Abstract
Attenuation of the secondary injury of spinal cord injury (SCI) can suppress the spread of spinal cord tissue damage, possibly resulting in spinal cord sparing that can improve functional prognoses. Granulocyte colony-stimulating factor (G-CSF) is a haematological cytokine commonly used to treat neutropenia. Previous reports have shown that G-CSF promotes functional recovery in rodent models of SCI. Based on preclinical results, we conducted early phase clinical trials, showing safety/feasibility and suggestive efficacy. These lines of evidence demonstrate that G-CSF might have therapeutic benefits for acute SCI in humans. To confirm this efficacy and to obtain strong evidence for pharmaceutical approval of G-CSF therapy for SCI, we conducted a phase 3 clinical trial designed as a prospective, randomized, double-blinded and placebo-controlled comparative trial. The current trial included cervical SCI [severity of American Spinal Injury Association (ASIA) Impairment Scale (AIS) B or C] within 48 h after injury. Patients are randomly assigned to G-CSF and placebo groups. The G-CSF group was administered 400 μg/m2/day × 5 days of G-CSF in normal saline via intravenous infusion for five consecutive days. The placebo group was similarly administered a placebo. Allocation was concealed between blinded evaluators of efficacy/safety and those for laboratory data, as G-CSF markedly increases white blood cell counts that can reveal patient treatment. Efficacy and safety were evaluated by blinded observer. Our primary end point was changes in ASIA motor scores from baseline to 3 months after drug administration. Each group includes 44 patients (88 total patients). Our protocol was approved by the Pharmaceuticals and Medical Device Agency in Japan and this trial is funded by the Center for Clinical Trials, Japan Medical Association. There was no significant difference in the primary end point between the G-CSF and the placebo control groups. In contrast, one of the secondary end points showed that the ASIA motor score 6 months (P = 0.062) and 1 year (P = 0.073) after drug administration tend to be higher in the G-CSF group compared with the placebo control group. The present trial failed to show a significant effect of G-CSF in primary end point.
Keywords: spinal cord injury, neuroprotection, G-CSF, clinical trial
Koda et al. present the results of a randomized phase 3 trial comparing granulocyte colony-stimulating factor versus placebo in patients with acute spinal cord injury. While the primary endpoint was not met, a sub-analysis revealed a trend towards superior efficacy of G-CSF versus placebo in an elderly population.
Introduction
Spinal cord injury (SCI) is a devastating injury caused by trauma including fracture, dislocation and torsion of the spinal column. Its annual incidence is ∼40 per million in Japan. Recent changes in the ageing demographics of Japanese society have led to increased numbers of aged patients that suffer from SCI, resulting in additional medical and social burdens (Katoh et al., 2014).
The pathophysiology of SCI consists of two sequential processes including primary and secondary injuries. The primary injury is defined as the mechanical injury caused by the trauma itself. Thus, the primary injury cannot be avoided except for advising precaution. The secondary injury is characterized by the biological reactions triggered by the primary injury, and includes haemorrhage, electrolyte shifts, excitotoxicity, ischaemia, inflammatory cytokine upregulation, infiltration of inflammatory cells, and disruption of axons and the myelin sheath, among others. The secondary injury is the main target for treatment and has been the core subject of extensive research for many years. Attenuation of the secondary injury can suppress the spread of spinal cord tissue damage, possibly resulting in spinal cord sparing which can improve functional prognoses (Yang et al., 2020).
Methylprednisolone sodium succinate (MPSS) is the only pharmaceutically approved SCI drug in Japan, its mechanism of action being to prevent secondary injury via anti-inflammatory effects and stabilization of the cell membrane (Bracken et al., 1990). However, recent reports showed that most orthopaedic surgeons and neurosurgeons do not use MPSS for SCI patients because MPSS has been shown to have only a modest effect on SCI, and also has adverse effects including infection and gastric ulcer (Hurlbert et al., 2015). Therefore, a novel drug that can attenuate secondary injury after SCI without substantial adverse events is greatly needed. Extensive laboratory and clinical studies have been conducted to establish the potential of novel neuroprotective agents for the acute phase of SCI.
Granulocyte colony-stimulating factor (G-CSF) is a haematological cytokine that induces development, survival and proliferation of granulocyte lineage cells, and is commonly used to treat neutropenia (Nicola et al., 1983). G-CSF also has non-haematopoietic functions and has been suggested as a treatment for neuronal injury (Roberts, 2005). Previous reports have shown that G-CSF promotes functional recovery in rodent models of SCI via various mechanisms of action including mobilization of bone marrow cells into the injured spinal cord, suppression of neuron and oligodendrocyte apoptosis, suppression of inflammatory cytokines and promotion of angiogenesis (Koda et al., 2007; Nishio et al., 2007; Kawabe et al., 2011; Kadota et al., 2012). Based on these results, we conducted a preliminary phase I/IIa clinical trial and confirmed the safety and feasibility of G-CSF as neuroprotective therapy in patients with acute SCI (Takahashi et al., 2012).
To validate the efficacy of G-CSF for SCI, a multicentre, prospective, non-randomized, non-blinded, comparative control study (phase 2 clinical trial) was conducted (Inada et al., 2014). Patients with acute cervical SCI (within 48 h post-injury) were enrolled in the trial and assigned to either the G-CSF group (G-CSF was administered at 10 μg/kg/day × 5 days via intravenous infusion) or the control group (without G-CSF administration) at each treatment facility. Nineteen patients in the G-CSF group and 26 patients in the control group were observed for 3 months after administration. Acquired points on the American Spinal Injury Association (ASIA) motor score (0 to 100 points) was compared between groups. Acquired ASIA motor scores 3 months after the injury from baseline values were 26.1 ± 18.9 in the G-CSF group and 12.2 ± 14.7 in the control group showing a significant improvement of motor paralysis in the G-CSF group (P < 0.01). In addition, in cases that could be followed for 1 year, a significant improvement in the ASIA motor score was still observed in the G-CSF group (Inada et al., 2014).
These lines of evidence demonstrate that G-CSF might have therapeutic benefits for acute SCI in humans. To confirm this efficacy and to obtain strong evidence for pharmaceutical approval of G-CSF therapy for SCI, we conducted a phase 3 clinical trial designed as a prospective, randomized, double-blinded and placebo-controlled comparative trial.
Materials and methods
The study design of the current trial was a prospective, multicentre, randomized, double-blinded, placebo-controlled comparative study (Koda et al, 2018). The registration number was UMIN000018752 (https://upload.umin.ac.jp/cgi-open-bin/ctr/ctr.cgi?function=brows&action=brows&type=summary&recptno=R000021694&language=J).
The current trial included cervical SCI [severity of ASIA Impairment Scale (AIS) B/C] within 48 h after injury. Patients were reassessed for neurological status at 48 ± 4 h post-injury, and those with a paralysis severity of AIS B or C at that time point were enrolled. The precise inclusion and exclusion criteria are shown in Box 1.
Box 1.
Inclusion and exclusion criteria
Inclusion criteria
Patients with cervical SCI (severity of AIS B/C) within 48 h of injury.
Patients reassessed for neurological status at 48 h after injury, and those whose palsy is AIS B/C will be enrolled.
Patients with neurological level of injury between C4 and C7.
Patients with age of 16 to 85 years.
Patients who agree to participate in the current trial and from whom informed consent was obtained orally and in writing.
Patients who can be followed up for 12 months after SCI.
Exclusion criteria
Patients with neurological recovery to AIS D at neurological reassessment 48 h after SCI, because only AIS B/C patients will be included to standardize the severity of paresis in order to stratify the patients at the initiation of drug administration.
Allergy to G-CSF.
Haematological malignancy
Within 6 months after invasive coronary intervention
Splenomegaly
Pregnancy
Consciousness impairment
Neurological disorders that can affect neurological evaluation in the present trial
Fracture of extremities that can affect the neurological evaluation
Massive dose administration of MPSS.
Exclusion criteria 2–6 are set for safety, criteria 1, and 7–9 are set to maintain homogeneity of the patient population enrolled, criteria 7–9 are set for maintenance of accuracy of functional assessment, criteria 7 is set to obtain patients' own informed consent upon participation to the trial and criteria 10 is set to omit the possible interference of MPSS on outcome assessment.
MPSS = methylprednisolone sodium succinate.
Patients were randomly assigned to the G-CSF or placebo control groups by a central registration system with dynamic randomization based on age (16–64 years of age, or 65–84 years of age) and severity of paralysis (AIS B or C) in a 1:1 ratio.
The G-CSF group was administered 400 μg/m2/day of G-CSF in normal saline via intravenous infusion for five consecutive days. The placebo control group was similarly administered a placebo, which consisted of the same solvent as the G-CSF solution. The dosage was determined based on the results from the early-phase clinical trials (Takahashi et al., 2012; Inada et al., 2014). In the current trial, the dosage of G-CSF is written as 400 μg/m2/day (=10 μg/kg/day) according to the instructions of the Japanese Pharmaceutical and Medical Device Agency (PMDA) for consolidation of product labelling.
Allocation was concealed between the blinded evaluators of efficacy/safety and those for laboratory data, as G-CSF markedly increases white blood cell counts that can reveal patient treatment. Efficacy and safety were thus evaluated by blinded observers.
Our primary end point was acquired ASIA motor points, which was calculated as change in ASIA motor score [using international standards for neurological classification of spinal cord injury (ISNSCI)] from baseline to 3 months after drug administration. Secondary end points were as follows: (i) acquired ASIA motor points, which was calculated as change in ASIA motor scores at 6 months and 12 months after G-CSF administration compared with baseline; (ii) acquired ASIA sensory points, which was calculated as change in ASIA pinprick score at 3, 6, and 12 months after G-CSF administration compared with baseline; (iii) percentage of patients whose AIS improved by 1 grade or more at 3 and 6 months post-administration compared with grade prior to administration of G-CSF; (iv) change in Spinal Cord Independence Measure (SCIM) scores at 3, 6, and 12 months after G-CSF administration compared with pretreatment; and (v) EQ-5D efficacy scores at 3, 6, and 12 months after G-CSF administration.
Each group included 44 patients (88 total patients). The necessary number of cases was calculated by a biostatistician based on data from the early-phase clinical trials. Our protocol was approved by the PMDA and this trial was funded by the Center for Clinical Trials, Japan Medical Association.
Statistical analyses
The background patient characteristics were compared using a chi-square test for categorical variables, and a t-test or Wilcoxon rank sum test for continuous variables. Analyses of the primary and secondary end points were performed as intention-to-treat analyses in the full analysis set (FAS), which includes all patients who were administered at least one course of treatment during the study, had no serious violation of the study protocol and had data collected after commencement of treatment. Additional analyses in the per protocol set (PPS), which included all patients who were administered the full drug regimen, were similarly performed to obtain complementary data. Missing data, including loss to follow-up and missing measurements were supplemented with the last observation carried forward method. The same method was applied to the secondary end points, change in ASIA motor/sensory scores, SCIM, and EQ-5D. A chi-squared test was applied to the ratio of responders based on AIS and the improvement in neurological level of injury. The frequency of adverse events was compared using a Fisher’s exact test. A P-value < 0.05 was considered statistically significant. All statistical analyses were performed using SAS software (version 9.4; SAS Institute, Cary, NC, USA).
Subanalyses
The acquired ASIA motor points, which was calculated as changes in ASIA motor score 3, 6 and 12 months after drug administration were compared between the G-CSF and control groups among the age subgroups, younger subgroup (16–64 years of age) and older subgroup (65–84 years of age), and paralysis severity subgroups, AIS B and AIS C subgroups. These subanalyses were performed for the PPS; the missing data including loss to follow-up and missing measurements were supplemented with the last observation carried forward method.
Adverse events
For the safety evaluation, adverse events, which refer to any symptom or disease signs in a participant after informed consent with or without causal relationship with G-CSF, were collected. Increases in white blood cell counts were considered an adverse event only when the count exceeded 50 000/µl from the perspective of a pharmacological effect of G-CSF. Anaphylaxis and adult respiratory distress syndrome are the most representative G-CSF-related severe adverse events to be given full attention. All adverse event-related terminology were coded by the investigators according to the ICH International Medical Dictionary for Regulatory Activities Japanese version (MedDRA/J).
Ethics
This study was approved by the Institutional Review Board (IRB) of each institution that participated in the present trial and was conducted according to the principles of the World Medical Association (WMA) Declaration of Helsinki-Ethical Principles for Medical Research Involving Human Subjects with the amendments made in Seoul, South Korea, October 2008, with a Note of Clarification on Paragraph 29 added by the WMA General Assembly, Washington 2002; Note of Clarification on Paragraph 30 added by the WMA General Assembly, Tokyo 2004, and in accordance with the Japanese Medical Research Involving Human Subjects Act (WMO) and other guidelines, regulations and acts.
Patient informed consent
The principal investigator prepared the informed consent form such that the informed consent was obtained from the patient and the patient's legal representative. In cases where the trial participant could not physically sign the informed consent form because of upper extremity palsy caused by SCI, allographs provided by the patients’ representatives were allowed.
Data availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Results
Patients demographics
Patient demographics are shown in Table 1. There were no significant differences in the male/female ratio, mean age at injury, ratio of patients under/over 65 years old, ratio of AIS B or C, or neurological level of injury. The ASIA motor score at the enrolment was 21.2 ± 21.8 in G-CSF group and 27.6 ± 21.1, showing no significant difference between both groups (Table 1).
Table 1.
Participant demographics
| G-CSF n = 43 | Placebo n = 45 | P-value | |
|---|---|---|---|
| Male: female | 36:7 | 21:115 | 0.55 |
| Mean age at injury, years | 64.3 ± 13.2 | 65.1 ± 13.1 | 0.76 |
| Under 65 years old | 16 (37.2%) | 18 (40.0%) | |
| Over 65 years old | 27 (62.8%) | 27 (60.0%) | 0.83 |
| AIS | 0.86 | ||
| A | 1 (2.3%) | 0 | |
| B | 12 (27.9%) | 11 (24.4%) | |
| C | 29 (67.4%) | 33 (73.3%) | |
| D | 1 (2.3%) | 1 (2.2%) | |
| ASIA motor score | 21.2 ± 21.8 | 27.6 ± 21.1 | 0.47 |
| Neurological level of injury | 0.12 | ||
| C4 | 26 (60.5%) | 21 (46.7%) | |
| C5 | 11 (25.6%) | 21 (46.7%) | |
| C6 | 4 (9.3%) | 3 (6.7%) | |
| C7 | 2 (4.7%) | 0 |
Enrolment
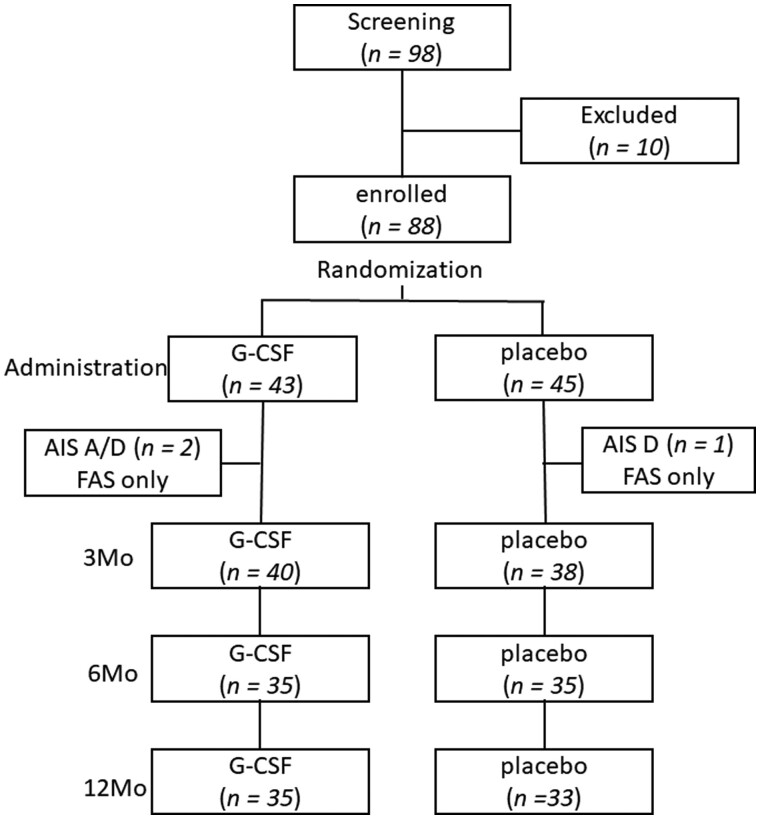
A total of 98 patients were screened for possible enrolment for the current trial. Ten of 98 patients were excluded because of the inappropriate degree of the paresis, patient refusal and concomitant diseases, etc. Three of 88 patients were diagnosed as AIS A or D [one patient in the G-CSF group was AIS A, two patients (each one patient in both the groups) were AIS D] after administration of the test drug. They were included in the FAS analyses whereas they were excluded from PPS analyses. One patient in the G-CSF group and six patients in the placebo group dropped out from follow-up examination 3 months after the drug administration. One year after the drug administration, six patients in the G-CSF group and 11 patients in the placebo group dropped out from follow-up. Therefore, the numbers of patients followed-up for 1 year were 35 in the G-CSF group and 33 in the placebo group (Fig. 1).
Figure 1.
Flow chart describing the enrolment process. A total of 98 patients were screened for possible enrolment for the current trial. Ten of 98 patients were excluded because of the inappropriate degree of the paresis, patient refusal and concomitant diseases, etc. Three of 88 patients were diagnosed as AIS A or D [one patient in the G-CSF group was AIS A, two patients (each one patient in both the groups) were AIS D] after the administration of test drug. They were included in FAS analyses whereas they were excluded from PPS analyses. One patient in the G-CSF group and six patients in the placebo group dropped out from follow-up examination 3 months after the drug administration. One year after the drug administration, six patients in the G-CSF group and 11 patients in the placebo group dropped out from follow-up. Therefore, the numbers of patients followed-up for 1 year were 35 in the G-CSF group and 33 in the placebo group.
G-CSF is well known to elevate white blood cell number. We counted the white blood cell number after administration of the test drugs. Although G-CSF apparently elevated the white blood cell number as expected, there was no severe adverse events directly related to the effect of G-CSF to increase white blood cells (Supplementary Tables 1 and 2).
Primary end point
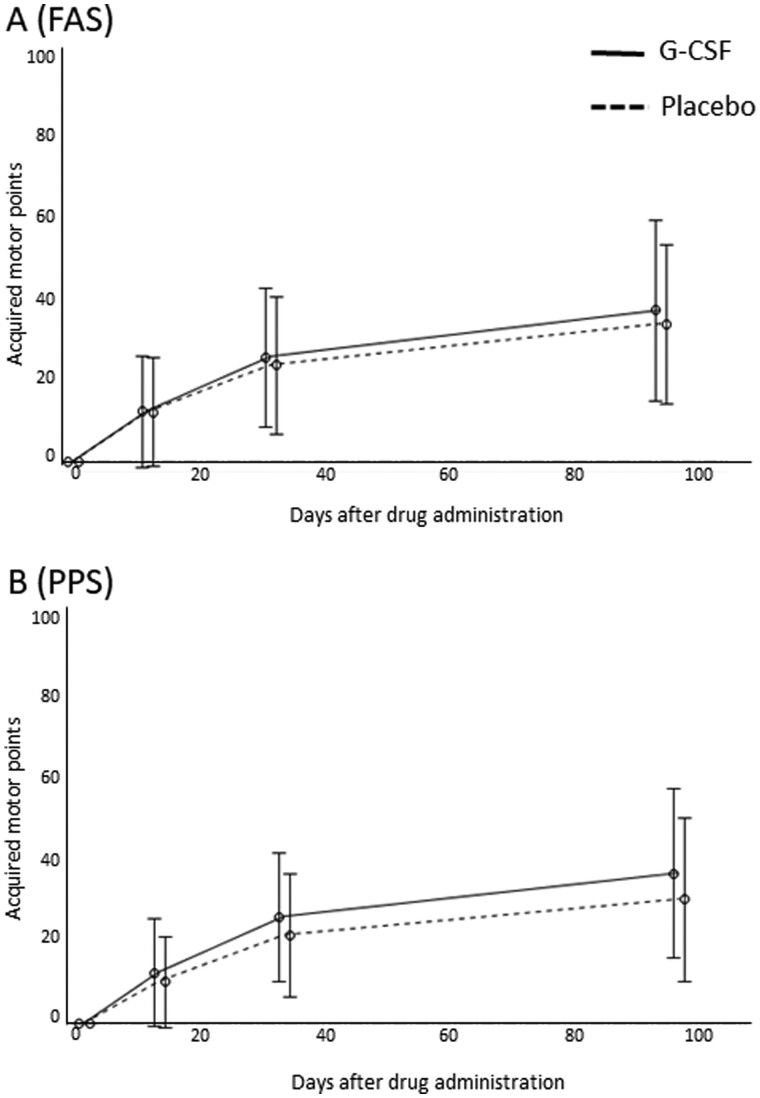
For the primary end point, there was no significant difference in acquired points on the ASIA motor score 3 months after drug administration between the G-CSF and control groups in either the FAS analysis (G-CSF group, n = 41, 34.5 ± 22.5; control group, n = 42, 30.8 ± 21.0; P = 0.40; Fig. 2A) or PPS analysis (G-CSF group, n = 40, 36.2 ± 21.8; control group, n = 39, 30.5 ± 21.1; P = 0.22; Fig. 2B).
Figure 2.
Primary end point. There was no significant difference in acquired points on the ASIA motor score 3 months after drug administration between the G-CSF and control groups in either (A) the FAS analysis (G-CSF group, n = 41, 34.5 ± 22.5; control group, n = 42, 30.8 ± 21.0; P = 0.40) or (B) PPS analysis (G-CSF group, n = 40, 36.2 ± 21.8; control group, n = 39, 30.5 ± 21.1; P = 0.22).
Secondary end points
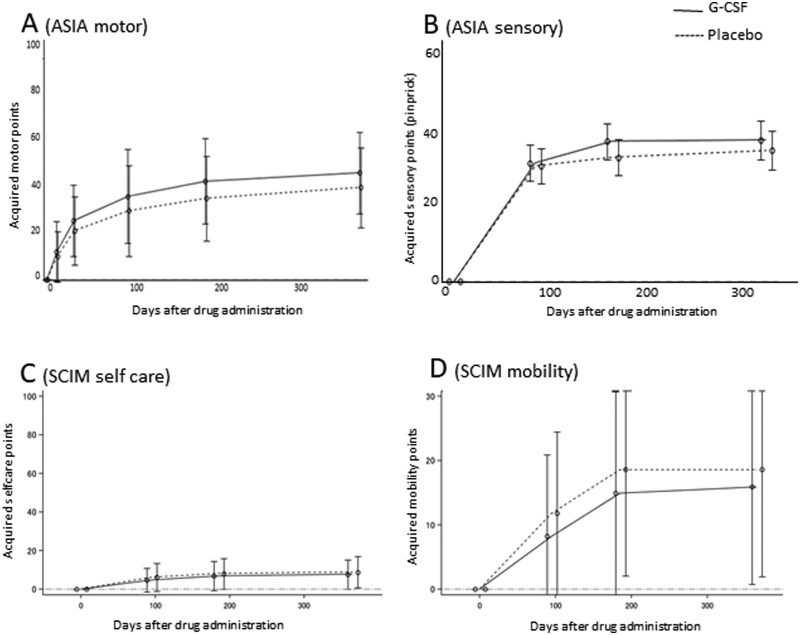
Acquired points on the ASIA motor score at 6 and 12 months after drug administration showed a trend towards improvement in the G-CSF group compared to the control group (6 months: G-CSF group, 40.5 ± 22.0, control group, 35.6 ± 22.6, P = 0.06; 12 months: G-CSF group, 41.8 ± 22.9, control group, 37.2 ± 20.9, P = 0.07; Fig. 3A) in PPS analysis with last observation carried forward supplementation.
Figure 3.
Secondary end points. (A) Acquired points on the ASIA motor score at 6 and 12 months after drug administration. There was a trend towards improvement in the G-CSF group compared to the control group (6 months: G-CSF group, 40.5 ± 22.0, and control group, 35.6 ± 22.6, P = 0.06; 12 months: G-CSF group, 41.8 ± 22.9, and control group, 37.2 ± 20.9, P = 0.07) in PPS analysis with last observation carried forward supplementation. (B) There was no significant difference in ASIA sensory score for pinprick between both groups. (C) changes in SCIM selfcare domain, 3 months: 4.8 ± 6.2 in the G-CSF group and 6.0 ± 7.0 in the placebo group, P = 0.57, 6 months: 4.8 ± 6.2 in the G-CSF group and 6.0 ± 7.0 in the placebo group, P = 0.80, 12 months: 4.8 ± 6.2 in the G-CSF group and 6.0 ± 7.0 in the placebo group, P = 0.79. (D) changes in SCIM mobility domain, 3 months: 4.8 ± 6.2 in the G-CSF group and 6.0 ± 7.0 in the placebo group, P = 0.50, 6 months: 15.3 ± 16.0 in the G-CSF group and 18.5 ± 15.9 in the placebo group, P = 0.19, 12 months: 15.9 ± 14.9 in the G-CSF group and 19.0 ± 16.3 in the placebo group, P = 0.57. There was no significant difference in change in SCIM self care score (C) and mobility score (D) between both groups.
There was no significant difference in the change in ASIA pinprick scores at either 3, 6, or 12 months between groups (Fig. 3B). The ratio of patients whose AIS improved at 3 and 6 months post-administration showed no significant difference between groups: 34/41 cases in the G-CSF group and 34/41 cases in the control group showed AIS improvement at 3 months post-administration, P = 1.0; 33/36 cases in the G-CSF group and 33/38 cases in the control group showed AIS improvement at 6 months post-administration, P = 0.71. There was also no significant difference in the change in selfcare and mobility domains of SCIM score at 3, 6, or 12 months after drug administration (selfcare domain: 3 months: 4.8 ± 6.2 in the G-CSF group and 6.0 ± 7.0 in the placebo group, P = 0.57; 6 months: 4.8 ± 6.2 in the G-CSF group and 6.0 ± 7.0 in the placebo group, P = 0.80; 12 months: 4.8 ± 6.2 in the G-CSF group and 6.0 ± 7.0 in the placebo group, P = 0.79; Fig. 3C) (mobility domain: 3 months: 4.8 ± 6.2 in the G-CSF group and 6.0 ± 7.0 in the placebo group, P = 0.50; 6 months: 15.3 ± 16.0 in the G-CSF group and 18.5 ± 15.9 in the placebo group, P = 0.19, 12 months: 15.9 ± 14.9 in the G-CSF group and 19.0 ± 16.3 in the placebo group, P = 0.57; Fig. 3D). EQ-5D scores at 3, 6, and 12 months post-drug administration also showed no significant differences between groups.
Adverse events
Safety evaluation showed no severe adverse events except for one patient that exhibited severe consciousness disorder in the G-CSF group. The cause of this severe adverse event was determined to be ‘not related to the test drug’ because brain CT revealed traumatic subarachnoid haemorrhage. Other than severe adverse event, there were high rate of adverse events in both groups (100% in the G-CSF group and 97.8% in the placebo group, P = 1.0, Supplementary Table 1). A precise list of all the adverse events are provided in Supplementary Table 1. The most frequent adverse events possibly related to the test drug were general disorder (fever) in five cases (11.6%) from the G-CSF group and respiratory disorder in three cases (6.7%) from the placebo group (Supplementary Table 1).
Subanalyses
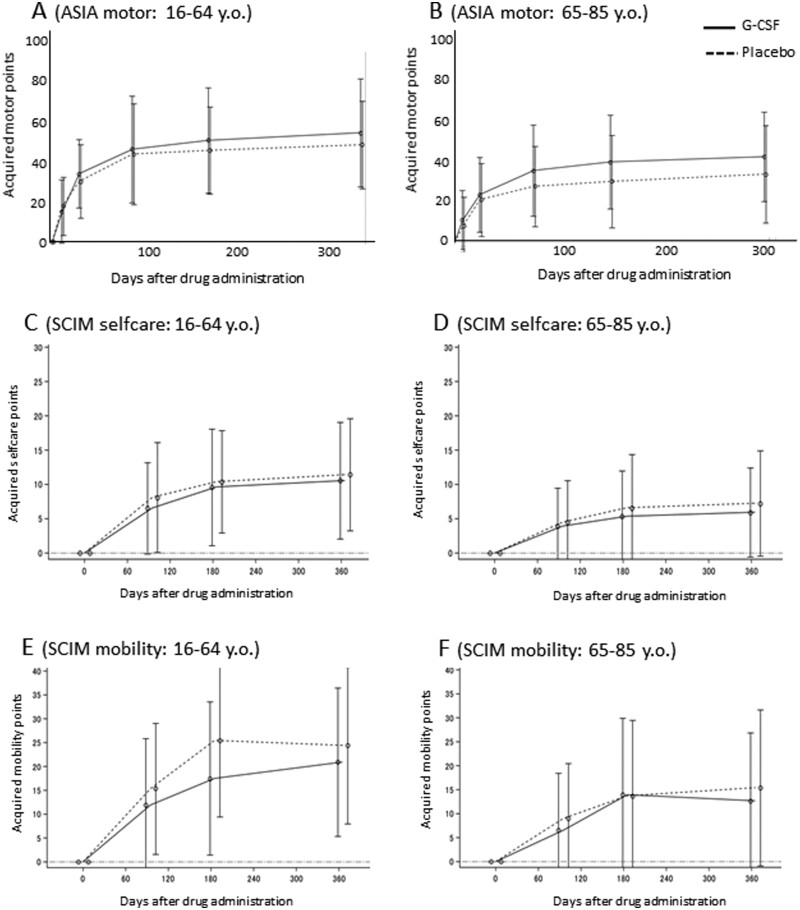
In the older subgroup (65–84 years of age), difference in the acquired ASIA motor points, which was calculated as change in ASIA motor score 6 and 12 months after drug administration between both groups nearly reached to statistical significance (6 months: G-CSF group, n = 26, 41.4 ± 21.2, and control group, n = 23, 29.3 ± 21.8, P = 0.056; 12 months: G-CSF group, n = 26, 43.9 ± 19.7, and control group, n = 23, 33.1 ± 23.3, P = 0.085, Fig. 4B). In contrast, there was no significant difference in acquired ASIA motor points, which was calculated as the change in ASIA motor score 6 and 12 months after drug administration in the younger subgroup (18–64 years of age; 6 months: G-CSF group, n = 14, 45.7 ± 23.8, and control group, n = 17, 41.2 ± 19.6, P = 0.56; 12 months: G-CSF group, n = 14, 49.1 ± 24.3, and control group, n = 17, 43.7 ± 19.7, P = 0.50, Fig. 4A). There was no significant difference in the change in ASIA motor score 6 or 12 months after drug administration in either the AIS B or C patient subgroups. Further, there was no significant difference in acquired SCIM selfcare and mobility points between the younger and older subgroups (Fig. 4C–F).
Figure 4.
Subanalyses. In the older subgroup (65–84 years of age), difference in the change in ASIA motor score 6 and 12 months after drug administration between both groups nearly reached to statistical significance (6 months: G-CSF group, n = 26, 41.4 ± 21.2, and control group, n = 23, 29.3 ± 21.8, P = 0.056; 12 months: G-CSF group, n = 26, 43.9 ± 19.7, and control group, n = 23, 33.1 ± 23.3, P = 0.085; Fig. 3B). In contrast, there was no significant difference in the change in ASIA motor score 6 and 12 months after drug administration in the younger subgroup (18–64 years of age; 6 months: G-CSF group, n = 14, 45.7 ± 23.8, and control group, n = 17, 41.2 ± 19.6, P = 0.56; 12 months: G-CSF group, n = 14, 49.1 ± 24.3, and control group, n = 17, 43.7 ± 19.7, P = 0.50; Fig. 3A). In addition, we compared the recovery between over and under 70’s and plotting recovery against age to elucidate the possible impact of age on the response to G-CSF. Comparison of recovery between patients aged ≤69 and those aged ≥70 revealed the similar trend to age over/under 65, which tend to be better in the group aged over 70 with G-CSF treatment 6 months after drug administration (Supplementary Fig. 1A and B). However, there was no significant correlation between acquired motor points and age in both groups (Supplementary Fig. 1C and D). Next, we analysed the recovery in patients with C4, 5 level injury to exclude the possible ceiling effects related to the lower level of injury (C6, 7). In C4, 5 level patients (n = 33 in the G-CSF group and n = 38 in the placebo group), the G-CSF group showed tendency for better recovery 6 months after drug administration (Supplementary Fig. 1E, P = 0.07), whereas there was no significant difference between both groups in C6, 7 level patients (Supplementary Fig. 1F).
In addition, we compared the recovery between patients aged ≤69 and those ≥70 and plotted recovery against age to elucidate the possible impact of age on the response to G-CSF. Comparison of recovery between patients aged ≤69 and those ≥70 revealed a similar trend to age over/under 65, which tend to be better in the over 70 group with G-CSF treatment 6 months after drug administration (Supplementary Fig. 1A and B). However, there was no significant correlation between acquired motor points and age in both groups (Supplementary Fig. 1C and D). Next, we analysed the recovery in patients with C4, 5 level injury to exclude the possible ceiling effects related to the lower level of injury (C6, 7). In patients with C4, 5 level injury (n = 33 in the G-CSF group and n = 38 in the placebo group), the G-CSF group showed tendency for better recovery 6 months after drug administration (Supplementary Fig. 1E, P = 0.07), whereas there was no significant difference between both groups in C 6, 7 patients (Supplementary Fig. 1F).
Discussion
Although the previous early phase clinical trial showed significantly better recovery by G-CSF treatment in acute SCI patients, the present study found no significant difference in the primary end point, acquired points on the ASIA motor score 3 months after drug administration, between the G-CSF and placebo control groups in either FAS or PPS analyses. This unexpected discrepancy between the previous results and the present one might to be attributed to the difference of study design between both trials. The study design of the previous trial was non-randomized, non-blinded comparative trial and the allocation between the G-CSF and the control group was based on the institute where the participant was treated, possibly resulting in selection bias and information bias. Moreover, in contrast to the unified timing of drug administration in the current trial, the timing of drug administration was not unified in the previous trial, of which timing was within 48 h after injury. Drug administration timing in the previous study varied from 6 h to 48 h after injury (Inada et al, 2014), possibly having a significant influence on the outcome.
There are several reasons why the present trial failed to show significant effects. It is possible that the time point selected for evaluation of the primary end point (3 months post-drug administration) was inappropriate. One of the secondary end points, change in ASIA motor score 6 and 12 months after drug administration, showed a strong tendency towards higher motor scores in the G-CSF group than in the placebo control group 6 months (P = 0.062) and 12 months (P = 0.073) after drug administration, whereas there was no significant difference in motor score recovery between these groups 3 months after drug administration (P = 0.13). Most of the previously reported clinical trials for SCI used 6 months post-injury as the time point for evaluating their primary end points (Bracken et al., 1990; Geisler et al., 2001; Tadié et al., 2003; Fehlings et al., 2016, 2018). We selected 3 months post-drug administration as the time point for evaluating our primary end point because an early-phase non-randomized, non-blinded comparative trial showed a significant difference between G-CSF-treated and control groups 3 months after SCI. We also had concerns that a large number of patients might drop-out from follow-up because of the Japanese clinical settings. Given that neurological recovery after SCI can occur 3 to 12 months after SCI, we should have selected 6 or 12 months post-treatment for the evaluation of the primary end point.
Another possible explanation for the lack of clear efficacy is that the inclusion criteria regarding patient age were suboptimal. In the subanalysis of patients aged over 65 years or 70 years, motor recovery tended to be better in the G-CSF treated group (Fig. 4B and Supplementary Fig. 1B) compared with the control group. Data of subanalysis also showed the worse recovery in placebo control group patients in older age subgroup compared with the placebo control group in younger age subgroup. This is in line with the previous reports showing worse recovery after SCI in elderly patients (Furlan and Fehling, 2009).
However, the underlying mechanisms behind the G-CSF-mediated enhanced outcomes in elderly SCI patients remain to be elucidated. Previous animal studies showed more severe inflammatory reactions in the spinal cords of older rodents compared with younger rodents (Hooshmand et al., 2014; Kamiya et al., 2017). Previous reports showed the chronic inflammation is related to the various kinds of ageing process including CNS (Pinti et al., 2016; Ventura et al., 2017). One of the major therapeutic targets of G-CSF is overexpression of inflammatory cytokines during the acute phase of SCI (Kadota et al., 2012). Thus, G-CSF might exert anti-inflammatory and/or immune-modulation effects against the more severe inflammatory reactions present in elderly patients. Recent studies revealed another possible G-CSF’s mode of action on the CNS. Guan et al. reported that G-CSF can attenuate cognitive dysfunction in elderly mice via regulation of autophagy and NF-κB signalling pathways (Guan et al., 2017), suggesting another possible mechanism of G-CSF. These lines of evidence suggest that the inclusion criteria might be limited to elderly patients especially given the current population distribution of SCI victims in Japan. Approximately two-thirds of the participants (54/88 cases, 61.3%) in the present trial were over 65 years. In a previous epidemiological report, 70 years of age represented the highest frequency of SCI in Japan (Katoh et al., 2014). Kudo et al. (2019) reported that the ratio of aged patients accounted for 75% of all cases of traumatic SCI in Akita prefecture, the northern part of Japan that has the oldest population (Kudo et al., 2019). Thus, the high percentage of elderly patients with SCI provides a rationale for limiting the inclusion criteria. For example, in the present trial, the acquired motor points at 6 and 12 months after drug administration in an age-limited population (patients over 65) nearly reached to the significant difference between the G-CSF-treated group and the placebo group (6 months: G-CSF group, n = 26, 41.4 ± 21.2; control group, n = 23, 29.3 ± 21.8; P = 0.056; 1 year: G-CSF group, n = 26, 43.9 ± 19.7; control group, n = 23, 33.1 ± 23.3; P = 0.085; Fig. 4).
Other than the aforementioned technical factors related to selecting the trial protocol, large variation in the participants’ neurological status could, contradictorily, result in significant differences between the groups. Large variation in neurological status is a specific characteristic of the acute phase of SCI for the following reasons: (i) early evaluations are most unstable for both general and neurological status, resulting in difficulty making precise neurological assessment; (ii) precise stratification is extremely difficult in the acute phase of SCI; and (iii) spinal shock makes precise neurological evaluation more difficult. It is well known that spinal shock in the acute phase of SCI masks the ‘true’ neurological severity of SCI, resulting in extreme difficulty in accurately determining injury severity and prognosis of the palsy. It is possible that these factors contribute to significant variation in the neurological status of acute SCI patients (Ditunno et al., 2004).
To minimize the influence from this large variation in neurological status, we used delayed drug administration, re-evaluation of neurological status and patient stratification. By delaying drug administration, a patient’s neurological status may be more stable than in the acute phase, resulting in more precise neurological evaluation and stratification (Fawcett et al., 2007). Thus, we selected 48 h post-SCI as the time point for drug administration. We determined the timing of drug administration based on both preclinical and early clinical data. In a rat model of contusive SCI, G-CSF promoted neurological recovery when administrated 24 h after injury. In a previous early-phase clinical trial in which drug administration was initiated within 48 h after SCI, patients who were administered G-CSF 48 h after SCI showed neurological recovery (Inada et al, 2014). These lines of evidence provided the rationale for selecting the timing of drug administration. Another method employed to reduce the influence of the wide variation in neurological status was neurological re-evaluation 48 h post-SCI, at the time of drug administration. We enrolled trial candidates within 48 h after injury, waited until 48 h post-SCI, then re-evaluated neurological status and only included patients who fulfilled the inclusion criteria at the 48-h time point, excluding patients who demonstrated neurological recovery to AIS D. This exclusion criteria resulted in three patients being excluded due to neurological deterioration to AIS A (one patient) and neurological recovery to AIS D (two patients) during the screening period. Patient stratification was another one of the additional methods utilized to reduce the influence of the wide variation in neurological status of the patients. We limited the inclusion criteria in the present trial to AIS B or C patients, meaning severe incomplete SCI, and excluding AIS A and D patients. However, even though we used these methods in an attempt to reduce patient variation in neurological status at drug administration, the standard deviation of the ASIA motor score at enrolment in the current trial was 21.8 points in the G-CSF group and 21.1 points in the placebo control group, showing the failure to reduce large variation. Similarly, data from the NASCIS 2 trial, one of the most well-known large scale clinical trials, showed a standard deviation in motor score at trial entry of 17.4 in the steroid-treated group and 19.6 in the placebo control group (Bracken et al., 1990), comparable to the variation reported in our current trial.
Considering the present results, extensive efforts to reduce the significant variation of heterogenous SCI patients must be considered for future acute phase SCI clinical trials, including stricter stratification of the participants, and a greater delay prior to drug administration. However, there are major obstacles to implementing these methods. Employing stricter stratification of the patients is a trade-off with patient enrolment, making it more difficult, and delaying the time of drug administration can limit the effectiveness of neuroprotective drugs. Further sophistication of trial protocols should be considered in order to obtain significant results in a clinical trial for acute phase SCI.
In conclusion, the present trial failed to show significant benefit from G-CSF in the primary end point.
Acknowledgements
We deeply appreciate Kyowa-Kirin Pharmaceuticals for preparing test drugs.
Funding
This trial was supported by Center for Clinical trials, Japan Medical Association, Japan (Grant number CCT-C-2746).
Competing interests
The authors report no competing interests.
Supplementary material
Supplementary material is available at Brain online.
Supplementary Material
Glossary
- AIS =
ASIA Impairment Scale
- AISA =
American Spinal Injury Association
- G-CSF =
granulocyte colony-stimulating factor
- PPS =
per protocol set
- SCI =
spinal cord injury
- SCIM =
Spinal Cord Independence Measure
References
- Bracken MB, Shepard MJ, Collins WF, Holford TR, Young W, Baskin DS, et al. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury: results of the Second National Acute Spinal Cord Injury Study. N Engl J Med 1990; 322: 1405–11. [DOI] [PubMed] [Google Scholar]
- Ditunno JF, Little JW, Tessler A, Burns AS.. Spinal shock revisited: a four-phase model. Spinal Cord 2004; 42: 383–95. [DOI] [PubMed] [Google Scholar]
- Fawcett JW, Curt A, Steeves JD, Coleman WP, Tuszynski MH, Lammertse D, et al. Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: spontaneous recovery after spinal cord injury and statistical power needed for therapeutic clinical trials. Spinal Cord 2007; 45: 190–205. [DOI] [PubMed] [Google Scholar]
- Fehlings MG, Nakashima H, Nagoshi N, Chow DSL, Grossman RG, Kopjar B.. Rationale, design and critical end points for the riluzole in acute spinal cord injury study (RISCIS): a randomized, double-blinded, placebo-controlled parallel multi-center trial. Spinal Cord 2016; 54: 8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehlings MG, Kim KD, Aarabi B, Rizzo M, Bond LM, McKerracher L, et al. Rho inhibitor VX-210 in acute traumatic subaxial cervical spinal cord injury: design of the SPinal Cord Injury Rho INhibition InvestiGation (SPRING) clinical trial. J Neurotrauma 2018; 35: 1049–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furlan JC, Fehling MG.. The impact of age on mortality, impairment and disability among adults with acute traumatic spinal cord injury. J Neurotrauma 2009; 26: 1707–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler FH, Coleman WP, Grieco G, Poonian D; Sygen Study Group. The Sygen multicenter acute spinal cord injury study. Spine (Phila Pa 1976) 2001; 26: S87–98. [DOI] [PubMed] [Google Scholar]
- Guan ZF, Tao YH, Zhang XM, Guo QL, Liu YC, Zhang Y, et al. G-CSF and cognitive dysfunction in elderly diabetic mice with cerebral small vessel disease: preventive intervention effects and underlying mechanisms. CNS Neurosci Ther 2017; 23: 462–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooshmand MJ, Galvan MD, Partida E, Anderson AJ.. Characterization of recovery, repair, and inflammatory processes following contusion spinal cord injury in old female rats: is age a limitation? Immun Agein 2014; 11:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurlbert RJ, Hadley MN, Walters BC, Aarabi B, Dhall SS, Gelb DE, et al. Pharmacological therapy for acute spinal cord injury. Neurosurgery 2015; 76: S71–83. [DOI] [PubMed] [Google Scholar]
- Inada T, Takahashi H, Yamazaki M, Okawa A, Sakuma T, Kato K, et al. A multicenter prospective non-randomized controlled clinical trial to prove neurotherapeutic effects of granulocyte colony-stimulating factor (G-CSF) for acute spinal cord injury: analyses of follow-up cases after at least one year. Spine 2014; 39: 213–9. [DOI] [PubMed] [Google Scholar]
- Kadota R, Koda M, Kawabe J, Hashimoto M, Nishio Y, Mannoji C, et al. Granulocyte colony-stimulating factor (G-CSF) protects oligodendrocyte and promotes hindlimb functional recovery after spinal cord injury in rats. PLoS One 2012; 7: e50391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya K, Furuya T, Hashimoto M, Mannoji C, Inada T, Ota M, et al. Exploration of spinal cord aging-related proteins using a proteomics approach. J Exp Neurosci 2017; 11: 117906951771301. 1179069517713019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh S, Enishi T, Sato N, Sairyo K.. High incidence of acute traumatic spinal cord injury in a rural population in Japan in 2011 and 2012: an epidemiological study. Spinal Cord 2014; 52: 264–7. [DOI] [PubMed] [Google Scholar]
- Kawabe J, Koda M, Hashimoto M, Fujiyoshi T, Furuya T, Endo T, et al. Neuroprotective effects of granulocyte colony-stimulating factor and relationship to promotion of angiogenesis after spinal cord injury in rats. J Neurosurg Spine 2011; 15: 414–21. [DOI] [PubMed] [Google Scholar]
- Koda M, Nishio Y, Kamada T, Someya Y, Okawa A, Mori C, et al. Granulocyte colony-stimulating factor (G-CSF) mobilizes bone marrow-derived cells into injured spinal cord and promotes functional recovery after compression-induced spinal cord injury in mice. Brain Res 2007; 1149: 223–31. [DOI] [PubMed] [Google Scholar]
- Koda M, Hanaoka H, Sato T, Fujii Y, Hanawa M, Takahashi S, et al. Study protocol for the G-SPIRIT trial: a randomised, placebo-controlled, double-blinded phase III trial of granulocyte colony-stimulating factor-mediated neuroprotection for acute spinal cord injury. BMJ Open 2018; 8: e019083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo D, Miyakoshi N, Hongo M, Kasukawa Y, Ishikawa Y, Ishikawa N, et al. An epidemiological study of traumatic spinal cord injuries in the fastest aging area in Japan. Spinal Cord 2019; 57: 509–15. [DOI] [PubMed] [Google Scholar]
- Nicola NA, Metcalf D, Matsumoto M, Johnson GR.. Purification of a factor inducing differentiation in murine myelomonocytic leukemia cells. Identification as granulocyte colony-stimulating factor. J Biol Chem 1983; 258: 9017–23. [PubMed] [Google Scholar]
- Nishio Y, Koda M, Kamada T, Someya Y, Kadota R, Mannoji C, et al. Granulocyte colony-stimulating factor (G-CSF) attenuates neuronal death and promotes functional recovery after spinal cord injury in mice. J Neuropathol Exp Neurol 2007; 66: 724–31. [DOI] [PubMed] [Google Scholar]
- Pinti M, Appay V, Campisi J, Frasca D, Fülöp T, Sauce D, et al. Aging of the immune system: focus on inflammation and vaccination. Eur J Immunol 2016; 46: 2286–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AW. G-CSF: a key regulator of neutrophil production, but that's no all!. Growth Factors 2005; 23: 33–41. [DOI] [PubMed] [Google Scholar]
- Tadié M, Gaviria M, Kamenka JM, Privat A, Carli P, Mathe JF, et al. Early care and treatment with a neuroprotecive drug, gacyclidine, in patientswith acute spinal cord injury. Rachis (Clichy) 2003; 15: 363–76. [Google Scholar]
- Takahashi H, Yamazaki M, Okawa A, Sakuma T, Kato K, Hashimoto M, et al. Neuroprotective therapy using granulocyte colony-stimulating factor for acute spinal cord injury: a phase I/IIa clinical trial. Eur Spine J 2012; 21: 2580–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura MT, Casciaro M, Gangemi S, Buquicchio R.. Immunosenescence in aging: between immune cells depletion and cytokines up-regulation. Clin Mol Allergy 2017; 15: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, Zhang F, Cheng F, Ying L, Wang C, Shi K, et al. Strategies and prospects of effective neural circuits reconstruction after spinal cord injury. Cell Death Dis 2020; 11: 439. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request.