Abstract
Coronavirus disease 2019 (COVID-19) was first identified at the end of 2019 as a cluster of pneumonia cases in Wuhan, China. By February 2020, this virus quickly spread, becoming a global pandemic. The spectrum of symptomatic infection severity can range from mild, severe, and critical disease. Many correlated comorbidities were established, including smoking, socioeconomic background, gender (male prevalence), hypertension, obesity, cardiovascular disease, chronic lung disease, diabetes mellitus, cancer, and chronic kidney disease. In an extensive literature search, post-COVID-19 necrotizing Staphylococcus aureus pneumonia with pneumothorax has not been recorded. We present a case about a 62-year-old male who presented with symptoms of COVID-19 with many underlying comorbidities, including hypertension and hyperlipidemia. He was on ventilatory support during his first week in the hospital and then received supplemental oxygenation as he recovered from his COVID-19 pneumonia. Nearly a month and a half after his initial presentation, he quickly decompensated and was started on supplemental oxygen and the necessary treatments. It was then, with the aid of lab work and imaging, that we determined that he had developed necrotizing Staphylococcus aureus pneumonia with pneumothorax. He was adequately treated, and once he was stable, he was discharged home and was told to continue his therapy.
Keywords: COVID-19, necrotizing pneumonia, Staphylococcus aureus, pneumothorax, community-acquired pneumonia
Introduction
With the increase in the number of COVID-19 cases, many different complications of COVID-19 pneumonia are now coming to light. In COVID-19 pneumonia, it is proposed that alveolar damage can lead to alveolar rupture. The pathophysiology of the air-leak has not yet been well recognized. In comparison, it is well-documented that a pneumothorax can arise as a mechanical ventilation complication. In a retrospective case series, data showed that 1% of the patients admitted to the hospital and 2% of those in the intensive care unit (ICU) with COVID-19 pneumonia develop pneumothorax as a complication. They further explain that it has been seen in patients without mechanical ventilation or pre-existing lung disease, and occurred at a higher rate in males than females at a ratio of 3.3:1.1
Concurrently, post-COVID-19 necrotizing Staphylococcus aureus pneumonia with pneumothorax has not yet been documented in the literature. In 2003, there was a reported increase of S. aureus superinfections with the severe acute respiratory syndrome coronavirus-1 (SARS-CoV-1) outbreak. Some of these cases led to necrotizing pneumonia, induced by Panton–Valentine leukocidin (PVL) secreting methicillin-susceptible S. aureus (MSSA). This strain of S. aureus has only been established in a few patients infected with COVID-19.2 A case-cohort study found that COVID-19 pneumonia patients had a higher risk of developing ICU bloodstream infections (ICU-BSIs) compared to patients who were critically ill without COVID-19 infection. The top eight microorganisms linked in these ICU-BSI were coagulase-negative Staphylococci (35.9%), Enterobacterales (12.8%), Pseudomonas aeruginosa (12.8%), Candida albicans (10.3%), Enterococcus spp (10.3%), S. aureus (7.7%), other Gram-positive (7.7%) and anaerobic bacteria (2.6%). In addition, their analyzed data concluded that COVID-19 patients receiving immune-modulatory therapies such as tocilizumab or anakinra had a significantly higher risk of ICU-BSI.3
Therefore, secondary ICU-BSIs occur at a higher rate in COVID-19 pneumonia patients, especially on immune-modulating therapies compared to those without COVID-19 pneumonia. The timeline of symptoms, lab work, and imaging all play a crucial role in determining whether complications such as a pneumothorax is secondary to COVID-19 pneumonia or to a BSI that has occurred concurrently or shortly after the COVID-19 pneumonia.
Case report
We present a case about a 62-year-old Hispanic male brought to the hospital’s emergency room due to increasing shortness of breath and dizziness. He had multiple comorbidities, including hypertension and hyperlipidemia. Before arrival at the emergency department (ED), emergency medical services (EMS) noted that his saturations were in the ’40s. They quickly placed him on 100% O2 via a non-rebreather, and they noticed improvement in his saturations to 91%. He was febrile with a 38.5°C temperature and had tachypnea recorded in the ’30s on 100% O2 high-flow nasal cannula (NC) in the ED. Due to his high oxygen needs and significant work of breathing, the patient was subsequently intubated.
When obtaining a history, he related that he had been getting progressively short of breath over 6 days. He also described high cyclical temperatures over the past 3 days, with the maximum recorded temperature of 38.9°C. When questioned further, he denied cough but did endorse anosmia starting 4 days earlier. A COVID-19 test was requested and returned positive for viral RNA with the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) RT-PCR. Labs revealed that his WBC and sodium were low at 4.2 × 109/L and 130 mmol/L, respectively. They also revealed high aspartate aminotransferase (AST), alanine aminotransferase (ALT), lactic acid, C-reactive protein (CRP), D-dimer, and fibrinogen at 160 U/L, 143 U/L, 3.4 mmol/L, 299 mg/L, 489 ng/mL, and 850 mg/dL, respectively. A chest X-ray was ordered and had positive findings for airspace disease with diffuse airspace opacities consistent with the findings of COVID-19 pneumonia.
The following was his disease progression during his stay in the hospital. On hospital day 1, he received tocilizumab and remdesivir. On the second day, he was given convalescent plasma. While reassessing the patient on day 5, there had been little improvement; his fever, shortness of breath, and anosmia persisted. He was started on a dose of methylprednisolone. By the seventh day, he was extubated, and he remained on 40%–50% O2 high-flow NC.
He started therapeutic enoxaparin on hospital day 14 due to persistently high oxygen requirements and elevated D-dimer. The following week, he remained symptomatic with a low-grade fever and increased oxygen requirements due to shortness of breath. On day 27, he was finally weaned down to 6 L of NC and was transferred to the step-down unit. He remained stable for about a week, only requiring oxygen, and his fever was no longer there. Hospital protocol indicated that a follow-up X-ray was necessary and revealed that his COVID-19 pneumonia was improving. On day 38 of his stay in the hospital, the Rapid Response Team was called. The patient decompensated significantly and required 100% O2 via high-flow NC.
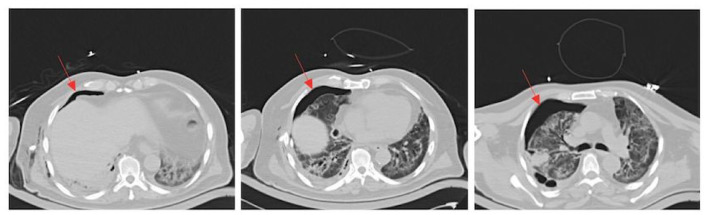
An EKG and enzymes were ordered and were unremarkable. A computed tomography (CT) of the chest was subsequently ordered due to his progressive shortness of breath with increased oxygenation requirements. The CT revealed an air-leak into the chest cavity, as seen in Figure 1. The red arrows depict a right-sided pneumothorax. The CT also demonstrated ground-glass opacities. It was believed that he developed this pneumothorax secondary to the COVID-19 pneumonia.
Figure 1.
Chest CT: the red arrows depict a right-sided pneumothorax. The CT also demonstrated ground-glass opacities.
Thoracic surgery was consulted because of the CT findings and the patient’s symptomatology. It was decided that a pigtail catheter should be inserted. A few hours after tube placement, the patient spiked a fever. Blood, urine, and sputum cultures were ordered. Two out of two sets of the blood cultures resulted in S. aureus. He was started on vancomycin and piperacillin/tazobactam until the sensitivities were conclusive. A total of three sets of blood cultures were ordered, and all came back positive for MSSA. The patient’s medications were adjusted to oxacillin accordingly.
After 48 h of receiving the new treatment, he was still having shortness of breath but no longer had a fever. A transesophageal echo was ordered and displayed no abnormalities. It was then that a repeat chest CT scan was ordered. The CT revealed that the patient continued to have persistent air-leaks for 1 week in addition to decreased parenchymal enhancement. In Figure 2, the yellow arrow points to the pneumothorax that has persisted due to the air-leak, and the blue arrow depicts the irregular cavities destroying the right upper lobe. The medical team concluded that the cause of these irregular cavities causing the pneumothorax was necrotizing S. aureus pneumonia that had developed secondary to his COVID-19 pneumonia.
Figure 2.
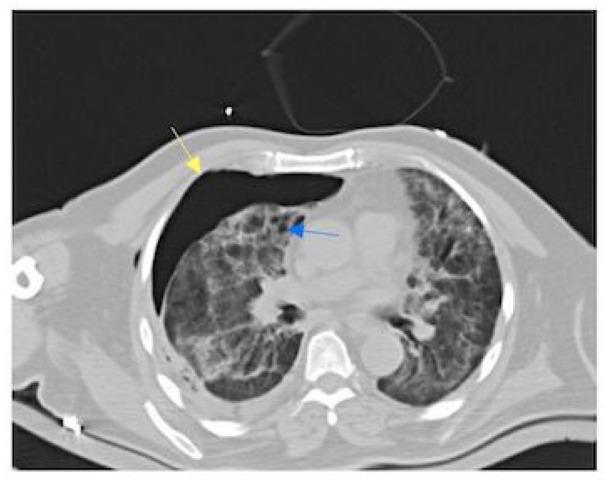
Follow-up chest CT: the yellow arrow points to the pneumothorax that has persisted due to the air-leak, and the blue arrow depicts the irregular cavities destroying the right upper lobe.
Due to the persistent leak visualized in the CT, he underwent right upper lobe endoscopic endobronchial valve. Shortly after valve placement, the air-leak stopped, and his shortness of breath resolved. Soon after, the chest tubes were clamped and subsequently removed. He had a peripherally inserted central catheter (PICC) line placed, and oxacillin treatment continued for 6 weeks. Once he was stabilized, he was discharged and was instructed to complete his antibiotics course.
Discussion
In December 2019, according to the World Health Organization (WHO), SARS-CoV-2, which causes COVID-19 pneumonia, was first reported in Wuhan, China. Subsequently, following the virus’s discovery and identification, more than 60,000,000 cases have been confirmed worldwide, along with more than 1,400,000 deaths.4 The increasing severity of the disease is correlated to many risk factors. These risk factors include age > 60 years, diabetes, hypertension, cardiac disease, chronic lung disease, cerebrovascular disease, chronic kidney disease, immunosuppression, and cancer.5 The Wuhan Data suggested that 8% of patients hospitalized with COVID-19 had a bacterial/fungal co-infection. The WHO had recommended not prescribing antimicrobials for COVID-19 pneumonia patients, especially if there is a low suspicion for bacterial infection. However, recent studies have shown that 1% of hospitalized COVID-19 patients and 2% of those in the ICU are at a greater risk of developing secondary BSIs. It is then that one must identify the secondary infection and treat it accordingly.1
Buetti et al.3 describe an increase in secondary and superinfections with SARS-CoV-2 similar to those seen in the early 2000s with the SARS-CoV-1 outbreak and the reported increase of S. aureus superinfections. In 1919, during the 1918–1919 influenza pandemic, S. aureus was recognized to cause secondary bacterial infection by Chickering. At that time, it was reported in healthy adults who did not have an underlying risk factor.5–7 According to Rothberg, if a viral influenza-like infection is followed by a period of complete resolution of symptoms, and then 4–14 days later was followed by a recurrence of fever, dyspnea, productive cough, and or pulmonary consolidation, most often this was due to S. pneumonia, S. aureus, or Haemophilus influenzae.7
S. aureus is considered an infrequent cause of community-acquired pneumonia (CAP). It accounts for about 3% of cases in which a bacterium is the identified cause. In comparison, S. aureus has been identified as one of the organisms that develop in the bloodstream at a rate of 7.7% in COVID-19 patients that develop these BSIs. However, it is a recognized cause of influenza-associated CAP.8–10 It is also noted that even though S. aureus pneumonia happens throughout the entire year, co-infections of influenza and S. aureus pneumonia commonly peak together.11,12
When S. aureus is detected as the cause of pneumonia, especially with an underlying influenza-like infection, such as SARS-CoV-2, it is usually associated with severe disease. Disease progression may lead to pulmonary necrosis, neutropenia, and shock.12 The proposed pathophysiology behind the development of this pulmonary necrosis is linked to PVL. PVL is a cytotoxin produced by S. aureus that can lead to necrotizing pneumonia due to a rapid increase of immune cells to lung tissues. Together, influenza-like diseases and the destruction of the cells by PVL ultimately damage the lungs’ epithelium.2 Furthermore, necrotizing pneumonia is described as a separate disease entity characterized by sudden onset and rapid worsening of the symptoms, leukopenia, airway hemorrhages, severe respiratory failure, necrotic destruction of vast areas of the lung, and a high mortality rate.13,14 This can ultimately lead to the development of a pneumothorax due to all of the damages occurring, as seen in our patient. When this happens, it is vital that the patients have lab work and appropriate imaging to assess both complications and treatment.
In comparison, in the setting of COVID-19 pneumonia, sudden respiratory decompensation with severe onset hypoxemia should also be investigated. Imaging revealing a pneumothorax is a well-established complication of mechanical ventilation, which may become evident.15 There have been documented cases of delayed pneumothorax and pneumomediastinum with mechanical ventilation and non-invasive ventilation such as high-flow NC. Less common is the pneumothorax’s delayed occurrence long after the discontinuation of mechanical ventilation.16,17 Martinelli et al. describe 60 cases of pneumothorax in which 58 had confirmed COVID-19 as the cause. They noted that not all of the patients had undergone mechanical ventilation and that inflammation and ischemic parenchymal damage were the causes of cyst formation leading to air-leaks. In addition, there was no need to operatively intervene in any of these cases, as the chest tube was sufficed enough.
Furthermore, blood, sputum, and urinary cultures can help narrow the cause of the lung trauma, whether from COVID-19 pneumonia or a secondary BSIs. Blood cultures help narrow the antibiotic therapy choices, while sputum cultures are also interpreted based on the clinical correlation and quantitation of the growth. Urinary antigen testing has been more sensitive and specific than Gram stains and sputum cultures, making them the most commonly done test.12 Specific lab values, including CRP, blood cultures, sputum cultures, and urinary antigens, play a vital role in diagnosing CAP. Findings that show a CRP > 40 mg/L, as seen in our patient, have a sensitivity of 70% and specificity of 90% for bacterial pneumonia. Ultimately, it must be interpreted in the context of the clinical presentation.
A secondary delayed necrotizing MSSA pneumonia presenting with pneumothorax from pulmonary necrosis has not been reported following COVID-19 pneumonia. In addition to imaging, further investigation may be required, especially if necrotizing S. aureus pneumonia is suspected to be the cause of the pneumothorax. During our patient’s hospital stay, he had a resolution of his COVID-19 pneumonia. He had been receiving tocilizumab, an immune-modulatory therapy, which significantly increased ICU-BSI risk. He then developed severe respiratory failure once more. All of the cultures, lab work, and imagining obtained from our patient demonstrated that he had developed MSSA necrotizing pneumonia. In addition, his MSSA pneumonia was further complicated with the development of a pneumothorax, for which he was treated correctly.
Conclusion
In brief, as the COVID-19 pandemic continues to evolve, we are continuously learning about possible complications from this new virus. S. aureus infection secondary to influenza-like illness, such as COVID-19, can lead to severe pulmonary compromise, including necrotizing pneumonia and pneumothorax. Therefore, in patients with COVID-19 pneumonia, especially those hospitalized, it is imperative to monitor the lungs and their overall function.
Footnotes
Author contribution: All authors contributed to investigating, writing, reviewing, and editing.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: Our institution does not require ethical approval for reporting individual cases or case series.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: Written informed consent was obtained from the patient(s) for their anonymized information to be published in this article.
ORCID iDs: Lidiya Didenko  https://orcid.org/0000-0003-3066-8135
https://orcid.org/0000-0003-3066-8135
Andrew Malek  https://orcid.org/0000-0001-9583-198X
https://orcid.org/0000-0001-9583-198X
References
- 1. Martinelli AW, Ingle T, Newman J, et al. COVID-19 and pneumothorax: a multicentre retrospective case series. Eur Respir J 2020; 56(5): 2002697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Duployez C, Le Guern R, Tinez C, et al. Panton-valentine leukocidin-secreting Staphylococcus aureus pneumonia complicating COVID-19. Emerg Infect Dis 2020; 26(8): 1939–1941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Buetti N, Ruckly S, de Montmollin E, et al. COVID-19 increased the risk of ICU-acquired bloodstream infections: a case-cohort study from the multicentric OUTCOMEREA network. Intensive Care Medicine 2021; 47(2): 180–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Coronavirus COVID-19. 2019-nCoV, 2020, https://www.arcgis.com/apps/opsdashboard/index.html?fbclid=IwAR15YZf9ZFqXmITAxmZxQVz1orlYaIJpKEX60TIGOsBdgvAFAn3v-ERUkFg (accessed 25 November 2020).
- 5. Guan WJ, Ni ZY, Hu Y, et al. China medical treatment expert group for Covid-19. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382(18): 1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Holshue ML, DeBolt C, Lindquist S, et al. Washington State 2019-nCoV case investigation team. First case of 2019 novel coronavirus in the United States. N Engl J Med 2020; 382(10): 929–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rothberg MB, Haessler SD, Brown RB. Complications of viral influenza. Am J Med 2008; 121(4): 258–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Martin CM, Kunin CM, Gottlieb LS, et al. Asian influenza A in Boston, 1957-1958. II. Severe staphylococcal pneumonia complicating influenza. AMA Arch Intern Med 1959; 103(4): 532–542. [DOI] [PubMed] [Google Scholar]
- 9. Chickering HT, Park JH. Staphylococcus aureus pneumonia. N Engl J Med 1919; 72: 617–626 [Google Scholar]
- 10. Fine MJ, Smith MA, Carson CA, et al. Prognosis and outcomes of patients with community-acquired pneumonia: a meta-analysis. JAMA 1996; 275(2): 134–141. [PubMed] [Google Scholar]
- 11. Self WH, Wunderink RG, Williams DJ, et al. Staphylococcus aureus community-acquired pneumonia: prevalence, clinical characteristics, and outcomes. Clin Infect Dis 2016; 63(3): 300–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bartlett JG, Breiman RF, Mandell LA, et al. Community-acquired pneumonia in adults: guidelines for management. The infectious diseases society of America. Clin Infect Dis 1998; 26(4): 811–838. [DOI] [PubMed] [Google Scholar]
- 13. Li HT, Zhang TT, Huang J, et al. Factors associated with the outcome of life-threatening necrotizing pneumonia due to community-acquired Staphylococcus aureus in adult and adolescent patients. Respiration 2011; 81(6): 448–460. [DOI] [PubMed] [Google Scholar]
- 14. Löffler B, Niemann S, Ehrhardt C, et al. Pathogenesis of Staphylococcus aureus necrotizing pneumonia: the role of PVL and an influenza coinfection. Expert Rev Anti Infect Ther 2013; 11(10): 1041–1051. [DOI] [PubMed] [Google Scholar]
- 15. Macia I, Moya J, Ramos R, et al. Spontaneous pneumomediastinum: 41 cases. Eur J Cardiothorac Surg 2007; 31(6): 1110–1114 [DOI] [PubMed] [Google Scholar]
- 16. Zhou C, Gao C, Xie Y, et al. COVID-19 with spontaneous pneumomediastinum. Lancet Infect Dis 2020; 20(4): 510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Manna S, Maron SZ, Cedillo MA, et al. Spontaneous subcutaneous emphysema and pneumomediastinum in non-intubated patients with COVID-19. Clin Imaging 2020; 67: 207–213. [DOI] [PMC free article] [PubMed] [Google Scholar]