2448 and total mTOR were unchanged. These findings reveal that PRMT5 regulates EMT through EGFR/Akt/GSK3β signaling pathway in human lung cancer.
Figure 4.
PRMT5 regulates EGFR/Akt axis to control EMT 35. (A) Western blotting analysis of indicated proteins in PRMT5 stable knockdown A549 36 cells. Representative pictures were shown. (B) The indicated proteins were quantified. 37 *P < 0.05 vs. Scr, n = 3. (C) PRMT5 stable knockdown A549 cells were transfected 38 with Flag-vector or Flag-PRMT5, and then the indicated proteins were detected by 39 Western blotting. Representative pictures were shown. (D) The indicated proteins were 40 quantified. *P < 0.05 vs. Flag-vector, n = 3.
Discussion
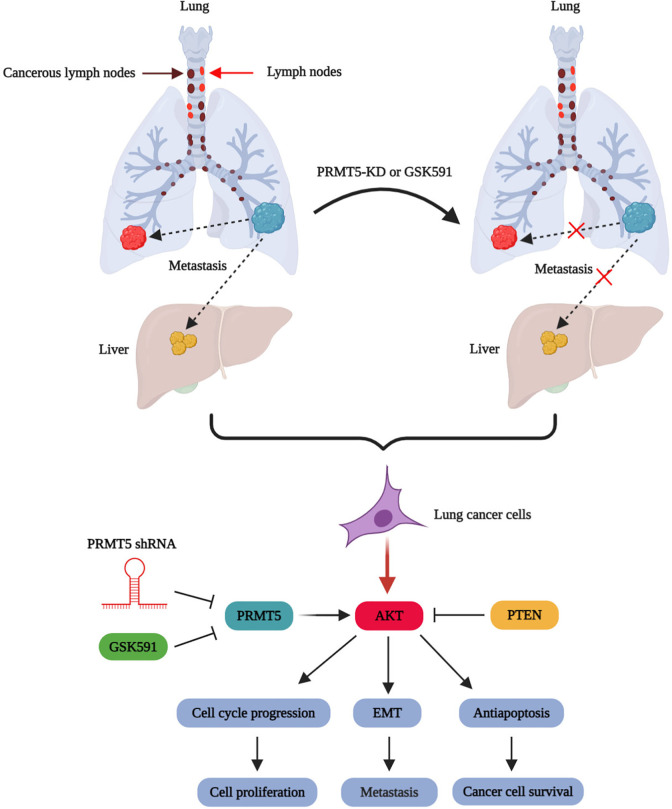
It has been well-known that dysfunction of PRMT5 was involved in different types of human cancer, and modulation of PRMT5 expression level or enzymatic activity was closely associated with cancer cell proliferation. Therefore, PRMT5 becomes a very promising target for the diagnosis and prognosis or treatment of human cancer. However, it is still unclear whether PRMT5 has participated in the EMT of human lung cancer. Additionally, the associated molecular mechanisms are also completely unknown. In the current study, we showed that the mRNA and protein expression levels of PRMT5 are highly expressed in human lung cancer tissues and cell lines (Fig. 1). Moreover, down-regulation of PRMTR5 by lentivirus-mediated shRNA or blocking PRMT5 enzymatic activity by specific inhibitor GSK591 repressed the expression of EMT-related markers (such as β-catenin, collagen I, vimentin, and ZEB1) both in mRNA and protein levels in vivo (Figs. 2 and 3). The loss-and-gain-of-function studies revealed that PRMT5 promotes EMT via regulation of the EGFR/Akt signaling pathway in human lung cancer cells (Figs. 4 and 5). Our findings indicate that PRMT5 is a vital upstream mediator in human lung cancer, and control of PRMT5 expression level or enzymatic activity may be a potential therapeutic strategy for the treatment of human lung cancer.
Figure 5.
The proposed model of PRMT5 promotes EMT in human lung cancer.
The emerging evidence has been shown that PRMT5 acted as an oncogene and engaged in cancer cell growth and proliferation. A recent study has been reported that PRMT5 overexpression promoted bladder cancer cell duplication, cell cycle progression, migration, and invasion via PI3K/Akt/mTOR signaling pathway16. Moreover, PRMT5 methylated and stabilized CDT1 to promoted cancer cell entry into the S phase17. PRMT5 also directly methylated transcriptional factor E2F1, a pivotal regulator for cell cycle, to promote cell cycle progression and led to cancer cell entry from G1 to S phase18, implying that the enzymatic activity of PRMT5 is required for cell cycle progression and proliferation in cancer cells. Further study showed that PRMT5 promoted colorectal cancer cell growth through arginine methylation of eIF4E and FGFR3, which was linked to mTOR/Akt/ERK signaling axis19. Besides, it has been reported that PRMT5 directly interacted with Akt and then controlled Akt activation in human lung cancer cells2. Besides, PRMT5 regulated FBW7 expression10 or EGFR/β-catenin signaling pathway to promote tumorigenesis in pancreatic cancer20. These observations suggest that PRMT5 plays a crucial role in various types of human cancer and regulates cancer cell proliferation, migration, and invasion via different signaling pathways. However, how does PRMT5 regulate EMT in human lung cancer, and the related mechanisms are still obscure. In the present study, we showed that PRMR5 was the ectopic expression in human lung cancer tissues and cell lines, and its enzymatic activity was also enhanced in lung cancer tissues (Fig. 1). Using the xenograft mouse model, we further showed that silencing PRMT5 by lentivirus-medicated shRNA or preventing PRMT5 enzymatic activity by specific inhibitor GSK591 reduced EMT-related markers both in mRNA and protein levels (Figs. 2 and 3). By loss-and-gain-of-function studies, we showed that PRMT5 regulates EMT-markers via EGFR/Akt signaling cascades (Fig. 4). Our findings not only confirm that PRMT5 is overexpressed in human lung cancer tissues and cells, but also reveal that PRMT5 is an important regulator for EMT in human lung cancer.
EMT plays an especial and vital role in human cancer progression and development. EMT is also closely related to the functions of various human tumors, such as cancer cell invasion, migration, and tumor initiation. The accumulated evidence has shown that PI3-K/Akt signaling cascades regulated EMT in different types of human cancer21, including lung cancer. Recently, many studies have shown that the PI3-K/Akt signaling cascades were the master signaling for cancer survival, proliferation, metabolism, cell cycle progression, and anti-apoptosis22. Additionally, the disorder of PI3-K/Akt signaling was involved in different human diseases, including cancer, autoimmune and cardiovascular disorders, metabolic diseases, and nerve disease22,23. Nevertheless, how does PRMT5 regulate EMT in human lung cancer cells and the underlying molecular mechanism remains unknown, although previous studies have shown that PRMT5 controlled PI3-K and PTEN hyperphosphorylation and then activated Akt in cancer cells24. So far, there was no evidence that PRMT5 modulated EMT via Akt signaling in lung cancer cells. In the present study, we reported that PRMT5 regulated both EGFR and Akt activation in lung cancer cells, which was independent of mTOR and PTEN signaling using PRMT5-stable depilation cells. Previous studies reported that down-regulation of PRMT5 dramatically decreased the phosphorylation level of ERK1/2 and mTOR in colorectal cancer cells19. Nevertheless, in our study, we did not detect any changes of the phosphorylation level of mTOR and total mTOR using PRMT5-stable depilation cells, suggesting that PRMT5 controlled EMT through EGFR/Akt signaling pathway, but not PTEN or mTOR. In addition, our results showed that down-regulation of PRMT5 or blocking PRMT5 enzymatic activity attenuated the EMT-related markers in vivo, indicating that PRMT5 expression level or enzymatic activity is required for EMT in human lung cancer cells. Importantly, our results also displayed novel signaling that PRMT5 promotes EMT by EGFR/Akt axis in lung cancer cells.
In summary, we demonstrated that PRMT5 was up-regulated in human lung cancer tissues and cell lines. Furthermore, our study discovered that PRMT5 promoted EMT in human lung cancer probably via EGFR/Akt signaling axis, but not the mTOR and PTEN pathway. The noteworthy feature of our study is that PRMT5 regulated the activation of EGFR and Akt at the same time, which then affected the expression of the downstream targets involved in EMT. More importantly, we established the relationship between PRMT5 and EGFR/Akt signaling axis in human lung cancer. The new insights into the regulation of the EGFR/Akt signaling pathway controlled by PRMT5 provided a novel mechanism of the carcinogenic function of PRMT5 in human lung cancer.
Supplemental Material
Supplemental Material, sj-doc-1-cll-10.1177_09636897211001772 for PRMT5 Promotes EMT Through Regulating Akt Activity in Human Lung Cancer by Jianhao Huang, Yonghua Zheng, Xiao Zheng, Bao Qian, Qi Yin, Jingjing Lu and Han Lei in Cell Transplantation
Supplemental Material, sj-docx-1-cll-10.1177_09636897211001772 for PRMT5 Promotes EMT Through Regulating Akt Activity in Human Lung Cancer by Jianhao Huang, Yonghua Zheng, Xiao Zheng, Bao Qian, Qi Yin, Jingjing Lu and Han Lei in Cell Transplantation
Footnotes
Author Contributing: Jianhao Huang and Yonghua Zheng are contributed equally to this work.
Ethical Approval: This study was approved by the Institutional Animal Care and Use Committee of Tongji University School of Medicine, Shanghai, China.
Statement of Human and Animal Rights: All procedures in this study were conducted in accordance with the Ethics Review Board of Shanghai East Hospital at Tongji University approved protocols.
Statement of Informed Consent: Informed consent was obtained from the human subjects used in this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work is supported by The Outstanding Clinical Discipline Project of Shanghai Pudong (PWYgy2018-06), Health Industry Clinical Research Project of Shanghai Health Family Planning Commission (201840222), and Project of Shanghai Health Commission (201940280).
ORCID iD: Yonghua Zheng  https://orcid.org/0000-0002-7799-9612
https://orcid.org/0000-0002-7799-9612
Jingjing Lu  https://orcid.org/0000-0003-2841-3117
https://orcid.org/0000-0003-2841-3117
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Huang L, Liu J, Zhang XO, Sibley K, Najjar SM, Lee MM, Wu Q. Inhibition of protein arginine methyltransferase 5 enhances hepatic mitochondrial biogenesis. J Biol Chem. 2018;293(28):10884–10894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhang S, Ma Y, Hu X, Zheng Y, Chen X. Targeting PRMT5/Akt signalling axis prevents human lung cancer cell growth. J Cell Mol Med. 2019;23(2):1333–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Li Y, Yang Y, Liu X, Long Y, Zheng Y. PRMT5 promotes human lung cancer cell apoptosis via Akt/Gsk3beta signaling induced by resveratrol. Cell Transplant. 2019;28(12):1664–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fong JY, Pignata L, Goy PA, Kawabata KC, Lee SC, Koh CM, Musiani D, Massignani E, Kotini AG, Penson A, Wun CM, et al. Therapeutic targeting of RNA splicing catalysis through inhibition of protein arginine methylation. Cancer Cell. 2019;36(2):194–209. e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pal S, Vishwanath SN, Erdjument-Bromage H, Tempst P, Sif S. Human SWI/SNF-associated PRMT5 methylates histone H3 arginine 8 and negatively regulates expression of ST7 and NM23 tumor suppressor genes. Mol Cell Biol. 2004;24(21):9630–9645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Scoumanne A, Zhang J, Chen X. PRMT5 is required for cell-cycle progression and p53 tumor suppressor function. Nucleic Acids Res. 2009;37(15):4965–4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ren J, Wang Y, Liang Y, Zhang Y, Bao S, Xu Z. Methylation of ribosomal protein S10 by protein-arginine methyltransferase 5 regulates ribosome biogenesis. J Biol Chem. 2010;285(17):12695–12705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tee WW, Pardo M, Theunissen TW, Yu L, Choudhary JS, Hajkova P, Surani MA. Prmt5 is essential for early mouse development and acts in the cytoplasm to maintain ES cell pluripotency. Genes Dev. 2010;24(24):2772–2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liu X, Zhang J, Liu L, Jiang Y, Ji J, Yan R, Zhu Z, Yu Y. Protein arginine methyltransferase 5-mediated epigenetic silencing of IRX1 contributes to tumorigenicity and metastasis of gastric cancer. Biochim Biophys Acta Mol Basis Dis. 2018;1864(9 Pt B):2835–2844. [DOI] [PubMed] [Google Scholar]
- 10. Qin Y, Hu Q, Xu J, Ji S, Dai W, Liu W, Xu W, Sun Q, Zhang Z, Ni Q, Zhang B, et al. PRMT5 enhances tumorigenicity and glycolysis in pancreatic cancer via the FBW7/cMyc axis. Cell Commun Signal. 2019;17(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Huang L, Pan D, Chen Q, Zhu LJ, Ou J, Wabitsch M, Wang YX. Transcription factor Hlx controls a systematic switch from white to brown fat through Prdm16-mediated co-activation. Nat Commun. 2017;8(1):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Musiani D, Bok J, Massignani E, Wu L, Tabaglio T, Ippolito MR, Cuomo A, Ozbek U, Zorgati H, Ghoshdastider U, Robinson RC, et al. Proteomics profiling of arginine methylation defines PRMT5 substrate specificity. Sci Signal. 2019;12(575):eaat8388. [DOI] [PubMed] [Google Scholar]
- 13. Shailesh H, Zakaria ZZ, Baiocchi R, Sif S. Protein arginine methyltransferase 5 (PRMT5) dysregulation in cancer. Oncotarget. 2018;9(94):36705–36718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Blanc RS, Richard S. Arginine methylation: the coming of age. Mol Cell. 2017;65(1):8–24. [DOI] [PubMed] [Google Scholar]
- 15. Karimi Roshan M, Soltani A, Soleimani A, Rezaie Kahkhaie K, Afshari AR, Soukhtanloo M. Role of AKT and mTOR signaling pathways in the induction of epithelial-mesenchymal transition (EMT) process. Biochimie. 2019;165:229–234. [DOI] [PubMed] [Google Scholar]
- 16. Tan L, Xiao K, Ye Y, Liang H, Chen M, Luo J, Qin Z. High PRMT5 expression is associated with poor overall survival and tumor progression in bladder cancer. Aging (Albany NY). 2020;12(9):8728–8741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Karkhanis V, Hu YJ, Baiocchi RA, Imbalzano AN, Sif S. Versatility of PRMT5-induced methylation in growth control and development. Trends Biochem Sci. 2011;36(12):633–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cho EC, Zheng S, Munro S, Liu G, Carr SM, Moehlenbrink J, Lu YC, Stimson L, Khan O, Konietzny R, McGouran J, et al. Arginine methylation controls growth regulation by E2F-1. EMBO J. 2012;31(7):1785–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang B, Dong S, Zhu R, Hu C, Hou J, Li Y, Zhao Q, Shao X, Bu Q, Li H, Wu Y, et al. Targeting protein arginine methyltransferase 5 inhibits colorectal cancer growth by decreasing arginine methylation of eIF4E and FGFR3. Oncotarget. 2015;6(26):22799–22811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ge L, Wang H, Xu X, Zhou Z, He J, Peng W, Du F, Zhang Y, Gong A, Xu M. PRMT5 promotes epithelial-mesenchymal transition via EGFR-beta-catenin axis in pancreatic cancer cells. J Cell Mol Med. 2020;24(2):1969–1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Xu W, Yang Z, Lu N. A new role for the PI3K/Akt signaling pathway in the epithelial-mesenchymal transition. Cell Adh Migr. 2015;9(4):317–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Manning BD, Toker A. AKT/PKB signaling: navigating the network. Cell. 2017;169(3):381–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mayer IA, Arteaga CL. The PI3K/AKT pathway as a target for cancer treatment. Annu Rev Med. 2016;67:11–28. [DOI] [PubMed] [Google Scholar]
- 24. Wei TY, Juan CC, Hisa JY, Su LJ, Lee YC, Chou HY, Chen JM, Wu YC, Chiu SC, Hsu CP, et al. Protein arginine methyltransferase 5 is a potential oncoprotein that upregulates G1 cyclins/cyclin-dependent kinases and the phosphoinositide 3-kinase/AKT signaling cascade. Cancer Sci. 2012;103(9):1640–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, sj-doc-1-cll-10.1177_09636897211001772 for PRMT5 Promotes EMT Through Regulating Akt Activity in Human Lung Cancer by Jianhao Huang, Yonghua Zheng, Xiao Zheng, Bao Qian, Qi Yin, Jingjing Lu and Han Lei in Cell Transplantation
Supplemental Material, sj-docx-1-cll-10.1177_09636897211001772 for PRMT5 Promotes EMT Through Regulating Akt Activity in Human Lung Cancer by Jianhao Huang, Yonghua Zheng, Xiao Zheng, Bao Qian, Qi Yin, Jingjing Lu and Han Lei in Cell Transplantation