Abstract
Within the hindbrain, serotonin (5-HT) functions as a modulator of the central glucagon-like peptide-1 (GLP-1) system. This interaction between 5-HT and GLP-1 is achieved via 5-HT2C and 5-HT3 receptors and is relevant for GLP-1-mediated feeding behavior. The central GLP-1 system is activated by various stressors, activates the hypothalamic pituitary adrenocortical (HPA) axis, and contributes to stress-related behaviors. Whether 5-HT modulates GLP-1’s role in the stress response in unknown. We hypothesized that the serotonergic modulation of GLP-1-producing neurons (i.e., PPG neurons) is stimuli-specific and that stressed-induced PPG activity is one of the modalities in which 5-HT plays a role. In this study, we investigated the roles of 5-HT2C and 5-HT3 receptors in mediating the activation of PPG neurons in the nucleus tractus solitarius (NTS) following exposure to three different acute stressors: lithium chloride (LiCl), noncontingent cocaine (Coc), and novel restraint stress (RES). Results showed that increased c-Fos expression in PPG neurons following LiCl and RES—but not Coc—is dependent on hindbrain 5-HT2C and 5-HT3 receptor signaling. Additionally, stressors that depend on 5-HT signaling to activate PPG neurons (i.e., LiCl and RES) increased c-Fos expression in 5-HT-expressing neurons within the caudal raphe (CR), specifically in the raphe magnus (RMg). Finally, we showed that RMg neurons innervate NTS PPG neurons and that some of these PPG neurons lie in close proximity to 5-HT axons, suggesting RMg 5-HT-expressing neurons are the source of 5-HT input responsible for engaging NTS PPG neurons. Together, these findings identify a direct RMg to NTS pathway responsible for the modulatory effect of 5-HT on the central GLP-1 system—specifically via activation of 5-HT2C and 5-HT3 receptors—in the facilitation of acute stress responses.
Keywords: Glucagon-like peptide-1, Serotonin, 5-HT2C receptor, 5-HT3 receptor, stress, Raphe Magnus
1. Introduction
Metabolic neuropeptide systems are influenced by a myriad of biological mechanisms, including signals not directly linked to energy balance, such as environmental stressors, anxiogenic factors, and exogenous substances of abuse (e.g., cocaine, alcohol) [for review see (Gülpinar and Yegen, 2004; Hernandez and Schmidt, 2019; Holt and Trapp, 2016; Kormos and Gaszner, 2013; Roubos et al, 2012)]. Disentangling the mechanism(s) by which different stimuli engage converging CNS systems is necessary to design more efficacious treatments for various clinical conditions, such as metabolic disorders (e.g., obesity and eating disorders), substance abuse, and mood disorders (e.g., anxiety and depression). An example of a metabolic system that is responsive to multiple cues is the GLP-1 system. In addition to glucoregulation and satiation (Barrera et al, 2011; Cabou et al, 2008; Müller et al, 2019), the GLP-1 system plays a role in the behavioral and physiological response to stress (Ghosal et al, 2013; Herman, 2018; Holt et al, 2016), nausea/emesis (De Jonghe et al, 2016; Kanoski et al, 2012; Kinzig et al, 2003), motivated behavior (Alhadeff et al, 2012; Dickson et al, 2012; Hayes and Schmidt, 2016; Hernandez et al, 2019), inflammation (Lee and Jun, 2016; Que et al, 2019), and neurogenesis (Athauda and Foltynie, 2016; Bae and Song, 2017; Grieco et al, 2019). Understanding the heterogeneity of the neural response that engages the GLP-1 system following exposure to different stimuli is therefore required to discern how the central GLP-1 system accomplishes its broad range of physiological functions.
The relationship between GLP-1 and stress is of particular interest because of the pervasive role stress mechanisms play in perturbing homeostasis and the close link that exists between stress and pathology. There is a wealth of literature highlighting the relationship between GLP-1 and stress. Indeed, GLP-1-producing (preproglucagon; PPG) neurons—expressed almost exclusively in the NTS (Larsen et al, 1997; Trapp and Cork, 2015)—are responsive to a variety of stressors (De Jonghe et al, 2016; Grill et al, 2004; Maniscalco et al, 2015; Rinaman, 1999b; Terrill et al, 2019). The GLP-1 system engages peripheral and central stress centers, the sympathetic nervous system (Baggio et al, 2017; Yamamoto et al, 2002) and the hypothalamic-pituitary-adrenal axis (HPA) (Gil-Lozano et al, 2010; Tauchi et al, 2008), respectively. Additionally, exogenous administration of GLP-1 and its agonists increase plasma levels of stress hormones such as ACTH (Kinzig et al, 2003) and corticosterone (Gil-Lozano et al, 2010; Schmidt et al, 2016). Further, GLP-1 receptor activation can induce behavioral responses similar to those invoked by stress including anorexia (Grill et al, 2004; Rinaman, 1999a; Terrill et al, 2018), anxiety- and depressive-like behaviors (Anderberg et al, 2016; Zheng et al, 2019). Previous efforts to understand the link between stress and the GLP-1 system have focused on a unidimensional comparison, which assesses the effect of a single stressor (e.g., LiCl, LPS, restraint, or cocaine) on a single metabolic system (i.e., GLP-1). This one-to-one comparison has laid the groundwork for most of what is known about the GLP-1 system in response to stress, however, it does not address the overlapping role that other stress/metabolic systems may play.
Serotonin (5-hydroxytryptamine; 5-HT) is implicated in the regulation of stress and energy balance mechanisms (Bagdy et al, 1989; Heisler et al, 2007; Mikkelsen et al, 2004; Pollock and Rowland, 1981; Tafet and Nemeroff, 2016) and its actions in the hindbrain have recently been shown to play a role in the modulation of the central GLP-1 system (Holt et al, 2017; Leon et al, 2019). Suppression of food intake and body weight, as well as pica, are the only GLP-1-driven effects that have been shown to depend on hindbrain 5-HT signaling of PPG neurons (Leon et al, 2019). Whether this serotonergic engagement of the central GLP-1 system is unique to feeding and nausea/malaise or represents a more ubiquitous relationship that influences other GLP-1-mediated functions remains to be determined, as does the source(s) of 5-HT that engages the central GLP-1 system. In this study, we set out to investigate whether 5-HT engages NTS PPG-neurons in response to different acute stressors and to identify the serotonergic sub-nucleus responsible for engaging the central GLP-1 system in the context of stress.
Of the plethora of available acute stressors, lithium chloride (LiCl), cocaine (Coc), and novel restraint (RES) were chosen for this study because all three stimuli engage the HPA axis (Bhatnagar and Dallman, 1998; Schmidt et al, 2016; Sugawara et al, 1988), activate PPG neurons (Rinaman, 1999b; Schmidt et al, 2016; Terrill et al, 2019), and are different in nature (interoceptive stress, drug of abuse, and psychogenic stress, respectively). These stimuli represent stressors that have been well characterized within the GLP-1 literature see (Ghosal et al, 2013; Hayes et al, 2016; Herman, 2018; Hernandez et al, 2019; Holt et al, 2016; Maniscalco and Rinaman, 2017). Of the 5-HTRs expressed in the NTS, mRNA transcripts for the 5-HT2C and 5-HT3 receptors are present in PPG neurons (Leon et al, 2019) making them ideal candidates to explore the 5-HT modulation of NTS PPG neurons.
Within the brain, 5-HT is produced primarily in the raphe nucleus (Berger et al, 2009; Hornung, 2012), which is comprised of multiple sub-nuclei that are categorized into two groups based on the orientation of their projections. The rostral raphe projects mostly to the forebrain and contains approximately 85% of all central 5-HT neurons, while the caudal raphe (CR) projects mainly to the brainstem and spinal cord and accounts for the remaining ~15% of central 5-HT neurons (Hornung, 2003). The CR is composed of the raphe pallidus (RPa), raphe obscurus (ROb), and raphe magnus (RMg). These nuclei are involved in a wide range of functions including cardiorespiratory, gastrointestinal, thermoregulatory, and motor control (Lovick, 1997; Ulhoa et al, 2013). Given the orientation of efferent projections from the caudal vs. rostral raphe and that the NTS lies caudally from the raphe, we hypothesized that the driver of 5-HT input into NTS PPG neurons is the CR.
The three main goals of this study are: (1) to assess the modulatory role of hindbrain 5-HT2C and 5-HT3 receptors in mediating NTS PPG activity following various stimuli known to engage stress centers, (2) to investigate whether 5-HT-expressing neurons within the CR are activated in response to stimuli that engage the central GLP-1 system, and (3) to determine whether neurons within the CR innervate NTS PPG neurons.
2. Materials and Methods
2.1. Animals
Male Sprague Dawley rats (275 g BW upon arrival; Charles River) were individually housed in hanging wire cages maintained at 23°C (12 h light/dark cycle) and given ad libitum chow (Purina LabDiet 5001) and autoclaved tap water. All procedures were approved by Institutional Animal Care and Use Committee at the University of Pennsylvania and were performed according to the guidelines determined by the National Institutes of Health.
2.2. Drugs
Central injections: Serotonin chloride (5-HT; Tocris, 3547) was dissolved in artificial cerebrospinal fluid (aCSF; Harvard Apparatus, 59–7316). The 5-HT3R antagonist, ondansetron (OND; Tocris) was dissolved in 100% DMSO to make a 12.5 μg/μl dose solution. This was delivered into the hindbrain via 4th ventricle (4V) administration at 2 μl volume (total dose 25 μg). The 5-HT2C antagonist, RS102221 (RS1; Tocris) was dissolved in 100% DMSO to make a 20 μg/μl dose solution and 2 μl (total dose 40 μg) was administered (4V).
Systemic injections: Lithium chloride (LiCl) was prepared as a 0.6M solution in 0.9% sterile saline (sal) and administered intraperitoneally (i.p.) in a volume of 5 ml/kg (total dose 127.2 mg/kg). Cocaine (Coc) was obtained from the National Institute on Drug Abuse (Rockville, MD) and prepared into a 15mg/kg solution in 0.9% sal, administered i.p. in a 1 ml/kg volume (total dose 15 mg/kg).
2.3. Acute Restraint Paradigm
Rats were handled daily for at least 7 d prior to test day. On the day of testing, rats were removed from their home cages and immediately placed into an adjustable restraint apparatus (Plas-Labs, 544-RR) for 30 min in individual plastic holding cages (not their home cage).
2.4. Blood Collection and CORT Assay
Blood (~300 ul) from the tail vein was collected (Hare et al, 2014) into Hep-coated tubes (Microvitte 22043975) and centrifuged for 10 min at 1000 x g. The plasma was then stored at −80C until processing. For corticosterone (CORT) assessment, samples were analyzed using a corticosterone enzyme-linked immunoassay (ELISA) (Enzo Scientific, 50658140; detection limit 27 pg/ml) per the manufacturer’s instructions. Sample CORT concentrations were determined by nonlinear regression from the standard curves. The mean coefficient of intra-assay variation for all plates was below 4%, this was determined by calculating the % coefficient of variance (CV) for each triplicate sample present on each plate and then averaging them. The mean coefficient of inter-assay variation was 3.91% and was determined by comparing the %CV of Standards 1–5 for each plate that was run.
2.5. Stereotaxic surgery
Fourth Ventricle (4V) Cannulation:
As previously described (Leon et al, 2019).
Viral Injections:
Animals received two viral injections during the same surgery. The first was a unilateral co-injection (60 nl) of AAV1-Cre (AAV1.hSyn.Cre.WPRE.hGH; Penn Vector Core; 3.28 × 1013 GC/mL) and cholera toxin B (CTB)-488 delivered into the RMg (coordinates: A/P −10.9 mm from Bregma, M/L 0, D/V 10.4 mm). This specific AAV1 is an anterograde viral tracer with a high rate of transduction in the CNS that exhibits trans-synaptic spread properties, jumping a single synapse (Suarez et al, 2018; Zingg et al, 2017). AAV1 was paired with Cre recombinase to increase the efficiency of trans-neuronal spread (Zingg et al, 2017). CTB was used to validate injection site via the presence of CTB immunoreactivity the RMg. The second injection was an AAV1-Flex-tdTomato (1:2 in 0.1M sodium phosphate-buffered saline, pH 7.4, AAV1.CAG.Flex.tdTomato.WPRE.bGH; diluted, 2.80 X 1013 GC/mL, Penn Vector Core) (Suarez et al, 2018) targeting the NTS (bilateral; 100nl/hemisphere) (coordinates: AP −1 mm form occipital, ML 0, DV 8.2 mm from skull). This second viral injection allows for the Cre-dependent expression of the orange fluorescent protein tdTomato. The animals received analgesics (meloxicam, 2 mg/kg s.c.) for 3 consecutive days following surgery.
2.6. Immunohistochemistry (IHC)
Hindbrain coronal sections (30 μm) were obtained using a cryostat (Leica, CM3050S), sections at the level of the NTS (~14.15–14.64 mm posterior to Bregma) and CR (9.2–14.6 mm posterior to Bregma) were collected, placed in cryoprotectant, and stored at −20°C until use. Briefly, the sections were dehydrated in 50% ethanol (30min at RT), rinsed in PBS (3X 5 min), incubated in 0.1% sodium borohydride (NaBH4) (20 min at RT), rinsed in PBS (3X 5 min), and blocked in PBS containing 5% normal donkey serum (NDS) and 0.2% Triton-X (60 min at RT). Sections were incubated in primary antibodies overnight at RT and, following three rinses in PBS, were incubated in secondary antibodies for 2h at RT. The primary antibodies used were rabbit anti-GLP-1 (1:2000; T-4363, Peninsula Laboratories), mouse anti-c-Fos (1:500; sc-271243; Santa Cruz), donkey anti-5-HT (1:500; ab66047; ABCAM), and mouse anti-beta subunit Cholera Toxin (1:500; ab62429; ABCAM). Secondary antibodies were donkey anti-rabbit Alexa Fluor 594 (1:500), donkey anti-mouse Alexa Fluor 488 (1:500), and donkey anti-donkey Alexa Fluor 405 (1:500) (all from Jackson Immuno Research). Sections were mounted onto glass slides (Fisher), coversliped with mounting media, and the edges sealed with nail polish. Slides were stored at 4oC until imaged.
2.7. Fluorescent In-Situ Hybridization (FISH) and Immunohistochemistry (IHC)
2.7.1. Tissue preparation:
Perfused hindbrain sections containing the NTS were used to perform FISH using a commercially available kit (Cat. No. 323100, RNAscope Multiplex Fluorescent v2 Assay, Advanced Cell Diagnostics).
2.7.2. FISH mRNA expression of PPG in the NTS.
NTS sections stored in cryoprotectant at - 20°C were rinsed in PBS and slide mounted. Following a 5min PBS rinse the slides were incubated for 30 min at 60oC in the HybEZ™ oven (ACD) and then post-fixed in 10% NBF for 15 min at 4oC. The sections were dehydrated in ascending ethanol solutions (5 min washes in 50, 70, 100, 100% ethanol). After the second 100% ethanol wash the slides were air-dried and a hydrophobic barrier was created on the slide surrounding the sections. The sections were treated with 0.3% H2O2 for 10min at RT, followed by diH20 washes (3X 1min). After the last rinse, the slides were transferred into a diH20 wash inside a steamer (temp >99°C) for 10s to acclimate before being incubated in the Target Retrieval Reagent (TRR; PN 322000) for 5min (also inside the steamer). The sections were removed from the steamer, rinsed in diH20, incubated in 100% ethanol for 3 min, and then air dried for 5 min at RT. The sections were incubated with Protease III (PN 322337) for 30 min at 40oC followed by diH20 washes (2X). Sections were then immediately incubated for 2h at 40oC using a probe designed by ACD to label PPG mRNA (Rn-GcgsC3; 315471-C3). Following rinses in 1X wash buffer (PN 310091) (2X 2 min) the sections went through a series of amplification steps at 40oC: 30min incubation in FL v2 AMP1, 30min incubation in FL v2 AMP2, 15min incubation in FL v2 AMP3 (with two rinses in wash buffer between each step). Following the amplification steps the sections were incubated in the Multiplex FL v2 HRP-C3 at 40oC for 15 min and then rinsed wash buffer (2X). The sections were then incubated in opal dye 690 (diluted 1:1000) at 40oC for 30 min, rinsed in wash buffer (2X), and then incubated with FLv2 HRP blocker for 15min at 40oC. The sections were rinsed one last time before starting the immunohistochemical processing.
2.7.3. IHC to visualize 5-HT fibers in NTS sections.
The IHC protocol was similar to the description above, the only difference was the concentration of the blocking solution (PBS containing 3% normal donkey serum and 0.3% Triton-X) and the incubation with primary antibodies was done at 4oC.
2.7.4. Image acquisition, processing, and quantification.
Sections were visualized with a Leica SP5 X confocal microscope using the 20 and 40 oil- immersion objectives and the 405, 488, 594, and 647 laser lines. Image z-stacks with the 40 oil-immersion were collected with a step size of 1 μm, while 2–3 optical zoom z-stack images using the same objective were collected with a step size of 0.5μm. All images were collected sequentially to avoid contamination of signals from other fluorophores. Co-localization of PPG neurons and TD-Tom positive neurons was quantified manually on Imaris following background subtraction. Three-dimensional rotational animations were rendered from the collected z-stack images using Imaris 9.5.0 (Bitplane).
2.8. Experimental procedures
2.8.1. Experiment 1: Effects of different stimuli on plasma levels of CORT.
Three different cohorts of animals were used. The first compared Sal, LiCl, and Coc (n=15); the second examined RES vs. no RES (n=12); the third compared 4V administration of 5-HT and aCSF (n=8). Testing for all cohorts began at ~1 hr before dark onset. Following an initial baseline blood drawn, rats were exposed to one of the following treatments: 30 min of novel restraint (RES), no RES, LiCl (127.2 mg/kg; i.p.), Coc (15 mg/kg; i.p.), Sal (5 ml/kg; i.p.), 5-HT (40μg; 4V), or aCSF (1 μl; 4V). Additional blood draws were conducted 30- and 60-min post stimuli exposure. In the case of RES, the blood draws were prior to the start of restraint (0 min), right after the end of the 30 min restraint session (30 min), and after 30 min of being back in their home cage (60 min). The blood was processed for CORT measurements as described in the method section 2.4.
2.8.2. Experiment 1: Effect of hindbrain 5-HTR blockade on c-Fos expression on NTS PPG neurons following LiCl, cocaine, and novel restraint.
Male rats (n=30) were pre-treated with 4V injections of the 5-HT2C receptor antagonist (RS1; 40μg), the 5-HT3 receptor antagonist (OND; 25 μg), or 100% DMSO (vehicle; 2 μl). These doses were chosen because they do not have an effect on food intake on their own but do block the intake suppressive effects of 5-HT (Leon et al, 2019). Given that these drugs were delivered into a ventricle, instead of the parenchyma, the volume used (2 μl) is within acceptable range as to not cause damage (Harris, 2017; Hayes et al, 2011; Noble et al, 2018). Fifteen minutes following 4V injections the animals were exposed to either a systemic injection of LiCl (127.2 mg/kg), Coc (15 mg/kg), or Sal (5ml/kg; vehicle). The animals were transcardially perfused 75min later and IHC analysis was conducted on caudal and mid NTS sections (14.2–15.7mm posterior to bregma) looking at co-localization between c-Fos (i.e. marker of neuronal activation) and GLP-1 immunoreactivity. Co-localization was quantified manually using Image J. A separate cohort was used to analyze the effects of 5-THR antagonists on PPG activity induced by novel restraint (RES) (Terrill et al, 2019). Fifteen minutes following 4V injections of 100% DMSO (veh), RS1, OND, or RS1+OND (Combo) male rats (n=33) were exposed to 30 min of novel restraint (RES) (methods section 2.4), the control groups were left uninterrupted in their cage for 30min (no RES). Similar to the previous cohort, the animals were transcardially perfused 75 min later and IHC analysis was conducted.
2.8.3. Experiment 2: Changes in c-Fos expression within the CR in response to LiCl, cocaine, and novel restraint.
Three separate cohorts were used to assess the effects of LiCl (n=12), Coc (n=8), or RES (n=10) on c-Fos expression in the CR. Seventy-five minutes after being exposed to either LiCl (or Sal), Coc (or Sal), or RES (or no RES) the animals were transcardially perfused and the brainstems were harvested and sectioned. Brain sections containing the RPa, ROb, and RMg (−9.6–13.5mm posterior to bregma) were collected and mounted. IHC analyses was performed to determine 1) neuronal activity in the CR subnuclei by assessing total changes in c-Fos expression and 2) correlation between neuronal activity (i.e. total c-Fos expression) and activation of 5-HT neurons in the CR subnuclei by quantifying co-localization between c-Fos and 5-HT positive neurons. In both cases, manual quantification of immunoreactive cells was conducted for each of the three sub-nuclei that make up the CR (RPa, ROb, and RMg) (n=3–5 sections per condition), these individual values were then combined to get an assessment of the entire CR (labeled in figures as Total CR). Total c-Fos and c-Fos/5-HT co-localization were quantified manually using Image J.
2.8.4. Experiment 3: Monosynaptic viral tracing from the RMg to the NTS.
Male rats (n=18 rats; 6 controls and 12 dual injections) received two viral injections (described above). Three weeks following the viral injections the animals were transcardially perfused and brains processed as described above. The site of the AAV1-Cre injection was verified using an anti-CTB antibody in the RMg, only animals that showed CTB immunoreactivity that exclusively localized within the RMg (i.e., positive control for accurate viral injection) were included (4 out of 12) in the analysis. A dual FISH/IHC protocol using an RNA probe for PPG neurons and a 5-HT antibody (described above) was used to assess monosynaptic projections from the RMg to NTS PPG neurons and proximity to 5-HT fibers.
2.9. Statistical analyses
All data are represented as mean ± SEM. Data were analyzed using either a two-way ANOVA, one-way ANOVA, or an unpaired t-test (specific for each dataset). Statistically significant effects were probed using either Bonferroni or Tukey post hoc analysis. For all statistical tests, a p-value less than 0.05 was considered significant.
3. Results
3.1. Plasma corticosterone levels are increased to comparable degrees following exposure to LiCl, cocaine, and novel restraint.
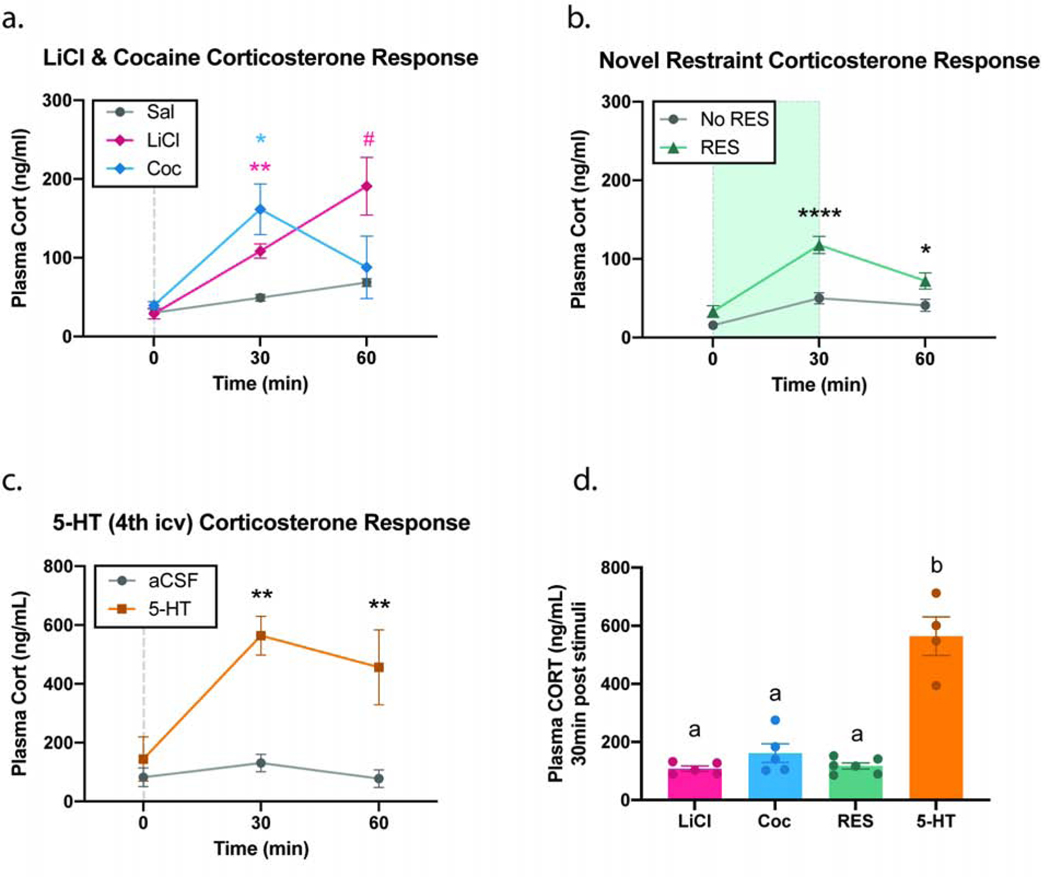
The stimuli employed in this study—namely LiCl, cocaine (Coc), and novel restraint (RES)—have all been shown to activate the central GLP-1 system and engage stress centers (Rinaman, 1999b; Schmidt et al, 2016; Terrill et al, 2019; Vahl et al, 2005). In order to properly categorize these three different stimuli as “stressors” in the current studies, we assessed their effects on plasma levels of corticosterone (CORT) (Fig. 1). Baseline levels of plasma CORT (0 min) did not differ between experimental and their relative control groups for any of the tested stimuli (Fig. 1 a–c). The first cohort of animals compared the effects of i.p. administration of Sal, LiCl, and Coc. There was a significant effect of time (F1,20≥17.91; p<0.0001), stimuli (F2,12≥4.315; p<0.05), and interaction (F4,24≥6.533; p<0.01). At 30min, plasma CORT of the LiCl and Coc groups were significantly higher when compared to the Sal group, p=0.0027 and 0.0481 respectively. At 60min, there was no significant difference between any of the groups, though there was a clear trend for the LiCl group (p=0.0557; represented with # on the graph) (Fig. 1a). In the novel restraint cohort, there was a significant effect of time (F2,20≥33.69; p<0.0001), stimuli (F1,10≥21.75; p<0.001), and interaction (F2,20≥6.533; p<0.01). The RES group had significantly higher CORT levels than the No RES group at 30min (p<0.0001) and 60min (p=0.0389) (Fig. 1b). It is worth noting, however, that the CORT levels for the RES group dropped significantly between 30 and 60min (p=0.0069), which is supported by the literature (Vahl et al, 2005).
Fig 1. Changes in circulating CORT levels in response to different stimuli.
Levels of plasma CORT were measured at 0, 30, and 60 min following exposure to LiCl (127.2 mg/kg; i.p.) (a), Coc (15 mg/kg; i.p.) (a), RES (30 min) (b), or 5-HT (40 μg; 4V) (c). All of these stimuli significantly increased plasma CORT 30 min post-exposure when compared to their relative controls (a-c). CORT levels at 30 min post stimuli-exposure were compared for all experimental groups, LiCl, Coc, and RES groups were all similar to each other but were significantly lower than the CORT levels of 5-HT-treated group (d). All data expressed as mean ± SEM (n = 4–6 per treatment group). Panels (a-c) were analyzed using a two-way ANOVA followed by Bonferroni post-hoc analysis (*P<0.05; **P<0.01; ****P<0.0001; #P=0.0557). Panel (d) was analyzed using a one-way ANOVA followed by Tukey post-hoc analysis (P<0.0001).
All three stimuli tested significantly increased plasma levels of CORT at the 30min timepoint. In order to provide a reference of the magnitude of relative CORT increase, 5-HT was infused centrally in a third cohort of rats. In the 5-HT cohort, there was a significant effect of time (F2,12≥4.14; p<0.05) and stimuli (F1,6≥134; p<0.001) but not of interaction (F2,12≥2.934; p=0.0918). When compared to its control, 5-HT increased plasma CORT at 30 and 60min, p=0.0011 and 0.0036 respectively (Fig. 1c). When we compared CORT levels induced by all 4 different stimuli, the degree to which 5-HT increases CORT (at 30-min post exposure), is significantly higher than the increase induced by LiCl, Coc, or RES, while no significant differences was observed between the latter 3 stimuli (Fig. 1d).
3.2. Pharmacological blockade of hindbrain 5-HT2C and 5-HT3 receptors decrease c-Fos in NTS PPG neurons following LiCl and novel restraint.
Here we set out to investigate the role of 5-HT2C and 5-HT3 receptors in mediating c-Fos expression in NTS PPG neurons following diverse acute stressors known to activate PPG neurons. To assess this, animals pre-treated with pharmacological antagonists for the 5-HT2CR (RS1) or the 5-HT3R (OND) were exposed to either LiCl, Coc, or RES. IHC analysis was then used to quantify changes in c-Fos and its co-localization with PPG neurons.
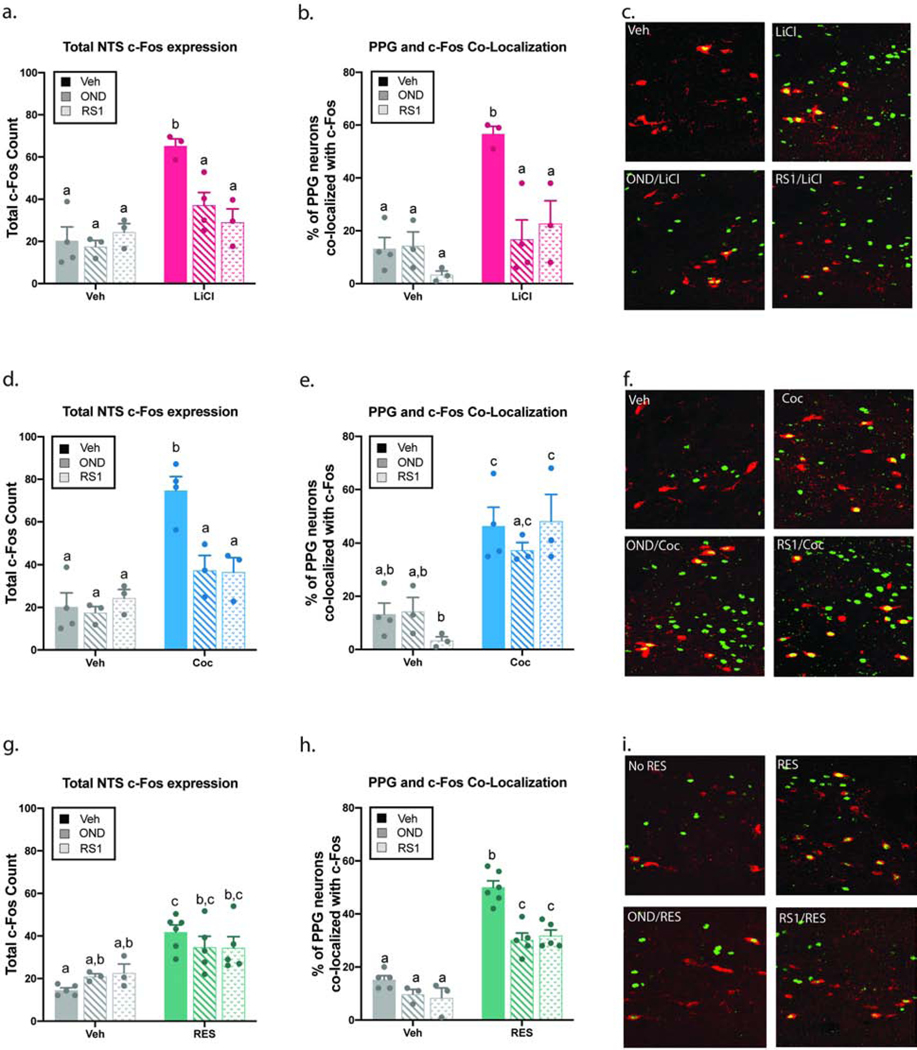
As expected, LiCl (magenta), Coc (blue), and RES (green) all increased total NTS c-Fos expression (all p<0.001, Fig. 2a, 2d, 2g) as well as activation of PPG neurons indicated by an increase in co-localization between c-Fos and GLP-1 immunoreactivity (all p<0.01, Fig. 2b, 2e, 2h). Administration of either antagonist, OND or RS1, had no significant effect on total c-Fos expression (Fig. 2a, 2d, 2g) or on c-Fos/GLP-1 co-localization (Fig. 2b, 12e, 2h) on its own. In the LiCl-treated groups, there was a significant interaction between LiCl and the 5HTR antagonists in the total expression of c-Fos (F2,14≥6.68; p<0.01) and in the PPG/c-Fos co-localization (F2,14≥6.63; p<0.01). Post hoc analyses revealed that treatment with either antagonist (OND or RS1) was sufficient to reverse the increase in total NTS c-Fos expression (Fig. 2a) as well as the increased c-Fos/GLP-1 co-localization (Fig. 2b–c). A zoomed-out image of the co-localization between PPG and c-Fos immunoreactivity can be found in S1a.
Fig 2. Pharmacological blockade of hindbrain 5-HT2C and 5-HT3 receptors decrease c-Fos expression in NTS PPG neurons following LiCl and novel restraint.
Pre-treatment with hindbrain (4V) RS1 (40 μg) or OND (20 μg) was followed by LiCl (127.2 mg/kg; i.p.) (a-c), Coc (15 mg/kg; i.p.) (d-f), or RES (30 min) (g-i). In the LiCl-treated groups, the increase in NTS c-Fos expression (a) and the increase in co-localization between c-Fos and GLP-1 immunoreactive cells (b) were both reversed by pre-treatment with either OND or RS1. Treatment with either 5-HTR antagonist reversed the increase in NTS c-Fos expression induced by Coc (d), however, the co-localization between c-Fos and GLP-1 immunoreactive cells was unaffected (e). The increase in NTS c-Fos expression induced by RES was not significantly affected by pre-treatment with OND or RS1 (g). Treatment with OND or RS1 caused an attenuation in co-localization between c-Fos and GLP-1 immunoreactive cells (h). Representative images of co-localization between c-Fos (green) and GLP-1 (red) immunoreactivity for the main treatment groups (c, f, i). All data expressed as mean ± SEM and were analyzed using a two-way ANOVA (n = 3–6 per treatment group). Different letters are significantly different from each other (p<0.05) according to Tukey post hoc analyses.
In the Coc groups, there was a significant interaction between Coc and the 5-HTR antagonists in the total expression of c-Fos (F2,14≥7.21; p<0.01). This degree of interaction was not detected in the co-localization between PPG and c-Fos immunoreactivity. Treatment with either 5-HTR antagonist reversed the increase in NTS c-Fos expression (Fig. 2d), but the increase in c-Fos/GLP-1 co-localization was unaffected (Fig. 2e–f; S1b). In the RES cohort, there was a significant interaction between RES and the 5-HTR antagonists in the co-localization between PPG and c-Fos immunoreactivity (F2,21≥5.00; p<0.05), but not in the total expression of c-Fos. Although neither antagonist caused a significant effect on total NTS c-Fos expression (Fig. 2g), PPG activity was attenuated by pre-treatment with either OND or RS1 (Fig. 2h–i; S1c). Animals that received the combo treatment (OND+RS1) did not see an additive effect in the attenuation of PPG activity. In fact, the attenuation in c-Fos/GLP-1 co-localization seen in the combo treated group (OND+RS1) was indistinguishable from the animals treated with a single antagonist (S2). Raw cell count of PPG, c-Fos, and co-localization of immunopositive neurons can be found in table S3. These results indicate that hindbrain 5-HT2C and 5-HT3 receptors play a role in mediating c-Fos expression in PPG neurons induced by LiCl and restraint but not cocaine supporting our hypothesis that the serotonergic modulation of the GLP-1 system is stimulus specific.
3.3. LiCl and novel restraint stress increase c-Fos expression in the RMg.
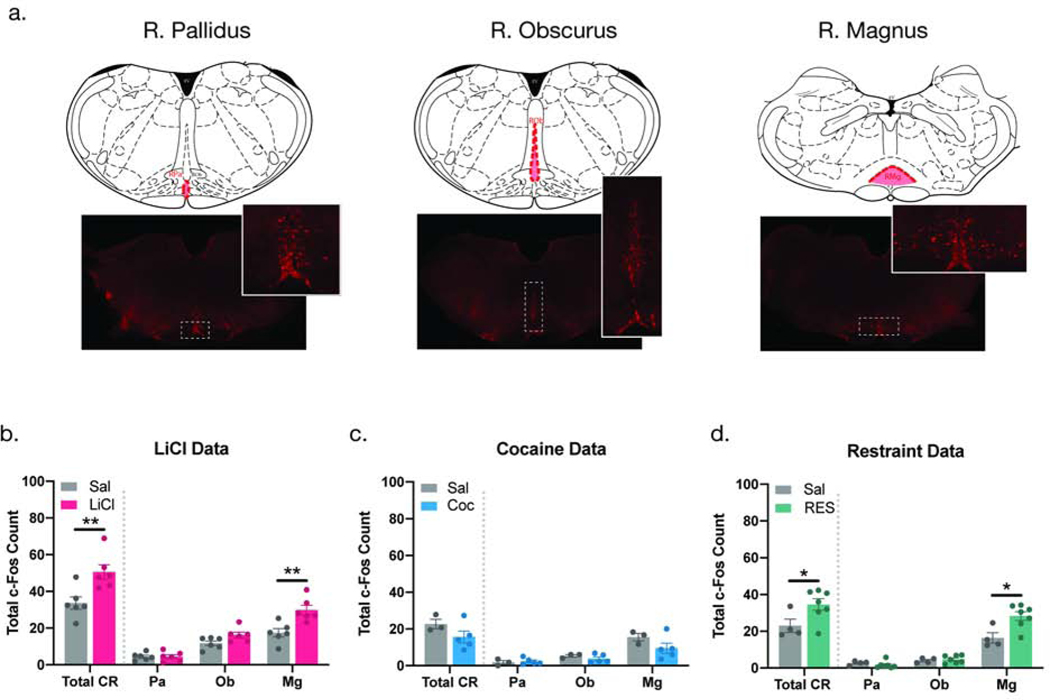
Next, we aimed to identify the potential source of 5-HT input responsible for engaging the GLP-1 system. Given the location of the NTS along the rostral-caudal plane and the orientation of efferent projections emerging from the raphe, we focused on the CR—and it’s three sub-nuclei (Fig 3a)—as the potential source of 5-HT input responsible for engaging the central GLP-1 system. Total c-Fos expression was quantified in the RPa, ROb, and RMg (total CR = RPa + ROb + RMg) of animals that had been treated with LiCl, Coc, or RES.
Fig 3. Increased c-Fos expression in the CR in response to stimuli that engage the central GLP-1 system is driven by the RMg.
Representative sections of the RPa, ROb, and RMg showing the presence of 5-HT neurons in each of the three CR sub-nuclei (a). Exposure LiCl and RES caused a significant increase in c-Fos immunoreactivity in the CR (Total CR), within the individual nuclei there was a significant increase in the RMg, but not in the RPa or ROb (b-c). Treatment with Coc had no effect on total c-Fos expression in any of the CR sub-nuclei (d). Total CR = RPa + ROb + RMg. All values are expressed as MEAN +/− SEM (n = 3–6 per treatment group). Data were analyzed using an unpaired t-test (p < 0.05).
LiCl increased total c-Fos expression in the CR (total CR) (p<0.01, Fig. 3b). A closer analysis revealed that the RMg drove this effect as it was the only sub-nuclei in which c-Fos expression was significantly elevated (p<0.01). The ROb and RPa had comparable c-Fos expression levels in the LiCl- and vehicle-treated animals (Fig. 3b). A single unsolicited Coc administration did not cause any changes in c-Fos expression in any of the three CR subnuclei (Fig. 3c). Similar to LiCl’s effects, RES also caused an increase in total c-Fos expression in the CR (total CR), again a RMg-driven effect (p<0.05) (Fig. 3d). These data suggest that the CR, specifically the RMg, is responsive to two stimuli that engage the GLP-1 system, namely LiCl and RES. This interpretation is supported by our NTS data showing that 5-HTRs in the NTS mediate the activation of PPG neurons induced by LiCl and RES but not Coc.
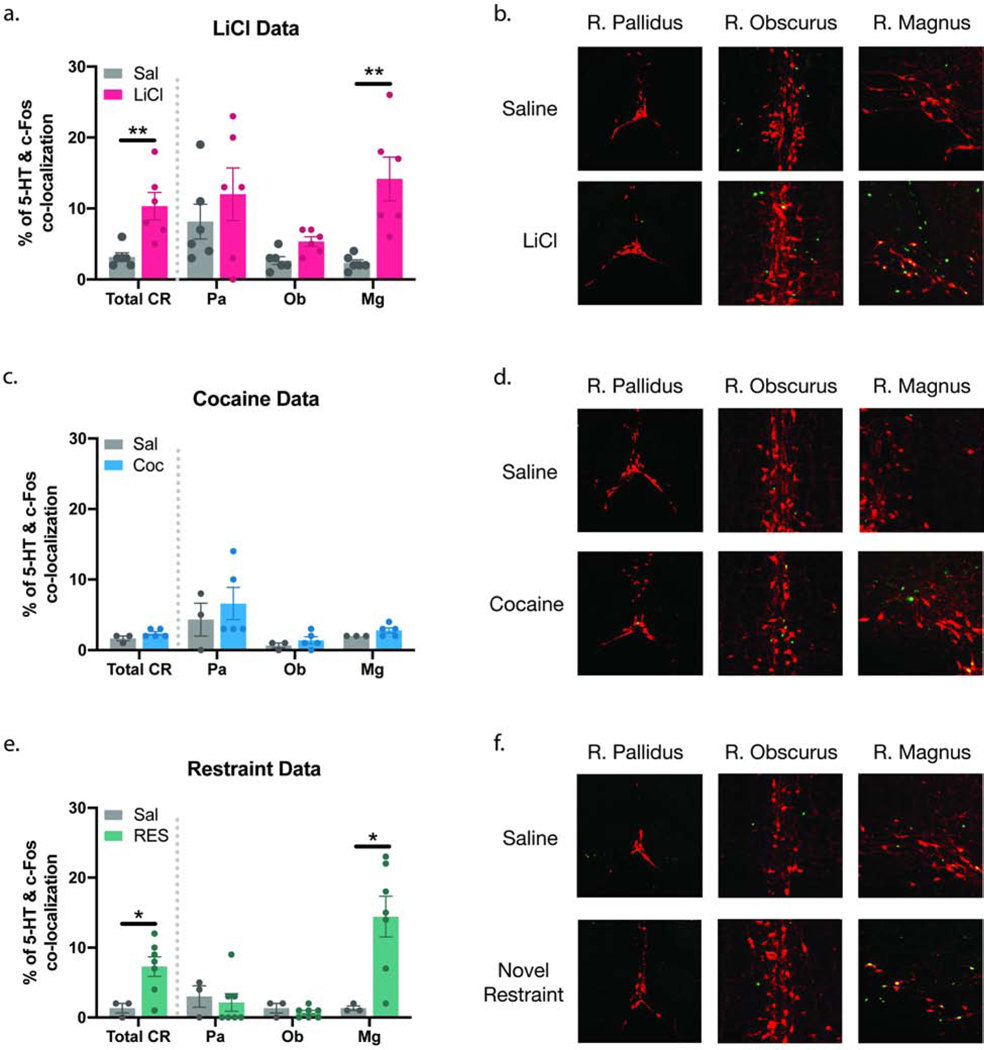
3.4. LiCl and novel restraint increase c-Fos expression in 5-HT neurons within the RMg.
Next, we tested whether the increase in c-Fos expression observed in the CR correlates with an increase in activation of 5-HT-expressing neurons. Co-localization between c-Fos and 5-HT immunoreactive cells in the CR was significantly increased in the LiCl group (Total CR) when compared to the control group (p<0.01, Fig. 4a). When analyzed individually, the c-Fos/5-HT colocalization in the LiCl treated brains was significantly higher in the RMg (p<0.005), but no difference was observed in the RPa or the ROb (Fig. 4a–b). A zoomed-out image of the co-localization between 5-HT and c-Fos immunoreactivity can be found in S4a. In the Coc group, there was no difference in the c-Fos/5-HT co-localization between the experimental and control groups in any of the CR sub-nuclei (Fig. 4c–d; S4b). The RES group saw a similar pattern to the LiCl group. There was a significant increase in c-Fos/5-HT co-localization in the CR (Total CR) (p<0.05, Fig. 4e). The RMg experienced an increase in c-Fos/5-HT colocalization following restraint (p<0.05), but no difference was observed in the RPa or the ROb (Fig. 4e–f; S4c). Raw cell count of 5-HT, c-Fos, and co-localization of immunopositive neurons can be found in table S5. Our data show that LiCl and RES engage the CR and increase activation of 5-HT neurons as read out by c-Fos, specifically in the RMg, identifying the RMg as the potential source of 5-HT input into NTS PPG neurons.
Fig 4. Systemic LiCl and novel restraint increase c-Fos expression in 5-HT neurons in the RMg.
LiCl (a-b) and RES (e-f) significantly increased co-localization between 5-HT and c-Fos immunoreactive cells in the CR (Total CR). In both groups the increase in co-colocalization was significant in the RMg but not in the ROb or RPa. Treatment with Coc did not cause significant changes in 5-HT/c-Fos co-localization in any of the CR sub-nuclei (c-d). Representative images of co-localization between c-Fos (green) and 5-HT (red) immunoreactivity for the main treatment groups in each of the CR sub-nuclei (b,d,f). Total CR = RPa + ROb + RMg. All values are expressed as MEAN +/− SEM (n = 3–6 per treatment group). Data were analyzed using an unpaired t-test (p<0.05).
3.5. Tracing mono-synaptic projections from the RMg to the NTS.
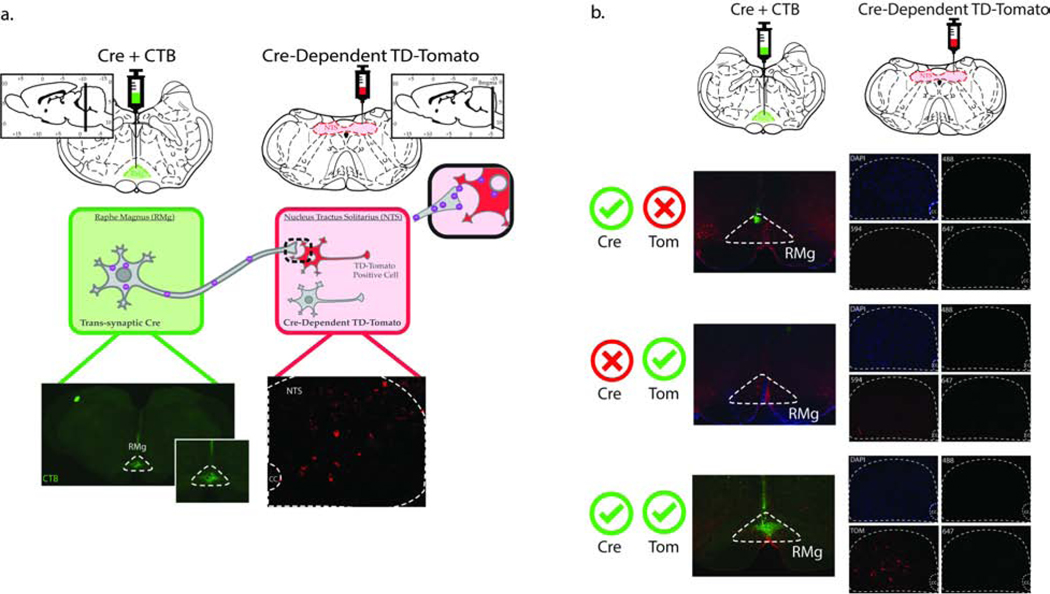
Male rats (n=18) received an anterograde tracer expressing Cre recombinase (AAV1.hSyn.Cre.WPRE.hGH) in combination with CTB-488 (used to validate injection site) into the RMg (Fig. 5a, top left) and an AAV1 expressing Cre recombinase-dependent TD-Tomato (AAV1.CAG.Flex.tdTomate. WPRE.bGH) into the NTS (Fig. 5a, top right) to determine whether RMg neurons directly innervate NTS PPG neurons. AAV1 exhibits anterograde transsynaptic spread properties, jumping a single synapse (Suarez et al, 2018; Zingg et al, 2017). This allows for viral particles taken up by neurons in the RMg to travel anterogradely down the axon and be transported onto downstream neurons, ultimately culminating in the expression of Cre recombinase in post-synaptic neurons. Cre recombinase-dependent TD-Tomato presence in the NTS allows for fluorescent tagging of NTS neurons that receive mono-synaptic projections from the RMg (Fig. 5a, bottom right).
Fig 5. Methods and controls for mono-trans-synaptic viral tracing from the RMg to the NTS.
Rats (n=18) received a cocktail injection containing an AAV1 Cre anterograde tracer and cholera toxin B (CTB)-488 (50nl) into the RMg and a Cre-dependent AAV1-FLEX-TD-Tomato (100nl /hemisphere) virus into the NTS (a). Three weeks following the viral injections the animals were transcardially perfused, brains were harvested, and tissue was processed for FISH/IHC analysis. The site of injection was verified using anti-CTB antibody in the RMg (a, bottom left). The trans-synaptic properties of the AAV1 Cre was confirmed via the presence of TD-Tomato-expressing neurons in the NTS (a, bottom right). Validation for injection site specificity (b, top panels), dependency on the presence of Cre for the expression of TD-Tomato (b, middle panels), and lack of bleed-through of TD-Tomato into other channels (b, bottom panels).
A series of different controls were first used for validation of our methods. The top panel in Fig. 5b shows our AAV1-Cre-positive controls animals (n=3), this group received the AAV1-Cre but not the Cre recombinase-dependent TD-Tomato. The accuracy of the injection site was corroborated by CTB immunoreactivity in the RMg (green). The lack of fluorescent signal in the NTS is expected as this group was not injected with AAV1.CAG.Flex.tdTomate.WPRE.bGH. The middle panel in Fig. 5b depicts a representative section of another control group, which received AAV1.CAG.Flex.tdTomate.WPRE.bGH injection in the NTS but no AAV1.hSyn.Cre.WPRE.hGH in the RMg (n=3). This group lacked both CTB presence in the RMg and TD-Tomato signal in the NTS, the latter corroborates that the expression TD-Tomato depends on the presence of AAV1-Cre. Finally, the bottom panel in Fig. 5b is showing a representative image of the RMg and NTS of animals that received both viral injections. Accuracy of the AAV1 Cre injection into the RMg was confined via CTB immunoreactivity, only animals in which CTB immunoreactivity was confined to the RMg were used in the analysis (4 out of 12) (Supplemental, Fig. S6). The presence of TD-Tomato-positive neurons in the NTS confirm the presence of a mono-synaptic connection from the RMg to the NTS and shows a lack of bleed-through of TD-Tomato into other channels. These results validate our methods demonstrating our ability to successfully trace direct synaptic connections from the RMg to the NTS.
3.6. NTS PPG neurons that receive mono-synaptic projections from the RMg lie in close proximity to 5-HT axons.
Next, we examined whether RMg neurons project mono-synaptically to PPG neurons. NTS sections from animals that accurately received both viral injections (n=4) were processed for FISH/IHC using a mRNA probe for PPG neurons (green) and a 5-HT antibody (blue) (Fig. 6). Following tissue processing required for the FISH/IHC protocol, the presence of TD-Tomato signal was visible and did not bleed into the other channels (Fig. 6b, NTS on bottom panel). At this rostral-caudal plane, 5-HT fibers were observed to preferentially target the NTS (Supplemental, Fig. S7).
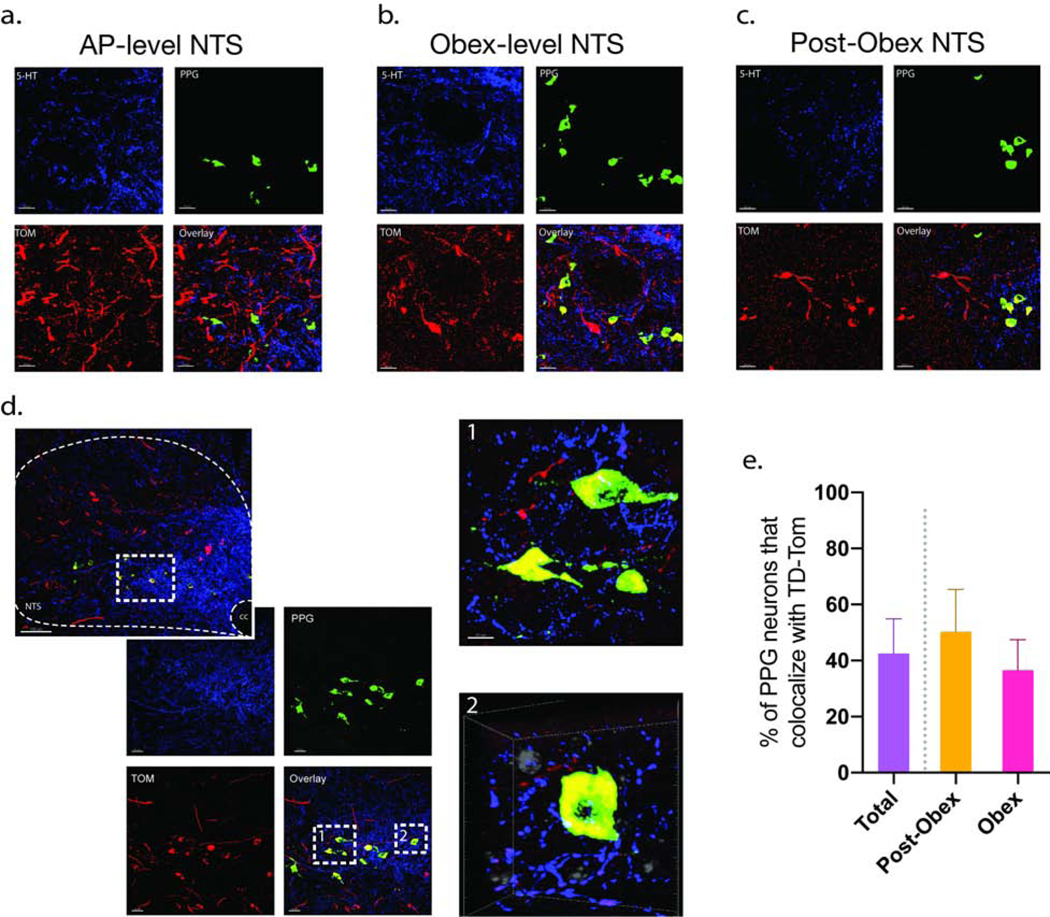
Fig 6. RMg neurons project mono-synaptically to PPG neurons in the NTS.
FISH/IHC was conducted on TD-Tom tagged NTS sections using an mRNA probe for PPG neurons (green) and a 5-HT antibody (blue) (n = 4). Neurons showing PPG/TD-Tom co-localization were identified in the AP- level NTS (a), Obex-level NTS (b), and Post-Obex NTS (c) sections. A representative obex-level NTS section at ×20 and x40 magnification is shown in (b), PPG are shown in green, TD-Tomato-expressing neurons in red, and 5-HT fibers in blue. Subpanel b-1 shows an optical zoom of two neurons co-localizing PPG and TD-Tom. Representative frame of three-dimensional rotational video showing PPG and TD-Tom co-expressing neurons in close opposition to 5-HT axons Subpanel b-2 (video can be found in the supplement data). The video was obtained from a z-stack collected from the mNTS at the level of the obex with the × 40 oil-immersion objective and a 4–5 optical zoom. A total of 43% of PPG neurons co-localized with TD-Tom, at the level of the pre-obex there was a 50% co-localization while at the obex-level there was a 37% co-localization (c).
TD-Tomato positive cells (red) were identified in at every rostral-caudal plane we analyzed, AP-level, obex, and post-obex, as were neurons that showed PPG and TD-Tomato co-localization (Fig. 6a–d). While there was a significant degree of co-localization, not every NTS neuron that received RMg input was PPG. Likewise, not every PPG neuron receives monosynaptic input from the RMg. Quantification of PPG/TD-Tomato co-localization revealed that 43% of PPG neurons receive monosynaptic input from RMg neurons, with the obex-level sections showing 37% co-localization, and the post-obex (caudal to obex) sections showing a 50% co-localization and (data were calculated as average expression per animal) (Fig. 6e). The low number of PPG neurons present at the AP-level of the NTS did not allow for proper quantification at this plane. There was no significant difference in PPG/TD-Tomato co-localization between hemispheres nor in the rostral-caudal, or medial-lateral planes. A subset of PPG neurons that co-localize with TD-Tomato were found to be in close proximity to 5-HT axons (Fig. 6d), this was corroborated with a three-dimensional rotation video of 40×z-stack overlays (Movie 1). Combined, these results suggest that the RMg projects mono-synaptically to NTS PPG neurons and that some of these projections may be from a 5-HT input.
4. Discussion
In this study we investigated whether different stressors engage the central GLP-1 system via 5-HT2CR and 5-HT3R signaling. Our results showed that activation of NTS PPG neurons following exposure to LiCl and novel restraint is dependent on 5-HT2CR and 5-HT3R signaling, whereas cocaine-induced activation of PPG neurons does not appear to rely on a hindbrain serotonergic mechanism. Changes in c-Fos expression within the CR identified the RMg as a potential source of 5-HT input into NTS PPG neurons. Finally, employing a viral tracing technique we showed that NTS PPG neurons are innervated directly by RMg neurons and that a sub-set of these neurons are in close proximity to 5-HT axons. These results support the hypothesis that the modulatory effect of 5-HT on the GLP-1 system extends beyond the realm of feeding to include modulation of PPG activity following some, but not all, acute stressors.
4.1. Effect of LiCl, cocaine, and novel restraint on plasma levels of CORT.
The increase in plasma CORT observed following LiCl, Coc, and RES provides a readout corroborating that these manipulations significantly increase levels of stress hormones and as such can be referred to as “stressors”. This data is supported by other groups that have found similar results (Hare et al, 2014; Schmidt et al, 2016; Sugawara et al, 1988). Further, a direct comparison of CORT levels at 30min across experimental groups revealed that LiCl, Coc, and RES elevate CORT to comparable levels (~150–200ng/ml). These results suggest that all three acute stressors employed in this study produce a similar release of CORT and on a comparable timeline, thus providing validity for the comparison of neuronal activity induced by these three stimuli.
Despite the congruence in levels of elevated CORT at 30min, there are differences in the overall temporal CORT response induced by these different stressors. Despite not reaching significance it is worth noting that the LiCl-treated animals showed a trend towards increased CORT at 60 min (p=0.0557) when compared to its control. It is likely that CORT levels induced by LiCl continue to increase after the 30 min of exposure. In fact, one study showed increase CORT up to 2 h post LiCl injection (Jacobs, 1978). The difference observed in our study could be due to differences in experimental design or individual variability. The effect of Coc on CORT levels is pronounced but short-lived, 60min post injection CORT levels were on par with the control group. Despite the increased CORT levels observed in the RES group at 60 min, this value was significantly lower than CORT levels measured at 30min. This is supported by the literature (Vahl et al, 2005) which indicates that CORT levels following novel restraint of 30 min peaks immediately after the animals are removed from the restraint apparatus.
4.2. Effect of hindbrain 5-HT2C and 5-HT3 receptors in mediating LiCl-induced PPG activity
LiCl is a well-established model of interoceptive stress, it induces pro-inflammatory effects and mimics patho-physiological (e.g. inhibition of gastric motility) and behavioral responses (e.g. anorexia) observed during systemic malaise (Kinzig et al, 2003; Kinzig et al, 2008; Koehnle and Rinaman, 2010; McCann et al, 1989; Nassar and Azab, 2014; Rinaman, 1999a, b; Sugawara et al, 1988). While the exact mechanisms through which LiCl engages stress centers remains elusive, activity of the GLP-1 (Rinaman, 1999a, b) and 5-HT (Limebeer et al, 2018; Sheard and Aghajanian, 1970) systems are both enhanced following systemic LiCl. The impact LiCl has on the central 5-HT system has been predominantly studied in forebrain structures (Limebeer et al, 2018; Sheard et al, 1970). To the best of our knowledge, the effect LiCl has on hindbrain 5-HT activity or the relationship this effect may have on the central GLP-1 system has not previously been studied.
In this study we showed that pharmacological blockade of hindbrain 5-HT2CRs or 5-HT3Rs was sufficient to block the LiCl-induced increase in total NTS c-Fos expression as well as the PPG/c-Fos co-localization. This indicates that hindbrain 5-HT2CRs and 5-HT3Rs mediate LiCl-induced PPG activation and are significant drivers of total NTS neuronal activity following LiCl. While it remains to be confirmed, this newfound association between 5-HT and GLP-1 likely underlies some of the behavioral effects induced by LiCl, such as hypophagia and malaise. Support for this comes from studies showing that systemic 5-HT3R antagonism reduces LiCl-induced gaping (i.e. a marker of visceral malaise) (Rock and Parker, 2013) and hindbrain 5-HT3R blockade reverses the acute anorectic effect (Leon et al, 2019) of LiCl. Additionally, blockade of GLP-1Rs attenuate LiCl-induced hypophagia (Rinaman, 1999a) and pica (i.e. kaolin consumption, a validated proxy for malaise) (Seeley et al, 2000). The ability of LiCl to activate the HPA axis and increase plasma levels of stress hormones (Jahng and Lee, 2015) could be explained by the engagement of the HPA via GLP-1 signaling, given that PPG neurons activated by LiCl project to the PVN (Rinaman, 1999b) and that central GLP-1 increases both ACTH and CORT levels (Kinzig et al, 2003). We have identified that LiCl engages the GLP-1 system via hindbrain 5-HTRs and discussed how this interaction may play a role in the behavioral and mechanistic stress response elicited by LiCl. However, future studies are required to decipher the mechanism through which LiCl activates the 5-HT system.
4.3. Effect of hindbrain 5-HT2C and 5-HT3 receptors in mediating PPG activity induced by novel restraint
Unlike LiCl, psychogenic stressors—such as acute, novel restraint—are not physically painful and thus rely on the perception of a threat (Doremus-Fitzwater et al, 2009; Herman and Cullinan, 1997). Restraint activates PPG neurons (Maniscalco et al, 2015; Terrill et al, 2019) and increases brain levels of 5-HT (McIntyre et al, 1999; Mo et al, 2008). Here, we show that blockade of hindbrain 5-HT2CRs and/or 5-HT3Rs attenuates the activation of PPG neurons induced by novel restraint without affecting total NTS neuronal activity. This indicates that hindbrain 5-HT2CRs and 5-HT3Rs play a role in mediating the specific engagement of the central GLP-1 system following exposure to novel restraint. The attenuation of RES-mediated PPG activity in the RS1+OND combo group was comparable to animals that received individual treatment of either antagonist. The observed lack of additive/synergistic effect could be explained by the significant overlap of 5-HT2C and 5-HT3 receptor expression among PPG neuronal populations. In fact, ~90% of PPG neurons express the 5-HT2C receptor and over 50% of PPG neurons co-express both the 5-HT2C and 5-HT3 receptors. Considering this degree of overlap, manipulation of either receptor type alone may be sufficient to engage the central GLP-1 system. There are two additional points worth discussing in more detail. First, in response to novel restraint— in contrast to LiCl— PPG activation is only partially dependent on hindbrain 5-HT signaling. Given the complexity of psychogenic stressors, it’s not too surprising that a single system (i.e., 5-HT) does not have an all-or-nothing effect on the neuronal activity produced by novel restraint. Second, the lack of effect on total NTS neuronal activity (total c-Fos) indicates that a significant portion of NTS neurons activated by novel restraint are not PPG in nature. This is substantiated by the inability of RS1 to significantly attenuate total NTS c-Fos expression despite 5-HT2CRs being present on ~90% of PPG neurons (Leon et al, 2019). Both points—partial dependence on 5-HT and RES activating non-PPG neurons—can be addressed by reports indicating that restraint engages other neuronal populations within the NTS, most notably noradrenergic neurons (Bundzikova-Osacka et al, 2015; Maniscalco et al, 2012). The lack of effect on total NTS c-Fos expression in response to 5-HT2CR and 5-HT3R blockade could result from restraint activating other neuronal populations within the NTS. This could also help explain the inability of hindbrain 5-HTR blockade to fully reverse RES-induced PPG activity as it is possible that PPG activation is a second- or third-order response to novel restraint. In one scenario, novel restraint could be engaging NTS neurons (e.g. NA, Glu) which then activate PPG neurons. However, it’s also possible that novel restraint engages PPG neurons via forebrain mechanisms such as projections from the central nucleus of the amygdala (Mohammad et al, 2000; Saha, 2005). The involvement of the 5-HT/GLP-1 interaction in mediating restraint-induced PPG activity opens the door to many questions regarding its role in modulating the physiological response to other psychogenic stressors [e.g. social defeat (Razzoli et al, 2015)].
4.4. Cocaine-induced PPG activity is not mediated by hindbrain 5-HT2C and 5-HT3 receptors
Despite their rewarding properties, drugs of abuse—such as ethanol, cocaine, and methamphetamine—cause aversive-like behaviors in rodents when administered in a noncontingent manner as was done in this study (Davies and Parker, 1990; Parker, 1993; Turenne et al, 1996). While cocaine is not considered a traditional stressor, its acute administration engages stress centers such as the HPA axis and increases plasma ACTH and CORT (Cleck and Blendy, 2008; Manetti et al, 2014; Schmidt et al, 2016). Cocaine engages the central GLP-1 and 5-HT systems, it increases endogenous 5-HT in the ventral pallidum (Matsui and Alvarez, 2018) and activates NTS PPG neurons (Schmidt et al, 2016). Additionally, GLP-1Rs in the ventral tegmental area (Hernandez et al, 2018) and nucleus accumbens (Hernandez et al, 2019) have been implicated in mediating cocaine-seeking behavior in rats. Our results showed that hindbrain blockade of either the 5-HT2CR or 5-HT3R following an acute noncontingent systemic infusion of cocaine attenuates total neuronal activation within the NTS but has no effect on the specific activation of PPG neurons. This indicates that hindbrain 5-HT2C and 5-HT3 receptors do not mediate cocaine-induced activation of PPG neurons, however, they do play a role in mediating cocaine’s actions on NTS neuronal activity, via a non-PPG mechanisms. It would be of interest in future studies to characterize the NTS neuronal population that is engaged by cocaine and susceptible to 5-HT modulation. Although the mechanism(s) through which cocaine engages PPG neurons remains unclear, glucocorticoids appear to be an attractive candidate. Schmidt et al showed that central administration of CORT attenuates cocaine self-administration, this effect was blocked by pretreatment with the GLP-1R antagonist, exendin (9–39) (Schmidt et al, 2016). Additionally, glucocorticoid receptors (GRs) are expressed extensively in the NTS (Härfstrand et al, 1986; Herman, 1993) and, thus, are likely to be present on PPG neurons. However, future experiments are needed to test this hypothesis.
4.5. Potential source of 5-HT driving engagement of NTS PPG neurons
Identifying the source of 5-HT responsible for mediating GLP-1 signaling is critical in better understanding how these two systems interact and the range of biological influence this interaction may have. Of the CR sub-nuclei, we focused on the RMg given that it contains the highest level of 5-HT neurons (Hornung, 2003). Here we showed that LiCl and novel restraint (stressors that require 5-HT2C and 5-HT3 receptors in order to engage NTS PPG neurons) increased total neuronal activity in the CR. This effect was driven by the RMg and correlated with an increase in 5-HT neuronal activity. This neuronal responsivity indicates that the CR, specifically the RMg, is engaged by stressors that activate the GLP-1 system. These data identify the RMg as a potential source of 5-HT responsible for modulating the central GLP-1 system—at least in response to certain stressful stimuli.
The RMg is known to project to the NTS (Thor and Helke, 1989), however, the neuronal population(s) it targeted remained unknown. Combining the viral tracing with a FISH/IHC protocol we showed that 43% of NTS PPG neurons receive mono-synaptic projections from the RMg. Additionally, we found a sub-set that were in close opposition to 5-HT axons. This suggests that at least a portion of the RMg input into NTS PPG neurons comes from a 5-HT source. A direct connection between PPG neurons and 5-HT fibers can only be confirmed using electron microscopy (EM) and remains a topic for future studies. In addition to confirming a direct synaptic connection between RMg 5-HT neurons and NTS PPG neurons it would be of interest to assess the behavioral relevance of 5-HT input onto PPG neurons on feeding or other stress-mediated responses. Assessing whether the interaction between 5-HT and GLP-1 is preserved between sexes was not addressed in this study and remains to be determined. Given the species-specific differences between mice and rats in regard to the central GLP-1 system (Cork et al, 2015; Lachey et al, 2005), it would be important to conduct electrophysiological studies aimed at characterizing the direct impact of 5-HT receptor activation on activity of NTS PPG neurons in rats once PPG-Cre transgenic rat lines become available.
Collectively, our results indicate that LiCl and novel restraint activate 5-HT neurons in the RMg of the CR and require 5-HT2C and 5-HT3 receptor signaling in the NTS in order to engage the central GLP-1 system. The stressors chosen in this study are different in nature and thus provide solid support for the idea that 5-HT modulates GLP-1 in response to stress. However, given that acute and chronic stress generate different neuroendocrine responses and, in some instances, opposing effects on feeding behavior (Rabasa and Dickson, 2016), it would be of interest to investigate whether the modulatory effect 5-HT has on central GLP-1 changes under long-term exposure to stress. While this study focused on 5-HT2C and 5-HT3 receptors, the role of other 5-HT receptor subtypes and their ability to modulate PPG activity following acute and chronic exposure to stress still needs to be investigated. In particular the 5-HT2A receptor, given its presence in the NTS (Hosford et al, 2015) and its well-established role in stress-related disorders including depression and post-traumatic stress disorder (Carr and Lucki, 2011; Murnane, 2019; Nutt, 2015).
5. Conclusion
Taken together, the NTS and CR data indicate that hindbrain 5-HT signaling—putatively originating in the RMg—acting through 5-HT2C and 5-HT3 receptors expressed on NTS PPG neurons mediate the neural activation of PPG neurons by LiCl and novel restraint, but not cocaine. These results support our initial hypothesis and show that the relationship between 5-HT and GLP-1 is not only relevant in the context of feeding (Leon et al, 2019), but that 5-HT also mediates GLP-1 in response to different acute stressors. The ability of 5-HT to control NTS PPG activity in response to different stressors highlights complexity and multidimensional nature of the central 5-HT/GLP-1 interaction.
Supplementary Material
Zoomed-out images from Figure 1 to help visualize c-Fos expression within an entire hemisphere of the NTS in each of the main treatment groups. Changes in PPG and c-Fos colocalization in the NTS following treatment with LiCl (a), Coc (b), or RES (c).
Animals received a combo pre-treatment (4V) of RS1 (40μg) and OND (25μg) followed RES (30min). The combo treatment caused an attenuation in co-localization between c-Fos and GLP-1 immunoreactive cells (a). Representative images of co-localization between c-Fos (green) and GLP-1 (red) immunoreactivity for the main treatment groups (b). All data expressed as mean ± SEM and was analyzed using a one-way ANOVA. Means with different letters are significantly different from each other (p<0.05) according to Tukey post hoc analyses.
Table 1. Number and distribution of PPG and/or c-Fos immunopositive neurons in the rat NTS following pre-treatment with either OND or RS1 and exposure to either LiCl, Coc, or RES.
Zoomed-out images from Figure 3 to help visualize c-Fos expression within an entire hemisphere of the NTS in each of the main treatment groups. Changes in 5-HT and c-Fos colocalization in the caudal raphe and its subniclei following treatment with LiCl (a), Coc (b), or RES (c). Total CR = RPa + ROb + RMg.
Table 2. Number and distribution of 5-HT and/or c-Fos immunopositive neurons in the rat CR following exposure to either LiCl, Coc, or RES.
Diagram illustrating representative injection sites for all 4 animals in which CTB immunoreactivity localized exclusively within the RMg. 4V = 4th ventricle; RMg = Raphe Magnus.
IHC was conducted on hindbrain NTS sections using a 5-HT antibody at three different rostral-caudal planes of the NTS, AP-level (a), Obex-level (b), and Post-Obex (c). Representative images of each rostral-caudal plane were taken at 10X (top) and 20X (bottom) magnifications. Representative images (a-c) show expression of 5-HT fibers (green) among the three rostral-caudal planes that were analyzed. 5-HT fibers were most densely aggregated in the NTS in all three rostral-caudal planes.
Highlights.
Hindbrain 5HT2C and 5HT3 receptors mediate activity of PPG neurons in response to lithium chloride and novel restraint.
LiCl and novel restraint activate 5-HT neurons in the Raphe Magnus (RMg)
RMg neurons monosynaptically innervate NTS PPG neurons
ACKNOWLEDGMENTS
The authors thank Dr. Yafang Zhang, Rinzin Lhamo, Jack Chen, Jane Gaisinky, Michael Hagan, and Christian Pinto for valuable technical assistance.
FUNDING AND DISCLOSURES
This research was supported by NIH R01-DK021397–41 (MRH), R01-DK112812 (BCD), R01 DA037897 and R21 DA045792 (HDS), SNF P400PB–186728 (TB), F32-DK118818 (LMS), and F31-DK118816 (RML). MRH has received research support, not used in the current studies, from investigator-initiated sponsored proposals from Eli Lilly & Co. and Boehringer-Ingelheim. HDS has received research support, not used in the current studies, from an investigator-initiated sponsored proposal from Novo Nordisk. BCD has received research support, not used in the current studies, from an investigator-initiated sponsored proposal from Pfizer.
Footnotes
The remaining authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Alhadeff AL, Rupprecht LE, Hayes MR (2012). GLP-1 neurons in the nucleus of the solitary tract project directly to the ventral tegmental area and nucleus accumbens to control for food intake. Endocrinology 153(2): 647–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderberg RH, Richard JE, Hansson C, Nissbrandt H, Bergquist F, Skibicka KP (2016). GLP-1 is both anxiogenic and antidepressant; divergent effects of acute and chronic GLP-1 on emotionality. Psychoneuroendocrinology 65: 54–66. [DOI] [PubMed] [Google Scholar]
- Athauda D, Foltynie T (2016). The glucagon-like peptide 1 (GLP) receptor as a therapeutic target in Parkinson’s disease: mechanisms of action. Drug Discov Today 21(5): 802–818. [DOI] [PubMed] [Google Scholar]
- Bae CS, Song J (2017). The Role of Glucagon-Like Peptide 1 (GLP1) in Type 3 Diabetes: GLP-1 Controls Insulin Resistance, Neuroinflammation and Neurogenesis in the Brain. Int J Mol Sci 18(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagdy G, Calogero AE, Murphy DL, Szemeredi K (1989). Serotonin agonists cause parallel activation of the sympathoadrenomedullary system and the hypothalamo-pituitary-adrenocortical axis in conscious rats. Endocrinology 125(5): 2664–2669. [DOI] [PubMed] [Google Scholar]
- Baggio LL, Ussher JR, McLean BA, Cao X, Kabir MG, Mulvihill EE, et al. (2017). The autonomic nervous system and cardiac GLP-1 receptors control heart rate in mice. Molecular metabolism 6(11): 1339–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrera JG, Sandoval DA, D’Alessio DA, Seeley RJ (2011). GLP-1 and energy balance: an integrated model of short-term and long-term control. Nature reviews Endocrinology 7(9): 507–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger M, Gray JA, Roth BL (2009). The expanded biology of serotonin. Annual review of medicine 60: 355–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar S, Dallman M (1998). Neuroanatomical basis for facilitation of hypothalamic-pituitary-adrenal responses to a novel stressor after chronic stress. Neuroscience 84(4): 1025–1039. [DOI] [PubMed] [Google Scholar]
- Bundzikova-Osacka J, Ghosal S, Packard BA, Ulrich-Lai YM, Herman JP (2015). Role of nucleus of the solitary tract noradrenergic neurons in post-stress cardiovascular and hormonal control in male rats. Stress 18(2): 221–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabou C, Campistron G, Marsollier N, Leloup C, Cruciani-Guglielmacci C, Penicaud L, et al. (2008). Brain glucagon-like peptide-1 regulates arterial blood flow, heart rate, and insulin sensitivity. Diabetes 57(10): 2577–2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr GV, Lucki I (2011). The role of serotonin receptor subtypes in treating depression: a review of animal studies. Psychopharmacology (Berl) 213(2–3): 265–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleck JN, Blendy JA (2008). Making a bad thing worse: adverse effects of stress on drug addiction. J Clin Invest 118(2): 454–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cork SC, Richards JE, Holt MK, Gribble FM, Reimann F, Trapp S (2015). Distribution and characterisation of Glucagon-like peptide-1 receptor expressing cells in the mouse brain. Molecular metabolism 4(10): 718–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies BT, Parker LA (1990). Novel versus familiar ethanol: a comparison of aversive and rewarding properties. Alcohol 7(6): 523–529. [DOI] [PubMed] [Google Scholar]
- De Jonghe BC, Holland RA, Olivos DR, Rupprecht LE, Kanoski SE, Hayes MR (2016). Hindbrain GLP-1 receptor mediation of cisplatin-induced anorexia and nausea. Physiology & behavior 153: 109–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson SL, Shirazi RH, Hansson C, Bergquist F, Nissbrandt H, Skibicka KP (2012). The glucagon-like peptide 1 (GLP-1) analogue, exendin-4, decreases the rewarding value of food: a new role for mesolimbic GLP-1 receptors. The Journal of neuroscience : the official journal of the Society for Neuroscience 32(14): 4812–4820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Varlinskaya EI, Spear LP (2009). Social and non-social anxiety in adolescent and adult rats after repeated restraint. Physiology & behavior 97(3–4): 484–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosal S, Myers B, Herman JP (2013). Role of central glucagon-like peptide-1 in stress regulation. Physiology & behavior 122: 201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil-Lozano M, Pérez-Tilve D, Alvarez-Crespo M, Martís A, Fernandez AM, Catalina PA, et al. (2010). GLP-1(7–36)-amide and Exendin-4 stimulate the HPA axis in rodents and humans. Endocrinology 151(6): 2629–2640. [DOI] [PubMed] [Google Scholar]
- Grieco M, Giorgi A, Gentile MC, d’Erme M, Morano S, Maras B, et al. (2019). Glucagon-Like Peptide-1: A Focus on Neurodegenerative Diseases. Frontiers in neuroscience 13: 1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill HJ, Carmody JS, Amanda Sadacca L, Williams DL, Kaplan JM (2004). Attenuation of lipopolysaccharide anorexia by antagonism of caudal brain stem but not forebrain GLP-1-R. Am J Physiol Regul Integr Comp Physiol 287(5): R1190–1193. [DOI] [PubMed] [Google Scholar]
- Gülpinar MA, Yegen BC (2004). The physiology of learning and memory: role of peptides and stress. Curr Protein Pept Sci 5(6): 457–473. [DOI] [PubMed] [Google Scholar]
- Hare BD, Beierle JA, Toufexis DJ, Hammack SE, Falls WA (2014). Exercise-associated changes in the corticosterone response to acute restraint stress: evidence for increased adrenal sensitivity and reduced corticosterone response duration. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 39(5): 1262–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Härfstrand A, Fuxe K, Cintra A, Agnati LF, Zini I, Wikström AC, et al. (1986). Glucocorticoid receptor immunoreactivity in monoaminergic neurons of rat brain. Proc Natl Acad Sci U S A 83(24): 9779–9783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris RBS (2017). Low-dose leptin infusion in the fourth ventricle of rats enhances the response to third-ventricle leptin injection. American journal of physiology Endocrinology and metabolism 313(2): E134–E147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes MR, Leichner TM, Zhao S, Lee GS, Chowansky A, Zimmer D, et al. (2011). Intracellular signals mediating the food intake-suppressive effects of hindbrain glucagon-like peptide-1 receptor activation. Cell metabolism 13(3): 320–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes MR, Schmidt HD (2016). GLP-1 influences food and drug reward. Curr Opin Behav Sci 9: 66–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisler LK, Pronchuk N, Nonogaki K, Zhou L, Raber J, Tung L, et al. (2007). Serotonin activates the hypothalamic-pituitary-adrenal axis via serotonin 2C receptor stimulation. The Journal of neuroscience : the official journal of the Society for Neuroscience 27(26): 6956–6964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP (1993). Regulation of adrenocorticosteroid receptor mRNA expression in the central nervous system. Cell Mol Neurobiol 13(4): 349–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP (2018). Regulation of Hypothalamo-Pituitary-Adrenocortical Responses to Stressors by the Nucleus of the Solitary Tract/Dorsal Vagal Complex. Cell Mol Neurobiol 38(1): 25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE (1997). Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci 20(2): 78–84. [DOI] [PubMed] [Google Scholar]
- Hernandez NS, Ige KY, Mietlicki-Baase EG, Molina-Castro GC, Turner CA, Hayes MR, et al. (2018). Glucagon-like peptide-1 receptor activation in the ventral tegmental area attenuates cocaine seeking in rats. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 43(10): 2000–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez NS, Schmidt HD (2019). Central GLP-1 receptors: Novel molecular targets for cocaine use disorder. Physiology & behavior 206: 93–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt MK, Llewellyn-Smith IJ, Reimann F, Gribble FM, Trapp S (2017). Serotonergic modulation of the activity of GLP-1 producing neurons in the nucleus of the solitary tract in mouse. Molecular metabolism 6(8): 909–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt MK, Trapp S (2016). The physiological role of the brain GLP-1 system in stress. Cogent Biol 2(1): 1229086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung J-P (2012). Chapter 11 - Raphe Nuclei. In: Mai JK, Paxinos G (eds). The Human Nervous System (Third Edition). Academic Press: San Diego, pp 401–424. [Google Scholar]
- Hornung JP (2003). The human raphe nuclei and the serotonergic system. Journal of chemical neuroanatomy 26(4): 331–343. [DOI] [PubMed] [Google Scholar]
- Hosford PS, Millar J, Ramage AG (2015). Cardiovascular afferents cause the release of 5-HT in the nucleus tractus solitarii; this release is regulated by the low- (PMAT) not the high-affinity transporter (SERT). J Physiol 593(7): 1715–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs JJ (1978). Effect of Lithium Chloride on Adrenocortical Function in the Rat. Proceedings of the Society for Experimental Biology and Medicine 157(2): 163–167. [DOI] [PubMed] [Google Scholar]
- Jahng JW, Lee JH (2015). Activation of the hypothalamic-pituitary-adrenal axis in lithium-induced conditioned taste aversion learning. Eur J Pharmacol 768: 182–188. [DOI] [PubMed] [Google Scholar]
- Kanoski SE, Rupprecht LE, Fortin SM, De Jonghe BC, Hayes MR (2012). The role of nausea in food intake and body weight suppression by peripheral GLP-1 receptor agonists, exendin-4 and liraglutide. Neuropharmacology 62(5–6): 1916–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinzig KP, D’Alessio DA, Herman JP, Sakai RR, Vahl TP, Figueiredo HF, et al. (2003). CNS glucagon-like peptide-1 receptors mediate endocrine and anxiety responses to interoceptive and psychogenic stressors. The Journal of neuroscience : the official journal of the Society for Neuroscience 23(15): 6163–6170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinzig KP, Hargrave SL, Honors MA (2008). Binge-type eating attenuates corticosterone and hypophagic responses to restraint stress. Physiology & behavior 95(1–2): 108–113. [DOI] [PubMed] [Google Scholar]
- Koehnle TJ, Rinaman L (2010). Early experience alters limbic forebrain Fos responses to a stressful interoceptive stimulus in young adult rats. Physiology & behavior 100(2): 105–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kormos V, Gaszner B (2013). Role of neuropeptides in anxiety, stress, and depression: from animals to humans. Neuropeptides 47(6): 401–419. [DOI] [PubMed] [Google Scholar]
- Lachey JL, D’Alessio DA, Rinaman L, Elmquist JK, Drucker DJ, Seeley RJ (2005). The role of central glucagon-like peptide-1 in mediating the effects of visceral illness: differential effects in rats and mice. Endocrinology 146(1): 458–462. [DOI] [PubMed] [Google Scholar]
- Larsen PJ, Tang-Christensen M, Holst JJ, Orskov C (1997). Distribution of glucagon-like peptide-1 and other preproglucagon-derived peptides in the rat hypothalamus and brainstem. Neuroscience 77(1): 257–270. [DOI] [PubMed] [Google Scholar]
- Lee YS, Jun HS (2016). Anti-Inflammatory Effects of GLP-1-Based Therapies beyond Glucose Control. Mediators Inflamm 2016: 3094642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon RM, Borner T, Reiner DJ, Stein LM, Lhamo R, De Jonghe BC, et al. (2019). Hypophagia induced by hindbrain serotonin is mediated through central GLP-1 signaling and involves 5-HT2C and 5-HT3 receptor activation. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 44(10): 1742–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limebeer CL, Rock EM, Sharkey KA, Parker LA (2018). Nausea-Induced 5-HT Release in the Interoceptive Insular Cortex and Regulation by Monoacylglycerol Lipase (MAGL) Inhibition and Cannabidiol. eNeuro 5(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovick TA (1997). The medullary raphe nuclei: a system for integration and gain control in autonomic and somatomotor responsiveness? Exp Physiol 82(1): 31–41. [DOI] [PubMed] [Google Scholar]
- Manetti L, Cavagnini F, Martino E, Ambrogio A (2014). Effects of cocaine on the hypothalamic-pituitary-adrenal axis. J Endocrinol Invest 37(8): 701–708. [DOI] [PubMed] [Google Scholar]
- Maniscalco JW, Kreisler AD, Rinaman L (2012). Satiation and stress-induced hypophagia: examining the role of hindbrain neurons expressing prolactin-releasing Peptide or glucagon-like Peptide 1. Frontiers in neuroscience 6: 199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniscalco JW, Rinaman L (2017). Interoceptive modulation of neuroendocrine, emotional, and hypophagic responses to stress. Physiology & behavior 176: 195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniscalco JW, Zheng H, Gordon PJ, Rinaman L (2015). Negative Energy Balance Blocks Neural and Behavioral Responses to Acute Stress by “Silencing” Central Glucagon-Like Peptide 1 Signaling in Rats. The Journal of neuroscience : the official journal of the Society for Neuroscience 35(30): 10701–10714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui A, Alvarez VA (2018). Cocaine Inhibition of Synaptic Transmission in the Ventral Pallidum Is Pathway-Specific and Mediated by Serotonin. Cell Rep 23(13): 3852–3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann MJ, Verbalis JG, Stricker EM (1989). LiCl and CCK inhibit gastric emptying and feeding and stimulate OT secretion in rats. Am J Physiol 256(2 Pt 2): R463–468. [DOI] [PubMed] [Google Scholar]
- McIntyre DC, Kent P, Hayley S, Merali Z, Anisman H (1999). Influence of psychogenic and neurogenic stressors on neuroendocrine and central monoamine activity in fast and slow kindling rats. Brain research 840(1): 65–74. [DOI] [PubMed] [Google Scholar]
- Mikkelsen JD, Hay-Schmidt A, Kiss A (2004). Serotonergic stimulation of the rat hypothalamo-pituitary-adrenal axis: interaction between 5-HT1A and 5-HT2A receptors. Ann N Y Acad Sci 1018: 65–70. [DOI] [PubMed] [Google Scholar]
- Mo B, Feng N, Renner K, Forster G (2008). Restraint stress increases serotonin release in the central nucleus of the amygdala via activation of corticotropin-releasing factor receptors. Brain research bulletin 76(5): 493–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammad G, Chowdhury I, Fujioka T, Nakamura S (2000). Induction and adaptation of Fos expression in the rat brain by two types of acute restraint stress. Brain Research Bulletin 52(3): 171–182. [DOI] [PubMed] [Google Scholar]
- Müller TD, Finan B, Bloom SR, D’Alessio D, Drucker DJ, Flatt PR, et al. (2019). Glucagon-like peptide 1 (GLP-1). Molecular metabolism 30: 72–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murnane KS (2019). Serotonin 2A receptors are a stress response system: implications for post-traumatic stress disorder. Behav Pharmacol 30(2 and 3-Spec Issue): 151–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassar A, Azab AN (2014). Effects of lithium on inflammation. ACS Chem Neurosci 5(6): 451–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble EE, Hahn JD, Konanur VR, Hsu TM, Page SJ, Cortella AM, et al. (2018). Control of Feeding Behavior by Cerebral Ventricular Volume Transmission of Melanin-Concentrating Hormone. Cell metabolism 28(1): 55–68.e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt D (2015). 5HT2a Receptors – a New Target for Depression? European Psychiatry 30: 35. [Google Scholar]
- Parker LA (1993). Taste reactivity responses elicited by cocaine-, phencyclidine-, and methamphetamine-paired sucrose solutions. Behav Neurosci 107(1): 118–129. [DOI] [PubMed] [Google Scholar]
- Pollock JD, Rowland N (1981). Peripherally administered serotonin decreases food intake in rats. Pharmacology, biochemistry, and behavior 15(2): 179–183. [DOI] [PubMed] [Google Scholar]
- Que Q, Guo X, Zhan L, Chen S, Zhang Z, Ni X, et al. (2019). The GLP-1 agonist, liraglutide, ameliorates inflammation through the activation of the PKA/CREB pathway in a rat model of knee osteoarthritis. J Inflamm (Lond) 16: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabasa C, Dickson SL (2016). Impact of stress on metabolism and energy balance. Current Opinion in Behavioral Sciences 9: 71–77. [Google Scholar]
- Razzoli M, McCallum J, Gurney A, Engeland WC, Bartolomucci A (2015). Chronic stress aggravates glucose intolerance in leptin receptor-deficient (db/db) mice. Genes Nutr 10(3): 458–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaman L (1999a). A functional role for central glucagon-like peptide-1 receptors in lithium chloride-induced anorexia. Am J Physiol 277(5 Pt 2): R1537–1540. [DOI] [PubMed] [Google Scholar]
- Rinaman L (1999b). Interoceptive stress activates glucagon-like peptide-1 neurons that project to the hypothalamus. Am J Physiol 277(2 Pt 2): R582–590. [DOI] [PubMed] [Google Scholar]
- Rock EM, Parker LA (2013). Effect of low doses of cannabidiolic acid and ondansetron on LiCl-induced conditioned gaping (a model of nausea-induced behaviour) in rats. British journal of pharmacology 169(3): 685–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roubos EW, Dahmen M, Kozicz T, Xu L (2012). Leptin and the hypothalamo-pituitary-adrenal stress axis. Gen Comp Endocrinol 177(1): 28–36. [DOI] [PubMed] [Google Scholar]
- Saha S (2005). Role of the central nucleus of the amygdala in the control of blood pressure: descending pathways to medullary cardiovascular nuclei. Clin Exp Pharmacol Physiol 32(5–6): 450–456. [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Mietlicki-Baase EG, Ige KY, Maurer JJ, Reiner DJ, Zimmer DJ, et al. (2016). Glucagon-Like Peptide-1 Receptor Activation in the Ventral Tegmental Area Decreases the Reinforcing Efficacy of Cocaine. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 41(7): 1917–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley RJ, Blake K, Rushing PA, Benoit S, Eng J, Woods SC, et al. (2000). The role of CNS glucagon-like peptide-1 (7–36) amide receptors in mediating the visceral illness effects of lithium chloride. The Journal of neuroscience : the official journal of the Society for Neuroscience 20(4): 1616–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheard MH, Aghajanian GK (1970). Neuronally activated metabolism of brain serotonin: effect of lithium. Life Sci 9(5): 285–290. [DOI] [PubMed] [Google Scholar]
- Suarez AN, Hsu TM, Liu CM, Noble EE, Cortella AM, Nakamoto EM, et al. (2018). Gut vagal sensory signaling regulates hippocampus function through multi-order pathways. Nat Commun 9(1): 2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara M, Hashimoto K, Hattori T, Takao T, Suemaru S, Ota Z (1988). Effects of lithium on the hypothalamo-pituitary-adrenal axis. Endocrinol Jpn 35(5): 655–663. [DOI] [PubMed] [Google Scholar]
- Tafet GE, Nemeroff CB (2016). The Links Between Stress and Depression: Psychoneuroendocrinological, Genetic, and Environmental Interactions. J Neuropsychiatry Clin Neurosci 28(2): 77–88. [DOI] [PubMed] [Google Scholar]
- Tauchi M, Zhang R, D’Alessio DA, Seeley RJ, Herman JP (2008). Role of central glucagon-like peptide-1 in hypothalamo-pituitary-adrenocortical facilitation following chronic stress. Exp Neurol 210(2): 458–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrill SJ, Holt MK, Maske CB, Abrams N, Reimann F, Trapp S, et al. (2019). Endogenous GLP-1 in lateral septum promotes satiety and suppresses motivation for food in mice. Physiology & behavior 206: 191–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrill SJ, Maske CB, Williams DL (2018). Endogenous GLP-1 in lateral septum contributes to stress-induced hypophagia. Physiology & behavior 192: 17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thor KB, Helke CJ (1989). Serotonin and substance P colocalization in medullary projections to the nucleus tractus solitarius: dual-colour immunohistochemistry combined with retrograde tracing. Journal of chemical neuroanatomy 2(3): 139–148. [PubMed] [Google Scholar]
- Trapp S, Cork SC (2015). PPG neurons of the lower brain stem and their role in brain GLP-1 receptor activation. Am J Physiol Regul Integr Comp Physiol 309(8): R795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turenne SD, Miles C, Parker LA, Siegel S (1996). Individual differences in reactivity to the rewarding/aversive properties of drugs: assessment by taste and place conditioning. Pharmacology, biochemistry, and behavior 53(3): 511–516. [DOI] [PubMed] [Google Scholar]
- Ulhoa MA, da Silva NF, Pires JG, Futuro Neto Hde A (2013). Raphe obscurus neurons participate in thermoregulation in rats. Arq Neuropsiquiatr 71(4): 249–253. [DOI] [PubMed] [Google Scholar]
- Vahl TP, Ulrich-Lai YM, Ostrander MM, Dolgas CM, Elfers EE, Seeley RJ, et al. (2005). Comparative analysis of ACTH and corticosterone sampling methods in rats. American journal of physiology Endocrinology and metabolism 289(5): E823–828. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Lee CE, Marcus JN, Williams TD, Overton JM, Lopez ME, et al. (2002). Glucagon-like peptide-1 receptor stimulation increases blood pressure and heart rate and activates autonomic regulatory neurons. J Clin Invest 110(1): 43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Reiner DJ, Hayes MR, Rinaman L (2019). Chronic Suppression of Glucagon-Like Peptide-1 Receptor (GLP1R) mRNA Translation in the Rat Bed Nucleus of the Stria Terminalis Reduces Anxiety-Like Behavior and Stress-Induced Hypophagia, But Prolongs Stress-Induced Elevation of Plasma Corticosterone. The Journal of Neuroscience 39(14): 2649–2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zingg B, Chou XL, Zhang ZG, Mesik L, Liang F, Tao HW, et al. (2017). AAV-Mediated Anterograde Transsynaptic Tagging: Mapping Corticocollicular Input-Defined Neural Pathways for Defense Behaviors. Neuron 93(1): 33–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Zoomed-out images from Figure 1 to help visualize c-Fos expression within an entire hemisphere of the NTS in each of the main treatment groups. Changes in PPG and c-Fos colocalization in the NTS following treatment with LiCl (a), Coc (b), or RES (c).
Animals received a combo pre-treatment (4V) of RS1 (40μg) and OND (25μg) followed RES (30min). The combo treatment caused an attenuation in co-localization between c-Fos and GLP-1 immunoreactive cells (a). Representative images of co-localization between c-Fos (green) and GLP-1 (red) immunoreactivity for the main treatment groups (b). All data expressed as mean ± SEM and was analyzed using a one-way ANOVA. Means with different letters are significantly different from each other (p<0.05) according to Tukey post hoc analyses.
Table 1. Number and distribution of PPG and/or c-Fos immunopositive neurons in the rat NTS following pre-treatment with either OND or RS1 and exposure to either LiCl, Coc, or RES.
Zoomed-out images from Figure 3 to help visualize c-Fos expression within an entire hemisphere of the NTS in each of the main treatment groups. Changes in 5-HT and c-Fos colocalization in the caudal raphe and its subniclei following treatment with LiCl (a), Coc (b), or RES (c). Total CR = RPa + ROb + RMg.
Table 2. Number and distribution of 5-HT and/or c-Fos immunopositive neurons in the rat CR following exposure to either LiCl, Coc, or RES.
Diagram illustrating representative injection sites for all 4 animals in which CTB immunoreactivity localized exclusively within the RMg. 4V = 4th ventricle; RMg = Raphe Magnus.
IHC was conducted on hindbrain NTS sections using a 5-HT antibody at three different rostral-caudal planes of the NTS, AP-level (a), Obex-level (b), and Post-Obex (c). Representative images of each rostral-caudal plane were taken at 10X (top) and 20X (bottom) magnifications. Representative images (a-c) show expression of 5-HT fibers (green) among the three rostral-caudal planes that were analyzed. 5-HT fibers were most densely aggregated in the NTS in all three rostral-caudal planes.