Abstract
Objectives:
To assess whether preoperative levels of physical activity predict the incidence of post-operative complications following anatomical lung resection.
Methods:
Levels of physical activity (daily steps) were measured for 15 consecutive days using pedometers in 90 consecutive patients (prior to admission). Outcomes measured were cardiac and respiratory complications, length of stay, and 30-day re-admission rate.
Results:
A total of 78 patients’ datasets were analysed (12 patients were excluded due to non-compliance). Based on steps performed they were divided into quartiles; 1 (low physical activity) to 4 (high physical activity). There were no significant differences in age, smoking history, COPD, BMI, percentage predicted FEV1 and KCO and cardiovascular risk factors between the groups. There were significantly fewer total complications in quartiles 3 and 4 (high physical activity) compared to quartiles 1 and 2 (low physical activity) (8 vs 22; P = .01). There was a trend (P > .05) towards shorter hospital length of stay in quartiles 3 and 4 (median values of 4 and 5 days, respectively) compared to quartiles 1 and 2 (6 days for both groups).
Conclusions:
Preoperative physical activity can help to predict postoperative outcome and can be used to stratify risk of postoperative complications and to monitor impact of preoperative interventions, ultimately improving short term outcomes.
Keywords: physical activity, daily step, pedometer, performance status; lung cancer
Introduction
Cancer incidence and mortality are increasing and lung cancer is the most common cause of cancer death worldwide.1 Around 85% of lung cancer occurring in the U.K. is non-small cell lung cancer (NSCLC) and of these 9% to 17% undergo surgical resection, according to the preoperative level of fitness for surgical intervention.2 Anatomical surgical resection with lymph node sampling remains the intervention of choice for stage I and II NSCLC, with a video-assisted approach preferred to open thoracotomy.3 Reflecting trends in the general population, patients undergoing anatomical lung resection for NSCLC tend to be older and suffer from significant cardio-pulmonary co-morbidities.4 Physical performance status is a widely accepted clinical parameter used by clinicians to assess fitness for surgery and to risk stratify patients preoperatively. It is one of many parameters, including pulmonary function test, echocardiogram, and cardiovascular comorbidities, which contribute to the preoperative assessment. In general, a poor physical performance status is associated with poor outcomes both with chemotherapy and surgery, as reflected also in the guidelines for radical management of patients with lung cancer.5 Sargent et al6 demonstrated how patients with poorer physical performance status undergoing chemotherapy for colorectal cancer show an increased risk of toxicities and mortality. Similar to these findings, Benzo et al.19 found that exercise capacity (expressed as maximal oxygen consumption) as an indirect measurement of performance status predicts postoperative cardiopulmonary complications after surgical resection in lung cancer patients. Several other studies7-9 indicated that the preoperative physiologic assessment in patients considered for lung resection is essential for identifying patients at increased risk of complications. In order to identify patients at higher risk of postoperative complications, Brunelli et al.23 suggested to systematically evaluate the physical performance before lung cancer resection through the stair-climbing test or the shuttle walk test. Alternatively, the Eastern Cooperative Oncology Group/World Health Organization (ECOG/WHO) Performance Status Scale and the Karnofsky Performance Status Scale have previously been proposed and currently used as simple methods to assess the functional status of a patient.10 However, these scales are subjective, also affected by a high inter-observer variability.10
A recent review focused on currently measures of physical activity showed that objective measures demonstrate less variability in properties of methodological effectiveness than self-reported measures.11 Kelly and Shahrokni10 have recently proposed the use of electronic activity monitoring systems (ie, wearable devices that objectively measure functional activity and can provide feedback to elicit self-monitoring of activity behavior) such as accelerometers, heart rate monitors, and pedometers to provide a better objective and accurate measurement of the functional performance in cancer patients. A recent study confirmed that daily step counts can predict clinical outcomes in patients treated with chemoradiation therapy for locally advanced non-small cell lung cancer.12 Furthermore, it has been documented that the preoperative level of physical activity predicted postoperative complications after colorectal cancer surgery13 and was also associated with a reduction in sick-leave after radical prostatectomy.14 However, only few randomized controlled trials evaluated whether preoperative physical activity can be used to predict postoperative complications after lung cancer resection.15 Although the results of these studies showed that preoperative exercise training may reduce postoperative length of hospital stay and the risk of developing a postoperative pulmonary complication, the level of evidence is still low and the number of patients enrolled is quite small.15
The aim of the present study is to determine in a large group of NSCLC patients the relationship between the preoperative physical activity level, objectivized by pedometer-recorded daily steps measurement, and the postoperative post-operative pulmonary and cardiac complications, following lung cancer resection.
Materials and Methods
Patients
Ninety consecutive patients undergoing anatomical lung resection for NSCLC at a single center from December 2015 to October 2017 were recruited. Patients who had surgery for benign disease were excluded. Patients with severe physical restrictions due to osteoarthritis or severe rheumatologic disease were excluded All underwent either Video Assisted Thorascopic Surgery (VATS) or thoracotomy.
All patients received a detailed explanation of the study and gave written informed consent prior to participation. The study conformed to the guidelines in the Declaration of Helsinki and was approved by the local ethics committee as a service evaluation project (Audit No. 5801).
Preoperative Physical Activity Assessment
All patients were instructed to wear a pedometer (3D TriSport, Realalt, London, UK) day and night for 15 days prior to the planned day of hospital admission. This pedometer was utilized considering the accuracy, the cost of the device and the light weight specified by the factory. This device was not used in any other previous studies. All patients were counseled, as routinely done in our unit preoperatively, regarding the importance of regular exercise and smoking cessation and encouraged to maximize their physical activity. Specifically they were asked to take a walk twice a day and to increase the distance walked each day. They were also issued a written copy of routine breathing exercises, however neither specific preoperative rehabilitation plans nor physiotherapy input was provided.
Data Collection Process
Physical activity data were collected for the 15 days prior to admission, over separate 24-hour interval periods. Average number of daily steps over 2 weeks was analysed.
Preoperative patients’ characteristics were recorded including gender, age, smoking history, history of COPD, cardiovascular disease, location of the tumor, and performance status according to the ECOG scale.10 Spirometry with gas transfer factors was also performed on all patients preoperatively and forced expiratory volume in 1 sec (FEV1), forced vital capacity (FVC) and transfer factor (TLCO) data were recorded.
Primary Outcomes
Patients were examined for post-operative complications and for 30 days re-admission rate. Complications were subdivided into respiratory and cardiac. Respiratory complications were identified by one or more of the following: chest X-ray or CT findings suggestive of lung consolidation or collapse; pneumonia characterized by chest X-ray or CT changes and a rise in inflammatory markers; respiratory failure with evidence of hypoxia on arterial blood gas analysis; requirement for invasive or non-invasive ventilation based on an acidemia on blood gas (pH<7.35), hypoxia (PaO2<8) despite maximal oxygen therapy, or hypercapnia respiratory failure (PaCO2>6kPa).
Cardiac complications including arrhythmias such as atrial fibrillation and flutter as well as acute ischemic events, represented by a rise in serial troponins were recorded.
Secondary Outcomes
Secondary outcomes included length of hospital stay and length of chest tube drainage. Prolonged air leak more than 5 days was also evaluated.
Statistical Analysis
Data were reported as absolute number and percentage or as mean and standard deviation (SD), unless otherwise specified. Patients were split into quartiles of physical activity based on the average number of steps taken per day. All potential prognostic indices were measured at the time of surgery and evaluated as categorical variables. Continuous data were reported with medians and ranges, while categorical data were reported with counts and percentages. Differences between patient groups were analyzed with Chi square test or two-tailed T-test when appropriate. Variables with a P < .1 were included in the multivariate analysis. Probability values <.05 were considered statistically significant. All analyses were conducted using the SPSS (SPSS Inc., USA) software package.
Results
Seventy-eight patients were eligible for the analysis (12 patients were excluded due to non-compliance, because they did not wear the pedometer every day for 14 days: Figure 1): 42 patients (45%) were male and 67 patients (86%) had a smoking history, with an average of 29 pack years. Pre-operative assessment defined a total of 23 respiratory co-morbidities (ie, presence of COPD) and 39 cardiac co-morbidities (ie, hypertension, history of cardiovascular disease or atrial fibrillation).
Figure 1.
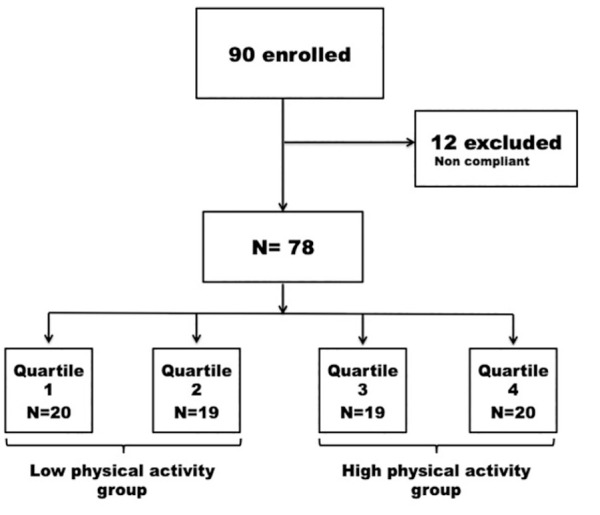
Patient enrollment flowchart and division into 4 groups according to the preoperative physical activity levels.
Physical Activity Levels
There was a mean of 3888 steps taken per day (median per day: 3354 steps, range 124-12 125 steps) during the 2 weeks before hospital admission. Twenty one patients (27%) achieved an average over 5000 steps/day with 4 patients achieving more than 10 000 steps/day. Patients were divided into 4 quartiles based on the number of daily steps taken based on the median and the 25% quartile (Figure 2). The first quartile (n = 20 patients) performed an average of 967 (SD±656) steps per day, the second (n = 19 patients) 2672 (SD±656) steps/day, the third (n = 19 patients) 4196 (SD±528) steps/day, and the fourth (n = 20 patients) 7673 (SD±2047) steps/day.
Figure 2.
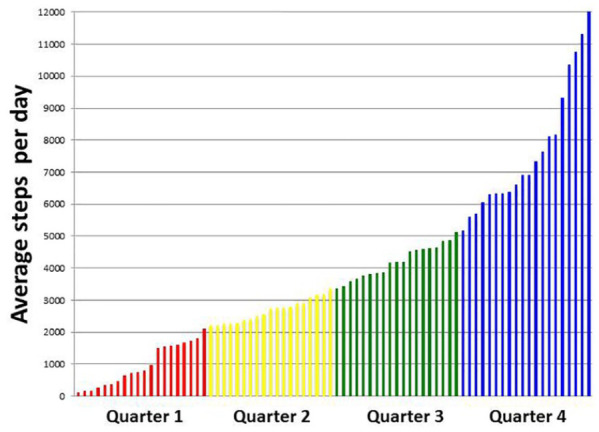
Bar chart displaying average daily steps by each subject. Quartiles of physical activity are delineated by different colors.
The 4 subgroups of patients showed comparable preoperative characteristics, including the ECOG performance status score, and comparable operative characteristics, including pathological stage (Table 1).
Table 1.
Preoperative and Operative Characteristics of Patients (Divided by Quartiles of Physical Activity) in the Study Population (n = 78).
| Variables | Group 1 |
Group 2 |
Group 3 |
Group 4 |
P value |
|---|---|---|---|---|---|
| N = 20 (%) | N = 19 (%) | N = 19 (%) | N = 20 (%) | ||
| Age (y, median) | 73 (46-79) | 71 (58-85) | 71 (60-83) | 66 (57-84) | .19 |
| Sex | .58 | ||||
| Male | 8 (40) | 11 (57.9) | 10 (52.6) | 12 (60) | |
| Female | 12 (60) | 8 (42.1) | 9 (47.4) | 8 (40) | |
| BMI (median range) | 25.4 (16.6-34.3) | 27.2 (20.4-34) | 26.2 (20.3-39.2) | 27.7 (22.7-33.7) | .7 |
| Comorbidities | |||||
| COPD | 7 (35) | 5 (26.3) | 6 (31.6) | 5 (25) | .89 |
| Cardiac comorbidities | .59 | ||||
| Hypertension | 7 (35) | 10 (52.6) | 9 (47.3) | 7 (35) | |
| AF | — | — | — | 1 (5) | |
| Ischemic heart disease | 2 (10) | 2 (10.5) | 1 (5.2) | — | |
| Diabetes | 2 (10) | 1 (5.2) | 2 (10.5) | 1 (5) | .86 |
| Chronic kidney disease | 1 (5) | — | — | — | .40 |
| Performance status | .12 | ||||
| 0 | 7 (35) | 9 (47.4) | 14 (73.7) | 14 (70) | |
| 1 | 11 (55) | 9 (47.4) | 5 (26.3) | 6 (30) | |
| 2 | 2 (10) | 1 (5.2) | — | — | |
| FEV1 (%) median | 83.9 | 85.8 | 91.7 | 77.2 | .8 |
| FVC (%) median | 100.4 | 106 | 99.3 | 100.7 | .5 |
| DLCO (%) median | 69 | 69 | 66 | 71.9 | .2 |
| Tumor side | .5 | ||||
| Right | 14 (70) | 9 (47.4) | 10 (52.6) | 12 (60) | |
| Left | 6 (30) | 10 (52.6) | 9 (47.4) | 8 (40) | |
| Surgical access | .6 | ||||
| VATS | 16 (84.2) | 17 (89.5) | 14 (73.7) | 17 (85) | |
| Thoracotomy | 3 (15.8) | 2 (10.5) | 5 (26.3) | 3 (15) | |
| Resection type | .26 | ||||
| Sublobar* | 5 (25) | 4 (22.2) | 2 (10.5) | 1 (5) | |
| Lobar resection | 15 (75) | 15 (77.8) | 17 (89.5) | 19 (95) | |
| TNM stage | .4 | ||||
| Stage Ia | 10 | 6 | 5 | 10 | |
| Stage Ib | 3 | 7 | 1 | 1 | |
| Stage IIa | 2 | 1 | 2 | 1 | |
| Stage IIb | 2 | 3 | 5 | 4 | |
| Stage IIIa | 3 | 2 | 4 | 1 | |
Abbreviations: AF, atrial fibrillation/flutter; BMI, body mass index; COPD, chronic obstructive pulmonary disease; DLCO, diffusing capacity of the lungs for carbon monoxide; FEV1, forced expiratory volume in the 1st second; FVC, forced vital capacity; ns, not significant; TNM, tumor nodal metastasis; VATS, video assisted thoracic surgery.
Sublobar includes wedge and segmentectomy for early stage lung cancer <2 cm.
Primary Outcomes
There were a total of 34 (43.6%) post-operative complications, of which 23 respiratory (ie, prolonged air leak or chest infection), 17 out of 23 in the low physical activity groups (Table 2).
Table 2.
Postoperative Complications Divided by Quartiles of Physical Activity.
| Quartile of physical activity | Group 1 |
Group 2 |
Group 3 |
Group 4 |
P value |
|---|---|---|---|---|---|
| N = 20 | N = 19 | N = 19 | N = 20 | ||
| Respiratory complications | 10 | 7 | 3 | 3 | .04 |
| Pneumonia/atelectasis | 8 | 4 | 1 | 2 | .03 |
| Prolonged air leak | 2 | 3 | 2 | 1 | .4 |
| Cardiac complications | 1 | 3 | 2 | 1 | .6 |
| Arrhythmia | 1 | 3 | 2 | 1 | .6 |
| Ischemic event | — | — | — | — | — |
| Post-operative bleeding | 1 | 1 | — | 1 | .5 |
| Ileus | 1 | — | — | — | |
| Patients with complication* | 12 (60) | 11 (57.9) | 4 (21) | 4 (20) | .007 |
| 30-day re-admission rate | 1 | 1 | 1 | 1 |
Some patients may more than one complications: 1 patient in group 1, 3, and 4 has a pneumonia and arrhythmia during the in hospital stay respectively.
Bold values indicate statistically significant differences.
There were significantly fewer post-operative cardio-respiraroty complications when comparing quartiles 3 and 4 (high physical activity groups) to quartiles 1 and 2 (low physical activity groups) (9 vs 21, P = .0008). Respiratory and cardiovascular complications were then analyzed separately. When comparing quartiles 3 and 4 (high physical activity groups) to quartiles 1 and 2 (low physical activity groups) 15.4% (n = 6) of patients versus 42.1% (n = 17) of patients had respiratory complications (P = .009). Specifically the pneumonia/lung atelectasis rate was 7.7% (n = 3) versus 32.4% (n = 12) respectively in the 2 subgroups (P = .008). The rate of cardiovascular complications in quartiles 3 and 4 (high physical activity groups) to quartiles 1 and 2 (low physical activity groups) was 10.5% (n = 4) versus 7.9% (n = 3) (P = .6).
At univariate analysis, the following other factors were then analyzed as potential risk factors for complications: age (P =.06), sex (51% in males vs 21.6% in female, P = .21), performance status (P =.01), respiratory comorbidities (P = .15), cardiac comorbidities (P = .18), diabetes (P = .7), chronic kidney disease (P = .2), BMI (P = .4), FEV1% (P = .36), FVC% (P = .6), TLCO% (P = .1), side of disease (P = .7), surgical access (P = .2), type of resection (P = .6). Only performance status correlated specifically with respiratory complications (P = .008), but not age (P = .16).
At multivariate analysis, performance status (P = .004, OR 1.267, 95% CI 1.096-1.437) and physical activity (P = .001, OR 0.700, 95% CI 0.501-0.899) were independent prognostic factors for postoperative complications.
Each quartile had 1 re-admission for varied reasons including: confusion, wound infection, episodes of absence and pain management. There was no difference in re-admission rate between the groups (P = .99).
Secondary Outcomes
The median length of chest drain and hospital stay was 2 days (range 1-20) and 5 days (range 1-30), respectively in the study population. High physical activity groups had a shorter length of stay (median values of 4 and 5 days for groups 3 and 4, respectively) compared to low physical activity groups (median values of 6 days for groups 1 and 2), although the difference between groups was not significant (P = .36 for all comparisons). There was also no significant difference in the length of chest tube drainage post-operatively between groups 1 and 4 (2.78 days vs 3.89 days, P = .22).
The number of patients who developed a prolonged air leak was comparable (P = .5) between the high physical activity groups (n = 3 patients in groups 3 and 4) and the low physical activity groups (n = 4 patients in groups 1 and 2).
Discussion
Level of physical activity and performance status correlate with survival in cancer patients. As recently shown by Cavalheri and Granger,15 exercise training reduces complications rate, but no objective data on physical activity are provided to stratify the patients who may benefit more from those interventions before surgery.
To the best of our knowledge this is the first study investigating the association between preoperative levels of physical activity, without direct preoperative intervention, and post-operative complications after anatomical lung resection. It is widely accepted that poor preoperative performance status is an independent predictor of poor outcome in cancer patients,6 and this was also confirmed in our series. However, it has been shown that the assessment of performance status may differ between nurses and physicians due to intra- and inter-observer variability.16 The majority of patients who undergo lung cancer surgery has a performance status of 0 to 1, but physical activity can help to further stratify those patients to possibly predict which patients have an increased risk of postoperative morbidity and mortality. We showed in the present study that preoperative physical activity is an independent prognostic factor for post-operative complications in patients with similar performance status. This tool may be used in the future to decide which patients may benefit from a preoperative dedicated rehabilitation program, also known as “prehabilitation.” Significant emphasis has been placed on optimizing preoperative fitness, however not without significant hurdles. A systematic review by Garcia et al17 suggested that prehabilitation may lead to a reduction in hospital stay and in post-operative complications. It was suggested that a rehabilitative intervention should begin 8-12 weeks prior to surgery, with length of treatment proportional to size of improvement. Prehabilitation programs require significant resources and time and it may be difficult to accommodate them into a cancer pathway when surgery cannot be delayed of 2 to 3 months. In U.K., patients are to be assessed and treated within 62 days of urgent referral.18 Patients’ perception of delaying treatment due to preoperative prehabilitation is also another important limitation and the adoption of a formal program may, understandably, cause distress and a lack of compliance if patients feel that their definitive treatment is being delayed. A study by Benzo et al19 stopped recruitment after a year, as patients did not want to delay surgical intervention to allow for 4 weeks of rehabilitation. In our study, physical activity within 2 weeks before surgery was evaluated as a tool to assess the impact in real life of physical activity on postoperative outcome in patients waiting for surgery. Two week physical activity level had an impact on post-operative complications and length of hospital stay. Patients in groups with low physical activity had a significantly higher number of respiratory complications, mainly chest infection and atelectasis. In the groups with low physical activity there were 17 respiratory complications vs only 6 in the groups with high physical activity. In thoracic surgery, the respiratory complications are the main cause of prolonged hospital stay and readmission in hospital. The length of stay was not significantly different between the groups, but it was shorter in patients with high physical activity. Our study suggests that 2 weeks seem to represent a convenient time to evaluate physical activity and it can be considered enough to begin a possible intervention to increase physical activity level avoiding delays in treatment and improving postoperative outcome, however, further comparison is required. Maddocks’20 study examined activity levels and steps taken over a week in a more heterogeneous population of lung and pleural cancers, with more advanced disease (100% Stage IIIB or IV). All patients were assessed as ECOG performance status score 0-2, however we had a larger proportion of patients with performance status score 0 (50% vs 19%). They recorded an average of 4200 steps per day and showed a significant difference in steps taken between performance status groups. Our data showed no significant difference between performance status groups, probably because our patients had limited disease.
The use of pedometers or other electronic activity monitors to monitor physical activity offers a more accurate and objective measurement of daily activity to objectively evaluate the preoperative performance status. Numerous studies have validated electronic activity monitors as accurate tools10,11 and research is on-going regarding the use of activity data in determining patient’s performance status and fitness for surgery.17,21 A criticism of pedometers is that there is inherent variation between subject’s gaits in factors such as stride length and speed, however Kelly’s review paper detailed a number of studies where consumer pedometers and electronic activity monitors were validated as accurate when compared to gait video analysis and direct observation of step count.10
Coats et al.22 showed that a home-based exercise program improved muscle strength and exercise tolerance in 16 pre-operative patients. However, they loaned and delivered exercise bikes to their patients, generating significant costs and making this approach challenging to replicate. In order to minimize the costs and increase the effectiveness of any intervention preoperatively, physical activity as measurement of prehabilitation while waiting for surgery should be carefully evaluated. Also, during a global pandemic, like the current COVID19 pandemic, where social distancing and restricting non-essential travel are necessary to minimize the viral spread, home-based activity monitoring may have additional benefit in pre-operative risk stratification.
There is no an ideal way to assess the functional status and in the literature, preoperative tests have been used to estimate the performance status and the postoperative risk of complications. Correlation between exercise capacity measured with 6 minute walking test or cardiopulmonary exercise test and complications is well established.23 Those more sophisticated and objective tests may further delay surgery and may increase the cost and pressure on hospital facilities. A simple measurement of the physical activity as we used in our study, can be used in daily clinical practice as a valid surrogate of clinical tests to stratify patient risk, containing costs and delays.
Previous studies showed that average daily steps can be combined with the diffusing capacity of the lungs for carbon monoxide percentage (DLCO) to accurately predict the V02Max in patients undergoing lung resection.24 In addition Varela’s group used age, activity levels and DLCO to create an estimated V02Max score, which was more predictive for the incidence of complications than an actual V02Max score.25 The majority of our patients did not meet the clinical criteria in order to undergo cardiopulmonary exercise testing, hence we did not perform this analysis. However, there was no significant variation in patients’ characteristics and spirometry values between quartiles, suggesting that cardiopulmonary exercise testing may not have added weight for comparison in our study population. Moreover this pilot study aimed to create a simple reproducible measurement with minimal impact on cost and tests before surgery.
There are inherent limitations within this study, which must be taken into account. Firstly, despite standard instruction, it is unclear whether the pedometers had an indirect impact on patient physical activity. This activity monitor has not been used in other studies and has to be further evaluate. Also 12 patients could not be evaluated due to not utilizing the pedometer regularly. Secondly, despite having the largest cohort of patients for comparison the effect stated requires further investigation with a larger patient population.
Conclusion
In conclusion, we observed an association between greater preoperative physical activity and fewer post-operative respiratory and cardiac complications. The use of pedometers offers an inexpensive and readily available tool to extrapolate and objectivize patients’ preoperative levels of activity, which can be utilized as a surrogate or added to complement the preoperative assessment. Furthermore, the use of pedometers could help setting a target of exercise tailored to each individual patient, potentially also serving as encouragement to optimize their performance in order to reduce morbidity and improve outcomes.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by The Thoracic Charity of Guy’s Hospital (A.B.) and by the Italian Ministry of Education, University and Research (MIUR) under the program “Dipartimenti di Eccellenza ex L. 232/2016” to the Department of Surgical Sciences, University of Torino (Italy) (M.A.M.).
ORCID iD: Andrea Billé  https://orcid.org/0000-0003-4709-4174
https://orcid.org/0000-0003-4709-4174
References
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [DOI] [PubMed] [Google Scholar]
- 2. Møller H, Coupland VH, Tataru D, et al. Geographical variations in the use of cancer treatments are associated with survival of lung cancer patients. Thorax. 2018;73:530-537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Howington JA, Blum MG, Chang AC, Balekian AA, Murthy SC. Treatment of stage I and II non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(suppl 5):e278S-e313S. [DOI] [PubMed] [Google Scholar]
- 4. Palma D, Visser O, Lagerwaard FJ, Belderbos J, Slotman B, Senan S. Treatment of stage I NSCLC in elderly patients: a population-based matched-pair comparison of stereotactic radiotherapy versus surgery. Radiother Oncol. 2011;101:240-244. [DOI] [PubMed] [Google Scholar]
- 5. Lim E, Baldwin D, Beckles M, et al. Guidelines on the radical management of patients with lung cancer. Thorax. 2010;65(suppl 3):iii1-27. [DOI] [PubMed] [Google Scholar]
- 6. Sargent DJ, Köhne CH, Sanoff HK, et al. Pooled safety and efficacy analysis examining the effect of performance status on outcomes in nine first-line treatment trials using individual data from patients with metastatic colorectal cancer. J Clin Oncol. 2009;27:1948-1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jones LW, Watson D, Herndon JE, 2nd, et al. Peak oxygen consumption and long-term all cause mortality in nonsmall cell lung cancer. Cancer. 2010;116:4825-4832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jones LW, Hornsby WE, Goetzinger A, et al. Prognostic significance of functional capacity and exercise behavior in patients with metastatic non-small cell lung cancer. Lung Cancer. 2012;76:248-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Arem H, Moore SC, Park Y, et al. Physical activity and cancer-specific mortality in the NIH-AARP Diet and Health Study cohort. Int J Cancer. 2014;135:423-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kelly CM, Shahrokni A. Moving beyond Karnofsky and ECOG performance status assessments with new technologies. J Oncol. 2016;2016:6186543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dowd KP, Szeklicki R, Minetto MA, et al. A systematic literature review of reviews on techniques for physical activity measurement in adults: a DEDIPAC study. Int J Behav Nutr Phys Act. 2018;15:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ohri N, Halmos B, Bodner WR, et al. Daily step counts: a new prognostic factor in locally advanced non-small cell lung cancer? Int J Radiat Oncol Biol Phys. 2019;105:745-751. [DOI] [PubMed] [Google Scholar]
- 13. Onerup A, Angenete E, Bonfre P, et al. Self-assessed preoperative level of habitual physical activity predicted postoperative complications after colorectal cancer surgery: a prospective observational cohort study. Eur J Surg Oncol. 2019;45:2045-2051. [DOI] [PubMed] [Google Scholar]
- 14. Angenete E, Angerås U, Börjesson M, et al. Physical activity before radical prostatectomy reduces sick leave after surgery - results from a prospective, non-randomized controlled clinical trial (LAPPRO). BMC Urol. 2016;16:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cavalher V, Granger C. Preoperative exercise training for patients with non-small cell lung cancer (review). Cochrane Database Syst Rev. 2017;6:CD012020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ando M, Ando Y, Hasegawa Y, et al. Prognostic value of performance status assessed by patients themselves, nurses, and oncologists in advanced non-small cell lung cancer. Br J Cancer. 2001;85:1634-1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Garcia RS, Brage MIY, Moolhuyzen EG, Granger CL, Denehy L. Functional and postoperative outcomes after preoperative exercise training in patients with lung cancer: a systematic review and meta-analysis. Interact Cardiovasc Thorac Surg. 2016;23:486-497. [DOI] [PubMed] [Google Scholar]
- 18. National Collaborating Centre for Cancer (UK). The Diagnosis and Treatment of Lung Cancer (Update). Cardiff: National Collaborating Centre for Cancer (UK); 2011. [PubMed] [Google Scholar]
- 19. Benzo R, Wigle D, Novotny P, et al. Preoperative pulmonary rehabilitation before lung cancer resection: results from two randomized studies. Lung Cancer. 2011;74:441-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Maddocks M, Wilcock A. Exploring physical activity level in patients with thoracic cancer: implications for use as an outcome measure. Support Care Cancer. 2012;20:1113-1116. [DOI] [PubMed] [Google Scholar]
- 21. Reilly CS. Can we accurately assess an individual’s perioperative risk? Br J Anaesth. 2008;101:747-749. [DOI] [PubMed] [Google Scholar]
- 22. Coats V, Maltais F, Simard S, et al. Feasibility and effectiveness of a home-based exercise training program before lung resection surgery. Can Respir J. 2013;20:e10-e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brunelli A, Kim AW, Berger KI. Physiological evaluation of the patient with lung cancer being considered for resectional surgery: diagnosis and management of lung cancer, 3rd edition: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013; 143(suppl 1):e166S-e190S. [DOI] [PubMed] [Google Scholar]
- 24. Novoa NM, Varela G, Jiménez MF, Ramos J. Value of the average basal daily walked distance measured using a pedometer to predict maximum oxygen consumption per minute in patients undergoing lung resection. Eur J Cardiothorac Surg. 2011;39:756-762. [DOI] [PubMed] [Google Scholar]
- 25. Vargas Fajardo Mdel C, Novoa Valentín NM, Jiménez López MF, Ramos Gonzalez J, Varela Simó G. An alternative method for predicting the risk of postoperative complications in lung resection. Arch Bronconeumol. 2014;50:87-92. [DOI] [PubMed] [Google Scholar]


