Abstract
Background
Both preterm and post-term births have been associated with neonatal morbidity and mortality, including adverse impact on neurodevelopment. Important neural maturational processes take place during sleep in newborns, but findings on gestational duration and sleep in early childhood are contradictory and often derive from small clinical samples. We studied the association of gestational age at birth with sleep duration in early childhood in three population-based cohorts.
Methods
Gestational age at birth and sleep duration were assessed in three population-based cohort studies in The Netherlands (n = 6471), Singapore (n = 862), and Canada (n = 583). Gestational age at birth was assessed using ultrasound in pregnancy in combination with date of birth, and caregivers repeatedly reported on child sleep duration at three, six, 24, and 36 months of age. Generalized estimating equations were used, which were adjusted for confounders, and findings were pooled in a meta-analysis.
Results
Children born preterm (<37 weeks of gestation) showed longer sleep duration than children born at term; and children born post-term (≥42 weeks of gestation) showed shorter sleep duration. The meta-analysis indicated a small negative effect of gestational age on child sleep duration (effect size −0.11), when assessed in children born at term only.
Conclusion
In early childhood, children with a lower gestational age have a longer sleep duration, even when they are born at term (37–42 weeks of gestation). These subtle yet consistent findings point to the importance of maturational processes during sleep, not only in premature children but also in children born at term after shorter gestational duration.
Keywords: Gestational age, Sleep, Sleep duration, Cohort studies, Premature birth, Post-term birth
Highlights
-
•
Children born after shorter gestational duration sleep longer than their term born peers.
-
•
Lower gestational age is linked to longer sleep duration in three cohorts, even in term born children (37-42 weeks gestation).
-
•
The association between gestational age and sleep duration is most prominent in the first year of life.
-
•
Our subtle yet consistent findings point to the importance of maturational processes during sleep.
-
•
Maturational processes underlying the interplay between gestational age and sleep duration should be investigated.
1. Introduction
Timely onset of labour is important for perinatal and postnatal health. Both preterm (<37 weeks of gestation) and post-term (≥42 weeks of gestation) births have been associated with neonatal morbidity and mortality, including adverse impact on neurodevelopment 1, 2, 3. Although sleep is crucial for the development of neural networks [4], and preterm birth affects the neural network that regulates sleep architecture [5], effects of gestational age at birth on postnatal sleep have not been well studied [6]. Moreover, findings have been contradictory. Some studies report more sleep problems and a shorter sleep duration in premature infants [7] and also after the first months of life [8]; while others report no differences in sleep duration between preterm and term infants 9, 10 or longer sleep duration in preterm infants 11, 12. Environmental and physiological mechanisms that might explain these contradictory effects are still unclear [6].
Sleep is critical for maturation in all areas of child development [13]. Current knowledge on the effects of gestational age at birth on sleep derives largely from clinical samples with (very) premature infants. Sample sizes are often small, and only a handful of studies used longitudinal designs. Furthermore, both obstetrician policy [1] and sleep habits differ among cultures [14], limiting the generalizability of available studies, mainly conducted in individuals of Caucasian/white ethnicity. In this study, using data from three population-based prospective cohorts across different continents, we explored the association of gestational age at birth and sleep duration in early childhood in the general population.
2. Methods
2.1. Study population
This study was conducted in three cohorts: the Generation R Study [15] (n = 6471; inclusion started in 2002 in The Netherlands); the Growing up in Singapore Towards healthy Outcomes cohort [16] (GUSTO, n = 862; inclusion started in 2009 in Singapore); and the Maternal Adversity, Vulnerability And Neurodevelopmental cohort [17] (MAVAN, n = 581; inclusion started in 2003 in Canada). The three study populations consist of children with information on gestational age at birth and sleep data at three, six, 24, and 36 months of age.
2.2. Gestational age at birth
Ultrasound measurements in early pregnancy (the Generation R Study) and early to mid-pregnancy (GUSTO, MAVAN) were used to determine gestational age to the nearest day in combination with date of birth 15, 16, 17. Preterm birth was defined as birth before 37 weeks gestation, term birth between 37 and 42 weeks gestation, and post-term birth after 42 weeks gestation.
2.3. Sleep assessments
In the all three cohorts, bedtimes, wake times and nap duration were reported by parents 15, 17, 18. Questions were derived from The Brief Infant Sleep Questionnaire (BISQ [19]), and include ‘How much time (on average) does your child spend in sleep during the night?’ and ‘How much time (on average) does your child spend in sleep (naps) during the day?’. Total sleep duration was calculated as hours of sleep per 24 h by adding nighttime and daytime sleep. Sleep duration was reported at ages three, six, 24, and 36 months; correlations between sleep duration at the four time points varied from r = 0.42 to 0.04 (time points closest to each other showed the highest correlations).
2.4. Statistical analyses
Although we had data at each of the time points, we combined all information using generalized estimating equations (GEE) to analyse the relation of gestational age at birth with sleep duration at four time points in three cohorts. With GEE analyses, symptoms repeatedly measured over time can be analyzed, taking into account that these repeated measurements within the same subject are correlated. Next, we compared the groups of preterm and post-term children, using term born children as the reference category (only in the Generation R Study; numbers in the other cohorts were too small: in MAVAN, n < 10 for preterm and post-term born children; in GUSTO, n = 0 for post-term born children). To test for effects of conceptional age, analyses were rerun including only term born children. If main effects were significant, time effects and gestational age × time interactions were tested.
All models (in all cohorts) were adjusted for potential confounders; birth weight and gender of the child, time points of sleep assessments, maternal age and education, and maternal smoking and drinking during pregnancy. In the Generation R Study and GUSTO, analyses were additionally adjusted for maternal ethnicity. Sleep duration was calculated in hours, with effect size B indicating the change in sleep duration per standard deviation change in gestational age. The mean proportion of missing values for confounders was 8.2%, 1.6%, and 8.0% in the Generation R, GUSTO, and MAVAN cohort, respectively, and multiple imputation was performed in 10 datasets. Only confounder variables were imputed. Finally, we performed a meta-analysis with a fixed-effects model (tests of heterogeneity indicated that samples were not heterogeneous).
3. Results
3.1. Gestational age and sleep duration in the three cohorts
Descriptive statistics for the three cohorts are presented in Table 1. Sleep duration for all ages and all cohorts can be found in Table 2. In the Generation R cohort, gestational age at birth was negatively related to total sleep duration (B = −0.13; 95% confidence interval [CI]: −0.18 to −0.07; p < 0.001), night sleep duration (B = −0.06; 95% CI: −0.10 to −0.03; p < 0.001), and nap duration (B = −0.03; 95% CI = −0.06 to −0.01; p < 0.017), indicating that children with lower gestational age sleep longer. A categorical approach showed similar results: preterm children were more likely to have longer sleep duration compared to term children (B = 0.26; 95% CI: 0.09 to 0.42; p = 0.003), whereas post-term children were more likely to have shorter sleep duration compared to term children (B = −0.15; 95% CI: −0.28 to −0.03; p = 0.019). When analyses were restricted to term born children (37–42 weeks of gestation) to address the potential effect of conceptional age, the association remained.
Table 1.
Demographic information of the three study populations.
| Generation R Study (n = 5637) | GUSTO (n = 862) | MAVAN (n = 581) | |
|---|---|---|---|
| Maternal characteristics | |||
| Maternal age at intake, y | 30.8 ± 0.06 | 30. 7 ± 0.17 | 30.7 ± 0.20 |
| Educational level | |||
| Primary education (%) | 8.4 | 4.2 | – |
| Secondary education (%) | 41.4 | 25.1 | – |
| Higher education (%) | 50.2 | – | – |
| High school or less (%) | – | – | 9.8 |
| Some community college (%) | – | – | 9.0 |
| Complete community college (%) | – | – | 32.4 |
| University degree (%) | – | – | 48.8 |
| Post-secondary education (%) | – | 11.5 | – |
| General certificate of education (GCE) (%) | – | 25.9 | – |
| University (%) | – | 33.3 | – |
| Maternal ethnicity | |||
| Dutch (%) | 57.5 | – | – |
| Non-Dutch western (%) | 8.6 | – | – |
| Non-Dutch non-western (%) | 33.9 | – | – |
| Canadian | – | – | 100 |
| Chinese (%) | – | 55.7 | – |
| Malay (%) | – | 26.6 | – |
| Indian (%) | – | 17.6 | – |
| Other (%) | – | 0.1 | – |
| Smoking habits | |||
| Never smoked in pregnancy (%) | 72.5 | 97.6 | 80.3 |
| Smoked in pregnancy (%) | – | 2.4 | – |
| Smoked in early pregnancy (%) | 13.0 | – | 9.8 |
| Smoked throughout pregnancy (%) | 14.5 | – | 9.9 |
| Drinking habits | |||
| Never drank in pregnancy (%) | 38.8 | 97.6 | 59.4 |
| Drank during pregnancy (%) | – | 2.4 | 40.6 |
| During early pregnancy (%) | 17.8 | – | – |
| Throughout pregnancy, occasionally (%) | 34.5 | – | – |
| Throughout pregnancy, frequently (%) | 8.9 | – | – |
| Child characteristics at birth | |||
| Sex of the child (% male) | 50.2 | 52.8 | 54.4 |
| Birth weight, g | 3422 ± 7.1 | 3097 ± 14.7 | 3376 ± 18.9 |
| Gestational age at birth, wk | 39.8 ± 0.02 | 38.4 ± 0.05 | 39.5 ± 0.05 |
| Range | 25.3–43.4 | 25.0–41.0 | 36.7–42.1 |
| Timing of birth | |||
| Term | 87.1 | 93.2 | 98.1 |
| Preterm | 5.8 | 6.8 | 0.9 |
| Post-term | 7.1 | – | 1.0 |
| Mode of delivery (%) | |||
| Vaginal delivery | 70.5 | 65.0 | – |
| Assisted vaginal delivery (forceps/vacuum) | 17.3 | 4.7 | – |
| Cesarian delivery | 12.2 | 30.8 | – |
| Apgar scores at birth | |||
| Apgar <7 at 1 min (%) | 5.4 | 2.6 | 6.4 |
| Apgar <7 at 5 min (%) | 1.0 | – | 1.2 |
All continuous variables are presented as means ± standard error; all categorical variables are presented as percentages.
ap Values are derived from analyses of variance for parametric continuous variables, Kruskal–Wallis tests for nonparametric continuous variables, and χ2.tests for categorical variables with the term born children as the reference group.
Table 2.
Total sleep duration in hours per day in the three cohorts.
| Generation R Study | GUSTO | MAVAN | |
|---|---|---|---|
| 3 Months | |||
| Mean ± SD | 14.6 ± 3.0 | 12.5 ± 3.5 | 14.2 ± 2.5 |
| Range | 6.0–22.0 | 6.0–22.0 | 7.0–21.0 |
| n | 4800 | 537 | 170 |
| % below recommendation (<12 h)a | 15.2 | 41.7 | 12.9 |
| 6 Months | |||
| Mean ± SD | 15.0 ± 2.7 | 12.1 ± 2.5 | 13.2 ± 1.9 |
| Range | 6.0–21.0 | 6.0–21.0 | 7.0–21.0 |
| n | 3691 | 647 | 483 |
| % below recommendation (<12 h)a | 11.3 | 42.0 | 20.3 |
| 24 Months | |||
| Mean ± SD | 13.4 ± 1.1 | 11.3 ± 1.4 | 12.5 ± 1.2 |
| Range | 6.4–19.0 | 6.5–19.0 | 8.0–16.5 |
| n | 5114 | 374 | 425 |
| % below recommendation (<11 h)a | 2.1 | 30.7 | 8.0 |
| 36 Months | |||
| Mean ± SD | 12.6 ± 1.2 | 11.0 ± 1.5 | 11.8 ± 1.2 |
| Range | 8.5–20.0 | 6.5–20.0 | 7.5–15.0 |
| n | 4847 | 170 | 409 |
| % below recommendation (<10 h)a | 0.4 | 10.6 | 4.9 |
Children with unreliable estimates of sleep duration (<6 h of sleep at all ages and maximum sleep duration of 22, 21 and 24 h at the age of three months, six months and 24/36 months) were excluded from the analyses.
SD, standard deviation.
Guidelines from the American Academy of Pediatrics (AAP) recommend the following ranges of sleep duration: infants 4–12 months of age: 12–16 h per 24 h (including naps); children 1–2 y of age: 11–14 h per 24 h (including naps); children 3–5 y of age: 10–13 h per 24 h (including naps).
A time effect was present; total sleep time and nap duration decreased as children grew older, whereas night sleep increased with age (data not shown). An interaction effect of gestational age × time was present (B = 0.03; 95% CI: −0.00 to −0.06; p = 0.036). Individual time points indicated that children born after shorter gestational duration slept longer at ages three and six months, but not at ages 24 and 36 months (data not shown). However, no interaction was found when restricting the sample to term children, suggesting that the effect was driven mainly by preterm children.
Analyses in the GUSTO cohort showed that gestational age was not associated with total sleep duration (B = −0.07; 95% CI: −0.24 to 0.11; p = 0.46), night sleep duration (B = −0.07; 95% CI: −0.24 to −0.11; p = 0.46), or nap duration (B = 0.07; 95% CI: −0.39 to 0.53; p = 0.77).
In the MAVAN cohort, no significant associations were observed between gestational age and total sleep (B = −0.04; 95% CI: −0.15 to 0.07; p = 0.46), night sleep (B = 0.01; 95% CI: −0.07 to 0.10; p = 0.75), or nap duration (B = −0.05; 95% CI: −0.13 to 0.02; p = 0.13).
3.2. Meta-analytic pooling
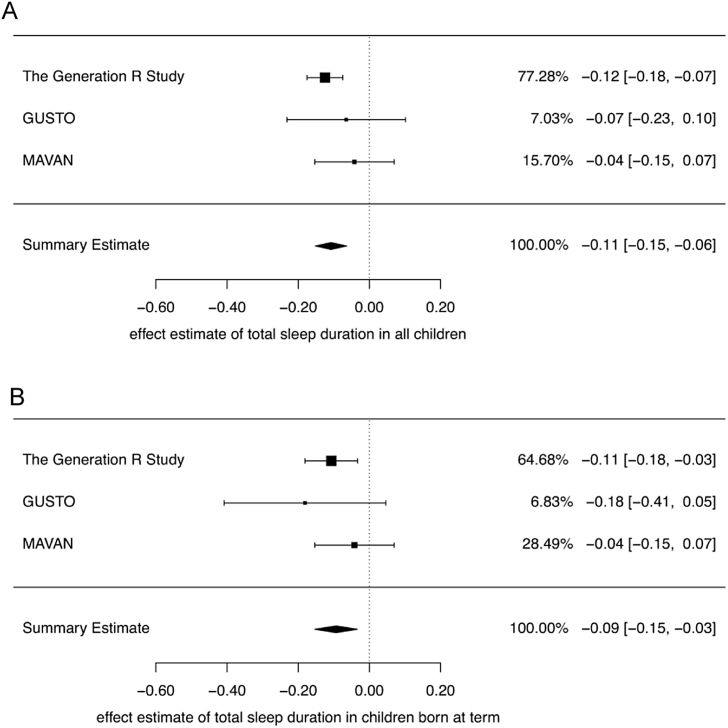
We performed meta-analyses to pool the effects for total sleep, night sleep, and nap duration across the three cohorts. Gestational age at birth was associated with total sleep duration (Bpooled = −0.11, 95% CI: −0.15 to −0.06), night sleep duration (Bpooled = −0.05, 95% CI: −0.08 to −0.02), and nap duration (Bpooled = −0.04, 95% CI: −0.06 to −0.01). When restricting the analyses to term children, the associations remained (Fig. 1).
Fig. 1.
Forest plot of effects of gestational age on sleep duration in early childhood in three population-based cohorts. (A) All children. (B) Term children only.
4. Discussion
Our study demonstrated that children born after shorter gestational duration slept longer than their term born peers. Moreover, the longer the gestational duration, the shorter children slept. The direction of effects aligned over three prospective cohort studies, even after controlling for confounders (such as birth weight) and also when tested in term born children only (37–42 weeks of gestation). Findings from the Generation R Study suggested that the association between gestational age and sleep duration is most prominent in the first year of life.
These results partly converge with those of earlier studies; some studies report shorter sleep 7, 8, some longer sleep 11, 12, and some no differences 9, 10. Most studies used clinical samples (children born at <32 weeks of gestation, low birth weight), and study samples rarely exceed 200 participants, limiting the power to detect small effects. No studies tested the effects of gestational duration in term born children only, or in children born post-term.
Our findings point to the importance of maturational processes during sleep, not only in premature children but also in children born at term after shorter gestational duration. In early childhood, sleep is a state involving maturation of the central nervous system [20], and children might progress on maturational processes, such as neural network connectivity, predominantly during sleep 4, 21. Our findings in post-term children in the Generation R Study point in the same direction: compared to their term born counterparts, children born post-term showed shorter sleep duration. Furthermore, results from the Generation R Study suggest that the effect of gestational age on sleep might be most pronounced in the first year of the child's life, particularly in preterm children. Catch-up in many developmental areas in the first year of life is common for children born preterm [3], and rapid catch-up has been suggested to predict better neurodevelopmental outcomes [22].
This study has multiple strengths. We were able to use repeated assessments from three large cohort studies from different continents, increasing power to find small effects, and increasing generalizability of findings to other populations. Differences in continents, ethnicities, and socio-economic backgrounds have been associated with differences in gestational age and sleep duration [18]. Indeed, in the current study, children from Singapore showed somewhat shorter sleep duration; parental beliefs about sleep may differ across cultures, which in turn may influence child sleep [14]. However, even when controlled for ethnic and demographic confounders, we found a small consistent effect across the cohorts.
Some study limitations should also be considered. Although the findings from the different cohorts align, significant findings were found only in one of the cohorts. The pooled effect size is small, which may limit clinical impact. With the current design, we were not able to assess whether differences in sleep duration may affect other developmental outcomes. Second, due to differences in local obstetrician policy and study design, we were able to study post-term birth only in the Generation R Study. Labour induction before 42 weeks of gestation has increased, but post-term births still occur (up to 5–10%) [1]. Third, the current study made use of caregiver reports of child sleep duration, and did not assess child sleep duration objectively. Ideally, sleep quantity, quality, and chronotype would be assessed with actigraphy or polysomnography [6].
5. Conclusion
In conclusion, meta-analytically pooled evidence from three international cohorts showed that children born after shorter gestational duration slept longer, even when they were born at term. Findings from the Generation R Study suggested catch-up sleep of preterm children in the first year of life. Further research is needed to determine how sleep duration and sleep quality early in life are related to neurodevelopmental outcomes, and which environmental, physiological, and (epi)genetic factors contribute to susceptibilities in child sleep architecture.
Funding
This work was supported by the Netherlands Organization for Health Research and Development [ZONMW Vici project 016.VICI.170.200], the European Union's Horizon 2020 research and innovation program [grant agreement No.633595 DynaHEALTH] and No.733206 LifeCycle].The Generation R Study is made possible by financial support from the Erasmus Medical Centre, the Erasmus University, and the Netherlands Organization for Health Research and Development.
The GUSTO cohort was supported by the Singapore National Research Foundation under its Translational and Clinical Research (TCR) Flagship Programme and administered by the Singapore Ministry of Health’s National Medical Research Council (NMRC), Singapore- NMRC/TCR/004-NUS/2008; NMRC/TCR/012-NUHS/2014. Additional funding was provided by the Singapore Institute for Clinical Sciences, Agency for Science, Technology and Research (A*STAR), Singapore.
Acknowledgements
The Generation R Study is conducted by the Erasmus Medical Centre in close collaboration with the School of Law and Faculty of Social Sciences of the Erasmus University Rotterdam, the Municipal Health Service Rotterdam area, the Rotterdam Homecare Foundation, and the Stichting Trombosedienst & Artsenlaboratorium Rijnmond (STAR), Rotterdam. We gratefully acknowledge the contribution of general practitioners, hospitals, midwives, and pharmacies in Rotterdam. We would like to thank the GUSTO study group and their participants. The GUSTO study group includes Allan Sheppard, Amutha Chinnadurai, Anne Eng Neo Goh, Anne Rifkin-Graboi, Anqi Qiu, Arijit Biswas, Bee Wah Lee, Birit F.P. Broekman, Boon Long Quah, Borys Shuter, Chai Kiat Chng, Cheryl Ngo, Choon Looi Bong, Christiani Jeyakumar Henry, Cornelia Yin Ing Chee, Yam Thiam Daniel Goh, Doris Fok, Fabian Yap, George Seow Heong Yeo, Helen Chen, Hugo P S van Bever, Iliana Magiati, Inez Bik Yun Wong, Ivy Yee-Man Lau, Jeevesh Kapur, Jenny L. Richmond, Jerry Kok Yen Chan, Joanna D. Holbrook, Joshua J. Gooley, Keith M. Godfrey, Kenneth Kwek, Kok Hian Tan, Krishnamoorthy Niduvaje, Leher Singh, Lin Lin Su, Lourdes Mary Daniel, Lynette P Shek, Marielle V. Fortier, Mark Hanson, Mary Foong-Fong Chong, Mary Rauff, Mei Chien Chua, Michael Meaney, Mya Thway Tint, Neerja Karnani, Ngee Lek, Oon Hoe Teoh, P. C. Wong, Peter D. Gluckman, Pratibha Agarwal, Rob M. van Dam, Salome A. Rebello, Seang-Mei Saw, Shang Chee Chong, Shirong Cai, Shu-E Soh, Sok Bee Lim, Chin-Ying Stephen Hsu, Victor Samuel Rajadurai, Walter Stunkel, Wee Meng Han, Wei Wei Pang, Yap-Seng Chong, Yin Bun Cheung, Yiong Huak Chan and Yung Seng Lee.
Footnotes
All authors have no conflict of interest to declare. There was no external grant or financial support for this study.
The ICMJE Uniform Disclosure Form for Potential Conflicts of Interest associated with this article can be viewed by clicking on the following link: https://doi.org/10.1016/j.sleepx.2019.100002.
Conflict of interest
The following is the supplementary data to this article:
Multimedia component 1
References
- 1.Seikku L., Gissler M., Andersson S. Asphyxia, neurologic morbidity, and perinatal mortality in early-term and postterm birth. Pediatrics. 2016;136 doi: 10.1542/peds.2015-3334. [DOI] [PubMed] [Google Scholar]
- 2.Brumbaugh J.E., Conrad A.L., Lee J.K. Altered brain function, structure, and developmental trajectory in children born late preterm. Pediatr Res. 2016;80:197–203. doi: 10.1038/pr.2016.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saigal S., Doyle L.W. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet. 2008;371:261–269. doi: 10.1016/S0140-6736(08)60136-1. [DOI] [PubMed] [Google Scholar]
- 4.Kurth S., Dean D.C., Achermann P. Increased sleep depth in developing neural networks: New insights from sleep restriction in children. Front Hum Neurosci. 2016;10:456. doi: 10.3389/fnhum.2016.00456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scher M.S., Johnson M.W., Ludington S.M., Loparo K. Physiologic brain dysmaturity in late preterm infants. Pediatr Res. 2011;70:524–528. doi: 10.1203/PDR.0b013e31822f24af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bennet L., Walker D.W., Horne R.S.C. Waking up too early – the consequences of preterm birth on sleep development. J Physiol. 2018;596:5687–5708. doi: 10.1113/JP274950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biggs S.N., Meltzer L.J., Tapia I.E. Sleep/wake patterns and parental perceptions of sleep in children born preterm. J Clin Sleep Med. 2016;12:711–717. doi: 10.5664/jcsm.5802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asaka Y., Takada S. Activity-based assessment of the sleep behaviors of VLBW preterm infants and full-term infants at around 12 months of age. Brain Dev. 2010;32:150–155. doi: 10.1016/j.braindev.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 9.Bilgin A., Wolke D. Regulatory problems in very preterm and full-term infants over the first 18 months. J Dev Behav Pediatr. 2016;37:298–305. doi: 10.1097/DBP.0000000000000297. [DOI] [PubMed] [Google Scholar]
- 10.Perkinson-Gloor N., Hagmann-von Arx P., Brand S. The role of sleep and the hypothalamic-pituitary-adrenal axis for behavioral and emotional problems in very preterm children during middle childhood. J Psychiatr Res. 2015;60:141–147. doi: 10.1016/j.jpsychires.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 11.Huang Y.-S., Paiva T., Hsu J.-F., Kuo M.-C., Guilleminault C. Sleep and breathing in premature infants at 6 months post-natal age. BMC Pediatr. 2014;14:303. doi: 10.1186/s12887-014-0303-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stangenes K.M., Fevang S.K., Grundt J. Children born extremely preterm had different sleeping habits at 11 years of age and more childhood sleep problems than term-born children. Acta Paediatr Int J Paediatr. 2017;106:1966–1972. doi: 10.1111/apa.13991. [DOI] [PubMed] [Google Scholar]
- 13.Spruyt K. A review of developmental consequences of poor sleep in childhood. Sleep Med. 2018 doi: 10.1016/j.sleep.2018.11.021. S1389-9457(18)30482-30489. [DOI] [PubMed] [Google Scholar]
- 14.Mindell J.A., Sadeh A., Wiegand B., How T.H., Goh D.Y. Cross-cultural differences in infant and toddler sleep. Sleep Med. 2010;11:274–280. doi: 10.1016/j.sleep.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 15.Kooijman M.N., Kruithof C.J., van Duijn C.M. The generation R Study: design and cohort update 2017. Eur J Epidemiol. 2016;31:1243–1264. doi: 10.1007/s10654-016-0224-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soh S.E., Saw S.M., Soh S.E. Cohort profile: Growing up in Singapore towards healthy outcomes (GUSTO) birth cohort study. Int J Epidemiol. 2014;43:1401–1409. doi: 10.1093/ije/dyt125. [DOI] [PubMed] [Google Scholar]
- 17.O’Donnell K.A., Gaudreau H., Colalillo S. The maternal adversity, vulnerability and neurodevelopment project: Theory and methodology. Can J Psychiatry. 2014;59:497–508. doi: 10.1177/070674371405900906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goh S.K.Y., Tham E.K.H., Goh D.Y.T. Infant night sleep trajectory from age 3–24 months: evidence from the Singapore GUSTO study. Sleep Med. 2017;33:82–84. doi: 10.1016/j.sleep.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 19.Sadeh A. A brief screening questionnaire for infant sleep problems: Validation and findings for an internet sample. Pediatrics. 2004;113:e570–e577. doi: 10.1542/peds.113.6.e570. [DOI] [PubMed] [Google Scholar]
- 20.Kocevska D., Verhoeff M.E., Meinderts S. Prenatal and early postnatal measures of brain development and childhood sleep patterns. Pediatr Res. 2018;83:760–766. doi: 10.1038/pr.2017.318. [DOI] [PubMed] [Google Scholar]
- 21.Sadeh A., Dark I., Vohr B.R. Newborns’ sleep-wake patterns: The role of maternal, delivery and infant factors. Early Hum Dev. 1996;44:113–126. doi: 10.1016/0378-3782(95)01698-8. [DOI] [PubMed] [Google Scholar]
- 22.Belfort M.B., Rifas-Shiman S.L., Sullivan T. Infant growth before and after term: effects on neurodevelopment in preterm infants. Pediatrics. 2011;128:e899–e906. doi: 10.1542/peds.2011-0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Multimedia component 1