Abstract
Background
Timely detection of the progression of the highly contagious coronavirus disease (COVID-19) is of utmost importance for management and intervention for patients in intensive care (ICU).
Aim
This study aims to better understand this new infection and report the changes in the various laboratory tests identified in critically ill patients and associated with poor prognosis among COVID-19 patients admitted to the ICU.
Methods
This was a retrospective study that included 160 confirmed SARS-CoV-2-positive patients.
Results
Elevated serum ferritin, D-dimer, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and nonconjugated bilirubin levels were present in 139 (96%), 131 (96%), 107 (68%), 52 (34%), and 89 (70%) patients, respectively. Renal parameters were abnormal in a significant number of cases with elevated creatinine and blood urea nitrogen in 93 (62%) and 102 (68%) cases, respectively. Hematological profiles revealed lower red blood cell count, hemoglobin, eosinophils, basophils, monocytes, and lymphocytes in 90 (57%), 103 (65%), 89 (62%), 105 (73%), 35 (24%), and 119 (83%) cases, respectively. The neutrophil count was found to increase in 71.3% of the cases. There was significantly higher mortality (83%) among patients older than 60 years (p=0.001) and in female patients (75%) (p=0.012). Patients with lung diseases had a poor outcome compared to patients with other comorbidities (p=0.002). There was a significant association between elevated D-dimer levels and increased mortality (p=0.003). Elevated levels of AST, creatinine, blood urea nitrogen, and bilirubin were significantly associated with unfavorable outcomes.
Conclusion
Different parameters can be used to predict disease prognosis, especially the risk of poor prognosis. Accurate diagnosis and monitoring of disease progression from the early stages will help in reducing mortality and unfavorable outcomes.
1. Background
Coronavirus disease (COVID-19) is highly contagious and was first reported in Wuhan, China, in December 2019. It has spread throughout the world and poses a great threat to global health [1]. It is caused by a novel coronavirus called severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [2]. SARS-CoV-2 belongs to a class of single-stranded RNA viruses, beta coronaviruses of the family Coronaviridae [3], and has a long incubation period, with human-to-human transmission having been confirmed in COVID-19 [2, 4]. Bats are postulated to be the primary source of SARS-CoV-2 as SARS-CoV-2 has similarities to bat coronaviruses [5]. SARS-CoV and Middle East respiratory syndrome-related coronavirus (MERS-CoV) were transmitted from market civets and dromedary camels, respectively, and both are believed to have originated in bats [6]. COVID-19 shows an alarming rate of transmission, and the World Health Organization has declared this to be a pandemic [7]. The SARS-CoV-2 infection has a variable presentation and high mortality rates in patients with comorbidities and immunocompromised states. Timely detection of the disease course and progression is of utmost importance for management and intervention [7]. Real-time reverse transcription-PCR (RT-PCR) assays are the gold standard in diagnosis, whereas rapid screening by antigen detection is also used to complement molecular diagnosis [7, 8].
Several studies have described the clinical characteristics and laboratory changes associated with COVID-19 patients [9–11]. The number of COVID-19 patients is increasing drastically, and treatment in intensive care units (ICUs) has become a challenge for the healthcare system [12]. SARS-CoV-2 infection can cause severe respiratory illness and may progress to clinically severe stages requiring ICU admission, extracorporeal membrane oxygenation (ECMO) therapy, and ventilator support [9, 13]. A pattern of abnormalities related to the hematologic, biochemical, inflammatory, and immune biomarkers has been identified in patients with a severe form of the disease compared to those with mild systemic disease [9–11, 13–16]. This study aimed to investigate the biochemical profiles and report the various predictors (laboratory tests) of poor prognosis among COVID-19 patients admitted to the ICU. A comparative analysis was also performed between the recovered (cured) and deceased patients based on their clinical, demographic, and laboratory parameters.
2. Methods
2.1. Study Design and Participants
This was a retrospective study conducted at Al Noor Specialist Hospital, Makkah, Saudi Arabia. The electronic records were searched retrospectively to identify RT-PCR-confirmed COVID-19 patients admitted to the ICU. All patients who were admitted to the ICU between March 21, 2020, and June 1, 2020, were included in this study. In total, 160 ICU-admitted patients were included in the study cohort.
2.2. Data Collection
Demographic data, laboratory findings, and clinical data were collected from hospital records. Laboratory test findings included results of hematological tests, including complete blood counts (CBCs), D-dimer, serum ferritin, C-reactive protein (CRP), blood urea nitrogen (BUN), serum creatinine, and liver function tests, performed on the day of admission to the ICU. The outcome data for patients were obtained from electronic medical records, with the primary outcome being either discharge due to recovery (cured) or death as a result of being infected with SARS-CoV-2.
2.3. Statistical Analyses
Data processing and analyses were carried out using the Statistical Package for the Social Sciences software (version 20.0). The chi-square test and Fisher's exact test were used for comparison, as appropriate. The odds ratio (OR), associated p value, and 95% confidence intervals (95% CI) were used to determine the association among demographic data, laboratory findings, clinical data, and primary outcomes. The Kaplan–Meier test was used to estimate the median and visualize survival time (in days), whereas the log rank test and associated p value were used for comparisons, as appropriate. The Cox regression model was applied to estimate the hazard ratio (HR), associated p value, and 95% CI. A p value of 0.05 was considered statistically significant for all statistical tests.
3. Results
3.1. Laboratory Findings among COVID-19 Patients Admitted in the ICU
Table 1 represents the patients' demographics and clinical and laboratory data. In total, 160 COVID-19 patients confirmed in the laboratory were included in this study. Forty (25%) were women and 120 (75%) were men, with a mean age of 56 ± 17 years. Comorbidities present in the patients were diabetes mellitus (DM) in 52 (32.5%), renal failure in 5 (3%), heart diseases in 43 (28%), and pulmonary disease in 78 (49%) patients. Out of 160, 93 (58%) succumbed to the disease and 67 (42%) were discharged after recovery.
Table 1.
Patients' demographics, clinical data, and laboratory data.
| Medians (interquartile ranges) | Frequencies | Cutoff∗ | ||
|---|---|---|---|---|
| N | % | |||
| Age (years) | M: 53 ± 15† F: 59 ± 20† All: 56 ± 17† |
|||
| 20–59 | 106 | 66.3 | ||
| ≥60 | 54 | 33.8 | ||
|
| ||||
| Sex | ||||
| Female | 40 | 25 | ||
| Male | 120 | 75 | ||
|
| ||||
| Diabetes | ||||
| Yes | 52 | 32.5 | ||
| No | 108 | 67.5 | ||
|
| ||||
| Renal failure | ||||
| Yes | 5 | 3 | ||
| No | 155 | 97 | ||
|
| ||||
| Pulmonary disease | ||||
| Yes | 78 | 49 | ||
| No | 82 | 51 | ||
|
| ||||
| Heart disease | ||||
| Yes | 43 | 28 | ||
| No | 109 | 72 | ||
|
| ||||
| Ferritin | 1229 (770–1853) | Male: 30–400 ug/L Female: 15–150 ug/L |
||
| Normal | 6 | 4 | ||
| Increased | 139 | 96 | ||
|
| ||||
| D-dimer | 4 (2–12) | All: 0–0.55 mg/L | ||
| Normal | 6 | 4 | ||
| Increased | 131 | 96 | ||
|
| ||||
| AST | 58 (29–99) | All: 15–37 U/L | ||
| Normal | 50 | 32 | ||
| Increased | 107 | 68 | ||
|
| ||||
| ALT | 42 (27–92) | All: 14–36 U/L | ||
| Normal | 103 | 66 | ||
| Increased | 52 | 34 | ||
|
| ||||
| ALP | 24 (20–30) | All: 46–120 U/L | ||
| Decreased | 146 | 100 | ||
| Normal | 0 | 0 | ||
|
| ||||
| Total protein | 68 (63–71) | All: 64–82 g/L | ||
| Decreased | 22 | 26 | ||
| Normal | 62 | 74 | ||
|
| ||||
| Bilirubin total | 13 (8–22) | All: 0–18.7 umol/L | ||
| Normal | 97 | 75 | ||
| Increased | 32 | 25 | ||
|
| ||||
| Bilirubin nonconjugated | 6 (3–15) | 3.4–12 umol/L | ||
| Normal | 38 | 30 | ||
| Increased | 89 | 70 | ||
|
| ||||
| Creatinine | 243 (114–508) | Male: 62–115 umol/L Female: 44–90 umol/L |
||
| Normal | 56 | 38 | ||
| Increased | 93 | 62 | ||
|
| ||||
| BUN | 24 (9–39) | All: 2.6–6.4 mmol/L | ||
| Normal | 47 | 32 | ||
| Increased | 102 | 68 | ||
|
| ||||
| CRP | 8 (2–13) | All: 0–6 mg/L | ||
| Normal | 53 | 42 | ||
| Increased | 74 | 58 | ||
|
| ||||
| RBCs | 4 (3–5) | Male: 4.5–5.5 10^12/L Female: 3.8–4.8 10^12/L |
||
| Decreased | 90 | 57 | ||
| Normal | 67 | 43 | ||
|
| ||||
| Hb | 97 (81–121) | Male: 130–170 g/L Female: 120–150 g/L |
||
| Decreased | 103 | 65 | ||
| Normal | 55 | 35 | ||
|
| ||||
| Neutrophils | 89 (80–92) | 2–7 10^9/L | ||
| Normal | 41 | 29 | ||
| Increased | 102 | 71 | ||
|
| ||||
| Eosinophils | 0 (0–2) | 0.1–0.8 10^9/L | ||
| Decreased | 89 | 62 | ||
| Normal | 54 | 38 | ||
|
| ||||
| Basophils | 0 (0–0) | 0.02–0.1 10^9/L | ||
| Decreased | 105 | 73 | ||
| Increased | 38 | 27 | ||
|
| ||||
| Monocytes | 4 (2–7) | 0.2–1 10^9/L | ||
| Decreased | 35 | 24 | ||
| Normal | 108 | 76 | ||
|
| ||||
| Lymphocytes | 7 (4–13) | 1–4 10^9/L | ||
| Decreased | 119 | 83 | ||
| Normal | 24 | 17 | ||
|
| ||||
| Discharge status | ||||
| Cured | 67 | 42 | ||
| Died | 93 | 58 | ||
| Overall | 160 | 100 | ||
N: number of patients; M: male; F: female; all: both male and female; SD: standard deviation; BUN: blood urea nitrogen; CRP: C-reactive protein; Hb: hemoglobin; AST: aspartate aminotransferase; ALT: alanine aminotransferase; ALP: alkaline phosphatase. †Mean ± SD.∗Cutoff for laboratory tests.
Elevated serum ferritin and D-dimer levels were present in 96% of the patients. Regarding the parameters for the liver function assessment, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and nonconjugated bilirubin were elevated in 107 (68%), 52 (34%), and 89 (75%) patients, respectively. The pattern of renal function assessments, including serum creatinine and BUN, varied in a significant number of cases, wherein serum creatinine and BUN were found to be elevated in 93 (62%) and 102 (69%) cases, respectively. However, hematological data suggest potential defects in erythropoiesis, as a decrease was noted in the case of red blood cell (RBC) count and hemoglobin in 90 (57.3%) and 103 (65%) cases, respectively. The white blood cell (WBC) count that include eosinophil, basophil, and lymphocyte counts were consistently decreased in 89 (62%), 105 (73%), and 119 (83.2%) cases, respectively. On the other hand, neutrophil counts were considerably higher in 102 (71.3%) cases.
3.2. Association between Data Variables and Outcome
Table 2 represents the pattern of association found among various clinical and laboratory parameters with regard to the primary outcome. When the patterns of mortality among the study population were considered, the data show that the overall percentage of death in male patients was 52.5%. There was a significantly high mortality rate among older patients aged above 60 years, with more than 80% deaths (p=0.001). The mortality rate was significantly higher in female (30, 75%) than in male patients (p=0.012). Patients with lung diseases had a poor outcome compared to patients with other comorbidities, such as DM, heart disease, or renal disease (p=0.002). Increased mortality was not significantly associated with elevated ferritin, whereas there was a significant association between elevated D-dimer levels and increased mortality (p=0.003).
Table 2.
Association between demographics, clinical data, laboratory test data, and primary outcomes.
| Discharge status | p value | Odds ratio (OR, 95% CI, p value) | ||
|---|---|---|---|---|
| Cured 67 (42%) | Died 93 (58%) | |||
| Age (years) | ||||
| 20–59 | 58 (55%) | 48 (45%) | <0.001 | 0.17, 0.074–0.373, <0.001 |
| ≥60 | 9 (17%) | 45 (83%) | ||
|
| ||||
| Sex | ||||
| Female | 10 (25%) | 30 (75%) | 0.012 | 2.7, 1.22–6.04, 0.015 |
| Male | 57 (47.5%) | 63 (52.5%) | ||
|
| ||||
| Diabetes | ||||
| Yes | 20 (38.5%) | 32 (61.5%) | 0.544 | — |
| No | 47 (43.5%) | 61 (56.5%) | ||
|
| ||||
| Renal failure | ||||
| Yes | 1 (20%) | 4 (80%) | 0.400 | — |
| No | 66 (43%) | 89 (57%) | ||
|
| ||||
| Pulmonary disease | ||||
| Yes | 23 (29.5%) | 55 (70.5%) | 0.002 | 2.8, 1.44–5.32, 0.002 |
| No | 44 (54%) | 38 (46%) | ||
|
| ||||
| Heart disease | ||||
| Yes | 21 (49%) | 22 (51%) | 0.291 | — |
| No | 43 (39%) | 66 (61%) | ||
|
| ||||
| Ferritin | ||||
| Normal | 1 (17%) | 5 (83%) | 0.401 | — |
| Increased | 59 (42%) | 80 (58%) | ||
|
| ||||
| D-dimer | ||||
| Normal | 6 (100%) | 0 (0%) | 0.003 | 21, 1.16–380.47, 0.039 |
| Increased | 50 (38%) | 81 (62%) | ||
|
| ||||
| AST | ||||
| Normal | 33 (66%) | 17 (34%) | <0.001 | 4.17, 2.04–8.5, 0.001 |
| Increased | 34 (32%) | 73 (68%) | ||
|
| ||||
| ALT | ||||
| Normal | 37 (36%) | 66 (64%) | 0.033 | 0.48, 0.24–0.95, 0.034 |
| Increased | 28 (54%) | 24 (46%) | ||
|
| ||||
| ALP | ||||
| Decreased | 58 (40%) | 88 (60%) | — | — |
| Normal | 0 (0%) | 0 (0%) | ||
|
| ||||
| Total protein | ||||
| Decreased | 0 (0%) | 22 (100%) | 0.001 | 26.8, 1.56–462.2, 0.023 |
| Normal | 23 (37%) | 39 (63%) | ||
|
| ||||
| Bilirubin total | ||||
| Normal | 42 (43%) | 55 (57%) | 0.065 | |
| Increased | 8 (25%) | 24 (75%) | ||
|
| ||||
| Bilirubin nonconjugated | ||||
| Normal | 24 (63%) | 14 (37%) | <0.001 | 4.2, 1.86–9.26, 0.0005 |
| Increased | 26 (29%) | 63 (71%) | ||
|
| ||||
| Creatinine | ||||
| Normal | 47 (84%) | 9 (16%) | <0.001 | 27.2, 11.01–66.94, <0.001 |
| Increased | 15 (16%) | 78 (84%) | ||
|
| ||||
| BUN | ||||
| Normal | 41 (87%) | 6 (13%) | <0.001 | 26.4, 9.87–70.37, <0.001 |
| Increased | 21 (21%) | 81 (79%) | ||
|
| ||||
| CRP | ||||
| Normal | 22 (41.5%) | 31 (58.5%) | 0.584 | |
| Increased | 27 (36.5%) | 47 (63.5%) | ||
|
| ||||
| RBCs | ||||
| Decreased | 25 (28%) | 65 (72%) | <0.001 | 4.4, 2.22–8.59, <0.001 |
| Normal | 42 (63%) | 25 (37%) | ||
|
| ||||
| Hb | ||||
| Decreased | 26 (25%) | 77 (75%) | <0.001 | 8.7, 4.09–18.4, <0.001 |
| Normal | 41 (74.5%) | 14 (25.5%) | ||
|
| ||||
| Neutrophils | ||||
| Normal | 33 (80.5%) | 8 (19.5%) | <0.001 | 6.7, 2.7–16.48, <0.001 |
| Increased | 22 (22%) | 80 (78%) | ||
|
| ||||
| Eosinophils | ||||
| Decreased | 23 (26%) | 66 (74%) | <0.001 | 4.2, 2.03–8.58, 0.001 |
| Normal | 32 (59%) | 22 (41%) | ||
|
| ||||
| Basophils | ||||
| Decreased | 31 (29.5%) | 74 (70.5%) | <0.001 | 4.1, 1.87–8.94, 0.0004 |
| Increased | 24 (63%) | 14 (37%) | ||
|
| ||||
| Monocytes | ||||
| Decreased | 7 (20%) | 28 (80%) | 0.01 | 3.2, 1.29–7.96, 0.0123 |
| Normal | 48 (44%) | 60 (56%) | ||
|
| ||||
| Lymphocytes | ||||
| Decreased | 35 (29%) | 84 (71%) | <0.001 | 12, 3.82–37.66, <0.001 |
| Normal | 20 (83%) | 4 (17%) | ||
BUN: blood urea nitrogen; CRP: C-reactive protein; Hb: hemoglobin; AST: aspartate aminotransferase; ALT: alanine aminotransferase; ALP: alkaline phosphatase. Symbol “—” indicates value that cannot be calculated due to zero cases/not significant value or incalculable odd ratio.
There was also a significant association between elevated levels of creatinine, BUN, and nonconjugated bilirubin in the deceased patients (p < 0.001). Additionally, AST level was significantly elevated in the deceased patients (p < 0.001) compared to in the recovered patients (Table 2). The hematological laboratory tests in the deceased patients showed a significant association with decreased lymphocyte, monocyte, eosinophil, and basophil counts, along with an increased neutrophil count, which was significant. In contrast, normal lymphocyte, monocyte, and neutrophil counts favored recovery (Table 2).
3.3. Predictors of Poor Prognosis among COVID-19 Patients Admitted in the ICU
The analyses indicated that the overall median survival time (time to death in ICU due to COVID-19) was 14 days with 95% CI between 11 and 17 days, whereas advanced age, the female sex, presence of DM, elevated levels of AST, nonconjugated bilirubin, creatinine, and BUN, an increased neutrophil count, and decreased eosinophil, basophil, monocyte, and lymphocyte counts were associated with an increased risk of death due to COVID-19 (Table 3, Figure 1). The Cox regression model, used for the multivariate analysis of demographics, clinical data, laboratory tests, and survival time of severe COVID-19 patients in the ICU, showed that patients' age (p < 0.05), sex (p=0.005), presence of DM (p=0.005), AST (p < 0.001), creatinine (p=0.007), and eosinophil count (p=0.017) demonstrated prognostic significance on a multivariate level (Table 4).
Table 3.
Median survival and 95% confidence interval (CI) by demographics, clinical data, and laboratory tests.
| Median survival time∗ | 95% CI | p value† | |
|---|---|---|---|
| Age (years) | |||
| 20–39 | 22 | 12–32 | 0.001 |
| ≥60 | 10 | 7–13 | |
|
| |||
| Sex | |||
| Female | 8 | 6–10 | 0.002 |
| Male | 17 | 13–21 | |
|
| |||
| Diabetes | |||
| Yes | 11 | 6–16 | 0.017 |
| No | 16 | 11–21 | |
|
| |||
| Renal failure | |||
| Yes | 17 | 6–28 | 0.563 |
| No | 14 | 11–17 | |
|
| |||
| Pulmonary disease | |||
| Yes | 12 | 9–15 | 0.925 |
| No | 22 | 14–30 | |
|
| |||
| Heart disease | |||
| Yes | 13 | 10–16 | 0.793 |
| No | 17 | 11–23 | |
|
| |||
| Ferritin | |||
| Normal | 13 | 1–39 | 0.925 |
| Increased | 15 | 12–18 | |
|
| |||
| D-dimer | |||
| Normal | — | — | — |
| Increased | 14 | 11–17 | |
|
| |||
| AST | |||
| Normal | 30 | 16–44 | <0.001 |
| Increased | 11 | 9–13 | |
|
| |||
| ALT | |||
| Normal | 13 | 10–16 | 0.218 |
| Increased | 19 | 10–28 | |
|
| |||
| ALP | |||
| Decreased | 14 | 11–17 | — |
| Normal | — | — | |
|
| |||
| Total protein | |||
| Decreased | 11 | 8–14 | <0.001 |
| Normal | 19 | 13–25 | |
|
| |||
| Bilirubin total | |||
| Normal | 18 | 14–22 | 0.075 |
| Increased | 12 | 10–14 | |
|
| |||
| Bilirubin nonconjugated | |||
| Normal | 36 | 20–52 | <0.001 |
| Increased | 12 | 10–14 | |
|
| |||
| Creatinine | |||
| Normal | 31 | 24–38 | <0.001 |
| Increased | 10 | 8–12 | |
|
| |||
| BUN | |||
| Normal | 36 | 26–46 | <0.001 |
| Increased | 11 | 9–13 | |
|
| |||
| CRP | |||
| Normal | 14 | 10–18 | 0.484 |
| Increased | 13 | 10–16 | |
|
| |||
| RBCs | |||
| Decreased | 13 | 10–16 | 0.091 |
| Normal | 30 | 2–58 | |
|
| |||
| Hb | |||
| Decreased | 12 | 9–15 | — |
| Normal | 30 | — | |
|
| |||
| Neutrophils | |||
| Normal | 36 | 24–48 | <0.001 |
| Increased | 11 | 9–13 | |
|
| |||
| Eosinophils | |||
| Decreased | 10 | 8–12 | <0.001 |
| Normal | 30 | 22–38 | |
|
| |||
| Basophils | |||
| Decreased | 12 | 10–14 | 0.015 |
| Increased | 17 | 7–27 | |
|
| |||
| Monocytes | |||
| Decreased | 10 | 7–13 | <0.001 |
| Normal | 17 | 10–24 | |
|
| |||
| Lymphocytes | |||
| Decreased | 12 | 9–15 | <0.001 |
| Normal | 36 | 1–75 | |
†Associated p value with the log rank test. Symbol “—” indicates value that cannot be calculated due to low number of events (death). ∗Survival time calculated in days. BUN: blood urea nitrogen; CRP: C-reactive protein; Hb: hemoglobin; AST: aspartate aminotransferase; ALT: alanine aminotransferase; ALP: alkaline phosphatase.
Figure 1.
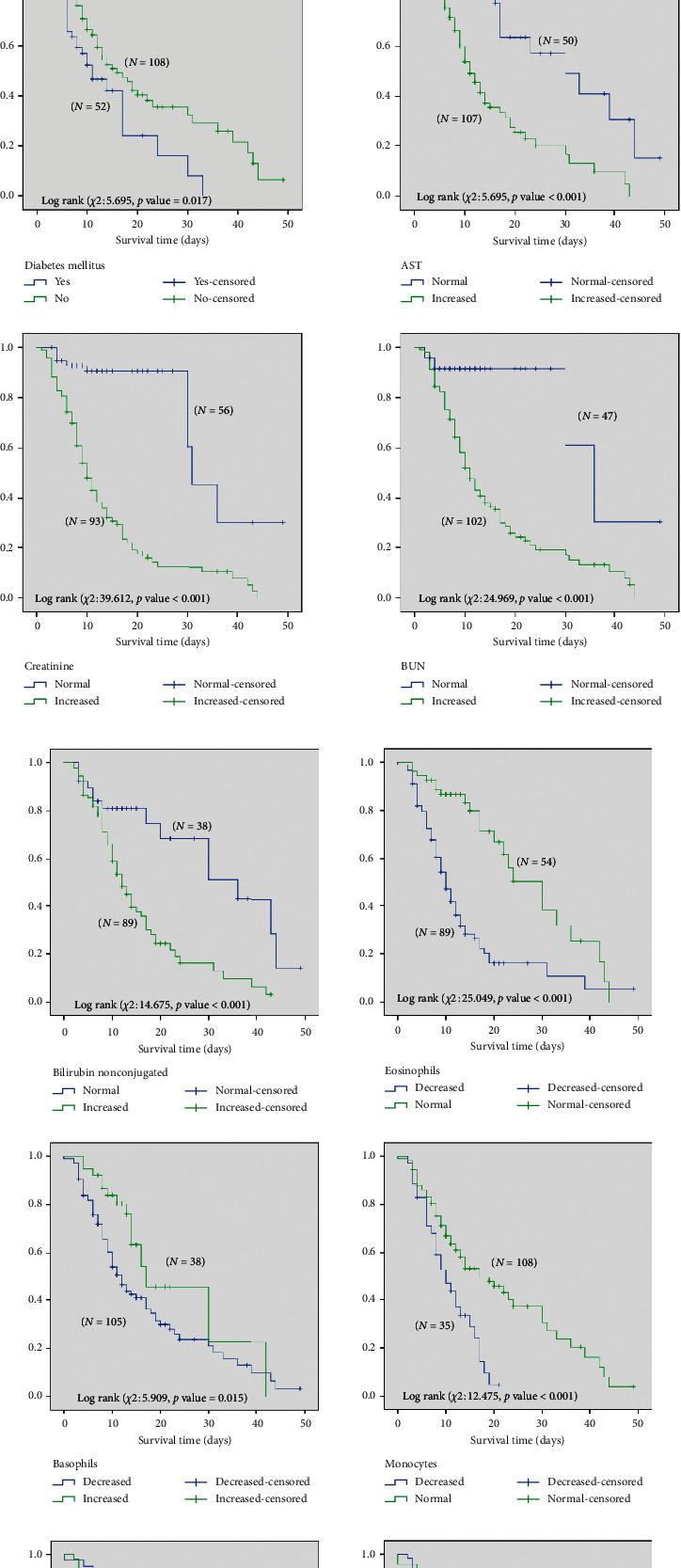
(a). Survival time (in days) of severe COVID-19 patients in ICU by age, sex, and clinical and laboratory test data, such as diabetes, AST, creatinine, and BUN. (b). Survival time (in days) of severe COVID-19 patients in ICU by clinical and laboratory test data, such as bilirubin eosinophils, basophils, monocytes, neutrophils, and lymphocytes.
Table 4.
Summary of the Cox regression model for the results of a multivariate analysis of demographics, clinical data, laboratory test, and survival time of severe COVID-19 patients in ICU.
| Factors | p value | Hazard ratio | 95% CI |
|---|---|---|---|
| Age (years) 20–39 | 0.04 | 2.73 | 1.046–7.195 |
| 40–59 | 0.024 | 2.532 | 1.133–5.657 |
| 60–79 | <0.001 | 4.447 | 1.948–10.153 |
| ≥ 80 | — | — | — |
| Sex (female) | 0.005 | 2.355 | 1.301–4.264 |
| Diabetes (yes) | 0.005 | 2.178 | 1.263–3.757 |
| AST (increased) | <0.001 | 4.346 | 2.088–9.044 |
| Bilirubin nonconjugated (increased) | 0.327 | 1.484 | 0.674–3.267 |
| Creatinine (increased) | 0.007 | 6.501 | 1.677–25.194 |
| BUN (increased) | 0.447 | 1.763 | 0.369–8.427 |
| Neutrophils (increased) | 0.634 | 1.302 | 0.401–4.480 |
| Eosinophils (decreased) | 0.017 | 2.211 | 1.155–4.234 |
| Basophils (decreased) | 0.368 | 1.426 | 0.658–3.093 |
| Monocytes (decreased) | 0.343 | 1.302 | 0.755–2.245 |
| Lymphocytes (decreased) | 0.894 | 1.124 | 0.202–6.244 |
Symbol “—” indicates unmeasurable values due to linearity issues in the original data. AST: aspartate aminotransferase; BUN: blood urea nitrogen.
3.4. Comparison between the COVID-19 Patients with and without Chronic Comorbidities
The COVID-19 patients with chronic comorbidities, such as DM, renal diseases (RD), pulmonary diseases (PD), and heart diseases (HD), were compared with the COVID-19 patients without comorbidities according to the level of laboratory findings, as shown in Table 5. There was a nonsignificant difference in the laboratory findings between the COVID-19 patients with and without comorbidities, except for D-dimer, liver function (AST, ALT), and renal functions (creatinine and BUN) in patients with RD (Table 5). The COVID-19 patients with HD showed significant differences in AST, ALT, and BUN compared to those with non-heart-related diseases.
Table 5.
Comparison between the COVID-19 patients with and without comorbidities, such as diabetes mellitus (DM), renal diseases (RD), pulmonary diseases (PD), and heart diseases (HD), and the laboratory test data.
| DM | RD | PD | HD | |||||
|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | Yes | No | |
| Age (years) | 60.6 ± 2.1 | 51.2 ± 1.5 | 63.6 ± 10.1 | 53.9 ± 1.3 | 57.2 ± 1.7 | 51.3 ± 1.8 | 53.1 ± 1.6 | 56.7 ± 2.2 |
| Ferritin | 1493 ± 112.8 | 1714 ± 211.2 | 1643 ± 370 | 1645 ± 154.7 | 1761 ± 184.8 | 1540 ± 231.8 | 1712 ± 211.2 | 1514 ± 160.1 |
| D-dimer | 8.5 ± 1.5 | 9.14 ± 1.6 | 19.5 ± 12.1 | 8.6 ± 1.1c | 10.7 ± 2.0 | 7.3 ± 1.3 | 8.2 ± 1.3 | 10.7 ± 2.3 |
| AST | 174.5 ± 84.3 | 196.3 ± 80.8 | 63.2 ± 13.2a | 193.3 ± 62.8 | 219.1 ± 107.0 | 161.2 ± 62.6 | 228.6 ± 88.8a | 107.3 ± 31.7 |
| ALT | 74.9 ± 19.6 | 128.2 ± 26.3 | 41.8 ± 13.7a | 113.3 ± 19.5 | 103.2 ± 26.3 | 118.5 ± 27.4 | 131.1 ± 27.4b | 70.02 ± 12.9 |
| ALP | 24.4 ± 0.9 | 25.41 ± 0.7 | 20.0 ± 1.9 | 25.2 ± 0.5 | 24.5 ± 0.8 | 25.5 ± 0.7 | 25.1 ± 1.2 | 25.8 ± 1.1 |
| Total protein | 65.6 ± 1.8 | 68.2 ± 1.0 | 69.3 ± 3.5 | 67.5 ± 0.9 | 67.3 ± 1.5 | 67.7 ± 1.0 | 67.1 ± 1.2 | 68.5 ± 1.3 |
| Bilirubin total | 14.8 ± 1.9 | 18.2 ± 2.2 | 18.4 ± 4.5 | 17.2 ± 1.6 | 21.0 ± 3.0 | 13.4 ± 1.0 | 16.6 ± 1.6 | 18.4 ± 3.6 |
| Bilirubin nonconjugated | 8.5 ± 1.6 | 11.2 ± 1.8 | 13.6 ± 4.5 | 10.2 ± 1.4 | 13.6 ± 2.6 | 7.1 ± 0.8a | 9.8 ± 1.4 | 11.3 ± 3.1 |
| Creatinine | 287.2 ± 36.2 | 288.3 ± 30.3 | 649.8 ± 216.3 | 275.4 ± 22.6a | 290 ± 33.5 | 286 ± 33.0 | 284 ± 26.7 | 296 ± 46.0 |
| BUN | 21.1 ± 2.4 | 54.4 ± 33.6 | 65.33 ± 45.1 | 21.4 ± 2.4a | 38.8 ± 12.1 | 43.2 ± 22.9 | 21.31 ± 1.9a | 86.0 ± 65.9 |
| CRP | 7.6 ± 1.0 | 8.6 ± 0.7 | 8.3 ± 4.6 | 8.3 ± 0.6 | 7.6 ± 0.8 | 8.8 ± 0.8 | 8.3 ± 0.7 | 8.2 ± 1.2 |
| RBCs | 3.8 ± 0.1 | 4.1 ± 1.0 | 3.4 ± 0.4 | 4.0 ± 0.08 | 3.9 ± 0.1 | 4.1 ± 0.1 | 4.1 ± 0.1 | 3.9 ± 0.1 |
| Hb | 105.6 ± 3.4 | 108.1 ± 2.5 | 87.4 ± 4.8 | 108.0 ± 2.1 | 105.0 ± 2.8 | 109.5 ± 2.8 | 109.0 ± 2.7 | 103.7 ± 3.2 |
| Neutrophils | 82.0 ± 2.2 | 81.5 ± 1.3 | 87.0 ± 2.6 | 81.5 ± 1.2 | 84.1 ± 1.3 | 79.2 ± 1.7 | 81.3 ± 1.4 | 82.5 ± 1.7 |
| Eosinophils | 0.8 ± 0.2 | 1.2 ± 0.2 | 2.0 ± 1.3 | 1.02 ± 0.2 | 0.9 ± 0.2 | 1.1 ± 0.2 | 1.0 ± 0.2 | 1.3 ± 0.36 |
| Basophils | 0.31 ± 0.08 | 0.27 ± 0.04 | 0.2 ± 0.2 | 0.28 ± 0.04 | 0.29 ± 0.06 | 0.27 ± 0.05 | 0.29 ± 0.05 | 0.27 ± 0.08 |
| Monocytes | 5.9 ± 3.3 | 5.2 ± 0.36 | 4.6 ± 1.2 | 5.3 ± 0.3 | 4.8 ± 0.4 | 5.6 ± 0.4 | 5.1 ± 0.4 | 5.6 ± 0.5 |
| Lymphocytes | 10.2 ± 0.1 | 11.4 ± 0.9 | 7.0 ± 1.2 | 11.1 ± 0.8 | 9.5 ± 0.9 | 12.3 ± 1.6 | 11.2 ± 0.9 | 10.5 ± 1.2 |
AST: aspartate aminotransferase; ALT: alanine aminotransferase; ALP: alkaline phosphatase; BUN: blood urea nitrogen; CRP: C-reactive protein; Hb: hemoglobin. ap value <0.001; bp value <0.01.
4. Discussion
SARS-CoV-2 is the third coronavirus after SARS-CoV and MERS-CoV, causing a health threat in the last decade [17]. This study reveals the clinical, demographic, and laboratory test results of a subset of critical patients with COVID-19 admitted to the ICU of a designated hospital in Makkah city, Saudi Arabia. Few reports have identified specific parameters to be useful predictors of a poor outcome. To the best of our knowledge, this is the first study from the Makkah region describing prognostic factors in critically ill COVID-19 patients hospitalized in the ICU.
In total, 160 patients were included in this study. The median age of the patients was 56 years. There were predominantly much older patients in the critically ill group, a finding also reported in other studies [5, 13–16]. According to the demographic data, age is a well-established factor for severe/critically ill COVID-19 patients aged above 60 years. Other studies have also reported that older patients have a faster disease progression than younger patients [18]. In addition, the majority of patients in our study were males, as found in most infectious diseases and related conditions, such as sepsis and septic shock, which predominantly involve the male sex and cause high mortality, as reported earlier [13, 15].
DM, chronic PD, HD, and renal insufficiency were the comorbidities present in the patients. Advanced age and underlying comorbidities were significant predictors in severely ill patients. Guan et al. reported that patients with severe disease were older than those with nonsevere disease, and the presence of any comorbidity was more common among patients with a severe disease than among those with a nonsevere disease [19]. There were 78 (49%) patients with chronic PD, which could be because smoking and chronic obstructive pulmonary disease (COPD) are more prevalent in this region [20, 21]. There is no proven relationship between smoking and SARS-CoV-2, whereas reports show the susceptibility of COPD patients and smokers to MERS-CoV [14]. Generally, older individuals have more health issues and comorbidities and are more susceptible to COVID-19; thus, they may develop a more severe disease than the younger population. Comorbidities, such as DM and hypertension, associated with old age, predispose them to immunological vulnerabilities [18]. Patients with severe disease are at a high risk of developing acute respiratory distress syndrome (ARDS) and being admitted to the ICU [17].
All patients had a severe form of the disease and presented with numerous clinical abnormalities. Disease severity in COVID-19 is associated with a cytokine storm due to higher concentrations of GCSF, IP10, MCP1, MIP1A, and TNF-α, which are associated with higher ICU admissions [6]. In our study, we did not test for these markers. Decreased total serum protein levels were also identified in all the deceased patients. Earlier, it was reported that liver aminotransferases and bilirubin were significantly elevated in severe COVID-19 patients requiring ICU admission [6]. Omrani-Nava et al. reported that there is also a higher risk of ICU admissions for patients with higher levels of ALT, AST, alkaline phosphatase (ALP), and bilirubin [22]. The incidence of death is also reported to be higher in COVID-19 patients with elevated creatinine levels [23]. This could be because SARS-CoV-2 targets the renal tubular epithelium by a mechanism similar to that seen in the lungs using the angiotensin-converting enzyme 2 (ACE2) protein receptors, which are expressed not only in type II alveolar, but also in other organs, such as the liver and kidneys [19].
SARS-CoV-2 appears to have a lower fatality rate when compared to SARS-CoV and MERS-CoV, and clinically, COVID-19 mimics SARS-CoV, with the dominant presentation being fever and cough [19]. More deaths were reported in older patient groups, especially those with one or more underlying diseases [15]. In this study, 93 (58%) patients died and 67 (42%) were discharged after recovery. The high mortality in our cohort may be due to the critical condition of patients, which in turn may be due to the rapidly progressive nature of the disease. It has been reported that viral clearance was observed in only a small proportion of patients admitted to the ICU. Uncontrolled viral replication in critically ill patients may also explain the persistent clinical and laboratory characteristics, lung damage, and disease progression [4]. Evidence also indicates that there may be an excessive host response that aids in disease progression [4].
Neutrophilic leukocytosis and lymphopenia were significant findings in the majority of our patients, similar to those in previous reports [13–16]. Neutrophilic leukocytosis may be due to secondary bacterial infection. Currently, little is known about the underlying lymphopenia caused by SARS-CoV-2 infection. A higher number of neutrophils and a lower number of lymphocytes were found in severely ill patients. The neutrophil-to-lymphocyte ratio is a well-known marker of infection and systemic inflammation [24].
MERS-CoV, but not SARS-CoV, can infect T cells from peripheral blood and human lymphoid organs and induce apoptosis of T cells [13]. Lymphocytes express ACE2, which is a receptor for SARS-CoV-2. The decrease in lymphocytes in peripheral circulation may be associated with immunosuppression and dysfunction [25].
There was a significant elevation in D-dimer levels along with CRP and ferritin in the majority of our patients, with a positive correlation. Many reports have described that severely ill patients have higher D-dimer, CRP, and ferritin values [1, 5, 6, 13–16]. When compared with patients with mild illness, higher D-dimer and fibrin degradation product (FDP) levels have been reported in severely ill COVID-19 patients [17]. Deranged coagulative mechanisms and an exaggerated inflammatory response have been reported in many studies. It has been reported that coagulation is activated in several infections, and it plays a role in immune function. However, excessive activation and an accelerated response with the consumption of coagulative factors may lead to disseminated intravascular coagulation and lead to an unfavorable outcome [17].
5. Conclusions
In this study, we investigated the clinical, demographic, and laboratory abnormalities in critical COVID-19 patients admitted to the ICU. Our findings were significant, including neutrophilia, lymphopenia, elevated D-dimer levels, increased CRP, AST, serum ferritin, creatinine, BUN, and decreased serum total protein. The most significant predictors of unfavorable outcomes were an advanced age with increased levels of AST, creatinine, BUN, bilirubin, and neutrophils and decreased eosinophils, monocytes, basophils, and lymphocytes. These predictors help predict the condition of severely ill patients and provide a picture of the degree of damage. There is a need to explore possible clinical mechanisms of this disease. Social distancing policies should be in place to slow down the rate of cases and prevent the overwhelming of healthcare resources. Accurate diagnosis and monitoring of disease progression from the early stages will help in reducing mortality and unfavorable outcomes.
Acknowledgments
This work was funded by the Deanship of Scientific Research at Umm Al-Qura University, Makkah, Saudi Arabia (Project No. 17-MED-1-01–0062).
Abbreviations
- ACE2:
Angiotensin-converting enzyme 2
- ALP:
Alkaline phosphatase
- ALT:
Alanine aminotransferase
- ARDS:
Acute respiratory distress syndrome
- AST:
Aspartate aminotransferase
- BUN:
Blood urea nitrogen
- CBCs:
Complete blood counts
- CI:
Confidence interval
- COPD:
Chronic obstructive pulmonary disease
- COVID-19:
Coronavirus disease
- CRP:
C-reactive protein
- ECMO:
Extracorporeal membrane oxygenation
- FDP:
Fibrin degradation product
- HR:
Hazard ratio
- ICU:
Intensive care unit
- MERS-CoV:
Middle Eastern respiratory syndrome-related coronavirus
- OR:
Odds ratio
- RT-PCR:
Real-time reverse transcription-polymerase chain reaction
- SARS-CoV-2:
Severe acute respiratory syndrome coronavirus 2
- WBC:
White blood cell.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon request.
Ethical Approval
This study was reviewed and approved by the Biomedical Ethics Committee of Umm Al-Qura University (Approval No. HAPO-02-K-012-2020-06-410).
Consent
Written informed consent was waived as there was no potential harm intended to the patients.
Disclosure
The funding organization had no role in the study design, data collection, analysis, interpretation, or manuscript writing.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Feng Y., Ling Y., Bai T., et al. COVID-19 with different severities: a multicenter study of clinical features. American Journal of Respiratory and Critical Care Medicine. 2020;201(11):1380–1388. doi: 10.1164/rccm.202002-0445OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deng Z., Zhang M., Zhu T., et al. Dynamic changes in peripheral blood lymphocyte subsets in adult patients with COVID-19. International Journal of Infectious Diseases. 2020;98:353–358. doi: 10.1016/j.ijid.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li X., Wang L., Yan S., et al. Clinical characteristics of 25 death cases with COVID-19: a retrospective review of medical records in a single medical center, Wuhan, China. International Journal of Infectious Diseases. 2020;94:128–132. doi: 10.1016/j.ijid.2020.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen J., Qi T., Liu L., et al. Clinical progression of patients with COVID-19 in Shanghai, China. Journal of Infection. 2020;80(5):e1–e6. doi: 10.1016/j.jinf.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang D., Hu B., Hu C., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. The Journal of the American Medical Association. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soraya G. V., Ulhaq Z. S. Crucial laboratory parameters in COVID-19 diagnosis and prognosis: an updated meta-analysis. Medicina Clínica (English Edition) 2020;155(4):143–151. doi: 10.1016/j.medcle.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang Y.-W., Schmitz J. E., Persing D. H., Stratton C. W. Laboratory diagnosis of COVID-19: current issues and challenges. Journal of Clinical Microbiology. 2020;58(6):e00512–20. doi: 10.1128/JCM.00512-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Skevaki C., Fragkou P. C., Cheng C., Xie M., Renz H. Laboratory characteristics of patients infected with the novel SARS-CoV-2 virus. Journal of Infection. 2020;81(2):205–212. doi: 10.1016/j.jinf.2020.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fan B. E., Chong V. C. L., Chan S. S. W., et al. Hematologic parameters in patients with COVID‐19 infection. American Journal of Hematology. 2020;95(6):E131–E134. doi: 10.1002/ajh.25774. [DOI] [PubMed] [Google Scholar]
- 11.Ponti G., Maccaferri M., Ruini C., Tomasi A., Ozben T. Biomarkers associated with COVID-19 disease progression. Critical Reviews in Clinical Laboratory Sciences. 2020;57(6):389–399. doi: 10.1080/10408363.2020.1770685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Velavan T. P., Meyer C. G. Mild versus severe COVID-19: laboratory markers. International Journal of Infectious Diseases. 2020;95:304–307. doi: 10.1016/j.ijid.2020.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen G., Wu D., Guo W., et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. Journal of Clinical Investigation. 2020;130(5):2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang J.-j., Dong X., Cao Y.-y., et al. Clinical characteristics of 140 patients infected with SARS‐CoV‐2 in Wuhan, China. Allergy. 2020;75(7):1730–1741. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 15.Du R.-H., Liu L.-M., Yin W., et al. Hospitalization and critical care of 109 decedents with COVID-19 pneumonia in Wuhan, China. Annals of the American Thoracic Society. 2020;17(7):839–846. doi: 10.1513/AnnalsATS.202003-225OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wan S., Xiang Y., Fang W., et al. Clinical features and treatment of COVID‐19 patients in northeast Chongqing. Journal of Medical Virology. 2020;92(7):797–806. doi: 10.1002/jmv.25783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han H., Yang L., Liu R., et al. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clinical Chemistry and Laboratory Medicine (CCLM) 2020;58(7):1116–1120. doi: 10.1515/cclm-2020-0188. [DOI] [PubMed] [Google Scholar]
- 18.Liu S., Luo H., Wang Y., et al. Clinical characteristics and risk factors of patients with severe COVID-19 in Jiangsu province, China: a retrospective multicentre cohort study. BMC Infectious Diseases. 2020;20(1):p. 584. doi: 10.1186/s12879-020-05314-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guan W.-j., Ni Z.-y., Hu Y., et al. Clinical characteristics of coronavirus disease 2019 in China. New England Journal of Medicine. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Al Ghobain M., Al-Hajjaj M. S., Wali S. O. Prevalence of chronic obstructive pulmonary disease among smokers attending primary healthcare clinics in Saudi Arabia. Annals of Saudi Medicine. 2011;31(2):129–133. doi: 10.5144/0256-4947.2011.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Al Ghobain M. The prevalence of chronic obstructive pulmonary disease in Saudi Arabia: where do we stand? Annals of Thoracic Medicine. 2011;6(4):185–186. doi: 10.4103/1817-1737.84770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Omrani-Nava V., Maleki I., Ahmadi A., et al. Evaluation of hepatic enzymes changes and association with prognosis in COVID-19 patients. Hepatitis Monthly. 2020;20(4):p. e103179. doi: 10.5812/hepatmon.103179. [DOI] [Google Scholar]
- 23.Cheng Y., Luo R., Wang K., et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney International. 2020;97(5):829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qin C., Zhou L., Hu Z., et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clinical Infectious Diseases. 2020;71(15):762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bonetti G., Manelli F., Patroni A., et al. Laboratory predictors of death from coronavirus disease 2019 (COVID-19) in the area of Valcamonica, Italy. Clinical Chemistry and Laboratory Medicine (CCLM) 2020;58(7):1100–1105. doi: 10.1515/cclm-2020-0459. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon request.


