Abstract
Beta‐2‐glycoprotein I (β2GPI) is the major antigen for the antiphospholipid antibodies in the antiphospholipid syndrome. The exposed epitope in domain I of β2GPI can be recognized by the anti‐β2GPI antibody. Here, we prepared the anionic di‐oleoyl‐phosphatidylserine (DOPS) and cardiolipin (CL) liposomes to interact with the β2GPI. The conformational changes of β2GPI upon binding with the liposomes were analyzed using hydrogen/deuterium exchange mass spectrometry. The exchange level of sequences 21–27 significantly increased after β2GPI had interacted with DOPS. This change indicated a reduced interaction between domain I and domain V, inferring to a protrusion of the sequences 21–27 from the ring conformation. After β2GPI had interacted with CL for 30 min, the exchange levels in 4 of the 5 domains increased significantly. The deuteration levels of sequences 1–20, 21–27, 196–205, 273–279 and 297–306 increased, suggesting that these regions had become more exposed, and the domain I was no longer in contact with domain V. The increasing deuteration levels in sequences 70–86, 153–162, 191–198, 196–205 and 273–279 indicated β2GPI undergoing conformational changes to expose these inner regions, suggesting a structural transition. Overall, DOPS and CL induced minor conformational changes of β2GPI at sequences 21–27 and forms an intermediate conformation after 10 min of interaction. After a complete protein–lipid interaction, high negatively charged CL membrane induced a major conformation transition of β2GPI.
Keywords: beta 2 glycoprotein I, cardiolipin, H/D exchange, mass spectrometry, phosphatidylserine
1. INTRODUCTION
The antiphospholipid syndrome is an autoimmune disease manifested with vascular thrombosis and obstetrical complications. 1 It has significant morbidity and mortality. 2 One of the disease characteristics is the binding of antiphospholipid antibody to proteins, through negatively charged phospholipids, such as prothrombin and β2‐glycoprotein I (β2GPI). 3 , 4 , 5 Thrombus formation in the antiphospholipid syndrome is via the binding of antiphospholipid antibody to β2GPI. 6 When the antiphospholipid antibody binds to β2GPI, the antibody complex interacts with a number of receptors, like annexin A2, Toll‐like receptor family, glycoprotein Ibα, low‐density lipoprotein receptor‐related protein 8 and low‐density lipoprotein receptor family. The result is the activation of endothelial cells, platelets, monocytes, 7 and trophoblasts. 8 , 9 The inflammation and clotting that follow could lead to vascular thrombosis or pregnancy‐related complications. 10 , 11
On β2GPI, the domain I is the antigenic epitope specifically bound by the antiphospholipid antibody. 12 , 13 , 14 , 15 In addition, other epitopes on β2GPI can also bind to the antiphospholipid antibody. 13 , 16 , 17 , 18 For example, antibodies binding to domain V of β2GPI were reported in other diseases, like leprosy and atopic dermatitis. 19 , 20 Since these other autoantibodies are not associated with clinical manifestations and therefore they are considered non‐pathogenic. Experiments have shown that the antibody binding can trigger β2GPI dimerization, which has stronger affinity for the phospholipid membrane. 21
β2GPI, also known as apolipoproteins H, contains 326 amino acids 22 and widely present in human plasma at ~200 μg/ml. 23 β2GPI contains high proportions of proline and cysteine, and a high glycosylation level. 24 , 25 β2GPI belongs to the complement control protein family with four short consensus repeats (SCRs). The SCR contains ~60 amino acids with four cysteines and one tryptophan. It is involved in the protein–protein and protein–carbohydrate interactions. 26
Levels of oxidative stress directly affect the structure and function of β2GPI. In the normal condition, the two disulfide bonds of β2GPI are located within Cys32‐Cys60 and Cys288‐Cys326 which are typically in the disconnected state. 27 , 28 Through the actions of the oxidoreductase thioredoxin‐1 and protein disulfide isomerase, these disulfide bonds are kept in the reduced form. 29 , 30 Under oxidative stress, the disulfide bonds form. The proportion of the oxidized β2GPI is reportedly elevated in patients with antiphospholipid syndrome. 28 Since the disulfide bond Cys32‐Cys60 is located close to the B cell epitope and Cys288‐Cys326 is close to the T cell epitope, the change of the redox state of β2GPI may affect the subsequent immune response they mediate. 31 , 32
β2GPI appears in at least two conformations. X‐ray crystallography analysis showed a J‐shape conformation. 33 Closed ring conformation as shown in electron microscopy is present in the absence of the anionic phospholipid. 34 , 35 An S‐shape modification of J‐shape crystal structure was detected by X‐ray small‐angle scattering experiments. Here, the epitope on domain I of the S‐shaped protein is blocked by glycosyl chains. 36 These different conformations may co‐exist and inter‐changed. 37 , 38
β2GPI was further suggested to interact with the mitochondrial phospholipid, cardiolipin (CL). CL externalization during apoptosis may lead to the expose of CL to β2GPI. 39 , 40 The Cys281‐Cys288 region of β2GPI was identified as the interaction site. 41 When β2GPI is not exposed to the negatively charged phospholipid membrane, domain V forms a closed ring with domain I. 34 When exposed to the negatively charged phospholipid membrane, domain V interacts with the negatively charged phospholipid surface and reduces the interaction with domain I, leading to the disconnection between domain I and V to promote circular unwinding. In the end, the protein expands into a J‐form and the epitopes Arg39 and Arg43 are exposed for binding with antiphospholipid antibodies. 37 Phosphatidylserine (PS) has also been shown to be involved in β2GPI binding to either apoptotic cells 42 or activated platelets, 43 both of which may contribute to the pathogenesis of antiphospholipid syndrome. 44 , 45
To explore the pathogenesis of antiphospholipid syndrome, we used hydrogen/deuterium exchange experiments 46 to investigate the conformational changes of β2GPI after interactions with different anionic phospholipids.
2. RESULTS
2.1. Identification of the proteolyzed β2GPI fragments
Analyzing β2GPI with HDXMS is a challenging procedure, because the protein contains 11 disulfide bonds and all five domains of β2GPI have at least two disulfide bonds, a condition which is particularly difficult for proteolysis. Although we had optimized pepsin digestion by introducing abundant reducing agent (1M of TCEP) to break the disulfide bonds in the quench solution, and extended digestion time to 20 min for better action, we still encountered significant loss of peptides and unstable digestion, especially in the later experiments containing lipids. Pepsin is a non‐specific digestion protease. The identification of the fragments would require tandem mass spectrometry. After optimization of proteolysis, we were able to identify 51 peptides, accounting for 80.3% of the 326‐residue native β2GPI (Figure 1).
FIGURE 1.
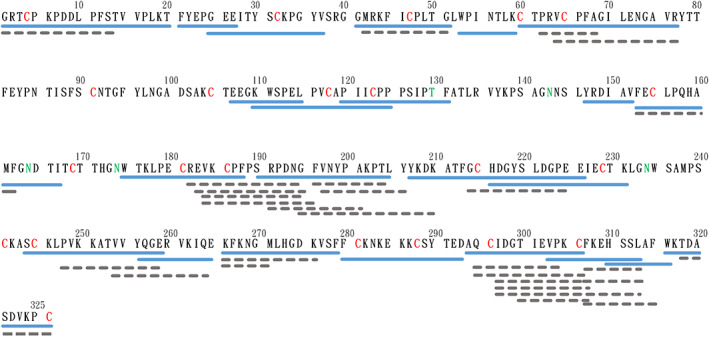
Peptide map of the pepsin digested β2GPI. Native β2GPI was hydrolyzed by pepsin for 20 minutes. The peptides were then separated by HPLC and fragmented by tandem mass spectrometry. Sequences of peptides were identified by X!Tandem. The blue solid line is the peptide used in the subsequent structural presentation, and the gray dotted line is the sequence identified and analyzed, but not embedded in the protein structural presentation. The red 'C' indicates the Cys forming disulfide bond, and the green 'T' and 'N' indicate the glycosylated Thr and Asn
2.2. H/D exchange of β2GPI
The native β2GPI protein was incubated in D2O buffer, pepsin‐digested and analyzed by mass spectrometry. The rate of H/D exchange was linearly dependent on the logarithmic scale of time. The time course 0, 10, 30, 100, 300, 1000, and 3000 s in log scale on the X‐axis can express the linear increases of deuteration through time. The H/D exchange experiments were all performed within 50 min to maintain the protein stability.
Peptides 110–125 containing part of domain II and the loop connecting domain III showed the highest exchange. The deuteration percentage of peptides 110–125 was set to indicate 100% deuteration level and non‐deuterated sample was defined as 0% deuteration level, against which other peptides were normalized accordingly. These normalized deuteration levels were presented in a color‐coded heat map (Figure 2(a)). The level of deuteration is defined as (number of incorporated deuteron)/(maximum exchangeable hydrogen) of a peptide. The original deuteration graphs with the full time course without normalization were shown in Figure S1. For each fragment, the first residue did not possess the peptide bond, and the amide on the second residue was too flexible to retain deuteron. Therefore, the deuteration regions on the heat map in Figure 2 were two‐residue shorter than the fragment shown in Figure 1. To avoid confusion, we used the identified peptide residues to represent the HDXMS region throughout the study. When β2GPI was deuterated for 10 s, the deuteration levels in all regions of the entire protein were < 60%. The peptides 207–226 had reached only 10.2% deuteration level at 10 s, a lowest exchange level. With extended times of H/D exchange, deuteration levels of fragments increased. At the exchange time of 3000 s, the exchange rate of the peptides 207–226 was 12.4% and was still the lowest among H/D exchanging fragments. In contrast, peptides 1–20, 110–125, 175–188, 244–259, and 294–306 had high deuteration levels at 62.9%, 96.1%, 71.2%, 58.9% and 51.0%, respectively, after 3000 s of H/D exchange.
FIGURE 2.
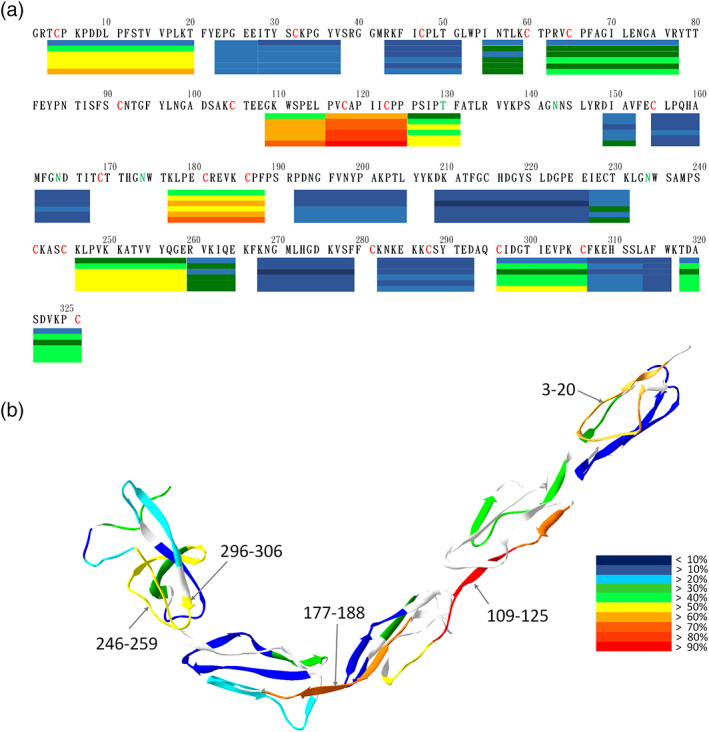
Deuteration level of β2GPI. β2GPI was deuterated for six time points: 0, 10, 30, 100, 300, 1000, and 3000 s. (a) Deuteration levels of the representative peptides in different regions are shown under their primary sequences in a bar graph. At each bar, its deuteration levels at the six time points are displayed from the top downward. Deuteration levels are indicated according to the heat map index. Connected bars without gaps indicate those fragments with overlapped residues. (b) Deuteration levels of the representative peptide of β2GPI at 3000 sec of panel (a) are mapped onto the crystal structure of β2GPI (PDB ID: 1C1Z)
Deuteration levels of β2GPI at longer H/D exchange time points showed greater differences in multiple regions. We further mapped the deuteration levels at 3000 s onto the X‐ray crystal structure (PDB ID: 1C1Z) for further structural display (Figure 2(b)). Domain IV was shown to have the lowest overall exchange rate among the five domains of β2GPI. The N‐terminal sequence 1–20 on domain I, the sequence 107–125 on domain II‐III, the sequence 175–188 on domain III‐IV, the sequence 244–259 on domain V and the C‐terminal sequence 294–306 all showed high levels of deuteration. Some of those highly deuterated regions (e.g., 107–125, 175–188 and 294–306) located on the connecting bridges among domains.
2.3. Phospholipid interactions with β2GPI
The β2GPI antibody and other β2GPI‐dependent antiphospholipid antibodies have been related to the antiphospholipid syndrome. 3 , 7 Negative charged phospholipids could interact with β2GPI, which would then be recognized by β2GPI antibody. Although both PS and CL phospholipids are negatively charged, they have significantly different molecular weight, head group, acyl chain number and charge in their molecular structure (Figure 3). Therefore, PS and CL may had different types of interactions with β2GPI to cause different levels of conformational changes. On the other hand, phosphatidylcholine is neutral in electrical charge and expected to exert no effect on β2GPI.
FIGURE 3.
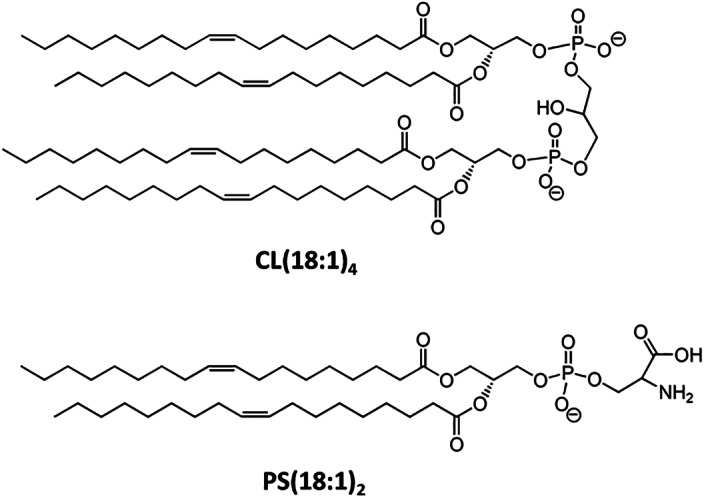
Structures of Tetraoleoyl‐Cardiolipin and Dioleoyl‐Phosphatidylserine (DOPS)
2.4. DOPS binding effects on the deuteration level of β2GPI
Because PS is an important component of anionic phospholipids in the cell membrane, 47 we hypothesized that PS may interact with β2GPI. To investigate the conformational changes and sites on β2GPI that bind with lipid membrane, we applied DOPS lipidosomes on β2GPI for 10 min and then performed the H/D exchange experiments. The original deuteration graphs with the full time course without normalization were shown in Figure S1.
Similar to the deuteration level in the absence of lipid membrane, the deuteration level of β2GPI treated with DOPS was mostly <50% at 10 s, except peptides 21–27. The regions 21–27 surged to a relatively high deuteration level (88.5%) at 10 seconds. At the exchange time of 3000 s, the presence of the lipid membrane mildly reduced deuteration levels at multiple regions: 1–20, 107–115, 120–131, 175–188, 197–206 and 297–307 (Figure 4(a)).
FIGURE 4.
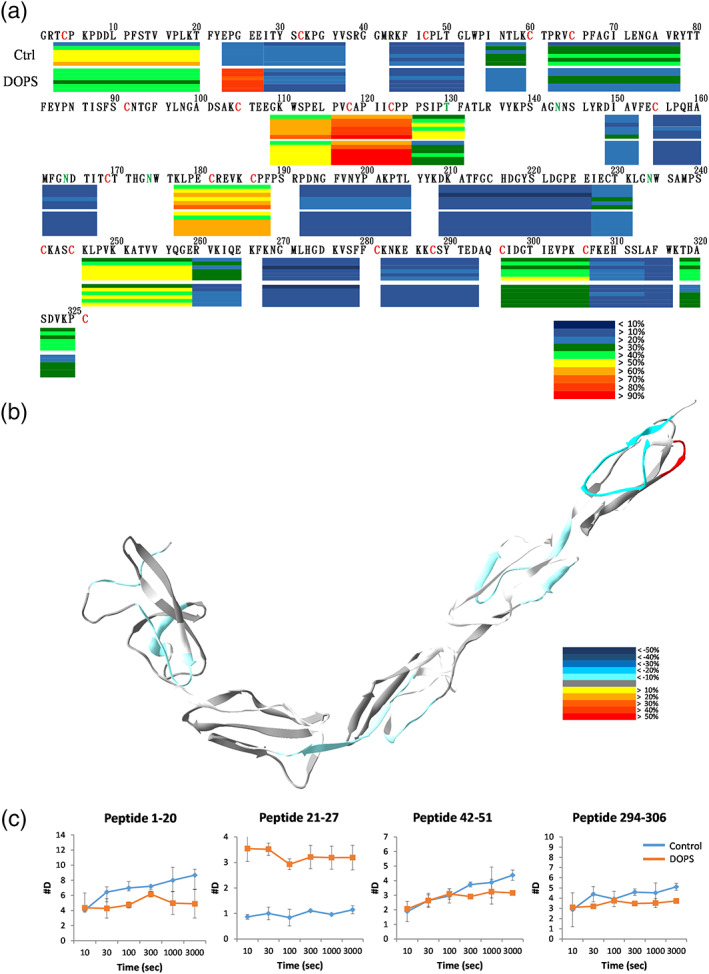
DOPS 10 min binding effects on β2GPI. β2GPI interacted with DOPS liposomes for 10 min and then deuterated for 0, 10, 30, 100, 300, 1000, 3000 s. (a) Deuteration levels of the representative peptides in different regions are shown underneath their primary sequences in a bar graph. In each bar, its deuteration levels at the six time points are displayed from top downward. Deuteration levels are indicated according to the heat map indices. Some fragments with overlapped regions are shown as connected bars. (b) Deuteration level differences of the representative peptide of β2GPI at 3000 s are mapped onto the crystal structure of β2GPI. (c) Deuteration of the representative peptides in duplicated experiments. Y‐axis shows the maximum deuteration level
Based on the results, longer time of H/D exchange revealed larger differences between control and the treatments. Therefore, 3000 s were chosen for the structural analysis. In the structural diagram showing the results of H/D exchange (Figure 4(b)), interactions between β2GPI and DOPS liposomes significantly increased the exchange rate of the region 21–27 (Figure 4(c)). It is likely that the tighten structure of β2GPI formed an exposed N‐terminus by interacting with the lipid membrane. When the protein and the lipid membrane came into contact, interactions between Domains I and V became weakened. Although this effect did not alter markedly the conformation of the entire protein, it enhanced greatly solvent accessibility of the sequence 21–27. The sequence 21–27 might have protruded from the original structure. Despite the increase in deuteration level at the N‐terminal, most other regions (like sequences 1–20, 42–51, 107–115, 120–131, 175–188, 197–206 and 294–306) showed marginally dropped levels of H/D exchange. These regions were likely flexible or the solvent‐exposed regions, which all showed high exchange rates in the absence of lipid liposomes. After interaction with DOPS liposomes, β2GPI probably formed a tighter structure, leading to the decreased exchange level. Due to the tighter structure, loops between the domains showed lower solvent accessibility and backbone flexibility. It is important to note that because of the experimental difficulty in the DOPS and β2GPI 30 min treatment, the effect of PS is not yet well‐characterized because of experimental difficulty.
2.5. Cardiolipin binding effects on β2GPI
β2GPI interacted with negatively charged phospholipid membrane through the positively charged surface of Domain V and a short hydrophobic loop. 24 CL is different from other negatively charged phospholipids in that it carries two negative charges. 48 Because β2GPI could interact with CL, 35 we applied HDXMS to detect conformational changes of the protein. β2GPI was first incubated with CL containing liposomes at 25°C for 10 min, and then the protein was subjected to H/D exchange experiments for 10, 30, 100, 300, 1000 or 3000 s. The original deuteration graphs with the full time course without normalization were shown in Figure S2. Upon binding with CL, β2GPI did not change its deuteration levels at most regions, except peptides 1–20 and 21–27 (Figure 5). The effect of increased deuteration level at peptide 21–27 upon CL binding was similar to the DOPS binding as mentioned above. Although peptide 21–27 was the most affected region after the binding to CL, this result indicated that more negatively charged lipid membrane significantly increased the solvent accessibility at peptide 21–27. The interactions between the lipid membrane and the protein would cause a weaken interaction between domain I and domain V. In addition, DOPS binding showed that the domain‐connecting loops had a slight decrease in deuteration levels, while in the 10 min CL reaction experiment, we found no change in the deuteration level. The results could be related to differences in electrostatic interaction or hydrogen bonding.
FIGURE 5.
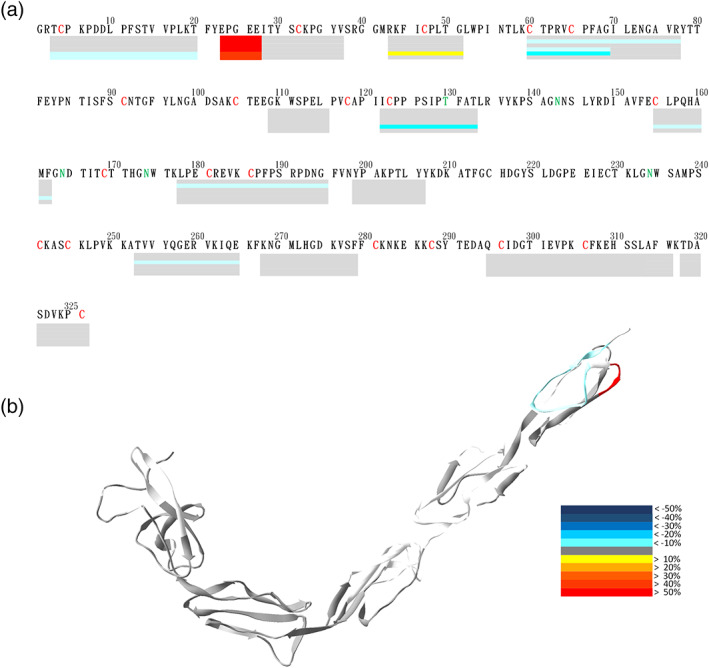
CL 10 min binding effects on β2GPI. β2GPI interacted with CL liposomes for 10 min and then deuterated for 0, 10, 30, 100, 300, 1000, and 3000 s. (a) Deuteration levels of the representative peptides in different regions shown under the primary sequence in a bar graph. In each bar, its deuteration levels of the six time points are displayed from the top downward. Deuteration levels are indicated in colors according to the heat map indices. (b) Deuteration levels of the representative peptides of β2GPI at 3000 s were mapped onto the crystal structure of β2GPI. The time course experiments were one experiment with five independent H/D exchange results
As the above 10 min CL binding experiment revealed no significant interactions between CL and the Domain V of β2GPI expect the N‐terminus, a finding which is inconsistent with the literature, 35 we therefore further performed a β2GPI and CL binding experiment at 25°C for 30 min. Here, 3000 s of H/D exchange showed a significant rise in the deuteration level of β2GPI, compared to the 10 min interaction (Figure 6). Deuteration levels of the sequences 1–20, 21–27, 196–205, 273–279 and 297–306 showed significant increases. In particular, the deuteration level of the N‐terminal sequence 1–20 originally showed a drop after short time CL interaction, now instead showed an increase by 17.3%. The HDXMS results were further mapped onto the ribbon structure and the surface of β2GPI (Figure 7). The Domains I, III, IV and IV all showed elevated deuteration levels. The constant exchange level of Domain II reflected the rigidity of Domain II, also suggesting that the relative positions among I, II, and III staying the same. The increases of the deuteration level on all four domains of β2GPI, and particularly the disconnection between domains I and V to expose the region 297–316 indicated a conformational change from a tight conformation to a loose conformation.
FIGURE 6.
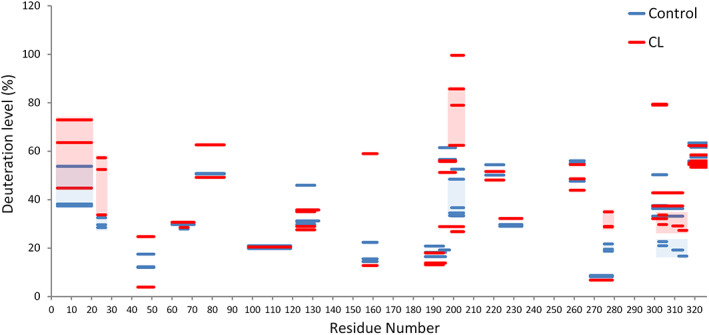
CL 30 min binding effects on β2GPI. β2GPI interacted with CL liposomes for 30 min and then deuterated for 3000 s. The deuteration levels are indicated in colors according to the heat map indices. Deuteration levels of the representative peptides of β2GPI at 3000 s are mapped onto the crystal structure of β2GPI. Significantly changed deuteration levels at different regions are shaded in red and blue. The experiments were performed in triplicates
FIGURE 7.
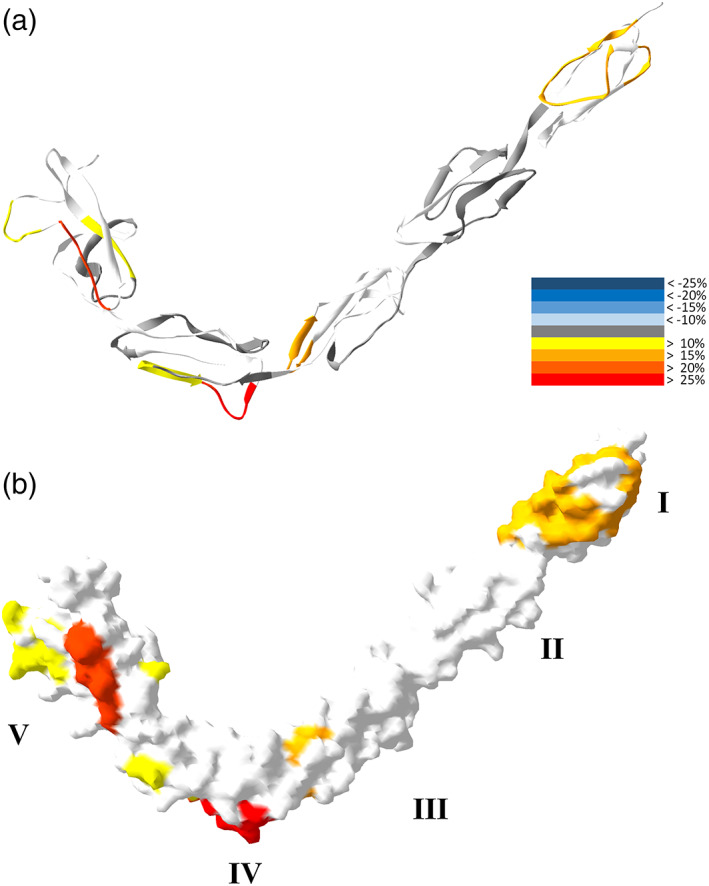
CL binding effects on the structure of β2GPI. β2GPI interacted with CL liposomes for 30 min and then deuterated for 3000 s. Deuteration levels of representative peptides of β2GPI at 3000 s are mapped onto the crystal structure of β2GPI in colors according to the heat map indices. The ribbon (a) and the surface (b) of the β2GPI structure are shown. Five domains of β2GPI are marked by roman numerals
3. DISCUSSION
The conformation of β2GPI is known to be altered by its interaction with anionic phospholipids, and the conformation changes are related to the recognition of cryptic epitopes by the antiphospholipid antibody. 10 Although we collected H/D exchange information from different conformations, currently only the J conformation of β2GPI has known X‐ray crystal structure. Therefore, we mapped H/D exchange data for all different conformations on the only available crystal structure in our study. Based on our results, we proposed a mechanism for β2GPI regarding phospholipid induction of the conformational changes (Figure 8).
FIGURE 8.
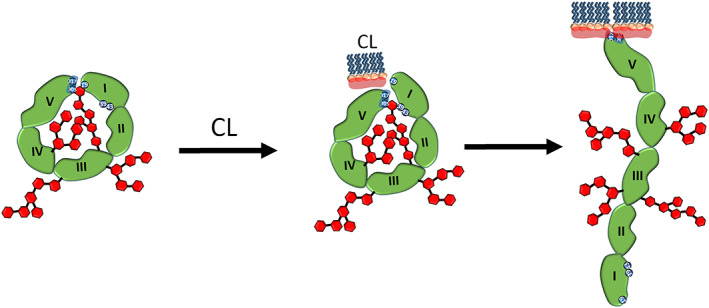
Mechanisms of the conformational changes of β2GPI upon interacting with DOPS and CL shown in schematic view. Blue numbers mark Lys/Arg patches, including Lys19, Arg39, Arg43, Lys305 and Lys317. Red hexagons represent polysaccharides. The head group of DOPS, shown in light red, carries weaker negative charges than CL. The domaina I, II, III, IV and V of β2GPI in green are also marked
The non‐treated β2GPI had the lowest rate of H/D exchange at the N‐terminal region on domain I. In this conformation, domain I is in contact with domain V, which hinders the regions from fast H/D exchanges. Further question is the location of the contact surface, which potentially can be targeted therapeutically. It is reasonable to assume the N‐terminus is involved in forming the ring structure with the C‐terminus. Although negatively charged Asp8 and Asp9 in this region could interact with the Lys patch in domain V, both residues are located on the side of the domain I on the β2GPI crystal structure. Plus, the N‐terminal sequences 1–3 and 7–11 are highly flexible loops with high rates of H/D exchange, consistent with their high deuteration levels. Therefore, the N‐terminus is less likely to be the region in connect with domain V. On the contrary, region 21–27 had a low exchange rate, which suggested a low solvent accessibility. This finding is related to the interactions with domain V in shielding it from H/D exchange or extensive hydrogen bonds in this region. We also found that Glu23, Glu26 and Glu27, forming a negatively charged patch, were located on the tip of the domain I ideally for closing the ring structure. Additionally, the rate of exchange at region 21–27 increased markedly after interacting with negatively charged phospholipids. In domain V, the Lys‐rich region (including the Lys282, Lys284, Lys286, and Lys287 regions) and the scattered Lys305, Lys308, and Lys317 form a positively charged tip of domain V for contacting domain I. The positively charged patch of Domain V has been shown to interact with the negatively charged molecules, such as heparin, DNA and plasma membrane. 24 , 33 The sequences 107–125, 175–188, and 244–259 are located at the junction of domain II‐III, III‐IV and IV‐V, respectively. They are long‐length β‐strands that act as bridges connecting adjacent domains. These regions are flexible and have fast H/D exchanges. Along with loop region, sequence 294–306 is a flexible loop on Domain V, located outside the J‐shape in the 3D structure.
The head group of DOPS, which has both a negatively charged carboxyl group and a positively charged amine group, is able to interact with β2GPI electrostatically, or form hydrogen bonds. In addition, β2GPI contains several polysaccharide groups and the hydroxyl groups in these polysaccharide groups are proper functional groups for forming hydrogen bonds, resulting in non‐specific interactions with DOPS. In the highly solvent accessible regions of β2GPI, a marginal drop in deuteration levels is observed after interactions with DOPS. The minor decreases of deuteration could be affected simultaneously by solvent accessibility and backbone flexibility.
CL is a phospholipid with strong negative charge, and tends to interact with the domain V that has strong positivity. β2GPI interacts either with weakly negatively charged DOPS or with strongly negatively charged CL. Deuteration levels of the sequence 21–27 grow high, indicating the solvent accessibility has been enhanced at sequence 21–27. Results suggest that the interaction between the lipid membrane and β2GPI weakens the interactions between domains I and V, but the weakening force is not enough to break the ring, leading to protruding peptide 21–27 out from the ring. This intermediate form may correspond to the sigma‐like structure observed under electron microscopy, as reported in an earlier study. 34 The binding between negatively phospholipids and β2GPI may not be similar to the ligand‐receptor binding. β2GPI may have multiple phospholipid interacting sites or non‐specifically interact with phospholipids with different affinities, orientation and rates. A previous study has demonstrated the occurrence of aggregation up to 30 min after β2GPI injection into cells. 49 Therefore, the intermediate conformation was observed after 10 min of CL liposome interaction.
Lys19, Arg39 and Arg43 of β2GPI are known to have poor solvent accessibility in the ring conformation to protect them from trypsin digestion. 34 Arg39 and Arg43 are presumably specific recognition residues of antiphospholipid antibody. After binding to the anionic lipid membrane, conformational changes of β2GPI expose the epitopes Arg39 and Arg43 from the shielding of the polysaccharide group. However, in the present study, we failed to observe a significant rise in deuteration levels near Arg39 and Arg43. The structure of β2GPI still maintains strong interactions between domains with the polysaccharide continuing shield the epitope, at least in part.
When the β2GPI is in the conformation before lipid interaction, the solvent accessibility is extremely low regarding sites Lys19, Arg39 and Arg43 on Domain I. When β2GPI interacts with negatively charged lipid membrane, its shape changes and all the deuteration levels at sequences 1–20, 21–27, and 41–51 (adjacent to the three sites of Lys19, Arg39, and Arg43) increase. On the other hand, in the conformation before lipid interaction, solvent accessibility at Lys305 and Lys317 on Domain V is also low. Our results also showed an increase of exchange rate at fragment 297–316 on domain V. Such increase could be due to the exposure of Lys305. Interestingly, in the conformation before lipid interaction, the Lys patch (including Lys282, Lys284, Lys286 and Lys287) located in the peptide 280–293, also showed a low rate of H/D exchange. After interaction has occurred between β2GPI and CL, peptide 280–293 is no longer available for mass spectrometry analysis, supporting either a change in its property or a shift in mass because of the CL binding. But the scattering lysine residues, Lys305, Lys308 and Lys317 can still be recognized by mass spectrometry and they showed elevated rates of H/D exchange. Findings are consistent with no direct association between these residues and CL. The above results indicated that after the interaction between β2GPI and CL lipid membrane, the protein has a significant conformational change. The interface between domain I and domain V is hence exposed. Interestingly, the recent evidences have shown that anti‐domain I but not anti‐domain V antibodies are pathogenic. 12 , 50 The conformational change induced by the lipid interactions may interfere with the production of antibody.
In conclusion, the H/D change experiment is a useful tool to investigate β2GPI‐lipid membrane interactions under physiological conditions, which corroborate previous findings on β2GPI conformational changes. Overall, β2GPI does not change the conformation during initial contact with anionic lipid membrane, but sequence 21–27 will be exposed. β2GPI continues to change its conformation drastically while staying on the more negatively charged CL membrane surface. Our results may help finding potential intervention sites therapeutically.
4. MATERIALS AND METHODS
4.1. Materials
Immobilized pepsin on 6% agarose resin was purchased from Thermo Fisher Scientific, United States. Beta 2‐glycoprotein I from human plasma (≥96%) was purchased from BBI solution (Cardiff, UK). The protein was purified from plasma via Cohn fractionation and Heparin chromatography. Deuterium oxide (99.9% D2O) was purchased from Cambridge isotope laboratories, Incorporation (Andover, MA). The 1,2‐dioleoyl‐sn‐glycero‐3‐phospho‐L‐serine (18:1 DOPS) and 1′,3′‐bis[1,2‐dioleoyl‐sn‐ glycero‐3‐phospho]‐sn‐glycerol (18:1 Cardiolipin) were purchased from Avanti Polar Lipid (Alabaster, AL). Tris(2‐carboxyethyl) phosphine hydrochloride (TCEP) was purchased from Sigma–Aldrich.
4.2. Sequence identification of the β2GPI proteolytic fragment
To analyze the HDXMS data, sequences of the detected pepsin‐digested fragments were first identified by UPLC‐Q‐TOF (Quadrupole time of flight mass spectrometry, QIII, Bruker). After proteolyzing β2GPI into fragments by immobilized pepsin, fragments were collected by a peptide trap (micro RP peptide trap, OPTI‐TRAPTM), and then separated by a reverse phase HPLC in a C18 column (BioBasic 18 LC Columns, Thermo Scientific). Mobile phase A was in 0.1% formic acid (pH 2.5), and the mobile phase B was in 80% Acetonitrile (ACN) and 0.02% formic acid. The linear gradient of mobile phase A was 95%–0% finished in 40 min at a flow rate of 0.1 ml/min. Most of the peptides were eluted between the periods of 15 to 30 min. Peptides were fragmented by tandem mass spectrometry (MS/MS), with the MS/MS data imported into the X! Tandem software to calculate the sequences of the fragments.
4.3. Sequence identification by X!Tandem
β2GPI MS/MS data were exported from Bruker DataAnalysis at an intensity threshold of 700. MS/MS data and the sequence were imported to X!Tandem for sequence identification. The mass error was set at 500 ppm. The fragment sequences were manually verified again based on the primary mass and the matched product ions under X!tandem‐parser‐1.7.7. The mass envelop of each non‐deuterated peptide matched with the theoretical mass envelop by the H/Dexaminer software and were carefully examined manually.
4.4. β2GPI HDXMS calculation
We loaded the information on sequences, retention times and charges of the identified peptide fragments into the H/D exchange software HDExaminer as the peptide pool. The data on non‐deuterated MS of β2GPI was then imported to HDExaminer to identify the peptide peak. Some low intensity peptides were excluded for further analysis, a step which served as a second filter for peptide identification. The masses of the fragment of the non‐deuterated β2GPI were used as the standards for all other H/D exchange samples. Then, MS data of the H/D exchange samples at all six time points were imported into HDExaminer. The average mass of each deuterated peptide at each time point was calculated. The shift of the average mass at a specific time point after H/D exchange was taken as the amount of deuteration. The level of deuteration is defined as the value of the number of incorporated deuteron divided by the maximum exchangeable amide hydrogen of a peptide. The peptide with the deuteration level was normalized to the full deuteration level in the heat map presentation.
4.5. Preparation of the phospholipid liposomes
We added 2.5 mg of 18:1 DOPS and 3.8 mg of 18:1 CL to different glass tubes, with organic solvent dried with nitrogen gas, and the with the addition of 1 ml of 100 mM KCl solution. We then sonicated the sample for 10 min in 55°C water. To avoid overheating, the sample went through a cycle of 30 sec sonication and 30 sec cooling on ice. After sonication, the phospholipid liposomes were placed at 25°C for 30 min to form stable liposomes. The final solution appeared transparent and colorless. Two different phospholipid liposome solutions were subjected to subsequent H/D exchange experiments.
4.6. H/D exchange of β2GPI
Interactions between β2GPI and phospholipids were initiated by mixing 1 μl of the prepared phospholipid liposomes and 10 μl of β2GPI (1 μg/μl). The mixture was then incubated at 25°C for 10 min. The ratio of lipid molecules to β2GPI was about 10:1 in molarity. The 20‐fold D2O dilution buffer was prepared using 2.5 M Tris base, 1 M NaCl, pH 7.5 in H2O. Heavy water (D2O) was mixed with a 20‐fold D2O buffer in a ratio of 19 μl:1 μl. D2O concentration was 95%. In the H/D experiment, 2 μl of β2GPI (1 μg/μl) was added to 40 μL of D2O buffer, and incubated at 25°C for 10, 30, 100, 300, 1000 or 3000 s. Pre‐ice cooled 150 μl of quench buffer (0.1% trifluoroacetic acid, 1 M TCEP) reduced the pH of the sample to pH 2.5 and cooling the sample down to terminate the H/D exchange reaction. Deuterated samples were added to the activated immobilized‐pepsin for proteolysis for 20 min, during which with brief vertex‐shaking every 30 s. The immobilized‐pepsin was activated by rinsing agarose beads three times with 0.1% TFA. All containing tubes, reagents and pipette tips were pre‐cooled in advance on ice or stored at 4°C. After completing hydrolysis, pepsin agarose beads were centrifuged and removed before applying the liquid‐chromatography mass spectrometry.
AUTHOR CONTRIBUTIONS
Kuo‐Tung Tang: Conceptualization; data curation; formal analysis; funding acquisition; project administration; resources; validation; writing‐original draft; writing‐review and editing. Ting‐Yuan Wu: Data curation; formal analysis; investigation; methodology; validation; writing‐original draft. Hsin‐Hua Chen: Project administration; resources; supervision; validation; writing‐review and editing. Chi‐Chien Lin: Project administration; resources; supervision; validation; writing‐review and editing. Yuan‐Hao Hsu: Conceptualization; data curation; formal analysis; funding acquisition; investigation; methodology; project administration; resources; software; supervision; validation; visualization; writing‐original draft; writing‐review and editing.
CONFLICT OF INTEREST
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Supporting information
Supplemental Figure 1 DOPS binding effects on β2GPI
β2GPI interacted with DOPS liposomes and then deuterated for 0, 10, 30, 100, 300, 1,000, 3,000 seconds. Deuteration number of all peptides in different regions are shown. Deuteration of the peptides were performed in duplicated experiments. Y‐axis showed the maximum deuteration level.
Supplemental Figure 2: CL 10‐min binding effects on β2GPI
β2GPI interacted with CL liposomes for 10 minutes and then deuterated for 0, 10, 30, 100, 300, 1,000, 3,000 seconds. Deuteration number of all peptides in different regions are shown. Deuteration of the peptides were performed in duplicated experiments. Y‐axis showed the maximum deuteration level.
ACKNOWLEDGEMENTS
This work was supported by Ministry of Science and Technology, Taiwan (MOST 106‐2113‐M‐029‐004, MOST 107‐2113‐M‐029‐008, MOST 108‐2113‐M‐029‐008 and MOST 106‐2923‐M‐029‐001‐MY3), and grants from the Taichung Veterans General Hospital/Tunghai University Joint Research Program (TCVGH‐T1067803), Taichung, Taiwan.
Tang K‐T, Wu T‐Y, Chen H‐H, Lin C‐C, Hsu Y‐HH. Cardiolipin interacts with beta‐2‐glycoprotein I and forms an open conformation–Mechanisms analyzed using hydrogen/deuterium exchange. Protein Science. 2021;30:927–939. 10.1002/pro.4054
Kuo‐Tung Tang and Ting‐Yuan Wu contributed equally to this study.
Funding information Ministry of Science and Technology, Taiwan, Grant/Award Numbers: 106‐2113‐M‐029‐004, 106‐2923‐M‐029‐001‐MY3, 107‐2113‐M‐029‐008, 108‐2113‐M‐029‐008; Taichung Veterans General Hospital, Grant/Award Number: TCVGH‐T1067803; Tunghai University
REFERENCES
- 1. Sciascia S, Amigo MC, Roccatello D, Khamashta M. Diagnosing antiphospholipid syndrome: 'extra‐criteria' manifestations and technical advances. Nat Rev Rheumatol. 2017;13:548–560. [DOI] [PubMed] [Google Scholar]
- 2. Ruiz‐Irastorza G, Egurbide MV, Ugalde J, Aguirre C. High impact of antiphospholipid syndrome on irreversible organ damage and survival of patients with systemic lupus erythematosus. Arch Intern Med. 2004;164:77–82. [DOI] [PubMed] [Google Scholar]
- 3. McNeil HP, Simpson RJ, Chesterman CN, Krilis SA. Anti‐phospholipid antibodies are directed against a complex antigen that includes a lipid‐binding inhibitor of coagulation: Beta 2‐glycoprotein I (apolipoprotein H). Proc Natl Acad Sci U S A. 1990;87:4120–4124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bevers EM, Galli M, Barbui T, Comfurius P, Zwaal RF. Lupus anticoagulant IgG's (LA) are not directed to phospholipids only, but to a complex of lipid‐bound human prothrombin. Thromb Haemost. 1991;66:629–632. [PubMed] [Google Scholar]
- 5. Wilson WA, Gharavi AE, Koike T, et al. International consensus statement on preliminary classification criteria for definite antiphospholipid syndrome: Report of an international workshop. Arthritis Rheum. 1999;42:1309–1311. [DOI] [PubMed] [Google Scholar]
- 6. Willis R, Pierangeli SS. Anti‐beta2‐glycoprotein I antibodies. Ann N Y Acad Sci. 2013;1285:44–58. [DOI] [PubMed] [Google Scholar]
- 7. de Groot PG, Urbanus RT. The significance of autoantibodies against beta2‐glycoprotein I. Blood. 2012;120:266–274. [DOI] [PubMed] [Google Scholar]
- 8. Mulla MJ, Brosens JJ, Chamley LW, et al. Antiphospholipid antibodies induce a pro‐inflammatory response in first trimester trophoblast via the TLR4/MyD88 pathway. Am J Reproduct Immunol. 2009;62:96–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Azuma H, Yamamoto T, Chishima F. Effects of anti‐beta2‐GPI antibodies on cytokine production in normal first‐trimester trophoblast cells. J Obstet Gynaecol Res. 2016;42:769–775. [DOI] [PubMed] [Google Scholar]
- 10. Giannakopoulos B, Krilis SA. The pathogenesis of the antiphospholipid syndrome. N Engl J Med. 2013;368:1033–1044. [DOI] [PubMed] [Google Scholar]
- 11. Abrahams VM, Chamley LW, Salmon JE. Emerging treatment models in rheumatology: Antiphospholipid syndrome and pregnancy: Pathogenesis to translation. Arthritis Rheumatol. 2017;69:1710–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. de Laat B, Derksen RH, van Lummel M, Pennings MT, de Groot PG. Pathogenic anti‐beta2‐glycoprotein I antibodies recognize domain I of beta2‐glycoprotein I only after a conformational change. Blood. 2006;107:1916–1924. [DOI] [PubMed] [Google Scholar]
- 13. Ioannou Y, Pericleous C, Giles I, Latchman DS, Isenberg DA, Rahman A. Binding of antiphospholipid antibodies to discontinuous epitopes on domain I of human beta(2)‐glycoprotein I: Mutation studies including residues R39 to R43. Arthritis Rheum. 2007;56:280–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ioannou Y, Romay‐Penabad Z, Pericleous C, et al. In vivo inhibition of antiphospholipid antibody‐induced pathogenicity utilizing the antigenic target peptide domain I of beta2‐glycoprotein I: Proof of concept. J Thromb Haemost. 2009;7:833–842. [DOI] [PubMed] [Google Scholar]
- 15. Iverson GM, Victoria EJ, Marquis DM. Anti‐beta2 glycoprotein I (beta2GPI) autoantibodies recognize an epitope on the first domain of beta2GPI. Proc Natl Acad Sci U S A. 1998;95:15542–15546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Andreoli L, Nalli C, Motta M, et al. Anti‐beta(2)‐glycoprotein I IgG antibodies from 1‐year‐old healthy children born to mothers with systemic autoimmune diseases preferentially target domain 4/5: Might it be the reason for their 'innocent' profile? Ann Rheum Dis. 2011;70:380–383. [DOI] [PubMed] [Google Scholar]
- 17. de Laat B, Derksen RH, Urbanus RT, de Groot PG. IgG antibodies that recognize epitope Gly40‐Arg43 in domain I of beta 2‐glycoprotein I cause LAC, and their presence correlates strongly with thrombosis. Blood. 2005;105:1540–1545. [DOI] [PubMed] [Google Scholar]
- 18. Iverson GM, Reddel S, Victoria EJ, et al. Use of single point mutations in domain I of beta 2‐glycoprotein I to determine fine antigenic specificity of antiphospholipid autoantibodies. J Immunol. 2002;169:7097–7103. [DOI] [PubMed] [Google Scholar]
- 19. Ambrozic A, Avicin T, Ichikawa K, et al. Anti‐beta(2)‐glycoprotein I antibodies in children with atopic dermatitis. Int Immunol. 2002;14:823–830. [DOI] [PubMed] [Google Scholar]
- 20. Arvieux J, Renaudineau Y, Mane I, Perraut R, Krilis SA, Youinou P. Distinguishing features of anti‐beta2 glycoprotein I antibodies between patients with leprosy and the antiphospholipid syndrome. Thromb Haemost. 2002;87:599–605. [PubMed] [Google Scholar]
- 21. Lutters BC, Meijers JC, Derksen RH, Arnout J, de Groot PG. Dimers of beta 2‐glycoprotein I mimic the in vitro effects of beta 2‐glycoprotein I‐anti‐beta 2‐glycoprotein I antibody complexes. J Biol Chem. 2001;276:3060–3067. [DOI] [PubMed] [Google Scholar]
- 22. Lozier J, Takahashi N, Putnam FW. Complete amino acid sequence of human plasma beta 2‐glycoprotein I. Proc Natl Acad Sci U S A. 1984;81:3640–3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. de Laat B, de Groot PG, Derksen RH, et al. Association between beta2‐glycoprotein I plasma levels and the risk of myocardial infarction in older men. Blood. 2009;114:3656–3661. [DOI] [PubMed] [Google Scholar]
- 24. Bouma B, de Groot PG, van den Elsen JM, et al. Adhesion mechanism of human beta(2)‐glycoprotein I to phospholipids based on its crystal structure. EMBO J. 1999;18:5166–5174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sheng Y, Kandiah DA, Krilis SA. Anti‐beta 2‐glycoprotein I autoantibodies from patients with the "antiphospholipid" syndrome bind to beta 2‐glycoprotein I with low affinity: Dimerization of beta 2‐glycoprotein I induces a significant increase in anti‐beta 2‐glycoprotein I antibody affinity. J Immunol. 1998;161:2038–2043. [PubMed] [Google Scholar]
- 26. O'Leary JM, Bromek K, Black GM, et al. Backbone dynamics of complement control protein (CCP) modules reveals mobility in binding surfaces. Protein Sci. 2004;13:1238–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ioannou Y, Zhang JY, Passam FH, et al. Naturally occurring free thiols within beta 2‐glycoprotein I in vivo: Nitrosylation, redox modification by endothelial cells, and regulation of oxidative stress‐induced cell injury. Blood. 2010;116:1961–1970. [DOI] [PubMed] [Google Scholar]
- 28. Ioannou Y, Zhang JY, Qi M, et al. Novel assays of thrombogenic pathogenicity in the antiphospholipid syndrome based on the detection of molecular oxidative modification of the major autoantigen beta2‐glycoprotein I. Arthritis Rheum. 2011;63:2774–2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Passam FH, Rahgozar S, Qi M, et al. Beta 2 glycoprotein I is a substrate of thiol oxidoreductases. Blood. 2010;116:1995–1997. [DOI] [PubMed] [Google Scholar]
- 30. Passam FH, Rahgozar S, Qi M, et al. Redox control of beta2‐glycoprotein I‐von Willebrand factor interaction by thioredoxin‐1. J Thromb Haemost. 2010;8:1754–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Arai T, Yoshida K, Kaburaki J, et al. Autoreactive CD4(+) T‐cell clones to beta2‐glycoprotein I in patients with antiphospholipid syndrome: Preferential recognition of the major phospholipid‐binding site. Blood. 2001;98:1889–1896. [DOI] [PubMed] [Google Scholar]
- 32. Reddel SW, Wang YX, Sheng YH, Krilis SA. Epitope studies with anti‐beta 2‐glycoprotein I antibodies from autoantibody and immunized sources. J Autoimmun. 2000;15:91–96. [DOI] [PubMed] [Google Scholar]
- 33. Schwarzenbacher R, Zeth K, Diederichs K, et al. Crystal structure of human beta2‐glycoprotein I: Implications for phospholipid binding and the antiphospholipid syndrome. EMBO J. 1999;18:6228–6239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Agar C, van Os GM, Morgelin M, Sprenger RR, Marquart JA, Urbanus RT, Derksen RH, Meijers JC, de Groot PG. Beta2‐glycoprotein I can exist in 2 conformations: Implications for our understanding of the antiphospholipid syndrome. Blood 2010;116:1336–1343. [DOI] [PubMed] [Google Scholar]
- 35. Hammel M, Schwarzenbacher R, Gries A, Kostner GM, Laggner P, Prassl R. Mechanism of the interaction of beta(2)‐glycoprotein I with negatively charged phospholipid membranes. Biochemistry. 2001;40:14173–14181. [DOI] [PubMed] [Google Scholar]
- 36. Hammel M, Kriechbaum M, Gries A, Kostner GM, Laggner P, Prassl R. Solution structure of human and bovine beta(2)‐glycoprotein I revealed by small‐angle X‐ray scattering. J Mol Biol. 2002;321:85–97. [DOI] [PubMed] [Google Scholar]
- 37. de Laat B, de Groot PG. Autoantibodies directed against domain I of beta2‐glycoprotein I. Curr Rheumatol Rep. 2011;13:70–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ninivaggi M, Kelchtermans H, Lindhout T, de Laat B. Conformation of beta2glycoprotein I and its effect on coagulation. Thromb Res. 2012;130(Suppl 1):S33–S36. [DOI] [PubMed] [Google Scholar]
- 39. Chu CT, Ji J, Dagda RK, et al. Cardiolipin externalization to the outer mitochondrial membrane acts as an elimination signal for mitophagy in neuronal cells. Nat Cell Biol. 2013;15:1197–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Esposti MD. Lipids, cardiolipin and apoptosis: A greasy licence to kill. Cell Death Differ. 2002;9:234–236. [DOI] [PubMed] [Google Scholar]
- 41. Hunt J, Krilis S. The fifth domain of beta 2‐glycoprotein I contains a phospholipid binding site (Cys281‐Cys288) and a region recognized by anticardiolipin antibodies. J Immunol. 1994;152:653–659. [PubMed] [Google Scholar]
- 42. Pittoni V, Ravirajan CT, Donohoe S, MacHin SJ, Lydyard PM, Isenberg DA. Human monoclonal anti‐phospholipid antibodies selectively bind to membrane phospholipid and beta2‐glycoprotein I (beta2‐GPI) on apoptotic cells. Clin Exp Immunol. 2000;119:533–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Vazquez‐Mellado J, Llorente L, Richaud‐Patin Y, Alarcon‐Segovia D. Exposure of anionic phospholipids upon platelet activation permits binding of beta 2 glycoprotein I and through it that of IgG antiphospholipid antibodies. Studies in platelets from patients with antiphospholipid syndrome and normal subjects. J Autoimmun. 1994;7:335–348. [DOI] [PubMed] [Google Scholar]
- 44. Rauch J, Subang R, D'Agnillo P, Koh JS, Levine JS. Apoptosis and the antiphospholipid syndrome. J Autoimmun. 2000;15:231–235. [DOI] [PubMed] [Google Scholar]
- 45. Meroni PL, Borghi MO, Raschi E, Tedesco F. Pathogenesis of antiphospholipid syndrome: Understanding the antibodies. Nat Rev Rheumatol. 2011;7:330–339. [DOI] [PubMed] [Google Scholar]
- 46. Hsu YH, Burke JE, Li S, Woods VL Jr, Dennis EA. Localizing the membrane binding region of group VIA Ca2+−independent phospholipase A2 using peptide amide hydrogen/deuterium exchange mass spectrometry. J Biol Chem. 2009;284:23652–23661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hirama T, Lu SM, Kay JG, et al. Membrane curvature induced by proximity of anionic phospholipids can initiate endocytosis. Nat Commun. 2017;8:1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Olofsson G, Sparr E. Ionization constants pKa of cardiolipin. PLoS One. 2013;8:e73040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gamsjaeger R, Johs A, Gries A, et al. Membrane binding of beta(2)‐glycoprotein I can be described by a two‐state reaction model: An atomic force microscopy and surface plasmon resonance study. Biochem J. 2005;389:665–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Durigutto P, Grossi C, Borghi MO, et al. New insight into antiphospholipid syndrome: Antibodies to beta(2)glycoprotein I‐domain 5 fail to induce thrombi in rats. Haematologica. 2019;104:819–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1 DOPS binding effects on β2GPI
β2GPI interacted with DOPS liposomes and then deuterated for 0, 10, 30, 100, 300, 1,000, 3,000 seconds. Deuteration number of all peptides in different regions are shown. Deuteration of the peptides were performed in duplicated experiments. Y‐axis showed the maximum deuteration level.
Supplemental Figure 2: CL 10‐min binding effects on β2GPI
β2GPI interacted with CL liposomes for 10 minutes and then deuterated for 0, 10, 30, 100, 300, 1,000, 3,000 seconds. Deuteration number of all peptides in different regions are shown. Deuteration of the peptides were performed in duplicated experiments. Y‐axis showed the maximum deuteration level.


