Abstract
We aimed to assess the association between urban/rural residence and the risk of ischemic stroke in individuals with newly diagnosed atrial fibrillation (AF), and to quantify the role of oral anticoagulation (OAC) initiation in the variation in stroke risk between urban and rural residents with AF. Using 5% random samples of Medicare claims, we identified fee-for-service beneficiaries who were diagnosed with AF between January 2014 and December 2015. Beneficiaries were followed for 1 year since their AF diagnosis, and were categorized according to their initiation of OAC within the year. We used the Rural-Urban Continuum Codes to define urban (levels 1–3) and rural (levels 4–9) areas. We applied marginal structural models to examine to what extent the difference in stroke risk between rural and urban areas were attributable to the difference in OAC initiation. In the year of AF diagnosis, 52% of those residing in urban areas and 56% residing in rural areas initiated an OAC (p <.001). Urban residence, compared to rural residence, was associated with a 22% (hazard ratio [HR] and 95% CI: 1.22 [1.13, 1.31]) increased risk of stroke. The HR attributed to urban residence decreased to 1.14 (1.01, 1.30) after accounting for the mediating role of lack of OAC initiation. Lack of OAC initiation explained 34% of the increased stroke risk observed in urban areas. In conclusion, urban residents with newly diagnosed AF were less likely to initiate OAC than rural counterparts, which explained one third of the increased risk of stroke observed in urban areas.
Keywords: atrial fibrillation, oral anticoagulation, urban, rural
Introduction
Atrial fibrillation (AF) is the main cause of ischemic stroke in elderly individuals.1 Oral anticoagulation (OAC) is indicated in stroke prevention in AF patients with a moderate or high risk of stroke.2 A large geographic variation in OAC use across the US exists, being lower in the Southeastern states, where stroke rates are higher.3 However, little is known about the potential urban/rural difference in stroke risk among patients with AF, and whether the regional variation in OAC use could explain the variability in stroke risk. We thus aimed to assess the association between urban/rural residence and the risk of ischemic stroke in elder individuals with newly diagnosed AF. We also quantified the extent to which the difference in OAC initiation between urban and rural residents may explain the difference in stroke risk. Since rural residents often face barriers to accessing healthcare,4–6 we hypothesized that AF patients residing in urban areas, versus rural areas, had a higher rate of OAC use and then a lower risk of stroke.
Methods
Claims data were obtained from 5% random samples of Medicare Part D beneficiaries from the Centers for Medicare and Medicaid Services (CMS). Using the CMS Chronic Condition Data Warehouse indicator of AF, we identified beneficiaries who were first diagnosed with AF between January 1, 2014 and December 31, 2015 (n = 72,306) (Figure 1). The index date was defined as the date of the first AF diagnosis. Individuals were excluded if they had a diagnosis of valvular disease, died within seven days of first AF diagnosis, were not continuously enrolled in Medicare Part D over the follow up period, or if the urban status of their county was unknown. The final sample included 42,874 beneficiaries and were followed from the index date to the end of the first year, or until the occurrence of ischemic stroke, or death.
Figure 1.
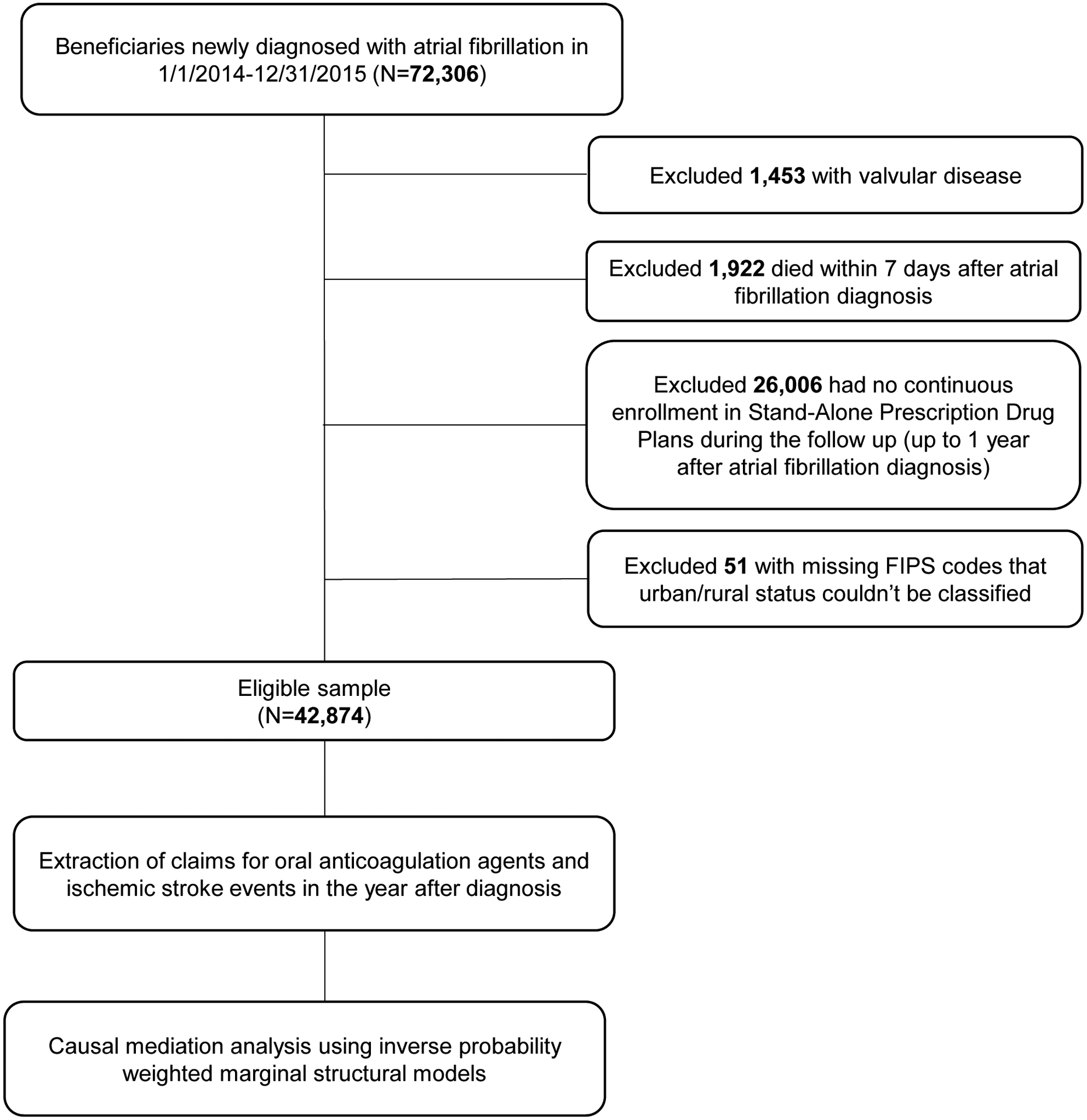
Overview of the study sample selection and analysis
The outcome was time to the first ischemic stroke event, which was defined as having one inpatient medical claim with primary or secondary International Classification of Diseases, Ninth Revision (ICD-9) diagnosis code 433.x1, 434.x1, or 436, or ICD-10 diagnosis code I63.x if after October 1st, 2015.7
The exposure of interest was urban versus rural residence, and the proposed mediator was OAC initiation in the year of AF diagnosis. We classified participants as living in an urban or rural area using the 9-level Untied State Department of Agriculture (USDA) Rural-Urban Continuum Codes.8 Consistent with the classification by USDA (Supplement, Table S1)8, in our primary analyses we dichotomized as urban patients living in levels 1–3, and as rural patients living in levels 4–9.8 Secondary analyses were performed where patients were categorized into 1 of 9 continuum levels. To define OAC initiation, we collected all prescriptions filled for OAC, including warfarin and approved direct oral anticoagulants (DOACs), i.e., apixaban, dabigatran, and rivaroxaban. We classified participants into the OAC initiation group if they had at least one prescription for OAC between the index date and the first ischemic stroke event, death or end of the study, or into the non-initiation group otherwise.
We collected information on demographics and clinical characteristics which were measured on or prior and proximal to the index date. Demographic characteristics included age, sex, race, and residence in four geographic regions, eligibility for Medicaid and for low income subsidy. Clinical characteristics included CHA2DS2-VASc score, HAS-BLED score, Alzheimer’s disease and related dementias, number of other CMS priority conditions (calculated as the sum of history of cataract, chronic obstructive pulmonary disease, depression, glaucoma, knee or hip replacement, osteoporosis, and rheumatoid arthritis or osteoarthritis), CMS priority types of cancer (i.e., breast cancer, colorectal cancer, endometrial cancer, and lung cancer). The CHA2DS2-VASc score was calculated using age, sex, history of stroke/transient ischemic attack, vascular disease, heart failure, hypertension, and diabetes.9 Because international normalized ratio (INR) levels are not available in Medicare claims data, the HAS-BLED score was calculated using all factors included in the risk score except labile INR.10 In defining each of the factors included in CHAD2S2-VASc and HAS-BLED scores, we used the CMS Chronic Condition Data Warehouse definitions when available, and 12 months of claims data before the AF diagnosis for remaining covariates (Supplement, Table S2).
We constructed marginal structural models with inverse probability weighting to estimate: (1) the association between urban versus rural residence and the risk of stroke; (2) the role of OAC initiation in this association. To do so, we first generated stabilized weights from modeling urban/rural residence and OAC initiation using logistic regression. The weights were then incorporated into two Cox proportional hazards regression models: (i) modeling the total association of urban versus rural residence with stroke; (ii) modeling the controlled direct association of urban versus rural residence with stroke by isolating the mediation role of OAC initiation (Figure 2). Standard errors were obtained using robust variance estimators, which accounted for the pseudo-clustering induced by the inverse probability weights.11,12 The difference between the total association and controlled direct association represented the effect of urban versus rural residence on stroke risk that was attributed to OAC initiation. Finally, the mediation proportion for lack of OAC initiation in the urban/rural residence-stroke association was calculated as follows: (Total effect − Controlled Direct Effect) / Total effect × 100%.
Figure 2.
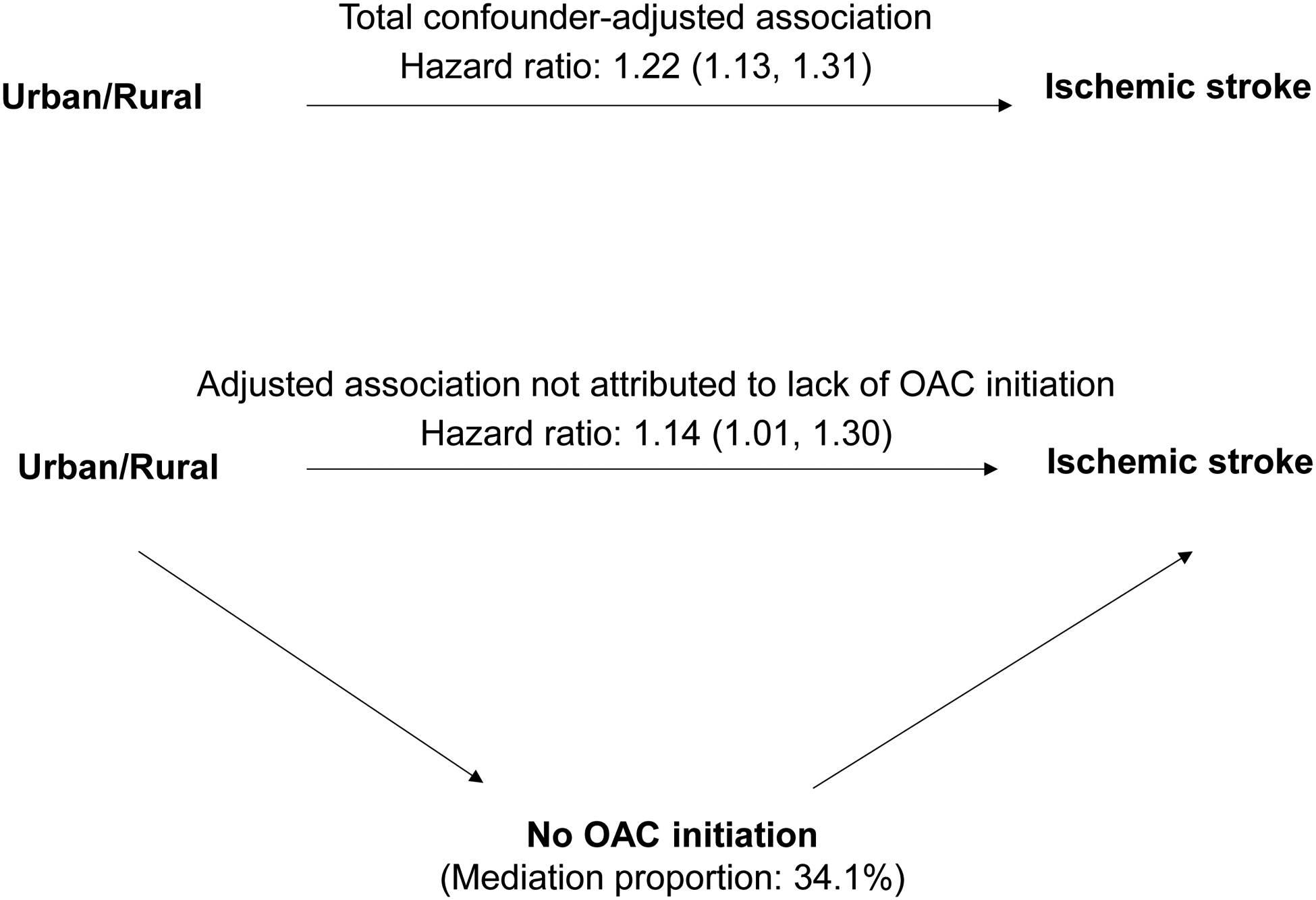
Diagram illustrating relationships between the urban/rural residence, oral anticoagulation initiation (OAC), and ischemic stroke risk in the year of atrial fibrillation diagnosis
We adjusted for the following baseline covariates to control for potential confounding factors when examining the association between urban/rural residence and stroke: age, sex, race, geographic region, Medicaid eligibility, low-income subsidy eligibility, CHA2DS2-VASc score, HAS-BLED score, Alzheimer’s disease and related dementias, number of other CMS priority conditions, and CMS priority types of cancer. The same set of covariates were adjusted when examining the association between OAC initiation and stroke.
Using the similar analytical strategy, in the secondary analyses we quantified the association between the 9-level Rural-Urban Continuum Codes and the stroke risk, and the mediation role of OAC initiation. In these analyses, we treated the 9 levels of the Rural-Urban Continuum Codes as a continuous variable and estimated the stroke risk for per level change towards metropolitan areas. In sensitivity analyses, we also applied the same methodology but adjusted for individual risk factors included in the calculation of CHA2DS2-VASc and HAS-BLED scores rather than for these two scores. Given that stroke history is an important risk factor of subsequent stroke that could potentially modify the key associations, we conducted similar mediation analysis stratified by the history of stroke. As exploratory analyses, we applied multiple logistic regressions to examine factors associated with OAC initiation in urban and rural residents, respectively.
A two-sided p <0.05 was considered significant. Analyses were performed using SAS v9.4 (SAS Institute, Cary, NC) and R version 3.5.3 (R Core Team, Vienna, Austria).
Results
Of the 42,874 participants, 78% were classified as residing in urban areas; 53% initiated an OAC drug within 1 year of their AF diagnosis. The mean age of the study cohort was 76 years. Over half (56%) were females and 87% were white. Compared to rural residents, urban residents were more likely to be non-white and live in the Northeast and West regions, and had slightly worse clinical risk profiles on average (Table 1). Compared to OAC initiators, non-initiators were older, more often non-white, more likely to receive Medicaid and low-income subsidy, and had substantially worse clinical risk profiles (Supplement, Table S3).
Table 1.
Study Participants Characteristics at the time of Atrial Fibrillation Diagnosis by Urban and Rural Residence.
| Variable | Rural (n=9,336, 21.8%) | Urban (n=33,538, 78.2%) | p value |
|---|---|---|---|
| Age (years) | 0.005 | ||
| <65 | 805 (8.6%) | 2582 (7.7%) | |
| 65–74 | 3420 (36.6%) | 12157 (36.2%) | |
| >=75 | 5111 (54.7%) | 18799 (56.1%) | |
| Women | 5237 (56.1%) | 18925 (56.4%) | 0.565 |
| White | 8634 (92.5%) | 28569 (85.2%) | <0.001 |
| Black | 441 (4.7%) | 2858 (8.5%) | |
| Hispanic | 39 (0.4%) | 627 (1.9%) | |
| Other | 222 (2.4%) | 1484 (4.4%) | |
| Eligibility for Medicaid | 2682 (28.7%) | 9130 (27.2%) | 0.004 |
| Eligibility for Low-income Subsidy | 3996 (42.8%) | 13755 (41.0%) | 0.002 |
| Region | <0.001 | ||
| Midwest | 3215 (34.4%) | 7231 (21.6%) | |
| Northeast | 978 (10.5%) | 9260 (27.6%) | |
| South | 4497(45.0%) | 11900 (35.5%) | |
| West | 946 (10.1%) | 5116 (15.3%) | |
| CHA2DS2-VASc score | <0.001 | ||
| 0–2 | 1316 (14.1%) | 4705 (14.0%) | |
| 3–4 | 3464 (37.1%) | 11541 (34.4%) | |
| ≥5 | 4556 (48.8%) | 17292 (51.6%) | |
| HAS-BLED scorea | <0.001 | ||
| 0–1 | 869 (9.3%) | 3124 (9.3%) | |
| 2–3 | 6000 (64.3%) | 20360 (60.7%) | |
| ≥4 | 2467 (26.4%) | 10054 (30.0%) | |
| Vascular diseaseb,c | 11,320 (30.0%) | 2436 (47.2%) | <0.001 |
| Alzheimer’s Disease or Dementiac | 1518 (16.3%) | 6816 (20.3%) | <0.001 |
| Chronic Kidney Diseasec | 3502 (37.5%) | 13275 (39.6%) | <0.001 |
| Heart Failurec | 4468 (47.9%) | 15772 (47.0%) | 0.155 |
| Diabetes Mellitusc | 4000 (42.8%) | 15164 (45.2%) | <0.001 |
| Hypertensionc | 8330 (89.2%) | 29700 (88.6%) | 0.071 |
| CMS priority Cancerd | 1159 (12.4%) | 4261 (12.7%) | 0.455 |
| Other CMS comorbiditiese | |||
| 0–1 | 2555 (27.4%) | 9851 (29.4%) | <0.001 |
| 2–3 | 4210 (45.1%) | 14314 (42.7%) | |
| ≥4 | 2571 (27.5%) | 9373 (27.9%) | |
| History of ischemic stroke | 887 (9.5%) | 3929 (11.7%) | <0.001 |
| Recent bleedingf | 1540 (16.5%) | 6870 (20.5%) | <0.001 |
| Recent Antiplatelet useg | 1308 (14.0%) | 4632 (13.8%) | 0.622 |
| Recent NSAID useh | 1303 (14.0%) | 4275 (12.7%) | 0.002 |
Abbreviations: CMS: Centers for Medicare and Medicaid Services; NSAIDs: Non-Steroidal Anti-inflammatory Drug.
Because Medicare claims data does not contain information on International Normalized Ratio (INR) levels, we calculated the HAS-BLED score as the sum of all factors except labile INR.
Vascular disease was identified by the diagnosis of prior myocardial infarction or peripheral arterial disease.
Prior myocardial infarction, Alzheimer’s Disease or related dementia, chronic kidney disease, heart failure, diabetes, hypertension, and a history of stroke or TIA were defined using the respective CMS Chronic Condition Warehouse definitions of each of these conditions.
CMS priority types of cancer include breast cancer, colorectal cancer, endometrial cancer, and lung cancer.
The number of other CMS priority comorbidities was calculated as the sum of history of cataract, chronic obstructive pulmonary disease, depression, glaucoma, knee or hip replacement, osteoporosis, and rheumatoid arthritis or osteoarthritis.
Recent bleeding was defined as having a claim with ICD-9 codes for bleeding events in the year before index date.
Recent antiplatelet use was defined as filling a prescription for aspirin, clopidogrel, prasugrel, dipyridamol, ticlopidine or ticagrelor in the six months before index date.
Recent NSAID use was defined as filling a prescription for diclofenac, ibuprofen, naproxen, ketoprofen, fenoprofen, flurbiprofen, piroxicam, meloxicam, mefenamic acid or indomethacin in the six months before index date
Compared to rural residents, urban residents were less likely to initiate an OAC (urban vs rural: 52% vs 56%, p <.001) in the first year of their AF diagnosis, and had a greater risk of incident ischemic stroke (cumulative incidence of stroke in urban vs rural areas: 14% vs 11%, p <.001). In analysis using 9-level Rural-Urban Continuum Codes, OAC initiation rate gradually increased (Figure 3) and stroke incidence gradually decreased (Table 2) with higher degree of rurality, both p trends <.001. More people initiated with warfarin rather than DOACs in rural areas, while more people initiated with DOACs rather than warfarin in urban areas (Figure 3).
Figure 3.
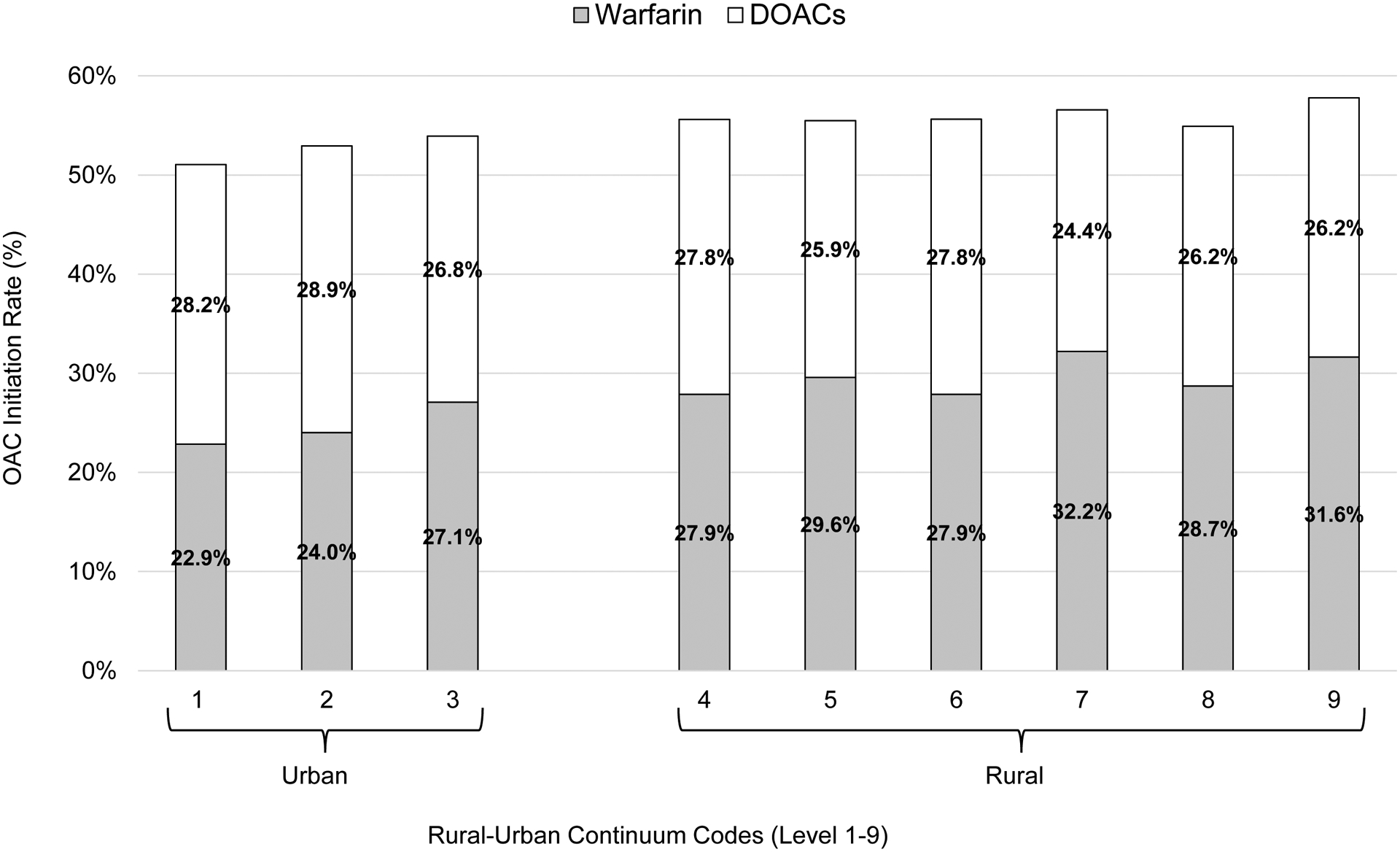
Oral anticoagulation initiation rate by Rural-Urban Continuum Codes in the year of atrial fibrillation diagnosis, stratified by warfarin and direct oral anticoagulants (DOACs)
Table 2.
Ischemic stroke risk in the year of atrial fibrillation diagnosis by the 9-level Rural-Urban Continuum Codes
| Rural-Urban | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| Continuum Codesa | |||||||||
| Total number of arterial fibrillation participants | 19,876 | 8,911 | 4,751 | 2,566 | 903 | 2,896 | 1,810 | 519 | 642 |
| Number of stroke events | 2,720 | 1,001 | 513 | 286 | 79 | 273 | 179 | 52 | 61 |
| Stroke rate | 13.68% | 11.23% | 10.80% | 11.15% | 8.75% | 9.43% | 9.89% | 10.02% | 9.50% |
Levels 1–3 are categorized as urban areas and levels 4–9 are categorized as rural areas
Urban residence, compared to rural residence, was associated with a 22% increased risk of ischemic stroke (Figure 2). When the mediating role of lack of OAC initiation was isolated, the increase in risk decreased to 14%. The lack of OAC initiation explained one-third of the increased stroke risk observed in urban areas. When we adjusted for individual risk factors instead of CHA2DS2-VASc and HAS-BLED scores, the results were similar (Supplement, Table S4). In the analysis using 9-level Rural-Urban Continuum Codes, each level of increase in the degree of urbanicity was associated with a 3% increased risk of ischemic stroke (Supplement, Table S5). In both subgroups with and without a history of stroke (Supplement, Table S6), urban residence, compared to rural residence, was associated with a significantly increased risk of stroke. The lack of OAC initiation partially explained the excess stroke risk observed in urban areas in both subgroups. In the exploratory analyses of examining factors associated with OAC initiation, only in urban areas, but not in rural areas, black race were associated with lower odds of initiating an OAC (Supplement, Table S7).
Discussion
Using a nationally representative sample of Medicare beneficiaries with incident AF, we compared the risk of stroke and the initiation of OAC therapy between urban and rural patients. Contrary to our hypothesis, urban residents were less likely to initiate an OAC and had a higher risk of ischemic stroke, compared to rural residents. The lower likelihood of OAC initiation in urban areas explained one-third of the higher risk of stroke observed among urban patients.
Prior studies reported a higher risk of stroke in rural areas than in urban areas in the general population.13,14 Our study, focusing on incident AF patients, suggested a significantly increased risk of ischemic stroke in urban areas compared to that seen in rural areas. The difference between our results and those of prior studies may be explained by different characteristics of the study cohorts. While previous results were mostly derived from general population13,14, our data focuses on elder individuals (≥65 years of age) with newly diagnosed non-valvular AF. The different risk factors for AF related stroke versus non-AF related stroke may partially explain the different distributions of stroke risk.15 A major contributor of AF related stroke is insufficient antithrombotic therapy,2 while vascular risk factors (i.e., hypertension and dyslipidemia), which may have higher prevalence in rural areas, play most important roles in the development of non-AF stroke. It is noteworthy that, in our analysis, the increased stroke risk in urban areas was consistent for the subgroups with and without a history of stroke, even though the magnitude of the relative risk was attenuated in the subgroup with stroke.
Our study also explored urban/rural variability in OAC initiation among patients with newly diagnosed AF. Although prior work has reported that rural residents face barriers to healthcare access in general,4–6 we found a lower rate of OAC initiation in urban areas than that seen in rural areas. Even more surprisingly, the likelihood of OAC initiation steadily decreased with the degree of urbanicity. Older studies showed conflicting data on regional difference of OAC use among American AF population.16–18 Using 1993–1996 data, Gage et al. found that patients discharged from a rural hospital were 1.7 times less likely to be prescribed with warfarin than those discharged from an urban hospital.16 However, a later report using 2006–2008 data observed no significant difference in warfarin use between urban and rural patients hospitalized for ischemic stroke or transient ischemic attack.17 All of these studies, however, leveraged data that preceded the approval of DOACs, and did not constrain analyses to an insured population, which may partially explain our apparently conflicting findings.
We observed that black race was associated with lower OAC initiation among urban but not among rural residents. Receipt of low-income subsidy, which serves as a proxy for socioeconomic status, was associated with 63% lower odds of OAC initiation in urban areas, and 53% in rural areas. The proportion of racial/ethnic minorities and of low-income beneficiaries is greater and has grown faster in urban than rural areas, which might partially explain the less likelihood of OAC initiation in urban areas.19 In turn, stroke rates are two times higher for Black patients than White patients, which may explain the higher stroke risk among urban AF patients.20 Future studies are needed to examine to what extent the differences in OAC use and stroke risk between urban and rural residents are attributable to racial composition.
Difference in OAC initiation between urban and rural residents are important because, as our study demonstrates, they have a significant impact on patient outcomes. Our data suggested that the lower OAC initiation observed in urban areas explains as much as one third of the excess stroke risk in these areas. Collectively, these findings reinforce that the underutilization of OAC for stroke prevention in AF remains a serious clinical challenge in real world clinical settings and will be useful in the orientation of targeted intervention programs to mitigate OAC underutilization and reduce regional disparities in stroke risk.
Several limitations of the present study should be noted. First, because we used claims data, we were not able to control for important clinical variables that impact stroke risk, such as blood pressure, INR measures, and the severity of comorbid conditions. Second, our study only included Medicare beneficiaries with continuous enrollment in Stand Alone Prescription Drug Plans. Thus, our results are not generalizable to AF patients younger than 65 years or those who obtain prescription drug coverage under Medicare Advantage, who tend to be younger and healthier than fee-for-service beneficiaries. Third, claims data only captures drugs purchased through insurance, so we were not able to observe warfarin purchased through $4 generic programs or over-the-counter aspirin use. Finally, because our study sample is mostly representative of elder individuals aged 65 years or above, our results may not be generalizable to younger populations.
In conclusion, urban residents newly diagnosed with AF were less likely to receive OAC than rural counterparts, which explained one third of the increased risk of ischemic stroke observed in urban areas.
Supplementary Material
Funding:
Research reported in this study was supported by the National Heart, Lung and Blood Institute (grant number K01HL142847).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures/Conflict of interests: Hernandez reports scientific advisory board fees from Pfizer, outside of the submitted work.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Ferro JM. Atrial fibrillation and cardioembolic stroke. Minerva Cardioangiol 2004;52:111–124. [PubMed] [Google Scholar]
- 2.Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med 2007;146:857. [DOI] [PubMed] [Google Scholar]
- 3.Hernandez I, Saba S, Zhang Y. Geographic variation in the use of oral anticoagulation therapy in stroke prevention in atrial fibrillation. Stroke 2017;48:2289–2291. [DOI] [PubMed] [Google Scholar]
- 4.Supina AL, Guirguis LM, Majumdar SR, Lewanczuk RZ, Lee TK, Toth EL, Johnson JA. Treatment gaps for hypertension management in rural Canadian patients with type 2 diabetes mellitus. Clin Ther 2004;26:598–606. [DOI] [PubMed] [Google Scholar]
- 5.Loftus J, Allen EM, Call KT, Everson-Rose SA. Rural-urban differences in access to preventive health care among publicly insured Minnesotans. J Rural Health 2018;34 Suppl 1:s48–s55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weeks WB, Kazis LE, Shen Y, Cong Z, Ren XS, Miller D, Lee A, Perlin JB. Differences in health-related quality of life in rural and urban veterans. Am J Public Health 2004;94:1762–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumamaru H, Judd SE, Curtis JR, Ramachandran R, Hardy NC, Rhodes JD, Safford MM, Kissela BM, Howard G, Jalbert JJ, Brott TG, Setoguchi S. Validity of claims-based stroke algorithms in contemporary Medicare data: reasons for geographic and racial differences in stroke (REGARDS) study linked with medicare claims. Circ Cardiovasc Qual Outcomes 2014;7:611–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.United States Department of Algriculture Economic Research Service. Rural-Urban Continuum Codes. 2013. Available at: https://www.ers.usda.gov/data-products/rural-urban-continuum-codes/. Accessed August 24, 2019.
- 9.Lip GYH, Nieuwlaat R, Pisters R, Lane DA, Crijns HJGM. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach. Chest 2010;137:263–272. [DOI] [PubMed] [Google Scholar]
- 10.Pisters R, Lane DA, Nieuwlaat R, Vos CB de, Crijns HJGM, Lip GYH. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest 2010;138:1093–1100. [DOI] [PubMed] [Google Scholar]
- 11.VanderWeele TJ. Marginal structural models for the estimation of direct and indirect effects. Epidemiology 2009;20:18–26. [DOI] [PubMed] [Google Scholar]
- 12.White H A heteroskedasticity-consistent covariance matrix estimator and a direct test for heteroskedasticity. Econometrica 1980;48:817. [Google Scholar]
- 13.Zeng D, You W, Mills B, Alwang J, Royster M, Anson-Dwamena R. A closer look at the rural-urban health disparities: Insights from four major diseases in the Commonwealth of Virginia. Soc Sci Med 2015;140:62–68. [DOI] [PubMed] [Google Scholar]
- 14.Howard G, Kleindorfer DO, Cushman M, Long DL, Jasne A, Judd SE, Higginbotham JC, Howard VJ. Contributors to the excess stroke mortality in rural areas in the United States. Stroke 2017;48:1773–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kamel H, Okin PM, Elkind MSV, Iadecola C. Atrial fibrillation and mechanisms of stroke. Stroke 2016;47:895–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gage BF, Boechler M, Doggette AL, Fortune G, Flaker GC, Rich MW, Radford MJ. Adverse outcomes and predictors of underuse of antithrombotic therapy in Medicare beneficiaries with chronic atrial fibrillation. Stroke 2000;31:822–827. [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez D, Cox M, Zimmer LO, Olson DM, Goldstein LB, Drew L, Peterson ED, Bushnell CD. Similar secondary stroke prevention and medication persistence rates among rural and urban patients. J Rural Heal 2011;27:401–408. [DOI] [PubMed] [Google Scholar]
- 18.Hsu JC, Maddox TM, Kennedy KF, Katz DF, Marzec LN, Lubitz SA, Gehi AK, Turakhia MP, Marcus GM. Oral anticoagulant therapy prescription in patients with atrial fibrillation across the spectrum of stroke risk: insights from the NCDR PINNACLE registry. JAMA Cardiol 2016;1:55–62. [DOI] [PubMed] [Google Scholar]
- 19.Pew Research Center. Demographic and economic trends in urban, suburban and rural communities. Available at: https://www.pewsocialtrends.org/2018/05/22/demographic-and-economic-trends-in-urban-suburban-and-rural-communities/. Accessed May 18, 2020. [Google Scholar]
- 20.Magnani JW, Norby FL, Agarwal SK, Soliman EZ, Chen LY, Loehr LR, Alonso A. Racial differences in atrial fibrillation-related cardiovascular disease and mortality the Atherosclerosis Risk in Communities (ARIC) study. JAMA Cardiol 2016;1:433–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


