Abstract
Context:
Whether the alcohol-based mouth rinses are as good as nonalcoholic mouth rinses as far as oral mucosal safety is concerned?
Aims:
The aim of the study was to investigate the oral mucosal safety of widely used alcohol- and nonalcohol-based mouth rinses at their recommended doses.
Settings and Design:
The clinical and cytological investigations were carried out by enrolling 120 systemically healthy volunteers fulfilling the inclusion criteria. The volunteers were subjected to a repeated mouth rinse for 60 days to either alcohol-based or alcohol-free mouth rinses at their recommended dosages. A comparative analysis for any clinical adverse response on the oral mucosa and efficacy, i.e., reduction of plaque and gingival index was done at the terminal of the exposure. The studies were also carried out to investigate the cytotoxicity and genotoxicity potential of alcohol-based and alcohol-free mouth rinses in the exposed mucosal cells.
Subjects and Methods:
The data have been presented in comparative account between alcohol-based and alcohol-free mouth rinses in the volunteers at day 0 and day 60. The cytotoxicity and genotoxicity potential of prescribed doses of alcohol- and alcohol-free mouth rinses have also been evaluated using tetrazolium bromide salt 3-(4, 5-dimethylthiazol-2-yl)-2,-diphenyl tetrazolium bromide, neutral red uptake, and trypan blue dye, micronucleus and chromosomal aberrations.
Results:
The study findings reveal no statistically as well as biologically significant adverse responses of both alcohol-based and alcohol-free mouth rinses at clinical and cytological level.
Conclusions:
Under cytological observation, repeated dose exposure up to 60 days of the mouth rinses (alcohol-based and alcohol-free) used in the study was found to be effective and safe at their prescribed dosages.
Keywords: Alcohol containing, cell viability, mouth rinses, mucosal cell genotoxicity, safety and efficacy
INTRODUCTION
In general, mouth rinses contain fluoride for caries prevention, chlorhexidine (CHX), and cetylpyridinium chloride for plaque control, i.e., inhibiting the microbial growth.[1] Mouth rinses are advised as an adjunct to mechanical oral hygiene in conditions such as post subgingival scaling and root planing, in patients having poor oral hygiene, after periodontal surgical procedures, for mentally or physically handicapped patients.[2] The available mouth rinses have variable concentrations of alcohol with highest up to 27%.[3] There is evidence in the literature showing moderate-to-severe side effects including oral pain,[4] burning sensation,[5] oral mucosal hypersensitivity and epithelial desquamation,[6] ulcerations, and petechiae[7] due to the use of alcohol-based mouth rinses (AM). On contrary, few other studies have shown no admissible adverse cytological effects due to AM when compared with nonalcohol-based mouth rinses.[8,9] Few sporadic reports speculated that some of the constituents of AM may be cytotoxic and genotoxic, and resultant to that induces irritation in the mucosal surfaces of the mouth.[10] Concerns have also been raised regarding the potential for AM for causing the adverse effects when compared with alcohol-free mouth rinses (AFM).[11]
In view of the inconclusive finding, whether alcohol or any other constituent of mouth rinses are having the potential of inducing the cellular damage in oral mucosa and subsequent adverse effect on the oral health or not; the present study was aimed to investigate the status of oral mucosal safety following the subchronic exposure (60 days) to recommended doses of certain widely used alcohol-based and alcohol-free mouth rinses. In routine practice, clinicians usually do not prescribe mouth rinses for more than 1 month without break, although it is not contraindicated, in this study, chronic exposure of 60 days was given as products that have not been evaluated in long-term clinical trials have no scientific evidence documenting safety and efficacy. In the clinicocytological studies, we analyzed the clinical changes in oral mucosa, plaque and gingival index (PI and GI) following 60 days of repeated exposure to AM and data compared to that of volunteers exposed to AFM rinses. The exposed mucosal cells (60 days exposure) were then examined for AM induced cytotoxic and genotoxic responses, if any, using the standard endpoints. The data generated were compared to that of cells exposed to AFM rinses and cells exposed to alcohol-containing mouth rinses.
SUBJECTS AND METHODS
A total of 120 systemically healthy human volunteers of 25–55 years age irrespective of gender having mild periodontitis with clinical attachment loss <4 mm were included, whereas volunteers having systemic diseases and pregnant and lactating women were excluded from the study. Sixty days of repeated exposure to either alcohol-based or alcohol-free mouth rinses was given randomly at recommended doses. The clinical changes in oral mucosa, PI, and GI were compared from the baseline values recorded at day 0. The data were also compared for changes between the groups receiving exposure to alcohol-based and alcohol-free mouth rinses. The studies were extended to investigate the adverse effect, if any, on mucosal cells at the cellular level. At the cellular level, exposed cells were examined for induction of cytotoxic and genotoxic responses. Distribution of volunteers was done by dividing systemically healthy human volunteers (120) into four groups [Table 1].
Table 1.
Distribution of groups
| Study groups | Sample size (n) | Type of mouth rinse | Composition |
|---|---|---|---|
| Group A* | 30 | AM | Active ingredients |
| Thymol, eucalyptol, methyl salicylate, menthol | |||
| Inactive ingredients | |||
| Alcohol (21.6%), sorbitol, sodium saccharin, sodium benzoate | |||
| Group B** | 30 | AM | Active ingredients |
| CHX digluconate (0.1%), chlorobutanol hemihydrate | |||
| Inactive ingredients | |||
| Alcohol (42.7%), sorbitol | |||
| Group C$ | 30 | AFM | CHX gluconate (0.2%), sodium monofluorophosphate, triclosan |
| Group D# | 30 | AFM | CHX gluconate (0.12%), saccharin, glycerin, peppermint, purified water |
*Listerine; **Eludril; $Hexidine; #Rexidine plus. AFM-Alcohol-free mouth rinse; AM-Alcohol-based mouth rinse; CHX-Chlorhexidine
All the selected subjects underwent phase-I therapy, which included oral hygiene instructions, full mouth supra, and subgingival scaling and root planing. The selected volunteers were randomly prescribed mouth rinse for 60 days.
Clinical parameters (PI and GI) and oral mucosal parameters (epithelial desquamation, ulcerations, and petechiae) were recorded at baseline (day 0) and after 60 days, whereas cytological changes were assessed after 60 days of use of AM and AFM by collecting buccal swab and processing on the same day. Buccal swab collection was done, by placing a wooden stick on the side of the cheek and gently rubbed the cheek for about 5–10 s [Figure 1]. Immediately after swabbing, the swab was placed directly in to DMEM/F-12 culture medium (Dulbecco's Modified Eagle Medium: Nutrient Mixture F-12) tubes, kept the samples into an icebox, and transported it to CSIR-Indian Institute of Toxicology Research (CSIR-IITR), Lucknow, for its processing. The samples were processed on the same day for endpoints of cytotoxicity and genotoxicity analysis as follows.
Figure 1.
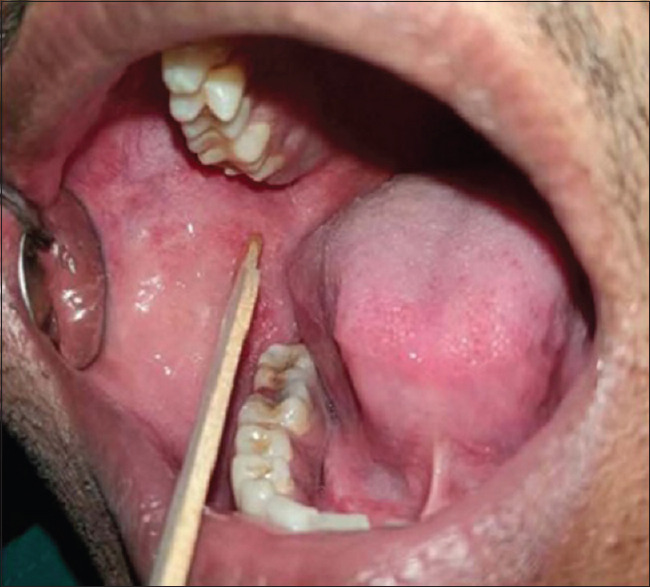
Buccal swab collection by a wooden stick
3-(4, 5-dimethylthiazol-2-yl)-2,-diphenyl tetrazolium bromide assay
Cytotoxicity assessment was done using 3-(4, 5-dimethylthiazol-2-yl)-2,-diphenyl tetrazolium bromide (MTT) assay following the protocol of Siddiqui et al.,[12] In brief, cells were cultured in 96-well plates for 24, 48, 72, and 96 h at 37° C. Tetrazolium salt (10 μl/well; 5 mg/ml of stock in phosphate-buffered saline [PBS]) was added 4 h before completion of respective incubation periods. The reaction mixture was then carefully taken out and 200 μl of culture grade dimethyl sulfoxide was added to each well. The content was mixed well and plates were incubated for 10 min at room temperature. The color change was read at 550 nm.
Neutral red uptake assay
The cells were seeded in poly-L-lysine precoated 96-well culture plates and allowed to adhere for 24 h. At the end of the incubation period, cells were washed with 1XPBS, and neutral red uptake (NRU) was added (50 μg/ml) in the medium. The NRU was removed after 3 h and cells were washed with 1% acetic acid and 50% ethanol. The cells were then incubated in 0.5% formaldehyde for 20 min at 37°C, and absorbance was taken at 540 nm.[12]
Trypan blue dye exclusion assay
The loss of membrane integrity was assessed following the protocols of Pant et al. 2001.[13] In brief, the cells were cultured for 24, 48, 72, and 96 h, followed by centrifugation at 600 rpm for 5 min and washing twice with sterile PBS (pH 7.4). The cells were re-suspended in PBS having 0.4% trypan blue and immediately observed under microscope. The live (unstained transparent) and dead (blue stained) cells were counted using a cell counter.
Micronucleus assay
Cells were grown on coverslips placed in 8-well plates in DMEM/F-12 medium. The cells were incubated up to 43–44 h in fresh medium and blocked for cytokinesis using cytochalasin-B. Finally, cells were dropped onto the slides and stained with 5% Giemsa in phosphate buffer (pH 6.8) for 15–20 min and mounted for microscopic examination. A minimum of 1000 bi-nucleated cells with well-defined cytoplasm in each slide was scored for the presence of MN using a Nikon Eclipse 80i upright microscope attached to a Nikon digital CCD cool camera.[14]
Chromosomal aberration assay
The cells were cultured in DMEM/F-12 medium in 48 well plates. Colcemid was added 4 h before harvesting the cells. After washing with HBSS, the cells were given a hypotonic shock in potassium citrate (0.8%) for 30 min. Thereafter, the cells were fixed in cold fixative (methanol: acetic acid, 5:2 ratios) and scored for the presence of CA using a Nikon Eclipse 80i upright microscope attached to a Nikon digital CCD cool camera.[14]
RESULTS
Data were summarized as mean ± standard error of the mean. Groups were compared by repeated measures of two-factor analysis of variance and the significance of mean difference within (intra) and between (inter) the groups was done by Newman–Keuls test after ascertaining normality by Shapiro–Wilk test and homogeneity of variance between groups by Levene's test. A two-tailed (α = 2) P < 0.05 was considered statistically significant. Analyses were performed on SPSS software (Statistical Package for Social Sciences) developed by IBM company, New York.
On clinical parameters, the effect of AM, i.e., (Group A and Group B) and AFM (Group C and Group D) were assessed, namely PI and GI to determine their efficacy. The analysis for PI and GI done at baseline (day 0) and after 60 days shows a marked decrease in mean PI and GI following the use of mouth rinse. This decrease was highly evident in Group B, followed by Group C, A, and D, respectively [Tables 2 and 3], whereas [Graph 1] illustrates the comparison of PI and GI between AM and AFM after 2 months and is observed that decrease in PI and GI was more evident in AM in comparison to that of AFM. However, the difference in reduction between the two groups in PI and GI index could not achieve the statistically significant levels.
Table 2.
Plaque index of four groups at two different periods (baseline and after 2 months) and comparison (P value) of difference in mean plaque index
| Groups | Baseline | 2 months | Comparison |
|---|---|---|---|
| A | 0.83±0.11 | 0.40±0.09 | <0.001 |
| B | 0.90±0.12 | 0.30±0.09 | <0.001 |
| C | 0.87±0.10 | 0.43±0.10 | <0.001 |
| D | 0.90±0.09 | 0.37±0.09 | <0.001 |
Newman-Keuls test showed significant (P<0.001) decrease in mean PI at 2 months. PI – Plaque index; P<0.001= Highly Statistically Significant
Table 3.
Gingival index of four groups at two different periods (baseline and after 2 months) and comparison (P value) of difference in mean gingival index
| Groups | Baseline | 2 months | Comparison |
|---|---|---|---|
| A | 0.97±0.10 | 0.53±0.11 | <0.001 |
| B | 1.03±0.09 | 0.47±0.10 | <0.001 |
| C | 1.00±0.13 | 0.60±0.09 | <0.001 |
| D | 1.03±0.13 | 0.53±0.10 | <0.001 |
Newman-Keuls test showed significant (P<0.001) decrease in mean GI at 2 months. GI – Gingival index; P<0.001= Highly Statistically Significant
Graph 1.
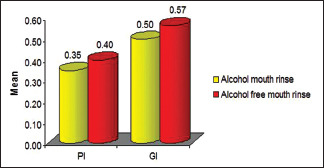
Comparison of plaque and gingival index (PI and GI) between alcohol-based mouth rinse and alcohol-free mouth rinse after 2 months
On oral mucosal change, no admissible adverse changes could be recorded in the oral mucosa, i.e., there was no epithelial desquamation, ulcerations, and petechiae when compared the findings of day 0 and day 60. There was slight discomfort in just 4 out of 120 patients with alcohol-based mouth rinses.
In terms of cytological changes, biosafety analysis of two alcohol-based (Groups A and B) and alcohol-free (Groups C and D) mouth rinses were assessed by percent cell viability using standard endpoints of cytotoxicity, namely tetrazolium bromide salt MTT assay, NRU assay, and trypan blue dye exclusion (trypan blue) assay in primary culture of oral mucosal cells obtained from different human samples that were pooled together and formed three groups. This was done to avoid heterogeneity due to the variable ethnic groups of the volunteers. On selected samples, post treatment DNA damage was also assessed by micronucleus (MN) frequency and chromosomal aberration (CA) frequency done using micronucleus assay, and CA assay, respectively. The loss of cell viability was recorded at (24 h, 48 h, 72 h, and 96 h) in the cells exposed to either alcohol-based or alcohol-free mouth rinses using MTT, NRU, and trypan blue dye exclusion assays. Both the alcohol-based mouth rinses (Groups A and B) were found to have more loss of cell viability than the alcohol-free mouth rinses (Groups C and D) [Table 4]. The comparative account for the percent cell viability loss at 24 h between AM and AFM assessed using MTT, NRU, and trypan blue dye exclusion assays is depicted in Graph 2. The cell viability was higher in case of AFM than that of AM at the time point of 24 h.
Table 4.
Posttreatment MTT, neutral red uptake, and trypan blue assay in showing percent cell viability of four groups over the periods (24 h, 48 h, 72 h, and 96 h)
| Group | 24 h | 48 h | 72 h | 96 h |
|---|---|---|---|---|
| A | 81.00±2.31 | 87.33±1.45 | 92.67±1.76 | 100.33±1.33 |
| B | 86.67±2.60 | 91.00±2.31 | 95.00±1.15 | 97.00±1.15 |
| C | 102.00±2.89 | 99.00±1.73 | 97.33±0.67 | 97.67±1.33 |
| D | 105.00±1.73 | 101.00±2.31 | 97.67±0.67 | 97.67±0.88 |
MTT-3-(4, 5-dimethylthiazol-2-yl)-2,-diphenyl tetrazolium bromide
Graph 2.
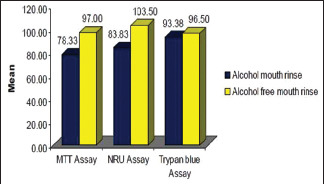
Comparison of percent cell viability at 24 h between alcohol mouth rinse and alcohol-free mouth rinse assessed using 3-(4, 5-dimethylthiazol-2-yl)-2 (MTT),-diphenyl tetrazolium bromide assay, neutral red uptake (NRU) assay, and trypan blue assay
In micronucleus and CA assays, though there was an increase in the frequency of generation of micronucleus and CA s in the cells exposed to alcohol-based mouth rinses than that of exposed to alcohol-free mouth rinses, this increase was found to be biologically insignificant [Graph 3].
Graph 3.
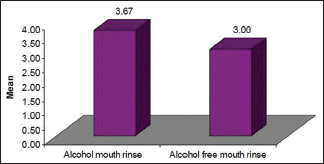
Increase in number of micronucleus and chromosomal frequency in alcohol-based mouth rinses in comparison to alcohol-free mouth rinses after 24 h
DISCUSSION
Mouth rinses are used widely worldwide, primarily for their capacity to control dental plaque and gingivitis. Daily use of mouth rinses has been recommended for the prevention of periodontal disease and control of caries.[15] The role of AM in oral tissues has been studied and found to be noncytotoxic with the absence of histopathological evidence of any adverse effects in oral tissues (Koschier et al.).[16]
However, few studies have reported to cause adverse effects on oral structures and functions, which includes the burning mouth, drying of the oral mucosa, softening effects on composite filling materials, and mucosal pain.[17,18] The accepted concentration of alcohol content in CHX mouth rinses by the Food and Drug Administration is 11.6%. Although Eludril has 42.7% alcohol while Listerine has 21.6% alcohol while nonalcohol containing CHX has fewer side effects, are less effective.[19] The present study was conducted to know whether and to what extent widely used mouth rinses containing alcohol and alcohol-free have direct effect, if any, on the clinical and cellular level of the oral mucosa.
The clinical parameters for the PI and GI were assessed and compared at baseline and after 60 days. The PI and GI for all four mouth rinses AM and AFM reduced significantly, but the difference in reduction between the two groups was found to be insignificant, so we can conclude that AM and AFM were shown to be equally effective as antiplaque and antigingivitis agents.
Kowitz et al. (1976) described some adverse effects after using mouthrinses containing alcohol, such as epithelium desquamation, ulceration, gingivitis, and petechiae, but in our study, no possible oral mucosal changes were seen.
Result findings of MTT, NRU, and trypan blue dye exclusion assays have suggested that there was more loss of percent cell viability in the cells isolated from the buccal cavity of volunteers using alcohol-containing mouthrinses than the volunteers using AFM rinse. Still, this loss could not reach the level of cytotoxicity. Cells allowed to grow for a longer period (48–96 h) in culture medium supplemented with growth factors were showing a recovery trend, i.e., the loss of percent cell viability got reduced in subsequent incubations of 48, 72, and 96 h and reached near to the basal level. As such, there was no significant reduction in percent cell viability reported in the cells following the exposure to all four mouth rinses used in the study.
The micronucleus and CA assays were done to assess the accumulation of genetic damage in the cells. There was no increase in the micronucleus (MN) frequency and no CA seen in cells exposed to any mouthwash. The minor decrease in 96 h might be due to autorecovery in the cells, and this change during 96 h was very obvious and routinely observed when we are using the biological system from human volunteers of different age, gender, ethnic group, etc.[20] Our findings demonstrated that all the test materials, i.e., alcoholic and nonalcoholic mouth rinses have no mutagenic potential even upto an incubation period of 96 h. Hence, the oral mouth rinses, i.e., AM and AFM were found to be nongenotoxic under experimental conditions.
CONCLUSION
This study concludes that both nonalcoholic and alcoholic mouth rinses are equally effective in controlling the plaque and gingivitis. Alcohol-containing mouth rinses induce greater cell damage when compared with alcohol-free mouth rinses, but none reached the level of cytotoxicity over 60 days of exposure. Since they result in some degree of cell damage, it may affect the healing of tissue in surgical cases; therefore, it should be used judiciously during and after surgical procedures. Further longitudinal studies with a larger sample size and longer duration may be required to assess the cytological changes by regular and long-term use of mouth rinses.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Baehni PC, Takeuchi Y. Anti-plaque agents in the prevention of biofilm-associated oral diseases. Oral Dis. 2003;9:23–9. doi: 10.1034/j.1601-0825.9.s1.5.x. [DOI] [PubMed] [Google Scholar]
- 2.Fischman SL. Guidelines for acceptance of chemotherapeutic products for the control of supragingival dental plaque and gingivitis: Council on dental therapeutics. J Am Dent Assoc. 1986;112:529–32. doi: 10.1016/s0002-8177(86)24021-0. [DOI] [PubMed] [Google Scholar]
- 3.Bhatti SA, Walsh TF, Douglas CW. Ethanol and pH levels of proprietary mouthrinses. Community Dent Health. 1994;11:71–4. [PubMed] [Google Scholar]
- 4.Gurgan CA, Zaim E, Bakirsoy I, Soykan E. Short-term side effects of 0.2% alcohol-free chlorhexidine mouthrinse used as an adjunct to non-surgical periodontal treatment: A double-blind clinical study. J Periodontol. 2006;77:370–84. doi: 10.1902/jop.2006.050141. [DOI] [PubMed] [Google Scholar]
- 5.Madan PD, Sequeira PS, Shenoy K, Shetty J. The effect of three mouthwashes on radiation-induced oral mucositis in patients with head and neck malignancies: A Randomized control trial. J Cancer Res Ther. 2008;4:3–8. doi: 10.4103/0973-1482.39597. [DOI] [PubMed] [Google Scholar]
- 6.Kowitz GM, Lucatorto FM, Cherrick HM. Effects of mouthwashes on the oral soft tissues. J Oral Med. 1976;31:47–50. [PubMed] [Google Scholar]
- 7.Bernstein ML. Oral mucosal white lesions associated with excessive use of listerine mouthwash: Report of two cases. Oral Surg Oral Med Oral Pathol. 1978;46:781–5. doi: 10.1016/0030-4220(78)90308-0. [DOI] [PubMed] [Google Scholar]
- 8.Bagan JV, Vera-Sempere F, Marzal C, Pellín-Carcelén A, Martí-Bonmatí E, Bagan L. Cytological changes in the oral mucosa after use of a mouth rinse with alcohol: A prospective double blind control study. Med Oral Patol Oral Cir Bucal. 2012;17:956–61. doi: 10.4317/medoral.18843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ros-Llor I, Lopez-Jornet P. Cytogenetic analysis of oral mucosa cells, induced by chlorhexidine, essential oils in ethanolic solution and triclosan mouthwashes. Environ Res. 2014;132:140–5. doi: 10.1016/j.envres.2014.03.032. [DOI] [PubMed] [Google Scholar]
- 10.Smigel K. High-alcohol mouthwashes are under scrutiny. J Natl Cancer Inst. 1991;83:751. doi: 10.1093/jnci/83.11.751. [DOI] [PubMed] [Google Scholar]
- 11.Reidy JT, McHugh EE, Stassen LF. A review of the role of alcohol in the pathogenesis of oral cancer and the link between alcohol-containing mouthrinses and oral cancer. J Ir Dent Assoc. 2011;57:200–2. [PubMed] [Google Scholar]
- 12.Siddiqui MA, Singh G, Kashyap MP, Khanna VK, Yadav S, Chandra D, et al. Influence of cytotoxic doses of 4-hydroxynonenal on selected neurotransmitter receptors in PC-12 cells. Toxicology In Vitro. 2008;22:1681–8. doi: 10.1016/j.tiv.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 13.Pant AB, Agarwal AK, Sharma VP, Seth PK. In vitro cytotoxicity evaluation of plastic biomedical devices. Hum Exp Toxicol. 2001;20:412–7. doi: 10.1191/096032701682692919. [DOI] [PubMed] [Google Scholar]
- 14.Srivastava RK, Rahman Q, Kashyap MK, Singh AK, Jain G, Jahan S, et al. Nano-titanium dioxide induces genotoxicity and apoptosis in human lung cancer cell line, A549. Hum Exp Toxicol. 2013;32:153–66. doi: 10.1177/0960327112462725. [DOI] [PubMed] [Google Scholar]
- 15.Kuyama K, Yamamoto H. A study of effects of mouthwash on the human oral mucosae: With special references to sites, sex differences and smoking. J Nihon Univ Sch Dent. 1997;39:202–10. doi: 10.2334/josnusd1959.39.202. [DOI] [PubMed] [Google Scholar]
- 16.Koschier F, Kostrubsky V, Toole C, Gallo MA. In vitro effects of ethanol and mouthrinse on permeability in an oral buccal mucosal tissue construct. Food Chem Toxicol. 2011;49:2524–9. doi: 10.1016/j.fct.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 17.Settembrini L, Penugonda B, Scherer W, Strassler H, Hittelman E. Alcohol-containing mouthwasheses: Effect on composite color. Oper Dent. 1995;20:14–7. [PubMed] [Google Scholar]
- 18.Weiner R, Millstein P, Hoang E, Marshall D. The effect of alcoholic and nonalcoholic mouthwashes on heat-treated composite resin. Oper Dent. 1997;22:249–53. [PubMed] [Google Scholar]
- 19.Jose A, Butler A, Payne D, Maclure R, Rimmer P, Bosma ML. A randomised clinical study to evaluate the efficacy of alcohol-free or alcohol-containing mouthrinses with chlorhexidine on gingival bleeding. Br Dent J. 2015;219:125–30. doi: 10.1038/sj.bdj.2015.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.White RE. High-throughput screening in drug metabolism and pharmacokinetic support of drug discovery. Annu Rev Pharmacol Toxicol. 2000;40:133–57. doi: 10.1146/annurev.pharmtox.40.1.133. [DOI] [PubMed] [Google Scholar]


