Abstract
Esophageal cancer has increased in incidence over the last several decades and is now the sixth leading cause of all cancer deaths, with more than 500,000 deaths in 2018. The 2 most common types of esophageal cancer, squamous cell cancer and esophageal adenocarcinoma, make up nearly 95% of diagnoses. Based on the global distribution of these histologic types, esophageal adenocarcinoma is more common in the United States while squamous cell cancer is more common throughout the world. For both the squamous cell cancer and esophageal adenocarcinoma variants of esophageal cancer, the most important step in determining prognosis and survival is accurate staging. Endoscopy, computed tomography, whole-body positron emission tomography with 18-fluorodeoxyglucose, and endoscopic ultrasound (EUS) all have important roles in the diagnosis and staging of esophageal cancer. EUS is key for locoregional staging and guides treatment planning of esophageal cancer in the absence of distant metastases. EUS has been shown to improve survival across various stages of esophageal cancer and to have a positive financial impact in cost-effectiveness analyses. This article describes current EUS technology and the role of EUS in esophageal cancer staging, as well as the applications, challenges, and limitations of EUS in the management of this disease.
Keywords: Esophageal cancer, endoscopic ultrasound, staging, Barrett esophagus, squamous cell cancer, esophageal adenocarcinoma
Despite advances in medical, endoscopic, and surgical therapies, the incidence of esophageal cancer has been on the rise over the last several decades, with an estimated 450,000 cases diagnosed worldwide annually.1,2 Esophageal cancer is the sixth leading cause of all cancer deaths, with more than 500,000 deaths in 2018.3 In the United States, it was estimated that there would be 17,650 new diagnoses of esophageal cancer and 16,080 deaths in 2019.4 The 5-year survival rate remains low, at approximately 15% to 20%.5
Squamous cell cancer and adenocarcinoma are the 2 most common types of esophageal cancer, making up nearly 95% of diagnoses.1,2 Worldwide, squamous cell cancer is more common, whereas in the United States, adenocarcinoma is more frequently diagnosed. Squamous cell cancer typically occurs in the midesophagus. Risk factors include tobacco and alcohol use as well as dietary factors, such as foods high in N-nitroso compounds (certain pickled vegetables) or high-temperature foods and beverages. Adenocarcinoma almost always arises in the distal esophagus and is associated with Barrett esophagus. The risk of developing esophageal adenocarcinoma is 30- to 40-fold higher in patients with Barrett esophagus.6
For both the squamous cell and adenocarcinoma variants of esophageal cancer, accurate staging has the most impact on prognosis and survival.7 Both cognitive and technical aspects of care are important in the evaluation and management of esophageal cancer, and are best achieved in a multidisciplinary setting where guidelines can be incorporated into patient care and consensus-based recommendations can be implemented. Endoscopy, computed tomography (CT), whole-body positron emission tomography with 18-fluorodeoxyglucose (FDG-PET), and endoscopic ultrasound (EUS) all have complementary roles in the diagnosis and staging of esophageal cancer. This article describes current EUS technology and the role of EUS in esophageal cancer staging, including the challenges and limitations of EUS in the management of this disease.
Current Guidelines for Staging Esophageal Cancer
Accurate pretreatment staging of esophageal cancer is important to provide patients with optimal and effective treatment options as well as for appropriate prognostication. In a recent review, Foley and colleagues recommended initial staging with contrast-enhanced CT scans of the chest and abdomen.8 If the tumor is found to extend beneath the diaphragm, a CT scan of the pelvis should also be completed. For potentially curable disease with no evidence of distant metastases, PET-CT is then recommended, followed by an EUS. For tumors involving the gastric cardia, diagnostic laparoscopy should also be performed.8 Several national societies have reported similar guidelines, including the National Comprehensive Cancer Network, which recommends a contrast-enhanced CT scan of the chest and abdomen to rule out distant metastases at the time of initial diagnosis. EUS with or without fine-needle aspiration (FNA) sampling of any extraesophageal targets should be performed once distant metastases have been excluded.9 The National Institute for Health and Care Excellence recommends EUS in cases where it is likely to change management, and PET-CT for all patients with potentially curative disease above stage T1a.10
Endoscopic Ultrasound Technology
EUS is the most accurate modality for locoregional staging of esophageal cancer. It is a minimally invasive procedure that uses high-frequency sound waves to visualize the layers of the esophageal wall and surrounding tissues, thereby evaluating the primary tumor as well as locoregional adenopathy. Over the last decade, EUS technology has rapidly evolved to allow for enhanced diagnostic and therapeutic interventions. This includes the development of devices and accessories for core biopsies, fiducials to help guide stereotactic radiotherapy, lumen-apposing metal stents for the drainage of fluid collections, and echo-endoscopes (such as forward-viewing echoendoscopes) to further support therapeutic interventions.
With respect to esophageal cancer, the primary modalities utilized in staging include the radial echoendo-scope, the curvilinear echoendoscope, and the probe-based EUS. The radial echoendoscope provides a 360° view for a circumferential evaluation of the esophageal wall and adjacent structures. Radial echoendoscopes operate at 7.5 to 12.0 MHz, providing excellent resolution to a depth of approximately 3 to 5 cm of surrounding tissue.5,11 In radial EUS, the ultrasound transducer is housed in the tip of the echoendoscope, and a fluid-filled balloon is often used to eliminate pockets of air around the transducer to enhance acoustic coupling. Staging evaluation is best performed by advancing the radial echoendoscope into the stomach and evaluating the celiac artery, liver, and perigastric stations. Then, the radial echoendoscope should be slowly withdrawn with the balloon slightly distended through the tumor in the esophagus, culminating in the evaluation of mediastinal adenopathy. Because the image created is perpendicular to the tip of the echoendoscope, image-guided interventions such as FNA or fine-needle biopsy (FNB) cannot be performed with the radial instrument. On the other hand, the curvilinear echoendoscope provides a 120° view of the esophageal wall and adjacent structures in the actual plane of the echoendoscope, allowing for image-guided interventions and fiducial placement.
Advances in diagnostic imaging include EUS elastography and contrast-enhanced EUS; however, there are very limited data on the application of these tools to esophageal cancer staging. Elastography may have a future role in the assessment of esophageal cancer with the potential to reduce the need for FNA of lymph nodes based on both sonographic appearance and elastography. This may be of particular benefit in diagnosing high-risk malignant periesophageal lymphadenopathy, where there may be intervening vasculature or malignancy. Contrast-enhanced EUS does not appear to offer much additional information because most esophageal tumors are not highly vascularized.5
Through-the-scope high-frequency EUS mini-probes (frequency of 20-30 MHz) have been developed and are in use for a variety of indications. EUS mini-probes are placed through a diagnostic endoscope and may be beneficial for staging smaller lesions and in patients with significant luminal stenosis, where passage of the echoendoscope is not possible. However, even though mini-probes may be able to traverse esophageal strictures, the high frequency of these catheters reduces sonographic tissue penetration, limiting their use in obstructive cancer staging.5
Tumor, Node, Metastasis Staging
Clinical staging for esophageal cancer is best performed using the tumor, node, metastasis (TNM) staging system (8th edition), which was developed by the Union for International Cancer Control (UICC) and American Joint Committee on Cancer (AJCC; Table).12 Tumor depth is well assessed via EUS due to detailed examination of the esophageal wall. Specifically, the wall layers of the esophagus are brighter (hyperechoic) or darker (hypoechoic) compared with surrounding tissue. The degree of involvement of the hypoechoic muscularis propria (4th layer) differentiates T1, T2, and T3 tumors. If the tumor is superficial to the muscularis propria with a clear plane, it is T1 (Figure 1). If the tumor invades into, but not through, the muscularis propria, it is T2 (Figure 2). If the tumor invades through the muscularis propria, it is at least T3 (Figure 3). If there is invasion into the adjacent structures or organs (eg, the aorta), the tumor is categorized as T4 (Figure 4).
Table.
Tumor, Node, Metastasis Staging Classification (8th Edition)12
| Primary Tumor | |
| TX | Primary tumor cannot be assessed. |
| T0 | No evidence of primary tumor |
| Tis | Carcinoma in situ |
| T1 | Tumor invades the lamina propria, muscularis mucosa, or submucosa. |
| T1a | Tumor invades the lamina propria or muscularis mucosa. |
| T1b | Tumor invades the submucosa. |
| T2 | Tumor invades the muscularis propria. |
| T3 | Tumor invades the adventitia. |
| T4 | Tumor invades the adjacent structures. |
| T4a | Tumor invades the pleura, pericardium, azygos vein, diaphragm, or peritoneum. |
| T4b | Tumor invades the other adjacent structures, such as the aorta, vertebral body, or trachea. |
| Regional Lymph Nodes | |
| NX | Lymph node status cannot be assessed. |
| N0 | No regional lymph node metastases |
| N1 | Metastasis in ≤2 regional lymph nodes |
| N2 | Metastasis in 3-6 regional lymph nodes |
| N3 | Metastasis in ≥7 regional lymph nodes |
| Distant Metastases | |
| MX | Presence of distant metastases cannot be assessed. |
| M0 | No distant metastases |
| M1 | Distant metastases |
Figure 1.
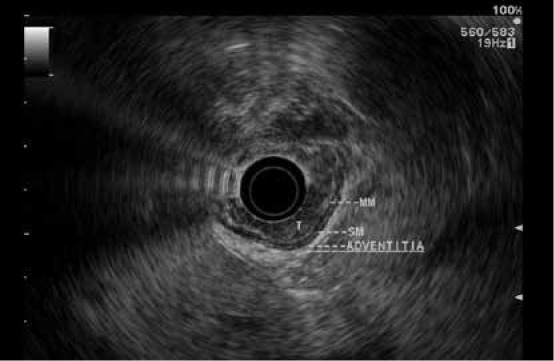
An early esophageal tumor limited to the mucosa consistent with a T1a lesion and amenable to endoscopic therapy. Endoscopic mucosal resection confirmed that the lesion’s stage was T1a.
Figure 2.
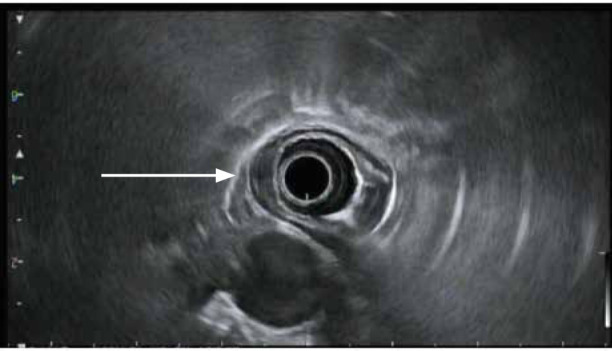
An esophageal tumor with invasion into (arrow), but not through, the muscularis propria. Findings are consistent with a T2 lesion.
Figure 3.
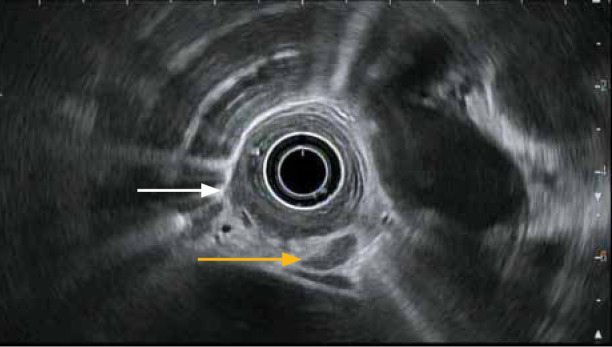
An esophageal tumor with invasion through the muscularis propria at the 8 o’clock position (white arrow). Findings are consistent with a T3 lesion. An enlarged, malignant-appearing lymph node (yellow arrow) is also seen in the region, consistent with the T3N1 stage per endoscopic ultrasound criteria.
Figure 4.
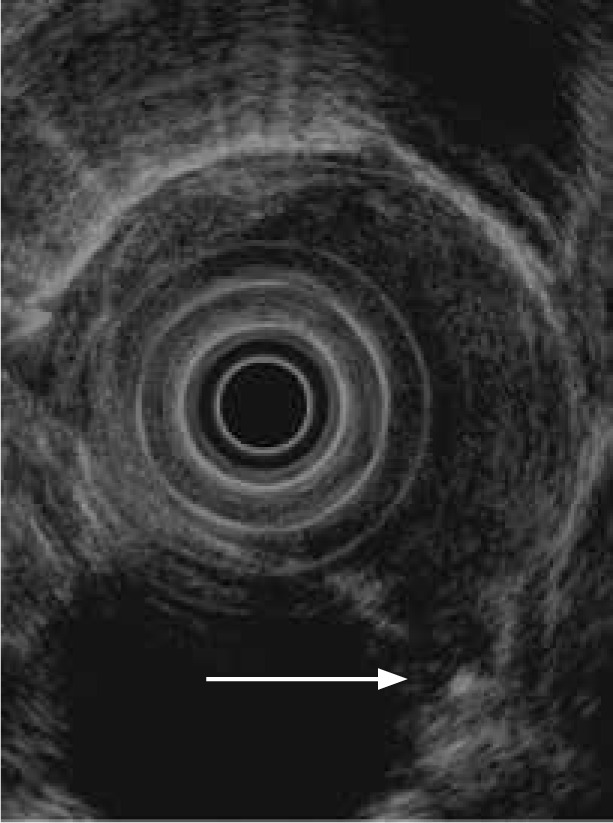
An esophageal tumor penetrating into the adjacent thoracic aorta (arrow), consistent with a T4 lesion.
Additionally, with high-frequency ultrasound imaging, it may be possible to distinguish T1a from T1b cancers (Figure 1). However, it should be noted that for T1 lesions and nodular Barrett esophagus, endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD) remain the best interventions to assess depth of invasion, as the accuracy of EUS is suboptimal in the precise staging of these superficial lesions. For T1b lesions, differentiating by depth of submucosal involvement (SM1, SM2, SM3) should also routinely be performed following EMR or ESD. Both procedures are highly effective in confirming T1N0MX lesions, differentiating T1a from T1b lesions, and determining SM classification.13-16
With respect to nodal staging, EUS, CT, and FDGPET all provide complementary locoregional lymph node imaging. These examinations assess the potential of a lymph node harboring metastases, but there are limitations to each of these modalities. For EUS, the criteria for a malignant lymph node include the features of round, hypoechoic, large (>1 cm), and discrete borders. With more features, the likelihood of malignancy within the lymph node is higher. With fewer features, nodal disease is less certain. Additionally, the staging of nodal disease is performed by determining the number of nodes present with malignant features (Table).
Accuracy of Endoscopic Ultrasound in Esophageal Cancer Staging
EUS has an overall accuracy of 90% for T and N staging of esophageal cancer.17 In the 2017 UICC/AJCC TNM classification system of esophageal and gastroesophageal tumors (Table),12 the distinction between T1a and T1b tumors is made. This distinction is important, as the risk for lymph node metastasis for T1a and T1b SM1 cancers is only 3% to 6%, compared with 21% to 24% for T1b SM2 and SM3 tumors.8 Accuracy for T staging with EUS is reported to be greater than 80%.8,18,19 Puli and colleagues reported the sensitivity and specificity of EUS for staging esophageal cancer to be 81.6% and 99.4% in T1 tumors, 81.4% and 96.3% in T2 tumors, 91.4% and 94.4% in T3 tumors, and 92.4% and 97.4% in T4 tumors, respectively, demonstrating that the accuracy of EUS in advanced esophageal cancer is higher compared with early cancer.14 In fact, in a systemic review of 27 studies evaluating performance, EUS was found to be highly effective at differentiating T1 or T2 cancers from T3 or T4 cancers (performance index, 0.89 for esophageal cancers and 0.91 for esophagogastric junction cancers).20 This is important, as T3 and T4 tumors often have nodal involvement and may require neoadjuvant therapy as compared with T1 or T2 tumors that could be managed with resection alone.21
EUS is also helpful in staging locoregional lymph-adenopathy using well-established imaging criteria for malignant lymph nodes or by using FNA or FNB. The overall accuracy of EUS for initial N staging in esophageal cancer is greater than 70%.18,19 Visible hypoechoic lymph nodes of at least 10 mm, discrete round shape (as opposed to oval or elliptical shape), sharp border, and absence of central intranodal vessels are suggestive of malignancy. If all 4 of these criteria are met, there is up to an 80% likelihood of metastasis; however, only 25% of cases meet all 4 criteria.22 A recent retrospective analysis of 123 patients with esophageal cancer improved EUS staging accuracy by adding 3 criteria to those listed above: lymph nodes near lesion, total number of lymph nodes, and T3/4 staging.23
The accuracy of EUS restaging after chemoradiation is poor, thought to be secondary to distortion of the architecture of the esophageal wall from post-treatment inflammation and fibrosis. A meta-analysis that included 593 patients who underwent EUS after neoadjuvant chemotherapy for re-evaluation of primary tumor status and 602 patients who underwent EUS for re-evaluation of locoregional lymph node status found that EUS had a sensitivity of 96.4% and a specificity of 10.9% for detecting residual cancer at the primary tumor site and 62.0% and 56.7%, respectively, for detecting residual cancer in locoregional lymph nodes.19
Clinical Application of Endoscopic Ultrasound
EUS is an important tool for esophageal cancer staging for many reasons. In early esophageal cancer, T staging may help select appropriate cases for minimally invasive treatment using EMR and ESD techniques. EUS also helps identify locoregional adenopathy that may be missed by CT and PET-CT, especially lymph nodes less than 1 cm in size. Endoscopic resection of early esophageal cancer is now an established intervention with long-term outcomes at par with or better than surgery, and EUS is a critical tool to help facilitate case selection.24,25
In early esophageal cancer with no nodal disease, EMR or ESD can be performed with histologic assessment to determine depth of invasion. Patients who are found to have T1b cancer on histopathology should be presented at a multidisciplinary tumor board and, in general, referred for surgical consultation. In some select patients with T1b disease, endoscopic management and observation may be acceptable (eg, SM1 depth, well-differentiated tumor, absence of lymphovascular invasion). Patients with T1a cancer and margin negative resection are deemed to be cured and can undergo subsequent endoscopic management as needed.25
In patients with more advanced esophageal cancer, EUS can confirm the presence of established indications for neoadjuvant treatment (T3 tumor and locoregional N1 disease). In addition, it can help more accurately stage occult metastatic disease that may be missed by cross-sectional imaging (liver lesions, celiac and abdominal adenopathy).26 In patients with obstructive tumors through which the echoendoscope may not pass, esophageal dilation may be performed to facilitate EUS staging. However, these patients are invariably candidates for neoadjuvant treatment or definitive chemoradiation, and risk-benefit analysis may favor the avoidance of dilation and aggressive efforts at staging in such cases. With respect to treatment of esophageal cancer, image-guided radiotherapy (IGRT) is a recent advancement and offers promising outcomes in a select group of patients with esophageal cancer. Fiducial placement provides for better localization and tracking, resulting in delivering precise high-dose radiotherapy to the target lesion. This results in an improved side-effect profile of radiotherapy due to minimized damage to surrounding normal tissue, safe use of higher doses of radiation, accelerated recovery due to fewer adverse effects, improved quality of life, and potential for intraoperative localization of primary tumor. Furthermore, groups, including at the University of Rochester, have shown the benefit of EUS-guided fiducial placement to facilitate IGRT of tumors in real time, thus minimizing damage to the surrounding radiosensitive organs at risk and maximizing therapy to the tumor.27,28
Controversies in Endoscopic Ultrasound Staging of Esophageal Cancer
There has been some controversy regarding the utilization of EUS in esophageal cancer staging. Findlay and colleagues reported that the risk of EUS in patients with advanced esophageal cancer (T2-T4) on CT scan outweighed the benefit.29 However, Hulshoff and colleagues found that EUS influenced primary treatment in 29% of patients who had initial staging with PET-CT.30 The study demonstrated that EUS was able to provide information to change the radiation field in 23% of patients, change staging from incurable to potentially curable in 2% of patients, influence the extent of lymph node resection in 17% of patients, and affect treatment decision-making with FNA in 9% of patients.30
A study by Wani and colleagues included 524 patients who underwent EUS staging and found that in all univariate comparisons, patients had improved survival for all stages (P<.0001) except for stage 0 disease.7 The addition of PET-CT did not add to the survival benefit.7 Recent research has found that CT and PET scans are not adequate for staging celiac and mediastinal lymphadenopathy, further supporting the use of EUS after ruling out distant metastasis.5 However, if distant metastatic disease is present, the role of EUS is limited and the procedure should be avoided for the purposes of staging.
Challenges and Limitations of Endoscopic Ultrasound Staging
Limitations of EUS staging include the inability to traverse high-grade malignant esophageal strictures for accurate T staging, with a reported failure rate of 30%, especially in advanced tumors.8 Dilation of malignant strictures has been reported with high complication rates in the past; however, more recent data suggest that this can be accomplished with acceptable complication rates. Jacobson and colleagues reported that approximately one-third of patients undergoing EUS staging for esophageal cancer required dilation of a malignant stricture.31 Staging was completed in 95% of cases after balloon dilation. Complications included 1 esophageal perforation, which was comparable to the rate observed during EUS staging without dilation.31 Another option is the use of EUS mini-probes to traverse esophageal strictures, although these probes have limited depth of penetration, reducing their effectiveness for cancer staging.
Another limitation of EUS is operator variability. An older study reported low accuracy and high interobserver variability for endosonographic staging of upper gastrointestinal cancers, even among experienced examiners.32 This is less of an issue at high-volume centers with experienced endosonographers.
Cost Analysis
Only a few studies have evaluated the cost implications of EUS in esophageal cancer management. For primary staging, EUS has been found to be less costly. Specifically, in a study by Hadzijahic and colleagues in 2000, T4 and/or M1 disease was diagnosed more frequently with EUS than with CT.26 However, recent advances in CT technology and TNM staging likely make this finding less relevant. In 2002, Harewood and Wiersema found EUS to be the most cost-effective esophageal cancer staging modality at $13,811, vs CT-guided FNA at $14,350 and surgery at $13,992.33 As such, EUS has emerged as the standard tissue sampling approach if and when potentially accessible target lesions are found in patients with esophageal cancer. A study comparing CT, EUS-FNA, PET, and mediastinoscopy/laparoscopy in esophageal cancer staging found that PET plus EUS-FNA was the most cost-effective approach, as it decreased overall cost by $3443 per patient by avoiding unnecessary neoadjuvant therapy or surgery in stage I and IV disease.34
Conclusion
Multimodality staging of esophageal cancer with histopathologic analysis, cross-sectional imaging, PET-CT, and EUS remains the current standard of care. In patients with evidence of distant metastasis, EUS is unlikely to provide any additional information and, therefore, is not indicated. However, if cross-sectional imaging is concerning for possible lymph node metastasis, EUS-FNA is important to accurately stage and guide treatment. EUS is also helpful for assessing the depth of tumor involvement when evaluating candidacy for endoscopic resection in early esophageal cancer or in patients who would benefit from neoadjuvant therapy. The overall safety profile of EUS in esophageal cancer staging is excellent, although risk may be increased if high-grade malignant strictures are aggressively dilated to facilitate EUS. The information gained from EUS staging is complementary to that obtained by cross-sectional and PET imaging, adds confidence to clinical staging, and helps guide appropriate management while avoiding unnecessary treatment in many patients.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet. 2013;381(9864):400–412. doi: 10.1016/S0140-6736(12)60643-6. [DOI] [PubMed] [Google Scholar]
- 3.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 4.American Cancer Society. Key statistics for esophageal cancer. https://www.cancer.org/cancer/esophagus-cancer/about/key-statistics.html Updated January 8, 2019. Accessed December 17, 2019.
- 5.DaVee T, Ajani JA, Lee JH. Is endoscopic ultrasound examination necessary in the management of esophageal cancer? World J Gastroenterol. 2017;23(5):751–762. doi: 10.3748/wjg.v23.i5.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang FL, Yu SJ. Esophageal cancer: risk factors, genetic association, and treatment. Asian J Surg. 2018;41(3):210–215. doi: 10.1016/j.asjsur.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 7.Wani S, Das A, Rastogi A et al. Endoscopic ultrasonography in esophageal cancer leads to improved survival rates: results from a population-based study. Cancer. 2015;121(2):194–201. doi: 10.1002/cncr.29043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foley K, Findlay J, Goh V. Novel imaging techniques in staging oesophageal cancer. Best Pract Res Clin Gastroenterol. 2018. pp. 36–37. 17-25. [DOI] [PubMed]
- 9.National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: esophageal and esophagogastric junction cancers. https://www.nccn.org/professionals/physician_gls/pdf/esophageal.pdf Published April 2019. Accessed December 17, 2019.
- 10.National Institute for Health and Care Excellence. Oesophago-gastric cancer: assessment and management in adults. NICE guideline [NG83]. https://www.nice.org.uk/guidance/ng83 Published January 2018. Accessed December 17, 2019. [PubMed]
- 11.Chapman CG, Siddiqui UD. New scopes, new accessories, new stents for interventional endoscopic ultrasound. Clin Endosc. 2016;49(1):41–46. doi: 10.5946/ce.2016.49.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rice TW, Kelsen DP, Blackstone EH. et al. Esophagus and esophagogastric junction. In: Amin MB, Edge SB, Greene FL. et al. AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer; 2017. 185 202 [Google Scholar]
- 13.Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T et al. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2015;47(9):829–854. doi: 10.1055/s-0034-1392882. [DOI] [PubMed] [Google Scholar]
- 14.Puli SR, Reddy JB, Bechtold ML, Antillon D, Ibdah JA, Antillon MR. Staging accuracy of esophageal cancer by endoscopic ultrasound: a meta-analysis and systematic review. World J Gastroenterol. 2008;14(10):1479–1490. doi: 10.3748/wjg.14.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thota PN, Sada A, Sanaka MR et al. Correlation between endoscopic forceps biopsies and endoscopic mucosal resection with endoscopic ultrasound in patients with Barrett’s esophagus with high-grade dysplasia and early cancer. Surg Endosc. 2017;31(3):1336–1341. doi: 10.1007/s00464-016-5117-1. [DOI] [PubMed] [Google Scholar]
- 16.Yang D, Coman RM, Kahaleh M et al. Endoscopic submucosal dissection for Barrett’s early neoplasia: a multicenter study in the United States. Gastrointest Endosc. 2017;86(4):600–607. doi: 10.1016/j.gie.2016.09.023. [DOI] [PubMed] [Google Scholar]
- 17.Lee WC, Lee TH, Jang JY et al. Staging accuracy of endoscopic ultrasound performed by nonexpert endosonographers in patients with resectable esophageal squamous cell carcinoma: is it possible? Dis Esophagus. 2015;28(6):574–578. doi: 10.1111/dote.12235. [DOI] [PubMed] [Google Scholar]
- 18.Kalaitzakis E, Meenan J. Controversies in the use of endoscopic ultrasound in esophageal cancer staging. Scand J Gastroenterol. 2009;44(2):133–144. doi: 10.1080/00365520802273066. [DOI] [PubMed] [Google Scholar]
- 19.van Rossum PSN, Goense L, Meziani J et al. Endoscopic biopsy and EUS for the detection of pathologic complete response after neoadjuvant chemoradio-therapy in esophageal cancer: a systematic review and meta-analysis. Gastrointest Endosc. 2016;83(5):866–879. doi: 10.1016/j.gie.2015.11.026. [DOI] [PubMed] [Google Scholar]
- 20.Kelly S, Harris KM, Berry E et al. A systematic review of the staging performance of endoscopic ultrasound in gastro-oesophageal carcinoma. Gut. 2001;49(4):534–539. doi: 10.1136/gut.49.4.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rice TW, Ishwaran H, Hofstetter WL et al. Esophageal cancer: associations with (pN+) lymph node metastases. Ann Surg. 2017;265(1):122–129. doi: 10.1097/SLA.0000000000001594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhutani MS, Hawes RH, Hoffman BJ. A comparison of the accuracy of echo features during endoscopic ultrasound (EUS) and EUS-guided fine-needle aspiration for diagnosis of malignant lymph node invasion. Gastrointest Endosc. 1997;45(6):474–479. doi: 10.1016/s0016-5107(97)70176-7. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y, He S, Dou L et al. Esophageal cancer N staging study with endoscopic ultrasonography. Oncol Lett. 2019;17(1):863–870. doi: 10.3892/ol.2018.9716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galey KM, Wilshire CL, Watson TJ et al. Endoscopic management of early esophageal neoplasia: an emerging standard. J Gastrointest Surg. 2011;15(10):1728–1735. doi: 10.1007/s11605-011-1618-3. [DOI] [PubMed] [Google Scholar]
- 25.Nurkin SJ, Nava HR, Yendamuri S et al. Outcomes of endoscopic resection for high-grade dysplasia and esophageal cancer. Surg Endosc. 2014;28(4):1090–1095. doi: 10.1007/s00464-013-3270-3. [DOI] [PubMed] [Google Scholar]
- 26.Hadzijahic N, Wallace MB, Hawes RH et al. CT or EUS for the initial staging of esophageal cancer? A cost minimization analysis. Gastrointest Endosc. 2000;52(6):715–720. doi: 10.1067/mge.2000.108481. [DOI] [PubMed] [Google Scholar]
- 27.Oliver JA, Venkat P, Frakes JM et al. Fiducial markers coupled with 3D PET/CT offer more accurate radiation treatment delivery for locally advanced esophageal cancer. Endosc Int Open. 2017;5(6):E496–E504. doi: 10.1055/s-0043-104861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aranez JL, Bittner K, Katz A et al. EUS-guided fiducial placement with stereo-tactic radiotherapy compared to conventional radiotherapy for esophageal cancer: x marks the spot! Gastrointest Endosc. 2019;89(6):AB299–AB300. [Google Scholar]
- 29.Findlay JM, Bradley KM, Maile EJ et al. Pragmatic staging of oesophageal cancer using decision theory involving selective endoscopic ultrasonography, PET and laparoscopy. Br J Surg. 2015;102(12):1488–1499. doi: 10.1002/bjs.9905. [DOI] [PubMed] [Google Scholar]
- 30.Hulshoff JB, Mul VEM, de Boer HEM et al. Impact of endoscopic ultra-sonography on 18F-FDG-PET/CT upfront towards patient specific esophageal cancer treatment. Ann Surg Oncol. 2017;24(7):1828–1834. doi: 10.1245/s10434-017-5835-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jacobson BC, Shami VM, Faigel DO et al. Through-the-scope balloon dilation for endoscopic ultrasound staging of stenosing esophageal cancer. Dig Dis Sci. 2007;52(3):817–822. doi: 10.1007/s10620-006-9488-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meining A, Rösch T, Wolf A et al. High interobserver variability in endosono-graphic staging of upper gastrointestinal cancers. Z Gastroenterol. 2003;41(5):391–394. doi: 10.1055/s-2003-39422. [DOI] [PubMed] [Google Scholar]
- 33.Harewood GC, Wiersema MJ. A cost analysis of endoscopic ultrasound in the evaluation of esophageal cancer. Am J Gastroenterol. 2002;97(2):452–458. doi: 10.1111/j.1572-0241.2002.05499.x. [DOI] [PubMed] [Google Scholar]
- 34.Shumaker DA, de Garmo P, Faigel DO. Potential impact of preoperative EUS on esophageal cancer management and cost. Gastrointest Endosc. 2002;56(3):391–396. doi: 10.1016/s0016-5107(02)70044-8. [DOI] [PubMed] [Google Scholar]


