People with obesity have an increased risk of severe COVID-19: a meta-analysis by Popkin and colleagues1 found that the odds ratio of people with obesity being hospitalised with COVID-19 was 2·13 when compared with those without obesity, and mortality was 48% higher in patients with obesity than in those without. This increased risk of severe disease is linked to higher rates of metabolic and cardiovascular complications.2 Another major contributing factor is the presence of substantial immune dysregulation and chronic systemic inflammation. Obesity is associated with increased levels of numerous inflammatory mediators, including interleukin (IL)-1, IL-6, IL-17, and tumour necrosis factor α.3 These cytokines are also implicated in the pathogenesis of COVID-19.4 In addition to inflammation, obesity is associated with important defects in immune cells tasked with host protection, including natural killer cells and mucosal associated invariant T (MAIT) cells.5, 6
Several publications have highlighted MAIT cells as potentially having a crucial role in the host response to SARS-CoV-2.7, 8, 9, 10 In each of these studies, reduced peripheral serum MAIT-cell frequencies were observed in a COVID-19 severity-dependent manner (ie, with lower frequency associated with more severe COVID-19). Conversely, increased numbers of MAIT cells were noted in the lungs of patients with COVID-19 together with higher expression of MAIT-cell chemoattractants,7, 8 and increased levels of activated MAIT cells producing granzyme B were noted in patients with COVID-19.9, 10 Furthermore, importantly, after co-culturing MAIT cells with SARS-CoV-2-infected macrophages, increased activity of the MAIT cells producing granzyme B was observed, suggesting a possible ability of MAIT cells to respond to or directly kill infected cells.9, 10
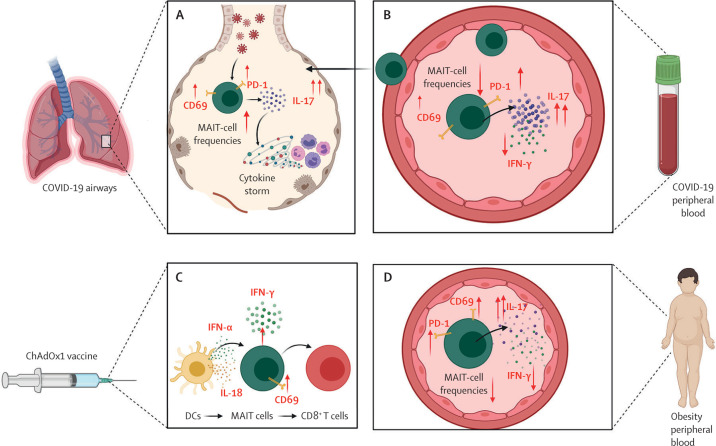
A striking observation across these studies is the COVID-19 severity-dependent increase in the activation marker CD69.7, 9, 10 MAIT-cell activation (via CD69) was associated with prolonged hospitalisation, reduced PaO2/FiO2 ratio, and increased Simplified Acute Physiology Score (SAPS II), a measure of mortality risk for patients in the intensive care unit. These associations with COVID-19 severity might be due to altered MAIT-cell activity driving a proinflammatory response in patients with COVID-19. Pulmonary MAIT cells increase their expression of IL-17A,7, 9 a cytokine implicated in the pathogenesis of COVID-19 and the development of acute respiratory distress syndrome (ARDS; figure A, B ).11, 12 Moreover, analysis of an alveolar lavage single-cell dataset by Parrot and colleagues9 showed that MAIT cells were the predominant T-cell source of IL-17A.8
Figure.
Role of MAIT cells in COVID-19 and obesity
MAIT cells in (A) the lungs and (B) the peripheral blood of patients with COVID-19. (C) MAIT-cell response to adenovirus-vectored SARS-CoV-2 vaccine. (D) MAIT cells in the peripheral blood of people with obesity. CD=cluster of differentiation. DC=dendritic cell. IFN=interferon. IL=interleukin. MAIT cell=mucosal associated invariant T cell. PD-1=programmed cell death protein 1.
A study by Provine and colleagues13 highlighted MAIT cells as an important mediator for adenovirus-vectored vaccine immunogenicity to COVID-19. The authors found that, after administration of the ChAdOx1 nCoV-19 vaccine, MAIT cells in both mice and humans had increased levels of the activation marker CD69. These ChAdOx1-activated MAIT cells also produced increased levels of the antiviral molecules interferon (IFN)-γ and granzyme B (figure C). Furthermore, MAIT cells, in response to IFN-α and IL-18, were shown to support vaccine-induced CD8+ T-cell immunity, providing an important link between innate and adaptive immunity.13
These studies highlight a potentially crucial role for MAIT cells in the pathogenesis of COVID-19. In addition, the COVID-19-associated alterations in MAIT cells closely reflect those changes in MAIT cells observed in people with obesity.6, 14, 15 We and others have reported reduced MAIT-cell frequencies and an activated phenotype (increased CD69 and PD-1 expression) in people with obesity.6 MAIT cells from people with obesity also display a loss of IFN-γ, a cytokine that is key for antiviral responses, and elevated levels of the inflammatory cytokine IL-17, an established driver of ARDS (figure D).6, 15 We propose two open questions. First, could the poor outcome in people with obesity and COVID-19 be the result of a second hit from SARS-CoV-2 on the already compromised, proinflammatory MAIT-cell population? Second, could the obesity-related defects in MAIT cells affect the immune bridge proposed by Provine and colleagues and lead to diminished vaccine efficacy? In addressing these questions, clinicians and scientists will need to consider the contribution of this novel population of T cells to the prevention, pathogenesis, and treatment of COVID-19. Only then will we be able to harness fully the potential of MAIT cells or their cytokine products as targets for modifying the course of disease caused by SARS-CoV-2.
We declare no competing interests. DOS and AEH are funded by the Health Research Board, Ireland. AEH is funded by the National Children's Research Centre, Ireland.
References
- 1.Popkin BM, Du S, Green WD, et al. Individuals with obesity and COVID-19: a global perspective on the epidemiology and biological relationships. Obes Rev. 2020;21 doi: 10.1111/obr.13128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO Obesity and overweight. April 1, 2020. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight
- 3.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 4.Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20:363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O'Shea D, Hogan AE. Dysregulation of natural killer cells in obesity. Cancers (Basel) 2019;11:e573. doi: 10.3390/cancers11040573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brien AO, Kedia-Mehta N, Tobin L, et al. Targeting mitochondrial dysfunction in MAIT cells limits IL-17 production in obesity. Cell Mol Immunol. 2020;17:1193–1195. doi: 10.1038/s41423-020-0375-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jouan Y, Guillon A, Gonzalez L, et al. Phenotypical and functional alteration of unconventional T cells in severe COVID-19 patients. J Exp Med. 2020;217 doi: 10.1084/jem.20200872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chua RL, Lukassen S, Trump S, et al. COVID-19 severity correlates with airway epithelium–immune cell interactions identified by single-cell analysis. Nat Biotechnol. 2020;38:970–979. doi: 10.1038/s41587-020-0602-4. [DOI] [PubMed] [Google Scholar]
- 9.Parrot T, Gorin J-B, Ponzetta A, et al. MAIT cell activation and dynamics associated with COVID-19 disease severity. Sci Immunol. 2020;5 doi: 10.1126/sciimmunol.abe1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flament H, Rouland M, Beaudoin L, et al. Outcome of SARS-CoV-2 infection linked to MAIT cell activation and cytotoxicity: evidence for an IL-18 dependent mechanism. medRxiv. 2020 doi: 10.1101/2020.08.31.20185082. published online Sept 2. (preprint). [DOI] [PubMed] [Google Scholar]
- 11.Wu D, Yang XO. TH17 responses in cytokine storm of COVID-19: an emerging target of JAK2 inhibitor fedratinib. J Microbiol Immunol Infect. 2020;53:368–370. doi: 10.1016/j.jmii.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muir R, Osbourn M, Dubois AV, et al. Innate lymphoid cells are the predominant source of IL-17A during the early pathogenesis of acute respiratory distress syndrome. Am J Respir Crit Care Med. 2016;193:407–416. doi: 10.1164/rccm.201410-1782OC. [DOI] [PubMed] [Google Scholar]
- 13.Provine NM, Amini A, Garner LC, et al. MAIT cell activation augments adenovirus vector vaccine immunogenicity. Science. 2021;371:521–526. doi: 10.1126/science.aax8819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carolan E, Tobin LM, Mangan BA, et al. Altered distribution and increased IL-17 production by mucosal-associated invariant T cells in adult and childhood obesity. J Immunol. 2015;194:5775–5780. doi: 10.4049/jimmunol.1402945. [DOI] [PubMed] [Google Scholar]
- 15.O'Brien A, Loftus RM, Pisarska MM, et al. Obesity reduces mTORC1 activity in mucosal-associated invariant T cells, driving defective metabolic and functional responses. J Immunol. 2019;202:3404–3411. doi: 10.4049/jimmunol.1801600. [DOI] [PubMed] [Google Scholar]