Abstract
Optimisation of skeletal mineralisation in childhood is important to reduce childhood fracture and the long-term risk of osteoporosis and fracture in later life. One approach to achieving this is antenatal vitamin D supplementation. The Maternal Vitamin D Osteoporosis Study is a randomised placebo-controlled trial, the aim of which was to assess the effect of antenatal vitamin D supplementation (1000 IU/day cholecalciferol) on offspring bone mass at birth. The study has since extended the follow up into childhood and diversified to assess demographic, lifestyle and genetic factors that determine the biochemical response to antenatal vitamin D supplementation, and to understand the mechanisms underpinning the effects of vitamin D supplementation on offspring bone development, including epigenetics. The demonstration of positive effects of maternal pregnancy vitamin D supplementation on offspring bone development and the delineation of underlying biological mechanisms inform clinical care and future public-health policies.
Keywords: bone mineral density, bone turnover markers, cholecalciferol, epigenetics, Maternal Vitamin D Osteoporosis Study, osteoporosis, pregnancy
Introduction
Optimising childhood bone health is important. Fracture in children and adolescents is common; approximately one third of boys and one fifth of girls will sustain a fracture by the age of 18 years,1 with implications for loss of education and parental earnings, pain, reduced physical functioning and use of healthcare resources.2 The majority of fractures in children occur in those with normal bone strength due to trauma. However, reduced bone strength due to impaired bone mineralisation and alterations in bone microarchitecture do increase the propensity to fracture.3,4
Bone mineralisation in childhood also establishes a trajectory for adult bone health. During childhood and adolescence, the skeleton grows in both length and width, resulting in an increase in bone mass [the composite of bone mineral content (BMC) and bone size]. Although final height is reached shortly after the end of puberty, bone mineral accrual continues into the third decade, with peak bone mass (PBM) being reached in the mid to late 20s. Thereafter, bone mass declines, with an acceleration in the rate of bone loss after the menopause in women. Whilst PBM is in part genetically determined, external factors that modify an individual’s ability to achieve their genetic potential might moderate osteoporosis risk, and indeed mathematical modelling has shown that a modest increase in PBM can substantially delay the onset of osteoporosis.5 Osteoporotic fractures in later life are associated with increased mortality,6 poorer quality of life and functional decline,7 and are a significant cause of healthcare spending.8 There is an urgent need for approaches to reducing this burden, and importantly, there is increasing evidence to support targeting early life skeletal development. One such potential intervention is antenatal vitamin D supplementation. In this review, we review and discuss the findings and clinical implications of the Maternal Vitamin D Osteoporosis Study (MAVIDOS), the largest randomised placebo-controlled trial of antenatal cholecalciferol supplementation specifically aiming to address the effects on offspring musculoskeletal health,9 in the context of the wider evidence base.
Foetal skeletal mineralisation
Skeletal development begins from 8 weeks to 12 weeks gestation and requires synchronisation of chondrogenesis, osteogenesis and synovial joint formation. Mineralisation of bone templates generated through intramembranous and endochondral ossification principally occurs during the third trimester when 80% of bone mineral is accreted. During pregnancy, maternal Ca2+ is actively transported across the placenta to the foetus, resulting in a greater plasma Ca2+ concentration in the foetus compared with the mother.10 A doubling of maternal fractional absorption of calcium through the intestine from as early as 12 weeks gestation and maintained until delivery, and to a lesser extent, resorption of the maternal skeleton during the third trimester facilitates the availability of Ca2+ to meet foetal demands.11–13 This is achieved through alterations to maternal calcitropic hormones, including an increase in parathyroid-related peptide and 1,25 dihydroxyvitamin D [1,25(OH)2D].12,14 During the final 6 weeks of gestation, calcium transfers across the placenta at a rate of 300 mg/day, and at term the average foetal skeleton will contain approximately 30 g of calcium in addition to 20 g phosphorus and 0.8 g magnesium.11 Limited availability of substrates for bone mineralisation, for example due to maternal diet, impaired maternal intestinal function, maternal vitamin D deficiency or impaired placental function/transfer are therefore likely to impact negatively on bone mineralisation during in utero life.
Vitamin D in pregnancy
Considering the importance of maternal vitamin D to the upregulation of intestinal calcium absorption, it is not surprising that antenatal vitamin D status has been explored as a possible approach to improving offspring skeletal mineralisation.
Vitamin D is a group of fat-soluble secosteroids, of which cholecalciferol (vitamin D3) and ergocalciferol (vitamin D2) are the most common forms. Vitamin D can be obtained from dietary sources including oily fish, eggs and fortified milk, but the majority is synthesised in the skin from the action of ultraviolet-B to convert 7-dehydrocholesterol to pre-vitamin D3. This is then hydroxylated in the liver to its circulating form, 25-hydroxyvitamin D [25(OH)D]. This circulating 25(OH)D acts as a reservoir for conversion to the active metabolite, 1,25(OH)2D, the classical function of which is calcium and phosphate homeostasis, although other non-classical functions including in immunological, muscular and neurological functions are increasingly documented.
Serum 25(OH)D levels rather than 1,25(OH)2D15 are currently the best available biomarker of vitamin D status due to the longer half-life and tight physiological regulation of 1,25(OH)2D in response to Ca2+ homeostasis. There is great variability in the recommended thresholds to define vitamin D deficiency (usually between 25 nmol/L and 50 nmol/L),16–18 but risk factors for low serum 25(OH)D are well recognised. This includes seasonal variation (nadir in winter months), residing at latitudes far from the equator, reduced cutaneous vitamin D synthesis due to dark skin pigmentation, extensive skin colouring or limited time outdoors, and high adiposity, due to sequestration of vitamin D in adipose tissue.
As in other population groups, biochemically low levels of 25(OH)D are common in pregnant women. For example, in a study of predominately White women in the south of the UK, 31% had a serum 25(OH)D <50 nmol/L and 18% <25 nmol/L in late pregnancy.19 In a more ethnically diverse population in London, 36% women had 25(OH)D <25 nmol/L in early pregnancy.20 In the UK, the Department of Health currently recommends supplementation with 400 IU/day cholecalciferol throughout pregnancy and lactation,21 and the Institute of Medicine and the Global Consensus on Prevention and Management of Nutritional Rickets suggest supplementation with 600 IU/day during this period.22,23 Such an approach is of demonstrable benefit in reducing the incidence of symptomatic neonatal hypocalcaemia,24–26 with increasing evidence for a benefit for offspring skeletal health and birth weight,27 and maternal obstetric complications, such as pre-eclamspia.28
Observational studies of maternal vitamin D status and offspring bone mineralisation
An ever-growing collection of observational studies have investigated the relationships between markers of maternal vitamin D status and offspring bone health, with inconsistent outcomes. Approaches have included utilising the known seasonal variation in maternal serum 25(OH)D status as an ecological marker of vitamin D status and direct measurement of blood 25(OH)D concentrations.
In 1998 a study from Korea found that infants born in winter months had both lower whole body BMC and umbilical cord serum 25(OH)D levels compared with those born in summer months.29 However, this contradicted the findings of a similar study by the same authors undertaken in the USA.30 Using the Avon Longitudinal Study of Parents and Children, Sayer and Tobias31 reported that in nearly 7000 mother–offspring pairs estimated maternal ultraviolet B (UVB) exposure in late pregnancy was positively associated with offspring whole-body less head (WBLH) BMC, bone area and bone mineral density (BMD) at 9 years of age. However, assessment of maternal serum 25(OH)D in a subset of this cohort subsequently did not reveal any associations with offspring bone mineralisation.32
Other studies using measurement of maternal or umbilical cord blood 25(OH)D have also reported inconsistent findings. However, the populations studied, including the distribution of vitamin D status, gestation at measurement of 25(OH)D, approach to defining 25(OH)D as a continuous or categorical outcome, and age and method at which bone mineralisation is quantified in the offspring, have varied considerably. For example, studies from Canada and Norway reported low maternal 25(OH)D status during pregnancy associated with reduced BMC in the neonatal period and at 14 months of age.33–35 In contrast, a cohort in The Gambia displayed no association between maternal 25(OH)D status and offspring whole-body BMC at birth or at several ages in the first year of life.36 In the latter of these cohorts, no mothers had a serum 25(OH)D <50 nmol/L, suggesting that maternal 25(OH)D levels may only negatively impact skeletal mineralisation in severe deficiency.
Similarly, findings from observational studies in later childhood are also inconsistent. In the Southampton’s Women’s Survey (SWS), a UK-based prospective cohort, which included 1030 maternal–offspring pairs, offspring born to mothers with a serum 25(OH)D <25 nmol/L in late pregnancy had lower whole-body bone area, BMC, areal BMD and lumbar spine BMC at 6 years compared with offspring born to mothers with a measurement above this level.37 Furthermore, positive associations between late pregnancy maternal 25(OH)D and offspring muscle strength at 4 years were observed in the same cohort.38 Given the importance of lean mass and muscle loading to bone mineralisation, this could represent a further mechanism for the effect of vitamin D supplementation on bone mineralisation. In another cohort from the same geographical area, maternal late pregnancy serum 25(OH)D was also associated with whole-body and lumbar spine BMC at 9 years.19 One study showed that at age 11 years, maternal 25(OH)D in early pregnancy, but not 28–32 weeks gestation, was associated with spine and WBLH BMD in boys, but not girls.39 The earlier age at commencement of puberty in girls might have been important to this observation. A study from Australia demonstrated the persistence of the relationship between maternal pregnancy vitamin D status and offspring bone mineralisation in young adulthood.40 In contrast, a study from The Netherlands with follow up at age 6 years did not support these findings.41 These inconsistent findings highlight the need for high-quality intervention studies to define the nature of these relationships.
The MAVIDOS trial
MAVIDOS was the first intervention study designed specifically to assess the effects of antenatal vitamin D supplementation in pregnancy on offspring bone mineralisation.42
Methodology
The trial recruited women in early pregnancy from three sites in the UK: University Hospital Southampton NHS Foundation Trust (latitude 50.9°N), John Radcliffe Hospital, Oxford (latitude 51.8°N) and Sheffield Hospitals NHS Trust (latitude 53.4°N). Women with a singleton pregnancy, not taking more than 400 IU/day vitamin D supplementation and, due to an ethical stipulation, with a baseline 25(OH)D between 25 nmol/L and 100 nmol/L were eligible to participate. Randomisation was to either 1000 IU/day cholecalciferol or matched placebo from 14 weeks gestation until delivery. Both participants and researchers were blinded to the randomisation. Detailed assessments of anthropometry, lifestyle, diet and blood sampling took place at 14 weeks and 34 weeks gestation (Table 1). At birth, infant anthropometric data were collected, and a whole-body dual-energy X-ray absorptiometry (DXA) scan performed within 2 weeks of birth. Follow up of the offspring occurred at regular intervals up to 6 years of age, including assessments of growth and bone health, as detailed in Table 1.
Table 1.
Schedule of data collection in the MAVIDOS trial.
| Pregnancy | Birth | Childhood follow up | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 14 weeks | 18–21 weeks | 34 weeks | Birth | 1 year | 2 years | 3 years | 4 years | 6–8 years | |
| Mother | |||||||||
| Anthropometry | x | x | x | ||||||
| Health, diet and lifestyle questionnaire | x | x | |||||||
| Blood sampling | x | x | |||||||
| Tablet count to assess compliance | x | x | x | ||||||
| Foetal ultrasonography | x | ||||||||
| DXA | x | x | |||||||
| pQCT | x | ||||||||
| Father | |||||||||
| Anthropometry | x* | x* | x* | ||||||
| Placenta | |||||||||
| Placental and cord tissue collected | x | ||||||||
| Offspring | |||||||||
| Anthropometry | x | x | x | x | x | x | |||
| Health, diet and lifestyle questionnaire | x | x | x | x | x | ||||
| DXA | x | x | x | ||||||
| pQCT | x | ||||||||
| HRpQCT | x | ||||||||
| Hand-grip strength | x | x | |||||||
| Blood sampling | x (cord blood) | x | x | ||||||
x denotes data collected at this time point.
Measurement obtained once at one of these visits.
DXA, dual-energy X-ray absorptiometry; HRpQCT, high-resolution peripheral quantitative computed tomography; MAVIDOS, Maternal Vitamin D Osteoporosis Study; pQCT, peripheral quantitative computed tomography.
Participant profile
A total of 1134 women were randomised into the study: 148 women were excluded pre-randomisation due to a screening of 25(O)H)D <25 nmol/L (n = 89) or >100 nmol/L (n = 59) and 965 (85.1%) remained in the study until delivery. These women were older, more likely of White ethnicity and better educated that those who withdrew. Over 94% of the women in the study were of White ethnicity. Just over 40% of the women were in their first pregnancy, 8% of the women smoked and nearly half were educated to degree level or higher. Of the 965 infants delivered into the study, 95% of infants were born at term; just over half were male. Compliance with the study medication, as assessed by tablet counts, was extremely high (>95%).
Strengths and limitations
The double-blind placebo-controlled nature of the MAVIDOS study has generated the highest quality evidence with low risk of bias, and the large number of participants provided higher statistical power than some of the earlier much smaller studies of gestational vitamin D supplementation. The detailed phenotyping and comprehensive assessments of the women and offspring has enabled both the primary outcome and a number of other secondary hypotheses to be addressed.
The main limitation of the MAVIDOS trial was the exclusion of women with very low levels of 25(OH)D in early pregnancy. This was due to ethics considerations that these women should receive supplementation despite allowing all participants to take up to 400 IU/day vitamin D, as advised by the UK Department of Health,21 if they wished. As many observational studies have suggested that any detrimental effects of low vitamin D only become apparent at very low levels of 25(OH)D, women with 25(OH)D <25 nmol/L are potentially more likely to benefit from supplementation.43–47 This stipulation therefore reduced the ability to discern effects in the most vitamin D-deficient population.
Over 95% of the participants in the MAVIDOS trial were of White ethnicity. This reflects the local populations from which the women were recruited and does give more homogeneity to the study population, but also limits the generalisability of the study to women from other ethnic groups.
Key findings of the MAVIDOS trial
The effect of antenatal cholecalciferol supplementation on offspring bone mineralisation
The primary outcome of MAVIDOS was to determine the effect of antenatal cholecalciferol supplementation on offspring bone mass at birth. DXA scans of 736 infants were included in the analysis. The primary outcome of neonatal whole BMC did not differ statistically significantly between babies born to vitamin D-supplemented versus placebo mothers. However, a number of interactions had been prespecified within the original analysis plan.42 In consideration of the recognised seasonal variation in serum 25(OH)D, this included analysis of the effect by season of birth. Here, amongst winter/early spring deliveries when background 25(OH)D concentrations tend to be lowest in the population, and vitamin D supplementation prevented the fall in 25(OH)D from early to late pregnancy that was observed in the placebo group, the intervention led to a 0.5 standard deviation (SD) increase in neonatal whole body BMC compared with placebo, with no differences apparent in other seasons. A smaller, but significant interaction was also evident between season and areal BMD (aBMD) (Figure 1).9 To place the observed effect sizes into a clinical context, these are substantially larger than those observed in children with and without fracture, and therefore have the potential to be clinically important if sustained into later childhood. These findings would support the notion that the last trimester is the critical window for foetal bone mineral accretion, and although supplementation with 1000 IU/day did not result in the same achieved 25(OH)D level in women who delivered in winter compared with summer months, supplementation did prevent the decline in 25(OH)D status from early to late pregnancy observed in mothers who delivered in winter and were randomised to placebo. Interim follow up of Southampton children at 4 years has demonstrated a persisting benefit for WBLH BMC and aBMD, unstratified by season, with a further suggestion of interactions with childhood calcium intake and physical activity.48
Figure 1.
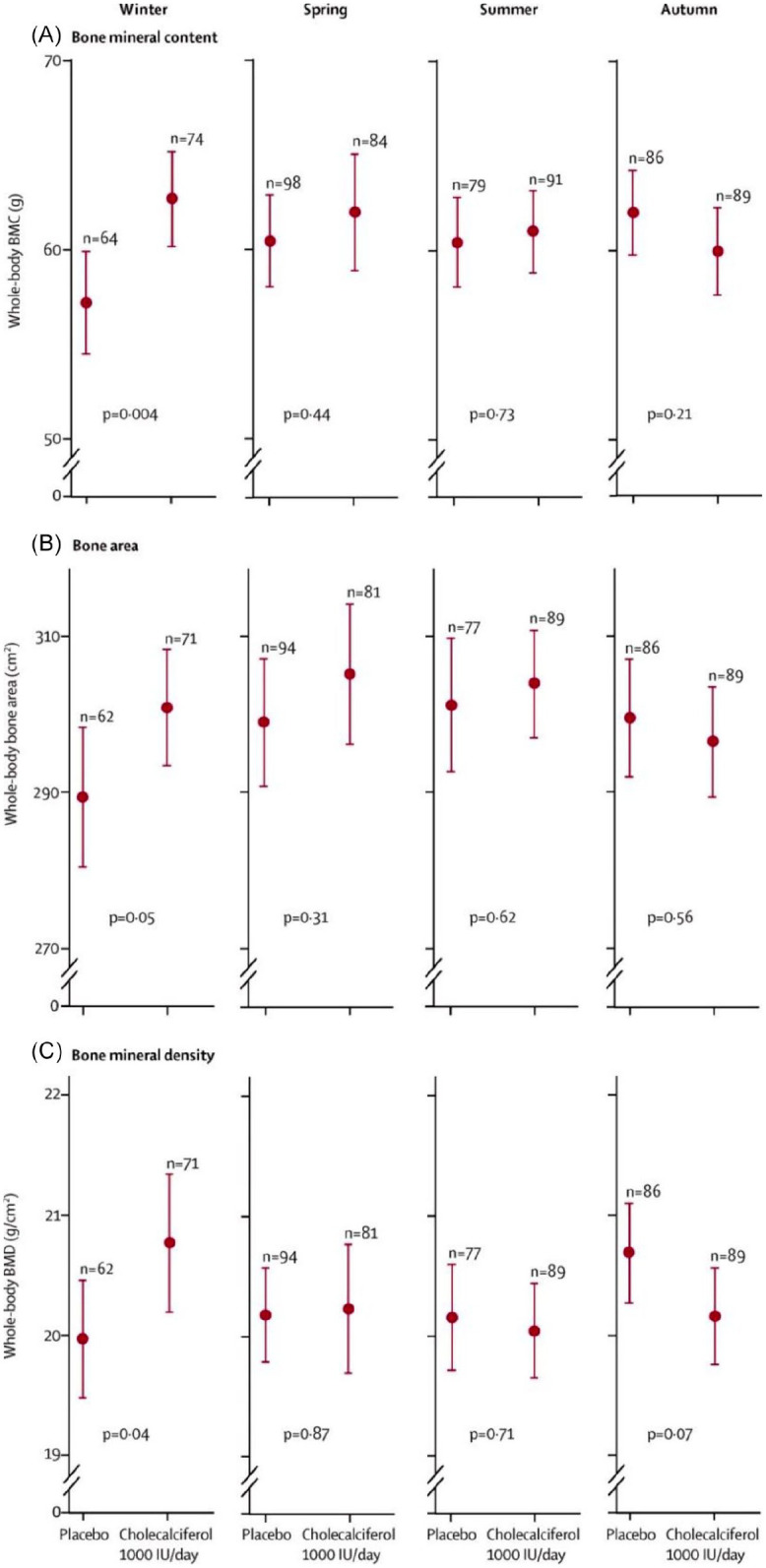
Neonatal whole-body BMC, bone area and BMD by intervention group and season of birth in the MAVIDOS trial.
Data are shown as mean and 95% confidence interval. Winter is December to February, spring is March to May, summer is June to August and autumn is September to November.
Reproduced with permission from Cooper et al.9
BMC, bone mineral content; BMD, bone mineral density; MAVIDOS, Maternal Vitamin D Osteoporosis Study.
This is consistent with recently published data from the Copenhagen Prospective Studies on Asthma in Childhood (COPSAC2010) trial.49 The Danish trial studied a higher dose of cholecalciferol supplementation (2800 IU/day versus 400 IU as control group), which was started later in gestation (24 weeks) but the observed differences in WBLH BMC and aBMD at 6 years of 0.15 and 0.2 SD, respectively, are of similar magnitude to the differences observed in MAVIDOS. The findings of MAVIDOS and COPSAC are in contrast to the findings of two small studies from India and Iran, which did not show a difference in infant bone mineralisation in response to antenatal cholecalciferol supplementation. However, major flaws in the methodology, substantial loss to follow up resulting in considerably smaller datasets (n = 52 and n = 25) and demographic differences between the randomisation groups limit the interpretation of these studies.50,51
Ongoing follow up of the MAVIDOS trial at 6 years of age will further assess the persistence of these findings, including analysis of both DXA and high-resolution peripheral quantitative computed tomography of the tibia.
Mechanistic understanding of the effect of antenatal vitamin D supplementation and offspring bone mass
Epigenetic and biochemical mechanisms
It is becoming clear that some of the residual variance in BMD and fracture risk in adulthood might be explained by the influence of the environment on gene expression, both in utero and in early life.52 It is widely recognised that genes effectively provide a library of information that can be read (expressed) differently in different cells and tissues according to function and need, often in response to environmental cues.53 These effects are likely to be underpinned by epigenetic mechanisms, processes by which gene expression is modified but without changes in the DNA code itself. Epigenetic mechanisms include DNA methylation, histone modification and non-coding RNAs, the most widely studied of which is DNA methylation, the transfer of a methyl group to a particular genomic location, usually at the 5′ carbon position of cytosine adjacent to a guanine base, or CpG site. Methylation at regions of the genome particularly rich in CpG sites, for example, at the 5′ end of genes in regions known as CpG islands, often near to the promoter of a gene, may have important influences on that gene’s expression.53–55 Earlier analyses in the SWS birth cohort study showed perinatal DNA methylation at two loci of interest, CDKN2A, a key element in cell senescence56 and the retinoid-X-receptor-A (RXRA) gene, were inversely associated with childhood BMC corrected for body size at 4 years of age.57,58 RXRA forms a heterodimer with the vitamin D receptor and is essential in the nuclear action of 1,25(OH)2-vitamin D. Methylation at one CpG site was related to an estimate of free 25(OH)D. Using the MAVIDOS trial to establish the influence of maternal vitamin D supplementation on methylation of RXRA in a randomised controlled trial setting, it was demonstrated that supplementation with cholecalciferol in pregnancy is associated with reduced methylation at specific regions near to the RXRA promoter in foetal DNA derived from the umbilical cord of the offspring (Figure 2).59 This was in keeping with previous findings in the SWS, raising the possibility of site specificity for a molecular interaction between 25(OH)D in pregnancy and DNA methylation.60 Whilst the exact nature of the mechanistic underpinnings of these findings remains to be elucidated, there are several routes by which maternal 25(OH)D status might influence perinatal RXRA methylation. As RXRA forms a heterodimer with several nuclear hormones known to influence bone metabolism, including 1,25(OH)2-vitamin D, maternal 25(OH)D status may play a permissive role in the transcriptional regulation of the RXRA gene. Studies have shown that vitamin D may interact with the epigenome on multiple levels61–65 and evaluation of public data from Encyclopedia of DNA elements (ENCODE) suggested that methylation at the studied CpG sites is likely to have functional relevance.
Figure 2.

Effect of maternal vitamin D supplementation in pregnancy and perinatal umbilical cord methylation at CpG sites in the RXRA locus.
Each bar comes from a separate linear regression. The outcomes are expressed in SDs.
Reproduced with permission from Curtis et al.59
CI, confidence interval; RXRA, retinoid-X-receptor-A; SD, standard deviation.
The MAVIDOS trial also offered the opportunity to study the impact of gestational vitamin D supplementation on the maternal skeleton, as maternal calcium homeostasis adapts to meet the calcium demands of the developing foetus,66–68 though its response to vitamin D supplementation is not well defined. Biochemical markers of bone turnover offer a noninvasive method of monitoring changes in bone resorption or formation during pregnancy;69 in the MAVIDOS trial maternal urinary C-terminal telopeptide of type I collagen (CTX) was measured at 14 weeks and 34 weeks gestation. At the population level, there is evidence of inverse associations between 25(OH)D concentrations and markers of bone resorption such as CTX.70–73 In the MAVIDOS cohort maternal gestational cholecalciferol supplementation was associated with a smaller gestational increase in bone resorption markers compared with placebo. Whilst maternal urinary CTX almost doubled from 14 weeks’ to 34 weeks’ gestation in both randomisation groups, the conditional increase in CTX from early to late pregnancy was lower in the cholecalciferol-supplemented group compared with the placebo group. Furthermore, late pregnancy CTX was inversely associated with postpartum measures of maternal bone from DXA.74 These findings are consistent with a protective effect of gestational vitamin D supplementation on maternal bone health, however, long-term follow up of both mothers and offspring, with repeat assessments of bone indices, is needed.
The effect of cholecalciferol supplementation on vitamin D status and determinants of the response to supplementation
The MAVIDOS trial has aided understanding of the biochemical response to supplementation. We clearly demonstrated that antenatal supplementation with 1000 IU/day increased maternal 25(OH)D status in late pregnancy; 83% of women randomised to cholecalciferol achieved a 25(OH)D >50 nmol/L at 34 weeks’ gestation compared with only 36% in the placebo group (Figure 3). No participant reported symptoms suggestive of vitamin D toxicity,75 although women with a baseline 25(OH)D >100 nmol/L, who might have been at higher risk of toxicity were excluded from participation. Nonetheless, this finding supports other studies that have also demonstrated a rise in biochemical vitamin D status in response to pregnancy supplementation, and even higher doses up to 4000 IU/day have not been associated with adverse clinical outcomes in other trials.76
Figure 3.

Proportion of women achieving vitamin D replete status (25(OH)D >50 nmol/L) in late pregnancy stratified by randomisation to placebo or 1000 IU/day cholecalciferol and season of delivery.
Winter was defined as December to May.
Importantly, despite high levels of compliance in this group, 17% of women did not achieve vitamin D repletion in late pregnancy, suggesting that perhaps higher doses are required to achieve this (Figure 3). However, interestingly, a previous study in New Zealand did not show a difference in repletion rates (25(OH)D >50 nmol/L) for doses of 1000 or 2000 IU/day cholecalciferol during pregnancy.77 Indeed, in the MAVIDOS trial evidence for a ceiling effect of supplementation was also present. Thus, there was a smaller difference in 25(OH)D concentrations at 34 weeks gestation between the placebo and cholecalciferol groups with increasing baseline 25(OH)D. This interaction between baseline 25(OH)D and randomisation group on achieved 25(OH)D was highly statistically significant (p < 0.001).
Using the MAVIDOS trial we identified a number of factors that were associated with the 25(OH)D response to cholecalciferol supplementation. Firstly, it is clear that the seasonal variation in 25(OH)D status at latitudes far from the equator78 is not abolished by this level of antenatal supplementation at latitudes within the UK75 (Figure 3). In addition, using multivariate analysis, compliance and baseline 25(OH)D were positively associated with the achieved 25(OH)D in late pregnancy following antenatal vitamin D supplementation, whereas weight gain during pregnancy was negatively associated.75 Similarly, Black and Minority Ethnic ethnicity was associated with a higher risk of not achieving vitamin D replete status in the women with supplementation, consistent with the finding of Hollis et al.76 that even with 4000 IU/day vitamin D during pregnancy, African-American women had lower 25(OH)D in late pregnancy than White or Hispanic women. Maternal weight gain during pregnancy was negatively associated with the response to cholecalciferol supplementation in the MAVIDOS trial. This finding is consistent with our earlier observation using data from the SWS birth cohort study, which showed greater gestational weight gain was negatively associated with the tracking of 25(OH)D from early to late pregnancy independent of supplement use.78 Other studies in nonpregnant adults have similarly demonstrated that over 50% of the variance in 25(OH)D increment in response to supplementation is explained by body weight.79
Genetic variation in the response to cholecalciferol was also identified in the MAVIDOS cohort. A number of single nucleotide polymorphisms (SNPs) within the vitamin D metabolism pathway, including in genes encoding 7-dehydrocholesterol reductase in the skin, 25-hydroxylase, 24-hydroxylase and vitamin D binding protein (DBP) have been identified as significantly associated with 25(OH)D status in nonpregnant populations.80,81 In women of White ethnicity in the MAVIDOS trial, SNPs in genes encoding 25-hydroxylase (CYP2R1) and DBP (GC) were associated with the achieved 25(OH)D after supplementation.82
A substudy of the MAVIDOS trial assessed psychological characteristics associated with compliance with the study medication, including self-efficacy, defined as the belief that one is capable of carrying out a specific behaviour. Women with higher self-efficacy experienced fewer practical problems with taking the supplement. Experiencing practical problems, having doubts and uncertainties about the medication and rate of compliance were all strongly associated with one another (p < 0.05 for all), and the latter with 25(OH)D achieved postsupplementation (Figure 4).83
Figure 4.
Conceptual model summarising the relationships between self-efficacy, vitamin D at 34 weeks, compliance with trial protocol, practical problems taking the study medication, uncertainty and doubts about taking the medication.
Reproduced with permission from Barker et al.83
Implications for clinical practice
Guidelines in the UK and USA recommend pregnant women should take 400–600 IU/day cholecalciferol.21,84 The findings of the MAVIDOS trial42,48 and COPSAC201049 suggest that higher doses of antenatal vitamin D supplementation have beneficial effects on offspring skeletal mineralisation. Attempts to replicate these findings in the Southampton Pregnancy Intervention for the Next Generation (SPRING) study is currently in progress,85 and ongoing follow up of the MAVIDOS trial at 6–8 years aims to demonstrate persistence of the observed effect further into childhood.
It is clear from the MAVIDOS trial that 1000 IU/day cholecalciferol does not abolish the seasonal variation in 25(OH)D status during late pregnancy and that a large proportion of women who were supplemented with this dose, and in particular those who delivered in winter months, will still have a 25(OH)D level in late pregnancy of <50 nmol/L. As such, if the aim of supplementation is to increase maternal 25(OH)D to >50 nmol/L, which is often considered the definition for repletion, then it is likely that 400 IU/day will not achieve this in many women. However, a change in public-health policy needs to be based on established benefits in high-quality randomised controlled trials, and whilst the findings of the MAVIDOS trial begin to demonstrate important clinical outcomes, consistent findings across more randomised controlled trials are required.
It is also important to be certain that in addition to benefits a higher dose will not be harmful. The literature with regards to falls risk in older individuals suggests that moderate doses of vitamin D (600–1000 IU/day) may have a beneficial effect whilst high bolus doses increase the risk of falls.86 There were no obvious side effects of 1000 IU/day during pregnancy in the MAVIDOS trial or up to 4000 IU/day in another pregnancy study,76 and there appears to be a ceiling effect to the achievable 25(OH)D following this level of supplementation when baseline 25(OH)D levels are high. However, a clear benefit of higher dose antenatal supplementation needs to be demonstrated before it can be recommended in routine clinical practice.
The MAVIDOS trial has confirmed that a number of maternal factors are associated with poorer biochemical response to supplementation, including compliance, low baseline 25(OH)D, non-White ethnicity and weight gain. In clinical practice, counselling women on the risk of vitamin D deficiency and need for supplementation is vital, particularly those with well-recognised risk factors for deficiency. This should be routinely reviewed at every antenatal appointment. In the UK, serum 25(OH)D is not routinely assessed in early pregnancy, and the additional economic cost of this might be difficult to justify in light of the low likelihood of harm from low-dose cholecalciferol supplementation. It perhaps needs to be demonstrated in research studies that dosing schedules based on baseline 25(OH)D will achieve higher vitamin D repletion in a greater number of women and improved clinical outcomes before measurement of 25(OH)D in early pregnancy could be deemed necessary. Similarly, it is clear that the degree of weight gain during pregnancy is associated with 25(OH)D status and the response to supplementation. Women with higher than recommended weight gain should be counselled on the need for vitamin D supplementation to maintain their 25(OH)D status.
Conclusion
The findings from the MAVIDOS trial of potential beneficial effects of maternal vitamin D supplementation during pregnancy on offspring bone mass, and the elucidation of possible underlying mechanisms, have increased our understanding of the role of vitamin D in pregnancy. Results from the MAVIDOS trial have informed policy from bodies such as the UK Scientific Advisory Committee on Nutrition87 and the National Institute for Health Research.88 These findings, and those from ongoing follow up, data analysis and substudies within the MAVIDOS trial, together with further independent trials such as SPRING, will be critical to future public-health advice on vitamin D supplementation in pregnancy.
Acknowledgments
We would like to thank the Medical Research Council (UK), National Institute for Health Research, Wellcome Trust, versus Arthritis, Royal Osteoporosis Society Osteoporosis and Bone Research Academy and International Osteoporosis Foundation for supporting this work. We are very grateful to all the members of the MAVIDOS trial group: Elaine Alexander, Nigel K. Arden, Linda Barron, Nicholas J. Bishop, Joanna Cantle, Andrew Carr, Patsy Coakley, Sue Collins, Vanessa Cox, Sarah R. Crozier, Stefania D’Angelo, Valerie Davill, Elaine M. Dennison, Caroline Doré, Richard Eastell, Roger Francis, Robert Fraser, Saurabh V. Gandhi, Keith M. Godfrey, Julia Hammond, Kate Hart, Doreen Hedger, Susan Higginbottom, Tina Horsfall, Hazel M. Inskip, M. Kassim Javaid, Wendy Johnson, Stephen Kennedy, Sue Macey, Pam Mahon, Karen McGill, Brenda Morgan, M. Zulf Mughal, Corinne Nisbet, Aris T. Papageorghiou, Mark Philips, Ann Prentice, David M. Reid, Chris Roberts, Sian Robinson, Inez Schoenmakers, Sarah Standfield, Deborah Symmons, Christine Taylor, Pat Taylor, Suzanne Wood.
Footnotes
Conflict of interest statement: NCH has received consultancy, lecture fees and honoraria from Alliance for Better Bone Health, Amgen, MSD, Eli Lilly, Servier, Shire, Consilient Healthcare and Internis Pharmaceuticals. CC has received consultancy, lecture fees and honoraria from Amgen, GlaxoSmithKline, Alliance for Better Bone Health, MSD, Eli Lilly, Pfizer, Novartis, Servier, Medtronic and Roche. EMC reports honoraria/travel support from Eli Lilly, UCB and Amgen outside the submitted work.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Rebecca J Moon  https://orcid.org/0000-0003-2334-2284
https://orcid.org/0000-0003-2334-2284
Contributor Information
Rebecca J. Moon, MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton General Hospital, Tremona Road, Southampton, Hampshire SO16 6YD, UK.
Elizabeth M. Curtis, MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton General Hospital, Southampton, Hampshire, UK
Stephen J. Woolford, MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton General Hospital, Southampton, Hampshire, UK
Shanze Ashai, MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton General Hospital, Southampton, Hampshire, UK.
Cyrus Cooper, MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton General Hospital, Southampton, Hampshire, UK; NIHR Southampton Biomedical Research Centre, University of Southampton and University Hospital Southampton NHS Foundation Trust, Southampton, UK; NIHR Biomedical Research Centre, University of Oxford, Oxford, UK.
Nicholas C. Harvey, MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton General Hospital, Southampton, Hampshire, UK NIHR Southampton Biomedical Research Centre, University of Southampton and University Hospital Southampton NHS Foundation Trust, Southampton, UK.
References
- 1. Moon RJ, Harvey NC, Curtis EM, et al. Ethnic and geographic variations in the epidemiology of childhood fractures in the United Kingdom. Bone 2016; 85: 9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Morris MWJ, Bell MJ. The socio-economical impact of paediatric fracture clinic appointments. Injury 2006; 37: 395–397. [DOI] [PubMed] [Google Scholar]
- 3. Clark EM, Ness AR, Tobias JH. Bone fragility contributes to the risk of fracture in children, even after moderate and severe trauma. J Bone Miner Res 2008; 23: 173–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Farr JN, Tomas R, Chen Z, et al. Lower trabecular volumetric BMD at metaphyseal regions of weight-bearing bones is associated with prior fracture in young girls. J Bone Miner Res 2011; 26: 380–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hernandez CJ, Beaupre GS, Carter DR. A theoretical analysis of the relative influences of peak BMD, age-related bone loss and menopause on the development of osteoporosis. Osteoporos Int 2003; 14: 843–847. [DOI] [PubMed] [Google Scholar]
- 6. Abrahamsen B, van Staa T, Ariely R, et al. Excess mortality following hip fracture: a systematic epidemiological review. Osteoporos Int 2009; 20: 1633–1650. [DOI] [PubMed] [Google Scholar]
- 7. Kim SM, Moon YW, Lim SJ, et al. Prediction of survival, second fracture, and functional recovery following the first hip fracture surgery in elderly patients. Bone 2012; 50: 1343–1350. [DOI] [PubMed] [Google Scholar]
- 8. Hernlund E, Svedbom A, Ivergard M, et al. Osteoporosis in the European Union: medical management, epidemiology and economic burden: a report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch Osteoporos 2013; 8: 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cooper C, Harvey NC, Bishop NJ, et al. Maternal gestational vitamin D supplementation and offspring bone health (MAVIDOS): a multicentre, double-blind, randomised placebo-controlled trial. Lancet Diabetes Endocrinol 2016; 4: 393–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Forestier F, Daffos F, Rainaut M, et al. Blood chemistry of normal human fetuses at midtrimester of pregnancy. Pediatr Res 1987; 21: 579–583. [DOI] [PubMed] [Google Scholar]
- 11. Kovacs CS. Maternal mineral and bone metabolism during pregnancy, lactation, and post-weaning recovery. Physiol Rev 2016; 96: 449–547. [DOI] [PubMed] [Google Scholar]
- 12. Cross NA, Hillman LS, Allen SH, et al. Calcium homeostasis and bone metabolism during pregnancy, lactation, and postweaning: a longitudinal study. Am J Clin Nutr 1995; 61: 514–523. [DOI] [PubMed] [Google Scholar]
- 13. More C, Bhattoa HP, Bettembuk P, et al. The effects of pregnancy and lactation on hormonal status and biochemical markers of bone turnover. Eur J Obstet Gynecol Reprod Biol 2003; 106: 209–213. [DOI] [PubMed] [Google Scholar]
- 14. Ardawi MS, Nasrat HA, HS BAA. Calcium-regulating hormones and parathyroid hormone-related peptide in normal human pregnancy and postpartum: a longitudinal study. Eur J Endocrinol 1997; 137: 402–409. [DOI] [PubMed] [Google Scholar]
- 15. Jones KS, Assar S, Harnpanich D, et al. 25(OH)D2 half-life is shorter than 25(OH)D3 half-life and is influenced by DBP concentration and genotype. J Clin Endocrinol Metab 2014; 99: 3373–3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Absoud M, Cummins C, Lim MJ, et al. Prevalence and predictors of vitamin D insufficiency in children: a Great Britain population based study. PLoS One 2011; 6: e22179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011; 96: 1911–1930. [DOI] [PubMed] [Google Scholar]
- 18. Aspray TJ, Bowring C, Fraser W, et al. National Osteoporosis Society vitamin D guideline summary. Age Ageing 2014; 43: 592–595. [DOI] [PubMed] [Google Scholar]
- 19. Javaid MK, Crozier SR, Harvey NC, et al. Maternal vitamin D status during pregnancy and childhood bone mass at age 9 years: a longitudinal study. Lancet 2006; 367: 36–43. [DOI] [PubMed] [Google Scholar]
- 20. McAree T, Jacobs B, Manickavasagar T, et al. Vitamin D deficiency in pregnancy – still a public health issue. Matern Child Nutr 2013; 9: 23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. National Institute for Health and Clinical Excellence. Antenatal care (NICE Clinical Guideline 62), www.guidance.nice.org.uk/cg622008 (accessed 1 August 2020)
- 22. Ross AC, Taylor CL, Yaktine AL, et al. Dietary reference intakes for calcium and vitamin D. Washington, DC: Institute of Medicine, 2011. [PubMed] [Google Scholar]
- 23. Munns CF, Shaw N, Kiely M, et al. Global consensus recommendations on prevention and management of nutritional rickets. J Clin Endocrinol Metab 2016; 101: 394–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Marya RK, Rathee S, Lata V, et al. Effects of vitamin D supplementation in pregnancy. Gynecol Obstet Invest 1981; 12: 155–161. [DOI] [PubMed] [Google Scholar]
- 25. Hashemipour S, Lalooha F, Zahir Mirdamadi S, et al. Effect of vitamin D administration in vitamin D-deficient pregnant women on maternal and neonatal serum calcium and vitamin D concentrations: a randomised clinical trial. Br J Nutr 2013; 110: 1611–1616. [DOI] [PubMed] [Google Scholar]
- 26. Cockburn F, Belton NR, Purvis RJ, et al. Maternal vitamin D intake and mineral metabolism in mothers and their newborn infants. BMJ 1980; 281: 11–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Roth DE, Leung M, Mesfin E, et al. Vitamin D supplementation during pregnancy: state of the evidence from a systematic review of randomised trials. BMJ 2017; 359: j5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fogacci S, Fogacci F, Banach M, et al. Vitamin D supplementation and incident preeclampsia: a systematic review and meta-analysis of randomized clinical trials. Clin Nutr 2020; 39: 1742–1752. [DOI] [PubMed] [Google Scholar]
- 29. Namgung R, Tsang RC, Lee C, et al. Low total body bone mineral content and high bone resorption in Korean winter-born versus summer-born newborn infants. J Pediatr 1998; 132: 421–425. [DOI] [PubMed] [Google Scholar]
- 30. Namgung R, Tsang RC, Specker BL, et al. Low bone mineral content and high serum osteocalcin and 1, 25-dihydroxyvitamin D in summer-versus winter-born newborn infants: an early fetal effect? J Pediatr Gastroenterol Nutr 1994; 19: 220–227. [DOI] [PubMed] [Google Scholar]
- 31. Sayers A, Tobias JH. Estimated maternal ultraviolet B exposure levels in pregnancy influence skeletal development of the child. J Clin Endocrinol Metab 2009; 94: 765–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lawlor DA, Wills AK, Fraser A, et al. Association of maternal vitamin D status during pregnancy with bone-mineral content in offspring: a prospective cohort study. Lancet 2013; 381: 2176–2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Weiler H, Fitzpatrick-Wong S, Veitch R, et al. Vitamin D deficiency and whole-body and femur bone mass relative to weight in healthy newborns. CMAJ 2005; 172: 757–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Viljakainen H, Korhonen T, Hytinantti T, et al. Maternal vitamin D status affects bone growth in early childhood – a prospective cohort study. Osteoporos Int 2011; 22: 883–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Viljakainen HT, Saarnio E, Hytinantti T, et al. Maternal vitamin D status determines bone variables in the newborn. J Clin Endocrinol Metab 2010; 95: 1749–1757. [DOI] [PubMed] [Google Scholar]
- 36. Prentice A, Jarjou LM, Goldberg GR, et al. Maternal plasma 25-hydroxyvitamin D concentration and birthweight, growth and bone mineral accretion of Gambian infants. Acta Paediatr 2009; 98: 1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Moon R, Harvey N, Davies J, et al. Vitamin D and bone development. Osteoporos Int 2015; 26: 1449–1451. [DOI] [PubMed] [Google Scholar]
- 38. Harvey NC, Moon RJ, Sayer AA, et al. Maternal antenatal vitamin D status and offspring muscle development: findings from the Southampton women’s survey. J Clin Endocrinol Metab 2014; 99: 330–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hyde NK, Brennan-Olsen SL, Mohebbi M, et al. Maternal vitamin D in pregnancy and offspring bone measures in childhood: the vitamin D in pregnancy study. Bone 2019; 124: 126–131. [DOI] [PubMed] [Google Scholar]
- 40. Zhu K, Whitehouse AJ, Hart P, et al. Maternal vitamin D status during pregnancy and bone mass in offspring at 20 years of age: a prospective cohort study. J Bone Miner Res 2014; 29: 1088–1095. [DOI] [PubMed] [Google Scholar]
- 41. Garcia AH, Erler NS, Jaddoe VW, et al. 25-hydroxyvitamin D concentrations during fetal life and bone health in children aged 6 years: a population-based prospective cohort study. Lancet Diabetes Endocrinol 2017; 5: 367–376. [DOI] [PubMed] [Google Scholar]
- 42. Harvey NC, Javaid K, Bishop N, et al. MAVIDOS maternal vitamin D osteoporosis study: study protocol for a randomized controlled trial. The MAVIDOS study group. Trials 2012; 13: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gernand AD, Simhan HN, Klebanoff MA, et al. Maternal serum 25-hydroxyvitamin D and measures of newborn and placental weight in a U.S. multicenter cohort study. J Clin Endocrinol Metab 2013; 98: 398–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wei SQ, Qi HP, Luo ZC, et al. Maternal vitamin D status and adverse pregnancy outcomes: a systematic review and meta-analysis. J Matern Fetal Neonatal Med 2013; 26: 889–899. [DOI] [PubMed] [Google Scholar]
- 45. Schneuer FJ, Roberts CL, Guilbert C, et al. Effects of maternal serum 25-hydroxyvitamin D concentrations in the first trimester on subsequent pregnancy outcomes in an Australian population. Am J Clin Nutr 2014; 99: 287–295. [DOI] [PubMed] [Google Scholar]
- 46. Eckhardt CL, Gernand AD, Roth DE, et al. Maternal vitamin D status and infant anthropometry in a US multi-centre cohort study. Ann Hum Biol 2015; 42: 215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Leffelaar ER, Vrijkotte TG, van Eijsden M. Maternal early pregnancy vitamin D status in relation to fetal and neonatal growth: results of the multi-ethnic Amsterdam born children and their development cohort. Br J Nutr 2010; 104: 108–117. [DOI] [PubMed] [Google Scholar]
- 48. Curtis EM, MR, D’Angelo S, Crozier SR, et al. Maternal pregnancy vitamin D supplementation is associated with greater offspring bone mineral density at 4 years: findings from the MAVIDOS trial. J Bone Miner Res, https://www.asbmr.org/meetings/2019-abstracts (2019, accessed 1 August 2020)
- 49. Brustad N, Garland J, Thorsen J, et al. Effect of high-dose vs standard-dose vitamin D supplementation in pregnancy on bone mineralization in offspring until age 6 years: a prespecified secondary analysis of a double-blinded, randomized clinical trial. JAMA Pediatr 2020; 174: 419–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sahoo SK, Katam KK, Das V, et al. Maternal vitamin D supplementation in pregnancy and offspring outcomes: a double-blind randomized placebo-controlled trial. J Bone Miner Metab 2016; 35: 64–471. [DOI] [PubMed] [Google Scholar]
- 51. Vaziri F, Dabbaghmanesh MH, Samsami A, et al. Vitamin D supplementation during pregnancy on infant anthropometric measurements and bone mass of mother-infant pairs: a randomized placebo clinical trial. Early Hum Dev 2016; 103: 61–68. [DOI] [PubMed] [Google Scholar]
- 52. Dennison EM, Arden NK, Keen RW, et al. Birthweight, vitamin D receptor genotype and the programming of osteoporosis. Paediatr Perinat Epidemiol 2001; 15: 211–219. [DOI] [PubMed] [Google Scholar]
- 53. Gluckman PD, Hanson MA, Cooper C, et al. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med 2008; 359: 61–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gicquel C, El-Osta A, Le Bouc Y. Epigenetic regulation and fetal programming. Best Pract Res Clin Endocrinol Metab 2008; 22: 1–16. [DOI] [PubMed] [Google Scholar]
- 55. Tang WY, Ho SM. Epigenetic reprogramming and imprinting in origins of disease. Rev Endocr Metab Disord 2007; 8: 173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hannou SA, Wouters K, Paumelle R, et al. Functional genomics of the CDKN2A/B locus in cardiovascular and metabolic disease: what have we learned from GWASs? Trends Endocrinol Metab 2015; 26: 176–184. [DOI] [PubMed] [Google Scholar]
- 57. Curtis EM, Murray R, Titcombe P, et al. Perinatal DNA methylation at CDKN2A is associated with offspring bone mass: findings from the Southampton women’s survey. J Bone Miner Res 2017; 32: 2030–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Harvey NC, Sheppard A, Godfrey KM, et al. Childhood bone mineral content is associated with methylation status of the RXRA promoter at birth. J Bone Miner Res 2014; 29: 600–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Curtis EM, Krstic N, Cook E, et al. Gestational vitamin D supplementation leads to reduced perinatal RXRA DNA methylation: results from the MAVIDOS trial. J Bone Miner Res 2019; 34: 231–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Jones PA. Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nat Rev Genet 2012; 13: 484–492. [DOI] [PubMed] [Google Scholar]
- 61. Carlberg C. Molecular endocrinology of vitamin D on the epigenome level. Mol Cell Endocrinol 2017; 453: 14–21. [DOI] [PubMed] [Google Scholar]
- 62. ENCODE Project Consortium. An integrated encyclopedia of DNA elements in the human genome. Nature 2012; 489: 57–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Takeyama K, Kato S. The vitamin D3 1alpha-hydroxylase gene and its regulation by active vitamin D3. Biosci Biotechnol Biochem 2011; 75: 208–213. [DOI] [PubMed] [Google Scholar]
- 64. Karlic H, Varga F. Impact of vitamin D metabolism on clinical epigenetics. Clin Epigenetics 2011; 2: 55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Fetahu IS, Höbaus J, Kállay E. Vitamin D and the epigenome. Front Physiol 2014; 5: 164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kovacs CS. Calcium and bone metabolism in pregnancy and lactation. J Clin Endocrinol Metab 2001; 86: 2344–2348. [DOI] [PubMed] [Google Scholar]
- 67. Givens MH, Macy IG. The chemical composition of the human fetus. J Biol Chem 1933; 102: 7–17. [Google Scholar]
- 68. Kumar R, Cohen WR, Silva P, et al. Elevated 1,25-dihydroxyvitamin D plasma levels in normal human pregnancy and lactation. J Clin Invest 1979; 63: 342–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Robins SP. Collagen crosslinks in metabolic bone disease. Acta Orthop Scand Suppl 1995; 266: 171–175. [PubMed] [Google Scholar]
- 70. Park H, Brannon PM, West AA, et al. Maternal vitamin D biomarkers are associated with maternal and fetal bone turnover among pregnant women consuming controlled amounts of vitamin D, calcium, and phosphorus. Bone 2017; 95: 183–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Thiering E, Bruske I, Kratzsch J, et al. Associations between serum 25-hydroxyvitamin D and bone turnover markers in a population based sample of German children. Sci Rep 2015; 5: 18138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Haliloglu B, Ilter E, Aksungar FB, et al. Bone turnover and maternal 25(OH) vitamin D3 levels during pregnancy and the postpartum period: should routine vitamin D supplementation be increased in pregnant women? Eur J Obstet Gynecol Reprod Biol 2011; 158: 24–27. [DOI] [PubMed] [Google Scholar]
- 73. O’Brien EC, Kilbane MT, McKenna MJ, et al. Calcium intake in winter pregnancy attenuates impact of vitamin D inadequacy on urine NTX, a marker of bone resorption. Eur J Nutr 2018; 57: 1015–1023. [DOI] [PubMed] [Google Scholar]
- 74. Curtis EM, Parsons C, Maslin K, et al. O29 bone turnover in pregnancy, measured by urinary C-terminal telopeptide of type I collagen (CTX), is influenced by vitamin D supplementation and is associated with maternal bone health: findings from the MAVIDOS trial. Rheumatology 2019; 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Moon RJ, Harvey NC, Cooper C, et al. Determinants of the maternal 25-hydroxyvitamin D response to vitamin D supplementation during pregnancy. J Clin Endocrinol Metab 2016; 101: 5012–5020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Hollis BW, Johnson D, Hulsey TC, et al. Vitamin D supplementation during pregnancy: double-blind, randomized clinical trial of safety and effectiveness. J Bone Miner Res 2011; 26: 2341–2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Grant CC, Stewart AW, Scragg R, et al. Vitamin D during pregnancy and infancy and infant serum 25-hydroxyvitamin D concentration. Pediatrics 2013; 133: e143–e153. [DOI] [PubMed] [Google Scholar]
- 78. Moon RJ, Crozier SR, Dennison EM, et al. Tracking of 25-hydroxyvitamin D status during pregnancy: the importance of vitamin D supplementation. Am J Clin Nutr 2015; 102: 1081–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Zittermann A, Ernst JB, Gummert JF, et al. Vitamin D supplementation, body weight and human serum 25-hydroxyvitamin D response: a systematic review. Eur J Nutr 2014; 53: 367–374. [DOI] [PubMed] [Google Scholar]
- 80. Wang TJ, Zhang F, Richards JB, et al. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet 2010; 376: 180–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Sollid ST, Hutchinson MY, Berg V, et al. Effects of vitamin D binding protein phenotypes and vitamin D supplementation on serum total 25(OH)D and directly measured free 25(OH)D. Eur J Endocrinol 2016; 174: 445–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Moon RJ, Harvey NC, Cooper C, et al. Response to antenatal cholecalciferol supplementation is associated with common vitamin D related genetic variants. J Clin Endocrinol Metab 2017; 102: 2941–2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Barker M, D’Angelo S, Ntani G, et al. The relationship between maternal self-efficacy, compliance and outcome in a trial of vitamin D supplementation in pregnancy. Osteoporos Int 2017; 28: 77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the institute of medicine: what clinicians need to know. J Clin Endocrinol Metab 2011; 96: 53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Baird J, Barker M, Harvey NC, et al. Southampton PRegnancy Intervention for the Next Generation (SPRING): protocol for a randomised controlled trial. Trials 2016; 17: 493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Dawson-Hughes B. Vitamin D and muscle function. J Steroid Biochem Mol Biol 2017; 173: 313–316. [DOI] [PubMed] [Google Scholar]
- 87. Scientific Advisory Committee on Nutrition. Vitamin D and health. London: The Stationary Office, 2016. [Google Scholar]
- 88. National Institute for Health Research. Better beginnings improving health for pregnancy. London: National Institute for Health Research, 2017. [Google Scholar]



