Significance
While the MAPK p38α is highly active in nonalcoholic fatty liver disease (NAFLD), whether its constitutive activity is a driver or mere consequence of pathology remains unclear. This was not answered due to the lack of methods for specific p38α activation in vivo. A transgenic mouse model allowing inducible expression of an intrinsically active p38α variant in a tissue-specific manner meets this challenge. Upon expression of the active p38α allele in the liver, mice develop lipidosis (fatty liver) within four weeks without gaining overweight. This study shows that fatty liver could occur in response to activation of a single protein and provides the first genetic model for revealing the etiology of fatty liver diseases from the point of their trigger.
Keywords: p38, active variants, transgenic mice, lipidosis, fatty liver
Abstract
One third of the western population suffers from nonalcoholic fatty liver disease (NAFLD), which may ultimately develop into hepatocellular carcinoma (HCC). The molecular event(s) that triggers the disease are not clear. Current understanding, known as the multiple hits model, suggests that NAFLD is a result of diverse events at several tissues (e.g., liver, adipose tissues, and intestine) combined with changes in metabolism and microbiome. In contrast to this prevailing concept, we report that fatty liver could be triggered by a single mutated protein expressed only in the liver. We established a transgenic system that allows temporally controlled activation of the MAP kinase p38α in a tissue-specific manner by induced expression of intrinsically active p38α allele. Here we checked the effect of exclusive activation in the liver. Unexpectedly, induction of p38α alone was sufficient to cause macrovesicular fatty liver. Animals did not become overweight, showing that fatty liver can be imposed solely by a genetic modification in liver per se and can be separated from obesity. Active p38α-induced fatty liver is associated with up-regulation of MUC13, CIDEA, PPARγ, ATF3, and c-jun mRNAs, which are up-regulated in human HCC. Shutting off expression of the p38α mutant resulted in reversal of symptoms. The findings suggest that p38α plays a direct causative role in fatty liver diseases and perhaps in other chronic inflammatory diseases. As p38α activity was induced by point mutations, it could be considered a proto-inflammatory gene (proto-inflammagene).
Inflammatory liver diseases form a serious epidemic in the Western world. Nonalcoholic fatty liver disease (NAFLD) has grown from a relatively unknown disease to the most common cause of chronic liver diseases in the world over the last two decades, with a global prevalence of 25% (1). NAFLD is characterized by steatosis of the liver, involving greater than 5% of parenchyma, with no evidence of hepatocyte injury. In nonalcoholic steatohepatitis (NASH), the liver cells become injured in a background of steatosis (2). NAFLD may further develop to NASH and, at a later stage, to liver cirrhosis that could eventually progress to liver cancer (3).
Many cases of liver diseases are associated with viral infections (hepatitis B and C and HIV), alcoholism, drugs, or autoimmunity. NAFLD and NASH are commonly associated with obesity, insulin resistance (IR), and type 2 diabetes. In addition, a large number of patients develop fatty liver with no known background (3, 4).
Regardless of the background conditions, the molecular basis of the triggering and the etiology of the diseases are unclear (3). As is the case for all other chronic inflammatory diseases, the current understanding of the underlying mechanism of the onset of NAFLD is that it involves some combination of genetic and environmental conditions. The latest theory is the “multiple hit” hypothesis, which suggests that a number of events, occurring in parallel in several tissues and systems, combined with environmental and genetic factors, lead to NAFLD (5–7). Such hits include IR, adipose tissue inflammation, hormones secreted from adipose tissue, nutritional factors, gut microbiota, and genetic and epigenetic factors (5, 8, 9). This explanation for the onset of NAFLD and other chronic inflammatory diseases stands in a sharp contrast to the molecular mechanism underlying the onset of cancer diseases, which are triggered by genetic alterations in proto-oncogenes and in tumor suppressor genes (10, 11). Namely, the “multiple hit” concept for the onset of NAFLD does not consider the possibility of proto-inflammatory genes that may become disease-causing genes (“inflammagenes”) following some genetic alteration. It is shown here, nonetheless, that fatty liver could be induced by a single mutated protein.
The lack of deep understanding of the molecular triggers of fatty liver diseases hinders the development of accurate experimental models (12). Current models rely on treatments that indirectly lead to accumulation of lipids in the liver. These models can be broadly categorized as diet induced, genetics, or a combination of more than one intervention. Diet-induced models include mainly the use of high-fat diet (HFD) that causes obesity and in turn NAFLD-like disease in mice. In these models, the development of the disease is slow as it must be preceded by a serious increase in body weight (13). In the genetic models, lipidosis (fatty liver) is enhanced in response to HFD as the animal is engineered to develop obesity faster. The principal genetic animal models of NASH are the ob/ob (leptin-deficient) and db/db (leptin receptor–deficient) mice, the Zucker (fa/fa) rat, foz mice (deficient in the Alstrom syndrome 1 gene) and several transgenic or conditional knockout mice (12). Exposure of the animals to drugs such as the hepatotoxin CCl4 to amplify injury and fibrosis also appears to closely resemble human NASH (12, 14). In all models, numerous biochemical pathways and systemic responses are induced in various tissues, making it difficult to extract the relevant activities that impose the liver disease. No genetic model exists in which fatty liver is induced solely by activation of a single protein by mutation or gene amplification, analogously to models of cancer diseases. By contrast, this study describes a transgenic system in which induction of a single mutated gene, exclusively in the liver, leads rapidly to the development of fatty liver, with no effect on body weight. The mutated gene encodes the mitogen-activated protein kinase (MAPK) p38α.
p38α, a member of the p38 family, which is composed of four isoforms (15), is abnormally active in chronic inflammatory diseases including NAFLD (15–18). Inhibition of p38α resulted in reversal of pathological symptoms in some experimental systems, suggesting that p38α plays a role in the maintenance of the disease and perhaps in its etiology as well (17, 18). Finally, mice knocked out for various MAPK phosphatases are prone to the development of fatty liver. Although these phosphatases affect all members of the MAPK family (i.e., p38s, JNKs, and ERKs) it seems that the effect on the liver is a result of overactive p38 (19–23).
In other inflammatory diseases in which p38α is abnormally active, including rheumatoid arthritis, asthma, and inflammatory bowel diseases (24–26), its role in these maladies, if any, is also not clear, similarly to the case of fatty liver diseases (18). We thus sought the establishment of an experimental model that will disclose the exact function of p38α in each tissue and may also point at its relative contribution to disease etiology. Achieving this goal necessitates in vivo activation of p38α per se in a tissue-specific and temporally controlled manner. Accomplishing such a task is far from trivial as, like all MAPKs, p38α manifests no basal catalytic activity when not activated, making an overexpression approach insufficient. Natural activation of p38α is obtained, in most cases, through a complex signaling cascade that culminates in p38α’s dual phosphorylation on a TGY motif located at its activation loop, a reaction catalyzed by the MAPK kinases MKK3 and MKK6 (27, 28). In the absence of this dual phosphorylation, p38α is catalytically impotent (27). Activation of upstream components of the p38 cascade induces the activity of various components in addition to p38α, including all p38 isoforms.
In this study, the challenge of activating p38α individually in a tightly controlled manner was met by combining a unique double cassette expression system in transgenic mice, with an intrinsically active mutant of p38α, p38αD176A+F327S (29, 30). p38αD176A+F327S was shown to be intrinsically catalytically active in vitro and in cell cultures, confirming its independence from MKK3/6 activation or any upstream induction (31–35). Importantly, apart from gaining intrinsic activity, p38αD176A+F327S maintained the biochemical, pharmacological, and physiological properties of naturally activated p38α molecules (35).
We describe a transgenic model in which p38α could be specifically activated in a reversible manner, in vivo, in any tissue of choice. This model could be applied for studying the role of p38α per se in various inflammatory diseases, and here, we apply it to reveal the role of p38α in the etiology of fatty liver. It was originally expected that activation of p38α in the liver would render the liver prone to stimuli that impose lipidosis, such as HFD, or CCl4. Unexpectedly, it was observed that expression of p38αD176A+F327S in liver of mice unprovoked by any treatment was sufficient to cause an increase in serum and hepatic triglycerides and a histological appearance that resembles macrovesicular lipidosis. This observation proposes a perspective on the onset of NAFLD.
Looking into the mechanism through which active p38α imposes fatty liver, it was assumed that p38α’s substrates would be strongly phosphorylated. Unexpectedly, the expression level and phosphorylation of the p38α’s substrate MAPKAPK2 (MK2) is down-regulated along with reduced phosphorylation of heat shock protein 27 (Hsp27) in mice expressing p38αD176A+F327S in the liver. This suggests that constitutive activity of p38α may impose fatty liver by causing down-regulation of downstream components, perhaps via a feedback inhibition mechanism.
Results
Generation of a “Carrier Mouse” Harboring an Induced Overexpression System for p38αD176A+F327S.
System outlines.
To achieve specific activation of p38α in liver only, we designed a double cassette system in a transgenic mouse, which allows induced overexpression of p38αD176A+F327S in a tissue-specific manner. The system is presented in Fig. 1 and is detailed below. The underlying principle is the establishment of a mouse in which the entire system exists but is silent (a “carrier” mouse). The silent system is composed of one cassette in which transcription of p38αD176A+F327S can be induced by the tetracycline-/doxycycline-dependent transcription factor rtTA and another cassette containing the rtTA gene. In the “carrier” mouse, rtTA is not expressed at all (Fig. 1A). The cassette harboring the rtTA gene can be activated to express rtTA only in cells expressing cre recombinase (Fig. 1B). The “carrier” mice do not harbor the cre gene, and therefore, expression of rtTA can be achieved only following mating of the “carrier” mice with mice strains expressing cre in a given tissue. Expression of p38αD176A+F327S thus requires the presence of both cre and doxycycline (dox) for activation of rtTA (Fig. 1C).
Fig. 1.
A schematic description of the expression system established in the transgenic mice used in this study. (A) The expression system as appears in the “carrier” mice. The bottom part of panel A shows an “rtTA expression cassette” at the 5′ region and a “transgene docking cassette” at the 3′ region. In “rtTA expression cassette,” the rtTA coding sequence (rtTA-M2) is driven by a strong and constitutively active CAGGS promoter but preceded by a transcription intervening sequence (STOP) that is bordered by flox sequences. Note that the gene encoding the rtTA could be transcribed only if the “STOP” is removed. The “transgene docking cassette” contains a PGK promoter driving hybrid selection marker (PACdTK) allowing for both positive and negative selection by puromycin and ganciclovir, respectively. The two cassettes were integrated into mouse ES cells at the Rosa26 locus via homologous recombination. The upper part of panel A shows the shuttle vector which contains the transgene expression cassette, comprised of the HA-tagged p38αD176A+F327S coding sequence driven by the tet-responsive promoter (tetOPcmv), a splicing acceptor/donor sequence (βII intron), and a polyadenylation signal sequence (bGHpA). The entire transgene expression cassette is flanked by an insulator sequence (Ins) and a FRT and FRT mutant (F3) sequence. The p38αD176A+F327S expression cassette is integrated into the transgene docking region by highly efficient recombinase-mediated cassette exchange (RMCE) process. Note that the tetOP-CMV promoter would be activated only if rtTA is expressed and is bound to doxycycline. (B) The expression system as appears in cells and tissues expressing cre, for example, in livers of p38αliver and p38αliver-Homo mice. The “STOP” sequence is removed by cre recombinase (shown in blue), and as a result, rtTA is transcribed and consequently translated (shown in pink). (C) The expression system as appears in cells and tissues expressing cre (in this study in liver) after dox is provided (shown in green). rtTA-dox complex is transcriptionally active and binds to the tet-responsive promoter, and the p38αD176A+F327S transgene is expressed.
As described below, the “carrier” mouse harboring the expression system was generated in two stages. First, the double cassette system was constructed in Rosa26 locus of mouse embryonic stem cells (mES cells). Second, the resulting ES cells were used to generate a “carrier” mouse.
Targeting p38αD176A+F327S to the designed expression system in ES cells.
To control p38αD176A+F327S expression in the mouse, we established ES cells that harbor two cassettes—the “rtTA expression cassette” and the “transgene docking cassette” (Fig. 1). Cassettes were integrated into the Rosa26 locus via homologous recombination. The “rtTA expression cassette” is comprised of a strong promoter located upstream of the rtTA gene. The rtTA gene is not transcribed however, due to the presence of a transcription-interfering sequence between the gene and the promoter (Fig. 1A; fragment marked as “stop”). The transcription-interfering sequence is flanked by two loxP sites. The “transgene docking cassette” accepts the transgene. When the gene of interest is introduced into cells on the proper shuttle vector, it can be integrated by a highly efficient recombinase-mediated cassette exchange process (Fig. 1A). Once the transgene is integrated, its transcription is controlled by a tetracycline-inducible promoter, namely, a promoter that could be induced by the rtTA transcription factor but only when the rtTA is bound to doxycycline. The human complementary DNA (cDNA) encoding p38αD176A+F327S, tagged with HA at its N terminus, was introduced into the mES cells, which harbor these cassettes. Clones in which p38αD176A+F327S was integrated properly into the “transgene docking cassette” were selected and confirmed.
Generation of p38αD176A+F327S-targeted mice.
To establish a transgenic mouse, the mES cells in which p38αD176A+F327S had been properly integrated into the “docking cassette” (Fig. 1) were injected into blastocysts which were later implanted into a pseudopregnant foster mother, thus obtaining chimeric mice (F0 generation). Heterozygous transgenic mice (F1 generation) that harbor one copy of the cassettes were obtained by breeding of the F0 chimeric mice with C57BL/6J mice. These mice are the “carriers.” When “carriers” are bred with mice expressing cre recombinase in a given tissue, the “stop” sequence is excised in the progeny only in that tissue, and consequently, rtTA is expressed exclusively in that tissue (Fig. 1B). However, the rtTA would activate transcription of the transgene only when bound to doxycycline, which can be provided in the chow (Fig. 1C).
Generation of Mice that Inducibly Express p38αD176A+F327S Specifically in the Liver.
To establish a mouse that inducibly expresses p38αD176A+F327S specifically in the liver, the carrier mouse was crossed with an Alb-cre driver homozygous mouse (36). In these mice, the cre recombinase is cloned under the Albumin enhancer/promoter and is therefore transcribed only in the liver.
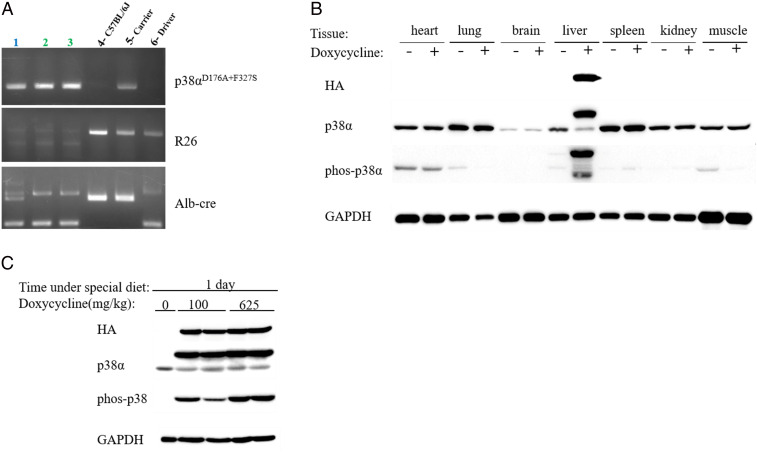
When Alb-cre mice are crossed with the p38αD176A+F327S carrier mice, in half of the progeny (i.e., those that harbor both the Alb-cre construct and the p38αD176A+F327S expression system), the “stop” fragment (that blocks transcription of rtTA) should be excised, and consequently, rtTA would be expressed only in the liver. These mice are heterozygous for the two transgenes. Upon providing doxycycline, p38αD176A+F327S should be expressed only in the liver. Mice that are positive for both the cDNA of p38αD176A+F327S and the Alb-cre were identified by PCR (SI Appendix, Fig. S1A) and termed p38αliver mice. p38αliver mice were further inbred to obtain mice that carry two copies of the p38αD176A+F327S allele and one or two copies of the Alb-cre allele (Fig. 2A). These mice were termed p38αliver-homozygous mice (from here on p38αliver-Homo) and were bred as such.
Fig. 2.
p38αD176A+F327S expression in p38αliver-Homo mice is specific to liver and responsive to the presence of doxycycline within 24 h. (A) A representative PCR of genotyping the progeny of inbreeding of p38αliver mice. Mouse 1 is homozygous for the p38αD176A+F327S transgene and heterozygous for Alb-cre: (p38αD176A+F327S +; R26 -; Alb-cre [390bp, 351bp]). Mice 2 and 3 are homozygous for the p38αD176A+F327S transgene and homozygous for Alb-cre: (p38αD176A+F327S +; R26 -; Alb-cre [390bp]). Mouse 4 is C57BL/6J: (p38αD176A+F327S -; R26 +; Alb-cre [351bp]). Mouse 5 is a carrier: (p38αD176A+F327S +; R26 +; Alb-cre [351bp]). Mouse 6 is a driver: (p38αD176A+F327S -; R26 +; Alb-cre [390bp]). (B) Western blot of protein lysates prepared from different organs as indicated, from the p38αliver-Homo mice provided with dox-supplemented (+) or regular (−) diet for 18 wk. The system is sensitive and rapid. (C) Western blot of protein lysates prepared from the liver of p38αliver-Homo mice provided with diet supplemented with either 0, 100, or 625 mg/kg doxycycline for 1 d.
p38αD176A+F327S Expression in the Transgenic Mice Is Liver Specific and Is Faithfully Responsive to Doxycycline.
To examine the operability of the transgene expression approach, a group of male p38αliver-Homo mice was provided with doxycycline (dox)-supplemented diet for 18 wk, while another group of identical mice were provided with regular diet. Mice were killed, and several tissues, including the liver, were analyzed by Western blot (Fig. 2B). This analysis revealed that p38αD176A+F327S was expressed specifically in the liver and not in any other of the tissues analyzed (heart, lung, brain, spleen, kidney, and skeletal muscle). Also, it was expressed only in mice that were fed with dox-supplemented diet (Fig. 2B).
To assess the promptness of the expression system and the degree of its sensitivity to doxycycline, mice were provided with chow supplemented with just 100 mg/kg of doxycycline for only 24 h. This dox concentration gave rise to similar expression level of p38αD176A+F327S in the liver to that observed with a concentration of 625 mg/kg within one day (Fig. 2C). In later experiments, it was confirmed that the expression system is not only rapidly responsive to low concentrations of dox but is also reversible, such that transferring mice from dox-supplemented to regular diet abolishes p38αD176A+F327S expression (see below). Notably, throughout the study, no expression whatsoever of the p38αD176A+F327S transgene was observed in any mouse or any tissue of the “carrier” mice colony or in any tissue, including the liver, of p38αliver-Homo or p38αliver (heterozygous) mice that were not exposed to doxycycline (see Figs. 3A, 7A, and 8A and SI Appendix, Fig. S1B). The transgenic expression system established here is thus reliable and strictly controlled. p38αD176A+F327S expression is dependent on both removal of the “stop” fragment and addition of dox (Fig. 1 B and C). It is faithfully restricted to liver and is sensitive, rapid, and readily reversible.
Fig. 3.
Expression of p38αD176A+F327S in the liver causes liver lipidosis without obesity. (A) Expression and phosphorylation of p38αD176A+F327S is stable and restricted to liver over a period of 33 wk, as observed in Western blots of protein lysates prepared from liver of mice that were fed with dox-supplemented (+) or regular (−) diet for 33 wk. (B) Representative H&E staining of liver cross sections from a WT mouse fed with dox-supplemented diet (Left) or from p38αliver-Homo mice provided with regular (Middle) or dox-supplemented (Right) diet for 4 wk (n = 3 to 8). (Scale bar, 100 µM.) Red arrow = hepatocellular (macrovesicular) fatty change (lipidosis). (C) A graph of average body weight of the mice in each group every week over a period of 33 wk (black: WT mice fed with dox-supplemented diet; pink: p38αliver-Homo mice fed with regular diet; and green: p38αliver-Homo mice fed with dox-supplemented diet). Data are presented as mean ± SEM.
Fig. 7.
p38αD176A+F327S expression causes down-regulation of MK2 and Hsp27 and does not affect p38γ, p38δ, and JNK. (A) Western blots of protein lysates prepared from the liver of p38αliver-Homo mice provided with dox-supplemented (+) or regular (−) diet for 1, 3, and 4 wk, using the indicated antibodies. (B) Levels of p38δ. Same protein lysates used in panel A were analyzed for expression of p38δ. As no expression of p38δ was observed in liver, the Western blot with anti p38δ antibodies included protein lysates prepared from small intestine as a positive control.
Fig. 8.
Liver-specific expression of p38αD176A+F327S is reversible. (A) Western blot of protein lysates prepared from livers of mice that were provided with dox-supplemented (+) or regular (−) diet for 28 wk or were provided with dox-supplemented diet for 22 wk and were then provided with regular diet for another 6 wk (+/−). (B) Representative H&E staining of a liver cross sections from a WT mouse provided with dox-supplemented diet and from p38αliver-Homo mice provided with dox-supplemented or regular diet for 28 wk. Bottom Right is a cross section of a p38αliver-Homo mouse that was fed with dox-supplemented diet for 22 wk and then with regular diet for another 6 wk (n = 3). (C) Representative H&E staining of a liver cross section from p38αliver-Homo mouse provided with dox-supplemented diet for 18 wk (Left), p38αliver-Homo mice provided with dox-supplemented or regular diet for 33 wk (Middle), and p38αliver-Homo mouse provided with dox-supplemented diet for 18 wk and then with regular diet for another 15 wk (Right) (n = 4). (Scale bar, 200 µM.) Red arrow in B and C = hepatocellular (macrovesicular) fatty change (lipidosis). (D) Representative micrograph showing Oil Red O staining of a liver cross section from p38αliver-Homo mouse provided with regular diet for 40 wk (Left), p38αliver-Homo mouse provided with dox-supplemented diet for 40 wk (Middle), and p38αliver-Homo mouse provided with dox-supplemented diet for 18 wk and then with regular diet for another 22 wk (Right) (n = 2). (Scale bar, 200 µM.)
p38αD176A+F327S Molecules Expressed Are Spontaneously Active in the Liver.
The critical question for using the mice as models for studying p38α biology and pathology in liver is whether the expressed p38α variant remains spontaneously activated. This concern is raised because the mechanism that renders p38αD176A+F327S spontaneously active in vitro and in cell cultures is its autophosphorylation/autoactivation capability (33–35, 37). Its activity is dependent, therefore, on its phosphorylation status that is easily maintained in vitro and in transient expression experiments in cell cultures because phosphatases either do not exist (in vitro) or are not fully activated (in the short transient experiments). In the context of the whole organism, however, tissues may activate phosphatases to compromise the constant activity of phosphorylated p38α molecules, which is potentially hazardous. The concern is strengthened by reports that active p38α is an activator of its own phosphatases in various cell types (38–40).
Testing the activation loop of the p38αD176A+F327S molecules expressed in the liver of p38αliver-Homo or p38αliver (heterozygous) mice revealed that it was spontaneously phosphorylated at high levels (see “phos-p38” in Fig. 2 B and C and SI Appendix, Fig. S1B). It implies that even if phosphatases are activated in the liver, the autophosphorylation capability of the mutant seems to dominate, maintaining the steady-state level of activation loop phosphorylation significantly higher than that of endogenous, wild-type (WT) p38α molecules. Thus, the p38αD176A+F327S molecules, which are spontaneously active in vitro and in cells in culture (29, 31, 33, 35), are also spontaneously activated in the liver of transgenic mice.
Experiments with larger numbers of mice further confirmed that the expression system is stable and reliable. Both expression and phosphorylation levels of p38αD176A+F327S in the liver of p38αliver-Homo mice provided with dox-supplemented diet were persistent at all time points tested, up to 33 wk (see details below; Fig. 3A).
It is concluded that the experimental model fulfills the goal for which it was designed (i.e., exclusive activation of p38α), only in liver in a tightly controlled manner.
p38αliver-Homo Mice Expressing p38αD176A+F327S in the Liver Develop Hepatocellular Macrovesicular Fatty Change (Lipidosis).
Thus far, no causative role has been assigned to any gene in NAFLD. In fact, no causative role for a mutated or overexpressed gene was proposed for any chronic inflammatory disease. We, therefore, did not expect that activation of p38α in liver would, by itself, cause pathological symptoms. The premise was that a constitutively active p38α in liver would render the mice prone to development of NAFLD that could be inflicted by common procedures, such as HFD or injection of CCl4 (41). Yet, we did wish to establish the baseline situation in the liver following induced expression of p38αD176A+F327S without any further treatments. To this purpose, we carried out four independent experiments that included three groups of male mice: 1) p38αliver-Homo mice provided with dox-supplemented diet (and therefore expressing p38αD176A+F327S), 2) identical mice provided with regular diet, and 3) C57BL/6J and/or p38αD176A+F327S “carrier” mice (that were not mated with Alb-cre driver mice and therefore cannot express the transgene) provided with dox-supplemented diet. The latter group (termed the WT group) serves as a control for any possible effect of dox itself.
These experiments confirmed that expression and phosphorylation of p38αD176A+F327S were stable over the entire period (up to 33 wk on dox) in the livers of p38αliver-Homo mice. No expression of the transgene was detected in the livers of the mice from the two control groups (Fig. 3A).
Unexpectedly, histopathological analysis, assisted by hematoxylin and eosin (H&E) staining, revealed the appearance of hepatocellular (macrovesicular) fatty change (lipidosis) in the p38αliver-Homo mice fed with dox-supplemented diet. Out of 30 mice 21 (70%) showed this phenotype, which was graded to a moderate level (score = 3) on average. None of the mice from the two control groups showed this phenotype (Fig. 3B). The macrovesicular lipidosis was observed as early as 4 wk after provision of dox and was maintained throughout the 33 wk of the experiment. The histological observations were supported by biochemical analyses of the liver and serum (described below).
In most experimental models used so far, fatty liver is obtained following the induction of obesity, imposed by feeding the mice with HFD (13). In our animals, the body weight of the mice from the three groups was essentially similar. In fact, not only did p38αliver-Homo mice provided with dox-supplemented diet not gain more weight than controls, they maintained a slightly lower body weight than identical mice that did not express p38αD176A+F327S, starting from 5 wk after provision of dox and throughout the 33 wk of the experiment (Fig. 3C). Also, in most other models, fatty liver develops 12 to 16 wk after introduction of HFD (19), while expression of p38αD176A+F327S causes macrovesicular lipidosis within 4 wk.
Thus, active p38α is both necessary and sufficient for the development of macrovesicular lipidosis in these mice.
Increased Hepatic Triglycerides in Mice Expressing p38αD176A+F327S in the Liver.
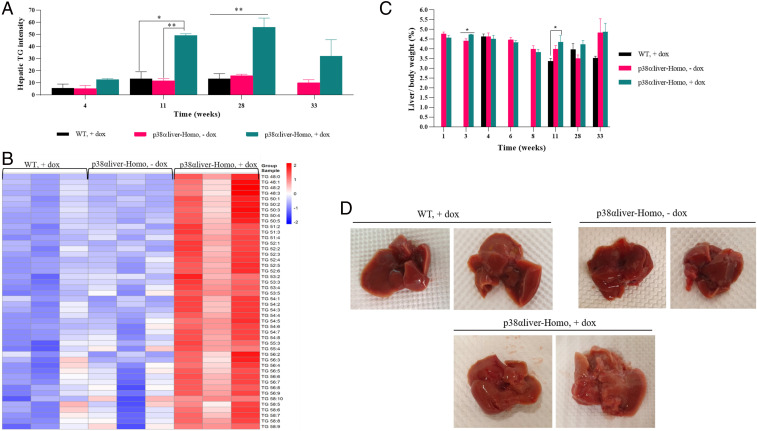
To test whether the observation of macrovesicular lipidosis at the histological level is also reflected at the biochemical level, we performed a mass spectrometry (MS)–based lipidomic assay to monitor the levels of total and specific triglyceride molecules. Significantly high levels of total triglycerides were observed in the p38αD176A+F327S-expressing mice (Fig. 4A). Detailed MS analysis showed that every single triglyceride species was up-regulated in the liver of these mice at the 28 wk time point (Fig. 4B). The high levels of triglycerides were not associated with changes in the liver weight (relative to body weight) (Fig. 4C). However, liver of mice expressing p38αD176A+F327S for 18 wk looked paler compared to the livers of p38αliver-Homo mice not provided with dox-supplemented diet and to WT mice provided with dox-supplemented diet for the same period of time (Fig. 4D).
Fig. 4.
High levels of hepatic triglycerides in mice expressing p38αD176A+F327S in the liver. (A) Liquid chromatography with tandem mass spectrometry (LC/MSMS) analysis for hepatic total triglycerides measured in WT mice fed with dox-supplemented diet (+) and in p38αliver-Homo mice fed with dox-supplemented (+) or regular (−) diet for 4, 11, 28, and 33 wk (n = 2 to 3). The intensity is normalized to internal standard and protein content. Data are presented as mean ± SEM. Statistical significance was determined by ordinary one-way ANOVA and is indicated as *P < 0.05, **P < 0.01. (B) Heat map showing up- and down-regulated species (46) of hepatic triglycerides measured by LC/MSMS in livers of WT mice fed with dox-supplemented diet (+) and of p38αliver-Homo mice fed with dox-supplemented (+) or regular (−) diet for 28 wk (n = 3). The intensity is normalized to internal standard and protein content. (C) Relative weight (liver to total body) of livers of mice (n = 2 to 9) from the three experimental groups 1, 3, 4, 6, 8, 11, 28, and 33 wk after the initiation of the experiment. Data are presented as mean ± SEM. Statistical significance was determined by unpaired two-tailed t test (for 1, 3, 6, and 8 wk time points) and by ordinary one-way ANOVA (for 4, 11, 28, and 33 wk time points) and is indicated as *P < 0.05. (D) Representative photos of whole livers from WT mice fed with dox-supplemented diet and from p38αliver-Homo mice fed with dox-supplemented (+) or regular (−) diet for 18 wk (n = 5 to 6).
Increased Triglycerides and Decreased Cholesterol and Albumin Levels in Serum of Mice Expressing p38αD176A+F327S in the Liver.
Liver functions, as assessed via testing serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST), were normal in mice expressing p38αD176A+F327S (Fig. 5A). Similarly, serum glucose and insulin levels (measured in nonfasted animals) were also not affected by p38α activation in the liver (SI Appendix, Fig. S2 A and B). Also, glucose tolerance tests, performed in fasted animals, did not show differences at the blood glucose levels in p38αliver-Homo mice provided or not with dox-supplemented diet, suggesting no diabetic symptoms (SI Appendix, Fig. S2C).
Fig. 5.
Increased triglyceride levels in the serum of mice expressing p38αD176A+F327S in the liver. (A) Serum AST and ALT levels monitored in mice (n = 2 to 8) 1, 3, 4, 6, 8, 11, 28, and 33 wk after the initiation of the experiment. Blue and black lines on the graphs represent the borders of the normal range of each parameter. (B) Serum triglycerides levels monitored in mice (n = 2 to 9) of the three indicated groups 1, 3, 4, 6, 8, 11, 28, 33, and 40 wk after the initiation of the experiment. The most right purple bar at the 28 wk time point represents a group of p38αliver-Homo mice that were provided with dox-supplemented diet for 22 wk and were then provided with regular diet for another 6 wk (n = 3). Data are presented as mean ± SEM. Statistical significance was determined by unpaired two-tailed t test (for 1, 3, 6, 8, and 40 wk time points) and by ordinary one-way ANOVA (for 4, 11, 28, and 33 wk time points) and are indicated as *P < 0.05, ***P < 0.001, ****P < 0.0001.
Equivalent to the observation in the liver, in serum too, higher levels of total triglycerides were monitored in 100% of the p38αliver-Homo mice that were provided with dox-supplemented diet (Fig. 5B). These high levels first appeared 3 wk after induction of p38αD176A+F327S expression and remained elevated during the entire experiment (Fig. 5B).
No differences were monitored in levels of nonesterified fatty acids in the serum of the three experimental groups (SI Appendix, Fig. S2D), but levels of serum albumin and cholesterol were significantly lower in mice expressing p38αD176A+F327S in the liver (Fig. 6 A and B). Hepatic cholesterol levels were also lower in the p38αliver-Homo mice provided with dox, but the differences between the three groups were not as significant as in the serum (Fig. 6 B and C). Serum high-density lipoprotein (HDL) and low-density lipoprotein/very-low density lipoprotein (LDL/VLDL) cholesterol levels were also lower in mice expressing p38αD176A+F327S (Fig. 6D).
Fig. 6.
Decreased cholesterol and albumin levels in the serum of mice expressing p38αD176A+F327S in the liver. (A) Serum albumin of mice (n = 2 to 8) 1, 3, 4, 6, 8, 11, 28, and 33 wk after the initiation of the experiment. Blue and black lines on the graph represent the borders of the normal range of albumin. (B) Serum cholesterol levels of mice (n = 2 to 9) from the three indicated groups 4, 6, 8, 11, 28, and 33 wk after the initiation of the experiment. The most right purple bar in A and B at the 28 wk time point represents a group of p38αliver-Homo mice that were provided with dox-supplemented diet for 22 wk and were then provided with regular diet for another 6 wk (n = 3). (C) Hepatic cholesterol levels of mice (n = 3 to 9) of the three indicated groups 4, 11, 28, and 33 wk after the initiation of the experiment. Data are presented as mean ± SEM. Statistical significance was determined by unpaired two-tailed t test (for 1, 3, 6, and 8 wk time points) and by ordinary one-way ANOVA (for 4, 11, 28, and 33 wk time points) and indicated as *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001. (D) Serum HDL and LDL/VLDL cholesterol levels of mice (n = 1 to 4) from the three indicated groups 4, 6, 28, and 33 wk after the initiation of the experiment. Data are presented as mean ± SEM. Statistical significance was determined by unpaired two-tailed t test and indicated as ***P < 0.001.
These measurements combined show that activation of p38α by itself results in dramatic changes in lipid composition in liver and serum: accumulation of triglycerides, appearance of numerous lipid droplets, and inability of the liver to provide sufficient albumin and cholesterol to the serum. These changes resemble some characteristics of NAFLD (Discussion).
Elevation of MUC13, CIDE A, PPARγ, ATF3, and c-jun mRNA Levels upon Expression of p38αD176A+F327S in the Liver.
The unexpected phenomenon that activation of a single enzyme by point mutations is sufficient to cause hepatocellular macrovesicular fatty change should facilitate the study of the molecular basis for fatty liver diseases. As a first step toward understanding which p38α-dependent downstream events lead to fatty liver, we monitored changes in the transcriptome of livers expressing p38αD176A+F327S for 1, 21, and 28 d. As is shown in the Venn diagram in SI Appendix, Fig. S3A, levels of 38 messsenger RNA (mRNA) molecules were significantly modified (up- or down-regulated by more than twofold) in p38αD176A+F327S-expressing mice compared to control mice at the three time points (SI Appendix, Fig. S3A). In total, 30 of the genes are known annotated genes (SI Appendix, Fig. S3B). We validated by individual qRT-PCRs some of the observations made via the global transcriptome analysis (i.e., the up-regulation of MUC13, Rsph1, WNK2, Ube2e2, and Gstp3 and the down-regulation of Slc10a, Fgl1, and Cyp2c44) (SI Appendix, Fig. S4). The prominent elevation of MUC13 mRNA in mice expressing p38αD176A+F327S in the liver (SI Appendix, Figs. S3B and S4 A, Left) is interesting given that MUC13 was reported to be up-regulated in hepatocellular carcinoma (HCC) (Discussion).
In addition to the 38 genes that were significantly modified in all three time points, we further considered genes that were significantly modified in one or two time points, some of which might be relevant to NAFLD and/or are known to be associated with the p38α pathway. For example, members of the cell death–inducing DEF45-like effector (CIDE) protein family, including CIDE A, CIDE B, and CIDE C (also known as FSP27), play important roles in the development of hepatic steatosis (42, 43). In the transcriptome analysis, CIDE A and CIDE C are found elevated in mice expressing p38αD176A+F327S (SI Appendix, Fig. S3C). This was confirmed via qRT-PCR that showed a significant increase of CIDE A levels in mice expressing p38αD176A+F327S, which was most prominent 33 wk after provision of dox-supplemented diet (SI Appendix, Fig. S5A). A slight increase in expression of CIDE C, but not of CIDE B, was also observed (SI Appendix, Figs. S3C and S5A).
Previous studies have shown that CIDE A is a direct mediator of peroxisome proliferator-activated receptor gamma (PPARγ)–dependent hepatic steatosis (19, 44). Indeed, levels of PPARγ mRNA were also high in p38αD176A+F327S-expressing mice at most time points analyzed (SI Appendix, Figs. S3C and S5B). Thus, active p38α can, by itself, induce the PPARγ–CIDE A module, known to be strongly associated with NAFLD (45, 46).
We also monitored levels of known target genes of the p38α pathway, mainly members of the AP-1 family, which are immediate early transcription factors (47). AP-1 is a group of homo- and heterodimers made up of members of the Jun, Fos, Maf, and ATF subfamilies (48). Several studies have shown an involvement of these proteins in different liver pathologies. Particularly, increased mRNA levels of ATF3 are closely associated with hepatic steatosis (49, 50). ATF3 expression in the livers of NAFLD patients is also positively correlated with expression of c-jun (50). In our mice system, we also observed a marked increase in mRNA levels of ATF3 and c-jun in the livers of mice expressing p38αD176A+F327S (SI Appendix, Fig. S6, Upper and SI Appendix, Fig. S3C). There were no marked differences in the expression levels of JunB, JunD, and c-Fos between livers of mice expressing or not expressing p38αD176A+F327S in the liver (SI Appendix, Fig. S6, Bottom and SI Appendix, Fig. S3C).
We further monitored mRNA levels of genes encoding proteins involved in endogenous synthesis, breakdown, or secretion of hepatic lipids (FASN, DGAT1, PPARα, MTTP, and TM6SF2) and apolipoproteins (APOA1, APOB, and APOE). Levels of these mRNAs didn’t differ significantly between the livers of the two groups of mice as observed in the microarray and the qRT-PCR assays (SI Appendix, Figs. S3C and S7) except for TM6SF2 which is known to be involved in VLDL secretion from the liver (51), and its expression was down-regulated in mice expressing p38αD176A+F327S in the liver (SI Appendix, Fig. S7, Right Bottom). This observation is in correlation with reduced levels of serum VLDL observed in these mice (Fig. 6D, Right).
High Levels of the Chemokines Gro-α, MCP-1, and RANTES in the Serum of Mice Expressing p38αD176A+F327S in the Liver.
Cytokines and adipocytokines play a major role in the orchestration of inflammatory processes throughout the body (52). The pathophysiology of various aspects of human NAFLD, for example, is associated with elevated levels of the proinflammatory cytokines TNFα (tumor necrosis factor) and IL-6 (5, 53). We thus monitored the levels of chemokines and cytokines in our model. Real-time PCR analysis revealed an increase of MCP-1/CCL-2 mRNA level in livers of mice expressing p38αD176A+F327S (SI Appendix, Fig. S8 A, Right Upper). As this result was not correlated with the results of the microarray (SI Appendix, Fig. S3C), in which slight reduction in the expression of MCP-1 was observed, we confirmed the reproducibility four times and also monitored elevation of the MCP-1 protein (in a multiplex analysis) (SI Appendix, Fig. S8 A, Right Upper and SI Appendix, Fig. S8B). Interleukin- 10 (IL-10), which was not observed in the microarray since its signal was too low, was slightly elevated in mice expressing p38αD176A+F327S, as detected by real-time PCR analysis (SI Appendix, Fig. S8 A, Right Bottom). No marked differences and/or slight down-regulation in the levels of TNFα, IL-6, IL-1β, and IFNγ mRNA between the two experimental groups (SI Appendix, Figs. S3C and S8A) were found both in the qRT-PCR and the microarray assays.
Multiplex analysis of 36 inflammatory cytokines and chemokines, using the Luminex system, identified higher levels of three chemokines in serum of mice expressing p38αD176A+F327S in the liver, Gro-α/KC, MCP-1, and RANTES (SI Appendix, Fig. S8B). Measures of the other 33 cytokines and chemokines were similar in the three experimental groups (SI Appendix, Table S1).
No Changes at the Expression and Phosphorylation Levels of Other MAPKs.
Although the model established in this study restricts activation of p38α per se, the possibility remains that the highly active p38α molecule leads to activation of other stress-activated kinases, such as other p38 isoforms and JNKs. This notion is based on the cross-talk that exists between p38 isoforms and between p38s and JNKs (31, 54) and on the fact that high JNK activity, especially of JNK1, is associated with NASH (55). It was observed however that expression of p38αD176A+F327S did not affect other stress kinases. p38γ is expressed in mouse liver, but its expression level and phosphorylation status were similar in the different groups of mice (Figs. 7A and 8A). Namely, no phospho-p38γ band, which should migrate slower on sodium dodecyl sulphate polyacrlylamide gel electrophoresis (SDS-PAGE), appears with the anti–phospho-p38 antibody. p38δ is not expressed in the liver and remained undetectable in livers of mice expressing or not expressing p38αD176A+F327S (Fig. 7B). JNK’s expression and phosphorylation levels were also similar in mice expressing or not expressing p38αD176A+F327S in the liver (Fig. 7A).
p38αD176A+F327S Expression Causes Down-Regulation of Its Substrate MK2 and of Hsp27 Phosphorylation.
What is the basis for the mechanism of action of p38αD176A+F327S expression that may cause fatty liver? To address this question, we analyzed the expression and phosphorylation levels of a major p38α’s substrate, MK2, and of its own substrate Hsp27 (56). The premise was that these p38α targets would be strongly activated. In contrast to this presumption, it was observed that in p38αliver-Homo mice expressing p38αD176A+F327S, MK2 expression and phosphorylation were down-regulated (Fig. 7A). Accordingly, the phosphorylation level of Hsp27 was also down-regulated, barely detectable in mice expressing p38αD176A+F327S for 4 wk (Fig. 7A). Interestingly, a mild reduction at the expression of endogenous p38α was noted as well in these mice compared to the control animals (Fig. 7A). A plausible model for the mechanism of action of p38αD176A+F327S would be, therefore, that this variant imposes down-regulation of its endogenous counterpart and downstream components of the pathway, either by direct activation of protein proteolytic systems or reduction of translation or indirectly via feedback mechanisms. Interestingly, the mutual relationships between p38α and MK2 were already observed in several systems, mainly in the MK2−/− and MK2/3−/− knockout mice in which it has been shown that MK2 and MK3 cooperate in stimulation of TNFα biosynthesis and in stabilization of p38 MAPK (57).
The Phenotypes Imposed on the Liver by p38αD176A+F327S Are Reversed upon Suppression of p38αD176A+F327S Expression.
To test whether the dox-driven expression of p38αD176A+F327S (and the phenotypes thereof) can be reversed by removing dox from the diet, p38α liver-Homo mice were provided with dox-supplemented diet for 22 wk and were then provided with regular diet for another 6 wk. Following this procedure, expression of the p38αD176A+F327S transgene and its phosphorylation could no longer be detected in the liver (Fig. 8A, lanes 10 through 12). The shut-off of p38αD176A+F327S expression was associated with some reversal of the symptoms. The levels of serum cholesterol, albumin, and triglycerides were restored to levels comparable with those in mice of the control groups (see the 28 wk time point [right purple bar] in Figs. 5B and 6 A and B). Also, mRNA levels of CIDEA, ATF3, c-jun, and MCP-1 returned to normal (SI Appendix, Figs. S5A, S6, and S8A [Upper]; see the 28 wk time point [right purple bar]). Importantly, although these molecular parameters were normal 6 wk after ending p38αD176A+F327S expression, lipidosis was still observed in the “reversed” mice (Fig. 8B). It could be that livers are so dramatically affected by p38αD176A+F327S that they cannot recover of lipidosis. It may also be that recovery requires more than 6 wk. To address this matter, in another experiment, we allowed 15 wk of recovery (providing regular diet) following 18 wk on dox-supplemented diet. Following this procedure, lipidosis wasn’t observed, as is reflected by absence of lipid droplets in H&E analysis (Fig. 8C) and negative staining with Oil Red O (Fig. 8D). It is concluded that livers can recover of p38αD176A+F327S-induced lipidosis but require a long time.
The above analysis suggests that the system is reversible, namely, p38α expression/activity can be readily turned on and off. It further suggests that the observed molecular changes and the outcome, lipidosis, are directly associated with the expression of the intrinsically active variant of p38α. Importantly, liver recovery is a very long process that requires turning p38α activity off for several months.
Discussion
The main observation of this study, that fatty liver readily develops in response to expression of a mutated gene, suggests that fatty liver diseases, and perhaps other chronic inflammatory diseases, could have a genetic basis. Current understanding of the onset of these diseases does not consider a single molecular or genetic alteration as a prominent causative event. Indeed, a few genetic risk factors have been proposed to increase the risk of NASH on the basis of genome-wide association studies, but none of them play a causative role, and they seem to require additional environmental drivers, such as adiposity (58). The best characterized NASH-associated single-nucleotide polymorphism (snp) was found in the PNPLA3 gene, which regulates lipolysis of hepatocyte lipid droplets (59). Another snp creates a splice variant of the TM6SF2 gene, which is likely required for normal VLDL secretion and also influences triglyceride secretion and hepatic lipid droplet content (60).
Here, we described a system in which a mutated protein plays an absolute causative role and is sufficient by itself to impose fatty liver. Previous studies reported a correlation between fatty liver diseases and activation of p38. It was shown, for example, that a molecule known as Regulator of G protein signaling 5, which directly binds TGF-β-activated kinase 1 (TAK1), prevents its hyperphosphorylation and activation of the downstream JNK/p38 signaling cascade, thereby protecting the liver against the progression of NAFLD (61). Also, p38α-deleted macrophages were shown to attenuate steatohepatitic changes in hepatocytes (17). In line with these observations, mice that are deficient in the general MAPK phosphatase 5 and manifest increased p38α phosphorylation in all tissues develop aging-associated hepatic lipid accumulation, inflammation, and fibrosis. When these mice are exposed to HFD, fatty liver is developed more readily (19). Several other MAPK phosphatases, such as the dual-specificity phosphatases DUSP9, DUSP12, DUSP14, and DUSP26, seem to protect the liver against NAFLD (20–23). The expression of the phosphatases is down-regulated in fatty livers. In these studies, which utilized hepatocyte-specific knockout or overexpression of the phosphatases, it was revealed that DUSP9 and DUSP12 bind and dephosphorylate ASK1 and inhibit its action on p38 and JNK (20, 22). On the other hand, DUSP14 and DUSP26 bind and dephosphorylate TAK1 to block the phosphorylation of p38 and JNK (21, 23). Accordingly, treatments that reduce the symptoms of NAFLD also cause reduction in p38α activity (62). These studies, together with our study that shows that active p38α actually causes fatty liver, assign a critical role for p38α in the etiology of liver inflammatory diseases.
Intriguingly, some studies described the opposite observation, namely, down-regulation of p38 activity in NAFLD. In a particular experimental setup, expression of MKP1 was reported to increase in mice fed with HFD, which was correlated with the development of hepatic steatosis, suggesting that hepatic p38α/β MAPK declines in states of obesity (63). Another study reported that hepatic p38α/β MAPK activity decreases in mice fed with HFD (64). Finally, suppression of p38 in liver or isolated hepatocytes led to elevated expression of lipogenic genes and increased triglyceride levels in both plasma and liver (65).
How could these contradictory studies be reconciled? A possible explanation is a dual role of p38α in NAFLD, as has already been shown in tumorigenesis. p38α may either facilitate or interfere with tumor development, depending on the context (18, 66). Another reason for the differences in the conclusions might reside in the fact that almost all studies mentioned above used pharmacological inhibitors of p38α/β and/or mice knocked out for DUSPs, which are not specific for p38α.
Interestingly, a recent study utilizing mice with hepatocyte-specific deletion of p38α pointed at a differential role of p38α in the liver, depending on the grade of steatosis. Weak p38α activation in fatty liver leads to up-regulation of genes involved in fatty acid β-oxidation through peroxisome proliferator-activated receptor alpha phosphorylation, thereby reducing steatosis. Conversely, strong p38α activation in NASH promoted caspase-3 cleavage, CCAAT-enhancer–binding proteins homologous protein expression, and B cell lymphoma 2 phosphorylation, thereby exacerbating hepatocyte death (67). The latter observation is in agreement with our model in which strong activation of the intrinsically active variant of p38α causes high degree of steatosis.
We have observed normal levels of serum ALT and AST in mice expressing p38αD176A+F327S. Although considered markers of hepatic dysfunction and usually elevated in NAFLD, several studies analyzing populations with NAFLD have shown individuals with normal ALT and AST values (68, 69).
Mice expressing the active variant of p38α in the liver had lower levels of serum albumin values. It was reported that an independent predictor of liver-related mortality in NAFLD patients was lower albumin levels (70). A more recent report has shown that the severity of liver steatosis was negatively correlated with serum albumin. Furthermore, the presence of NAFLD and low serum albumin levels were strong predictors of poor outcome in elderly hemodialysis patients (71).
NAFLD is mostly associated with high serum and hepatic cholesterol levels (72, 73). However, a study of A. Trojak et al. suggests that NAFLD is associated with visceral obesity and low HDL-cholesterol in patients with type 2 diabetes (74), which is in correlation with our observation of low serum HDL levels in mice expressing p38αD176A+F327S.
Transcriptome analysis revealed up-regulation of MUC13, CIDEA, PPARγ, ATF3, and c-jun. It has been shown that overexpression of MUC13 plays a critical role in the development and progression of HCC by activating Wnt signaling (75). Moreover, in ovarian cancer, MUC13 promotes cell motility and proliferation through modulation of JNK-, PK1-, and p38-signaling pathways (76). Elevation of MUC13, c-jun, and ATF3 at early stages of p38αD176A+F327S-induced lipidosis may suggest that not only does activation of p38α per se cause fatty liver, it also induces proto-oncogenic components that may contribute to the initiation of the NAFLD-to-HCC process.
Combining the different observations, a plausible mechanism for p38αD176A+F327S-imposed fatty liver may involve down-regulation of MK2 expression and activity that in turn leads to reduction of Hsp27 phosphorylation, which activates the PPARγ–CIDEA module. It has been shown previously that phosphorylated Hsp27 promotes hepatic lipid clearance and stimulates autophagy (77).
Hepatic lipid accumulation may be induced by four separate mechanisms: 1) increased hepatic uptake of circulating fatty acids, 2) increased hepatic de novo fatty acid synthesis, 3) decreased hepatic beta oxidation, and (4) decreased hepatic lipid export (78). It remains to be revealed which of these mechanisms is used by p38α to cause NAFLD. Constructing a system for activation of p38α per se was hindered by its tight regulation and by the fact that mutations that render MAPKs unregulated have not been identified in diseases [reviewed in (15, 18)]. Therefore, isolation of intrinsically active variants required a particular type of large-scale screening (29, 32, 33, 35). These screens provided intrinsically active variants of all p38 and Erk isoforms (34, 35, 79, 80), enabling the generation of transgenic systems, similar to the one presented here, for studying each of these proteins.
To confirm the general application of the transgenic system described here, we have already applied it to generate mice that inducibly express p38αD176A+F327S specifically in lung (SI Appendix, Fig. S9A) or in skeletal muscle (SI Appendix, Fig. S9B). We are currently studying the effects of specific activation of p38α on these tissues.
Materials and Methods
Transgenic Mice.
The carrier p38αD176A+F327S transgenic mice were generated in the Animal Gene Editing Laboratory (AGEL), Biological Resource Centre, Agency for Science, Technology and Research (A*STAR) under institutional animal care and use committee (IACUC) #151101. The p38αD176A+F327S targeted ES cells were generated by two-step cell transfection. Alb-cre driver homozygous mice (Jackson Laboratories, stock: 003574) were purchased from JAX laboratories. C57BL/6JInv (Jax) mice were purchased from InVivos.
Supplementary Material
Acknowledgments
We thank Dr. Allan Bar-Sinai and Dr. Ido Goldstein for useful comments on the manuscript. We also thank Prof. Shlomo Sasson for critical comments on the manuscript and for useful advice on lipids analysis. The study was supported by the Israel Science Foundation, Grant 1463/18 (to D.E.) and by the Singapore National Research Foundation under its HUJ-NUS partnership program in the Campus for Research Excellence and Technology Enterprise (to D.E. and W.S.F.W.). D.E. holds a Wolfson family chair in Biochemistry.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2018069118/-/DCSupplemental.
Data Availability
All study data are included in the article and/or SI Appendix.
Change History
April 05, 2021: The PDF has been updated.
References
- 1.Younossi Z., et al., Global perspectives on nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology 69, 2672–2682 (2019). [DOI] [PubMed] [Google Scholar]
- 2.Kanwar P., Kowdley K. V., The metabolic syndrome and its influence on nonalcoholic steatohepatitis. Clin. Liver Dis. 20, 225–243 (2016). [DOI] [PubMed] [Google Scholar]
- 3.Benedict M., Zhang X., Non-alcoholic fatty liver disease: An expanded review. World J. Hepatol. 9, 715–732 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharma A., Nagalli S., “Chronic liver disease” in StatPearls (Treasure Island, FL, 2020). [Google Scholar]
- 5.Cai J., Zhang X. J., Li H., Progress and challenges in the prevention and control of nonalcoholic fatty liver disease. Med. Res. Rev. 39, 328–348 (2019). [DOI] [PubMed] [Google Scholar]
- 6.Tilg H., Adolph T. E., Moschen A. R., Multiple parallel hits hypothesis in NAFLD - revisited after a decade. Hepatology 73, 833–842 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tilg H., Moschen A. R., Evolution of inflammation in nonalcoholic fatty liver disease: The multiple parallel hits hypothesis. Hepatology 52, 1836–1846 (2010). [DOI] [PubMed] [Google Scholar]
- 8.Azzu V., Vacca M., Virtue S., Allison M., Vidal-Puig A., Adipose tissue-liver cross talk in the control of whole-body metabolism: Implications in nonalcoholic fatty liver disease. Gastroenterology 158, 1899–1912 (2020). [DOI] [PubMed] [Google Scholar]
- 9.Sookoian S., Pirola C. J., Valenti L., Davidson N. O., Genetic pathways in nonalcoholic fatty liver disease: Insights from systems biology. Hepatology 72, 330–346 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang L. H., Wu C. F., Rajasekaran N., Shin Y. K., Loss of tumor suppressor gene function in human cancer: An overview. Cell. Physiol. Biochem. 51, 2647–2693 (2018). [DOI] [PubMed] [Google Scholar]
- 11.Vogelstein B., Kinzler K. W., Cancer genes and the pathways they control. Nat. Med. 10, 789–799 (2004). [DOI] [PubMed] [Google Scholar]
- 12.Santhekadur P. K., Kumar D. P., Sanyal A. J., Preclinical models of non-alcoholic fatty liver disease. J. Hepatol. 68, 230–237 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Velázquez K. T., et al., Prolonged high-fat-diet feeding promotes non-alcoholic fatty liver disease and alters gut microbiota in mice. World J. Hepatol. 11, 619–637 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedman S. L., Neuschwander-Tetri B. A., Rinella M., Sanyal A. J., Mechanisms of NAFLD development and therapeutic strategies. Nat. Med. 24, 908–922 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cuenda A., Rousseau S., p38 MAP-kinases pathway regulation, function and role in human diseases. Biochim. Biophys. Acta 1773, 1358–1375 (2007). [DOI] [PubMed] [Google Scholar]
- 16.Jiménez-Castro M. B., Cornide-Petronio M. E., Gracia-Sancho J., Casillas-Ramírez A., Peralta C., Mitogen activated protein kinases in steatotic and non-steatotic livers submitted to ischemia-reperfusion. Int. J. Mol. Sci. 20, 1785 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang X., et al., Macrophage p38α promotes nutritional steatohepatitis through M1 polarization. J. Hepatol. 71, 163–174 (2019). [DOI] [PubMed] [Google Scholar]
- 18.Gupta J., Nebreda A. R., Roles of p38α mitogen-activated protein kinase in mouse models of inflammatory diseases and cancer. FEBS J. 282, 1841–1857 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang P., et al., Protective function of mitogen-activated protein kinase phosphatase 5 in aging- and diet-induced hepatic steatosis and steatohepatitis. Hepatol. Commun. 3, 748–762 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang Z., et al., Dual specificity phosphatase 12 regulates hepatic lipid metabolism through inhibition of the lipogenesis and apoptosis signal-regulating kinase 1 pathways. Hepatology 70, 1099–1118 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang S., et al., Hepatocyte DUSP14 maintains metabolic homeostasis and suppresses inflammation in the liver. Hepatology 67, 1320–1338 (2018). [DOI] [PubMed] [Google Scholar]
- 22.Ye P., et al., Dual-specificity phosphatase 9 protects against nonalcoholic fatty liver disease in mice through ASK1 suppression. Hepatology 69, 76–93 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ye P., et al., Dual-specificity phosphatase 26 protects against nonalcoholic fatty liver disease in mice through transforming growth factor beta-activated kinase 1 suppression. Hepatology 69, 1946–1964 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feng Y. J., Li Y. Y., The role of p38 mitogen-activated protein kinase in the pathogenesis of inflammatory bowel disease. J. Dig. Dis. 12, 327–332 (2011). [DOI] [PubMed] [Google Scholar]
- 25.Schett G., Zwerina J., Firestein G., The p38 mitogen-activated protein kinase (MAPK) pathway in rheumatoid arthritis. Ann. Rheum. Dis. 67, 909–916 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barnes P. J., Kinases as novel therapeutic targets in asthma and chronic obstructive pulmonary disease. Pharmacol. Rev. 68, 788–815 (2016). [DOI] [PubMed] [Google Scholar]
- 27.Raingeaud J., et al., Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase activation by dual phosphorylation on tyrosine and threonine. J. Biol. Chem. 270, 7420–7426 (1995). [DOI] [PubMed] [Google Scholar]
- 28.Zarubin T., Han J., Activation and signaling of the p38 MAP kinase pathway. Cell Res. 15, 11–18 (2005). [DOI] [PubMed] [Google Scholar]
- 29.Bell M., Capone R., Pashtan I., Levitzki A., Engelberg D., Isolation of hyperactive mutants of the MAPK p38/Hog1 that are independent of MAPK kinase activation. J. Biol. Chem. 276, 25351–25358 (2001). [DOI] [PubMed] [Google Scholar]
- 30.Engelberg D., Livnah O., Isolation of intrinsically active mutants of MAP kinases via genetic screens in yeast. Methods 40, 255–261 (2006). [DOI] [PubMed] [Google Scholar]
- 31.Beenstock J., et al., p38β mitogen-activated protein kinase modulates its own basal activity by autophosphorylation of the activating residue Thr180 and the inhibitory residues Thr241 and Ser261. Mol. Cell. Biol. 36, 1540–1554 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yaakov G., Bell M., Hohmann S., Engelberg D., Combination of two activating mutations in one HOG1 gene forms hyperactive enzymes that induce growth arrest. Mol. Cell. Biol. 23, 4826–4840 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Diskin R., Lebendiker M., Engelberg D., Livnah O., Structures of p38alpha active mutants reveal conformational changes in L16 loop that induce autophosphorylation and activation. J. Mol. Biol. 365, 66–76 (2007). [DOI] [PubMed] [Google Scholar]
- 34.Avitzour M., et al., Intrinsically active variants of all human p38 isoforms. FEBS J. 274, 963–975 (2007). [DOI] [PubMed] [Google Scholar]
- 35.Askari N., et al., Hyperactive variants of p38α induce, whereas hyperactive variants of p38γ suppress, activating protein 1-mediated transcription. J. Biol. Chem. 282, 91–99 (2007). [DOI] [PubMed] [Google Scholar]
- 36.Postic C., et al., Dual roles for glucokinase in glucose homeostasis as determined by liver and pancreatic beta cell-specific gene knock-outs using Cre recombinase. J. Biol. Chem. 274, 305–315 (1999). [DOI] [PubMed] [Google Scholar]
- 37.Askari N., Beenstock J., Livnah O., Engelberg D., p38α is active in vitro and in vivo when monophosphorylated at threonine 180. Biochemistry 48, 2497–2504 (2009). [DOI] [PubMed] [Google Scholar]
- 38.Owens D. M., Keyse S. M., Differential regulation of MAP kinase signalling by dual-specificity protein phosphatases. Oncogene 26, 3203–3213 (2007). [DOI] [PubMed] [Google Scholar]
- 39.Slack D. N., Seternes O. M., Gabrielsen M., Keyse S. M., Distinct binding determinants for ERK2/p38alpha and JNK map kinases mediate catalytic activation and substrate selectivity of map kinase phosphatase-1. J. Biol. Chem. 276, 16491–16500 (2001). [DOI] [PubMed] [Google Scholar]
- 40.Hutter D., Chen P., Barnes J., Liu Y., Catalytic activation of mitogen-activated protein (MAP) kinase phosphatase-1 by binding to p38 MAP kinase: Critical role of the p38 C-terminal domain in its negative regulation. Biochem. J. 352, 155–163 (2000). [PMC free article] [PubMed] [Google Scholar]
- 41.Van Herck M. A., Vonghia L., Francque S. M., Animal models of nonalcoholic fatty liver disease-A starter’s guide. Nutrients 9, 1072 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou L., et al., Cidea promotes hepatic steatosis by sensing dietary fatty acids. Hepatology 56, 95–107 (2012). [DOI] [PubMed] [Google Scholar]
- 43.Jinno Y., et al., Cide-a and Cide-c are induced in the progression of hepatic steatosis and inhibited by eicosapentaenoic acid. Prostaglandins Leukot. Essent. Fatty Acids 83, 75–81 (2010). [DOI] [PubMed] [Google Scholar]
- 44.Viswakarma N., et al., Transcriptional regulation of Cidea, mitochondrial cell death-inducing DNA fragmentation factor alpha-like effector A, in mouse liver by peroxisome proliferator-activated receptor alpha and gamma. J. Biol. Chem. 282, 18613–18624 (2007). [DOI] [PubMed] [Google Scholar]
- 45.Liss K. H., Finck B. N., PPARs and nonalcoholic fatty liver disease. Biochimie 136, 65–74 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wolf Greenstein A., et al., Hepatocyte-specific, PPARγ-regulated mechanisms to promote steatosis in adult mice. J. Endocrinol. 232, 107–121 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shaulian E., Karin M., AP-1 as a regulator of cell life and death. Nat. Cell Biol. 4, E131–E136 (2002). [DOI] [PubMed] [Google Scholar]
- 48.Mechta-Grigoriou F., Gerald D., Yaniv M., The mammalian jun proteins: Redundancy and specificity. Oncogene 20, 2378–2389 (2001). [DOI] [PubMed] [Google Scholar]
- 49.Nagahara R., et al., Gene expression analysis of the activating factor 3/nuclear protein 1 axis in a non-alcoholic steatohepatitis mouse model. Yonago Acta Med. 62, 36–46 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim J. Y., et al., Activating transcription factor 3 is a target molecule linking hepatic steatosis to impaired glucose homeostasis. J. Hepatol. 67, 349–359 (2017). [DOI] [PubMed] [Google Scholar]
- 51.Borén J., et al., Effects of TM6SF2 E167K on hepatic lipid and very low-density lipoprotein metabolism in humans. JCI Insight 5, 144079 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marra F., Bertolani C., Adipokines in liver diseases. Hepatology 50, 957–969 (2009). [DOI] [PubMed] [Google Scholar]
- 53.Byrne C. D., Targher G., NAFLD: A multisystem disease. J. Hepatol. 62(suppl. 1), S47–S64 (2015). [DOI] [PubMed] [Google Scholar]
- 54.Wagner E. F., Nebreda A. R., Signal integration by JNK and p38 MAPK pathways in cancer development. Nat. Rev. Cancer 9, 537–549 (2009). [DOI] [PubMed] [Google Scholar]
- 55.Akazawa Y., Nakao K., To die or not to die: Death signaling in nonalcoholic fatty liver disease. J. Gastroenterol. 53, 893–906 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu L., Chen S., Bergan R. C., MAPKAPK2 and HSP27 are downstream effectors of p38 MAP kinase-mediated matrix metalloproteinase type 2 activation and cell invasion in human prostate cancer. Oncogene 25, 2987–2998 (2006). [DOI] [PubMed] [Google Scholar]
- 57.Ronkina N., et al., The mitogen-activated protein kinase (MAPK)-activated protein kinases MK2 and MK3 cooperate in stimulation of tumor necrosis factor biosynthesis and stabilization of p38 MAPK. Mol. Cell. Biol. 27, 170–181 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stender S., et al., Adiposity amplifies the genetic risk of fatty liver disease conferred by multiple loci. Nat. Genet. 49, 842–847 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pingitore P., Romeo S., The role of PNPLA3 in health and disease. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1864, 900–906 (2019). [DOI] [PubMed] [Google Scholar]
- 60.Kozlitina J., et al., Exome-wide association study identifies a TM6SF2 variant that confers susceptibility to nonalcoholic fatty liver disease. Nat. Genet. 46, 352–356 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang J., et al., Hepatic regulator of G protein signaling 5 ameliorates NAFLD by suppressing TAK1-JNK/p38 signaling. Hepatology 73, 104–125 (2021). [DOI] [PubMed] [Google Scholar]
- 62.Musolino V., et al., Bergamot polyphenols improve dyslipidemia and pathophysiological features in a mouse model of non-alcoholic fatty liver disease. Sci. Rep. 10, 2565 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lawan A., et al., Hepatic mitogen-activated protein kinase phosphatase 1 selectively regulates glucose metabolism and energy homeostasis. Mol. Cell. Biol. 35, 26–40 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee J., et al., p38 MAPK-mediated regulation of Xbp1s is crucial for glucose homeostasis. Nat. Med. 17, 1251–1260 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xiong Y., et al., p38 mitogen-activated protein kinase plays an inhibitory role in hepatic lipogenesis. J. Biol. Chem. 282, 4975–4982 (2007). [DOI] [PubMed] [Google Scholar]
- 66.Igea A., Nebreda A. R., The stress kinase p38α as a target for cancer therapy. Cancer Res. 75, 3997–4002 (2015). [DOI] [PubMed] [Google Scholar]
- 67.Hwang S., et al., Protective and detrimental roles of p38α MAPK in different stages of nonalcoholic fatty liver disease. Hepatology 72, 873–891 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Uslusoy H. S., Nak S. G., Gülten M., Biyikli Z., Non-alcoholic steatohepatitis with normal aminotransferase values. World J. Gastroenterol. 15, 1863–1868 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mofrad P., et al., Clinical and histologic spectrum of nonalcoholic fatty liver disease associated with normal ALT values. Hepatology 37, 1286–1292 (2003). [DOI] [PubMed] [Google Scholar]
- 70.Rafiq N., et al., Long-term follow-up of patients with nonalcoholic fatty liver. Clin. Gastroenterol. Hepatol. 7, 234–238 (2009). [DOI] [PubMed] [Google Scholar]
- 71.Mikolasevic I., et al., Nonalcoholic fatty liver disease (NAFLD) - a new factor that interplays between inflammation, malnutrition, and atherosclerosis in elderly hemodialysis patients. Clin. Interv. Aging 9, 1295–1303 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ioannou G. N., The role of cholesterol in the pathogenesis of NASH. Trends Endocrinol. Metab. 27, 84–95 (2016). [DOI] [PubMed] [Google Scholar]
- 73.Ballestri S., Nascimbeni F., Romagnoli D., Lonardo A., The independent predictors of non-alcoholic steatohepatitis and its individual histological features.: Insulin resistance, serum uric acid, metabolic syndrome, alanine aminotransferase and serum total cholesterol are a clue to pathogenesis and candidate targets for treatment. Hepatol. Res. 46, 1074–1087 (2016). [DOI] [PubMed] [Google Scholar]
- 74.Trojak A., Waluś-Miarka M., Woźniakiewicz E., Małecki M. T., Idzior-Waluś B., Nonalcoholic fatty liver disease is associated with low HDL cholesterol and coronary angioplasty in patients with type 2 diabetes. Med. Sci. Monit. 19, 1167–1172 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dai Y., et al., Overexpression of MUC13, a poor prognostic predictor, promotes cell growth by activating Wnt signaling in hepatocellular carcinoma. Am. J. Pathol. 188, 378–391 (2018). [DOI] [PubMed] [Google Scholar]
- 76.Chauhan S. C., et al., Expression and functions of transmembrane mucin MUC13 in ovarian cancer. Cancer Res. 69, 765–774 (2009). [DOI] [PubMed] [Google Scholar]
- 77.Shen L., et al., Phosphorylated heat shock protein 27 promotes lipid clearance in hepatic cells through interacting with STAT3 and activating autophagy. Cell. Signal. 28, 1086–1098 (2016). [DOI] [PubMed] [Google Scholar]
- 78.Geisler C. E., Renquist B. J., Hepatic lipid accumulation: Cause and consequence of dysregulated glucoregulatory hormones. J. Endocrinol. 234, R1–R21 (2017). [DOI] [PubMed] [Google Scholar]
- 79.Levin-Salomon V., Kogan K., Ahn N. G., Livnah O., Engelberg D., Isolation of intrinsically active (MEK-independent) variants of the ERK family of mitogen-activated protein (MAP) kinases. J. Biol. Chem. 283, 34500–34510 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Smorodinsky-Atias K., Soudah N., Engelberg D., Mutations that confer drug-resistance, oncogenicity and intrinsic activity on the ERK MAP kinases-current state of the art. Cells 9, 129 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or SI Appendix.