Abstract
Background:
Platelet-rich fibrin (PRF) is the second-generation platelet concentrate first described by Choukron et al. It incorporates leukocytes, platelets, and growth factors within dense fibrin matrix, can be used in periodontal regeneration alone or in combination with bone grafts.
Aim:
This study assesses bone fill in intrabony defects, following the use of β tricalcium phosphate (TCP) bone graft with and without PRF.
Materials and Methods:
Thirty sites with intrabony defects in periodontitis patients were selected, randomly allotted into three groups: Group A open flap debridement (OFD), Group B OFD with β TCP with PRF, and Group C β TCP. Clinical parameters such as plaque index, gingival index, sulcus bleeding index, and PPD recorded at baseline and 6 months. Radiographic parameters include cementoenamel junction (CEJ) to base of defect, CEJ to alveolar crest, depth of defect, and bone fill assessed using the cone-beam computed tomography (CBCT). The comparison between the test group and control group in terms of clinical and radiographical parameters was assessed using the independent sample t-test.
Results:
Significant reduction in probing depth measurements, defect fill observed in both β TCP with PRF and β TCP alone groups compared to OFD. However, intergroup comparison assessed using the independent sample t-test found to be statistically nonsignificant (P < 0.05 is considered significant).
Conclusion:
All three treatment strategies resulted in significant reduction in probing depth and bone fill at 6 months. Bone fill achieved in β TCP with PRF was more compared to β TCP alone and OFD at 6 months follow-up. CBCT can be accurately used to assess the morphology of intrabony defect and also in evaluating bone fill.
Keywords: Cone-beam computed tomography, intrabony defects, platelet-rich fibrin, β tricalcium phosphate
INTRODUCTION
Periodontitis is a disease of the periodontium characterized by the irreversible loss of connective tissue attachment and supporting alveolar bone.[1] Loss of attachment due to periodontal disease can be regenerated with definitive periodontal therapy. Intraosseous periodontal defects of varying morphology may have a varying regenerative potential depending on the extent of the source of the cells of periodontium.[2]
The periodontal regeneration requires an orchestrated sequence of biological factors and techniques for its successful outcome.[3,4] The combination of various regenerative biologic agents and techniques has attracted the interest of researchers in the field of reconstructive periodontal surgery. Autogenous bone grafting is considered the gold standard for grafting, although it has limitations.[5]
Traditional synthetic bone grafts are ceramics of hydroxyapatite, tricalcium phosphate (TCP) or combination of two which are resorbable materials.[6] Among the tested bone substitutes, β TCP showed significantly higher percentage of bone fill at 24 weeks of healing.[7] It is well tolerated and has no adverse affects such as allergic reactions.[8]
Platelet-rich fibrin (PRF), which belongs to the second-generation platelet concentrate, was first developed in France by Choukroun et al.[9] The natural fibrin clot in PRF seems to be responsible for the slow release of growth factors for an extended period.[10] Because of its strict autologous nature, extended growth factor release, and cost-effectiveness, PRF may be considered as a better treatment option compared to platelet-rich plasma – a first-generation platelet concentrate.[11]
Researchers reported successful bone regeneration, and bone fill was achieved using PRF in periodontal sites. However, human clinical studies using PRF in periodontal therapy are limited. Since β-TCP is tolerated well and has no adverse effects such as allergic reactions, it is of biologic and clinical interest to use β-TCP as a bone substitute in conjunction with PRF. It might be hypothesized that β-TCP may resorb, thus allowing the newly forming periodontal tissues to fill the available space.[12]
The purpose of the present study to evaluate bone fill in intra bony defects following regeneration with (βTCP) with and without PRF.
MATERIALS AND METHODS
Patient selection
A total of 30 sites with intrabony defects in periodontitis patients were selected from the outpatient department of periodontics and implantology, after ethical and research approval, written consent was obtained from the participants of the study. Sites with the presence of probing depth >5 mm after nonsurgical therapy and radiographical presence of an intra bony defect ( Intraoral Periapical Radiograph (IOPA) taken to detect intra bony defects, only 3 wall defects were included) were selected for the study. Patients with systemic disease, pregnant and lactating women, smokers >20 cigarettes per day, tobacco users, allergy to β TCP, and previous periodontal surgery performed teeth were excluded.
After evaluating the oral hygiene after routine nonsurgical therapy cases proceeded for surgical therapy.
Clinical and radiographic measurements
Clinical parameters: plaque index (Silness and Loe),[13] gingival index (Loe and Silness),[13] sulcus bleeding index (Muhlemann and son),[14,15] and probing depth were recorded at baseline and after 6 months.
Radiological parameters cementoenamel junction (CEJ) to base of defect and bone fill measured at baseline and after 6 months using the cone-beam computed tomography (CBCT). Thirty intrabony defects were divided into three groups: Group A, B, and Group C. Group A treated with open-flap debridement (OFD) alone, Group B with β TCP with PRF, and Group C OFD with β TCP alone. Treatment order followed was random and blinding of observer/examiner as well as statistician was done. Single observer performed all clinical measurements preoperatively as well as postoperatively without the knowledge of treatment groups. Patients were not aware of the treatment group.
Radiographic assessment of intrabony defect was recorded by the CBCT images.
The measurements of CBCT images (in millimeters) were recorded in sagittal section views.
Cemento enamel junction to base of the defect at baseline [Figure 1].
Figure 1.
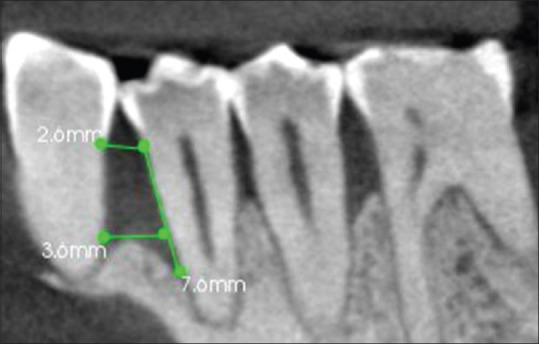
Open flap debridement with β tricalcium phosphate group, Sagittal section of CBCT showing intrabony defect mesial to 44, cementoenamel junction to base of defect at baseline (A) = 7.6 mm
Cemento enamel junction to alveolar crest [Figure 2].
Figure 2.
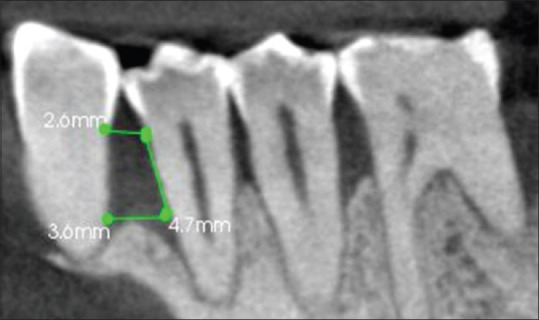
Cementoenamel junction to alveolar crest (B) = 4.7 mm
Cemento enamel junction to base of defect at 6 months [Figure 3].
Figure 3.
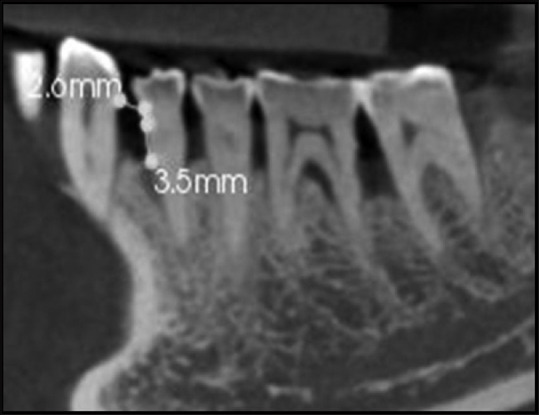
Cemento enamel junction to base of defect at 6months 3.5mm (D) Bone fill A-D 7.6-3.5= 4.1mm
were as follows
CEJ to base of defect at baseline “A,” CEJ to alveolar crest “B,” Depth of defect C = A−B, CEJ to base of defect at 6 months “D,” Bone fill E = A−D.
Preparation of platelet-rich fibrin
PRF was prepared by drawing 6 ml intravenous blood from the antecubital fossa of the patient as per Choukroun's standard protocol[16] (using a centrifuge at 3000 rpm for 10 min). The PRF clot/gel located in the middle of the tube soaked in acellular plasma was removed with tweezers and used as graft material at the experimental site.
Surgical procedure
Kirkland flap was chosen as the flap of choice, and a full-thickness mucoperiosteal flap was reflected till the exposure of the base of the osseous defect. Then, a thorough surgical debridement and root planning were performed. The PRF was prepared by following the protocol developed by Choukroun et al. (Choukroun et al., 2000) PRF was mixed with β TCP (SYBOGRAF-T) of particle size 300–400 micron.[17] βTCP bone graft mixed with PRF placed in intrabony defect. This mixed mass was properly condensed into the defect site for Group B or Group C, β TCP bone graft alone was packed in the intrabony defect. Defect closed without any graft in Group A. Flaps were adapted back to their original position and suturing were done using 3-0 silk sutures. A periodontal dressing (Coe-pack) was applied to the surgical sites.
Postoperative care
All patients received systemic antibiotic therapy (amoxicillin 500 mg thrice daily) for 5 days, Flagyl (metronidazole 400 mg twice daily for 5 days), and analgesic therapy (Acecloren-P 100 mg/500 mg tablets twice daily) for 3 days to reduce discomfort and postoperative pain and edema. Postsurgical instructions were given, and patients were recalled after 7 days for the removal of periodontal dressing and sutures.
Clinical and radiological parameters were recorded after 6 months.
Statistical analysis
Intragroup comparison of clinical and radiological parameters at different time periods analyzed using the paired t-test. Intergroup comparison of parameters in between three groups analyzed using the unpaired t-test. P < 0.001 used for statistical analysis.
RESULTS
Thirty intrabony defects were treated with either OFD + β TCP + PRF; β TCP alone or OFD alone. No adverse reactions seen during the study period.
Plaque index, gingival index, and sulcus bleeding index showed significant reduction from baseline to 6 months [Tables 1-3].
Table 1.
Mean comparison of plaque index between baseline and 6 months visit in Group A, Group B, and Group C
| Plaque index | ||||||
|---|---|---|---|---|---|---|
| Groups | Visits | Mean±SD | Mean±SD difference | Percentage of decrease | t | P |
| Group A | Baseline | 2.48±0.53 | 1.61±0.32 | 64.92 | 8.864 | <0.001* |
| 6 months | 0.87±0.21 | |||||
| Group B | Baseline | 2.42±0.52 | 1.64±0.29 | 67.77 | 8.630 | <0.001* |
| 6 months | 0.78±0.23 | |||||
| Group C | Baseline | 2.43±0.47 | 1.58±0.07 | 65.02 | 6.256 | <0.001* |
| 6 months | 0.85±0.40 | |||||
Values expressed as mean±SD. Paired t-test, *P<0.001 significant. SD – Standard deviation; t – T value measured using Paired t test; P – Probability value
Table 2.
Mean comparison of gingival index between baseline and 6 months in Group A, Group B, and Group C
| Gingival index | ||||||
|---|---|---|---|---|---|---|
| Groups | Visits | Mean±SD | Mean±SD difference | Percentage of decrease | t | P |
| Group A | Baseline | 2.41±0.48 | 1.67±0.26 | 69.29 | 9.554 | <0.001* |
| 6 months | 0.74±0.22 | |||||
| Group B | Baseline | 2.40±0.46 | 1.89±0.25 | 78.75 | 12.549 | <0.001* |
| 6 months | 0.51±0.21 | |||||
| Group C | Baseline | 2.25±0.54 | 1.58±0.24 | 70.22 | 9.214 | <0.001* |
| 6 months | 0.67±0.30 | |||||
Values expressed as mean±SD. Paired t-test, *P<0.001 significant. SD – Standard deviation; t – T value measured using Paired t test; P – Probability value
Table 3.
Mean comparison of sulcus bleeding index between baseline and 6 months visit in Group A, Group B, and Group C
| Sulcus bleeding index | ||||||
|---|---|---|---|---|---|---|
| Groups | Visits | Mean±SD | Mean±SD difference | Percentage of decrease | t | P |
| Group A | Baseline | 2.45±0.47 | 1.60±0.15 | 65.31 | 10.667 | <0.001* |
| 6 months | 0.85±0.32 | |||||
| Group B | Baseline | 2.70±0.26 | 2.14±0.02 | 79.26 | 24.026 | <0.001* |
| 6 months | 0.56±0.28 | |||||
| Group C | Baseline | 2.60±0.39 | 2.07±0.05 | 79.62 | 10.363 | <0.001* |
Values expressed as mean±SD. Paired t-test, *P<0.001 significant. SD – Standard deviation; t – T value measured using Paired t test; P – Probability value
All the three treatment groups showed significant reduction in probing pocket depth (PD) at 6 months. PPD in OFD + β TCP + PRF group at 6 months was 1.8 ± 0.26 mm compared to 2.15 ± 0.63 mm in β TCP group and in OFD 2.3 ± 0.48 mm [Table 4]. Intergroup comparison of plaque index, gingival index, and sulcus bleeding index between treatment groups showed no significant difference at different time periods [Table 5].
Table 4.
Mean comparison of probing pocket depth among baseline and 6 months visit in Group A, Group B, and Group C
| Probing pocket depth | ||||
|---|---|---|---|---|
| Groups | Visit | Mean±SD | F | P |
| Group A | Baseline | 5.60±0.97 | 70.109 | <0.001* |
| 1 month | 2.90±0.39 | |||
| 6 months | 2.30±0.48 | |||
| Group B | Baseline | 6.10±0.99 | 147.832 | <0.001* |
| 1 month | 2.30±0.26 | |||
| 6 months | 1.80±0.26 | |||
| Group C | Baseline | 5.60±0.97 | 63.142 | <0.001* |
| 1 month | 2.40±0.66 | |||
| 6 months | 2.15±0.63 | |||
Values expressed as mean±SD. Paired t-Test, *P<0.001 significant. SD – Standard deviation; F – Fisher test value; P – Probability value
Table 5.
Inter group comparison of plaque index, gingival index, sulcus bleeding index, probing pocket depth from baseline to 6 months
| Visit | Baseline to 6 months, mean±SD difference | |||
|---|---|---|---|---|
| Plaque index | Gingival index | Sulcus bleeding index | Probing pocket depth | |
| Group A | 1.61±0.32 | 1.67±0.26 | 1.60±0.15 | 3.30±0.49 |
| Group B | 1.64±0.29 | 1.89±0.25 | 2.14±0.02 | 4.30±0.73 |
| t | 0.012 | −1.135 | −1.135 | −2.424 |
| P | 0.990 (NS) | 0.272 (NS) | 0.272 (NS) | 0.027 (S) |
| Group A | 1.61±0.32 | 1.67±0.26 | 1.60±0.15 | 3.30±0.49 |
| Group C | 1.58±0.07 | 1.58±0.24 | 2.07±0.05 | 3.45±0.34 |
| t | 0.003 | 0.413 | 0.413 | −0.917 |
| P | 0.998 (NS) | 0.684 (NS) | 0.684 (NS) | 0.370 (NS) |
| Group B | 1.64±0.29 | 1.89±0.25 | 2.14±0.02 | 4.30±0.73 |
| Group C | 1.58±0.07 | 1.58±0.24 | 2.07±0.05 | 3.45±0.34 |
| t | −0.008 | 1.633 | 1.633 | 1.624 |
| P | 0.994 (NS) | 0.120 (NS) | 0.120 (NS) | 0.122 (NS) |
Values expressed as mean±SD. Independent sample t-test, Statistically significant if P<0.05. NS – Not significant; SD – Standard deviation; S – Significant; t – T value measured using Paired t test; P – Probability value
Significant amount of bone fill and percentage bone fill recorded for the three treatment groups. Mean bone fill in β TCP + PRF group was 2.51 ± 0.91 mm compared to 2.02 ± 0.64 mm in β TCP and 1.23 ± 0.22 mm in OFD group [Table 6]. Intergroup comparison of bone fill was not statistically significant at 6 months between the Group B and Group C.
Table 6.
Mean comparison of bone fill from baseline to 6 months in Group A, B, and Group C
| Radiographic parameters: CEJ to base of defect | |||||||
|---|---|---|---|---|---|---|---|
| Groups | Visits | Mean | SD | Amount of bone fill | Percentage of bone fill | t | P |
| Group A | Baseline | 5.69 | 0.97 | 1.23±0.22 | 21.62 | 6.804 | <0.001 (S) |
| 6 months | 4.46 | 1.19 | |||||
| Group B | Baseline | 5.60 | 1.43 | 2.51±0.91 | 44.82 | 7.062 | <0.001 (S) |
| 6 months | 3.09 | 0.52 | |||||
| Group C | Baseline | 6.08 | 1.51 | 2.02±0.64 | 33.22 | 5.232 | <0.001 (S) |
| 6 months | 4.06 | 0.87 | |||||
Values expressed in mean±SD. Paired t-test. Statistically significant if P<0.05. S – Significant; SD – Standard deviation; CEJ – Cemento enamel junction; t – T value measured using Paired t test; P – Probability value
Percentage bone fill in β TCP with PRF group was 44.82% and 33.22% in β TCP compared to 21.52% in OFD group. Mean bone fill and percentage bone fill were greater in β TCP + PRF group than in β TCP and OFD group [Tables 6 and 7].
Table 7.
Intergroup comparison of bone fill in cone-beam computed tomography
| Bone fill | |||
|---|---|---|---|
| Visit | Mean±SD difference | t | P |
| Group A | 1.23±0.22 | -2.888 | 0.010 (S) |
| Group B | 2.51±0.91 | ||
| Group A | 1.23±0.22 | -2.153 | 0.044 (S) |
| Group C | 2.02±0.64 | ||
| Group B | 2.51±0.91 | 0.457 | 0.653 (NS) |
| Group C | 2.02±0.64 | ||
Values expressed in mean±SD. Paired t-test, P<0.05 significant, S – Significant; NS – Non significant; CEJ – Cementoenamel junction; SD – Standard deviation; t – T value measured using Paired t test; P – Probability value
DISCUSSION
Periodontitis is an inflammatory disease that causes pathological alteration in tooth supporting tissues which can lead to tooth loss if left untreated. The ultimate goal of periodontal therapy is to regenerate the lost periodontal tissues.[18] Bone grafts, root biomodification, and guided tissue regeneration (GTR) techniques used to gain these therapeutic benefits. β TCP is biocompatible osteointegrative, osteoconductive material that is widely used in intrabony defects. The graft particles are composed of a highly porous matrix with 100–300 μm pore size.[19] Osteoconductive property of βTCP bone graft is responsible for bone fill in intrabony defects PRF described by Choukroun et al.[9] is a second-generation platelet concentrate which contains platelets and growth factors. PRF has sustained release of growth factors for 1 week[10,20] and up to 28 days.[21] Leukocytes produce large amounts of vascular endothelial growth factor to promote angiogenesis and serve as a biological healing matrix by supporting cell migration and cytokine release.[22] Hence, PRF can be used as potential adjunct in the treatment of intrabony defects. The results of the present study indicate that combination of PRF with β TCP had greater bone fill compared to OFD and OFD with β TCP.
Clinical parameters also evaluated between treatment groups. In the present study, plaque index, gingival index, and sulcus bleeding index improved in all the three treatment groups from baseline to 6 months. However, intergroup comparison of these indices was not statistically significant.
In the present study, smokers <20 cigarettes per day were included. There is no significant difference in tissue gained in smokers and nonsmokers. This is in accordance with study conducted by Tonetti et al.[23] in which they evaluated the effect of cigarette smoking on the healing response following GTR in deep infrabony defects. However, there was difference in probing attachment gain at 1 year between smokers (2.1 ± 1.2 mm) and nonsmokers (5.2 ± 1.9 mm) in their study.
PD reduction in β TCP with PRF group was found to be 4.3 ± 0.73 mm compared to β TCP alone (3.45 ± 0.34 mm) and (3.3 ± 0.34 mm) in OFD group. This is in accordance with a study conducted by Sharma and Pradeep[24] in which they evaluated autologous PRF in treatment of intrabony defects where mean PD reduction was 4.55 ± 1.87 mm in the test group and 3.21 ± 1.64 mm in the control group. There was greater PD reduction and bone fill at sites treated with PRF with conventional open-flap debridement compared to conventional open-flap debridement alone.
In the present study, vertical bone fill measured on CBCT and compared between the groups. Mean bone fill in OFD with PRF with β TCP group was 2.51 ± 0.97 mm compared to 2.02 ± 0.64 mm in OFD with β TCP alone and 1.23 ± 0.22 mm in OFD group.
Chadwick et al.[25] evaluated the use of Demineralized Freeze-Dried Bone Allograft (DFDBA) versus PRF for the treatment of periodontal intrabony defects. PRF group had mean radiographic bone fill of 1.10 ± 1.01 mm and DFDBA 1.14 ± 0.88 mm. There was significant gain in bone fill after 6 months of healing, with no significant difference between PRF and DFDBA In the present study, osteoconductive nature of β TCP bone graft is responsible for greater bone fill in β TCP group compared to OFD alone. However, when PRF is combined with β TCP has still more additive effect on bone fill as it promotes wound healing, bone growth maturation, graft stabilization, hemostasis, and improves handling properties of graft material. PRF contains PDGF and TGF-β growth factors which help in protein synthesis in osseous tissues, stimulates angiogenesis, and enhance woven bone formation. The present study has certain limitations. Long-term effect of the treatment options need to be assessed with larger sample size and longer study period. As the study is not a split-mouth study, inter patient variation may affect the prognosis of the treatment.
CONCLUSION
Within the limitations of the present study, it can be concluded that the combination of β TCP with PRF resulted in greater bone fill compared to β TCP alone and OFD in the treatment of intrabony defects. CBCT offers accurate evaluation of bone fill and can be used for the diagnosis and treatment of intrabony defects.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet. 2005;366:1809–20. doi: 10.1016/S0140-6736(05)67728-8. [DOI] [PubMed] [Google Scholar]
- 2.Manson JD. Bone morphology and bone loss in periodontal disease. J Clin Periodontol. 1976;3:14–22. doi: 10.1111/j.1600-051x.1976.tb01847.x. [DOI] [PubMed] [Google Scholar]
- 3.Caffesse RG, Quinones CR. Polypeptide growth factors and attachment proteins in periodontal wound healing and regeneration. Periodontol 2000. 1993;1:69–79. [PubMed] [Google Scholar]
- 4.Carranza FA., Jr . A Textbook on Clinical Periodontology. 10th ed. Philadelphia: W.B Saunders; 1996. Reconstructive osseous surgery; pp. 269–78. [Google Scholar]
- 5.Nasr HF, Aichelmann-Reidy ME, Yukna RA. Bone and bone substitutes. Periodontol 2000. 1999;19:74–86. doi: 10.1111/j.1600-0757.1999.tb00148.x. [DOI] [PubMed] [Google Scholar]
- 6.Bansal R, Patil S, Chaubey KK, Thakur RK, Goyel P. Clinical evaluation of hydroxyapatite and β-tricalcium phosphate composite graft in the treatment of intrabony periodontal defect: A clinic-radiographic study. J Indian Soc Periodontol. 2014;18:610–7. doi: 10.4103/0972-124X.142455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buser D, Hoffmann B, Bernard JP, Lussi A, Mettler D, Schenk RK. Evaluation of filling materials in membrane-protected bone defects. A comparative histomorphometric study in the mandible of miniature pigs. Clin Oral Implants Res. 1998;9:137–50. doi: 10.1034/j.1600-0501.1998.090301.x. [DOI] [PubMed] [Google Scholar]
- 8.Mellonig JT. Autogenous and allogeneic bone grafts in periodontal therapy. Crit Rev Oral Biol Med. 1992;3:333–52. doi: 10.1177/10454411920030040201. [DOI] [PubMed] [Google Scholar]
- 9.Choukroun J, Adda F, Schoeffler C, Vervelle A. PRF: An opportunity in perio-implantology. Implantodontie. 2001;42:55–62. [Google Scholar]
- 10.Dohan Ehrenfest DM, de Peppo GM, Doglioli P, Sammartino G. Slow release of growth factors and thrombospondin-1 in Choukroun's platelet-rich fibrin (PRF): A gold standard to achieve for all surgical platelet concentrates technologies. Growth Factors. 2009;27:63–9. doi: 10.1080/08977190802636713. [DOI] [PubMed] [Google Scholar]
- 11.Pradeep AR, Rao NS, Agarwal E, Bajaj P, Kumari M, Naik SB. Comparative evaluation of autologous platelet-rich fibrin and platelet-rich plasma in the treatment of 3-wall intrabony defects in chronic periodontitis: A randomized controlled clinical trial. J Periodontol. 2012;83:1499–507. doi: 10.1902/jop.2012.110705. [DOI] [PubMed] [Google Scholar]
- 12.Pinipe J, Mandalapu NB, Manchala SR, Mannem S, Gottumukkala NV, Koneru S. Comparative evaluation of clinical efficacy of ß-tri calcium phosphate (Septodont-RTR)™ alone and in combination with platelet rich plasma for treatment of intrabony defects in chronic periodontitis. J Indian Soc Periodontol. 2014;18:346–51. doi: 10.4103/0972-124X.134573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Löe H. The gingival index, the plaque index and the retention index systems. J Periodontol. 1967;38:610–6. doi: 10.1902/jop.1967.38.6.610. [DOI] [PubMed] [Google Scholar]
- 14.Nasr HF, AichelmannReidy ME, Yukna RA. Bone and bone substitutes. Periodontol 2000. 1999;19:74–86. doi: 10.1111/j.1600-0757.1999.tb00148.x. [DOI] [PubMed] [Google Scholar]
- 15.Benqué E, Zahedi S, Brocard D, Oscaby F, Justumus P, Brunel G. Guided tissue regeneration using a collagen membrane in chronic adult and rapidly progressive periodontitis patients in the treatment of 3-wall intrabony defects. J Clin Periodontol. 1997;24:544–9. doi: 10.1111/j.1600-051x.1997.tb00227.x. [DOI] [PubMed] [Google Scholar]
- 16.Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J, et al. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part II: Platelet-related biologic features. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:e45–50. doi: 10.1016/j.tripleo.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 17.Zaner DJ, Yukna RA. Particle size of periodontal bone grafting materials. J Periodontol. 1984;55:406–9. doi: 10.1902/jop.1984.55.7.406. [DOI] [PubMed] [Google Scholar]
- 18.He l, Lin Y, Hu X. A comparative study of platelet rich fibrin (PRF) and platelet rich plasma (PRP) on the effect of ploliferation and differentiation of rat osteoblasts invitro. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108(105):572–9. doi: 10.1016/j.tripleo.2009.06.044. [DOI] [PubMed] [Google Scholar]
- 19.Rai JJ, Kalantharakath T. Biomimetic ceramics for periodontal regeneration in infrabony defects: A systematic review. J Int Soc Prev Community Dent. 2014;4:S78–92. doi: 10.4103/2231-0762.146207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J, Gogly B, et al. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part III. Leuco-cyte activation: A new feature for platelet concentrates? Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:E51–55. doi: 10.1016/j.tripleo.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 21.He L, Lin Y, Hu X, Zhang Y, Wu H. A comparative study of platelet-rich fibrin (PRF) and platelet-rich plasma (PRP) on the effect of proliferation and differentiation of rat osteoblasts in vitro. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:707–13. doi: 10.1016/j.tripleo.2009.06.044. [DOI] [PubMed] [Google Scholar]
- 22.Dohan Ehrenfest DM, Rasmusson L, Albrektsson T. Classification of platelet concentrates: From pure plateletrich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF) Trends Biotechnol. 2008;27:158–67. doi: 10.1016/j.tibtech.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 23.Tonetti MS, Pini-Prato G, Cortellini P. Effect of cigarette smoking on periodontal healing following GTR in infrabony defects. A preliminary retrospective study. J Clin Periodontol. 1995;22:229–34. doi: 10.1111/j.1600-051x.1995.tb00139.x. [DOI] [PubMed] [Google Scholar]
- 24.Sharma A, Pradeep AR. Treatment of 3-wall intrabony defects in patients with chronic periodontitis with autologous platelet.rich fibrin: A randomized controlled clinical trial. J Periodontol. 2011;82:1705–12. doi: 10.1902/jop.2011.110075. [DOI] [PubMed] [Google Scholar]
- 25.Chadwick JK, Mills MP, Mealey BL. Clinical and radiographic evaluation of demineralized freeze-dried bone allograft versus platelet-rich fibrin for the treatment of periodontal intrabony defects in humans. J Periodontol. 2016;87:1253–60. doi: 10.1902/jop.2016.160309. [DOI] [PubMed] [Google Scholar]


