Abstract
Background:
High-sensitivity cardiac troponin (hs-cTn) assays have different analytic characteristics.
Objectives:
The goal of this study was to quantify differences between assays for common analytical benchmarks and to determine whether they may result in differences in the management of patients with suspected acute coronary syndrome (ACS).
Methods:
We included patients with suspected ACS enrolled in the Rule Out Myocardial Infarction/ Ischemia Using Computer-Assisted Tomography (ROMICAT) I and II trials, with blood samples taken at ED presentation (ROMICAT-I and II) or at two and four hours thereafter (ROMICAT-II). Hs-cTn concentrations were measured using three assays (Roche Diagnostics, Elecsys 2010 platform; Abbott Diagnostics, ARCHITECT i2000SR; Siemens Diagnostics, HsVista). Per blood sample, we determined concordance across analytic benchmarks (<limit of detection [<LOD]/LOD-99th percentile/>99th percentile). Per-patient, we determined concordance of management recommendations (rule-out/observe/rule-in) per the 0/2 hour algorithm, and their association with diagnostic test findings (coronary artery stenosis >50% on coronary CT angiography or inducible ischemia on perfusion imaging) and ACS.
Results:
Among 1,027 samples from 624 patients (52.8 ± 10.0 years, 39.4% female), samples classified as <LOD (56.3% vs 10.4% vs 41.2%; p<0.001), LOD-99th percentile (36.5% vs 83.5% vs 52.6; p<0.001) >99th percentile (7.2% vs 6.0% vs 6.2%) by Roche, Abbott, and Siemens, respectively. 37.4% (n=384/1,027) of blood samples were classified into the same analytical benchmark category, with low concordance across benchmarks (<LOD 11.1%; LOD-99th percentile 29.3%; >99th percentile 43.6%). Serial samples were available in 242 patients (40.1% female; mean age: 52.8±8.0 years). The concordance of management recommendations across assays was 74.8% (n=181/242) considering serial hs-cTn measurements. 19.6–21.1% of patients who were recommended to discharge had positive diagnostic test findings and 2.8–4.3% had ACS at presentation.
Conclusion:
Caregivers should be aware that there are significant differences between hs-cTn assays in stratifying individual samples and patients with intermediate likelihood of ACS according to analytical benchmarks that may result in different management recommendations.
Keywords: High-sensitivity cardiac troponin, High-sensitivity cardiac troponin assays, Acute coronary syndrome, Concordance
CONDENSED ABSTRACT
High-sensitivity cardiac troponin (hs-cTn) assays have known differences in analytic performance. We evaluated the concordance between three hs-cTn assays in patients with intermediate likelihood of ACS enrolled in the ROMICAT I and II trials for analytical benchmarks (<LOD, LOD-99th percentile, and >99th percentile) and management recommendations per the 0/2 hour algorithm. Overall, 37.4% (n=384/1,027) of blood samples were classified into the same analytical benchmark and 74.8% (n=181/242) into the same management recommendations category. Caregivers should be aware that there are significant differences between hs-cTn assays in stratifying individual samples and patients according to analytical benchmarks that may result in different management recommendations.
INTRODUCTION
Cardiac troponin measurement and clinical assessment are the cornerstones of early risk stratification for patients presenting to the emergency department (ED) with suspicion of acute coronary syndrome (ACS). Analytical advancements have led to the development of high-sensitivity cardiac troponin (hs-cTn) assays, which allow the detection of very low levels and small changes in troponin concentration, already within one hour.(1–4) Because even in the absence of ACS most patients have a measurable troponin concentrations, the binary nature of information derived by conventional assays (positive or negative) has evolved more into a continuous measure, requiring more nuanced interpretation.(5)
Several diagnostic algorithms for various hs-cTn assays have been developed for the diagnosis of ACS. These diagnostic protocols may 1) set the threshold for early rule-out after a single blood testing to the limit of detection (LOD), 2) use the 99th percentile cut point to define abnormal troponin values (as defined by the Fourth Universal Definition of Myocardial Infarction [MI](6)) and 3) recommend serial testing at one, two or three hours in patients with measurable troponin below the 99th percentile and recommend management based on the change in troponin concentration.(7,8)
Three assays referred clinically as high sensitivity assays (Roche Diagnostics, Elecsys 2010; Abbott Diagnostics, ARCHITECT i2000SR; Siemens Diagnostics, HsVista) cleared by the Food and Drug Administration (FDA) are now clinically available.(9–11) Diagnostic algorithms, such as the 0/2 hour algorithms (12–15), developed for these three assays acknowledge differences in performance characteristics and recommend assay-specific cut points instead of generally applicable thresholds for clinical decisions.
Factors that contribute to the need for assay specific thresholds are the known differences in the assays’ analytic characteristics e.g. their analytic sensitivities (as per the LOD) and the use of different reference populations to derive each assay’s 99th percentile.(16) This ultimately leads to difficulties in establishing general patient management rules across assays.
While many published studies, including those based on analytical benchmarks or assay specific diagnostic algorithms have reported excellent negative predictive values (99.5 to 99.8%) to rule out and high specificities (95.0 to 99.0%) to rule in MI for the individual assays(12–14,17); it remains unknown whether differences in assay sensitivity and derivation of the 99th percentile affect classification of blood samples into analytic categories, or render different management recommendations. To address these uncertainties, we measured troponin using three hs-cTn assays in patients with suspected ACS in the Rule Out Myocardial Infarction/Ischemia Using Computer Assisted Tomography (ROMICAT) I and II trials.
METHODS
Patient population and study design
We included patients with suspected ACS enrolled in the ROMICAT-I and II trials (18,19) (NCT00990262 and NCT01084239) who were referred to further non-invasive diagnostic testing after inconclusive initial ED triage, defined as negative conventional troponin measurement (<99th upper reference limit) and non-ischemic electrocardiogram (ECG), detailed in the Supplemental Appendix.
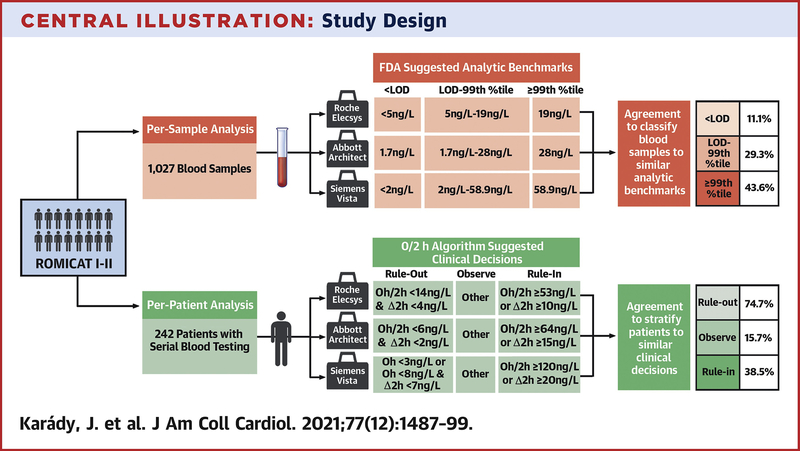
All included patients provided written informed consent, and the studies were approved by the local institutional review board. In both studies, ACS was defined as either MI or UAP and adjudicated by an independent events committee. In this sub-study of the ROMICAT trials, we included patients who consented to blood draw and whose blood samples were analyzed with three hs-cTn assays (Central Illustration).
Central illustration: Study design.
Outline of the per-sample and per-patient analysis to assess the concordance between the three assays. Hs-cTn=High-Sensitivity Cardiac Troponin; LOD=Limit of Detection; %tile=Percentile.
High-sensitivity cardiac troponin measurements
Blood samples
In the ROMICAT-I trial (23) a single blood draw was performed at the time of CT angiography, at a median of 4.2 hours from initial presentation, while in ROMICAT-II (24) sequential blood testing was performed at the time of ED presentation and at two- and four hours thereafter.(20–22) Blood was collected into tubes containing EDTA and immediately centrifuged and stored in microcentrifuge tubes at −80°C until sample assessment. For the analysis serum was used. All blood samples were tested with three assays: Roche Elecsys Cobas Gen 5 assays (ROMICAT-I: e2010, ROMICAT-II: e411; Roche Diagnostics, Penzberg, Germany), Abbott ARCHITECT (Abbott Laboratories, Irving, TX), and a pre-commercial Siemens Vista (Siemens Diagnostics, Newark, DE) (Central Illustration).(9–11) The analytic properties of assays are summarized in Table 1. All blood samples were analyzed in a blinded fashion for clinical information.
Table 1.
Analytic benchmarks of high-sensitivity troponin assays.
| FDA recommended analytic benchmarks (9–11) | %CV at the 99th %tile (49) | Epitopes recognized by antibodies (49) | ||||||
|---|---|---|---|---|---|---|---|---|
| LOD | LOQ | 99th %tile | Sex-specific 99th %tiles | |||||
| 99th %tile of males | 99th %tile of females | Detection | Capture | |||||
| Roche Elecsys, TnT, ng/L | 5 | 6 | 19 | 22 | 14 | <10.0% | 23–29 | 87–91 |
| Abbott ARCHITECT, TnI, ng/L | 1.7 | 3.5 | 28 | 35 | 17 | 4.0% | 41–49 | 24–40 |
| Siemens Vista, TnI, ng/L | 2.0 | 3 | 58.9 | 78.5 | 53.7 | <5.0% | 41–56 | 27–32 |
References used for FDA recommended analytic benchmarks: (6–8); Reference for the %CV at the 99th percentile and Epitopes recognized by antibodies: (9).
AMI=Acute Myocardial Infarction; TnI=Troponon I; TnT=Troponin T. LOD=Limit of detection; LOQ=Limit of quantification; CV=Coefficient of variance; %tile=Percentile.
Analytic benchmarks
We defined analytic benchmarks along assay specific analytic characteristics (table 1): <LOD, LOD to 99th percentile and >99th percentile. Non-sex-specific 99th percentile were used for primary analysis while sex-specific 99th percentiles as recommended by both laboratory guidelines and Fourth Universal Definition of MI were used for secondary analyses.(6,23) In a per-sample analysis, we determined the agreement across assays to classify blood samples obtained in the ROMICAT-I and ROMICAT-II trials, independent of the timing of blood drawn (treated as independent blood samples), according to analytic benchmarks (Central Illustration).
Patient management recommendations
As a primary analysis, we applied 0/2 hour algorithms developed for the evaluated assays (12–14) to determine management recommendation of rule-out or rule-in for MI or observation: 1) rule-out: patients with low likelihood for non-ST segment MI (NSTEMI) with low baseline levels and lack of a relevant increase in serial hs-cTn, 2) rule-in: patients with a high likelihood for NSTEMI with a moderately elevated hs-cTn concentration at presentation or clearly rising hs-cTn concentrations, and 3) observe: any patient who cannot be ruled-out or ruled-in. We determined the agreement across assays to stratify patients with serial blood samples available from the ROMICAT-II trial for management recommendations according to 0/2 hour algorithms defined assay specific thresholds (Central Illustration).
As a secondary analysis, we applied the 99th percentile threshold based on the Fourth Universal Definition of MI(6) to determine the agreement across assays to stratify patients with serial blood samples from the ROMICAT-II trial according to clinical management recommendations.
Non-invasive diagnostic testing
In ROMICAT-II patients with serial blood samples and who underwent coronary CTA or nuclear myocardial stress perfusion imaging (SPECT), we assessed the association of management recommendations with test findings. Coronary CTA was defined as positive if obstructive coronary artery disease (luminal narrowing >50%) was detected; SPECT was defined as positive if stress induced ischemia (reversible myocardial perfusion defect) was detected.
Acute coronary syndrome
Diagnosis of ACS was adjudicated by an external, independent clinical events committee, blinded to the hs-cTn results, based on prospectively collected data on patients’ demographics, cardiovascular risk factors, symptoms and clinical presentation, serial ECG and conventional troponin measurements as well as diagnostic testing results during the index hospital admission.(18,19) ACS was defined as either MI or unstable angina pectoris (UAP) (Supplemental Appendix).
Statistical analysis
Continuous variables are presented as means and standard deviations, while categorical variables as numbers and percentages. Baseline characteristics of subjects from the ROMICAT-I and II trials were compared by using the Fisher exact test, Student T test, or Wilcoxon rank sum test as appropriate.
hs-cTn measurements were classified as <LOD, from LOD to 99th percentile, and >99th percentile for each assay (Central Illustration). We determined the concordance of classification between assays using McNemar’s test (pairwise comparison), Cochran’s Q test (overall comparison between all three assays) and provided Kappa values to indicate the statistical strength of concordance/discordance.
We determined the concordance of patient stratification along patient management recommendations (rule-out/observe/rule-in) based on the 0/2 hour algorithms with assay specific thresholds (Central Illustration). We used McNemar’s test for pairwise comparison and Cochran’s Q test for overall comparison of management recommendations between assays.
Statistical analyses were performed using Stata 14.2 (StataCorp LP). For all analyses, a 2-tailed p value <0.05 was required to reject the null hypothesis.
Results
Study Population
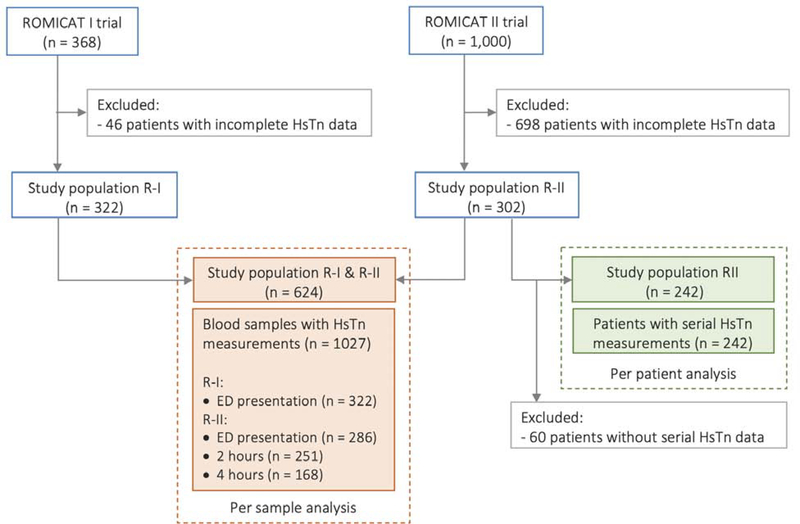
We included 322/368 patients enrolled to the ROMICAT-I trial and 302/1,000 ROMICAT-II patients who contributed altogether 1,027 individual blood samples (608 obtained at arrival, 251 at two hours and 168 at four hours) (Figure 1). Patients were 52.8±10.0 years old, 39.4% were female, most had a low Thrombolysis in MI (TIMI) risk score (TIMI score 0 or 1: 84.8%; n=529/624), and 7.9% (n=49/624) had an adjudicated diagnosis of ACS. Among patients referred to non-invasive testing, 20.5% (n=98/479) had obstructive CAD on coronary CTA and 24.3% (n=46/189) had inducible myocardial ischemia on SPECT (Table 2). The time since chest pain onset was 3.1 hours (IQR:1.5–9.2) in the ROMICAT I population. Characteristics of ROMICAT-I and ROMICAT-II patients did not show clinically meaningful differences in demographics, cardiovascular risk factors, TIMI score, or rate of ACS during index hospitalization. Patients included in this sub-study were not significantly different from those not included (Supplemental Table 1).
Figure 1. Study Population enrollment, exclusion, and inclusion.
The flow chart summarizes the selection of the patient population included in the per-sample and per-patient analyses. ED=emergency department; Hs-cTn=High-sensitivity cardiac troponin; R-I=ROMICAT-I; R-II=ROMICAT-II; ROMICAT=Rule Out Myocardial Infarction/Ischemia Using Computer-Assisted Tomography II.
Table 2.
Demographic data.
| Total (n=624) |
ROMICAT-I (n=322) |
ROMICAT-II (n=302) |
P value | |
|---|---|---|---|---|
| Age, years | 52.8 ± 10.0 | 52.6 ± 11.7 | 52.9 ± 7.8 | 0.70 |
| Female sex, n (%) | 246 (39.4) | 121 (37.6) | 125 (41.4) | 0.37 |
| BMI, kg/m2 | 28.9 ± 5.4 | 28.9 ± 5.9 | 28.9 ± 4.7 | 0.91 |
| Cardiovascular risk factors | ||||
| Hypertension, n (%) | 286 (45.8) | 128 (39.8) | 158 (52.3) | 0.002 |
| Diabetes mellitus, n (%) | 82 (13.1) | 37 (11.5) | 45 (14.9) | 0.24 |
| Dyslipidemia, n (%) | 249 (39.9) | 121 (37.6) | 128 (42.4) | 0.25 |
| Former/current smoker, n (%) | 303 (48.6) | 155 (48.1) | 148 (49.0) | 0.87 |
| Family history of premature CAD, n (%) | 193 (30.9) | 80 (24.8) | 113 (37.4) | 0.001 |
| Number of cardiovascular risk factors, n (%) |
0.003 | |||
| 0–1 | 268 (43.0) | 159 (49.4) | 109 (36.1) | |
| 2–3 | 307 (49.2) | 142 (44.1) | 165 (54.6) | |
| ≥4 | 49 (7.9) | 21 (6.5) | 28 (9.3) | |
| TIMI score, n (%) | <0.001 | |||
| 0 | 342 (54.8) | 154 (47.8) | 188 (62.3) | |
| 1 | 187 (30.0) | 101 (31.4) | 86 (28.5) | |
| 2 | 75 (12.0) | 50 (15.5) | 25 (8.3) | |
| ≥3 | 20 (3.2) | 17 (5.3) | 3 (1.0) | |
| Prior medication | ||||
| Aspirin, n (%) | 171 (27.4) | 103 (32.0) | 68 (22.5) | 0.009 |
| Beta-blocker, n (%) | 127 (20.4) | 75 (23.3) | 52 (17.2) | 0.07 |
| Statin, n (%) | 174 (27.9) | 91 (28.3) | 83 (27.5) | 0.86 |
| Non-invasive diagnostic testing | ||||
| Positive test, n (%) | 125/517 (24.2) | 80/322 (24.8) | 45/195 (23.1) | 0.67 |
| Positive coronary CTA*, n (%) | 98/479 (20.5) | 58/322 (18.0) | 40/157 (25.5) | 0.07 |
| Positive SPECT†, n (%) | 46/189 (24.3) | 38/132 (28.8) | 8/57 (14.0) | 0.041 |
| Clinical events | ||||
| ACS, n (%) | 49 (7.9) | 24 (7.5) | 25 (8.3) | 0.77 |
| AMI, n (%) | 11 (1.8) | 5 (1.6) | 6 (2.0) | 0.77 |
| UAP, n (%) | 38 (6.1) | 19 (5.9) | 19 (6.3) | 0.87 |
Positive coronary CTA: >50% luminal narrowing
Positive SPECT: evidence of stress induced ischemia defined as reversible myocardial perfusion defect.
ACS=Acute coronary syndrome; BMI=Body mass index; CAD=Coronary artery disease; MI=Myocardial infarction; UAP=Unstable angina pectoris.
Agreement between hs-cTn assays in classifying blood samples according to analytic benchmarks
The proportion of samples <LOD and between LOD to 99th percentile was significantly different between all assays (<LOD: 56.3% [n=578/1,027] vs 10.4% [n=107/1,027] vs 41.2% [n=423/1,027]; LOD to 99th percentile: 36.5% [n=375/1,027] vs 83.5% [n=858/1,027] vs 52.6% [n=540/1,027] for Roche Elecsys, Abbott Architect and Siemens Vista, respectively, p<0.001). The proportion of samples classified >99th percentile on the other hand did not differ significantly (7.2% [n=74/1,027] vs 6.0% [n=62/1,027] vs 6.2% [n=64/1,027], p=0.114) (Table 3).
Table 3.
Agreement between assays in classifying blood samples according to analytic benchmarks.
| Analytic benchmarks: | Roche Elecsys n (%) | Abbott Architect n (%) | Siemens Vista n (%) | P values* | |||
|---|---|---|---|---|---|---|---|
| Roche vs. Abbott | Roche vs. Siemens | Abbott vs. Siemens | Overall Comparison | ||||
| < LOD | 578 (56.3) | 107 (10.4) | 423 (41.2) | <0.001 | <0.001 | <0.001 | <0.001 |
| LOD - 99th %tile | 375 (36.5) | 858 (83.5) | 540 (52.6) | <0.001 | <0.001 | <0.001 | <0.001 |
| > 99th %tile | 74 (7.2) | 62 (6.0) | 64 (6.2) | 0.064 | 0.157 | 0.670 | 0.114 |
| Total | 1,027 (100.0) | 1,027 (100.0) | 1,027 (100.0) | N/A | N/A | N/A | N/A |
Indicating the differences between the assays. LOD=Limit of detection; %tile=Percentile.
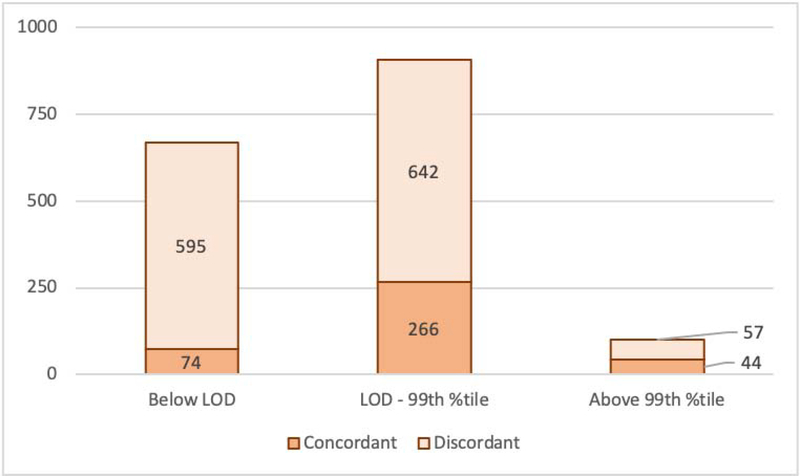
The proportion of samples concordantly classified into the same analytic benchmarks was low with 37.4% (n=384/1,027; Kappa: 0.22). The highest concordance occurred for classification of samples >99th percentile (43.6%, n=44/101; Kappa: 0.70); however, among the 57 discordant blood samples, 9 were classified simultaneously as <LOD by at least one assay. Concordance was lower for LOD to 99th percentile (29.3%, n=266/908; Kappa: 0.15), where 56 discordant cases classified >99th percentile in parallel, while the rest was overlapping with the benchmark of <LOD resulting in a very low concordance for <LOD (11.1%, n=74/669; Kappa: 0.16) (Figure 2). In pairwise comparison, Roche vs Abbott agreed in 47.8% (overall: n=491/1027; Kappa=0.17; <LOD: 15.9%, n=94/591; Kappa=0.12; LOD to 99th percentile: 39.6%, n=350/883; Kappa=0.12; >99th percentile: 52.8%, n=47/89; Kappa=0.67), Roche vs Siemens in 64.0% (overall: n=657/1027; Kappa=0.37; <LOD: 50.8%, n=337/664; Kappa=0.38; LOD to 99th percentile: 43.2%, n=276/639; Kappa=0.30; >99th percentile: 46.8%, n=44/94; Kappa=0.61) and Abbott vs Siemens in 62.2% (overall: n=639/1027; Kappa=0.27; <LOD: 18.3%, n=82/448; Kappa=0.17; LOD to 99th percentile: 56.6%, n=505/893; Kappa=0.22; >99th percentile: 70.3%, n=52/74; Kappa=0.81), respectively (Supplemental Figures 1A–C).
Figure 2.
Agreement between assays in classifying blood samples along analytic benchmarks using non-sex specific 99th percentile. Concordant is defined as agreement between all three assays, anything else is considered as discordant*. LOD=Limit of detection; 99th%tile=99th percentile.*Discordant cases are classified to more than one analytic benchmark, therefore the overall sum of the columns is not equal to the overall sum of studied blood samples (n=1,027), but it is higher because of the redundancy.
When using sex-specific thresholds, significant disagreement was observed between the assays. As stratified by sex, the overall rate of measurable samples was 50.3%, 92.7%, and 65.0% in males and 33.7%, 84.8%, and 49.4% in females for Roche, Abbott and Siemens, respectively. There were significant differences between the proportion of samples <LOD and LOD to 99th percentile; however, for the >99th percentile no differences were identified for males, and significant difference were seen for females (Supplemental Table 2A–C).
When using the 99th percentile as a binary threshold, the proportion of blood samples above the 99th percentile was similar for all assays when non-sex specific 99th percentiles were applied (7.2%, 6.0% and 6.2% per Roche, Abbott and Siemens, respectively) but differed between the Roche vs Siemens (7.4%vs 4.9%, p<0.001) and Abbott vs Siemens (7.0% vs 4.9%, p<0.001) for sex-specific 99th percentiles. Stratified by sex, no differences were identified for males, and significant difference were seen for females (Supplemental Table 3A–D).
Agreement between hs-cTn assays to risk stratify patients according to 0/2 hour algorithms
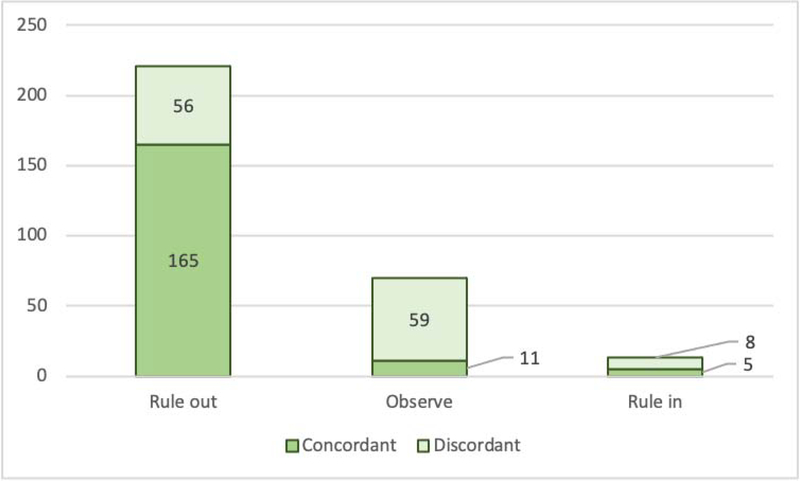
The proportion of patients with similar management recommendation was significantly different between the assays for both rule-out (87.2%, 73.1%, and 78.5%) and observe (9.5%, 24.0%, 17.8%, respectively; p<0.001 for both) recommendations; there was no significant difference for rule-in strata (3.3%, 2.9%, and 3.7%; all p=0.687) (Table 4). All three assays were concordant for management recommendations in 74.8% (n=181/242). When stratified by sex, we observed similar results as in the overall group (Supplemental Table 4A–B). Within the management strata, concordance was 74.7% for rule-out, 15.7% for observe, and 38.5% for admission (Figure 3).
Table 4.
Agreement between assays in stratifying patients based on the 0/2 h algorithm using serial hs-cTn measurements.
| Clinical decision | Roche Elecsys n (%) | Abbott Architect n (%) | Siemens Vista n (%) | P values* | |||
|---|---|---|---|---|---|---|---|
| Roche vs. Abbott | Roche vs. Siemens | Abbott vs. Siemens | Overall comparison | ||||
| Rule-out | 211 (87.2) | 177 (73.1) | 190 (78.5) | <0.001 | <0.001 | 0.007 | <0.001 |
| Observe | 23 (9.5) | 58 (24.0) | 43 (17.8) | <0.001 | 0.002 | 0.004 | <0.001 |
| Rule-in | 8 (3.3) | 7 (2.9) | 9 (3.7) | 0.655 | 0.706 | 0.317 | 0.687 |
| Total | 242 (100.0) | 242 (100.0) | 242 (100.0) | N/A | N/A | N/A | N/A |
Indicating the differences between the assays.
Figure 3.
Agreement between assays in patient management recommendations based on the 0/2 hour algorithm. Concordant is defined as agreement between all three assays, anything else is considered as discordant*. *Discordant cases are stratified to more than one analytic benchmark, therefore the overall sum of the columns is not equal to the overall sum of studied patients (n=242), but it is higher because of the redundancy.
As a secondary analysis, we assessed the agreement between the assays based on the Fourth Universal Definition of MI (99th percentile).(6) Patient risk stratification based on serial testing was similar across the three assays: rate of patients above the 99th percentile was 6.6% (n=16/242) vs 5.4% (n=13/242) vs 5.4% (n=13/242) per Roche, Abbott and Siemens, respectively, p=0.50; using sex-specific 99th percentiles similarly resulted in no differences between the assays (Supplemental Tables 5A–B).
Hs-cTn, non-invasive diagnostic testing, and acute coronary syndrome
Overall, n=148/242 patients received non-invasive testing (n=104/148 CTA, n=29/148 SPECT, n=15/148 CTA and SPECT) (Supplemental Table 6A). Among patients in whom serial hs-cTn suggested rule-out, 21.1% (n=28/133), 19.6% (n=22/112) and 21.0% (n=25/119) for Roche, Abbott and Siemens, respectively, had positive non-invasive testing finding. Among those patients, who were ruled out by at least one assay, 4.3% (n=9/211), 2.8% (n=5/177) and 3.7% (n=7/190) were diagnosed with ACS (MI: 0.5% [1/211], 0.0% [0/177], 0.0% [0/190]; UAP: 3.8% [8/211], 2.8% [5/177)], 3.7% [7/190]). Additionally, in those with a troponin measurement <LOD, 17.7% (n=15/85), 33.3% (n=5/15) and 22.2% (n=12/54) had a positive test finding as tested with Roche, Abbott and Siemens, respectively (Supplemental Table 6B). Among 221 patients in whom at least one assay met the clinical decision of rule-out, 9 patients (4.1%) had ACS (1 NSTEMI, 8 UAP).
Discussion
We provide a head-to-head comparison of three hs-cTn assays cleared by the FDA in patients with intermediate likelihood of ACS who were referred to non-invasive diagnostic testing after inconclusive initial triage. Our data suggest substantial differences between the assays both in terms of stratification into common analytical benchmarks with the potential for differences in management. We report four major findings: 1) on a blood sample basis, the agreement between the three assays based on analytic benchmarks was low at 37.4%; 2) on a per-patient basis, the agreement based on 0/2 hour algorithm was 74.7% for rule-out, 15.7% for observe, and 38.5% for admission for rule-in ACS; 3) assays were concordant to classify samples >99th percentile when the general 99th percentile was applied, but significantly disagreed when sex-specific 99th percentiles were used and 4) obstructive CAD/myocardial ischemia was found in around 20% of patients with serial hs-cTn who had troponin measurements <LOD.
Analytic benchmarks as threshold: Limit of detection
Differences in assay analytic performance, predominantly on the level of sensitivity, have been recognized previously.(24) Our data extend these observations by quantifying the differences for analytic benchmarks, suggesting a two-fold difference between assays in the proportion of samples with measurable values (43.7%, 89.6% and 58.8% samples with measurable troponin for Roche, Abbott and Siemens, respectively, p<0.001). This may highlight e.g. the reduced sensitivity of Roche assay at lower concentrations of analyte with an inability to resolve differences between 3 and 6ng/L and may justify restrictions placed on reporting of this assay by the FDA to only reporting concentrations above the limit of quantification.(25) Notably however, throughout the pairwise comparison we discovered poor agreement between Abbott vs Siemens (<LOD: 18.3%), suggesting that Roche was not the only driver of the observed discordances below the LOD.
99th percentile and Fourth Universal Definition of MI to define ACS
The 99th percentile of hs-cTn is defined as the threshold for myocardial injury by the Fourth Universal Definition of MI(6), with an emphasis on sex-specific differences. However, there are identified concerns regarding the use of the 99th percentile as a general threshold.
One issue is that there is a substantial degree of discordance between assays when their respective 99th percentiles are used to define MI. Ungerer et al. assessing all-comer ED patients, described rates of misclassification ranging between 3–17% across four hs-cTn platforms (Abbott Archiect i2000SR, Beckman Coulter Access2, Roche Cobas e601, and Siemens ADVIA Centaur XP) when their respective 99th percentile was used to diagnose MI.(26) In fact, it has been shown that re-derivation of the 99th percentile permits more concordant diagnosis of AMI between two assays (Abbott Architect i2000R and Roche Cobas 601).(27) In our per-sample analysis, the studied FDA cleared hs-cTn assays were concordant when non-sex specific 99th percentile thresholds were used, as recommended by the FDA, but were discordant when the sex-specific 99th percentiles were applied. This does not exclude the possibility that using sex-specific percentiles improves accuracy for detection of MI for each assay; for example, clinical data suggests that sex-specific thresholds help to overcome the under-diagnosis of MI in women.(28) Nevertheless, according to our results, if the recommended sex-specific thresholds are applied, then different assays might stratify patients substantially differently potentially translating into clinically meaningful discordances between triaging centers, depending on which assay is used. Interestingly, our sex-stratified analysis indicated the discordance stems from differences between the assays among females (supplemental tables 3A–D). One of the possible reasons for this finding could be differences in the representation of men and women in the various reference populations used for each assay, strengthening the argument for a common reference biobank to derive the 99th percentile for assays.(24) A further important consideration is that a >50% analytical sensitivity is recommended for both men and women. A critical difference between the evaluated assays, is that while the Abbott and Siemens assays fulfil this criterion, Roche has a lower sensitivity for men (50%) and fails to detect troponin in over 50% in women.(23) However, this difference in the evaluated platforms’ analytical properties rendered no differences in clinical performance: e.g. altogether 4 men and 1 woman had clinically adjudicated diagnosis of an AMI; of the 4 men all three assays classified 1 individual below the 99th percentile and 3 above the 99th percentile, while the 1 female was classified above the 99th percentile by all three assays – when using both non-sex specific and sex-specific 99th percentiles. Important to note, that because of the relatively low number of events, concluding that these differences in analytical sensitivity do not have an impact on clinical performance is unsubstantiated.
Another concern in using the 99th percentile as a threshold for MI is that for the same assay, different 99th percentile thresholds have been published based on different reference cohorts. According to the vendor’s recommendations and FDA approval document for the Roche Elecsys assay, a cut-off of 19ng/L is provided as 99th percentile, based on data from 1,301 healthy individuals in the US (50.4% female; median 48 years [IQR: 21–89 years]).(29) Using the same assay, in 616 healthy volunteers from Germany (49.8% female; 44±14 years), a 99th percentile of 13.5ng/L was reported.(30) While deriving 99th percentiles from the Dallas Heart Study suggested 18ng/L, the Atherosclerosis Risk in Communities Studies reported 22ng/L, and the Cardiovascular Health Study found 36ng/L. One potential reason for the variation in 99th percentiles is the different representation of genders in the reference populations, which is suggested to be resolved with the use of sex specific thresholds. However, the use of sex-specific cut-off values were shown to reclassify only a small percentage of patients compared to a common cut-off.(31)
To overcome concerns regarding the 99th percentile, a universal sample bank has been created to provide a common reference to define the 99th percentile across all hs-cTn assays.(4,32,33) Whether a single reference cohort is sufficient to ensure generalizability across age, sex, and race, or whether unique 99th percentile definitions are required with multiple reference cohorts, it is still unanswered. Moreover, recommendations specify various details for the derivation of the 99th percentile (e.g. use of non-parametric method, sample size of at least 300 male and 300 female healthy subjects etc.) – although these recommendations exist, the lack of adherence to follow such designs may introduce variation.(23)
0/2 hour algorithm: Rule-out, Rule-in and Observe
Assay-specific cut-off values provided by the 0/2 hour algorithm to rule out an MI have been shown to have excellent sensitivity.(12–14,17) Our study provides a direct comparison of hs-cTn assays in a cohort with suspected ACS referred to further non-invasive testing, suggesting a 74.8% (n=181/242) overall agreement between the three assays. We observed, that across three commercially available hs-cTn assays the rate of patients ruled-out (87.2% vs 73.1%, vs 78.5%) differ significantly.
Applying thresholds proposed by the 0/2 hour algorithm, we found good agreement for rule-in decision across assays (3.3% vs 2.9% vs 3.7%). Throughout the per-patient analysis, two patients who were classified as rule-out by either Siemens or Abbott were classified as rule-in by Roche indicating a still considerable degree of disagreement.
This may translate into substantial differences in patient care between hospitals: e.g. by using a troponin-I assay (Abbott or Siemens) the rate of those who cannot be discharged nor can be admitted is around double compared to Roche (24.0% and 17.8% vs 9.5%), leading to an almost two-fold difference between these assays. It is not surprising thus, that we observed the poorest agreement within observe management strata with 15.7% concordance between all three assays (Figure 3).
Hs-cTn, CAD, myocardial ischemia and ACS
Our results highlight prior findings, that about 20% of patients (70% in agreement by all three assays) stratified to rule-out for ACS had a positive test finding. In our study the majority of patients with ACS in whom ACS was ruled out by at least one assay were diagnosed with UAP (Roche: 1 AMI, 8 UAP; Abbott: 0 AMI, 5 UAP; Siemens: 0 AMI, 7 UAP). Importantly, the 0/2 hour algorithm and 99th percentile were validated to identify MIs. However, because the known short-term (30-day) and long-term prognostic significance of obstructive CAD and inducible myocardial ischemia, it is important to take into account the degree of underlying disease potentially unrecognized via hs-cTn testing. Overall, around 4% (2.8–4.3%) of patients who were ruled-out (<LOD) by the three assays were diagnosed with ACS. This is in contrast to previous publications reporting almost perfect negative predictive value for ACS for these assays (Roche: 99.0%; Abbott: 100.0%; Siemens: 98.9%(34–36)). Notably, these studies differ from our analysis in that we defined ACS as both NSTEMI and UAP, while most of the other studies defined ACS synonymous with MI. The clinical significance of UAP is debated, however it is recognized that patients with UAP have increased risk for future cardiovascular events compared to those without, thus patients with unidentified UAPs could constitute an important subgroup.(37)
Comparison of hs-cTn assays measuring troponin-I vs T
Finally, it could be argued whether comparing troponin-I with T assays is feasible, because of the known differences in the behavior of the two isotypes of troponin molecules.(12) For example troponin-I concentration increases earlier when cardiomyocyte injury occurs (38), while troponin-T concentrations follow diurnal changes but troponin-I does not (39), and in renal failure troponin-T clearance is affected in a greater extent.(40) However, as both the analytic benchmarks and the 0/2 hour thresholds are assay specific, these differences are assumed to be incorporated and reflected by the recommended thresholds. A pairwise comparison between assays on a per-sample level showed that the concordance between troponin-I assays was not higher compared to troponin-I vs troponin-T assays (Abbott vs Siemens: 62.2%; Abbott vs Roche: 47.8%; Siemens vs Roche: 64.0%). These results are in line with previously reported findings demonstrating a discordance rate of 18.2% between the Roche and the Abbott assays to diagnose patients with AMI as assessed among all-comer ED patients.(41) In our per-patient analysis, the all three assays differed significantly for rule-out (Roche: 87.2% vs Abbott: 73.1% vs Siemens: 78.5%, p<0.001), suggesting that the observed discordances are not likely the result of differences in release dynamics between isotypes, but perhaps reflect a threshold-related disagreement.
Patient population
We report variability between hs-cTn assays in a highly selected population eligible for further non-invasive testing with coronary CTA, representing about 10–20% of all comers, and excluding high-risk groups e.g. those with a prior history of CAD, kidney disease, or those with objective signs of ACS.(18) Thus, the ACS rate in this population (between 2–7%; (18,42–44)) is lower than reported in all-comers (ranging from 10–30% (12,31,45,46)) and troponin values tend to be lower as well. Nevertheless, we report substantial discordance of three high-sensitivity troponin assays to risk-stratify patients with suspected ACS, in a particularly challenging subgroup of the ED chest pain population.
Implications and future directions
Our data suggest that in patients with suspected ACS, clinical management based hs-cTn assay-specific thresholds such as the 99th percentile might deviate significantly when using three hs-cTn assays. This might lead to differences in referral to further non-invasive diagnostic testing and detection of underlying CAD. Because the reported discrepancies are not likely to be attributable to differences in diagnostic performance of the studied assays, as these were all reported to have excellent diagnostic properties, it is more probable that differences in reference populations used for derivation of thresholds factored in. It is known, that age and gender distribution of reference populations could impact substantially the upper limit of detection of normal healthy population.(16,33) Therefore, potential solutions include endorsement of thresholds (99th percentile) derived with the use of a standardized reference population for all hs-cTn platforms (24,33) or methods to express likelihood for ACS in a probabilistic fashion.(47,48)
Limitations
This study has limitations. First, our patient cohort does not reflect the entire spectrum of patients presenting with suspicion for ACS to ED’s in the US. However, we included patients at intermediate likelihood of ACS, who were referred to further noninvasive diagnostic testing after inconclusive initial triage (normal conventional troponin-T and non-ischemic ECG). This group represents about 20% of all comers with suspicion of ACS presenting to US ED’s and moreover, poses the highest diagnostic challenge for safe and efficient triage. Second, only patients in ROMICAT-II, but not ROMICAT-I, underwent serial troponin measurements limiting the assessments of guideline-based management recommendation. Third, the 0/2 hour algorithm was developed for the Siemens Centaur platform, but not for the Siemens Dimension Vista. Moreover, the assay we used was a pre-commercial assay and is likely different from the platform that has been commercially implemented. Given that the differences between the two assays are relatively small with the Centaur being a slightly more sensitive assay(49), a small proportion of samples and patients may be reclassified; however, it is unlikely that this would have altered our results substantially in terms of agreement between the three assays. Our data on the Siemens assay, however, warrant replication with the commercially available platform. Fourth, both the 0/2 hour algorithms and the 99th percentile analytic benchmarks were derived and validated to identify MIs, while the majority of ACS in the ROMICAT trials was UAP. It is important to note that this adjudication was based on conventional non-highly sensitive assays and further analyses have shown that about 40% of UAP would have been adjudicated as MI using hs-cTn. (29). Nevertheless, this constitutes a limitation of the data.
Conclusion
Caregivers should be aware of the substantial discordance between commercially available hs-cTn assays in stratifying patients with intermediate likelihood of ACS according to standard analytical benchmarks that may result in different management recommendations.
Supplementary Material
CLINICAL PERSPECTIVES.
Competency in Patient Care:
Comparison of three commercially available high-sensitivity blood troponin (hs-cTn) assays in patients with suspected acute coronary syndromes (ACS) found up to 2-fold differences in results that could influence the proportion of patients referred for further observation or investigation.
Translational Outlook:
Additional work is necessary to create a universal sample bank encompassing all hs-cTn assays and standardize thresholds to guide triage of patients with suspected ACS.
Acknowledgments
DISCLOSURE
The content of this manuscript is solely the responsibility of the authors and does not necessarily reflect the views of the National Heart, Lung, and Blood Institute, the National Institutes of Health, or the United States Department of Health and Human Services.
Dr. Karády has received grant support from the Fulbright Visiting Researcher Grant (E0583118); and the Rosztoczy Foundation.
Dr. Ferencik was supported by the grant from the American Heart Association (Fellow to Faculty Award #13FTF16450001).
Dr. Peacock has received grant support from Abbott, Boehringer Ingelheim, Braincheck, CSL Behring, Daiichi-Sankyo, Immunarray, Janssen, Ortho Clinical Diagnostics, Portola, Relypsa, Roche, Salix, and Siemens; consulting income from Abbott, Astra-Zeneca, Bayer, Beckman, Boehrhinger-Ingelheim, Ischemia Care, Dx, Immunarray, Instrument Labs, Janssen, Nabriva, Ortho Clinical Diagnostics, Relypsa, Roche, Quidel, Salix, and Siemens; expert testimony from Johnson and Johnson; and reports stock/ownership interest for AseptiScope Inc, Brainbox Inc, Comprehensive Research Associates LLC, Emergencies in Medicine LLC, Ischemia DX LLC.
Dr. Nagurney has received research funds from Roche Diagnostics, Ortho Diagnostics, and Alere/Biosite to the Massachusetts General Hospital.
Dr. Januzzi is a Trustee of the American College of Cardiology, is a Board member of Imbria Pharmaceuticals, has received grant support from Novartis Pharmaceuticals, Roche Diagnostics, Abbott, Singulex and Prevencio; consulting income from Abbott, Janssen, Novartis, Pfizer, Merck, and Roche Diagnostics, and participates in clinical endpoint committees/data safety monitoring boards for Abbott, AbbVie, Amgen, Boehringer-Ingelheim, Janssen, and Takeda.
Dr. Koenig reports personal fees from AstraZeneca, Novartis, Pfizer, The Medicines Company, DalCor, Kowa, Amgen, Corvidia, Berlin-Chemie, and Sanofi, grants and non-financial support from Beckmann, Singulex, Abbott, and Roche Diagnostics, outside the submitted work.
Dr. Hoffmann reported receiving research support on behalf of his institution from Duke University (Abbott), HeartFlow, Kowa Company Limited, and MedImmune/Astrazeneca; and receiving consulting fees from Duke University (NIH), and Recor Medical unrelated to this research.
The remaining authors have reported nothing to disclose.
ABBREVIATIONS
- ACS
Acute Coronary Syndrome
- AMI
Acute Myocardial Infarction
- CTA
Computed Tomographic Angiography
- ED
Emergency Department
- ECG
Electrocardiogram
- FDA
Food and Drug Administration
- Hs-cTn
High-Sensitivity Cardiac Troponin
- LOD
Limit of Detection
- ROMICAT
Rule Out Myocardial Infarction/Ischemia Using Computer Assisted Tomography
- SPECT
Single Photon Emission Computed Tomography
- TIMI
Thrombolysis in Myocardial Infarction
- UAP
Unstable Angina Pectoris
Footnotes
Clinical Trial Registration: NCT00990262; NCT01084239.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Januzzi JL Jr., Suchindran S, Coles A et al. High-Sensitivity Troponin I and Coronary Computed Tomography in Symptomatic Outpatients With Suspected Coronary Artery Disease: Insights From the PROMISE Trial. JACC Cardiovasc Imaging 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Westermann D, Neumann JT, Sorensen NA, Blankenberg S. High-sensitivity assays for troponin in patients with cardiac disease. Nat Rev Cardiol 2017;14:472–483. [DOI] [PubMed] [Google Scholar]
- 3.Apple FS, Sandoval Y, Jaffe AS, Ordonez-Llanos J, Bio-Markers ITFoCAoC. Cardiac Troponin Assays: Guide to Understanding Analytical Characteristics and Their Impact on Clinical Care. Clin Chem 2017;63:73–81. [DOI] [PubMed] [Google Scholar]
- 4.Apple FS, Collinson PO, Biomarkers ITFoCAoC. Analytical characteristics of high-sensitivity cardiac troponin assays. Clin Chem 2012;58:54–61. [DOI] [PubMed] [Google Scholar]
- 5.Twerenbold R, Jaffe A, Reichlin T, Reiter M, Mueller C. High-sensitive troponin T measurements: what do we gain and what are the challenges? Eur Heart J 2012;33:579–86. [DOI] [PubMed] [Google Scholar]
- 6.Thygesen K, Alpert JS, Jaffe AS et al. Fourth Universal Definition of Myocardial Infarction (2018). J Am Coll Cardiol 2018;72:2231–2264. [DOI] [PubMed] [Google Scholar]
- 7.Twerenbold R, Jaeger C, Rubini Gimenez M et al. Impact of high-sensitivity cardiac troponin on use of coronary angiography, cardiac stress testing, and time to discharge in suspected acute myocardial infarction. Eur Heart J 2016;37:3324–3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pickering JW, Than MP, Cullen L et al. Rapid Rule-out of Acute Myocardial Infarction With a Single High-Sensitivity Cardiac Troponin T Measurement Below the Limit of Detection: A Collaborative Meta-analysis. Ann Intern Med 2017;166:715–724. [DOI] [PubMed] [Google Scholar]
- 9.Review memorandum Elecsys® Troponin T - FDA.
- 10.Review memorandum ARCHITECT STAT High Sensitivity Troponin-I - FDA.
- 11.Review memorandum Dimension Vista® High-Sensitivity Troponin I (TNIH) Assay.
- 12.Reichlin T, Cullen L, Parsonage WA et al. Two-hour algorithm for triage toward rule-out and rule-in of acute myocardial infarction using high-sensitivity cardiac troponin T. Am J Med 2015;128:369–79 e4. [DOI] [PubMed] [Google Scholar]
- 13.Boeddinghaus J, Reichlin T, Cullen L et al. Two-Hour Algorithm for Triage toward Rule-Out and Rule-In of Acute Myocardial Infarction by Use of High-Sensitivity Cardiac Troponin I. Clin Chem 2016;62:494–504. [DOI] [PubMed] [Google Scholar]
- 14.Boeddinghaus J, Twerenbold R, Nestelberger T et al. Clinical Validation of a Novel High-Sensitivity Cardiac Troponin I Assay for Early Diagnosis of Acute Myocardial Infarction. Clin Chem 2018;64:1347–1360. [DOI] [PubMed] [Google Scholar]
- 15.Collet JP, Thiele H, Barbato E et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J 2020. [DOI] [PubMed] [Google Scholar]
- 16.Collinson PO, Heung YM, Gaze D et al. Influence of population selection on the 99th percentile reference value for cardiac troponin assays. Clin Chem 2012;58:219–25. [DOI] [PubMed] [Google Scholar]
- 17.Nestelberger T, Boeddinghaus J, Greenslade J et al. Two-Hour Algorithm for Rapid Triage of Suspected Acute Myocardial Infarction Using a High-Sensitivity Cardiac Troponin I Assay. Clin Chem 2019;65:1437–1447. [DOI] [PubMed] [Google Scholar]
- 18.Hoffmann U, Bamberg F, Chae CU et al. Coronary computed tomography angiography for early triage of patients with acute chest pain: the ROMICAT (Rule Out Myocardial Infarction using Computer Assisted Tomography) trial. J Am Coll Cardiol 2009;53:1642–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoffmann U, Truong QA, Fleg JL et al. Design of the Rule Out Myocardial Ischemia/Infarction Using Computer Assisted Tomography: a multicenter randomized comparative effectiveness trial of cardiac computed tomography versus alternative triage strategies in patients with acute chest pain in the emergency department. Am Heart J 2012;163:330–8, 338 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Januzzi JL, Sharma U, Zakroysky P et al. Sensitive troponin assays in patients with suspected acute coronary syndrome: Results from the multicenter rule out myocardial infarction using computer assisted tomography II trial. Am Heart J 2015;169:572–8 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferencik M, Mayrhofer T, Lu MT et al. High-Sensitivity Cardiac Troponin I as a Gatekeeper for Coronary Computed Tomography Angiography and Stress Testing in Patients with Acute Chest Pain. Clin Chem 2017;63:1724–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferencik M, Liu T, Mayrhofer T et al. hs-Troponin I Followed by CT Angiography Improves Acute Coronary Syndrome Risk Stratification Accuracy and Work-Up in Acute Chest Pain Patients: Results From ROMICAT II Trial. JACC Cardiovasc Imaging 2015;8:1272–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu AHB, Christenson RH, Greene DN et al. Clinical Laboratory Practice Recommendations for the Use of Cardiac Troponin in Acute Coronary Syndrome: Expert Opinion from the Academy of the American Association for Clinical Chemistry and the Task Force on Clinical Applications of Cardiac Bio-Markers of the International Federation of Clinical Chemistry and Laboratory Medicine. Clin Chem 2018;64:645–655. [DOI] [PubMed] [Google Scholar]
- 24.Apple FS, Wu AHB, Sandoval Y et al. Sex-Specific 99th Percentile Upper Reference Limits for High Sensitivity Cardiac Troponin Assays Derived Using a Universal Sample Bank. Clinical Chemistry 2020. [DOI] [PubMed] [Google Scholar]
- 25.Caposino PV, Kondratovich MV. Considerations for Single-Measurement Risk-Stratification Strategies for Myocardial Infarction Using Cardiac Troponin Assays. J Am Coll Cardiol 2019;74:283–284. [DOI] [PubMed] [Google Scholar]
- 26.Ungerer JP, Marquart L, O’Rourke PK, Wilgen U, Pretorius CJ. Concordance, variance, and outliers in 4 contemporary cardiac troponin assays: implications for harmonization. Clin Chem 2012;58:274–83. [DOI] [PubMed] [Google Scholar]
- 27.Kimenai DM, Henry RM, van der Kallen CJ et al. Direct comparison of clinical decision limits for cardiac troponin T and I. Heart 2016;102:610–6. [DOI] [PubMed] [Google Scholar]
- 28.Shah AS, Griffiths M, Lee KK et al. High sensitivity cardiac troponin and the under-diagnosis of myocardial infarction in women: prospective cohort study. BMJ 2015;350:g7873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fitzgerald R, Defilippi CR, Hollander J et al. OVERALL AND SEX-SPECIFIC REFERENCE RANGES FOR HIGH-SENSITIVITY TROPONIN T GEN 5 STAT IN THE UNITED STATES. Journal of the American College of Cardiology 2018;71:233. [Google Scholar]
- 30.Giannitsis E, Kurz K, Hallermayer K, Jarausch J, Jaffe AS, Katus HA. Analytical validation of a high-sensitivity cardiac troponin T assay. Clin Chem 2010;56:254–61. [DOI] [PubMed] [Google Scholar]
- 31.Rubini Gimenez M, Badertscher P, Twerenbold R et al. Impact of the US Food and Drug Administration-Approved Sex-Specific Cutoff Values for High-Sensitivity Cardiac Troponin T to Diagnose Myocardial Infarction. Circulation 2018;137:1867–1869. [DOI] [PubMed] [Google Scholar]
- 32.Wu AHB, Apple F, Love SA, Koch D, Myers GL, Christenson RH. Creation of a Universal Sample Bank for Determining the 99th Percentile for Cardiac Troponin Assays. The Journal of Applied Laboratory Medicine 2017;1:711–719. [DOI] [PubMed] [Google Scholar]
- 33.Apple FS, Ler R, Murakami MM. Determination of 19 cardiac troponin I and T assay 99th percentile values from a common presumably healthy population. Clin Chem 2012;58:1574–81. [DOI] [PubMed] [Google Scholar]
- 34.Body R, Burrows G, Carley S et al. High-sensitivity cardiac troponin t concentrations below the limit of detection to exclude acute myocardial infarction: a prospective evaluation. Clin Chem 2015;61:983–9. [DOI] [PubMed] [Google Scholar]
- 35.Boeddinghaus J, Nestelberger T, Twerenbold R et al. Direct Comparison of 4 Very Early Rule-Out Strategies for Acute Myocardial Infarction Using High-Sensitivity Cardiac Troponin I. Circulation 2017;135:1597–1611. [DOI] [PubMed] [Google Scholar]
- 36.Jaeger C, Wildi K, Twerenbold R et al. One-hour rule-in and rule-out of acute myocardial infarction using high-sensitivity cardiac troponin I. Am Heart J 2016;171:92–102 e1–5. [DOI] [PubMed] [Google Scholar]
- 37.Eggers KM, Jernberg T, Lindahl B. Unstable Angina in the Era of Cardiac Troponin Assays with Improved Sensitivity-A Clinical Dilemma. The American journal of medicine 2017;130:1423–1430 e5. [DOI] [PubMed] [Google Scholar]
- 38.Rubini Gimenez M, Twerenbold R, Reichlin T et al. Direct comparison of high-sensitivity-cardiac troponin I vs. T for the early diagnosis of acute myocardial infarction. Eur Heart J 2014;35:2303–11. [DOI] [PubMed] [Google Scholar]
- 39.Klinkenberg LJ, Wildi K, van der Linden N et al. Diurnal Rhythm of Cardiac Troponin: Consequences for the Diagnosis of Acute Myocardial Infarction. Clin Chem 2016;62:1602–1611. [DOI] [PubMed] [Google Scholar]
- 40.Artunc F, Mueller C, Breidthardt T et al. Sensitive troponins--which suits better for hemodialysis patients? Associated factors and prediction of mortality. PLoS One 2012;7:e47610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wildi K, Gimenez MR, Twerenbold R et al. Misdiagnosis of Myocardial Infarction Related to Limitations of the Current Regulatory Approach to Define Clinical Decision Values for Cardiac Troponin. Circulation 2015;131:2032–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Litt HI, Gatsonis C, Snyder B et al. CT angiography for safe discharge of patients with possible acute coronary syndromes. N Engl J Med 2012;366:1393–403. [DOI] [PubMed] [Google Scholar]
- 43.Hoffmann U, Truong QA, Schoenfeld DA et al. Coronary CT angiography versus standard evaluation in acute chest pain. The New England journal of medicine 2012;367:299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Singer AJ, Domingo A, Thode HC Jr. et al. Utilization of coronary computed tomography angiography for exclusion of coronary artery disease in ED patients with low- to intermediate-risk chest pain: a 1-year experience. Am J Emerg Med 2012;30:1706–11. [DOI] [PubMed] [Google Scholar]
- 45.Peacock WF, Baumann BM, Bruton D et al. Efficacy of High-Sensitivity Troponin T in Identifying Very-Low-Risk Patients With Possible Acute Coronary Syndrome. JAMA Cardiol 2018;3:104–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Than M, Cullen L, Aldous S et al. 2-Hour accelerated diagnostic protocol to assess patients with chest pain symptoms using contemporary troponins as the only biomarker: the ADAPT trial. J Am Coll Cardiol 2012;59:2091–8. [DOI] [PubMed] [Google Scholar]
- 47.Than MP, Pickering JW, Sandoval Y et al. Machine Learning to Predict the Likelihood of Acute Myocardial Infarction. Circulation 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Neumann JT, Twerenbold R, Ojeda F et al. Application of High-Sensitivity Troponin in Suspected Myocardial Infarction. N Engl J Med 2019;380:2529–2540. [DOI] [PubMed] [Google Scholar]
- 49.Collinson PO, Saenger AK, Apple FS, Ifcc CC. High sensitivity, contemporary and point-of-care cardiac troponin assays: educational aids developed by the IFCC Committee on Clinical Application of Cardiac Bio-Markers. Clin Chem Lab Med 2019;57:623–632. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.