Abstract
Background:
Collagen fibers are the main element of gingival connective tissue and contribute a leading role in the preservation of structural integrity and tissue function. Hence, its degradation is regarded as the main marker of periodontal disease progression.
Aim:
The aim of this study is to analyze and compare collagen fibers, their birefringence pattern in healthy and in diseased gingival tissues stained using picrosirius red stain (PRS) and the polarizing microscope.
Materials and Methods:
A total of 90 participants screened were divided into the control group (healthy gingiva) and experimental group (moderate periodontitis and severe periodontitis) based on the clinical parameters. Gingival tissue sections were stained with PRS and observed under the polarized microscope to assess the type of collagen fibers in healthy and diseased gingival tissue. Statistical analysis was performed using the one-way ANOVA and Tukey multiple comparison test.
Results:
The healthy group revealed well-packed collagen in a parallel pattern with a strong birefringence, whereas in severe periodontitis group showed loosely packed collagen fibers in a haphazard pattern suggestive of severe destruction of the extracellular matrix. The moderate periodontitis group had a blended mixture of thick and thin fibers.
Conclusions:
Collagen fibers showed birefringence property when stained with PRS that helps in a better understanding of normal and pathological conditions.
Keywords: Birefringence, collagen, extracellular matrix, pathogenesis, periodontitis
INTRODUCTION
Periodontitis, the most prevalent and immune-mediated inflammatory disease affects periodontal tissues. Understanding disease pathogenesis is very essential as various treatment modalities depend on the same.
The causative factors responsible for periodontitis are heterogeneous, including complex microorganisms, genetic influences, individual behavioral factors, and so on.[1] Bacterial complexes and their products are the primary etiological agents responsible for periodontal tissue destruction, that initiates the underlying immune-inflammatory response and generate self-destruction of the connective tissue of the periodontium. This unbalanced release of bacterial and host substances results in the degradation of the extracellular matrix.[2]
Collagen fibers are the main element of gingival connective tissue. Its deposition and degradation occur in a well-regulated manner for maintaining tissue architecture. The significant changes and loss of the collagen component reflect the severity of the periodontal disease. Hence, collagen degradation is regarded as the main marker of periodontal disease progression.[3]
Different staining techniques are used to differentiate the different types of collagen fibers, among which picrosirius red stain (PRS) is more accurate and extremely specific for staining of collagen fibers which also displays polarizing characteristics.[4]
The elongated dye molecules are aligned to the collagen fiber in such a way that their long axis is parallel. This parallel relationship between dye and collagen permits the property of an enhanced birefringence. Collagen has the inherent property of birefringence. Optical technologies such as the polarized microscope are popular tools to assess the birefringence architecture of the tissue, indicative of the organization of collagen fibers in connective tissues in health and disease.[5] A combination of staining and enhancement of birefringence by sirius red offers a simple, specific, sensitive method to distinguish between normal and pathological collagen.
A better perception of the first changes that happen in the gingiva can be beneficial to ascertain the course of damage occurring to other periodontal tissues. Histochemical research on collagen enables the biology of this family of macromolecules to be mastered better.
The current study aimed at analyzing and comparing collagen fibers, their birefringence pattern in healthy and in diseased gingival tissues using PRS, the polarizing microscope as well as image–analyzer software for a better understanding of collagen fibers in health and pathology.
MATERIALS AND METHODS
A total of 90 participants in an age group of 30–50 years were screened and divided into the following groups based on the clinical parameters, including the color, bleeding on probing, probing depth, clinical attachment loss, and gingival indices.
Group A (control group): Consisted of 30 systemically healthy patients with gingival index less than 1.0.
Group B (experimental groups): Consisted of 60 patients with chronic periodontitis and was further divided into two groups based on the international workshop for the classification of periodontal diseases and conditions 1999.[6]
Group B1–30 patients with moderate periodontitis
Group B2–30 patients with severe periodontitis.
With the patient's consent, gingival tissue samples that would have been discarded were obtained from the patients of the control group (following extraction for an orthodontic reason, extraction of the noninfected impacted tooth, and crown lengthening procedures) and experimental group (following periodontal surgical procedures).
All gingival samples (0.3 cm × 0.2 cm) were obtained from buccal marginal tissues and one biopsy was taken per patient. Approval was obtained from the Institutional Ethics Committee before the commencement of the research study.
Tissue preparation and collagen staining for histopathological analysis
An excised gingival tissue sample was fixed in 10% neutral-buffered formalin for 24 h, followed by routine paraffin-embedded technique, and two sections of 5 μm thickness were taken. One slide was stained with hematoxylin and eosin (H and E) and observed under light microscopy to confirm the clinical diagnosis and evaluate inflammatory components and the second one was stained with PRS according to Junqueria et al.[7] and observed under polarizing microscopy (POLYVAR2 research microscope) to evaluate the arrangement, type, and polarization color of collagen fiber bundles in the connective tissues [Figure 1].
Figure 1.

POLYVAR2 research microscope-polarizing microscope
Examination of polarization colors of picrosirius stained collagen fibers under a polarizing microscope
The identification and evaluation of polarization colors of thin (0.8 μm or less) and thick collagen fibers (1.6–2.4 μm) were done with the aid of TC-capture image analyzer software. In each section, three separate high-power fields with at least 50 fibers were examined, and the mean value was recorded.
Collagen when stained with PSR and when viewed under polarized light microscopy normally shows polarization colors (birefringence pattern):
Green birefringence ranging from blue to green
Yellow birefringence ranging from yellowish green and yellowish orange
Red birefringence ranging from orange red.
Under polarized light, the thickest collagen fibers appeared yellow/orange through orange to red while the finest are green to greenish yellow.
Statistical analysis
To eliminate subjective bias, two observers autonomously evaluated all cases. Descriptive statistics and mean and standard deviation were calculated for the continuous variables. A comparison between thick and thin collagen fibers was done using the one-way ANOVA and Tukey multiple comparison test. P < 0.05 was considered to be statistically significant.
RESULTS
Histopathology-hematoxylin and eosin staining
Tissue samples from the healthy group (Group A) showed long rete pegs, thick collagen fibers in the deeper lamina propria, arranged in a parallel pattern, containing dense and plump fibroblasts. Inflammatory cells in a scarce (<15 inflammatory cells/field) in diffuse form were seen between collagen fibers, located more frequently near the blood vessels. Small-caliber blood vessels or even capillaries were seen in deep connective tissue, proving to be quite vascularized [Figure 2].
Figure 2.
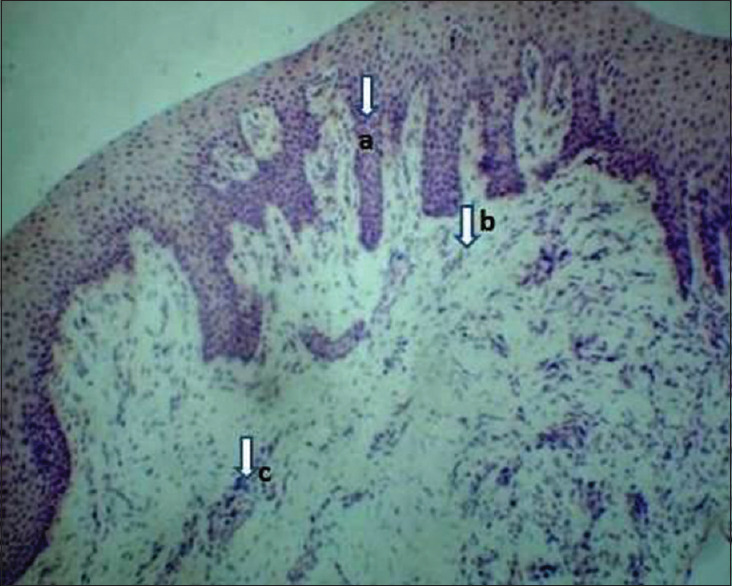
Photomicrograph showing healthy gingival tissue section (Group A), H and E, ×10. (a) Long rete pegs, (b) Collagen fibers containing dense and plump fibroblasts, (c) Blood vessels
Tissue samples from a moderate periodontitis group (Group B1) showed dilated blood vessels; long rete pegs, localized mixed inflammatory cells (15–50 inflammatory cells/field) of which chronic inflammatory cells are predominant, more of lymphocytes, plasma cells with few acute inflammatory cells. Collagen fibers were slightly loosely arranged, but the parallel pattern was retained [Figure 3].
Figure 3.
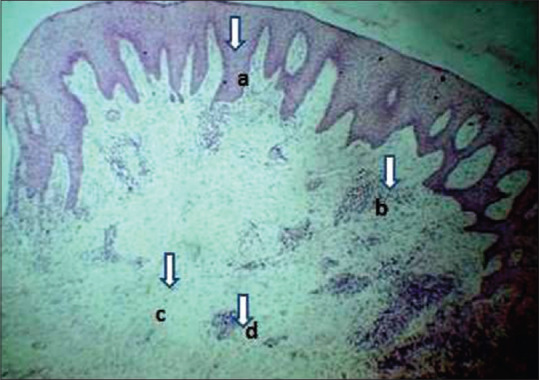
Photomicrograph showing a case of moderate periodontitis gingival tissue section (Group B1) H and E, ×10. (a) Long rete pegs, (b) Localized inflammatory cells, (c) Loosely arranged collagen fibers, (d) Dilated blood vessels
Tissue samples from the chronic periodontitis group (Group B2) showed a disruption of the extracellular matrix organization, collagen fibers were loosely arranged and showed the haphazard pattern, vacuolated area in-between the connective tissue with very few fibroblasts and a large number of inflammatory cells (>50 inflammatory cells/field) were scattered throughout the tissue more dilated blood vessels characterizing a chronic inflammation of the tissue. There was an apparent decrease in total fibers [Figure 4].
Figure 4.
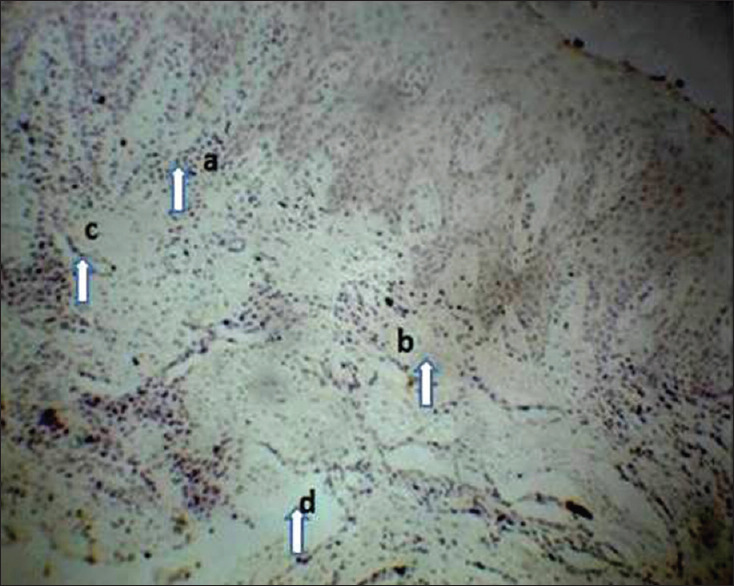
Photomicrograph showing a case of severe periodontitis gingival tissue section (Group B2), H and E, ×10. (a) Inflammatory cells, (b) Loosely arranged collagen fibers, (c) Dilated blood vessels, (d) Vacuolated areas
Histopathology-picrosirius red
This technique allowed an optimal visualization of collagen fibers and their organization within the extracellular matrix. In the control group, collagen bundles revealed a strong birefringence with yellowish–red/green color, and the majority were thick fibers [Figure 5]. The moderate periodontitis group showed yellowish-green birefringence and had a blended mixture of thick and thin fibers [Figure 6]. Greenish birefringence was observed in the severe periodontitis group, and the majority of collagen fibers were predominantly thin and represent loosely packed collagen [Figure 7].
Figure 5.
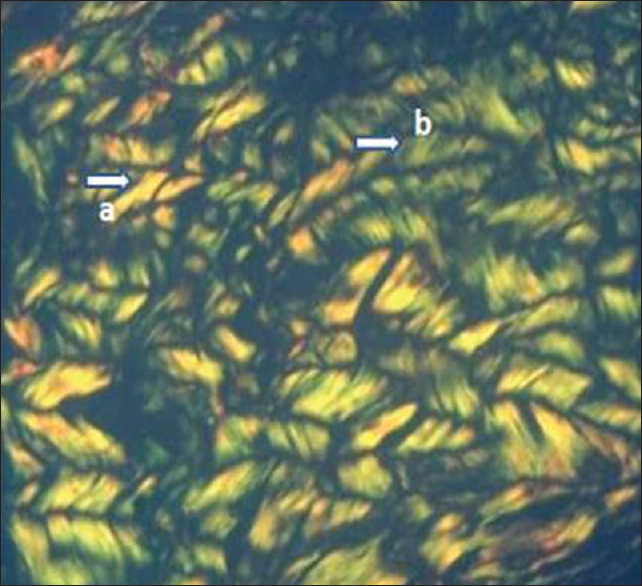
Picrosirius red-stained healthy gingival tissue section (Group A) in × 40 magnification showing collagen fibers with strong birefringence arranged in the parallel fascicular pattern. (a) Thick collagen fibers with yellowish–red birefringence, (b) thick fibers with yellowish-green birefringence
Figure 6.

Picrosirius red-stained moderate periodontitis gingival tissue section (Group B1) in × 40 magnification showing collagen fibers with a blended combination of thick and thin fibers. (a) Thick collagen fibers with yellowish–red birefringence, (b) thin fibers with greenish birefringence
Figure 7.

Picrosirius red stained severe periodontitis gingival tissue section (Group B2) in × 40 magnification. (a) loosely packed collagen fibers with greenish birefringence
The mean percentage of thick fibers is more in the control group (Group A) – 85.73%, followed by moderate periodontitis Group (Group B1) 58% and is less in severe periodontitis (Group B2) – 24.86%, whereas the mean percentage of thin fibers is more in severe periodontitis group (75.133%) and is significant statistically [Table 1].
Table 1.
Crosstab given below reveals the percentage of thick and thin fibers in healthy and periodontitis groups
| Groups | N | Mean | Sth. Deviation | (ANOVA) | P | |
|---|---|---|---|---|---|---|
| Percentage of thick fibres | GROUP A | 30 | 85.7333 | 3.35213 | 1239.787* | <0.001 |
| GROUP B1 | 30 | 58 | 4.95497 | |||
| GROUP B2 | 30 | 24.8667 | 6.00421 | |||
| Total | 90 | 56.2 | 25.48417 | |||
| Percentage of thin fibres | GROUP A | 30 | 14.2667 | 3.35213 | 1239.787* | <0.001 |
| GROUP B1 | 30 | 42 | 4.95497 | |||
| GROUP B2 | 30 | 75.1333 | 6.00421 | |||
| Total | 90 | 43.8 | 25.48417 |
N-number of subjects in each group. P-level of significance, P<0.05 is considered to be statistically significant
The overall polarization colors were determined for all 90 cases, and the results were as follows: 51% in the yellow color range, 29% in the red color range, and 16% of thick fibers were in the green color range. Among thin fibers 34% were in the green color range, 34% in the yellow color range, 7% in the red color range and were statistically significant [Graphs 1 and 2].
Graph 1.
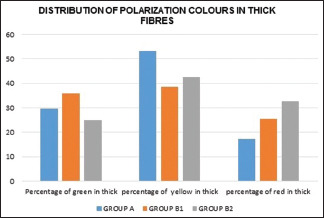
Graphical representation of the distribution of polarization colors of thick fibers in the healthy and periodontitis groups
Graph 2.
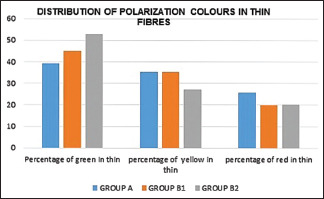
Graphical representation of the distribution of polarization colors of thin fibers in the healthy and periodontitis group
DISCUSSION
The present study comprises participants with an age group of 30–50 years. Histological examination of healthy gingival samples in this study group illustrates healthy aspects of epithelium and connective tissue, and no variation was found in the pattern of collagen fibers. According to a study by Andreescu et al.,[8] participants over 65 years old illustrated modification both in the epithelium and connective tissue. Age-related changes in the mucosa are still controversial, and age alone is not considered a risk factor in developing periodontitis.
The inflammatory mediators produced as a consequence of the host-immune response to the bacteria by the resident and the migrating inflammatory cells results in the degradation of the extracellular matrix.[9]
According to Junqueira et al., the use of the polarizing microscope to identify collagen in the PRS tissue section significantly improves the specificity, sensitivity, and resolution of the techniques.[7] Costa et al.[10] and Arora et al.[11] used PRS as a tool for the detection of collagen fibers in tissue sections.
Considering this evidence, the present study was done to analyze and compare the characterization of collagen fibers in healthy and disease. The inflammatory components were also evaluated histopathologically to different clinical stages of periodontitis.
The findings from the present study of picrosirius-stained collagen fibers under a polarizing microscope showed the following pattern:
In a healthy group, the fibers were dense in a parallel fascicular pattern showing intense birefringence with yellowish–red/yellowish-green color, and the majority were thick fibers. Yellowish red or yellowish green represents well-packed collagen [Figure 5]. This parallel arrangement relatively organized pattern in the healthy group is in agreement with those found by several authors Almedia et al.[12] and Ejeil et al.[3]
In the moderate periodontitis group, the fibers were still distributed in a similar arrangement of healthy group samples, but there was a blended combination of thick and thin fibers and are relatively organized and showed yellowish-green birefringence. Thin fibers showed greenish birefringence suggesting that some areas are loosely packed, and destruction is evident in those regions. Yellowish red/green regions represent the densely packed fibers [Figure 6].
In severe periodontitis, tissue samples showed greenish birefringence, and the majority of collagen fibers were predominantly thin and represent loosely packed collagen that suggests severe destruction of the extracellular matrix [Figure 7]. Ejeil et al.[3] in their study also concluded that the area occupied by the collagen in severely inflamed gingival tissues has been reduced by 20% compared to normal gingival tissues.
These findings were in accordance with Gupta et al.,[4] Junqueira et al.,[8] Dayan et al.,[13] and Lattouf et al.[14] findings. The very thin collagen fibrils which are undetectable under normal microscopy become visible, as a source of light against the dark background.
The color exhibited by these fibers is due to the variability of fiber size, alignment, packing, and molecular organization. Usually green to greenish-yellow represent poorly packed collagen and orange-red originate from well-packed collagen.[15] Junqueira et al. observation was that the fiber thickness plays a significant role in determining the wavelength of polarization colors of PRS sections.[7]
Gupta et al.[4] in their study observed that PRS had enhanced birefringence, longer shelf life and the staining intensities were maintained for many months. The parallel orientation of collagen and Sirius red molecules increase the natural birefringence of collagen and helps in determining the orientation.
Seguier et al.[16] noted that the quantity and quality of collagen fibers are inversely related to the extent of the inflammatory lesion and a significant increase in the number of macrophages in the periodontitis group suggests that these cells are implicated in the course of periodontal disease. Joachim et al.[17] observations were that increased collagen destruction was associated with an increase in plasma cell percentage density.
Macrophages and neutrophils have a common feature of elaboration of a tissue collagenase which is capable of causing the hydrolytic breakdown of peptide bonds in the helical region of collagen. Collagenolytic activity is least in normal and mildly inflamed tissue and greatest in severely inflamed tissue and hyperplastic tissue, thus the inflammatory cell count affects the arrangement of collagen. These modifications in the quantity and quality of collagen fibers result can contribute to accelerating the periodontal disease progression.[18]
However, contrary to the above-mentioned studies, Pierard[19] in his study concluded that the color of fibers appearing after the Picrosirius red polarization method is unrelated to their molecular nature as it may vary according to the orientation of the slide on the stage of the microscope and did not reveal specifically collagen fibers.
Although the present study offers originality and provides evidence regarding morphological and qualitative aspects of collagen fibers in healthy and diseased human gingival tissue, as well as the inflammatory components histopathologically to different clinical stages of periodontitis, had some limitations such as quantitative assessment of the different type of collagen is not carried out, which might limit the extrapolation of results.
CONCLUSIONS
To conclude, the collagen fibers showed birefringence property when stained with PRS and visualized under the polarized microscope and even small fibers could be readily identified. It helps in a better understanding of normal and pathological conditions. Such histochemical research enables the biology of collagen macromolecules to be mastered better, which helps in early detection of collagen destruction and in the development of newer treatment strategies that can prevent such destruction involved in disease progression.
Thus further studies have to be conducted with larger and more homogenous samples to clarify the differences between the presence and distribution of more types of collagen in healthy and diseased human gingival tissue.
The current outcome has enhanced the knowledge of the pathogenesis of periodontal disease and thus thorough studies on histopathological aspects during the development of periodontal disease helps to establish new techniques that may assist in treatment planning and prognosis. The polarization method should be further explored and that it has potential usefulness in the biology and pathology of connective tissue.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Cekici A, Kantarci A, Hasturk H, Van Dyke TE. Inflammatory and immune pathways in the pathogenesis of periodontal disease. Periodontol 2000. 2014;64:57–80. doi: 10.1111/prd.12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kinane DF. Causation and pathogenesis of periodontal disease. Periodontol 2000. 2001;25:8–20. doi: 10.1034/j.1600-0757.2001.22250102.x. [DOI] [PubMed] [Google Scholar]
- 3.Ejeil AL, Igondjo-Tchen S, Ghomrasseni S, Pellat B, Godeau G, Gogly B. Expression of matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) in healthy and diseased human gingiva. J Periodontol. 2003;74:188–95. doi: 10.1902/jop.2003.74.2.188. [DOI] [PubMed] [Google Scholar]
- 4.Gupta S, Aggarwal R, Gupta V, Vij R, Tyagi N, Misra A. Picrosirius red: A better polarizing stain. J Histotechnol. 2017;40:46–53. [Google Scholar]
- 5.Montes GS, Junqueira LC. The use of the picrosirius-polarization method for the study of the biopathology of collagen. Mem Inst Oswaldo Cruz. 1991;86(Suppl 3):1. doi: 10.1590/s0074-02761991000700002. [DOI] [PubMed] [Google Scholar]
- 6.Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999;4:1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- 7.Junqueira LC, Bignolas G, Brentani RR. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J. 1979;11:447–55. doi: 10.1007/BF01002772. [DOI] [PubMed] [Google Scholar]
- 8.Andreescu CF, Mihai LL, Răescu M, Tuculină MJ, Cumpătă CN, Ghergic DL. Age influence on periodontal tissues: A histological study. Rom J Morphol Embryol. 2013;54:811–5. [PubMed] [Google Scholar]
- 9.Garlet GP, Cardoso CR, Silva TA, Ferreira BR, Avila-Campos MJ, Cunha FQ, et al. Cytokine pattern determines the progression of experimental periodontal disease induced by Actinobacillus actinomycetemcomitans through the modulation of MMPs, RANKL, and their physiological inhibitors. Oral Microbiol Immunol. 2006;21:12–20. doi: 10.1111/j.1399-302X.2005.00245.x. [DOI] [PubMed] [Google Scholar]
- 10.Costa GM, Araujo SL, Xavier Júnior FA, Morais GB, Silveira JA, Viana DD, et al. Picrosirius red and Masson's trichrome staining techniques as tools for detection of collagen fibers in the skin of dogs with endocrine dermato pathologies. Braz J Vet Res Anim Sci. 2019;20:1–10. [Google Scholar]
- 11.Arora KS, Nayyar A, Kaur P, Arora KS, Goel A, Singh S. Evaluation of collagen in leukoplakia, oral submucous fibrosis and oral squamous cell carcinomas using polarizing microscopy and immunohistochemistry. Asian Pac J Cancer Prev. 2018;19:1075–80. doi: 10.22034/APJCP.2018.19.4.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Almeida T, Valverde T, Martins-Júnior P, Ribeiro H, Kitten G, Carvalhaes L. Morphological and quantitative study of collagen fibers in healthy and diseased human gingival tissues. Rom J Morphol Embryol. 2015;56:33–40. [PubMed] [Google Scholar]
- 13.Dayan D, Hiss Y, Hirshberg A, Bubis JJ, Wolman M. Are the polarization colors of pirosirius red-stained collagen determined only by the diameter of the fibres? Histochemistry. 1989;93:27–9. doi: 10.1007/BF00266843. [DOI] [PubMed] [Google Scholar]
- 14.Lattouf R, Younes R, Lutomski D, Naaman N, Godeau G, Senni K, et al. Picrosirius red staining: A useful tool to appraise collagen networks in normal and pathological tissues. J Histochem Cytochem. 2014;62:751–8. doi: 10.1369/0022155414545787. [DOI] [PubMed] [Google Scholar]
- 15.Velidandla S, Gaikwad P, Ealla KK, Bhorgonde KD, Hunsingi P, Kumar A. Histochemical analysis of polarizing colors of collagen using picrosirius red staining in oral submucous fibrosis. J Int Oral Health. 2014;6:33–8. [PMC free article] [PubMed] [Google Scholar]
- 16.Séguier S, Godeau G, Brousse N. Immunohistological and morphometric analysis of intra-epithelial lymphocytes and Langerhans cells in healthy and diseased human gingival tissues. Arch Oral Biol. 2000;45:441–52. doi: 10.1016/s0003-9969(00)00018-2. [DOI] [PubMed] [Google Scholar]
- 17.Ranney RR, Debski BF, Tew JG. Pathogenesis of gingivitis and periodontal disease in children and young adults. Pediatr Dent. 1981;3:89–100. [Google Scholar]
- 18.Seguier S, Godeau G, Brousse N. Collagen fibres and inflammatory cells in healthy and diseased human gingival tissues. A comparative and qualitative study by Immuno histochemistry and automated image analysis. J Periodontol. 2000;71:1079–85. doi: 10.1902/jop.2000.71.7.1079. [DOI] [PubMed] [Google Scholar]
- 19.Piérard GE. Sirius red polarization method is useful to visualize the organization of connective tissues but not the molecular composition of their fibrous polymers. Matrix. 1989;9:68–71. doi: 10.1016/s0934-8832(89)80021-6. [DOI] [PubMed] [Google Scholar]


