Abstract
Fine particulate air pollution <2.5 μm in diameter (PM2.5) is a major environmental threat to global public health. Multiple national and international medical and governmental organizations have recognized PM2.5 as a risk factor for cardiopulmonary diseases. A growing body of evidence indicates that several personal-level approaches that reduce exposures to PM2.5 can lead to improvements in health endpoints. Novel and forward-thinking strategies including randomized clinical trials are important to validate key aspects (e.g., feasibility, efficacy, health benefits, risks, burden, costs) of the various protective interventions, in particular among real-world susceptible and vulnerable populations. This paper summarizes the discussions and conclusions from an expert workshop, Reducing the Cardiopulmonary Impact of Particulate Matter Air Pollution in High Risk Populations, held on May 29 to 30, 2019, and convened by the National Institutes of Health, the U.S. Environmental Protection Agency, and the U.S. Centers for Disease Control and Prevention.
Keywords: cardiopulmonary disease, cardiovascular disease, fine particulate air pollution, portable air cleaner, randomized clinical trials
Air pollution is a heterogeneous mixture of particulate matter (PM) and gases derived from multiple sources, including fossil fuel combustion (1–6). PM itself is an amalgam of pollutants (e.g., carbon species, sulfates, nitrates, metals) ranging in size from a few nanometers to several microns. While a variety of gases (e.g., ozone) have been linked to adverse health effects, the largest body of evidence supports PM ≤2.5 μm in diameter (PM2.5) as a major environmental threat to global public health. Indeed, PM2.5 ranks among the leading risk factors for global mortality, accounting for roughly 8.9 million premature deaths per year in recent estimates—with 213,000 in North America alone (2).
PM2.5 has been associated with wide-ranging adverse health effects including neurologic (e.g., dementia), metabolic (e.g., diabetes mellitus [DM]), allergic (e.g., rhinitis), kidney, inflammatory, and auto-immune disorders; lower respiratory infections; and several cancers (e.g., lung) (Figure 1) (7). However, from a public health standpoint, the impact on cardiopulmonary diseases is of paramount importance (1). Exposures over the short-term contribute to increased asthma and chronic obstructive pulmonary disease (COPD) exacerbations, whereas over the long-term, they can worsen lung function and may promote the incidence of COPD (7). More than one-half of all PM2.5-related deaths are from cardiovascular causes (1). Short-term exposures increase the risk for myocardial infarction (MI), stroke, heart failure, and sudden death (6,8–11). A 10-μg/m3 increase in PM2.5 ambient levels increases these event rates by up to 1% to 2% in the population during the ensuing few days. Chronic exposures over months to years increase these risks to an even greater degree (≥10% per 10-μg/m3 increase). Additionally, longer-term exposures have been associated with poorer health status in patients with cardiovascular disease (CVD) (12). Numerous mechanisms have been shown to contribute to the adverse cardiovascular outcomes including: vascular dysfunction, elevated blood pressure (BP), metabolic derangements (e.g., insulin resistance), enhanced thrombosis-coagulation, heightened arrhythmia potential, as well as increased atherosclerosis and plaque vulnerability (3–6). How PM2.5 exposure elicits this host of extrapulmonary responses remote from the site of inhalation has also been intensely investigated. Broad mediating pathways potentially responsible include the triggering of systemic inflammation and oxidative stress, autonomic imbalance, neuro-hormonal activation, and/or the release of secondarily-generated endogenous factors (e.g., oxidized lipids) or pollutant constituents (e.g., metals, nanoparticles) from the pulmonary into the systemic circulation. As such, the American Heart Association (5), European Society of Cardiology (6), the American Thoracic Society, and European Respiratory Society (13), as well as the U.S. Environmental Protection Agency (EPA) (14) have recognized PM2.5 as a causal risk factor for pulmonary disease and CVD.
FIGURE 1. Subset of Diseases Associated With Fine Particulate Air Pollution by Organ System.
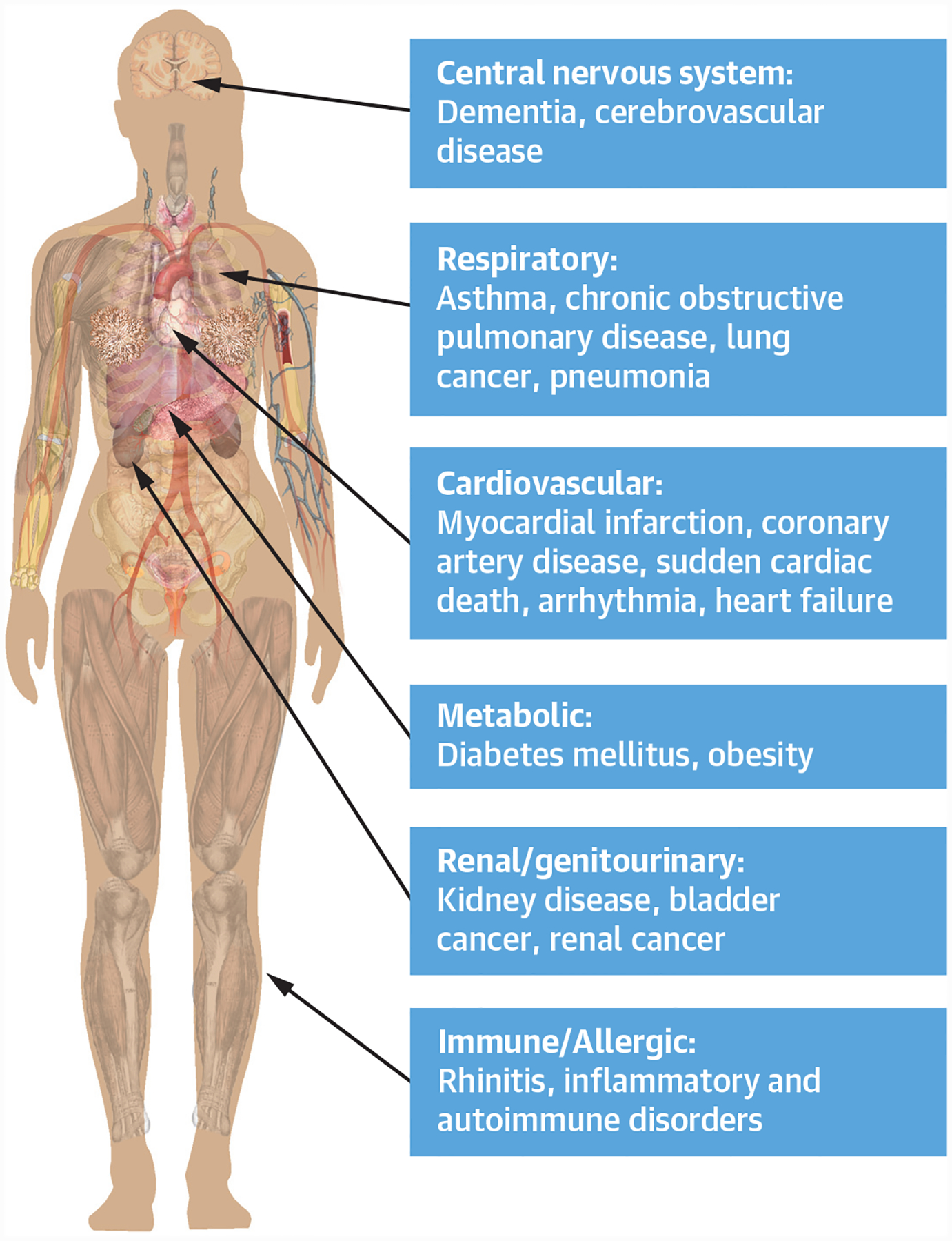
Diseases associated with fine particulate air pollution organized by organ system. This figure compiles data from multiple observational and retrospective studies to show the heterogeneity of diseases associated with fine particulate air pollution exposure.
Recent epidemiological evidence has greatly enhanced our understanding of the scope of the threat posed by PM2.5. Both short- and long-term exposures to low concentrations increase the risks for morbidity and mortality (15–21). The shape of the population exposure-risk relationship does not appear to have a lower “safe” threshold even down to background levels (2 to 3 μg/m3) (2). At the other end of the spectrum of the exposure-response function, extremely poor air quality (PM2.5 levels >50 to 100 μg/m3) faced by hundreds of millions of people across Asia and specific low- to middle-income countries on a daily basis, poses significant health risks that may be even greater than previously estimated (21–23). Studies also show that certain subgroups of people are more susceptible to PM2.5, including older adults, lower socioeconomic and minority populations, and individuals with pre-existing chronic pulmonary or cardiometabolic (e.g., DM, coronary artery disease) diseases. Indeed, the cardiovascular risks from PM2.5 exposures are likely much higher among MI survivors (e.g., 20% to 64% per 10 μg/m3) than the general population (24–26).
A growing body of evidence also supports that reductions in PM2.5 levels can result in demonstrable benefits to population health (27–30). The improvement in air quality across the United States over the past few decades has been independently associated with increased life expectancy. These results parallel the observations of rapid decreases in cardiovascular risk following bans of public smoking (31). Finally, an increasing number of studies have reported that personal-level and some building-level approaches to reduce exposure to PM2.5 can produce improvements in surrogate markers of cardiopulmonary and metabolic risk. At this time, the candidate interventions that might be most feasibly implemented in large populations are indoor portable air cleaners (PACs) and/or face masks (e.g., N95 respirators). Intermediate health endpoints shown to improve with use of 1 of these interventions include BP, ST-segment depression with activity, systemic inflammation, stress hormones, and insulin sensitivity (3,32).
The rationale for formally studying the efficacy and health benefits of personal-level interventions to reduce PM2.5 exposures in a clinical outcome trial is several-fold (3,4,32,33). First, tens of thousands of deaths and cardiopulmonary events likely related to particulate matter occur annually in the United States (1,2). Novel and forward-thinking strategies are therefore essential to help protect the population (particularly high-risk individuals) and reduce the residual public health toll from present-day levels of air pollution—particularly in “hot-spots” (e.g., urban or near-roadway locations). The scientific testing of building and personal-level strategies could demonstrate the public health potential to reduce CVD events through these interventions. Moreover, medical societies (e.g., American Heart Association) often ascribe grades of the level of evidence in their guidelines that support the use of any intervention in clinical practice. Positive results from randomized clinical trials provide the highest level of evidentiary support and are often required for formal top-tier recommendations. Although compelling observational data can be supportive, contemporary clinical practice patterns are rarely changed without robust results from clinical outcome trials. Such evidence could be the most instrumental in fostering a widespread and evidence-based approach in clinical medicine for personal interventions protecting against PM2.5. Second, hundreds of thousands of deaths and morbid events occur per year in heavily polluted regions (e.g., China and South Asia) where the air quality is likely to remain unhealthy for many years (1,2). At-risk individuals who reside in (or travel to) these locations could benefit from validated options proven to help protect their health (33). Third, although cardiovascular morbidity and mortality in the United States have decreased substantially over the past several decades, there has been a recent plateauing—and possibly a reversal—of this reduction in some groups (34,35). It is possible that current levels of PM2.5 contribute to residual CVD risk and may partially explain our inability to further reduce cardiovascular events despite pharmacological and procedural advances in cardiovascular care. Fourth, clinical trials can best validate key aspects the various interventions (e.g., feasibility, efficacy, health benefits, risks, burden, costs) in real-world populations. Finally, the effectiveness of an intervention to reduce PM2.5 exposures in preventing cardiovascular events would provide further experimental evidence in support of a causal relationship between this air pollutant and CVD.
Herein, we summarize discussions from a recent expert workshop held on May 29 to 30, 2019: Reducing the Cardiopulmonary Impact of Particulate Matter Air Pollution in High Risk Populations, convened by the National Heart, Lung, and Blood Institute and the National Institute of Environmental Health Sciences of the National Institutes of Health, the U.S. EPA, and the U.S. Centers for Disease Control and Prevention. Details on the meeting goals and structure are available online (36). The stated objective was to “Discuss feasible trials or other research designs that will address the effectiveness of personal air pollution interventions in reducing mechanistic and surrogate endpoints, and adverse cardiovascular and respiratory health outcomes in high risk populations” (36). The organizing committee believes the overall awareness of the serious health threats posed by indoor and outdoor sources of air pollution remains low among health care providers. Concerted efforts are needed to highlight the importance and prioritization of research efforts seeking to mitigate the health risks of air pollutants. They represent prudent actions based upon precautionary principles and expert opinions. Clinical trials have the potential to significantly bolster support for these and other actions.
Given the growing number of small studies and feasibility/adaptability of some interventions to clinical trial settings, the organizing committee thought it reasonable and important to discuss the plausibility and potential designs of future outcome trials to test whether health benefits can be derived from specific interventions to reduce air pollution exposures in subsets of higher-risk individuals. Due to the enormous population adversely affected by PM2.5, the implementation of proven protective measures could offer an unparalleled potential to benefit global public health. Trials of appropriate interventions in at-risk populations yielding positive or null results would both be helpful to guide clinicians and inform the public.
WORKSHOP DESCRIPTION
On May 29 to 30, 2019, the National Heart, Lung, and Blood Institute, EPA, National Institute of Environmental Health Sciences, and Centers for Disease Control and Prevention held a workshop at the National Institutes of Health’s Natcher Conference Center to discuss feasible trials or other research designs to address the effectiveness of personal-level interventions to reduce air pollution exposures and improve cardiovascular and respiratory clinical and/or surrogate endpoints in high risk populations (36). The mechanistic pathways underpinning the association between PM2.5 and cardiopulmonary diseases were not discussed in detail and were beyond the scope of the workshop agenda, as they have been reviewed in detail previously. Workshop members were provided background information regarding the epidemiology and mechanisms of air pollution induced health effects prior to attending the conference (3). To address the conference aim and develop cross-disciplinary dialogue, attendees included experts in air pollution exposure assessment and epidemiology, cardiovascular and pulmonary medicine, clinical trials and epidemiology, building engineering and health sciences, and health care disparities and outcomes in minorities and under-represented populations. Although it was recognized that gaseous pollutants (e.g., ozone and gaseous traffic-related air pollutants) promote cardiovascular and pulmonary diseases, the workshop focused on PM2.5. This was because PM2.5 poses the greatest public health threat and there is more evidence regarding personal-level protective strategies (1–6). The workshop structure and agenda has been described (36). Paper drafts and findings were reviewed by workshop participants.
POTENTIAL INTERVENTIONS
A key focus of the workshop was on existing interventions that could be tested in a clinical trial (Figure 2). To date, no personalized intervention has been evaluated in a large-scale randomized controlled clinical trial addressing hard clinical endpoints. However, 3 sets of empirical findings should increase our confidence in previously modeled estimates of benefits (37). First, robust data to date support an association between exposure to particles of ambient origin and mortality including ischemic heart disease mortality (3). Second, there is strong evidence that filtration interventions can reduce exposure to particles (37). Third, there is emerging evidence that filtration improves markers that predict future adverse coronary events (32,38), and can improve respiratory health in small-scale studies of children and adults with asthma (39,40). The use and efficacy of these interventions in reducing personal exposure may vary considerably (as would any derived benefit), depending on the context of exposure (indoor vs. outdoor) and a number of personal, ecological, and exposure-related factors. Although several interventions, including lifestyle changes (e.g., reducing traffic exposure) along with commonsense approaches such as closing house and car windows and using automobile cabin filters/air conditioning, may be effective and have been reviewed previously, they are generally not amenable to testing in the clinical trial context (32). However, this should not discount these and other strategies from being important options for intervention and targets for society and for broader and societal regulations. The mission of this workshop was to focus on potential personal interventions that are applicable to be studied in trials, in particular randomized, blinded, clinical outcome trials. It is important to note that several pharmacological interventions (omega-3 fatty acids, statins), dietary changes (Mediterranean diet), and exercise may help to mitigate air pollution-induced health effects, as reviewed elsewhere (3,32). These interventions were also not the focus of this workshop.
FIGURE 2. Approaches to Limit Fine Particulate Air Pollution Exposure.
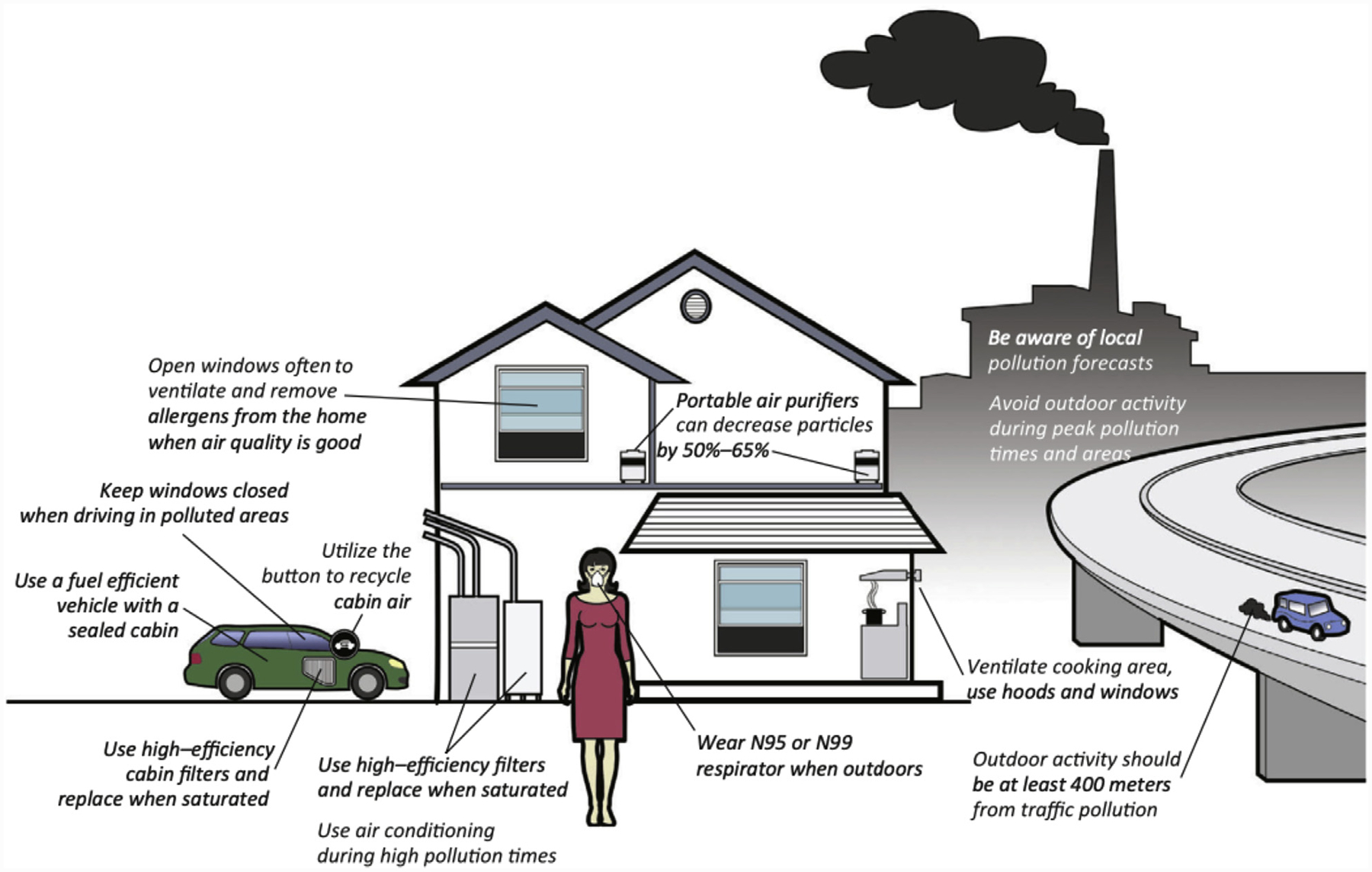
Portable air purifiers, N95 respirator and high-efficiency filters discussed in the paper as testable in a randomized trial. Additional exposure reduction and mitigation strategies are displayed. Figure labels as indicated. Republished with permission from Bard et al. (32).
RESPIRATORY PROTECTION EQUIPMENT.
While inexpensive cloth, cotton, gauze, or procedural (e.g., surgical) masks are widely available, they are not designed nor validated to be effective at reducing PM2.5 exposures and are therefore not recommended (41). They also lack an airtight facial seal when worn, and as such, even if particles are filtered to some variable degree (e.g., 30% to 70%) by the various materials, there can be no reliable reduction in the inhaled dose. Conversely, there are forms of personal protective equipment (PPE) such as filtering facepiece respirators (e.g., N95 respirators) that are validated to reduce exposures to PM2.5 and are usually also widely available. They form an air-tight facial seal when worn correctly, and their material is specifically designed to filter at least 95% of particles at the 0.3-μm size range. Larger and small PM size fractions are typically filtered with even greater effectiveness. These and other types of respirators are certified by the National Institutes of Occupational Safety and Health (NIOSH) typically for the workplace (42). Small studies have demonstrated a beneficial impact on some health outcomes (Supplemental Table 1). Despite these findings, extended use of respiratory protective equipment over protracted periods (weeks to months) and in the general public outside of workplace settings (i.e., without facial fit and seal testing) may be less practical and effective and has not been formally tested.
HIGH-EFFICIENCY HOME AIR FILTRATION.
Household air pollution can encompass a range of particles that originate not only from outdoor ambient pollutants that penetrate indoors, but also from indoor sources. Building-level filters include high-efficiency media that trap fine particles and can be added to pre-existing heating, ventilation, and air conditioning systems. If properly installed and maintained, and provided that cycle times are high enough, particle filtration systems in homes and buildings can be highly effective (50% to 85% reduction in PM2.5) in reducing indoor particle concentrations (43–45). However, such systems only reduce exposures while people remain indoors. A number of variables can influence their effectiveness including the operation time of the fan, often determined by heating or cooling demand; nominal (rated) efficiency of the building filter; tightness of the building enclosure including any open windows; filter installation (e.g., properly fit gasket); and frequency of filter change. There are no current studies demonstrating changes in cardiovascular surrogates with use of building-level filtration systems. The expenses involved in reconfiguring heating, ventilation, and air conditioning units will vary depending upon several factors including the building and pre-existing system, which may not be prohibitive for many individual households (e.g., $150 for installing larger filter slots and $100 to $200 per year for filters and added energy costs). In addition to the aforementioned limitations, other difficulties of this intervention type include ensuring participant blinding and enrolling a broad and representative population. While building system interventions may prove difficult to test in a clinical trial, it is possible that such an intervention could serve as a natural experiment, especially in large-scale communities.
PORTABLE AIR CLEANERS.
PACs can be affordable and effective in reducing indoor PM2.5 by as much as 50% to 60% in carefully controlled studies (27,46–49). PACs not only lower indoor PM levels in a designated room where they are positioned, but have been shown to reduce the average exposure over a 24-h period by roughly 40% (measured by wearing personal monitors) among individuals not otherwise restricted in activities outside their household (28,50). However, it is important to note that the filtration efficacy can be undermined by a number of variables (open windows or leaky enclosures, high levels of in-room air exchange, significant indoor sources, large space beyond the capacity of device to filter, and very high outdoor levels). Extreme levels of outdoor ambient PM2.5 (>100 to 500 μg/m3) as is common in many heavily-polluted countries (e.g., India, China), may result in persistently unhealthy indoor particle concentrations, even assuming PACs remain capable of providing a >50% reduction in indoor levels at this high level of pollution (51). Their effectiveness to help protect against the harmful effects of wildfire smoke has been reviewed elsewhere (52).
Although PAC use can provide some degree of protection, it may not be equally effective across all global regions or in all households. Most notably, PACs can only reduce exposures while people remain indoors in proximity to the filtration devices. The U.S. EPA identifies 3 types of PACs (53):
Ultraviolet light air cleaners sterilize some biological pollutants in indoor air and are not recommended for PM2.5 reduction, unless when used in conjunction with filters. Some ultraviolet devices may circulate and/or generate ozone.
Electronic or electrostatic air cleaners ionize an incoming stream of particles, depositing them on an oppositely charged metal plate and/or to enhance deposition to a traditional filter media. These devices may produce ozone and thus are not recommended.
Mechanical air filters capture particles on filter materials. Media filtration methods vary from true high-efficiency particulate arrestance (HEPA) filters that, by definition, filter particles 0.3 μm in diameter (the most difficult particle size to filter) by at least 99.97% versus other less-effective filters. Detailed descriptions of the filtering media and technologies are provided by the EPA (53).
Until approximately 2008, health research using PACs was mainly focused on respiratory outcomes in asthma studies (53). Thereafter, outcomes other than lung function, such as BP, heart rate variability, endothelial function, and plasma oxidative stress/inflammatory markers, have been explored. Studies of PACs have been reviewed elsewhere (32), and a summary table is included online (Supplemental Table 2). The available evidence from surrogate endpoint trials suggests that the use of PACs may improve cardiometabolic health, in particular BP, by reducing particulate exposures (32). However, due to several key limitations (e.g., small sample sizes, brief durations) of nearly all studies, the findings only represent a proof-of-principle at the current time. The magnitude of reduction in clinical respiratory and CVD events potentially gained over several years in high-risk individuals cannot be directly calculated solely from these results. Nonetheless, data from these studies can be used together with other results to help formulate estimations of effect and samples sizes for future outcome trials.
ISSUES TO CLARIFY MOVING FORWARD
The workshop identified 4 main categories of issues for evaluation to inform an air pollution intervention trial (Table 1). An overarching question of the workshop was whether vanguard-style smaller trials could help to address some or many of these potential issues prior to undertaking a full-scale outcome trial.
TABLE 1.
Issues to Address to Inform on the Design of an Air Pollution Intervention Trial
| Issue Category | Important for Preliminary Data or Questions to Address |
|---|---|
| Clinical |
|
| Air pollution exposure |
|
| Personal intervention(s) |
|
| Determinants of adherence |
|
CLINICAL TRIAL DESIGN CONSIDERATIONS.
This category focuses on target populations for a trial, such as individuals “at-risk” for the health effects of air pollution (Figure 3). This includes both biological susceptibility (i.e., worse health responses to the same exposures) and/or increased vulnerability (i.e., higher levels or increased toxicity of exposures). Pre-existing cardiometabolic disease, including ischemic heart disease, heart failure, and DM, are important determinants of biological susceptibility to highlight in the design of future intervention studies (3). Other groups with greater susceptibility to the health effects of PM2.5 exposure include older adults, individuals of lower socioeconomic status, and populations traditionally underrepresented in clinical trials (e.g., African Americans) or those with comorbid pulmonary conditions (15,16). Other considerations for a future intervention study/trial include populations with socioeconomic disparities and disproportionate air pollution exposures who may be particularly vulnerable to the cardiovascular effects of air pollution exposure (54). An additional consideration for a future intervention study/trial is that concomitant medications, severity of other comorbid disease, and other clinical characteristics may modify the effects of air pollution exposure on cardiovascular outcomes. The role of patient barriers for testing and use of personal air pollution interventions is also a concern. Other notable clinical concerns include determinations of optimal locations for air cleaner or filtration technology, such as community housing versus single family homes and urban locations versus trial protocols without location restrictions. The “scalability” of interventions from a clinical trial to more widespread use was also discussed as important. There is also a recognized need to bridge the gap between assessment of air filtration efficacy in a clinical trial to long-term measurements of intervention effectiveness when used in a community setting.
FIGURE 3. Potential Design Aspects of Clinical Trials.
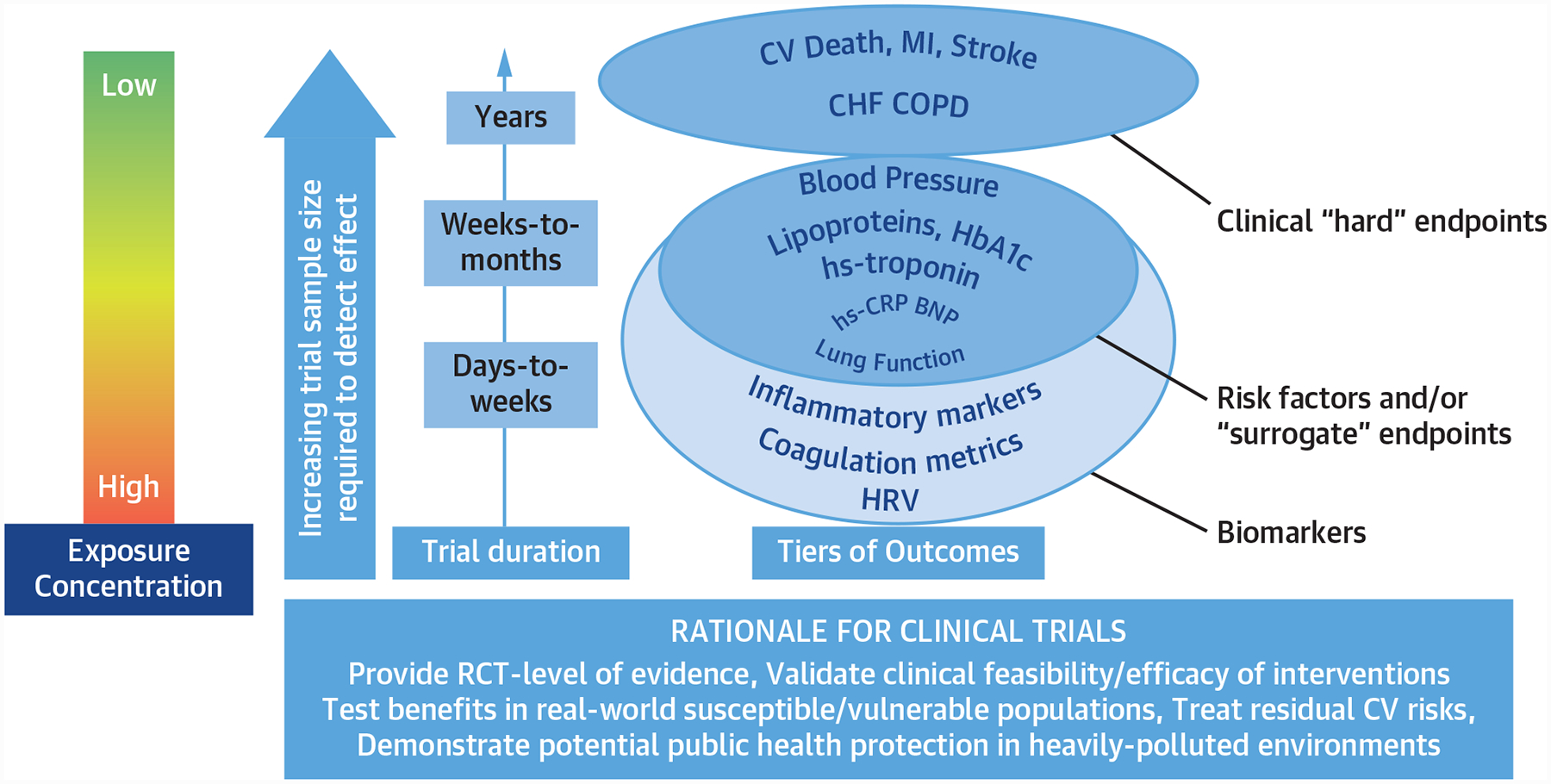
Rationale for clinical trials of the cardiovascular effects of reductions in fine particulate air pollution. The purpose of randomized trials is to provide RCT-level evidence and validate feasibility and efficacy of interventions, and to test benefits in real-world susceptible/vulnerable populations. CHF = congestive heart failure; COPD = chronic obstructive pulmonary disease; CV = cardiovascular; HbA1c = hemoglobin A1c; HRV = heart rate variability; hs = high-sensitivity; MI = myocardial infarction; RCT = randomized controlled trial.
AIR POLLUTION EXPOSURE.
To design an appropriately powered clinical trial, there is a definite need to determine the expected magnitude of relative and absolute reductions in PM2.5 exposure projected with any intervention. Estimates from recent studies suggest relative reductions of 30% to 60% can be achieved by PAC usage, whereas reduction in inhaled pollutants is less certain and more variable through face mask use (e.g., N95 respirators vs. surgical or cloth/improvised face masks) (32). Nevertheless, reductions of the magnitude observed with PACs have been associated with improvement in both short- and long-term health outcomes (32). The absolute reduction in PM2.5 exposure will thereby be highly dependent upon baseline ambient indoor and outdoor PM2.5 concentrations. For example, populations in Asia that often face daily levels above 50 to 100 μg/m3 could experience much larger decreases in absolute exposures in response to the same intervention (e.g., a PAC that yields a 50% reduction) compared with those living in regions such as the United States and Canada, with average daily PM2.5 levels around 5 to 35 μg/m3 (1,2). This demonstrates that a larger sample size would be required for a trial in regions with lower baseline levels of exposure (such as the United States) compared with regions with higher levels (e.g., China, India). However, given the risk for cardiovascular events and mortality from PM2.5 that continues at levels below current annual average concentrations typical for North America (i.e., 8 to 12 μg/m3), there is reason to expect that an intervention that even further decreases exposures could provide significant reductions in clinically meaningful cardiovascular outcomes (2,21).
An important consideration for future intervention trials to reduce air pollution exposure is the need for monitoring of individual-level exposures and reduction of exposures with interventions. Performing a trial with no exposure monitoring might be analogous to conducting a trial of antihypertensive therapy without measuring BP. Failure to derive a health benefit may be due to an inadequacy of the specific intervention to meaningfully reduce exposures and not a failure of exposure reduction per se to yield health benefits. Therefore, some effort to ensure the success of the intervention, at the very least in a representative subgroup, is greatly important. The need for individual-level exposure monitoring and reduction is juxtaposed against the increases in participant burden and complexity with these measures in a large-scale trial. The use of mobile global positioning systems and other low-cost sensors, crowdsourcing, and other novel exposure assessment methods warrant exploration for use in future clinical trials. In addition, the contribution of gaseous copollutants, such as ozone and nitrogen oxides (3–6), may be a target for future trials. Finally, the risks posed by indoor versus outdoor exposures and potential heterogeneity of effect on clinical outcomes remains unclear. Participants that travel or move from their initial study location also pose a challenge for monitoring and filtration in the context of a clinical trial.
PERSONAL INTERVENTIONS.
There are advantages and disadvantages of any intervention, such as indoor PACs compared with face masks. First, there is well-described variation in the technologies and usage of both PACs and face mask types (32), highlighting the importance of selecting a practical yet effective intervention for use in a clinical trial. Given their efficacy, the evidence thus far from small trials, and the fact that they do not create ozone (unlike some ionizing air cleaners), workshop members believed that indoor PACs using HEPA filtration are the most favorable existing technology to adopt for clinical trials. Although N95 respirators reduce PM2.5 inhalation by 95%, they are uncomfortable, require a tight facial seal to be fully effective, and are in general not worn during sleep, which can be a meaningful exposure period, for example, to residential wood-smoke in some regions (55). Their practicality, compliance rates, and effectiveness in real-world settings remain to be validated, particularly over longer periods of time. Procedure masks are less expensive and easier to wear; however, as stated previously, they offer variable facial seal and are much less effective and variable in their efficacy (32). The aggressiveness of intervention required and likelihood of acceptance by the population varies by the study location. Conversely, indoor PACs are likely the most viable approach for the United States due to their ability to reduce PM2.5 exposures even at the low end of ambient concentrations coupled with their nonobtrusive nature and the characteristics (e.g., more air-tight) of many (but not all) households nationwide that support their viability. Their usefulness in locations such as China or India is less certain due to very high PM2.5 levels (e.g., unclear effectiveness over protracted periods). In order to fully reduce exposures in heavily-polluted locations to levels below or even near air quality guidelines, combination interventions (e.g., indoor PACs plus N95 respirators worn outdoors) may be required. However, this would complicate any trial expense and design and may not be essential for success. As stated previously, most recent estimations support that there should still be health benefits by reducing exposures 30% to 50% even if post-intervention levels remain above current air quality guideline thresholds.
Second, the setting and scope in which a clinical trial intervention will be evaluated needs to be clearly defined (28,47,56). For example, if testing a PAC intervention, considerations include the area of use (e.g., room), hours of usage, seasons of use, and a schedule for filter change and use of high/low settings. Additionally, window opening and limitation of sources of exposures (e.g., traffic) may be evaluated in a subset of trial participants. This may also allow for targeting vulnerable populations at risk for the adverse health effects of PM2.5 exposure in a home environment (e.g., older adults or very young). Third, the duration of the intervention, adherence, and estimations of drop-out or reductions in adherence during the trial are critical design considerations, and may differ substantially across chosen personal interventions (32). Fourth, for any clinical trial, careful preparation and blinding for sham versus active filtration may be desirable for studies of both face mask and air cleaner interventions. Related to blinding will be maintenance protocols for air filtration and/or replacement of face masks over the duration of the trial. Although sham air cleaners may be relatively easy to develop, an indistinguishable (yet ineffective) sham (placebo) face mask is much more difficult to design.
DETERMINANTS OF ADHERENCE.
General estimates of the adherence and persistence for any intervention strategy are important. For example, to define air filtration effectiveness, adherence with air cleaner usage to reduce PM2.5 exposures is important for any future clinical trial and will affect the sustainability of the intervention to improve cardiovascular outcomes. Several factors in the study population, such as participant age, socioeconomic status, and CVD prevalence, could influence adherence. The effects of enrolling vulnerable populations (e.g., urban, under-represented minority populations) on adherence is largely unknown and may play an important role. Another important dimension to adherence will be the balance of patient burden and trial engagement. For example, use of a PAC may be less of a burden for some—but not all—participants than wearing a respirator or other face mask. Because adherence to an air filter intervention may require changes in multiple dimensions of participant behavior, such as use or nonuse of air conditioning, window integrity, use of incense, second-hand cigarette exposure, and electronic cigarette use, maintaining patient engagement while minimizing burden will be relevant for future intervention studies. Engagement may increase if patients and their families view the intervention as potentially beneficial to other family members, including children with asthma or elderly household members with comorbid respiratory illnesses.
POTENTIAL TRIAL DESIGNS
The discussion of potential clinical trial designs to evaluate the effectiveness of personal-level interventions to reduce exposure to PM2.5 and improve subclinical and clinical cardiovascular outcomes was a primary focus of the workshop (Figure 3, Central Illustration). There were 6 domains discussed to inform the design of future intervention studies (Table 2).
CENTRAL ILLUSTRATION. Burden, Strategies, and Needs to Address the Cardiovascular Effects of Exposure to Fine Particulate Air Pollution.
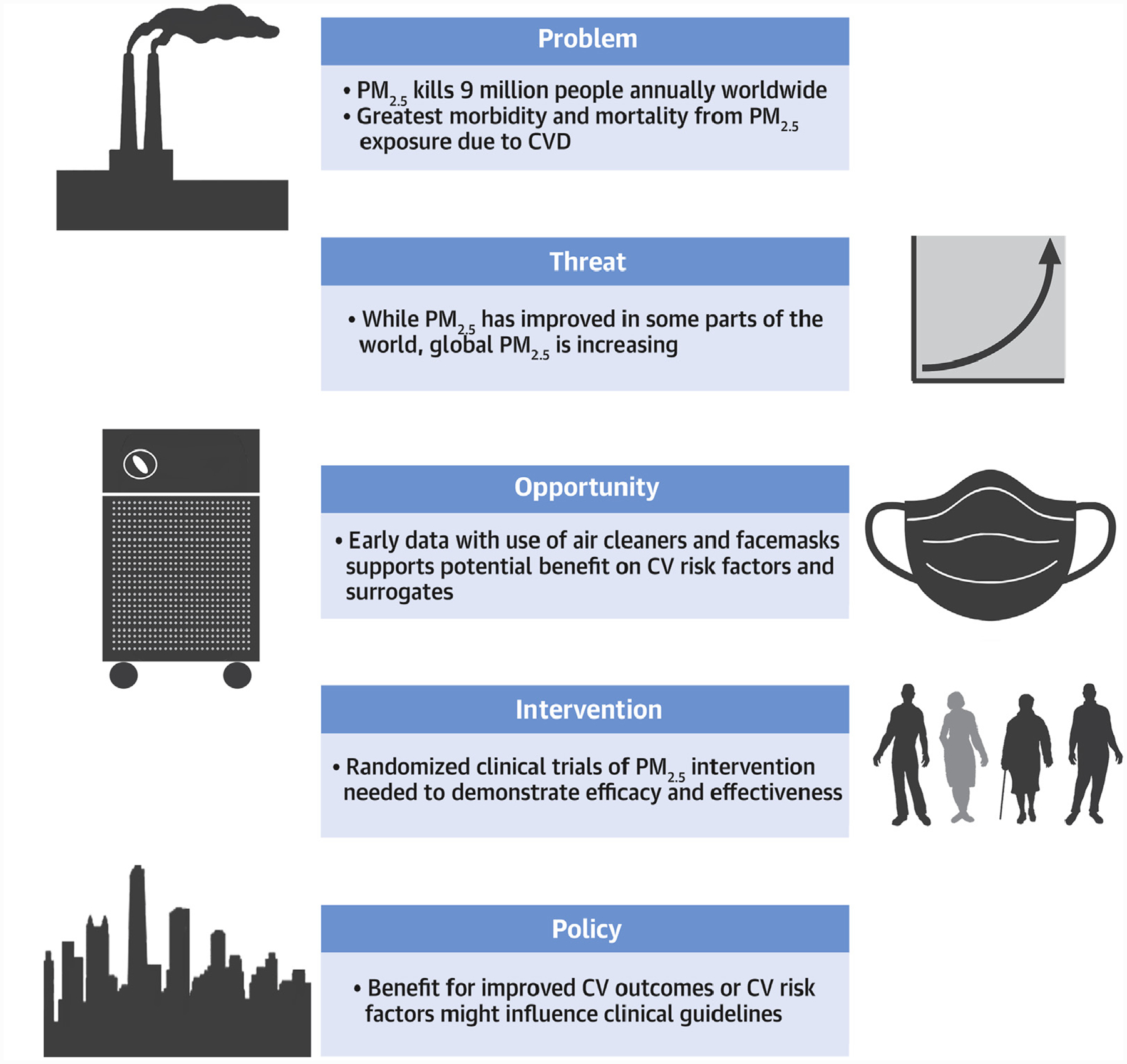
The scope of the problem from fine particulate air pollution exposure, the threat of problem, the opportunity to address this problem with early data supporting reductions in CV events with air cleaners and face masks, along with broad needs for future randomized clinical trials and policy interventions. CVD = cardiovascular disease.
TABLE 2.
Design Considerations for a Future Intervention Trial to Reduce the Cardiovascular Effects of PM2.5
| Trial Characteristic | Design Considerations |
|---|---|
| Population |
|
| Sample size |
|
| Exposure levels |
|
| Duration |
|
| Outcomes |
|
| Other design issues |
|
COPD = chronic obstructive pulmonary disease; CVD = cardiovascular disease; eGFR = estimated glomerular filtration rate; HbA1c% = HemoglobinA1c; hs-CRP = high-sensitivity c-reactive protein; hs-troponin = high-sensitivity troponin; LDL-C = low-density lipoprotein-cholesterol; PAD = peripheral artery disease; PM2.5 = fine particulate matter.
POPULATION(S)
Discussants focused on the importance of enrolling a population with increased susceptibility and vulnerability to the cardiovascular effects of PM2.5. This improves the feasibility (e.g., sample size, power) of a trial and its external relevance. Overall, it was felt that the most relevant population to consider is patients with ischemic CVD (e.g., prior MI or stroke) for whom a trial of a PAC intervention could rapidly lead to improvements in cardiometabolic risk. There was discussion that PM2.5 is also associated with heart failure. It might be possible to enroll a subset of heart failure patients with reduced as well as preserved ejection fraction. The latter population has few proven effective interventions and thus merits special interest. Given the importance of air pollutants for pulmonary health, a trial involving patients with COPD could also be considered (7). The potential to enroll a large population of patients with or at risk for both cardiovascular and pulmonary diseases was also discussed; however, this trial design has rarely been conducted. Finally, future trials should strive to balance the efficacy of the intervention with the potential for its equitable scalability and public health benefit outside the context of a controlled clinical trial. This means that minority populations (e.g., African Americans) and individuals living in at-risk communities (e.g., urban settings) must be adequately represented in any trial. These populations are established to be both more susceptible and vulnerable to air pollution exposures (54).
TRIAL SAMPLE SIZE.
Recent pharmacological cardiovascular outcome trials (57–59) randomized participants at elevated baseline risk of CVD (aggregate trial event rate 7% to 22%), with a median follow-up of approximately 3.5 years and study sizes ranging from over 7,000 to nearly 28,000 participants (57–60). The effect size of potential interventions (e.g., PACs) on reducing cardiovascular events is not well quantified at present. In addition to knowing the absolute event rate in the population, the expected relative risk reduction afforded by the intervention is required for sample size calculations. PACs and face masks can reduce PM2.5 exposures by roughly 50% (32). One way to estimate the effect size would be to presume that CVD events will be reduced commensurate with the known epidemiological exposure-risk curve per absolute decrease in PM2,5 exposures (2). In this scenario, knowing baseline PM2.5 concentrations would also be important. Assuming that a 1-μg/m3 decrease in PM2.5 will result in a 1% decrease in CVD mortality (as per the population-wide risk curve), then an absolute decrease of 5 to 10 μg/m3 (estimating a mean daily range of 5 to 35 μg/m3 in the United States) will translate into a 5% to 10% decrease in CVD events in the general population. The sample size required to detect this small of an effect size would likely be prohibitively large. Conversely, other studies have shown much larger health risks and suggest that this is an overly conservative estimate (61), particularly if the endpoints are extended beyond mortality. The risks for nonfatal events (e.g., a composite CVD endpoint commonly used in modern clinical trials) may occur in relation to PM2.5 exposures at much greater rates than mortality alone (3,12,24). Moreover, higher-risk patients, particularly those with established CVD, are at greater risk of adverse health outcomes from air pollution. For example, a recent study in Ontario showed that a 10-μg/m3 increase in PM2,5 was associated with a 64% increased risk of future fatal MI among MI survivors (24). In this scenario, enrolling a high-risk population and focusing the primary outcome on a composite endpoint of fatal and nonfatal events (e.g., cardiovascular mortality, MI, stroke, heart failure) could yield a much more realistic effect size of a 20% to 30% relative risk reduction by lowering PM2.5 exposure by 5 to 10 μg/m3. Such a trial would be feasible in a contemporary population of well-treated patients at high residual risk. Evaluation of the feasibility of a definitive outcome trial would require further study of sustained adherence to the intervention, along with the feasibility of recruiting a large high-risk population. Given this gap in knowledge, workshop attendees did not conclude either way if an outcome trial is currently realistic to consider or undertake. In contrast, there was more uniform enthusiasm for the opportunities provided by launching smaller (n ≈ 100 to 1,000 participants) intervention trials (that are nonetheless larger than prior studies) with the primary endpoint being pathologically relevant cardiovascular biomarkers and/or risk factors. Multicenter studies focusing on surrogate endpoints of proven prognostic relevance (e.g., BP) alone or as part of a vanguard phase trial could significantly inform the feasibility and design (i.e., size, outcomes) of future clinical outcome trials. Sample sizes for trials enrolling other patients, such as those with heart failure or COPD, and focusing on disease-related endpoints were not specifically discussed.
POLLUTION EXPOSURE LEVELS.
For clinical trial results to have the greatest impact on clinical care in the United States, it is desirable for a future PAC trial to be conducted at levels of PM2.5 exposures relevant to the current U.S. population, as opposed to an area with markedly elevated PM2.5 exposures (1,2). There was discussion that if a PAC improved relevant intermediate cardiovascular outcomes at the relatively low levels (from a global perspective) in the United States, such an intervention may also (but not assuredly) be effective in areas facing far higher PM2.5 levels (e.g., China, India). Exposure-response relationships between PM2.5 levels and cardiovascular events, including mortality, support the assertion that reductions in PM2.5 exposures, even from contemporary low U.S. levels, should translate into reductions in CV events (21,62). There was discussion regarding the likely greater impact on clinical recommendations of demonstrating effectiveness of interventions in a U.S. population compared with an intervention in a highly polluted country. Although the global population health importance of air pollution in Asia and other heavily polluted regions was acknowledged, it was felt that given logistical and other difficulties and the residual morbidity and mortality in the United States due to PM2.5 even at present-day levels, the research need to focus initial trials in the United States (or North America and Western Europe) was great. Concomitant trials in areas with higher exposures also present important research opportunities. It is possible that a PAC could yield a much larger absolute decrease in exposure (e.g., 25 to 50 μg/m3) if the intervention is proven effective in regions with poor air quality experiencing PM2,5 >50 to 100 μg/m3 on a daily basis. Several small studies in China have indeed found this magnitude of exposure reduction is possible with PACs and, if proven true on a larger-scale, would markedly decrease the study sample size needed in an outcome trial (32). Ultimately, conducting clinical trials in both pollution settings would be optimal to help combat the global public health threat.
TRIAL DURATION.
It was discussed that a cardiovascular outcomes trial, even if sufficiently large, typically requires 3 to 5 years of follow-up for the number of requisite events to occur. In contrast, a smaller-sized trial focused on the effects of personal PM2.5 filtration on clinically relevant cardiovascular, metabolic, and/or pulmonary biomarkers and cardiovascular risk factors, such as BP, could be performed in a much shorter timeline (28). The effect of study duration on adherence with PACs or face masks needs to be estimated for future trials. Although several small and short-term studies with intermediate biomarkers have already been performed (Supplemental Tables 1 and 2), they have all been very small and brief (days to weeks). There remain many unclear issues, as previously reviewed. Prior to launching full-scale outcome trials, multicenter studies of intermediate duration on the order of weeks to months could provide useful information, including the persistence of exposure reduction and biomarker benefits as well as anticipated adherences and pitfalls over a longer period of intervention.
POTENTIAL OUTCOMES AND OTHER DESIGN ISSUES.
Before designing and launching a full-scale endpoint trial, cardiometabolic biomarkers could serve as surrogate endpoints in a trial. Relevant biomarkers are probably associated with PM2.5 exposures and also linked to an adverse cardiovascular prognosis. Potential biomarkers include those for systemic inflammation (e.g., high-sensitivity [hs] c-reactive protein), myocardial damage (e.g., hs-troponin), heart failure (e.g., brain natriuretic peptide), and insulin resistance (e.g., percent glycated hemoglobin). Other biomarker endpoints could also be considered. Some biomarkers have been independently associated with cardiovascular outcomes and CVD pathogenesis, and may be implicated in relevant causal pathways for the health effects of PM2.5 exposures.
In addition to CVD outcomes, the workshop discussed the potential and merits of separate trials of PACs or HEPA home filtration in COPD patients focusing on pulmonary endpoints, changes in forced expiratory volume in 1 s, or COPD exacerbations. However, it was felt that because the largest global public health burden from PM2.5-induced mortality is due to CVD, and that even among people with COPD the most common cause of death is CVD-related, the first priority of an intervention trial could focus on a CVD-enriched population and target a CVD-related endpoint. However, this does not obviate the potential benefits of a trial focusing on COPD patients in general, particularly at a later time.
PM2.5 has been linked to elevations in BP and an increased incidence of hypertension (3–5). In a Detroit study, PACs lowered systolic BP by 3.2 mm Hg over a few days among elderly adults living in a low-income senior facility (28). A recent meta-analysis of 10 randomized blinded controlled trials (n = 604) demonstrated that PAC use lowers systolic BP by an average of 3.94 mm Hg (95% confidence interval: −7.00 to −0.89; p = 0.01) over a median of 13.5 days (63). High BP is a potent, widespread, and modifiable CVD risk factor, and is well-validated as a “surrogate endpoint” (64,65) because a reduction in BP nearly always leads to a proportionate reduction in CVD events. During the workshop discussion, trials in appropriate populations that focused on BP as a primary outcome were discussed. In addition, other clinical risk factor targets for PACs discussed included lipoprotein levels, blood glucose and glycemic control, and parameters of renal function. Each factor plays an independent role in CVD pathogenesis and may partially mediate the adverse cardiometabolic effects of PM2.5 exposures (3–5,32).
A significant portion of the workshop was devoted to discussing potential clinical endpoints for a future CV outcome trial, with the choice of endpoints dependent on the enrolled population. Demonstrating a reduction in “hard” clinical outcomes by an intervention would have the largest impact and provide the most compelling evidence to engender meaningful changes in the clinical care of at-risk patients. In this era of evidence-based medicine and the reliance on outcome trials to formulate clinical guidelines, we believe such trials have the greatest potential to influence health care practices moving forward. Observational studies and improvements in surrogate endpoints can still have an impact, albeit with less compelling classes of recommendation and levels of evidence in clinical guidelines. Therefore, a long-term goal would be to demonstrate that 1 intervention (or more) to reduce PM2.5 exposures actually translates into improved clinical outcomes in germane populations. As discussed earlier and like most contemporary trials, a composite primary endpoint would be most relevant and feasible. There was some debate in this regard during the workshop. However, the greatest amount of evidence links PM2.5 with ischemic cardiovascular events including MIs, strokes, and cardiovascular death. Therefore, a defensible endpoint would be a composite involving these outcomes. Whether or not to include additional “soft” events (e.g., revascularization) requires further considerations. Given the high event rate, one potential design would be to enroll patients at high risk for cardiovascular events (e.g., patients with recent acute coronary syndrome or MI). To increase the event rates, the population could be enriched for other high-risk conditions (e.g., DM, chronic kidney disease, or heart failure). In this case, a composite of ischemia-related fatal and nonfatal events, including MI, stroke, sudden cardiac death, heart failure, and urgent revascularization for refractory angina, could be relevant. Composite endpoints are important outcomes for recurrent events in at-risk populations and are more common than major adverse cardiovascular events such as death or MI alone. Another option discussed was to further supplement enrollment with patients also with heart failure—particularly patients with heart failure and a preserved ejection fraction (HFpEF) (66). The advantage of studying patients with HFpEF is that there are few evidence-based treatments that show outcome benefits, and PM2.5 has been linked to exacerbations of heart failure suggesting a potential benefit to testing PACs in this population. Heart failure with reduced ejection fraction frequently occurs in ischemic heart disease patients and could be included as part of an expanded endpoint. However, concerns were also expressed regarding the heterogeneity of the HFpEF population, including uncontrolled risk factors such as hypertension and DM, disadvantaged socioeconomic conditions, and potential difficulties with adherence in this subpopulation of patients. There may be another disadvantage of competition between endpoint types in a time-to-first event clinical trial. For example, if HFpEF patients represent a large subgroup, heart failure events may overwhelm ischemic events in this population. This could lessen the robustness of observing significant reductions in any specific subtype of clinical event (commonly declared as secondary endpoints) in the whole study cohort. Finally, workshop members also discussed the possibility of including “hard” pulmonary endpoints (e.g., COPD hospitalization or death). This would require enrolling patients with or at risk for both CVD and COPD (or 2 subsets of patients each with or at risk for one or the other condition). This design is intriguing, because lowering PM2.5 exposures is one of the few interventions that has the clear potential to improve both cardiac and pulmonary health. The breadth of the population impacted by the trial results would therefore be enhanced. On the negative side, competition between subtypes of events would occur. We are also aware of only 1 previous trial that undertook this type of design to include patients at risk for both cardiovascular and pulmonary endpoints (67). Lack of precedent may make this design more at risk for unexpected pitfalls.
There was discussion on relevant trial design features, including utilizing an adaptive design feature to test and update the study population along with clinical and subclinical targets of the intervention (68). Pragmatic designs were also discussed favorably, in which real-world effectiveness of PACs could be more accurately evaluated. Future studies including evaluation of adherence and effectiveness with proposed PAC interventions were viewed favorably.
NEXT STEPS
There was some discussion on the appropriate course of action in the context of reviewing the present state of the evidence and opinions voiced during the workshop. Because PM2.5 air pollution remains a serious public health problem in the United States as well as globally, novel strategies, such as personal-level interventions and coordinated effects, involving governmental agencies and the private sector are desirable to help reduce the burden of air pollution–related diseases. No definitive conclusion was reached on the single best first approach and whether a full-scale clinical trial is the next important research opportunity. However, there was indeed general agreement that many unanswered issues should be clarified to optimally design and launch a full-scale clinical outcome trial. Workshop members did see smaller-scale studies, albeit multicenter and larger than prior studies, as presenting important near-term research opportunities. Such trials could focus on changes in validated surrogate health endpoints over weeks to months of intervention and thereby provide clinically important information and help address key points required to design and validate the feasibility of full-scale clinical outcome trials. Positive studies would further bolster support for the merits of performing a large-scale trial. As to the intervention type, there was general agreement that in the United States (as well as North America and Western Europe) the most viable overall approach would be to test PACs. Finally, additional workshops in the future could help assure that this research moves forward in a coordinated fashion and remains well informed by experts across the multiple relevant scientific fields (Central Illustration).
IMPACT OF CORONAVIRUS DISEASE-2019
In early 2020, the pandemic due to the severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) has fundamentally altered nearly all aspects of human society. Medical care and clinical trials have faced numerous unprecedented obstacles to ensure patient health and safety. Members of the workshop organizing committee felt it was important to discuss interactions between SARS-CoV-2 and fine particulate air pollution, as well as the potential impact upon the design of clinical trials discussed in this workshop. This section was added in the spring of 2020 and reviewed by all members of the workshop.
First, a national study has suggested that chronic PM2.5 exposures predispose to increased SARS-CoV-2 mortality (69). It is plausible that interventions that lower pollution exposures might reduce the pulmonary manifestations of coronavirus disease-2019. Second, mask use is more ubiquitous across the United States than ever before. This presents a difficulty in studying the cardiopulmonary benefits of N95 respirators and PACs. Although it has not been quantified, it is likely there is a high degree of variability in the effects of mask usage, compliance, and efficacy on personal-level PM2.5 exposures (e.g., facial fit, N95 respirator vs. surgical mask). This would make it difficult to accurately estimate patients’ true particulate exposures. Widespread mask use might also compound difficulties in the detection of health benefits associated with PAC use. However, unanticipated opportunities may also be present. If mask usage is needed long-term to protect from coronavirus disease- 2019 in the United States, it is possible to envision studying the efficacy of various mask types (N95 respirator vs. surgical mask) alone or on top of PACs to prevent the adverse cardiopulmonary effects of exposure to SARS-CoV-2 as well as PM2.5. This is a rapidly evolving medical and public health crisis that will require adaptability of trial designs over time.
CONCLUSIONS
PM2.5 air pollution is a leading risk factor for global morbidity and mortality, with cardiovascular events being the single largest contributor. Although air quality has generally improved across the United States over the past few decades, PM2.5 still poses significant threats to public health, particularly among susceptible populations such as patients with cardiovascular and pulmonary diseases. Moreover, many countries (e.g., China, India) continue to face extremely poor air quality with very high levels of PM2.5 likely to persist into the foreseeable future. There is a need to further reduce air pollution in countries with both high and low current ambient exposure levels. Strategies that focus on preventing and reducing exposures at the personal level, among at-risk individuals, deserve further research, including trials involving surrogate and hard clinical outcomes, to more precisely determine if such strategies can prevent adverse health consequences.
Supplementary Material
HIGHLIGHTS.
Particulate air pollution is a threat to global public health, particularly for cardiopulmonary diseases.
Personal-level approaches that reduce air pollution exposure can lead to improved health endpoints.
Trials of personal strategies to reduce air pollution exposure and improve health outcomes are warranted.
ACKNOWLEDGMENT
The authors thank Sharine Wittkopp, MD, PhD, for her assistance with the drafting of Figure 3.
AUTHOR DISCLOSURES
The views expressed in this paper are those of the authors and do not necessarily represent the views of the National Institutes of Health (NIH), National Heart, Lung, and Blood Institute (NHLBI), National Institute of Environmental Health Sciences, Environmental Protection Agency, Centers for Disease Control and Prevention, or the United States Department of Health and Human Services. Drs. Bhatt and Brooks served as co-chairs of this NIH/NHLBI/EPA/CDC Expert Workshop on air pollution. Dr. Newman has received grant support from the NIH (K23HL125991). Dr. Bhatt has served on the advisory board of Cardax, CellProthera, Cereno Scientific, Elsevier Practice Update Cardiology, Level Ex, Medscape Cardiology, PhaseBio, PLx Pharma, and Regado Biosciences; has served on the Board of Directors for Boston VA Research Institute, Society of Cardiovascular Patient Care, and TobeSoft; has served as chair of the American Heart Association Quality Oversight Committee, NCDR-ACTION Registry Steering Committee, and VA CART Research and Publications Committee; has served on the data monitoring committees of Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Cleveland Clinic (including for the ExCEED trial, funded by Edwards), Contego Medical (Chair, PERFORMANCE 2), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi-Sankyo), and the Population Health Research Institute; has received honoraria from the American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Vice-Chair, ACC Accreditation Committee), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE-DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim; AEGIS-II executive committee funded by CSL Behring), Belvoir Publications (Editor-in-Chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial, funded by Ferring Pharmaceuticals), HMP Global (Editor-in-Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), K2P (Co-Chair, interdisciplinary curriculum), Level Ex, Medtelligence/ReachMD (CME steering committees), MJH Life Sciences, Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and USA national co-leader, funded by Bayer), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), and WebMD (CME steering committees); has served as Deputy Editor of Clinical Cardiology; has received research funding from Abbott, Afimmune, Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Cardax, Chiesi, CSL Behring, Eisai, Ethicon, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, Idorsia, Ironwood, Ischemix, Lexicon, Lilly, Medtronic, Pfizer, PhaseBio, PLx Pharma, Regeneron, Roche, Sanofi, Synaptic, and The Medicines Company; has received royalties from Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald’s Heart Disease); has served as site co-investigator for Biotronik, Boston Scientific, CSI, St. Jude Medical (now Abbott), and Svelte; served as a trustee of the American College of Cardiology; and has received unfunded research from FlowCo, Merck, Novo Nordisk, and Takeda. Dr. Balmes is the Physician Member of the California Air Resources Board. Dr. Brook has received an investigator-initiated grant from RB, Inc.; and has received support from NIH awards 2R01-ES019616 and 2R01-NR014484. Dr. Brauer has received grant support from the Health Effects Institute, the Canadian Institutes of Health Research, The Terry Fox Research Institute, and the Wellcome Trust. Dr. Hansel has received grant support from the NIH, EPA, NHLBI, National Institute of Environmental Health Sciences, COPD Foundation, and Boehringer Ingelheim; and has participated in advisory boards for GlaxoSmithKline, AstraZeneca, and Mylan. Dr. Hernandez has received research funding from American Regent, AstraZeneca, Boehringer Ingelheim, Merck, Novartis, and Verily; and has served as a consultant for Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Boston Scientific, Merck, Novartis, and Relypsa. Dr. Hochman has received grant support from the NHLBI for the ISCHEMIA Trial, for which there were in-kind donations for participating sites from Abbott Vascular, Medtronic, Inc., St. Jude Medical, Inc., Volcano Corporation, Arbor Pharmaceuticals, AstraZeneca Pharmaceuticals, Merck Sharp & Dohme Corp., Omron Healthcare, Inc., and Amgen Inc; has received financial donations from Arbor Pharmaceuticals and AstraZeneca Pharmaceuticals; and has served as PI for the ZEDS (Long-term Suppressive Valacyclovir Treatment for Herpes Zoster Ophthalmicus) Study, supported by NIH/National Eye Institute. Dr. Kaufman has received grant support from the National Institutes of Health and the Environmental Protection Agency. Dr. Malik is supported by the NHLBI of the NIH under Award Number T32HL110837. Dr. Peel has received research funding from the NIH (1UM1HL134590 and R01ES023688). Dr. Rajagopalan has received research funding from the NIH; and has served as a consultant to Novo Nordisk, Takeda, GlaxoSmithKline, and AstraZeneca. Dr. Zhang’s research has been partly funded by the NIH, Underwriter’s Laboratory, and Natural Science Foundation of China (via Duke Kunshan University in China). All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
ABBREVIATIONS AND ACRONYMS
- BP
blood pressure
- COPD
chronic obstructive pulmonary disease
- CVD
cardiovascular disease
- DM
diabetes mellitus
- MI
myocardial infarction
- PAC
portable air cleaner
- PM
particulate matter
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
APPENDIX For supplemental tables, please see the online version of this paper.
REFERENCES
- 1.Cohen AJ, Brauer M, Burnett R, et al. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the Global Burden of Diseases Study 2015. Lancet 2017;389:1907–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burnett R, Chen H, Szyszkowicz M, et al. Global estimates of mortality associated with long-term exposure to outdoor fine particulate matter. Proc Natl Acad Sci U S A 2018;115:9592–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rajagopalan S, Al-Kindi SG, Brook RD. Air pollution and cardiovascular disease: JACC State-of-the-Art Review. J Am Coll Cardiol 2018;72: 2054–70. [DOI] [PubMed] [Google Scholar]
- 4.Brook RD, Newby DE, Rajagopalan S. Air pollution and cardiometabolic disease: an update and call for clinical trials. Am J Hypertens 2017;31: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brook RD, Rajagopalan S, Pope CA, et al. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation 2010; 121:2331–78. [DOI] [PubMed] [Google Scholar]
- 6.Newby DE, Mannucci PM, Tell GS, et al. Expert position paper on air pollution and cardiovascular disease. Eur Heart J 2014;36:83–93b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schraufnagel DE, Balmes JR, Cowl CT, et al. Air pollution and noncommunicable diseases: a review by the Forum of International Respiratory Societies’ Environmental Committee, Part 2: Air Pollution and Organ Systems. Chest 2019;155: 417–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shah AS, Langrish JP, Nair H, et al. Global association of air pollution and heart failure: a systematic review and meta-analysis. Lancet 2013; 382:1039–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mustafić H, Jabre P, Caussin C, et al. Main air pollutants and myocardial infarction. JAMA 2012; 307:713–21. [DOI] [PubMed] [Google Scholar]
- 10.Wing JJ, Sánchez BN, Adar SD, et al. Synergism of short-term air pollution exposures and neighborhood disadvantage on initial stroke severity. Stroke 2017;48:3126–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cesaroni G, Forastiere F, Stafoggia M, et al. Long term exposure to ambient air pollution and incidence of acute coronary events: prospective cohort study and meta-analysis in 11 European cohorts from the ESCAPE Project. BMJ 2014;348: f7412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malik AO, Jones PG, Chan PS, Peri-Okonny PA, Hejjaji V, Spertus JA. Association of long-term exposure to particulate matter and ozone with health status and mortality in patients after myocardial infarction. Circ Cardiovasc Qual Outcomes 2019;12:e005598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thurston GD, Kipen H, Annesi-Maesano I, et al. A joint ERS/ATS policy statement: what constitutes an adverse health effect of air pollution? An analytical framework. Eur Respir J 2017;49: 1600419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.U.S. EPA. Integrated science assessment (ISA) for particulate matter (final report, 2019). Available at: https://cfpub.epa.gov/ncea/isa/recordisplay.cfm?deid=347534. Accessed April 14, 2020.
- 15.Di Q, Wang Y, Zanobetti A, et al. Air pollution and mortality in the Medicare population. N Engl J Med 2017;376:2513–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Di Q, Dai L, Wang Y, et al. Association of short-term exposure to air pollution with mortality in older adults. JAMA 2017;318:2446–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crouse DL, Peters PA, van Donkelaar A, et al. Risk of nonaccidental and cardiovascular mortality in relation to long-term exposure to low concentrations of fine particulate matter: a Canadian national-level cohort study. Environ Health Perspect 2012;120:708–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pinault L, Tjepkema M, Crouse DL, et al. Risk estimates of mortality attributed to low concentrations of ambient fine particulate matter in the Canadian community health survey cohort. Environ Health 2016;15:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weichenthal S, Villeneuve PJ, Burnett RT, et al. Long-term exposure to fine particulate matter: association with nonaccidental and cardiovascular mortality in the Agricultural Health Study Cohort. Environ Health Perspect 2014;122: 609–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thurston GD, Ahn J, Cromar KR, et al. Ambient particulate matter air pollution exposure and mortality in the NIH-AARP Diet and Health Cohort. Environ Health Perspect 2016;124:484–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu C, Chen R, Sera F, et al. Ambient particulate air pollution and daily mortality in 652 cities. N Engl J Med 2019;381:705–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yin P, Brauer M, Cohen A, et al. Long-term fine particulate matter exposure and nonaccidental and cause-specific mortality in a large national cohort of Chinese men. Environ Health Perspect 2017;125:117002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li T, Zhang Y, Wang J, et al. All-cause mortality risk associated with long-term exposure to ambient PM2$5 in China: a cohort study. Lancet Public Health 2018;3:e470–7. [DOI] [PubMed] [Google Scholar]
- 24.Chen H, Burnett RT, Copes R, et al. Ambient fine particulate matter and mortality among survivors of myocardial infarction: population-based cohort study. Environ Health Perspect 2016;124: 1421–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tonne C, Wilkinson P. Long-term exposure to air pollution is associated with survival following acute coronary syndrome. Eur Heart J 2013;34: 1306–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tibuakuu M, Michos ED, Navas-Acien A, Jones MR. Air pollution and cardiovascular disease: a focus on vulnerable populations worldwide. Curr Epidemiol Rep 2018;5:370–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maestas MM, Brook RD, Ziemba RA, et al. Reduction of personal PM2.5 exposure via indoor air filtration systems in Detroit: an intervention study. J Expo Sci Environ Epidemiol 2018;29: 484–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morishita M, Adar SD, D’Souza J, et al. Effect of portable air filtration systems on personal exposure to fine particulate matter and blood pressure among residents in a low-income senior facility. JAMA Intern Med 2018;178:1350–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boldo E, Linares C, Lumbreras J, et al. Health impact assessment of a reduction in ambient PM2. 5 levels in Spain. Environ Int 2011;37:342–8. [DOI] [PubMed] [Google Scholar]
- 30.Brusselen DV, de Oñate WA, Maiheu B, et al. Health impact assessment of a predicted air quality change by moving traffic from an urban ring road into a tunnel. The case of Antwerp, Belgium. PLOS ONE 2016;11:e0154052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frazer K, Callinan JE, McHugh J, et al. Legislative smoking bans for reducing harms from secondhand smoke exposure, smoking prevalence and tobacco consumption. Cochrane Database Syst Rev 2016;2:CD005992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bard RL, Ijaz MK, Zhang J, et al. Interventions to reduce personal exposures to air pollution a primer for health care providers. Global Heart 2019;14:47–60. [DOI] [PubMed] [Google Scholar]
- 33.Brook RD, Newby DE, Rajagopalan S. The global threat of outdoor ambient air pollution to cardiovascular health: time for intervention. JAMA Cardiology 2017;2:353–62. [DOI] [PubMed] [Google Scholar]
- 34.Gregg EW, Hora I, Benoit SR. Resurgence in diabetes-related complications. JAMA 2019;321: 1867–8. [DOI] [PubMed] [Google Scholar]
- 35.Mensah GA, Wei GS, Sorlie PD, et al. Decline in cardiovascular mortality. Circ Res 2017;120: 366–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.National Heart, Lung, and Blood Institute. Reducing the cardiopulmonary impact of particulate matter air pollution in high-risk populations. Available at: https://www.nhlbi.nih.gov/events/2019/reducing-cardiopulmonary-impact-particulate-matter-air-pollution-high-risk-populations. Accessed April 9, 2020.
- 37.Fisk WJ, Chan WR. Effectiveness and cost of reducing particle-related mortality with particle filtration. Indoor Air 2017;27:909–20. [DOI] [PubMed] [Google Scholar]
- 38.Münzel T, Sørensen M, Gori T, et al. Environmental stressors and cardio-metabolic disease: part I–epidemiologic evidence supporting a role for noise and air pollution and effects of mitigation strategies. Eur Heart J 2017;38:550–6. [DOI] [PubMed] [Google Scholar]
- 39.Butz AM, Matsui EC, Breysse P, et al. A randomized trial of air cleaners and a health coach to improve indoor air quality for inner-city children with asthma and secondhand smoke exposure. Arch Pediatr Adolesc Med 2011;165: 741–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cui X, Li Z, Teng Y, et al. Association between bedroom particulate matter filtration and changes in airway pathophysiology in children with asthma. JAMA Pediatr 2020;174:533–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shakya KM, Noyes A, Kallin R, Peltier RE. Evaluating the efficacy of cloth facemasks in reducing particulate matter exposure. J Expo Sci Environ Epidemiol 2016;27:352–7. [DOI] [PubMed] [Google Scholar]
- 42.CDC. National Institute for Occupational Safety and Health (NIOSH). Approved particulate filtering facepiece respirators 2020. Available at: https://www.cdc.gov/niosh/npptl/topics/respirators/disp_part/default.html. Accessed April 9, 2020.
- 43.Brugge D, Simon MC, Hudda N, et al. Lessons from in-home air filtration intervention trials to reduce urban ultrafine particle number concentrations. Build Environ 2017;126:266–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Siegel JA. Primary and secondary consequences of indoor air cleaners. Indoor Air 2015;26: 88–96. [DOI] [PubMed] [Google Scholar]
- 45.Fisk WJ. Health benefits of particle filtration. Indoor Air 2013;23:357–68. [DOI] [PubMed] [Google Scholar]
- 46.Spilak MP, Karottki GD, Kolarik B, Frederiksen M, Loft S, Gunnarsen L. Evaluation of building characteristics in 27 dwellings in Denmark and the effect of using particle filtration units on PM2.5 concentrations. Building and Environment 2014;73:55–63. [Google Scholar]
- 47.Weichenthal S, Mallach G, Kulka R, et al. A randomized double-blind crossover study of indoor air filtration and acute changes in cardiorespiratory health in a First Nations community. Indoor Air 2013;23:175–84. [DOI] [PubMed] [Google Scholar]
- 48.Wheeler AJ, Gibson MD, MacNeill M, et al. Impacts of air cleaners on indoor air quality in residences impacted by wood smoke. Environ Sci Technol 2014;48:12157–63. [DOI] [PubMed] [Google Scholar]
- 49.Chen R, Zhao A, Chen H, et al. Cardiopulmonary benefits of reducing indoor particles of outdoor origin a randomized, double-blind crossover trial of air purifiers. J Am Coll Cardiol 2015;65: 2279–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bard RL, Rubenfire M, Fink S, et al. Reduced fine particulate matter air pollution exposures using in-home portable air cleaners: pilot results of the Cardiac Rehabilitation Air Filter Trial (CRAFT). J Cardiopulm Rehabil Prev 2020;40: 276–9. [DOI] [PubMed] [Google Scholar]
- 51.Vyas S, Srivastav N, Spears D. An experiment with air purifiers in Delhi during winter 2015–2016. PLOS ONE 2016;11:e0167999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barn PK, Elliott CT, Allen RW, Kosatsky T, Rideout K, Henderson SB. Portable air cleaners should be at the forefront of the public health response to landscape fire smoke. Environ Health 2016;15:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.U.S. EPA. Indoor Air Quality (IAQ): air cleaners and air filters in the home. 2014. 2014. Available at: https://www.epa.gov/indoor-air-quality-iaq/air-cleaners-and-air-filters-home-0. Accessed April 9, 2020.
- 54.Bowe B, Xie Y, Yan Y, Al-Aly Z. Burden of cause-specific mortality associated with PM2.5 air pollution in the United States. JAMA Netw Open 2019;2:e1915834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ries FJ, Marshall JD, Brauer M. Intake fraction of urban wood smoke. Environ Sci Technol 2009; 43:4701–6. [DOI] [PubMed] [Google Scholar]
- 56.Li H, Cai J, Chen R, et al. Particulate matter exposure and stress hormone levels. Circulation 2017;136:618–27. [DOI] [PubMed] [Google Scholar]
- 57.Bhatt DL, Steg PG, Miller M, et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med 2019;380: 11–22. [DOI] [PubMed] [Google Scholar]
- 58.Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373: 2117–28. [DOI] [PubMed] [Google Scholar]
- 59.Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med 2017;376: 1713–22. [DOI] [PubMed] [Google Scholar]
- 60.Wright JT Jr., Williamson JD, Whelton PK, et al. , for the SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015;373: 2103–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Miller KA, Siscovick DS, Sheppard L, et al. Long-term exposure to air pollution and incidence of cardiovascular events in women. N Engl J Med 2007;356:447–58. [DOI] [PubMed] [Google Scholar]
- 62.Hadley MB, Baumgartner J, Vedanthan R. Developing a clinical approach to air pollution and cardiovascular health. Circulation 2018;137: 725–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Walzer D, Gordon T, Thorpe L, et al. Effects of home particulate air filtration on blood pressure. Hypertension 2020;76:44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lassere MN, Johnson KR, Schiff M, Rees D. Is blood pressure reduction a valid surrogate endpoint for stroke prevention? an analysis incorporating a systematic review of randomised controlled trials, a by-trial weighted errors-in-variables regression, the surrogate threshold effect (STE) and the biomarker-surrogacy (BioSurrogate) evaluation schema (BSES). BMC Medical Research Methodology 2012;12:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines [Published correction in J Am Coll Cardiol 2018;71:2275–9]. J Am Coll Cardiol 2018; 71:e127–248. [DOI] [PubMed] [Google Scholar]
- 66.Ward-Caviness CK, Weaver AM, Buranosky M, et al. Associations between long-term fine particulate matter exposure and mortality in heart failure patients. J Am Heart Assoc 2020;9: e012517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vestbo J, Anderson JA, Brook RD, et al. Fluticasone furoate and vilanterol and survival in chronic obstructive pulmonary disease with heightened cardiovascular risk (SUMMIT): a double-blind randomised controlled trial. Lancet 2016;387:1817–26. [DOI] [PubMed] [Google Scholar]
- 68.Bhatt DL, Mehta C. Adaptive designs for clinical trials. N Engl J Med 2016;375:65–74. [DOI] [PubMed] [Google Scholar]
- 69.Wu X, Nethery RC, Sabath BM, Braun D, Dominici F. Exposure to air pollution and COVID-19 mortality in the United States: a nationwide cross-sectional study. medRxiv 2020. April 7 [E-pub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


