Abstract
Objectives:
To characterize the progression of mid-frequency sensorineural hearing loss (MFSNHL) over time.
Methods:
A retrospective chart review spanning 2012 to 2017 was performed at a tertiary care audiology and neurotology center. Our cohort included 37 patients met the criteria for MFSNHL also known as “cookie bite hearing loss”. It was defined as having a 1, 2, and 4 kHz average pure tone audiometry greater than 10 dB in intensity compared to the average threshold at 500 Hz and 8 kHz.
Results:
Average age at initial presentation was 11.8 years (range: 8 months to 70 years). Across all individuals, the average mid-frequency threshold was 47 dB, compared to 27 dB at 500 Hz and 8 kHz. Twenty-three patients (62%) had multiple audiograms with 4-year median follow up time. Average values across all frequencies (0.5, 1, 2, 4, 8 kHz) in the initial audiogram was 37 dB, compared to an average of 39 dB demonstrated on final audiogram. Of those with serial audiograms, only 5 patients demonstrated threshold changes of 10 dB or more. Of these 5 patients, only one was found to have clinical worsening of MFSNHL.
Conclusions:
MFSNHL is an uncommon audiometric finding with unspecified long-term outcomes. We demonstrated that most patients (96%) with MFSNHL do not experience clinical worsening of their hearing threshold over almost 4 years of follow up. Future prospective studies aimed at collecting longer-term data are warranted to further elucidate the long-term trajectory of MFSNHL patients.
Keywords: Mid-frequency sensorineural hearing loss, Cookie bite hearing loss, Pediatric, Audiometry
Introduction
Mid-frequency sensorineural hearing loss (MFSNHL) is an uncommon audiometric finding with a reported prevalence of 0.7% (1). Its characteristic appearance is often described as U-shaped, saucer or cookie bite shape, making it easily recognized on audiogram. MFSNHL is characterized by an average pure tone threshold of 1, 2, and 4 kHz that is greater than the threshold at 0.5 and 8 kHz by at least 10 dB (2). Numerous etiologies have been proposed for MFSNHL, including dominant non-syndromic familial deafness, congenital deafness associated with Turner’s syndrome, bilateral congenital SNHL with fluctuant hearing and episodic vertigo, and small vestibular schwannomas (3-6).
MFSNHL is most often due to a variety of genetic mutations, with a variation of the TECTA gene (encodes alpha-tectorin) cited as the most frequent cause (7). Despite the numerous proposed etiologies, the long-term consequences of MFSNHL remain underreported. Only one study in the literature has described the long-term prognostic and clinical outcomes of MFSNHL (1). However, no study to date has characterized MFSNHL patients over time with serial audiograms. As such, we aimed to report the long-term outcomes of MFSNHL patients at our institution.
Methods
A retrospective chart review spanning 2012 to 2017 was performed with IRB approval at a tertiary care audiology and neurotology center. MFSNHL was identified when the average pure tone thresholds of 1, 2, and 4 kHz were 10 dB greater than the average of thresholds at 0.5 and 8 kHz. To exclude audiograms with classic “noise notches”, patients with worst hearing at 4 kHz haven’t been included in the cohort. Chart review did not reveal any included patients to have suffered from loud noise, explosion, or head trauma leading to sudden hearing loss. All patients were seen by an audiologist and received comprehensive audiologic testing including audiogram, otoacoustic emissions (OAEs), speech recognition threshold (SRT), word recognition score (WRS), and otoscopic examination. If there was a difference between the bone conduction and air conduction threshold, the bone conduction threshold was used. All patients included had normal otoscopic examination and normal tympanometry at 226 Hz. The first audiogram of each patient was used to diagnose MFSNHL. The difference in MFSNHL was clinically significant if hearing threshold changed by 10 dB or more from first to last audiogram in patients receiving serial audiograms. Clinically significant serial audiograms were then reviewed by the senior author (H.D.) and assessed to determine the validity of the clinical mid-frequency hearing change over time. All statistical analyses were performed using PASW 18.0 (SPSS Inc., Chicago, IL). A p value of 0.05 or less was considered statistically significant.
Results
Overall, 37 patients met criteria for MFSNHL. Of those, 20 (54%) were males with an average age of 11.8 ± 12.3 years (range: 8 months-70 years). This cohort is composed of mostly pediatric patients, with only two patients above the age of 16, at 45 and 70 years of age. Although etiology was not assessed, five (14%) endorsed a family history of MFSNHL, five (14%) had a documented history of otitis media, while seven (19%) had previously undergone myringotomy and tube placement (Table 1). Average SRT and WRS in all MFSNHL patients were 30 dB and 90%, respectively. Across all individuals, average mid-frequency threshold was 47 dB compared to 27 dB measured at 500 Hz and 8 kHz averages (p < 0.01). Figure 1 demonstrates a box plot of all the audiograms in the study. Bilateral MFSNHL was observed in 27 (73%) patients. Average SRT was 30 dB both in patients who demonstrated bilateral and unilateral MFSNHL.
Table 1.
Characteristics of all 37 mid-frequency sensorineural hearing loss patients.
| Age at diagnosis (mean ± SD) | 11.8 ± 12.3 years |
| No. of male patients | 20 (54%) |
| Right ear hearing loss only | 5 (14%) |
| Left ear hearing loss only | 5 (14%) |
| Bilateral MFSNHL | 27 (73%) |
| Age of patients with family history of hearing loss (mean ± SD) | 8.5 ± 5.7 years |
| No. of patients with family history of hearing loss | 5 (14%) |
| History of myringotomy and tube | 7 (19%) |
| History of otitis media | 5 (14%) |
| Average SRT of all audiograms | 30 dB |
| Average WRS of all audiograms | 90% |
| Number of patients with serial audiograms | 23 |
| Follow-up duration in patients with serial audiograms (mean ± SD) | 3.7 ± 2.4 years |
SD: standard deviation; MFSNHL: mid-frequency sensorineural hearing loss; SRT: speech recognition threshold; WRS: word recognition score
Figure 1.
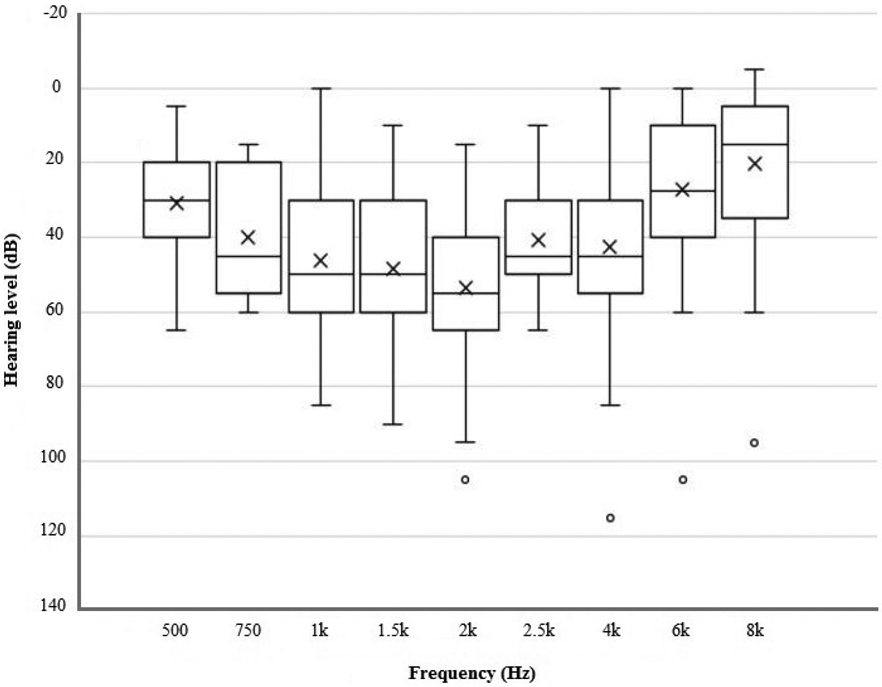
The distribution of all audiograms included in the cohort. The horizontal line in the middle of each box represents the respective median, and the “×” indicates the mean. Whiskers represent the range (minimum to maximum) unless there is an outlier (circles). Outliers are defined as any data points exceeding 1.5 times of interquartile range (between quartile 1 and 3) below or beyond the 1st or 3rd quartile, respectively.
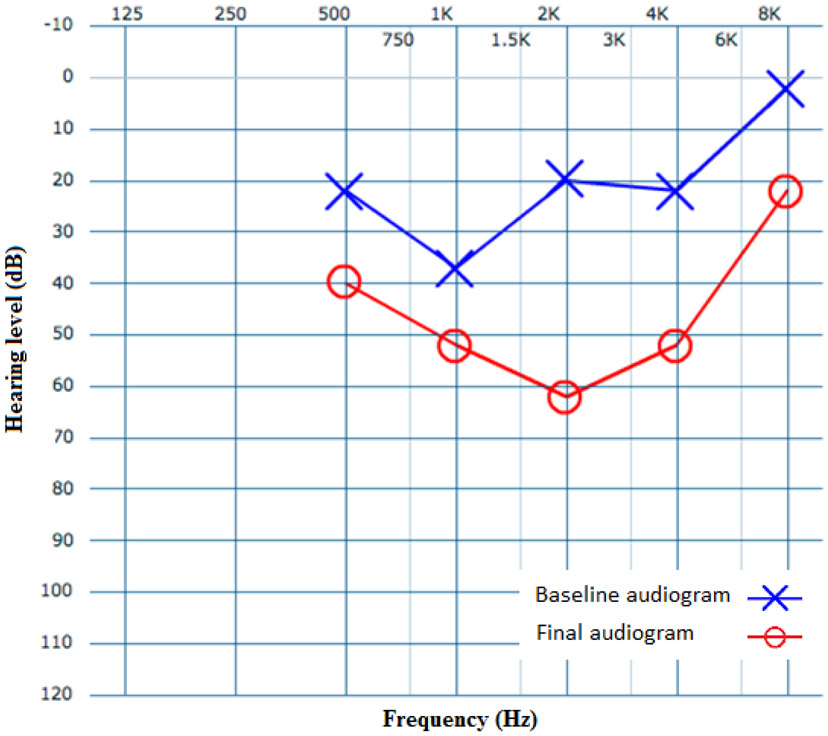
Serial audiograms were obtained and reviewed in 23 (62%) patients, allowing the study team to track changes in hearing loss for an average time of 3.7 ± 2.4 years (median: 4 years). The average thresholds of all frequencies (0.5, 1, 2, 4, 8 kHz) on initial and final audiograms were 37 dB and 39 dB, respectively (p = 0.54). Over time, 5 patients experienced mid-frequency changes greater than or equal to the 10 dB. These changes were reviewed for clinical significance by the senior author. In total, for three patients, thresholds worsened over time by 10, 13, and 21 dB. The remaining 2 patients’ thresholds improved by 11 and 22 dB. Of these 5 patients with changes 10 dB or more on mid-frequency (1, 2, and 4 kHz) hearing thresholds, only 1 was deemed to have a clinically significant change over time (Figure 2). Of note, this patient was subsequently diagnosed with Alport syndrome.
Figure 2.
Baseline (blue) and final (red) audiograms for the patient with worsening MFSNHL. Blue X and red O do not represent left and right ear audiograms.
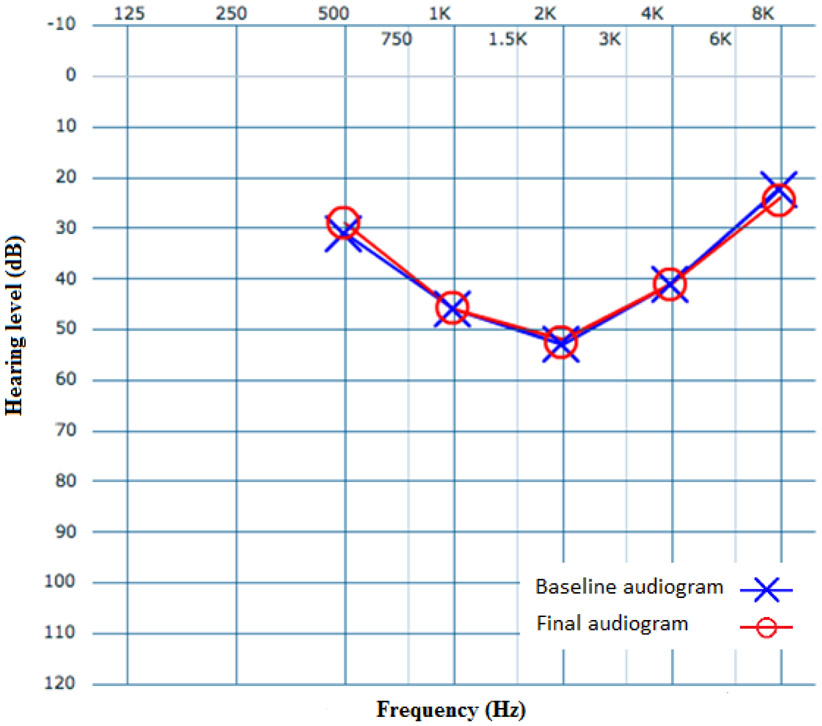
With only 1 clinically significant change over time, the remaining 22 patients receiving serial audiograms demonstrated clinically insignificant changes (Figure 3). The mean age in the 22 patients without any clinically significant hearing changes was 9 years, compared to 5 years in the patient who worsened over time. Pure tone average (PTA) in patients whose mid-frequency hearing thresholds remained constant was 42 dB, compared to a PTA of 53 dB in the one MFSNHL patient experiencing clinically worsened hearing thresholds (Table 2). As shown in Table 2, average SRT in the worsening MFSNHL patient was 43 dB, while average SRT in the 22 patients with no clinical change in hearing was 29 dB. Average WRS in both groups was 92%. Average change in mid-frequency (1, 2, and 4 kHz) hearing thresholds in patients who remained constant was 1.0 dB, compared to a 21.0 dB in the patient whose MFSNHL worsened over time.
Figure 3.
Average baseline of all cookie bite audiograms included in study (blue) and average of final audiograms in 22 patients with unchanged MFSNHL (red). Blue X and red O do not represent left and right ear audiograms.
Table 2.
Characteristics of the 23 patients with cookie bite hearing loss with serial audiograms.
| Unchanged MFSNHL |
Worsened MFSNHL |
|
|---|---|---|
| No. of patients | 22 (96%) | 1 (4%) |
| Mean age at diagnosis | 9 years | 5 years |
| Bilateral MFSNHL | 17 (77%) | 1 (100%) |
| Right ear hearing loss only | 3 (13%) | 0 |
| Left ear hearing loss only | 2 (10%) | 0 |
| No. of patients with family history of hearing loss | 5 (23%) | 0 |
| Average SRT of all audiograms | 29 dB | 43 dB |
| Average WRS of all audiograms | 92% | 92% |
| Time between first and last audiograms | 3.7 years | 4.3 years |
| Average PTA of all audiograms | 42 dB | 53 dB |
| Change in mid-frequency (1, 2, 4 kHz) hearing thresholds over time | 1.0 dB | 21.0 dB |
MFSNHL: mid-frequency sensorineural hearing loss; SRT: speech recognition threshold; WRS: word recognition score; PTA: pure tone average
Discussion
This study demonstrates that only 4% of MFSNHL patients with serial audiograms experienced clinically significant worsening mid-frequency hearing thresholds over time. This finding, while limited in sample size and follow-up duration, demonstrates a stable level of hearing after the initial hearing loss in almost all of our MFSNHL patient population. Originally, five patients were thought to have demonstrable changes in hearing thresholds with time. However, after careful assessment of serial audiograms in these patients, only one of them experienced true clinical worsening of mid-frequency hearing thresholds. The changes in hearing thresholds in the remaining four patients, deemed clinically insignificant are thought to be attributed to the test-retest variability inherent to all audiograms and audiograms performed by different audiologists. Careful examination of these clinically insignificant changes included a return to baseline on subsequent audiograms, improvement in one frequency and decrease in an adjacent frequency, and change in air conduction without changes in bone conduction.
Although the precise etiologies of our MFSNHL cohort were not assessed, the variety of etiologies known to cause MFSNHL may contribute to the reason why MFSNHL can change over time. The stratification of long-term outcomes of MFSNHL patients based on etiology may offer enhanced granularity on the prognosis of this patient cohort. The four genes identified that are known to cause non-syndromic MFSNHL are TECTA (encodes alpha-tectorin protein involved in tectorial membrane) (8), COL11A2 (encodes for one of the two alpha chain proteins in collagen XI) (9), CCDC50 (encodes a protein “Ymer” known to be associated with the inner ear) (8), and EYA4 (encodes EYA4 protein involved in organ of Corti maturation) (10). Of these genes, there are seven loci known to cause MFSNHL. Some mutations result in hearing loss pre-lingually (11-17), while others affect hearing loss post-lingually that can begin as late as the third decade of life (18-21). Forms of autosomal dominant MFSNHL that began pre-lingually have been demonstrated to be stable and not progressive over time. In contrast, those who experience hearing loss post-lingually demonstrate worsening MFSNHL overtime (22). Thus, progression may be dependent on the specific genetic mutation.
Another etiology of MFSNHL can be sudden onset hearing loss that is not hereditary. These causes can be attributed to various causes, including an idiopathic nature or secondary to vestibular schwannoma, head trauma, or infection. For instance, there are literature reports of MFSNHL occurring after cordless telephone injury, acoustic reflex test, or head trauma (23-25). In patients with small vestibular schwannomas and MFSNHL, progression slowly worsens (26). In Turner’s syndrome, MFSNHL progresses with time to affect higher frequencies (4). In contrast, one study showed that patients with sudden onset MFSNHL of an idiopathic nature improved after treatment with anti-viral medication and steroids (5). However, this study did not expand far into the group of MFSNHL patients (n = 4) who improved over time. These four patients were designated as “U-shaped;” however, this study did not mention the degree of hearing loss or the hearing threshold difference between low and high frequencies compared to mid-frequencies used to definitively identify MFSNHL. In our own experience of over 400 sudden hearing loss patients, we have yet to experience a patient with MFSNHL.
This study is the second to describe an MFSNHL cohort and the first to demonstrate a primarily lack of change in hearing loss as a function of time. The previous study which had a higher mean age (35 years) with reported mid-frequency threshold means of 17 dB and 20 dB higher than thresholds at 0.5 kHz and 8 kHz did not provide results over time (1). Although great care was taken to ensure the accuracy and validity of this study, a number of limitations exist. First, MFSNHL is rare, with only 37 patients meeting our inclusion criteria at a high-volume audiology and neurotology center over the study period. While more patients were tracked in this study compared to the previous study by Shah et al. (1), it is likely the sample size in our study is still limited in number. Second, our patient population consisted of mainly pediatric patients. This is significant because the test-retest variability inherent to all audiograms is more evident in pediatric patients (27). Moreover, audiograms were performed by multiple audiologists at different times, causing some degree of test-retest variability. Furthermore, the follow-up period in this study is limited to an average of 3.7 years (median: 4 years), which may not be long enough to truly understand the long-term trajectory of MFSNHL. This study can serve as the foundation for any future follow-up of these patients for a longer period of time in order to better understand this entity. Prospective studies with longer follow-up periods are warranted to better characterize the long-term outcomes of this patient population. Lastly, we were unable to identify the precise etiology of each MFSNHL patient in our study. Better delineation of etiology may better define why the one patient in our study worsened.
Conclusion
The prognosis of MFSNHL is contingent, in part, upon its diagnosed etiology and age of symptom onset. In our study, 96% of MFSNHL patients experienced no clinical progression of their hearing loss. We observed that, while uncommon, it is possible for patients to experience worsening MFSNHL and need hearing amplification in the future. Long-term follow-up and better delineation of the etiology of hearing loss in these patients may provide better prognostic data to help with patient education.
Footnotes
Financial Disclosure: None
Conflict of Interests: None
References
- 1.Shah RK, Blevins NH, Karmody CS. Mid-frequency sensorineural hearing loss: Aetiology and prognosis. J Laryngol Otol 2005; 119(7): 529–33. [DOI] [PubMed] [Google Scholar]
- 2.Martini A, Milani M, Rosignoli M, Mazzoli M. Audiometric patterns of genetic non-syndromal sensorineural hearing loss. Audiology 1997; 36(4): 228–36. [DOI] [PubMed] [Google Scholar]
- 3.Saunders JE, Luxford WM, Devgan KK, Fetterman BL. Sudden hearing loss in acoustic neuroma patients. Otolaryngol Head Neck Surg 1995; 113(1): 23–31. [DOI] [PubMed] [Google Scholar]
- 4.Sculerati N, Oddoux C, Clayton CM, Lim JW, Oster H. Hearing loss in Turner syndrome. Laryngoscope 1996; 106(8): 992–7. [DOI] [PubMed] [Google Scholar]
- 5.Zadeh MH, Storper IS, Spitzer JB. Diagnosis and treatment of sudden-onset sensorineural hearing loss: A study of 51 patients. Otolaryngol Head Neck Surg 2003; 128(1): 92–8. [DOI] [PubMed] [Google Scholar]
- 6.Halstead L, Karmody C. Mid-frequency Sensorineural Hearing Loss. In: Eastern Section Meeting of the Triological Society Boston; 1987. [Google Scholar]
- 7.Yamamoto N, Mutai H, Namba K, et al. Prevalence of TECTA mutation in patients with mid-frequency sensorineural hearing loss. Orphanet J Rare Dis 2017; 12(1): 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xia W, Liu F, Ma D. Research progress in pathogenic genes of hereditary non-syndromic mid-frequency deafness. Front Med 2016; 10(2): 137–42. [DOI] [PubMed] [Google Scholar]
- 9.Kuivaniemi H, Tromp G, Prockop DJ. Mutations in fibrillar collagens (types I, II, III, and XI), fibril-associated collagen (type IX), and network-forming collagen (type X) cause a spectrum of diseases of bone, cartilage, and blood vessels. Hum Mutat 1997; 9(4): 300–15. [DOI] [PubMed] [Google Scholar]
- 10.Zhang X, Liu Y, Zhang L, et al. Associations of genetic variations in EYA4, GRHL2 and DFNA5 with noise-induced hearing loss in Chinese population: a case- control study. Environ Health 2015; 14: 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Govaerts PJ, De Ceulaer G, Daemers K, et al. A new autosomal-dominant locus (DFNA12) is responsible for a nonsyndromic, midfrequency, prelingual and nonprogressive sensorineural hearing loss. Am J Otol 1998; 19(6): 718–23. [PubMed] [Google Scholar]
- 12.Verhoeven K, Van Laer L, Kirschhofer K, et al. Mutations in the human alpha-tectorin gene cause autosomal dominant non-syndromic hearing impairment. Nat Genet 1998; 19(1): 60–2. [DOI] [PubMed] [Google Scholar]
- 13.Meyer NC, Nishimura CJ, Mcmordie S, Smith RJH. Audioprofiling identifies TECTA and GJB2-related deafness segregating in a single extended pedigree. Clin Genet 2007; 72(2): 130–7. [DOI] [PubMed] [Google Scholar]
- 14.Kobayashi M, Miyagawa M, Nishio S, et al. WFS1 mutation screening in a large series of Japanese hearing loss patients: Massively parallel DNA sequencing-based analysis. PLoS One 2018; 13(3): e0193359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iwasaki S, Harada D, Usami S, Nagura M, Takeshita T, Hoshino T. Association of clinical features with mutation of TECTA in a family with autosomal dominant hearing loss. Arch Otolaryngol Head Neck Surg 2002; 128(8): 913–7. [DOI] [PubMed] [Google Scholar]
- 16.Plantinga RF, Cremers CW, Huygen PL, Kunst HP, Bosman AJ. Audiological evaluation of affected members from a Dutch DFNA8/12 (TECTA) family. J Assoc Res Otolaryngol 2007; 8(1): 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Heer AR, Pauw RJ, Huygen PL, Collin RW, Kremer H, Cremers CW. Flat threshold and mid-frequency hearing impairment in a Dutch DFNA8/12 family with a novel mutation in TECTA. Some evidence for protection of the inner ear. Audiol Neurotol 2009; 14(3): 153–62. [DOI] [PubMed] [Google Scholar]
- 18.Kunst H, Marres H, Huygen P, et al. Non-syndromic autosomal dominant progressive non-specific mid-frequency sensorineural hearing impairment with childhood to late adolescence onset (DFNA21). Clin Otolaryngol Allied Sci 2000; 25(1): 45–54. [DOI] [PubMed] [Google Scholar]
- 19.Modamio-Høybjør S, Moreno-Pelayo MA, Mencía A, et al. A novel locus for autosomal dominant nonsyndromic hearing loss (DFNA44) maps to chromosome 3q28-29. Hum Genet 2003; 112(1): 24–8. [DOI] [PubMed] [Google Scholar]
- 20.Van Beelen E, Oonk AMM, Leijendeckers JM, et al. Audiometric Characteristics of a Dutch DFNA10 Family with Mid-Frequency Hearing Impairment. Ear Hear 2016; 37(1): 103–11. [DOI] [PubMed] [Google Scholar]
- 21.Moreno-Pelayo M, del Castillo I, Villamar M, et al. A cysteine substitution in the zona pellucida domain of alpha-tectorin results in autosomal dominant, postlingual, progressive, mid frequency hearing loss in a Spanish family. J Med Genet 2001; 38(5): E13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bitner-Glindzicz M Hereditary deafness and phenotyping in humans. Br Med Bull 2002; 63: 73–94. [DOI] [PubMed] [Google Scholar]
- 23.Orchik DJ, Schmaier DR, Shea JJ Jr, Emmett JR, Moretz WH Jr, Shea JJ 3rd. Sensorineural hearing loss in cordless telephone injury. Otolaryngol Head Neck Surg 1987; 96(1): 30–3. [DOI] [PubMed] [Google Scholar]
- 24.Hunter LL, Ries DT, Schlauch RS, Levine SC, Ward WD. Safety and clinical performance of acoustic reflex tests. Ear Hear 1999; 20(6): 506–14. [DOI] [PubMed] [Google Scholar]
- 25.Scott AM, Bauch CD, Olsen WO. Head trauma and mid-frequency hearing loss. Am J Audiol 1999; 8(2): 101–5. [DOI] [PubMed] [Google Scholar]
- 26.Kanzaki J, Ogawa K, Shiobara R, Toya S. Hearing preservation in acoustic neuroma surgery and postoperative audiological findings. Acta Otolaryngol 1989; 107(5-6): 474–8. [DOI] [PubMed] [Google Scholar]
- 27.Beck RM, Ramos BF, Grasel SS, et al. Comparative study between pure tone audiometry and auditory steady-state responses in normal hearing subjects. Braz J Otorhinolaryngol 2014; 80(1): 35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]