Abstract
Objectives:
To evaluate the efficacy of a multi-modal migraine prophylaxis therapy for patients with hyperacusis.
Methods:
In a prospective cohort, patients with hyperacusis were treated with a multi-modal step-wise migraine prophylactic regimen (nortriptyline, verapamil, topiramate, or a combination thereof) as well as lifestyle and dietary modifications. Pre- and post-treatment average loudness discomfort level (LDL), hyperacusis discomfort level measured by a visual analogue scale (VAS), and scores on the modified Khalfa questionnaire for severity of hyperacusis were compared.
Results:
Twenty-two of the 25 patients (88%) reported subjective resolution of their symptoms following treatment. Post-treatment audiograms showed significant improvement in average LDL from 81.3 ± 3.2 dB to 86.4 ± 2.6 dB (p < 0.001), indicating increased sound tolerability. The VAS discomfort level also showed significant improvement from a pre-treatment average of 7.7 ± 1.1 to 3.7 ± 1.6 post-treatment (p < 0.001). There was also significant improvement in the average total score on modified Khalfa questionnaire (32.2 ± 3.6 vs. 22.0 ± 5.7, p < 0.001).
Conclusions:
The majority of patients with hyperacusis demonstrated symptomatic improvement from migraine prophylaxis therapy, as indicated by self-reported and audiometric measures. Our findings indicate that, for some patients, hyperacusis may share a pathophysiologic basis with migraine disorder and may be successfully managed with multimodal migraine prophylaxis therapy.
Keywords: Hyperacusis, Migraine, Migraine treatment, Quality of life, Modified Khalfa questionnaire
Introduction
Hyperacusis is a clinical phenomenon that broadly encompasses various adverse reactions to sound. Determining the prevalence of hyperacusis was identified as a major research question in a 2018 review of gaps in knowledge regarding hyperacusis.1 The reported prevalence of hyperacusis in children and adolescents has ranged between 3.2% and 17.1%.2 Tyler et al., in their review of the literature, defined four subtypes of hyperacusis that may occur singly or in combination. These are: “loudness hyperacusis”, where normal volume sounds are perceived as loud; “annoyance hyperacusis”, where sounds may cause mood disturbance; “fear hyperacusis”, where there are perseverative thoughts about loud sounds and consequent avoidant behavior; and “pain hyperacusis”, where there is a lowered threshold for sound-induced pain.3 Left untreated, hyperacusis has been shown to be associated with significant emotional and behavioral consequences.4,5
Given the variability in the presentations of hyperacusis, it is unsurprising that hyperacusis is also associated with many different disorders. Hyperacusis is a common feature of hearing loss disorders, neurodevelopmental disorders, and various psychiatric and chronic pain disorders such as Meniere’s disease, tinnitus, autism spectrum disorder, depression, post-traumatic stress disorder, fibromyalgia, complex regional pain syndrome, and chronic migraine.6-10 Treatment options for hyperacusis include avoidance of provocative stimuli, cognitive behavioral therapy, tinnitus retraining therapy, hearing amplification, and surgical reinforcement of the round and oval windows.11-14 Hyperacusis as it relates to chronic migraine is well described and is typically called “phonophobia” in the migraine literature. It is a symptom commonly found in patients with migraine and is strongly associated with the severity of headache.15 While many studies have focused on the auditory disturbances of migraine headache, to our knowledge there have not been any studies in which patients presenting for hyperacusis were evaluated for migraine. In this cohort, we aimed to describe the relationship of hyperacusis and migraine in patients presenting for the evaluation of hyperacusis and to assess the impact of a multi-modal migraine prophylactic regimen on hyperacusis severity.
Methods
In this cohort, we describe patients with hyperacusis who were referred to our tertiary care neurotology practice from 2015 to 2018. Following Institutional Review Board approval, patients with the subjective complaint of hypersensitivity to sound were asked to fill out a modified Khalfa questionnaire on severity of hyperacusis symptoms. Patients with a modified Khalfa score of >28 were considered hyperacoustic and those with a score ≤28 were considered normal. Audiograms and imaging of the brain and internal auditory canals were obtained to rule out underlying causes. Patients were included in the study if their symptoms were persistent for a minimum of six months and their average loudness discomfort level (LDL) was ≤85 dB.16 By contrast, most normal patients have an average LDL between 86-98 dB.16 Average LDL was calculated by averaging the LDL at 0.125, 0.25, 0.5, 1, 2, 4, and 8 kHz.17 Patients with misophonia or history of only temporary sensitivity to sound (e.g., only during episodic migraine headache) were excluded from the study.
Twenty-five patients with hyperacusis were included in this study and treated with a multi-modal migraine prophylactic regimen. As part of the migraine prophylaxis, patients were counseled on implementing lifestyle modifications. This included dietary modifications, which consisted of avoiding foods containing certain preservatives, fermented products, chocolate, nuts, eggs, alcohol, fresh breads/yeast products, aged/processed meats, certain beans, certain fruit (high histamine), and pickled or preserved fruits/vegetables. In addition, dietary supplementation with magnesium 400 mg bid and riboflavin (vitamin B2) 200 mg bid was prescribed. We did not restrict sodium intake as long as the patient stayed well-hydrated. Patients were also instructed to eat three meals and sleep on a regular schedule on weekends and weekdays to avoid fatigue, hunger, and dehydration.18
The patients were also prescribed pharmacologic migraine prophylaxis in a step-wise agent- and dose-escalating manner illustrated in Figure 1. The patients were most commonly started on nortriptyline 25 mg PO qhs and gradually escalated by 25 mg every three weeks to a maximum dose of 75 mg if symptoms had not improved. Nortriptyline was indicated for patients who had difficulty sleeping, interrupted sleep, or endorsed significant stress or anxiety. Nortriptyline was not used for patients already taking an antidepressant. If patients had comorbid hypertension or nortriptyline was contraindicated, verapamil 120 mg PO qhs was started and escalated by 60 mg every two weeks to a maximum dose of 240 mg if symptoms were not improved. Verapamil was not used for patients with systolic blood pressure <100 mmHg or heart rate < 60 BPM. Patients who failed to improve on a single agent were started on a second agent (either topiramate or verapamil) and dose-escalated to effect, unless contraindicated. If the first-line therapy was ineffective or contraindicated, topiramate 25 mg PO qhs with weekly escalation of 25 mg up to 150 mg was prescribed. Patients were instructed to maintain the dosage of their current regimen once they achieved satisfactory control of their symptoms.
Figure 1.
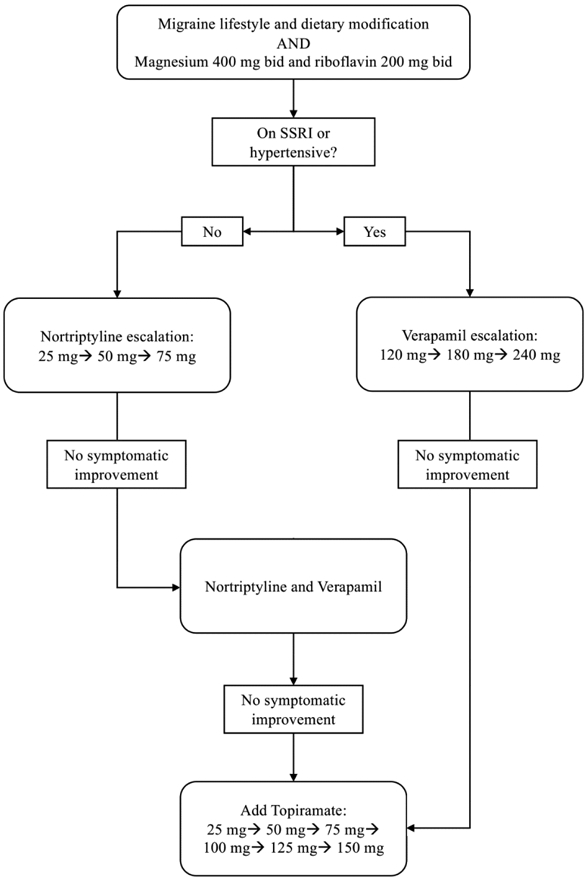
Algorithm for step-wise migraine prophylactic regimen.
A modified Khalfa questionnaire was administered to assess the severity of hyperacusis in terms of impairment of quality of life (QOL).19 The questionnaire was modified from the author’s original version by removing the QOL domains that did not demonstrate satisfactory internal consistency reliability in the original study. The resulting modified questionnaire utilized Likert-type scales to assess QOL in three domains: attention, emotional, and social. The possible total score ranged from zero to 42; scores above 28 indicate hyperacusis and scores below or equal to 28 indicate non-hyperacusis or normal.19 Additionally, we recorded a baseline subjective discomfort level using a visual analog scale (VAS). Patients were then prescribed multi-modal migraine prophylaxis and followed at 3-month intervals. All patients were seen at three and six months. A final evaluation was performed at the six-month visit. Paired sample t-test was performed to compare pre- and post-treatment scores. Independent sample t-test was used to compare post-treatment improvement in hyperacusis discomfort measured by VAS between the sub cohorts. SPSS 17.0 software (SPSS Inc., Chicago, IL, USA) was used for statistical analysis with a 0.05 alpha considered significant.
Results
The average age of patients was 48.5 ± 12.0 years (range, 23-71 years). There were 19 female (76%) and 6 male participants (24%), female to male ratio 3:1. The prevalence of migraine-related symptoms are shown in Table 1. At the time of the final visit, 15 patients (60%) were on a single agent, 9 patients (36%) were on two agents, and 1 patient (4%) was on three agents (Table 2). Post-treatment testing showed significant improvement in average loudness discomfort level (LDL) from 81.3 ± 3.2 dB to 86.4 ± 2.6 dB (p < 0.001), indicating increased sound tolerability. A total of 22 patients (88%) reported subjective resolution of their symptoms after treatment.
Table 1.
Prevalence of migraine-related symptoms and family history of migraine-related disorders in the cohort.
| Clinical feature | No. of patients | Proportion of the cohort |
|---|---|---|
| Previous history of | ||
| Visual motion sensitivity | 18 | 72% |
| Light sensitivity | 19 | 76% |
| Head motion sensitivity | 14 | 56% |
| Odor sensitivity | 13 | 52% |
| Weather change sensitivity | 8 | 32% |
| Motion sickness | 18 | 72% |
| Mental confusion (head/brain fog) | 20 | 80% |
| Previous medication for migraine | 10 | 40% |
| Sinus pain, facial pressure, or headache when exposed to wind or air conditioner | 18 | 72% |
| Scalp or face allodynia | 5 | 20% |
| Ice cream headache (brain freeze) | 15 | 60% |
| Sinus headache | 18 | 72% |
| Unilateral neck stiffness | 18 | 72% |
| Tinnitus | 16 | 64% |
| Daily headache | 5 | 20% |
| Frequent headache (≥ one per week) | 20 | 80% |
| Sleep problems | 7 | 28% |
| Family history of | ||
| Migraine headache | 4 | 16% |
| Meniere's disease | 1 | 4% |
| Motion sickness | 1 | 4% |
Table 2.
Eventual medication regimen at the six-month follow-up visit.
| Medication | No. of patients | Proportion of the cohort using medication |
|---|---|---|
| Nortriptyline | 7 | 28% |
| Verapamil | 6 | 24% |
| Topiramate | 2 | 8% |
| Nortriptyline + Verapamil | 5 | 20% |
| Nortriptyline + Topiramate | 4 | 16% |
| Nortriptyline + Verapamil + Topiramate | 1 | 4% |
As shown in Figure 2, there was significant improvement between pre- and post-treatment scores in all three domains of the Khalfa questionnaire: emotional domain (9.5 ± 1.4 vs. 6.5 ± 2.2, p < 0.001), social domain (13.1 ± 1.6 vs. 9.5 ± 2.3, p < 0.001), and attention domain (9.6 ± 1.3 vs. 5.9 ± 2.1, p < 0.001). Additionally, the average total Khalfa score improved significantly from 32.2 ± 3.6 pre-treatment to 22.0 ± 5.7 post-treatment (p < 0.001), indicating improvement to non-hyperacusis. The VAS subjective discomfort level also showed significant improvement from a pre-treatment average of 7.7 ± 1.1 to 3.7 ± 1.6 post-treatment (p < 0.001). Post-treatment improvement in VAS discomfort level for patients with and without a history of migraine (4.1 ± 2.2 vs. 3.9 ± 2.1, p = 0.822), those with and without tinnitus (4.5 ± 1.2 vs. 2.9 ± 2.5, p = 0.040), and those whose headache frequency did and did not improve as defined by at least 50% decrease in headache frequency (4.2 ± 1.1 vs. 2.8 ± 2.1, p = 0.066) are shown in Table 3.
Figure 2.
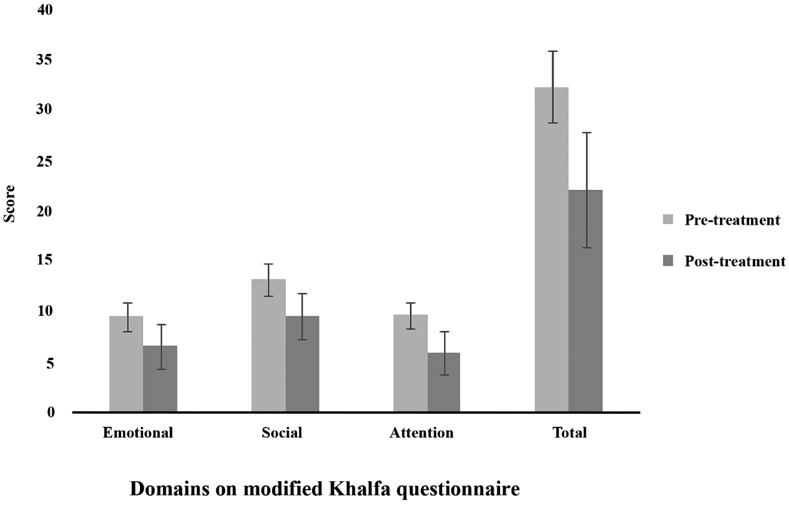
Pre- and post-treatment average scores on different domains of modified Khalfa questionnaire.
Table 3.
Comparison of average post-treatment improvement in hyperacusis discomfort measured by a visual analog scale.
| Clinical Feature Present? | |||
|---|---|---|---|
| Yes | No | p value | |
| History of migraine | 4.1 ± 2.2 | 3.9 ± 2.1 | 0.822 |
| Tinnitus | 4.5 ± 1.2 | 2.9 ± 2.5 | 0.040 |
| *Improvement in headache frequency | 4.2 ± 1.1 | 2.8 ± 2.1 | 0.066 |
Improvement defined by ≥ 50% decrease in headache frequency.
Discussion
We found a significant history of migraine-related symptoms among patients presenting for evaluation of hyperacusis (Table 1). The majority of our patients with hyperacusis showed significant improvement in QOL, subjective resolution of their symptoms, and audiometric results following treatment with our multi-modal migraine prophylactic regimen. The average pre-treatment score on the modified Khalfa questionnaire showed hyperacoustic status which significantly improved to an average score well below the threshold post-treatment.
Various treatments for hyperacusis have been described; however, more randomized controlled trials are needed to evaluate the efficacy of these treatment modalities.20 In 2016, Silverstein et al. described minimally invasive surgical reinforcement of the round and oval windows with temporalis fascia or tragal perichondrium in a series of six patients. The authors cautioned that the test-retest reliability of the LDL measurement can depend on the consistency of instructions provided by the tester, however other studies have reported that the LDL was a reliable tool for follow-up.12 Recently, sound therapy has been used to improve the LDL of patients with tinnitus and co-morbid hyperacusis and recent reviews have confirmed modest benefit.11,21 The present study is the first to describe treatment of hyperacusis with a multi-modal migraine prophylactic regimen. It is the experience of our practice that migraine prophylaxis generally yields better results than sound generators, and that all patients presenting for treatment of persistent hyperacusis should be offered a trial of migraine prophylaxis along with lifestyle and dietary modifications as first-line therapy.
The selected dosages of nortriptyline, verapamil, and topiramate have previously been shown to be both safe and effective for treatment of other otologic conditions.22-25 However, side effects are a possibility and patients should be counseled and monitored accordingly. Nortriptyline most commonly can cause lightheadedness, dizziness, and sedation, and baseline EKG with QTc monitoring is a consideration. Verapamil can also cause lightheadedness and dizziness as well as constipation. Topiramate can cause mild tingling in the extremities, mild appetite suppression, and mild taste disturbance. Our most commonly reported side effect was hypotension while on verapamil due to individuals beginning concomitant anti-hypertensive use; these individuals were switched to topiramate only or to nortriptyline and topiramate (Table 2).
While the mechanisms underlying hyperacusis remain unknown, it is theorized that different types of hyperacusis may have distinct pathophysiological mechanisms.6,8,13 For example, the evidence has supported the phenomenon of “excessive central gain enhancement” as the etiological factor in loudness hyperacusis and tinnitus. The central gain model posits that tinnitus and hyperacusis result from a compensatory increase in gain or neural amplification in the central auditory system to compensate for a loss of sensory input from the cochlea.26,27 Hyperacusis associated with chronic pain has been theorized to be caused by abnormal activity in the thalamus, locus coeruleus, and other convergent sites in the brainstem.28
A hallmark of migraine pathophysiology is altered perception of normal sensory stimuli such as sound, light, smell, and touch.29,30 Central sensitization is the process by which trigeminal and cervical nociceptors become especially sensitive to normal stimuli leading to allodynia and migraine.31,32 It is believed that in migraine, like in other chronic pain conditions, a sensitization of peripheral and nociceptive pathways can spread to higher central circuits and compromise auditory modulation mechanisms, leading to hyperacusis. In fact, the sites of convergence of both migraine and chronic pain syndromes are in the locus coeruleus and its ascending projections to the thalamus. Convergence of these sensory pathways results in symptom amplification such that light, for example, can cause increased firing in the trigeminal sensory fibers.28 When this sensitization occurs specifically for sound, hyperacusis may be the expected result, particularly in patients with migraine and migraine-related otologic disorders like vestibular migraine and Meniere’s disease.18,23
The interrelation of the migraine and chronic pain pathways may also shed light on the types of hyperacusis that is best targeted by the migraine treatments explored in this paper. It is likely that, of the various types of hyperacusis including loudness, annoyance, fear, and pain, migraine prophylactic treatment will best target loudness and pain hyperacusis. Loudness and pain hyperacusis have the closest relation with alterations in the threshold of hearing. By effectively lowering the threshold for firing of sensory neurons, migraine can increase the perceived noise level of specific sounds.8 Furthermore, via the convergence of migraine and pain pathways, this altered sensitivity to sound can transform into a perception of pain as well. By helping treat migraine and the subsequent changes in hearing threshold, these medications should reduce both the perceived loudness of sound and any pain associated with it. It is possible that annoyance and fear hyperacusis, which are defined more so by their mood disturbances, would be improved by these medications (particularly nortriptyline) as well, however further studies are needed to elucidate the efficacy of migraine treatments for different types of hyperacusis.
Interestingly, many parallels exist between hyperacusis and tinnitus, which may explain the significance in post-treatment improvement of VAS discomfort level in patients with concurrent tinnitus compared to those without tinnitus (Table 3). Similar to hyperacusis, the neural pathways involved in tinnitus also overlap with mood and pain disorders.33-35 Additionally, tinnitus shares a strong association with migraine.36 It is not surprising then that these medications can reduce the perception of both hyperacusis and tinnitus, likely by acting on the same root cause or shared neural pathways.
The main limitation of this study was the lack of a control group. Although patients were only included if their symptoms had persisted for six months, we cannot say with certainty that the improvements shown were solely due to our treatments because of the possibility of placebo effect. However, the long duration of symptoms prior to presentation and a response rate of 88% is suggestive of a treatment effect. Another limitation is the inherently subjective nature of the outcome measures of hyperacusis. The VAS, QOL survey, and even audiometric LDL testing are patient-reported scores and vulnerable to response bias. Additionally, the dietary and lifestyle modifications required for migraine prophylaxis are rigorous. The variability in adherence to our prescribed regimen was not quantified. Our evidence suggests our approach may be efficacious for certain types of hyperacusis but may not be effective for all subtypes. For example, we did not have any subjects with hearing loss in our study cohort and so could not investigate the difference in treatment efficacy for patients with hearing loss and tinnitus versus those with normal hearing and no tinnitus. A larger study will be needed to adequately evaluate the treatment effect in all subtypes of hyperacusis. Finally, due to limited study duration we are unable to assess the long-term efficacy of our treatment. Future studies will require the addition of a placebo control group, and the development of a valid objective measure of hyperacusis to demonstrate efficacy.
Conclusions
The majority of patients with hyperacusis demonstrated symptomatic improvement from migraine prophylaxis therapy, as indicated by self-reported and audiometric measures. Our findings indicate that, for some patients, hyperacusis may share a pathophysiologic basis with migraine disorder and may be successfully managed with multimodal migraine prophylaxis therapy.
Acknowledgments
Financial Disclosure: Mehdi Abouzari, MD, PhD; is supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant TL1TR001415-04.
Footnotes
Conflicts of Interest: None
References
- 1.Baguley DM, Hoare DJ. Hyperacusis: major research questions. HNO. 2018;66(5):358–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosing SN, Schmidt JH, Wedderkopp N, Baguley DM. Prevalence of tinnitus and hyperacusis in children and adolescents: a systematic review. BMJ Open. 2016;6(6):e010596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tyler RS, Pienkowski M, Roncancio ER, et al. A review of hyperacusis and future directions: part I. Definitions and manifestations. Am J Audiol. 2014;23(4):402–419. [DOI] [PubMed] [Google Scholar]
- 4.Paulin J, Andersson L, Nordin S. Characteristics of hyperacusis in the general population. Noise Health. 2016;18(83):178–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Juris L, Andersson G, Larsen HC, Ekselius L. Psychiatric comorbidity and personality traits in patients with hyperacusis. Int J Audiol. 2013;52(4):230–235. [DOI] [PubMed] [Google Scholar]
- 6.Auerbach BD. Physiological mechanisms of hyperacusis: an update. ENT & Audiology News. 2019; 27(6) https://www.entandaudiologynews.com/features/audiology-features/post/physiological-mechanisms-of-hyperacusis-an-update. [Google Scholar]
- 7.Wilson US, Sadler KM, Hancock KE, Guinan JJ, Lichtenhan JT. Efferent inhibition strength is a physiological correlate of hyperacusis in children with autism spectrum disorder. J Neurophysiol. 2017;118(2):1164–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suhnan AP, Finch PM, Drummond PD. Hyperacusis in chronic pain: neural interactions between the auditory and nociceptive systems. Int J Audiol. 2017;56(11):801–809. [DOI] [PubMed] [Google Scholar]
- 9.Gurkov R, Jerin C, Flatz W, Maxwell R. Clinical manifestations of hydropic ear disease (Meniere's). Eur Arch Otorhinolaryngol. 2019;276(1):27–40. [DOI] [PubMed] [Google Scholar]
- 10.Aazh H, Moore BCJ. Prevalence and Characteristics of Patients with Severe Hyperacusis among Patients Seen in a Tinnitus and Hyperacusis Clinic. J Am Acad Audiol. 2018;29(7):626–633. [DOI] [PubMed] [Google Scholar]
- 11.Pienkowski M. Rationale and Efficacy of Sound Therapies for Tinnitus and Hyperacusis. Neuroscience. 2019;407:120–134. [DOI] [PubMed] [Google Scholar]
- 12.Silverstein H, Ojo R, Daugherty J, Nazarian R, Wazen J. Minimally Invasive Surgery for the Treatment of Hyperacusis. Otol Neurotol. 2016;37(10):1482–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aazh H, McFerran D, Salvi R, Prasher D, Jastreboff M, Jastreboff P. Insights from the first international conference on hyperacusis: Causes, evaluation, diagnosis and treatment. Noise Health. 2014;16(69):123–126. [DOI] [PubMed] [Google Scholar]
- 14.Juris L, Andersson G, Larsen HC, Ekselius L. Cognitive behaviour therapy for hyperacusis: A randomized controlled trial. Behav Res Ther. 2014;54:30–37. [DOI] [PubMed] [Google Scholar]
- 15.Kelman L, Tanis D. The relationship between migraine pain and other associated symptoms. Cephalalgia. 2006;26(5):548–553. [DOI] [PubMed] [Google Scholar]
- 16.Knobel KA, Sanchez TG. Loudness discomfort level in normal hearing individuals. Pro Fono. 2006;18(1):31–40. [DOI] [PubMed] [Google Scholar]
- 17.Sheldrake J, Diehl PU, Schaette R. Audiometric characteristics of hyperacusis patients. Front Neurol. 2015;6:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abouzari M, Abiri A, Djalilian HR. Successful treatment of a child with definite Meniere's disease with the migraine regimen. Am J Otolaryngol. 2019;40(3):440–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khalfa S, Dubal S, Veuillet E, Perez-Diaz F, Jouvent R, Collet L. Psychometric normalization of a hyperacusis questionnaire. ORL J Otorhinolaryngol Relat Spec. 2002;64(6):436–442. [DOI] [PubMed] [Google Scholar]
- 20.Fackrell K, Potgieter I, Shekhawat GS, Baguley DM, Sereda M, Hoare DJ. Clinical Interventions for Hyperacusis in Adults: A Scoping Review to Assess the Current Position and Determine Priorities for Research. Biomed Res Int. 2017;2017:2723715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park JM, Kim WJ, Ha JB, Han JJ, Park SY, Park SN. Effect of sound generator on tinnitus and hyperacusis. Acta Otolaryngol. 2018;138(2):135–139. [DOI] [PubMed] [Google Scholar]
- 22.Ghavami Y, Mahboubi H, Yau AY, Maducdoc M, Djalilian HR. Migraine features in patients with Meniere's disease. Laryngoscope. 2016;126(1):163–168. [DOI] [PubMed] [Google Scholar]
- 23.Ghavami Y, Haidar YM, Moshtaghi O, Lin HW, Djalilian HR. Evaluating Quality of Life in Patients With Meniere's Disease Treated as Migraine. Ann Otol Rhinol Laryngol. 2018;127(12):877–887. [DOI] [PubMed] [Google Scholar]
- 24.Sullivan M, Katon W, Russo J, Dobie R, Sakai C. A randomized trial of nortriptyline for severe chronic tinnitus. Effects on depression, disability, and tinnitus symptoms. Arch Intern Med. 1993;153(19):2251–2259. [PubMed] [Google Scholar]
- 25.Moshtaghi O, Mahboubi H, Haidar YM, Sahyouni R, Lin HW, Djalilian HR. Resolution of Persistent Post-Stapedotomy Vertigo With Migraine Prophylactic Medication. Otol Neurotol. 2017;38(10):1500–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Auerbach BD, Rodrigues PV, Salvi RJ. Central gain control in tinnitus and hyperacusis. Front Neurol. 2014;5:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zeng FG. An active loudness model suggesting tinnitus as increased central noise and hyperacusis as increased nonlinear gain. Hear Res. 2013;295:172–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Noseda R, Kainz V, Jakubowski M, et al. A neural mechanism for exacerbation of headache by light. Nat Neurosci. 2010;13(2):239–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harriott AM, Schwedt TJ. Migraine is Associated With Altered Processing of Sensory Stimuli. Curr Pain Headache Rep. 2014;18(11). [DOI] [PubMed] [Google Scholar]
- 30.Kelman L The triggers or precipitants of the acute migraine attack. Cephalalgia. 2007;27(5):394–402. [DOI] [PubMed] [Google Scholar]
- 31.Guven H, Cilliler AE, Comoglu SS. Cutaneous allodynia in patients with episodic migraine. Neurol Sci. 2013;34(8):1397–1402. [DOI] [PubMed] [Google Scholar]
- 32.de Tommaso M, Sciruicchio V, Delussi M, et al. Symptoms of central sensitization and comorbidity for juvenile fibromyalgia in childhood migraine: an observational study in a tertiary headache center. J Headache Pain. 2017;18(1):59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roberts LE, Eggermont JJ, Caspary DM, Shore SE, Melcher JR, Kaltenbach JA. Ringing ears: the neuroscience of tinnitus. J Neurosci. 2010;30(45):14972–14979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wager TD, Rilling JK, Smith EE, et al. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science. 2004;303(5661):1162–1167. [DOI] [PubMed] [Google Scholar]
- 35.Ploghaus A, Tracey I, Gati JS, et al. Dissociating pain from its anticipation in the human brain. Science. 1999;284(5422):1979–1981. [DOI] [PubMed] [Google Scholar]
- 36.Hwang JH, Tsai SJ, Liu TC, Chen YC, Lai JT. Association of Tinnitus and Other Cochlear Disorders With a History of Migraines. JAMA Otolaryngol Head Neck Surg. 2018;144(8):712–717. [DOI] [PMC free article] [PubMed] [Google Scholar]


